(C) 2013 Odalisca Breedy. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Breedy O, Williams GC, Guzman HM (2013) Two new species of gorgonian octocorals from the Tropical Eastern Pacific Biogeographic Region (Cnidaria, Anthozoa, Gorgoniidae). ZooKeys 350: 75–90. doi: 10.3897/zookeys.350.6117
The gorgoniid Eugorgia is exclusively an eastern Pacific genus. It has a wide geographic and bathymetric range of distribution, found from California to Perú and extends down to 65 m deep. Two new species are herein described. The morphological characters were analyzed and illustrated by light and scanning electron microscopy. Eugorgia beebei sp. n. can be distinguished by its white, ascending, sparse colony growth. Eugorgia mutabilis sp. n. can be distinguished by its white colony that changes color after collection, and the conspicuous sharp-crested disc sclerites. From a morphological point of view the new species are related to the daniana-group, the rubens-group and the siedenburgae-group of Eugorgia; their affiliations, and the proposal of a new group are discussed. These new species increases the number of species in the genus to 15, and contribute to the knowledge of the eastern Pacific octocoral biodiversity.
Eugorgia, eastern Pacific, gorgonian, soft corals, taxonomy, white species
Eugorgia is a gorgonian octocoral (family Gorgoniidae) with 13 valid species. The genus is considered to be exclusively eastern Pacific and is distributed from southern California to Perú, and found in oceanic islands. It presents a wide bathymetric range of distribution, found in shallow waters (down to 40 m), and in the mesophotic region (down to 65 m) (
Eugorgia is recognised for their bright colored colonies. The white color has been reported only for one species, Eugorgia alba Bielschowsky, 1929 in the ampla-group (
CAS California Academy of Science, California, USA
UCR Museo de Zoología, Universidad de Costa Rica
STRI Smithsonian Tropical Research Institute, Panama
USNM National Museum of Natural History, Washington, USA
The specimens used in this study belong to the octocoral collections of the above cited museums.
Preserved specimens were photographed for later detailed observation. Sclerites were obtained by dissolving tissues from branches with 3.5% sodium hypochlorite (household bleach). Sclerites were rinsed many times with distilled water then 100% ethanol, dried, and mounted on stubs for scanning electron microscopy (SEM), and coated with 60–80 nm Pt/Pd. They were observed and photographed using an Hitachi 3700 SEM operated at 15kV. For light microscopy, clean sclerites were mounted in water or glycerin and observed and photographed using an Olympus LX 51 inverted stereoscope.
We followed
Morphological characters of colonies and the most abundant sclerite types of the species examined here are presented in Table 1. The most abundant sclerites in these species are disc-spindles and double discs that present various degrees of tubercle fusion. The illustrations of the sclerites are presented in different planes to provide a better idea of their architecture (Figs 3, 5). Comparison is made with the closest morphological groups, in this case, the daniana-, siedenburgae-, and rubens-groups (Table 1).
Comparative features of the new species, Eugorgia beebei sp. n. and Eugorgia mutabilis sp. n. within the daniana- group, the rubens-group, and the siedenburgae-group. Characteristics are based on the holotypes and lectotypes (
| Species | Colony growth | Branching type | Max number. of branching | Pinna-like arr branchlets | Branchlet distance | Branchlet diameter | Branchlet length | Polyp distribution | Double disc | Capstans | Disc-spindle | Spindles | Bent spindles | Crosses | Anth. rods | Colour of colony | Bicolour colony | Coenenchymal sclerite colour | Colour rings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eugorgia aurantiaca (Horn, 1860) | fla | irr-pi | 6 | X | 1.5–8 | 1–2.5 | 6–30 | irr | 0.04–0.07 | 0.07 | no | 0.11 | X | 0.06 × 0.06 | not found | do, r | r, y | y | |
| Eugorgia daniana Verrill, 1868 | fla | irr-pi | 7 | X | 1–4 | 1–1.5 | 1–15 | irr | 0.065–0.08 | 0.07 | 0.13 | 0.13 | X | 0.075 × 0.065 | not found | r | r, y | ||
| Eugorgia multifida Verrill, 1870 | fla | irr-pi | 7 | X | 1–4 | 1–1.5 | 1–10 | reg | 0.07 | 0.07 | 0.13 | 0.13 | X | 0.06 × 0.06 | 0.08 mm | do, r | r, y | ||
| Eugorgia rubens Verrill, 1868 | spa | lb | 5 | X | 6–20 | 1.5–2 | 2–30 | reg | 0.06–0.07 | no | 0.10 | 0.10 | no | not found | p | p | |||
| Eugorgia siedenburgae Breedy & Guzman, 2013 | bu | irr-pi | 10 | no | 1–15 | 1–1.5 | 2–30 | irr | 0.08–0.05 | 0.07 | 0.11 | 0.11 | 0.08 × 0.07 | not found | p, o | X | p, y | ||
| Eugorgia mutabilis sp. n. | fla | irr-pi | 7 | X | 1–4 | 1–2 | 1–10 | irr | 0.045–0.075 | no | 0.15 | 0.14 | no | not found | w | w | |||
| Eugorgia beebei sp. n. | spa | irr-pi | 10 | no | 5–13 | 1–2.5 | 2–50 | irr | 0.07–0.06 | no | 0.14 | 0.14 | 0.08 × 0.06 | not found | w | w |
Colony growth: bu, bushy; fla, planar growth, flabelliform; spa, sparse growth.
