(C) 2013 Anna Emilia Hjalmarsson. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Hjalmarsson AE, Bukontaite R, Ranarilalatiana T, Randriamihaja JH, Bergsten J (2013) Taxonomic revision of Madagascan Rhantus (Coleoptera, Dytiscidae, Colymbetinae) with an emphasis on Manjakatompo as a conservation priority. ZooKeys 350: 21–45. doi: 10.3897/zookeys.350.6127
We review the diving-beetle genus Rhantus Dejean of Madagascar (Coleoptera, Dytiscidae, Colymbetinae) based on museum collection holdings and recently collected expedition material. Both morphology and DNA is used to test species boundaries, in particular whether newly collected material from the Tsaratanana mountains in the north represent a new species or are conspecific with Rhantus manjakatompo Pederzani and Rocchi 2009, described based on a single male specimen from the central Ankaratra mountains. DNA of the holotype of R. manjakatompo was successfully extracted in a non-destructive way and sequenced. The general mixed Yule coalescent model applied to an ultrametric tree constructed from mitochondrial cytochrome c oxidase subunit I (COI) sequence data delimited three species. Morphological characters supported the same species unambiguously. We therefore recognise three species of Rhantus to occur in Madagascar: R. latus (Fairmaire, 1869), R. bouvieri Régimbart, 1900 and R. manjakatompo Pederzani and Rocchi, 2009. All three species are endemic to Madagascar and restricted to the highlands of the island. Rhantusstenonychus Régimbart, 1895, syn. n., is considered a junior synonym of R. latus. We designate lectotypes for R. bouvieri and R. goudoti Sharp, 1882, the latter a junior synonym of R. latus. We provide descriptions, a determination key, SEM-images of fine pronotal and elytral structures, distribution maps, habitus photos, and illustrations of male genitalia and pro- and mesotarsal claws. We discuss the role of the Manjakatompo forest as a refugium for Madagascan Rhantus diversity and other endemics of the montane central high plateau.
Diving beetles, Madagascar, GMYC, species delimitation, refugium, lectotype designation, new synonymy
Rhantus Dejean is a large cosmopolitan genus of medium-sized aquatic diving beetles. Several studies have pointed out that the genus as presently defined is clearly paraphyletic and will likely be split in the future (
The first Rhantus species described from Madagascar was Rhantus latus (
All specimens examined in this study are registered in the NHRS collection objects database (interface via www.naturarv.se) but are deposited in the following collections and referred to by the abbreviations:
BMNH Museum of Natural History, London, Great Britain;
MNHN Muséum National d’Histoire Naturelle, Paris, France;
NMW Naturhistorisches Museum Wien, Austria;
NHRS Swedish Museum of Natural History, Stockholm, Sweden;
DEUA Departement d’Entomologie, Université d’Antananarivo, Antananarivo, Madagascar.
Measurements were taken on specimens in a horizontal position. The following measurements were taken: ML, maximum length from head to tip of elytra; MW, maximum width; LP, length of pronotum medially; WPB, pronotal width at base; LE, length of elytra from tip of scutellum to apex. The measurements were taken using an Olympus SZX12 stereomicroscope with an Infinity X camera and a calibrated ruler tool in the software DeltaPix Insight 2.0. Environmental scanning electron microscopy was done using a FEI/Philips XL30 FEG ESEM at the Institute for Surface Chemistry, Stockholm, Sweden. The images were generated at 350× magnification, with a gaseous secondary electron detector in low vacuum mode; the accelerating voltage of the electron beam was 17 kV. In opposite to a backscattered detector, a gaseous secondary electron detector depicts depressions of the surface brightly.
DNA was extracted from legs of ethanol-preserved material collected after 2004 using Qiagen DNeasy 96 Tissue kit (see Table 1 for specimen information). For the dry-mounted holotype of Rhantus manjakatompo, (collected 2001) the QIAamp® DNA Micro kit was used, following the protocol for animal tissue with incubation at 56°C overnight. A single hindleg was carefully removed and after incubation re-glued to the body. Two fragments of the gene cytochrome c oxidase subunit I (COI) were sequenced for analysis. Primers used for amplification and sequencing were derived from several sources (Table 2).
Data on specimens sequenced for COI and Genbank accession numbers. First accession number is for the 3’ end (patdyt-jerry) and second accession number is for the 5’ end (lco-hco) of COI.
| Cat. ID | Species | Locality | Date | Collector | Mus. | GB Acc. No. |
|---|---|---|---|---|---|---|
| BMNH-743391 | Rhantus bouvieri | Andringitra | 9.V.2006 | Bergsten et al | NHRS | KF548613, KF548639 |
| BMNH-743392 | Rhantus bouvieri | Andringitra | 9.V.2006 | Bergsten et al | BMNH | KF548614, KF548640 |
| BMNH-829990 | Rhantus latus | Ambalavao, 15km SW of | BMNH | KF548615, KF548641 | ||
| NHRS-JLKB000000089 | Rhantus latus | Ambohijanahary | 19.XII.2009 | Bergsten et al | NHRS | KF548616, KF548642 |
| NHRS-JLKB000000090 | Rhantus latus | Ambohijanahary | 19.XII.2009 | Bergsten et al | NHRS | KF548617, KF548643 |
| BMNH-741961 | Rhantus latus | Ambositra, 34km S of | 06.V.2006 | Bergsten et al | BMNH | KF548618, KF548644 |
| BMNH-829991 | Rhantus latus | Andasibe | 04.I.2007 | Isambert et al | BMNH | KF548619, KF548645 |
| BMNH-742090 | Rhantus latus | Andringitra | 9.V.2006 | Bergsten et al | BMNH | KF548620, KF548646 |
| BMNH-729860 | Rhantus latus | Col des Tapia | 8.XII.2004 | Balke et al | BMNH | KF548621, KF548647 |
| BMNH-729861 | Rhantus latus | Col des Tapia | 8.XII.2004 | Balke et al | BMNH | KF548622, KF548648 |
| BMNH-729862 | Rhantus latus | Col des Tapia | 8.XII.2004 | Balke et al | BMNH | KF548623, KF548649 |
| BMNH-729863 | Rhantus latus | Col des Tapia | 8.XII.2004 | Balke et al | BMNH | KF548624, KF548650 |
| BMNH-729864 | Rhantus latus | Col des Tapia | 8.XII.2004 | Balke et al | BMNH | KF548625, KF548651 |
| BMNH-829992 | Rhantus latus | Col des Tapia | 8.XII.2004 | Balke et al | BMNH | KF548626, KF548652 |
| BMNH-741810 | Rhantus latus | Isalo | 11.V.2006 | Bergsten et al | BMNH | KF548627, KF548653 |
| BMNH-742359 | Rhantus latus | Sendrisoa | 7.V.2006 | Bergsten et al | BMNH | KF548628, KF548654 |
| BMNH-741979 | Rhantus latus | Zombitse | 14.V.2006 | Bergsten et al | BMNH | KF548629, KF548655 |
| BMNH-742639 | Rhantus latus | Zombitse | 15.V.2006 | Bergsten et al | BMNH | KF548630, KF548656 |
| BMNH-742640 | Rhantus latus | Zombitse | 15.V.2006 | Bergsten et al | BMNH | KF548631, KF548657 |
| BMNH-742641 | Rhantus latus | Zombitse | 15.V.2006 | Bergsten et al | BMNH | KF548632, KF548658 |
| BMNH-729851 | Rhantus latus | BMNH | KF548633, KF548659 | |||
| NHRS-JLKB000030412 | Rhantus manjakatompo | Manjakatompo | 8.X.2001 | Gerecke & Goldschmidt | NMW | KF548634, na |
| BMNH-672725 | Rhantus manjakatompo | Tsaratanana | 20–24.XII.2004 | Lees & Ranaivosolo | BMNH | KF548635, KF548660 |
| BMNH-672726 | Rhantus manjakatompo | Tsaratanana | 20–24.XII.2004 | Lees & Ranaivosolo | BMNH | KF548636, KF548661 |
| BMNH-672730 | Rhantus manjakatompo | Tsaratanana | 20–24.XII.2004 | Lees & Ranaivosolo | NHRS | KF548637, KF548662 |
| BMNH-672731 | Rhantus manjakatompo | Tsaratanana | 20–24.XII.2004 | Lees & Ranaivosolo | NHRS | KF548638, KF548663 |
Primers used for the PCR to amplify two fragments of COI.
