(C) 2013 Canella Radea. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Radea C, Parmakelis A, Papadogiannis V, Charou D, Triantis KA (2013) The hydrobioid freshwater gastropods (Caenogastropoda, Truncatelloidea) of Greece: new records, taxonomic re-assessments using DNA sequence data and an update of the IUCN Red List Categories. ZooKeys 350: 1–20. doi: 10.3897/zookeys.350.6001
Hydrobioid freshwater gastropods were collected from mainland and insular Greece. Several threatened taxa, such as Graecoanatolica vegorriticola, Pseudamnicola negropontina, Pseudamnicola pieperi, Pseudobithynia eubooensis and Pseudoislamia balcanica, were recorded from new localities. Trichonia trichonica, which has been considered extinct from its type locality for the last twenty eight years, was re-discovered, whereas the presence of Daphniola exigua, G. vegorriticola, Marstoniopsis graeca, P. pieperi and Pseudobithynia trichonis in their type localities was verified. The taxonomic status of P. negropontina and the newly discovered populations of G. vegorriticola was elucidated using COI sequence data. The new data recorded during this survey indicate that the IUCN status of some Greek endemic hydrobioids needs to be updated.
Hydrobioids, Rissooidea, Truncatelloidea, Gastropoda, Greece, freshwater, taxonomy, IUCN status, conservation
Hydrobioid gastropods include the family Hydrobiidae Troschel, 1857 and several other families of Rissooidea s.l. that resemble Hydrobiidae in general features (
Greek freshwater ecosystems are widely recognized as hotspots of European freshwater biodiversity (e.g.
Almost all described Greek hydrobioid species are included in the IUCN Red List of Threatened Species (2012) and 55% of them have been classified as threatened. Graecoanatolica macedonica Radoman & Stankovic, 1978 is characterized as Extinct, and is followed by twenty four species that have been classified as Critically Endangered, five as Endangered, nine as Vulnerable, three as Near Threatened, five as Least Concern and twenty two as Data Deficient.
During 2012, several localities across Greece were sampled by the authors for hydrobioid freshwater gastropods. The sampling took place following the goals of the research project “Species on the brink of extinction” that was funded by the public benefit foundation “John S. Latsis”. The goals of this project was a) to assess and evaluate the population status of 10 freshwater snails species of Greece (9 endemics) which, according to the recent report from the International Union for Conservation of Nature (IUCN), are classified as either Extinct or Critically Endangered, b) to evaluate the status of the wetlands these species are present in and to assess the main anthropogenic regime of threats. In the network of localities sampled for the purposes of the project, we added several more localities hosting water bodies that are frequently reported in the freshwater literature of Greece or are in the vicinity of the primarily targeted localities. Therefore, we do not consider our fieldwork to be exhaustive; rather it is focused on freshwater localities that have been searched before, are frequently reported in the literature, as hosting (or having hosted) threatened species, and we complemented these localities with surrounding ones that could have served as the refuges of the threatened species.
Some of the findings of this survey as well as suggestions for the IUCN status update of some hydrobioids collected, are presented and discussed herein. Additionally, using COI sequence data generated from some specimens, we aimed to elucidate the taxonomic status of:
(i) The Greek endemic taxon of the genus Pseudamnicola Paulucci, 1878, namely Pseudamnicola (Pseudamnicola) macrostoma negropontina (Clessin, 1878). Based on the slight morphological and anatomical differences between Pseudamnicola (Pseudamnicola) macrostomanegropontina and Pseudamnicola (Pseudamnicola) macrostoma macrostoma (Küster, 1853),
(ii) Two new populations of the genus Graecoanatolica Radoman, 1973, which were recorded in Sterea Ellada (Voiotia). The morphological and anatomical studies were not conclusive in assigning these populations to the known Greek Graeconatolica species, Graeconatolicavegorriticola (Schütt, 1962) and Graecoanatolica macedonica, or even to a new species of Graecoanatolica. Therefore, COI sequence data were used to compare the Graecoanatolica vegorriticola specimens collected from the type locality of the species, with those of the newly located southern populations.
Overall, the current study offers new distributional data and, in the light of these, is evaluating the conservation status of some hydrobioid species of Greece. Furthermore, using newly generated COI sequence data, we are resolving taxonomic uncertainties that could not be elucidated based on morphology alone.