| Primer | Direction | Sequence (5’-3’) |
|---|---|---|
| PatDyt |
Reverse | TCATTGCACTAATCTGCCATATTAG |
| Jerry |
Forward | CAACATTTATTTTGATTTTTTGG |
| LCO |
Forward | GGTCAACAAATCATAAAGATATTGG |
| HCO |
Reverse | TAAACTTCAGGGTGACCAAAAAATCA |
1
2
3
DNA fragments were PCR amplified using “Ready-to-go” PCR Beads from Pharmacia Biotech and Phire Hot Start II PCR Master mix following the manufacture’s standard protocols. The thermal cycling profile for “Ready-to-go” PCR was 95°C for 5 min, followed by 40 cycles of 95°C for 30 s, 50°C for 30 s, 72°C for 1 min and finally 72°C for 8 min. PCR cycling for Phire Hot Start PCR was 98°C for initial denaturation 30 s, followed by 40 cycles of 98°C for 5 s, 53°C for 5 s, 72°C for 15 s, 72°C for 1 min. Product yield, specificity of amplification and contamination were investigated using agarose gel electrophoresis. PCR products were purified using ExoFAP Cleanup mix and cycle sequenced using the same primers used to amplify. For sequencing reactions the ABI BigDye Terminator kit ver. 3.1 was used. Sequencing products were purified using the DyeEx 96 kit and fragments were analysed on an ABI377xl analyser from Applied Biosystems. Gene regions were sequenced in both directions. The contigs were assembled from the forward and reverse reads and primers were trimmed in Sequencher 5.0 (
Sequence data for 26 specimens were aligned in ClustalX 2.1 (
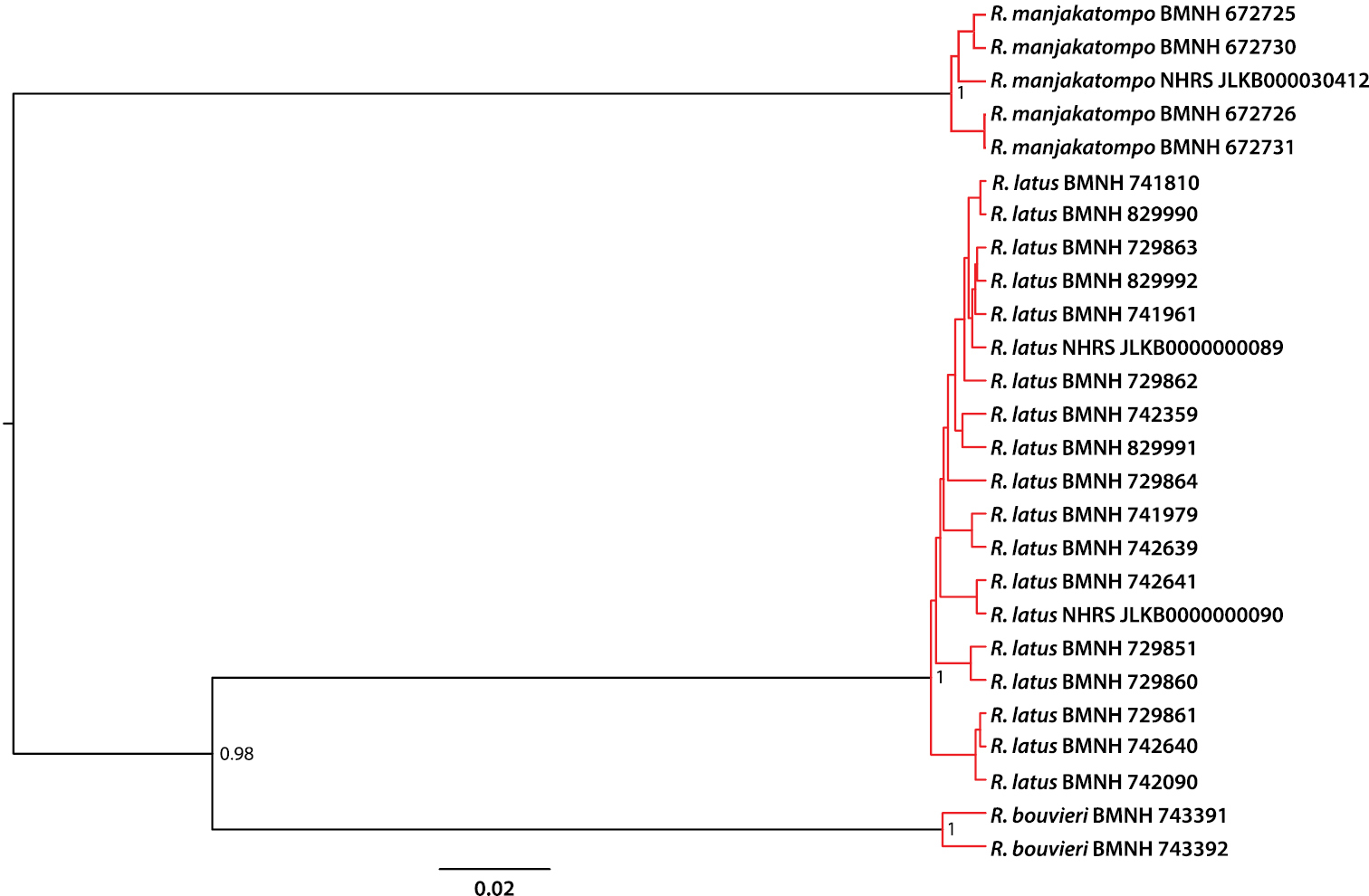
The GMYC model was significantly better than a single coalescence model with a likelihood ratio test (lgL GMYC: 158.9, lgL single coalescence: 149.4, p = 0.0003), and divided the terminals into tree separately coalescing units: Rhantus manjakatompo, Rhantus latus and Rhantus bouvieri (Figure 1). No other solution had a log-likelihood value within 2 units (an approximate confidence interval) of the maximum likelihood solution. The holotype of Rhantus manjakatompo (NHRS-JLKB000030412) from Ankaratra mountains was nested within the single coalescing unit represented by the four specimens from the Tsaratanana mountains (Figure 1). The genetic distance between the holotype from Ankaratra and the specimens from Tsaratanana was 0.008-0.011, which was within the range among the Tsratanana specimens alone (0-0.012). The within-species genetic variation was 0.015 for Rhantus bouvieri and 0-0.019 (mean = 0.009) for Rhantus latus. The distances between the three species were almost an order of magnitude greater: 0.11-0.13 between Rhantus bouvieri and Rhantus latus, 0.17 between Rhantus bouvieri and Rhantus manjakatompo and 0.16-0.17 between Rhantus latus and Rhantus manjakatompo.
Ultrametric tree obtained from Bayesian analysis of of the two concatenated COI gene fragments. Red branches indicate separately coalescing clusters corresponding to species. Node values show posterior probability values and scale bar indicates the expected number of substitutions per site. “Rhantus manjakatompo NHRS–JLKB000030412” is the holotype of Rhantus manjakatompo.
http://species-id.net/wiki/Rhantus_manjakatompo
Figures 2a–b, 3a–c, 4a, 5a–b, 6a–b, 7a–b, gMadagascar, Antananarivo province, Ankaratra mountains, Manjakatompo reserve.
Type material (NMW). Holotype ♂ “Madagascar, Ankaratra (Antananarivo), Reserve Manjakatompo, spring stream exp. SE. N deviation to Analamitana (left affl. MD 107) m 1850 asl, 8.x.2001, 16.0°C, 0.003 mS/cm. Gerecke & Goldschmidt collectors BMNH (E) 2004-46”, “Holotype Rhantus manjakatompo Pederzani & Rocchi 2008”, “Data in NHRS-JLKB 000030412” “DNA Voucher”.
Additional material studied (NHRS, BMNH, DEUA, see Appendix): Sofia region (former provinces: Mahajanga): 2♂, 2♀ (Cat. No. BMNH-672731, 672730, 672726, 672725): Tsaratanana [Antetykalambazaha Camp], 14.1824S, 48.9448E, 1700m, 20–24.xii.2004, Malaisetrap, Leg. Lees, Ranaivosolo.