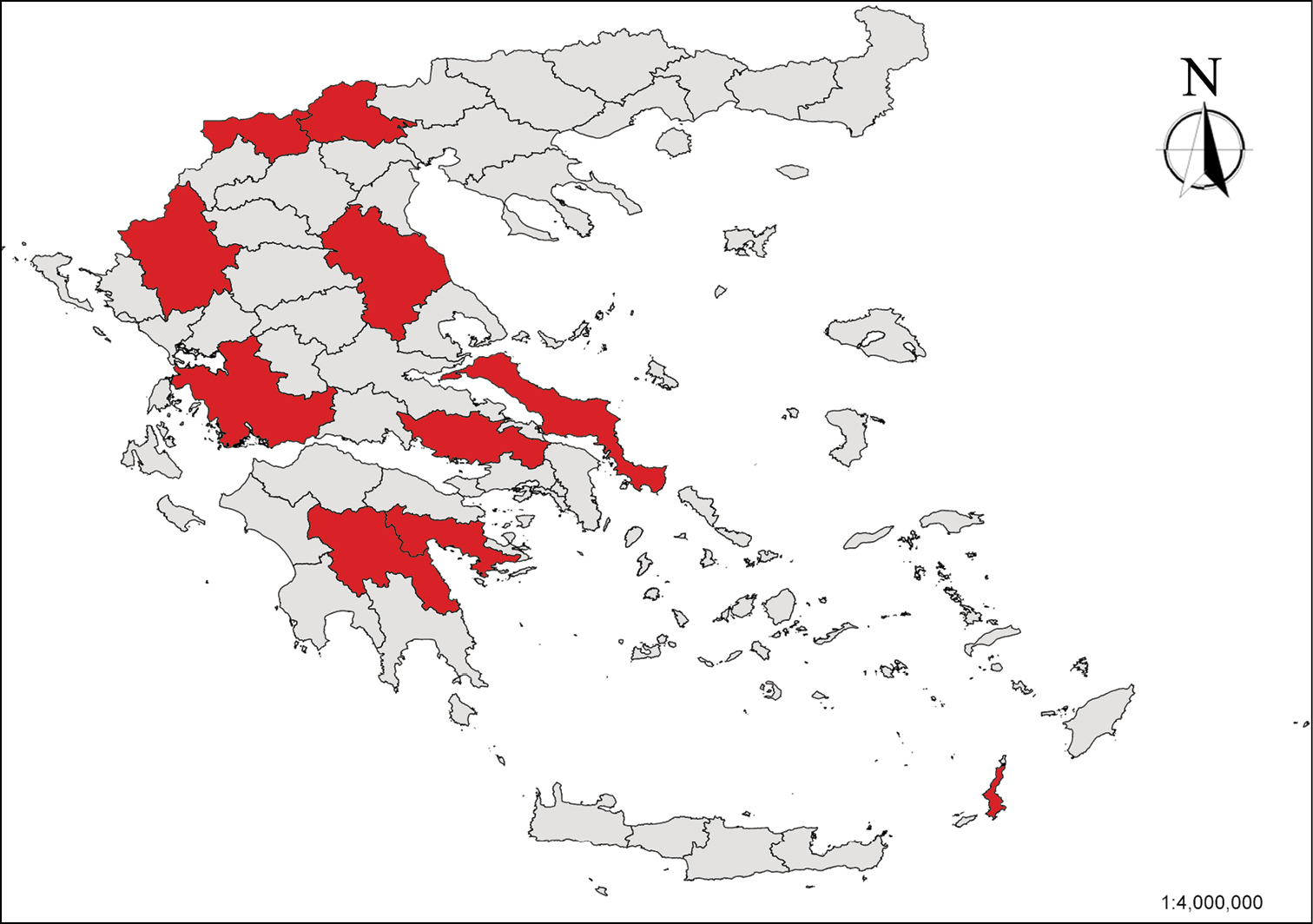
The freshwater localities of almost all the threatened hydrobioids of Greece were carefully sampled (Fig. 1). The snails were hand collected from stones, gravel and dead leaves (Fig. 2). Specimens were placed into vials filled with water and were transported alive to the laboratory. A portion of the specimens collected from each locality was stored in -20°C for molecular analysis, whereas others were preserved un-relaxed in 70% ethanol for morphological and anatomical studies.
Map showing the administrative units of Greece where “hydrobioid” localities were sampled during the fieldwork of this study.
Sampling localities. A Ag. Sophia, Aitoloakarnania B Olympos, Karpathos C Stoupaioi, Evvoia D Peraia, Lake Vegorritis E Megali Vrysi, Argolida. The arrows point the exact site where the specimens were found.
Shell characters (shell height and width, apertural height and width) were measured using the micrometer of a stereomicroscope (Stemi 2000-C, Zeiss). Digital pictures were taken using a Canon EOS 1000D camera that was attached to the stereomicroscope. During this procedure, the specimens were submerged into water in order to avoid the malformation of important taxonomic features that can be caused by the long-term tissue preservation buffers.
Details of the specimens used in the molecular analyses are provided in Table 1. The entire individuals were used for total genomic DNA isolation. In total, we extracted DNA from one specimen of Pseudamnicola macrostoma macrostoma and sixteen specimens of Graecoanatolica from Pella and Voiotia.