Vakinankaratra region (former provinces: Antananarivo): 4spp. (Cat. No. NHRS-JLKB000021018) Manjakatompo [Analamitana] [swamp near stream][MJK12-02], 19.3640S, 47.2991E, 1760m, 22.i.2012, Leg. Ranarilalatiana, Randriamihaja, 1sp. (Cat. No. NHRS-JLKB000021019) Manjakatompo [Tavolatara][swamp near stream][MJK12-08], 19.3491S, 47.2784E, 2050m, 24.i.2012, Leg. Ranarilalatiana, Randriamihaja, 5spp. (Cat. No. NHRS-JLKB000021020) Manjakatompo [Tavolatara][pool near stream][MJK12-09], 19.3491S, 47.2780E, 2050m, 24.i.2012, Leg. Ranarilalatiana, Randriamihaja, 11spp. (Cat. No. NHRS-JLKB000021021) Manjakatompo [Tavolatara][swamp near source][MJK12-10], 19.3496S, 47.2779E, 2050m, 24.i.2012, Leg. Ranarilalatiana, Randriamihaja, 3spp. (Cat. No. NHRS-JLKB000021022) Manjakatompo [Anosiarivo][lake near source][MJK12–13], 19.3449S, 47.3041E, 2070m, 24.i.2012, Leg. Ranarilalatiana, Randriamihaja, 1spp. (Cat. No. NHRS-JLKB000021023) Manjakatompo [Andongolongo][pool near source][MJK12–12], 19.3536S, 47.3006E, 1900m, 24.i.2012, Leg. Ranarilalatiana, Randriamihaja.
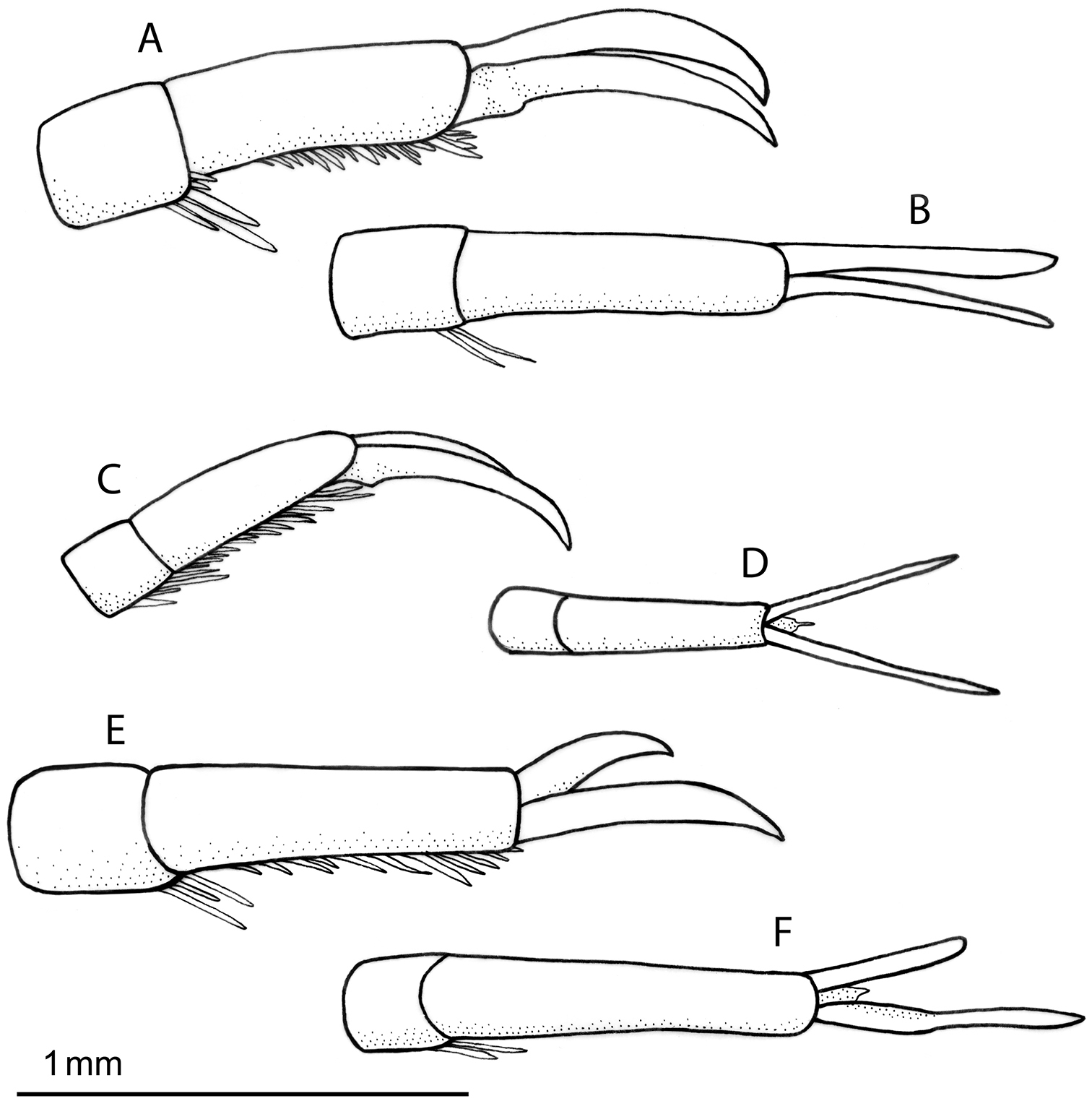
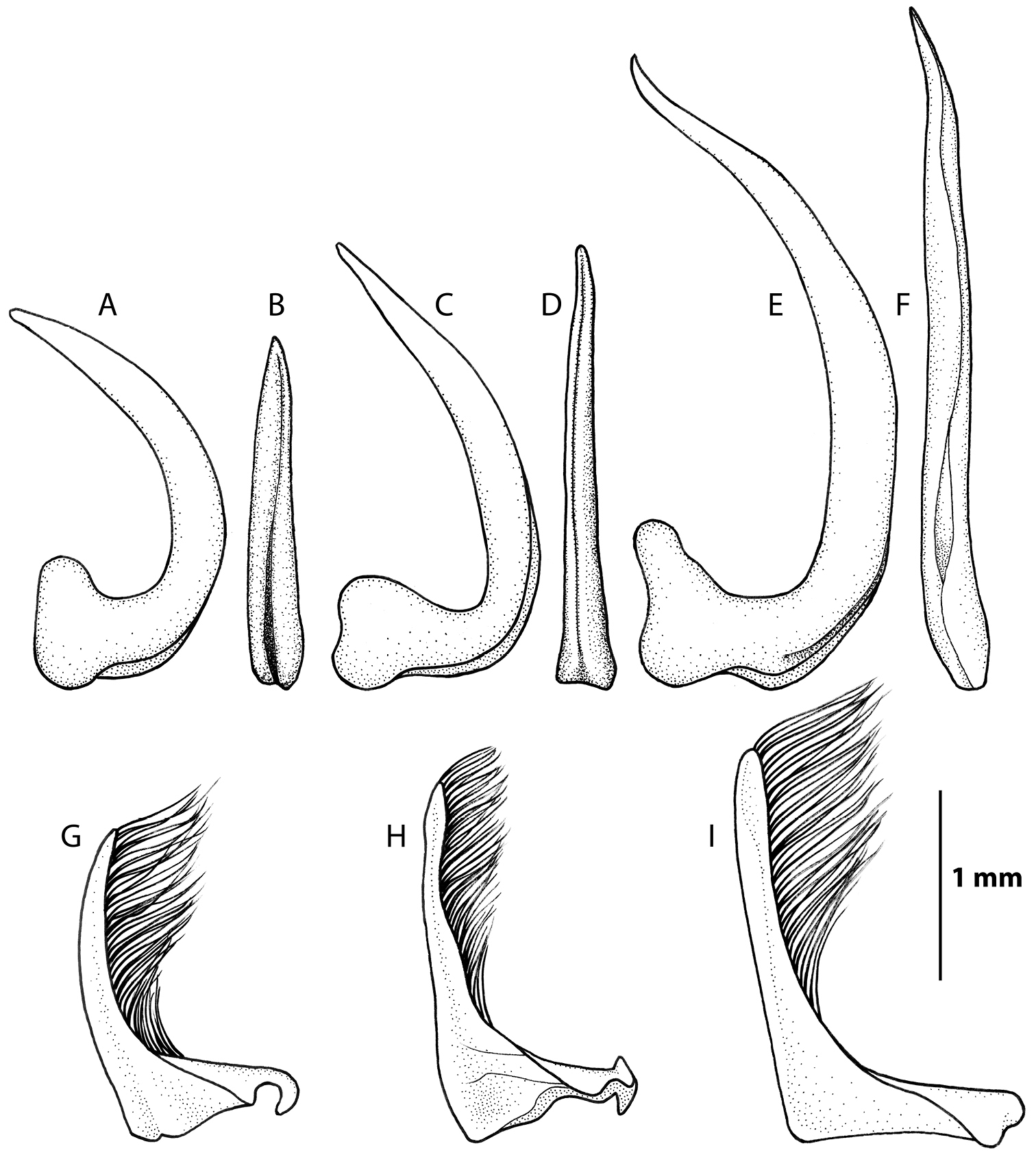
Pronotum entirely testaceous or with two small dark spots medially (Figure 2a–b). Black irrorations of elytra somewhat confluent subapically (Figure 2a–b). Male protarsal claws equally long, evenly curved, apex acute (Figure 5a–b). Male mesotarsal claws equally long but posterior claw distinctly thicker than anterior claw (Figure 6a–b). Penis short and robust (Figure 7a–b), Parameres evenly curved and tapering to apex (Figure 7g). 11.5–12.5 mm long.