Details on the origin of the specimens used in the COI sequence divergence analyses.
| Species | Number of specimens | Nomos | Exact locality | Habitat | Latitude, Longitude | Sample code | COI Accession Numbers |
|---|---|---|---|---|---|---|---|
| Graecoanatolica vegorriticola | 6 | Voiotia | Orchomenos, Pigi Chariton | Spring | 38°29'41"N, 22°58'23"E | GraVeg_Cha | KF758767-72 |
| Graecoanatolica vegorriticola | 2 | Voiotia | Livadia, Spring of Krya | Spring | 38°25'49"N, 22°52'22"E | GraVeg_Kr1 | KF758773-74 |
| Graecoanatolica vegorriticola | 8 | Pella | Pella, Vegorritis Lake | Lake | 40°44'38"N, 21°49'07"E | GraVeg_Veg | KF758775-82 |
| Pseudamnicola macrostoma macrostoma | 1 | Attiki | Marathon, Kato Souli | Stream | 38°09'28"N, 24°00'19"E | PsdMac_KaSou | KF758783 |
| Pseudamnicola macrostoma negropontina | 1 | Evvoia | Marmaris | - | - | EF061915 ( |
† According to the GenBank registration records the locality of origin of EF061915 is Kato Souli. However, this must be an erroneous record since in the reference (
DNA was extracted using the Purelink Genomic DNA mini kit (Invitrogen) following the manufacturers’ protocol. A fragment of the mitochondrial gene cytochrome oxidase subunit I (COI) was amplified from each specimen using the universal primers LCO1490 and HCO2198 (
The primers in the sequencing reactions were the same as in the PCR amplifications. Sequences generated for this study were deposited in GenBank under the accession numbers provided in Table 1.
The newly generated sequences were viewed and edited using CodonCode Aligner v. 2.06 (Genecodes Corporation). The authenticity of the mtDNA sequences and the homology with the targeted mitochondrial gene were evaluated by a BLAST search in the NCBI genetic database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). All sequences alignments were performed with CodonCode Aligner v. 2.06 by implementing the Clustal algorithm.
For deciphering whether the two Pseudamnicola taxa represent two different species or if they are subspecies of a single species, we estimated the sequence divergence separating them by using the Kimura 2-parameter (K-2p) model (
In the case of the newly discovered Graeconatolica populations from southern Greece, based on the K-2p model, we estimated the COI sequence divergence of these specimens from the Graecoanatolica vegorriticola specimens sampled from the type locality of the species.
An alphabetical sequence of families, genera and species applies to the hydrobioid list below. Abbreviations used: Nom.= Nomos (administrative unit).
Nom. PELLAS: Lake Vegorritis, stony bank close to Peraia, ca 515 m asl, 40°44'38"N, 21°49'07"E, 15.xi.2012, Radea and Parmakelis.
Marstoniopsis graeca was described from Lake Vegorritis as Parabythinella graeca Radoman, 1978 and, according to
Nom. EVVOIAS: pool with Nasturtium sp. and Platanus orientalis, 3.8 km NE of Paradeisi to Ag. Dimitrios, ca 335 m asl, 38°05'26"N, 24°23'57"E, 25.xi.2012, Radea and Constantinidis; spring close to the road towards Ag. Dimitrios beach, ca 120 m asl, 38°08'26"N, 24°27'01"E, 25.xi.2012, Radea and Constantinidis; spring close to the road from Ag. Dimitrios to Kalianoi, ca 295 m asl, 38°07'21"N, 24°26'30"E, 25.xi.2012, Radea and Constantinidis; cistern, ca 0.7 km NNW of Kalianoi, ca 205 m asl, 38°07'22"N, 24°29'26"E, 26.xi.2012, Radea and Constantinidis; spring in Myloi village, ca 205 m asl, 38°01'56"N, 24°26'09"E, 26.xi.2012, Radea and Constantinidis; spring close to Stoupaioi, ca 255 m asl, 38°07'21"N, 24°18'51"E, 26.xi.2012, Radea and Constantinidis; stream with Nasturtium sp. and Helosciadium sp., on coastal flats 3.2 km NW of Marmari, ca 0 m asl, 38°04'16"N, 24°18'06"E, 26.xi.2012, Radea and Constantinidis.
Nom. VOIOTIAS: Krya spring in Livadia, ca 240 m asl, 38°25'49"N, 22°52'22"E, 17.iv.2012, Radea and Constantinidis; spring Pigi Chariton close to Orchomenos, ca 170 m asl, 38°29'41"N, 22°58'23"E, 17.iv.2012, Radea and Constantinidis.
The populations of Bythinella found in Evvoia and Voiotia have morphological and anatomical similarity to Bythinella charpentieri, which is the only known species of the genus inhabiting Attiki, Evvoia and Parnassos Mt. (
http://species-id.net/wiki/Pseudobithynia_euboeensis
Figure 3Nom. EVVOIAS: stream with dense vegetation composed mainly by Nasturtium sp. and Helosciadium sp., on coastal flats 3.2 km NW of Marmari, ca 0 m asl, 38°04'16"N, 24°18'06"E, 26.xi.2012, Radea and Constantinidis.
Pseudobithynia euboeensis was collected in 1985 for the first time and it was described by
So far, the species was known only from its type locality. The new locality is probably close to the type locality and is likely influenced by touristic activity during summer. Several specimens of Pseudobithynia euboeensis were found either on plant material or under stones.