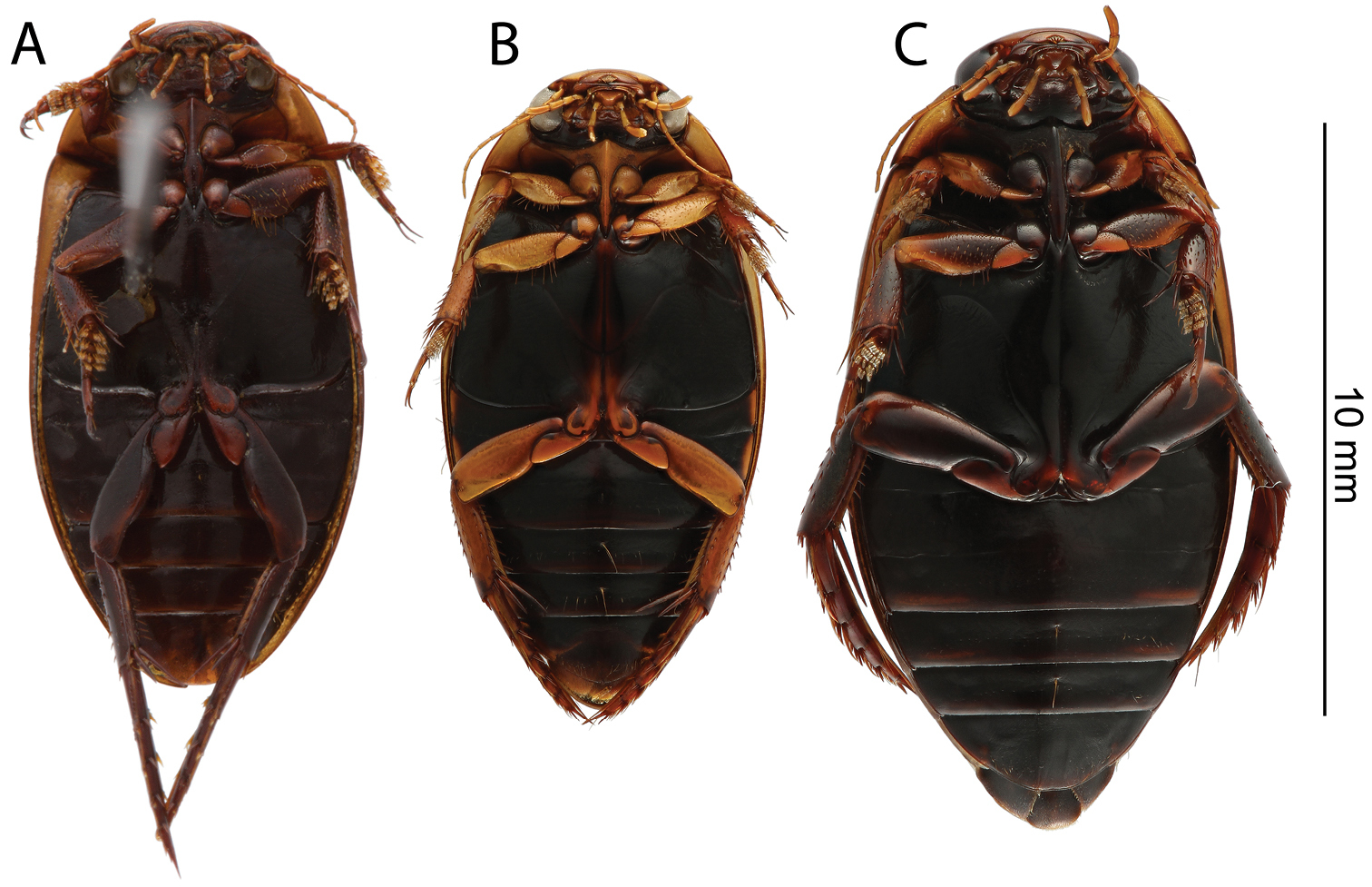
Habitus, dorsal view: A Rhantus manjakatompo holotype (Ankaratra mountains) B Rhantus manjakatompo (Tsaratanana mountains) C Rhantus bouvieri D Rhantus latus.
Size: ML 11.5–12.5 mm; MW 5.7–5.9 mm; Lp 1.4 mm; Epb 4.7–4.9 mm; Le 8.4–9.0 mm (n = 5).
Head. Testaceous with black areas posteriorly and inside eyes. Interocular black pattern narrowly separated medially (Figure 2a–b). Dense reticulation of well impressed meshes, very fine punctuation within and at edges of meshes.
Pronotum. Testaceous to ferrugineous with two small dark spots medially (Figure 2b), which may be absent (Figure 2a). Rim at lateral margin clearly visible to indistinct. Dense reticulation of well impressed meshes, very fine punctuation within and at edges of meshes (Figure 3a).
Elytra. Testaceous with black irrorations, leaving paler sides and yellow sutural lines (Figure 2a–b). Black irrorations somewhat confluent subapically. Reticulation of polygonal meshes simple anteriorly and double at middle and posteriorly, meshes are somewhat less impressed than on pronotum and larger posteriorly. Very fine punctuation within and at edges of meshes (Figure 3b–c).
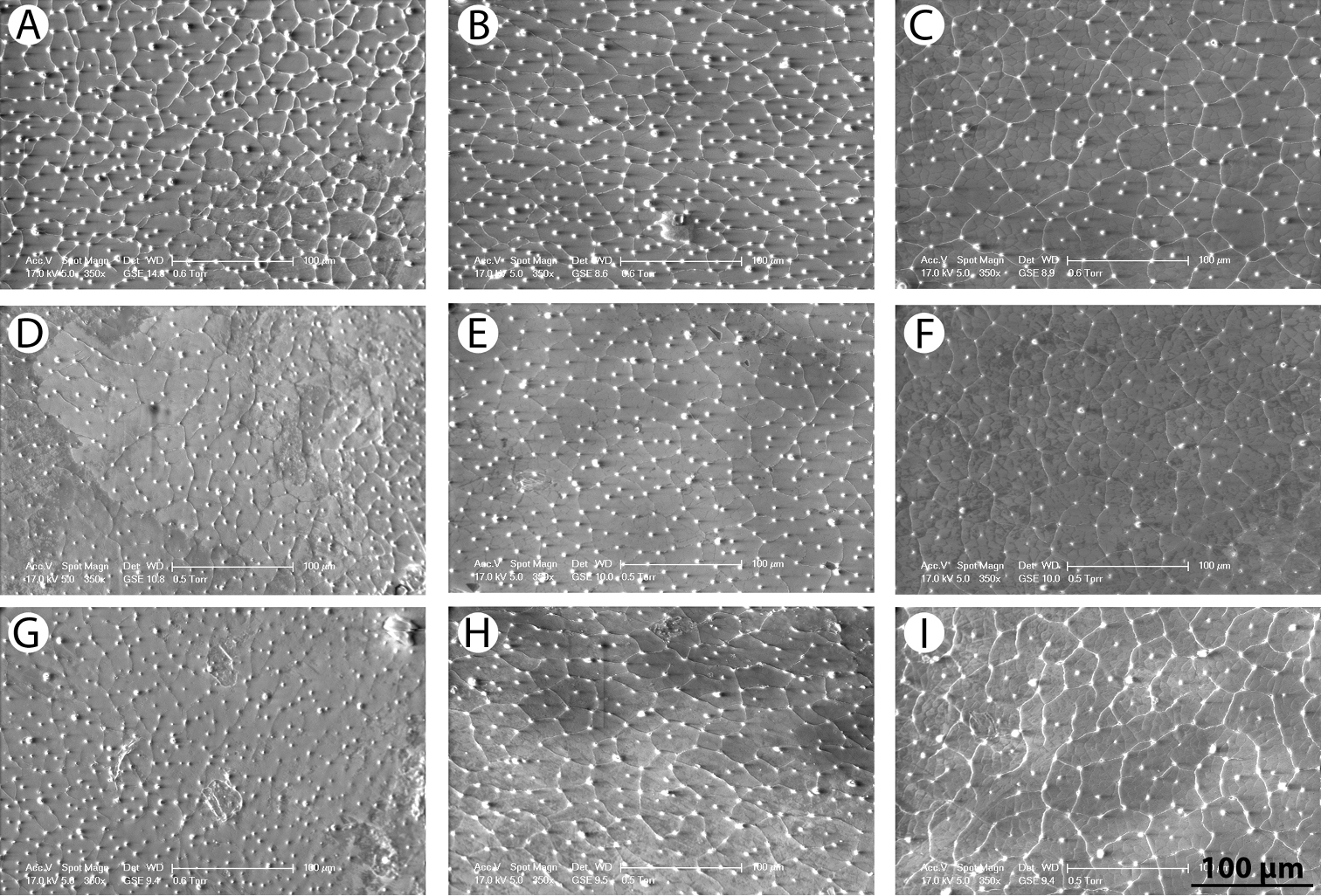
SEM images of pronotal and elytral microstructure: A–C Rhantus manjakatompo pronotum (A) anterior part of elytron (B) and posterior part of elytron (C) D–F Rhantus bouvieri pronotum (D) anterior part of elytron (E) and posterior part of elytron (F) G–I Rhantus latus pronotum (G) anterior part of elytron (H) and posterior part of elytron (I).