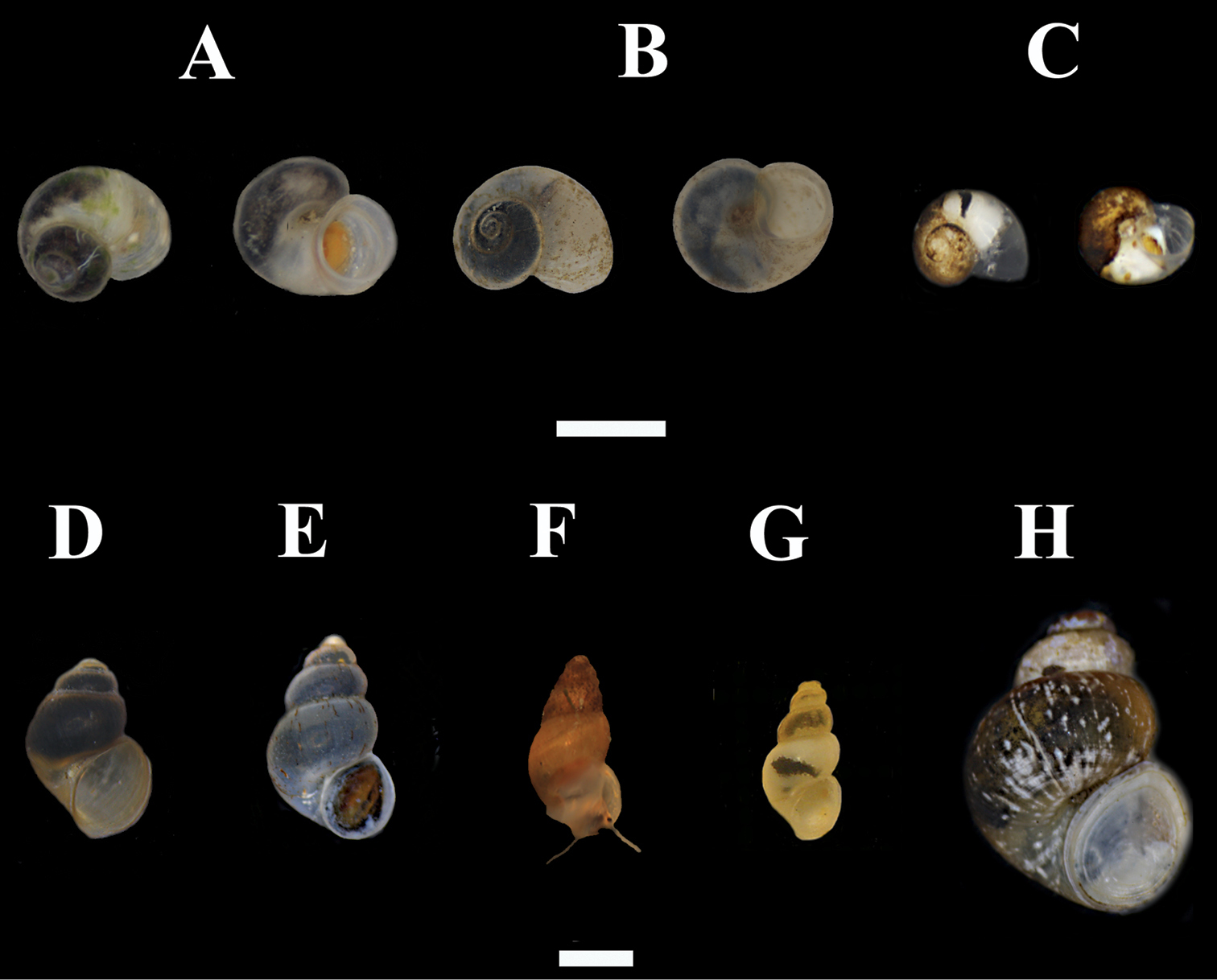
Hydrobioids collectedduring the survey in mainland and insular Greece. A Daphniola exigua (dorsal and ventral view) B Isimerope semele (dorsal and ventral view, Megali Vrysi) C Pseudoislamia balcanica (dorsal and ventral view, Ag. Sophia) D Pseudamnicola pieperi (Olympos) E Radomaniola cf. curta (spring of Louros river) F Radomaniola cf. curta (Ag. Sophia spring) G Trichonia trichonica H Pseudobithynia eubooensis. Scale bar 1 mm.
Nom. AITOLOAKARNANIAS: Lake Trichonis, NE rocky and stony shore close to Loutra Myrtias, ca 15 m asl, 38°33'34"N, 21°37'33"E, 09.iii.2012, Radea, Charou, Papadogiannis, Parmakelis.
The species was described by
A large population of Daphniola exigua was found in Ag. Paraskevi spring, Tempi valley, Nom. Larissas where anthropogenic activity is high. Ag. Paraskevi spring is one of the two known localities where the species is distributed (
http://species-id.net/wiki/Graecoanatolica_vegorriticola
Figure 4Nom. VOIOTIAS: Krya spring in Livadia, ca 240 m asl, 38°25'49"N, 22°52'22"E, 17.iv.2012, Radea and Constantinidis; spring Pigi Chariton close to Orchomenos, ca 170 m asl, 38°29'41"N, 22°58'23"E, 17.iv.2012, Radea and Constantinidis.