Ventral side. Dark brown to black. Epiplura testaceous. Metafemora infuscated (Figure 4a).
Habitus, ventral view: A Rhantus manjakatompo holotype B Rhantus bouvieri C Rhantus latus.
Male. Protarsal claws equally long, evenly curved, apex acute (Figure 5a–b). Mesotarsal claws equally long but posterior claw distinctly thicker than anterior claw (Figure 6a–b). Penis short and robust, shape as Figure 7a–b. Parameres evenly curved and tapering to apex (Figure 7g).
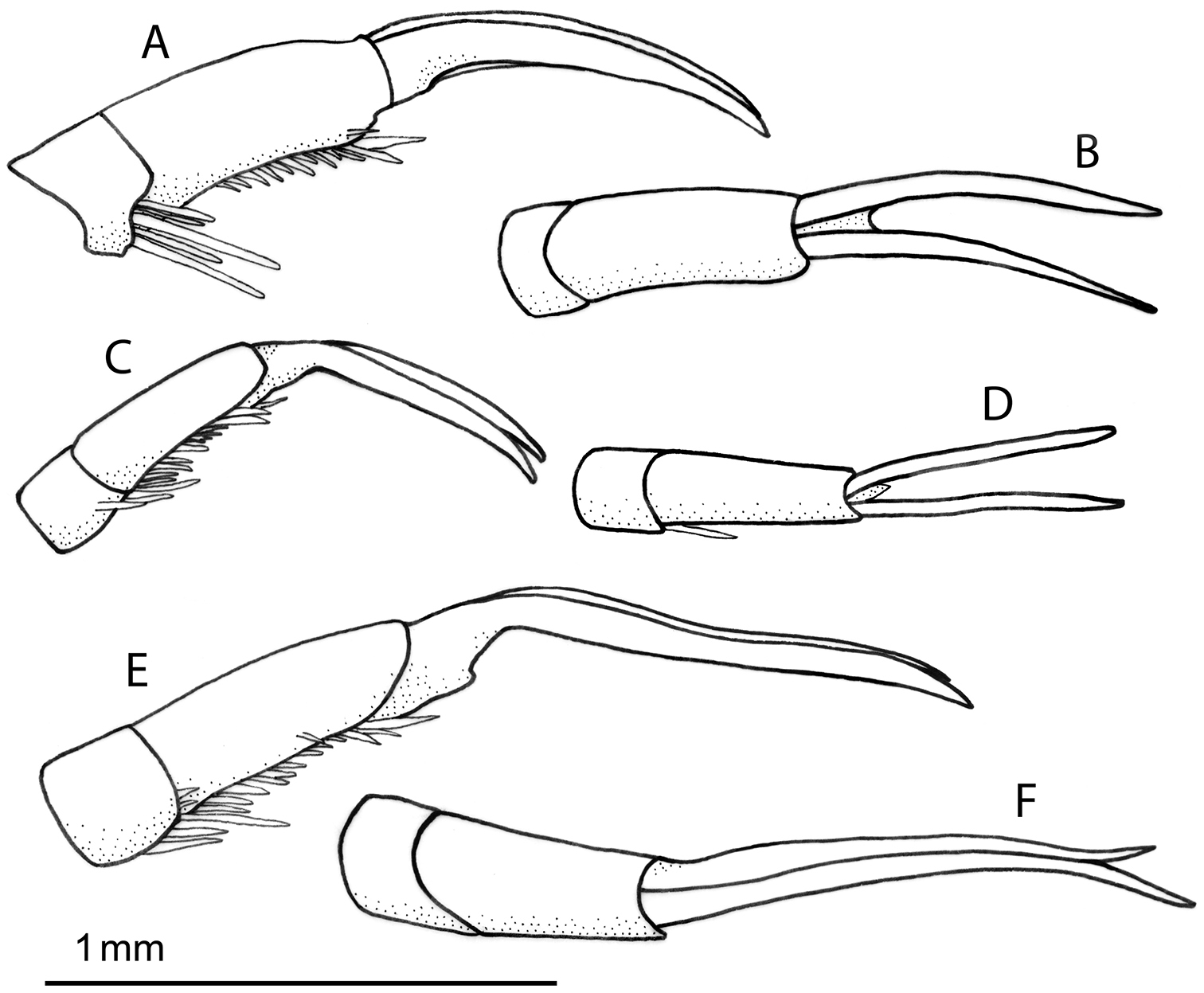
Left protarsal claws: A–B Rhantus manjakatompo, lateral (A) and dorsal (B) view C–D Rhantus bouvieri, lateral (C) and dorsal (D) view E–F Rhantus latus, lateral (E) and dorsal (F) view.
Left mesotarsal claws: A–B Rhantus manjakatompo, lateral (A) and dorsal (B) view C–D Rhantus bouvieri, lateral (C) and dorsal (D) view E–F Rhantus latus, lateral (E) and dorsal (F) view.
Male genitals, penis in lateral and dorsal view: A–B Rhantus manjakatompo C–D Rhantus bouvieri E–F Rhantus latus. Parameres in lateral view G Rhantus manjakatompo H Rhantus bouvieri I Rhantus latus.
In 2004 David Lees and Ravomiarana Ranaivosolo collected material of what seemed to be a new Rhantus species from the mountain massif of Tsaratanana, North Madagascar. Rhantus manjakatompo was described in 2009 based on a single male specimen from Ankaratra mountains, 70km south of Antananarivo in central Madagascar. Despite variation in color and impression of a lateral rim of pronotum, molecular data indicate that the studied material from Ankaratra and Tsaratanana are conspecific or at least not an old enough divergence to be recognised based on COI sequence (genetic distance 0.008–0.011). As also male tarsal claws, aedeagus and parameres were identical, we consider the specimens from Tsaratanana mountains conspecific with the holotype of Rhantus manjakatompo.
Associated with sources and streams, and surrounding water assemblages like nearby pools and marshes at altitudes between 1700 to 2070 m a.s.l. In Manjakatompo, the species was most numerous at elevations above 2000 m.
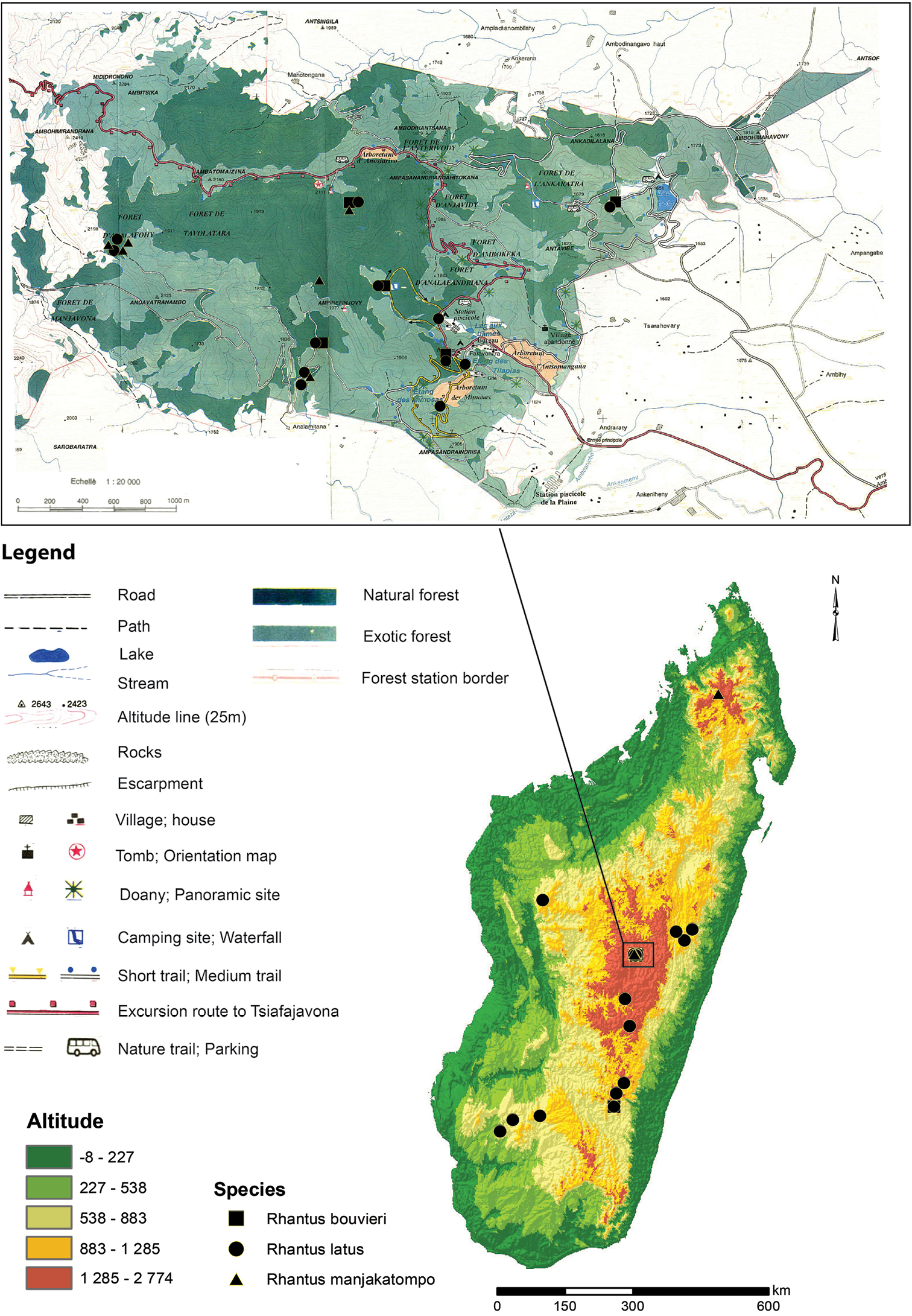
Endemic to Madagascar. Only known from Tsaratanana mountains and Manjakatompo forestry station in the Ankaratra mountains (Figure 8).
Distribution and all known records of the three Rhantus species of Madagascar with a special emphasis on Manjakatompo. Inset map of Manjakatompo adapted from
http://species-id.net/wiki/Rhantus_bouvieri
Figures 2c, 3d–f, 4b, 5c–d, 6c–d, 7c–d, hMadagascar, Fianarantsoa.
(MNHN). Lectotype ♂, here designated to fix the concept of Rhantus bouvieri and to ensure the universal and consistent interpretation of the same: “Data in NHRS-JLKB000030144”, “Rhantus Bouvieri Rég. M. Régimbart det.”, “Museum Paris Madagascar Fianarantsoa Grandidier 1852–91”, ”Rhantus bouvieri Régimbart, 1900 Det. J. Bergsten 2011” our lectotype label.
Paralectotypes 2♀: “Data in NHRS-JLKB000030405 and Data in NHRS-JLKB0000304283”, “Museum Paris Madagascar Fianarantsoa Grandidier 1852–91”, our paralectotype labels.
Additional material studied (NHRS, BMNH, MNHN, NMW, DEAU, see Appendix):
Matsiatra Ambony (Haute Matsiatra) region (former provinces: Fianarantsoa): 2♂ (Cat. No. BMNH-743391, 743392), Andringitra NP [Zomandao river, by bridge on road to camp Belamba], 22.1043S, 46.9207E, 1420m, 09.v.2006, Bottletrap, Leg Bergsten et al.
Vakinankaratra region (former provinces: Antananarivo): 1♂, 1♀ (Cat. No. NHRS-JLKB000030410, 30411), Manjakatompo [pond by], 10.x.1968, Leg. Starmühlner, 2spp. (Cat. No. NHRS-JLKB000010272), Manjakatompo [Analafandriana, 500m S fish farm by the road][grassy pond][MAD11–14], 19.3619S, 47.3150E, 1730m, 03.xi.2011, Leg. Bergsten, Ranarilalatiana, Randriamihaja, Bukontaite, 8spp. (Cat. No. NHRS-JLKB000010275), Manjakatompo [500m E Lac Froid by the road][pond and inlet stream][MAD11–16] 19.3449S, 47.3338E, 1620m, 04.xi.2011, Leg. Bergsten, Ranarilalatiana, Randriamihaja, Bukontaite, 16spp. (Cat. No. NHRS-JLKB000010276), Manjakatompo [500m E Lac Froid by the road][pond and inlet stream][MAD11-16], 19.3449S, 47.3338E, 1620m, 04.xi.2011, Leg. Bergsten, Ranarilalatiana, Randriamihaja, Bukontaite, 10spp. (Cat. No. NHRS-JLKB000010273), Manjakatompo [2km NE Amparandraindrisa][pond and stream][MAD11-18], 19.3607S, 47.3009E, 1770m, 05.xi.2011, Leg. Bergsten, Ranarilalatiana, Randriamihaja, Bukontaite, 4spp. (Cat. No. NHRS-JLKB000021024), Manjakatompo [Anosiarivo][lake near source][MJK12-13], 19.3449S, 47.3041E, 2070m, 24.i.2012, Leg. Ranarilalatiana, Randriamihaja, 7spp. (Cat. No. NHRS-JLKB000021025), Manjakatompo [Poste][lake with grass][MJK12-14], 19.3542S, 47.3081E, 1800m, 24.i.2012, Leg. Ranarilalatiana, Randriamihaja.
Madagascar (region indecisive): 3♂, 6♀ (Cat. No. NHRS-JLKB000030139, 30140, 30141, 30142, 30144, 30400, 30401, 30402, 30403), Madagascar, Leg. Catat, 2♀ (Cat. No. NHRS-JLKB000030404, 30143) Madagascar [Centre-Sud], 1901, Leg. Alluaud, 1♀ (Cat. No.: NHRS-JLKB000030138) Antananarivo [city or province, indecisive], Leg. Sikora, 1♀ (Cat. No. NHRS-JLKB000030287) Forêt Tanala [province indecisive], Leg. Alluaud
Pronotum with two elongated rectangular dark spots, narrowly (or partly) separated in middle (Figure 2c). Male protarsal claws equally long, straight in lateral view apart from at base and apex (Figure 5c–d). Male mesotarsal claws curved in lateral view, equally thin, the anterior claw somewhat longer than the posterior (Figure 6c–d). Penis in dorsal view evenly tapering towards apex, in lateral view with a relatively sharp angle at the base (Figure 7c–d). Parameres with inner margin undulated (Figure 7h). 9.4–10.8 mm long.
Size: ML, 9.4-10.8 mm; MW, 5.2–5.7 mm; Lp, 1.0–1.4 mm; Wp, 3.8–4.1 mm; Le, 7.2–8.1 mm (n = 18).
Head. Testaceous with black areas inside and posterior of eyes. Interocular black pattern tapering towards the interior and narrowly separated (Figure 2c). Dense micropunctation and incomplete microreticulation.
Pronotum. Testaceous ferrugineous with two elongated rectangular black areas that are narrowly separated medially (Figure 2c). Lateral rim present, distinct. Dense micropunctation and incomplete microreticulation (Figure 3d).
Elytra. Testaceous with black irrorations, leaving paler sides and yellow sutural lines (Figure 2c). Somewhat confluent to form two small black areas subapically. Microreticulation double but somewhat indistinct. Very fine punctuation within and at edges of meshes (Figure 3e–f).
Ventral side. Dark brown to black, abdominal segments with testaceous spots along the lateral edges. Epiplura and legs, including metafemur, testaceous (Figure 4b).
Male. Protarsal claws equally long, medially straight in lateral view (Figure 5c-d). Mesotarsal claws curved in lateral view, equally thin, the anterior claw somewhat longer than the posterior (Figure 6c–d). Penis in dorsal view evenly tapering towards apex (Figure 7d). in lateral view not evenly curved but almost angulate at base (Figure 7c). Parameres with inner margin undulating (Figure 7h).