Nom. PELLAS: Lake Vegorritis, stony bank close to Peraia, ca 515 m asl, 40°44'38"N, 21°49'07"E, 15.xi.2012, Radea and Parmakelis.
Graecoanatolica vegorriticola was initially described as Hydrobia vegorriticola by
According to
We found a low abundance population of Graecoanatolica vegorriticola on the banks of Lake Vegorritis south of Arnissa town. Furthermore, two high abundance populationsof Graecoanatolica cf. vegorriticola with many mature individuals were discovered in Livadia and Orchomenos, Central Greece, ca 270 km away from the type locality of Graecoanatolica vegorriticola.
COI sequence data generated from specimens that have been collected from Lake Vegorritis, Livadia and Orchomenos were used to elucidate the taxonomic status of the populations inhabiting the latter two localities. In total, we obtained eight COI sequences of Graecoanatolica vegorriticola specimens from Lake Vegorritis, two sequences of Graecoanatolica from Livadia and six sequences from Orchomenos (Table 1). The COI sequence divergence separating specimens from these populations and those of Graecoanatolica vegorriticola from Lake Vegorritis is 1.7%. The two newly discovered localities are heavily influenced by tourism and agriculture.
During the field survey, we devoted significant effort in finding and collecting alive specimens of the other Balkan species of this genus, Graecoanatolica macedonica, in the Greek part of Lake Dojran. Unfortunately, only empty shells (some of the specimens looking to have recently died) were retrieved.
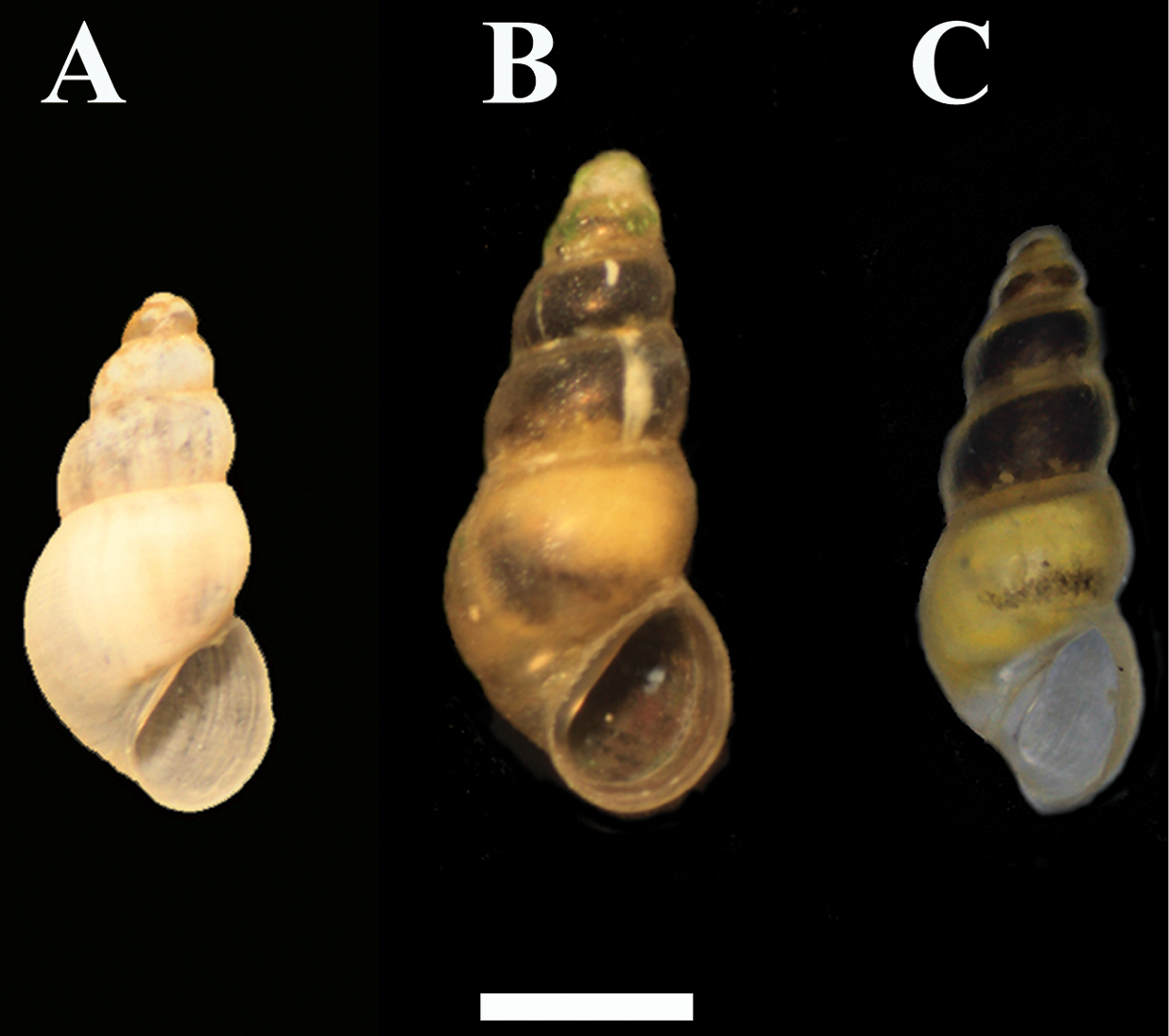
Graecoanatolica vegorriticola A Krya spring, Voiotia B Pigi Chariton, Voiotia C Lake Vegorritis, Pella. Scale bar 1 mm.
Isimerope semele was found in three localities of Peloponnisos, two in Nom. ARGOLIDAS (Megali Vrysi and “Second Spring”) and one in Nom. ARKADIAS (Elissonas River, Piana) (
http://species-id.net/wiki/Pseudamnicola_negropontina
Figure 5Nom. EVVOIAS: stream with dense vegetation composed mainly by Nasturtium sp. and Helosciadium sp., on coastal flats 3.2 km NW of Marmari, ca 0 m asl, 38°04'16"N, 24°18'06"E, 26.xi.2012, Radea and Constantinidis.
According to
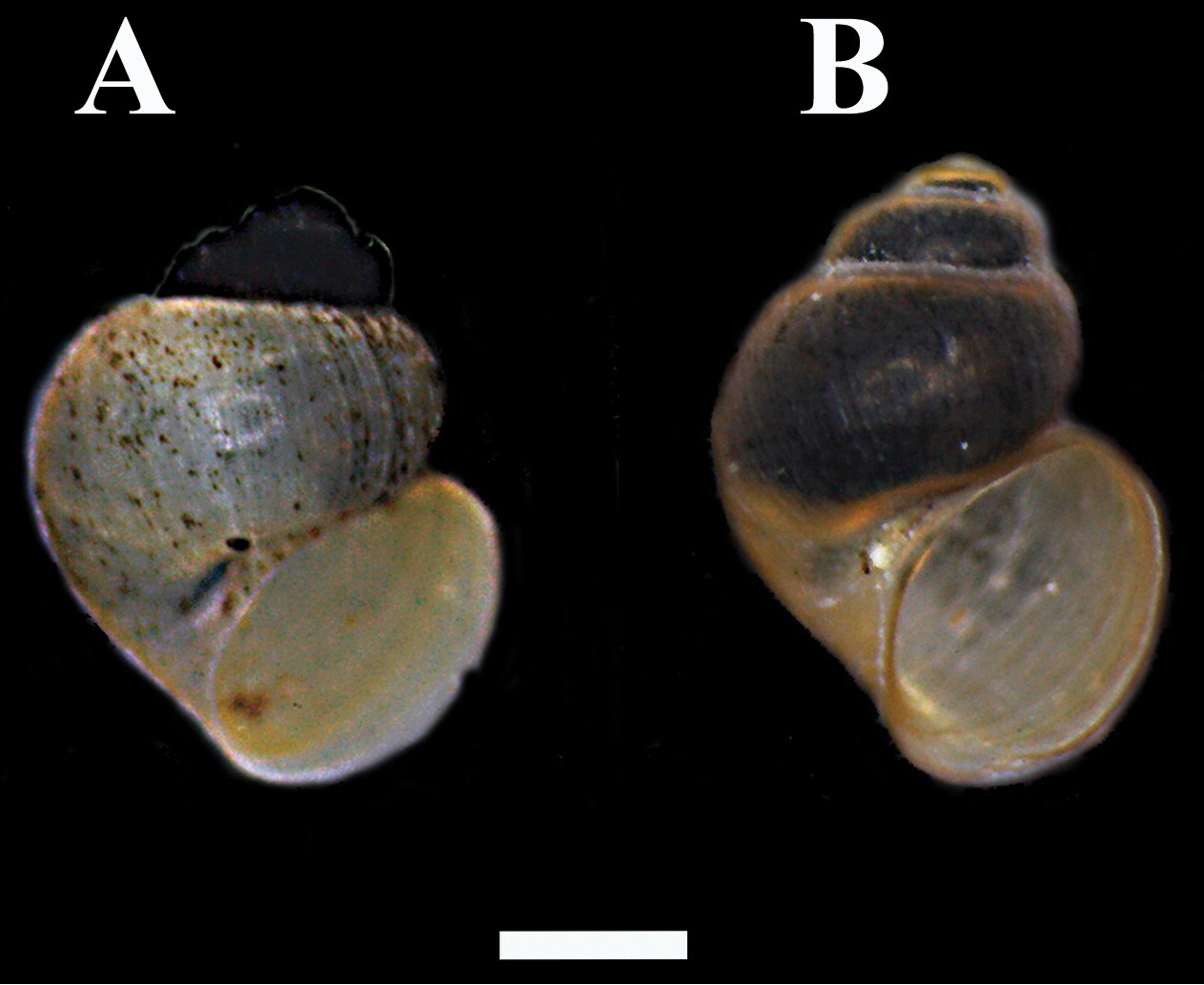
The latter authors consider Pseudamnicola macrostoma negropontina as a distinct species, Pseudamnicola negropontina. The molecular analysis that we performed after finding fresh specimens of Pseudamnicola macrostoma in Kato Souli, Attiki, showed that the COI sequence divergence separating the two taxa is 5.8% (K-2p model).