In the original description, Régimbart refers to specimens from Fianarantsoa collected by Grandidier, but the holotype is not designated. The three specimens labelled “Fianarantsoa Grandidier” at MNHN are therefore considered to be syntypes and a male with genitalia extracted is designated here as the lectotype. Lectotype and paralectotypes were labelled as such.
Known from a river in Andringitra and streams and grassy ponds in Manjakatompo, in both cases at altitudes between 1420 to 2070 m a.s.l.
Endemic to Madagascar. Precise localities only known from Manjakatompo forestry station in the Ankaratra mountains and the Andringitra mountains (Figure 8). Forêt Tanala, Fianarantsoa and Tananarive are less precise region descriptions which includes Manjakatompo (Antananarivo) and Andingitra (Fianarantsoa, Forêt Tanala).
http://species-id.net/wiki/Rhantus_latus
Figures 2d, 3g–i, 4c, 5e–f, 6e–f, 7e–f, iTypematerial of Colymbetes latus could not be localized in the Paris collections. We have no reasons to believe the type material is lost or destroyed, but to localize and identify the material that Fairmaire studied proved difficult as it was not found in Fairmaire’s collection. The type material was collected by medicine doctor and entomologist Charles Coquerel who died in 1867.
Rhantus stenonychus: Holotype ♂ for Rhantus stenonychus (MNHN): “Madagascar, Lac Ambodo, R.P. Camboue”, “Data in NHRS-JLKB000030296”.
Rhantus goudoti: Lectotype ♂ for Rhantus goudoti, here designated to fix the concept of Rhantus goudoti Sharp and to ensure the universal and consistent interpretation of the same (BMNH): “905”, “C. latus Fairm. seems very close to this, but is a little shorter in form, +paler” [handwritten note on the specimen], “Sharp Coll. 1905–313.”, “Type”, “Type 905 Goudoti Dej. Madagascar.” “Data in NHRS-JLKB 000030415“, our lectotype label. Paralectotype ♀ (BMNH) “Sharp Coll. 1905–313.”, “Co-type”, “Madagascar. 905” “Data in NHRS-JLKB 000030413“, our paralectotype label. Paralectotype ♂ (BMNH) “37” “Co-type”, “Madagascar 905”, “Sharp Coll. 1905–313.” “Data in NHRS-JLKB 000030414“our paralectotype label. 1♂ Paralectotype (MNHN): Data in NHRS-JLKB 000030297, Colymbetes goudotii mihi Madagascar, D. Sharp Monogr., Ex Musæo Dejean, our paralectotype label.
Additional material studied (for full details see Appendix).
Vakinankaratra region (former provinces: Antananarivo): 15spp. (Cat No. NHRS-JLKB000010277), Manjakatompo [2km NE Amparandraindrisa][pond and stream][MAD11-18], 19.3607S, 47.3009E, 1770m, 05.xi.2011, Leg. Bergsten, Ranarilalatiana, Randriamihaja, Bukontaite, 24 spp. (Cat. No. NHRS-JLKB000010278), Manjakatompo [500m E Lac Froid by the road][pond and inlet stream][MAD11-16], 19.3449S, 47.3338E, 1620m, 04.xi.2011, Leg. Bergsten, Ranarilalatiana, Randriamihaja, Bukontaite, 12 spp. (Cat. No. NHRS-JLKB000010274), Manjakatompo [Analafandriana, 500m S fish farm by the road][grassy pond][MAD11-14], 19.3619S, 47.3150E, 1730m, 03.xi.2011, Leg. Bergsten, Ranarilalatiana, Randriamihaja, Bukontaite, 1sp. (Cat. No. NHRS-JLKB000010269), Manjakatompo [Analamitana, by bridge close to SKOL facility][stream and stagnant pool][MAD11-19], 19.3646S, 47.2989E, 1750m, 05.xi.2011, Leg. Bergsten, Ranarilalatiana, Randriamihaja, Bukontaite, 2sp. (NHRS-JLKB000010270), Manjakatompo [Analafandriana close to fish farm][stream and wet field][MAD11-13], 19.3581S, 47.3140E, 1730m, 03.xi.2011, Leg. Bergsten, Ranarilalatiana, Randriamihaja, Bukontaite, 1sp. (Cat. No. NHRS-JLKB000021026), Manjakatompo [Tavolatara][pool near stream][MJK12-09], 19.3491S, 47.2780E, 2050m, 24.i.2012, Leg. Ranarilalatiana, Randriamihaja, 2spp. (Cat. No. NHRS-JLKB000021027), Manjakatompo [Analamitana] [swamp near stream][MJK12-02], 19.3640S, 47.2991E, 1760m, 22.i.2012, Leg. Ranarilalatiana, Randriamihaja, 2spp. (Cat. No. NHRS-JLKB000021028), Manjakatompo [Tavolatara][swamp near source][MJK12-10], 19.3496S, 47.2779E, 2050m, 24.i.2012, Leg. Ranarilalatiana, Randriamihaja, 3spp. (cat. No. NHRS-JLKB000021029), Manjakatompo [Anosiarivo][lake near source][MJK12-13], 19.3449S, 47.3041E, 2070m, 24.i.2012, Leg. Ranarilalatiana, Randriamihaja, 2spp. (Cat. No. NHRS-JLKB000021030), Manjakatompo [Poste][lake with grass][MJK12-14], 19.3542S, 47.3081E, 1800m, 24.i.2012, Leg. Ranarilalatiana, Randriamihaja, 5sp. (Cat. No. NHRS-JLKB000021031), Manjakatompo [Andohariana][small lake][MJK12-07], 19.3677S, 47.3143E, 1710m, 24.i.2012, Leg. Ranarilalatiana, Randriamihaja, 10spp. (Cat. No. NHRS-JLKB000021032), Manjakatompo [near camping][big temp. lake][MJK12-15], 19.3630S, 47.3171E, 1710m, 25.i.2012, Leg. Ranarilalatiana, Randriamihaja.
Alaotra Mangoro region (former provinces: Toamasina): 1♂ (Cat. No. NHRS-JLKB000030407), Mantadia, NP, Rianila Basin, [affluent non nommé riv.], [PO670], 18.935S, 48.4167E, 920m, 29.xi.1996, Leg. Legrand, Randriamasimanana, 1sp. (Cat. No. NHRS-JLKB000010268), Mantadia NP [3km from park entrance][open pond with vegetation][MAD11-42], 18.8526S, 48.4272E, 920m, 13.xi.2011, Leg. Bergsten, Ranarilalatiana, Randriamihaja, Bukontaite, 1♀ (Cat. No. BMNH-829991), Andasibe NP [entry to park, Anamalozaotra river and pond][P61Bi01], 18.9348S, 48.4175E, 950m, 04.i.2007, Leg. Isambert et al., 2♂ (Cat. No. NHRS-JLKB000030295), Antsianaka, 1892, Leg. Perrot, Perrot, 1♂ (Cat. No. NHRS-JLKB000030289), Ambatosoratra, env. Tananarive, vii.1934, Leg. Olsoufieff.
Matsiatra Ambony (Haute Matsiatra) region (former provinces: Fianarantsoa): 1sp. (Cat. No. BMNH-742359), Sendrisoa, S of Ambalavao, [P38], 22.0098S, 46.9504E, 1160m, 07.v.2006, Leg. Bergsten et al., 1sp. (Cat. No. BMNH-742090), Andringitra NP [Zomandao river, by bridge on road to camp Belamba], 22.1043S, 46.9207E, 1420m, 09.v.2006, Leg. Bergsten et al., 1♂ (Cat. No. BMNH-829990), Ambalavao, 15km SW of, RN7.
Amoron’i Mania region (former provinces: Fianarantsoa): 6spp. (Cat. No. BMNH-729860, 729861, 729862, 729863, 729864, 829992), Col des Tapia, 48 km N Ambositra, RN7, [P30MD33], 20.2388S, 47.1002E, 1440m, 08.xii.2004, Leg. Balke et al., 1♂ (Cat. No. BMNH-741961), Ambositra, 34km S of, RN7, 20.7719S, 47.1810E, 1690m, 06.v.2006, Leg. Bergsten et al.