A Pseudamnicola negropontina B Pseudamnicola macrostoma. The first whorls of the shells of Pseudamnicola negropontina wereheavily encrusted with epibionts. Scale bar 1 mm.
http://species-id.net/wiki/Pseudamnicola_pieperi
Figure 3KARPATHOS island: spring close to Olympos, ca 260 m asl, 35°44'00"N, 27°10'01"E, 30.xi.2012, Radea, Bazos, Contantinidis; spring close to Prasteio, ca 220 m asl, 35°43'01"N, 27°11'00"E, 01.xii.2012, Radea, Bazos, Contantinidis; spring, Vananta, ca 0 m asl, 35°46'00"N, 27°12'01"E, 02.xii.2012, Radea, Bazos, Contantinidis; spring close to Spoa, ca 295 m asl, 35°38'01"N, 27°08'01"E, 03.xii.2012, Radea, Bazos, Contantinidis; spring close to Pyles, ca 305 m asl, 35°31'01"N, 27°07'01"E, 03.xii.2012, Radea, Bazos, Contantinidis; stream crossing the secondary road leading to Ag. Nikolaos temple, ca 740 m asl, 35°34'36"N, 27°09'39"E, 15.iv.2013, Radea and Constantinidis; discharge from the pumps of a water intake built on the spring close to Ag. Nikolaos temple, ca 705 m asl, 35°34'26"N, 27°09'57"E 15.iv.2013, Radea and Constantinidis.
This species was collected for the first time by Pieper in 1977 from Aperi in Karpathos Island and described later by
In 2012, we found several specimens of Pseudamnicola pieperi in the type locality. The seven new localities, where the species was found, are located in the central and northern part of the island. In the majority of the new localities, the population abundance was medium to high.
Nom. AITOLOAKARNANIAS: Lake Trichonis, N shore close to Dougri, ca 15 m asl, 38°36'01"N, 21°34'10"E, two specimens on leaves and stems of Myriophyllum sp., depth 2–4 m, 09.iii.2012, Radea, Charou, Papadogiannis, Parmakelis; spring close to Ag. Sophia, 3 km NW from Thermos, ca 305 m asl, 38°34'59"N, 21°38'56"E, three mature and some immature specimens on stones, 10.iii.2012, Radea, Charou, Papadogiannis, Parmakelis.
Pseudoislamia balcanica is an endemic species previously known only from its type locality, the NE rocky banks of Lake Trichonis near Myrtia (
Figure 3
Nom. AITOLOAKARNANIAS: Spring close to Ag. Sophia, 3 km NW from Thermos, ca 305 m asl, 38°34'59"N, 21°38'56"E, 10.iii.2012, Radea, Charou, Papadogiannis, Parmakelis. Nom. IOANNINON: Lake Toumpa, ca 650 m asl, 39°43'31"N, 20°44'53"E, 29.iv.2012, Parmakelis and Triantis; springs of Louros river, ca 285 m asl, 39°25'56"N, 20°50'25"E, 30.iv.2012, Parmakelis and Triantis; spring in Chani Terovo, ca 205 m asl, 39°23'48"N, 20°50'54"E, 30.iv.2012, Parmakelis and Triantis; springs in Ag. Georgios, ca 105 m asl, 39°16'09"N, 20°51'01"E, 30.iv.2012, Parmakelis and Triantis.
Radomaniola was proposed by
Recently,
Despite the fact that our sampling localities (Fig. 1) are situated very close to those of
Nom. AITOLOAKARNANIAS: Lake Trichonis, N shore close to Dougri, ca 15 m asl, 38°36'01"N, 21°34'10"E, three specimens on leaves and stems of Myriophyllum sp. and Potamogeton sp., depth 2-4 m, 09.iii.2012, Radea, Charou, Papadogiannis, Parmakelis.
Trichonia trichonica was described by
Among other findings of this study, we found Graecoanatolica vegorriticola in two new localities quite distant from all the known localities of this taxon. In our effort to properly assign these populations to the species they belong to, we realized that the morphological and anatomical studies were not conclusive. To overcome this issue, COI sequence data were used to compare the Graecoanatolica vegorriticola specimens collected from the type locality of the species, with those of the newly located southern populations. The level of COI sequence divergence (1.7%) between the population from the type locality and those from the new localities is well within the range separating conspecific populations of Hydrobiidae genera, e.g. Pyrgulopsis 0–3.44% (
Due to insufficient morphological and anatomical differentiation, the nominal subspecies Pseudamnicola macrostoma macrostoma (Küster, 1853) and Pseudamnicola macrostoma negropontina cannot be discriminated. However,
During the field survey undertaken for this study, several threatened taxa, such as Graecoanatolica vegorriticola, Pseudamnicola negropontina, Pseudamnicola pieperi, Pseudobithynia eubooensis and Pseudoislamia balcanica, were recorded from new localities. Trichonia trichonica, which has been considered extinct from its type locality for the last twenty eight years (
Graecoanatolica vegorriticola. Our findings indicate that none of the criteria of the category Critically Endangered are met since the extent of occurrence (EOO) and the area of occupancy (AOO) become >100 km2 and >10 km2, respectively. Therefore, this species may be down-listed to the category Endangered if the criteria of the category Critically Endangered continue to not be met for the next five years. Additionally, (a) and b(iii) are met because the number of locations is ≤ 5 and a continuing decline is observed in the quality of the habitat, respectively; c(iv) is also met because extreme fluctuation in the number of mature individuals has been recorded (
Scale of endemism of the hydrobioids collected and the suggested transfers between IUCN Red List Categories (with bold our sampling localities).
| Scale of Endemism | IUCN Red List Category (2012) ver. 3.1 | Transfers | |
|---|---|---|---|
| Family Amnicolidae | |||
| Marstoniopsis graeca | EPELLA (LAKE VEGORRITIS) | Critically Endangered B1ab(i, iii) | - |
| Family Bythinellidae | |||
| Bythinella charpentieri, Bythinella cf. charpentieri | EATTIKI+EVVOIA+PARNASSOS Mt. EVVOIA+VOIOTIA |
Least Concern | - |
| Family Bithyniidae | |||
| Pseudobithynia euboeensis | EEVVOIA (SPRING) | Critically Endangered B2ab(iii) | - |
| Pseudobithynia trichonis | EAITOLOAKARNANIA (LAKE TRICHONIS+LAKE LYSIMACHEIA) | Endangered B1ab(iii) | - |
| Family Hydrobiidae | |||
| Daphniola exigua | ETHESSALIA (AG. PARASKEVI SPRING+DAPHNI SPRING) | Endangered B2ab(iii) | - |
| Graecoanatolica vegorriticola | EPELLA (LAKE VEGORRITIS+LAKE PETRON)+VOIOTIA (SPRINGS) | Critically Endangered B1ab(i, iii, iv)c(iv)+2ab(i, iii, iv)c(iv) | Endangered B1ab(iii)c(iv) |
| Isimerope semele | EARGOLIDA (SPRINGS)+ARKADIA (RIVER+SPRING) | Not Evaluated | Endangered B1ab(iii) |
| Pseudamnicola negropontina | EEVVOIA (SPRING) | Not Evaluated | Critically Endangered B2ab(ii, iii) |
| Pseudamnicola pieperi | EKARPATHOS (SPRINGS) | Vulnerable D2 | - |
| Pseudoislamia balcanica | EAITOLOAKARNANIA (LAKE TRICHONIS+SPRING) | Critically Endangered B1ab(iii) | Endangered B1ab(iii) |
| Radomaniola curta, Radomaniola cf. curta | EALBANIA+GREECE AITOLOAKARNANIA+IOANNINA |
Least Concern | - |
| Trichonia trichonica | EAITOLOAKARNANIA (LAKE TRICHONIS) | Critically Endangered B2ab(i, iii) | Critically Endangered B1ab(iii) |
Pseudoislamia balcanica. The discovery of this species in a new locality increases the extent of its occurrence (EOO), which becomes >100 km2. Therefore, Pseudoislamia balcanica may be down- listed to the category Endangered if it continues to thrive in other locality(ies), apart from Lake Trichonis, at least for the next five years. Additionally, (a) and b(iii) are met because the number of locations is ≤ 5 and a continuing decline is observed in the quality of the habitat, respectively.
Trichonia trichonica. We re-discovered this species in Lake Trichonis. On the contrary, no single specimen or even empty shells were recorded in Krya’s spring (Lake Pamvotis) due to the water intake built on it. Therefore, the criterion B1, the extent of occurrence (EOO) <100 km2, is met for Trichonia trichonica. Additionally, (a) and b(iii) are met because the number of locations is 1 and a continuing decline is observed in the quality of the habitat, respectively.
Pseudamnicola negropontina has not yet been evaluated because it is now elevated to species level (newly split). The area of occupancy of this species is < 10 km2 and, consequently, the criterion B2 is met. Additionally, (a) and b(ii, iii) are met because the number of locations is 1 and a continuing decline is observed in the area of occupancy and the quality of the habitat, respectively.
Isimerope semele has not yet been evaluated because it is a newly described species (newly described). The extent of occurrence of this species is 100 km2<EOO<5000 km2 and therefore the criterion B1 for the category Endangered is met. Additionally, (a) and b(iii) are met because the current distribution of the species is severely fragmented and a continuing decline is observed in the quality of the habitat, respectively.
During the field survey we ascertained that many of the “hydrobioid” localities in Greece are heavily influenced by various human activities such as tourism, agriculture, livestock, industry, housing development and forestry. Thus, a decline or even loss of local freshwater gastropods is expected and, in some cases, it has already been reported (
Against the loss of hydrobioids due to the declining number of suitable habitats, a taxonomically accurate record of taxa, especially for those thriving in springs and spring brooks (crenobionts), will contribute significantly in assigning high conservation priorities (
This project was funded by the “John S. Latsis” Public Benefit Foundation. We thank Th. Constantinidis and I. Bazos for their help in the fieldwork and A. Oikonomou for comments on a previous version of the manuscript.