Melaky region (Former provinces: Mahajanga): 1♂, 1♀ (Cat. No. NHRS-JLKB000000089, 000000090), Ambohijanahary NP [MAD09-76], 18.2685S, 45.4635E, 910m, 19.xii.2009, Leg. Bergsten, Ranarilalatiana, Randriamihaja, Jönsson
Atsimo Andrefana region (former provinces: Toliara): 3spp. (Cat. No. BMNH-742641, 742639, 742640), Zombitse-Vohibasia NP [Isoky, Ranomena, muddy pool in river basin], 22.6401S, 44.8644E, 580m, 15.v.2006, Leg. Bergsten et al., 1sp. (Cat. No. BMNH-741979), Zombitse-Vohibasia NP [edge of, Ambiamena, stagnant zebu-visited marshland, muddy & vegetation], 22.8601S, 44.6173E, 530m, 14.v.2006, Leg. Bergsten et al.
Ihorombe region (former provinces: Fianarantsoa): 1sp. (cat. No. BMNH-741810), Isalo NP [Menamaty river][river with vegetation][P41AM01], 22.5500S, 45.4012E, 760m, 11.v.2006, Leg. Bergsten et al.
Analamanga region (former provinces: Antananarivo): 1♂ (Cat. No. NHRS-JLKB000030282), Antananarivo [city or province, indecisive], 31.v.1947, Leg. Clement, 1♂ (Catl. No. NHRS-JLKB000030288), Lac Tsimbazaza, Antananarivo, 18.9333S, 47.5333E, 1410m, vii.1934, Leg. Vadon, 1♂ (Cat. No. NHRS-JLKB000030284), Andrang, Leg. Sikora, 1♂ (Cat. No. NHRS-JLKB000030285), Ambohibeloma, Leg. Sikora.
Madagascar (region indecisive): 2♂, 1♀ (Cat. No. NHRS-JLKB000030406, 30408, 30409), Madagascar, vii.1968 – ix.1968, Leg. Starmühlner, 1sp. (Cat. No. BMNH-729851), Madagascar, 1♂ (Cat. No. NHRS-JLKB000030286), Madagascar [Centre-Sud], Leg. Alluaud.
Pronotum somewhat infuscated and with one wide black spot, not divided medially (Figure 2d). Maleprotarsal claws long and slender, almost twice the length of last tarsal segment, somewhat sinuated and unequal, anterior claw longer (Figure 5e–f). Male mesotarsal claws very unequal, the anterior almost twice the length, and very broadened, compared to posterior (Figure 6e–f). Penis long and slender, apically upturning in lateral view, in dorsal view with the apex curved to the left and sharply pointed (Figure 7e–f). Parameres with dorsal edge straight (Figure 7i). 11.6–13.0 mm long.
Size:ML, 11.6–13.0 mm; MW, 6.2–7.0 mm; Lp, 1.2–1.6 mm; Wp, 4.7–5.3 mm; Le, 9.3–10.1 mm (n = 12).
Head. Testaceous with black areas inside and posterior of eyes. Interocular black pattern often rather broadly separated medially (Figure 2d). Dense micropunctuation but no reticulation.
Pronotum. Somewhat infuscated and with one wide dark spot which is not medially divided (Figure 2d). No rim at lateral margin. Micropunctation and vague microreticulation (Figure 3g).
Elytra. Testaceous ferrugineous with black irrorations, leaving paler sides and yellow sutural lines (Figure 2d). Black irrorations regular throughout, even posteriorly with no sign of preapical black areas. Microreticulation double, meshes are well impressed posteriorly but vague anteriorly. Micropunctation within and at edges of meshes (Figure 3h–i).
Ventral side. Dark brown to black. Epiplura testaceous. Metafemur infuscated (Figure 4c).
Male. Protarsal claws long and slender, almost twice the length of last tarsal segment, somewhat sinuated and unequal, anterior claw longer (Figure 5e–f). Mesotarsal claws curved and unequal, the anterior claw is almost twice as long as the posterior and much broader (Figure 6e–f). Penis long and slender, apically upturning in lateral view, in dorsal view with the apex curved to the left and sharply pointed (Figure 7e–f). Parameres with dorsal edge straight (Figure 7i).
The interpretation of Fairmaire’s name Colymbetes latus is unambiguous following common usage (e.g.
The holotype is as judged by morphological characters conspecific with Rhantus latus. Already in the original description similarities with Rhantus latus are obvious (also see
Occurs in a quite wide range of habitats, like streams and rivers, muddy waterpools and grassy ponds. Of the three Rhantus species it is the only one that can be found below 1000 m altitude and known range include 530 to 2070 m.
Possibly endemic to Madagascar as we have not been able to verify the records from mainland Africa by
| 1a | Smaller (ML 9.4–10.8 mm), legs including metafemur mostly yellow (Figure 4b), pronotum yellow with two elongated rectangular black fields narrowly divided in middle (Figure 2c) | Rhantus bouvieri |
| 1b | Larger (ML: 11.5–13.0 mm), legs mostly infuscated especially metafemur (Figure 4a, c), pronotum yellow or infuscated with or without black markings which, if present, are either not medially divided or are not elongated but small dots (Figure 2a–b, d). | 2 |
| 2a | Pronotum entirely yellow without black markings (Figure 2a) or with two small black dots, medially divided (Figure 2b). Elytral black irrorations somewhat confluent subapically to form small dark fields (Figure 2a–b) | Rhantus manjakatompo |
| 2b | Pronotum somewhat infuscated and with a single medial elongated rectangular black field not medially divided (Figure 2d). Black elytral irrorations even throughout, not forming denser black areas subapically (Figure 2d) | Rhantus latus |
| 1a | Male mesotarsal claws very unequal in length, anterior claw broad and almost twice as long as posterior claw (Figure 6e–f). Male protarsal claws very long, slender and sinuate, almost twice the length of last protarsal segment (Figure 5e–f). Penis long and slender, apically twisted (Figure 7e–f) | Rhantus latus |
| 1b | Male mesotarsal claws equal or somewhat unequal, anterior claw thin (Figure 6a–d). Male protarsal claws shorter and not sinuated (Figure 5a–d). Penis shorter and not apically twisted (Figure 7a–d) | 2 |
| 2a | Smaller (ML 9.4–10.8 mm), Male mesotarsal claws subequal, posterior claw somewhat shorter but equally thin as anterior claw (Figure 6c–d). Pronotum with two wide rectangular dark spots (Figure 2c). Penis in lateral view not evenly curved but almost angulate at base (Figure 7c). Parameres with inner margin undulating (Figure 7h) | Rhantus bouvieri |
| 2b | Larger (ML: 11.5–12.5 mm), Male mesotarsal claws equally long but posterior claw distinctly thicker than anterior claw (Figure 6a–b). Pronotum with two small dark spots (Figure 2b), or spots are absent (Figure 2a). Penis in lateral view short robust and evenly curved (Figure 7a). Parameres evenly curved and tapering to apex (Figure 7g) | Rhantus manjakatompo |
On Madagascar, Rhantus is a genus of the highland plateau. Like the Rhantus diversity in southeast Asia, Indonesia and Melanesia (
Manjakatompo is located in the province of Antananarivo, region Vakinakaratra and district of Ambatolampy, at 17 km to the west of the city Ambatolampy (19°22'S, 47°16'E). It lies on the eastern slope of the Ankaratra mountain massif of Quaternary volcanic origin. The altitude ranges between 1550 and 2643 m with the highest peak, Tsiafajavona, being the third highest on Madagascar. Forests are humid and the climate follows a pattern of cold and dry austral winter and a warm and wet austral summer (annual rainfall around 2000mm) (
The forest relics have been kept partly thanks to legal protection more or less effectively exerted by the agents of the Forestry Station, with its status as Integral Reserve (
Fieldwork and collecting was conducted thanks to support by MICET and the Entomology Department at Antananarivo University, and under the following permits: No.175MINENVEF/SG/DGEF/DPB/SCBLF (2004), No.82/06/MINENV.EF/SG/DGEF/DPB/SCBLF/RECH (2006), No.250/06/MINENV.EF/SG/DGEF/DPB/SCBLF/RECH (2006-2007), No 266/09/MEF/SG/DGF/DCB.SAP/SLRSE (2009), No 250/11/MEF/SG/DGF/DCB.SAP/SCB (2011-2012).
We are very grateful to Rafaralahy, forest agent at Manjakatompo forest station, for help with collecting and guiding in Manjakatompo. We also thank Antoine Mantilleri (MNHN) and Manfred Jäch (NMW) for the loan of type material. Rodrigo Robinson at Institute for Surface Chemistry, Stockholm is thanked for help with SEM imaging. Financial support came from Riksmusei Vänner, Kungliga Vetenskapsakademien and Vetenskapsrådet in Sweden.
Supplementary table include detailed information of all examined specimens. (doi: 10.3897/zookeys.350.6127.app) File format: Microsoft Excel file (xls).