Branching type: irr-pi, irregularly pinnate; lb, laterally branched.
Polyp distribution: irr, arrangement mostly in irregular longitudinal rows; reg, arrangement mostly in regular longitudinal rows.
Colors: dark orange (do), orange (o), pink (p), red (r), yellow (y), white (w).
X: character present.
Blank space: character absent or not found.
http://zoobank.org/8B75AC49-5089-4BEF-BE80-B76198B9D0E8
http://species-id.net/wiki/Eugorgia_beebei
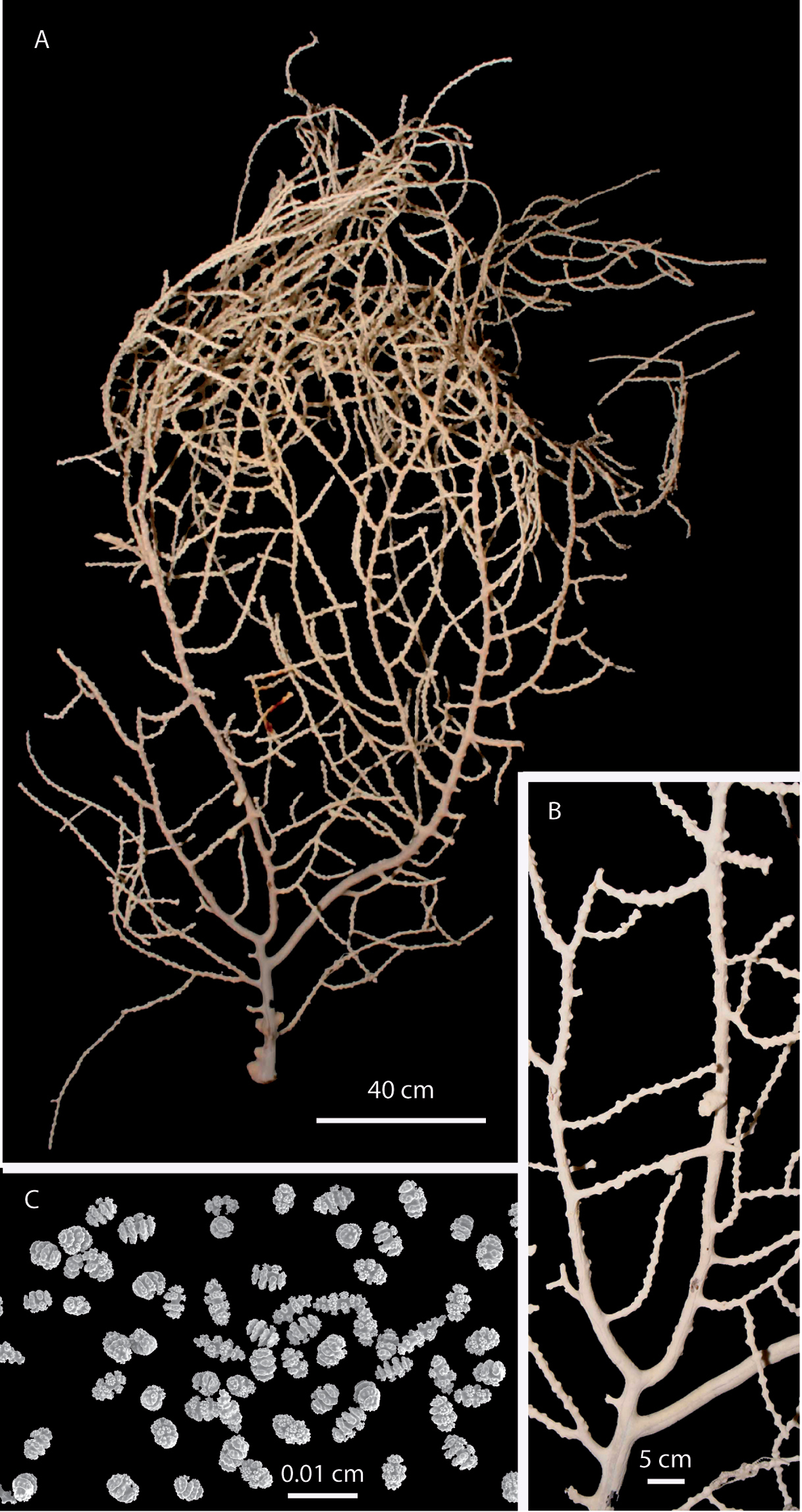
Figs 1–3Holotype. CASIZ 75783, ethanol preserved, Los Frailes, Baja California sur, México, 52 m, coll. R. Adcock, 18 June 1979.
Eugorgia beebei (CASIZ 75783) holotype. A entire colony B detail of branches C SEM sclerites.
Paratypes. MCZ 36106, dry, Paita, Piura, Perú, no more data available. USNM 56879, ethanol preserved, El Alto, Piura, Perú, 1860-1815 m but depth data dubious (F. M. Bayer’s note on label: ‘specimen probably from previous shallow station’), Anton Bruun Cruise, 18B, Sta. 766, 4°10'S, 81°27'W, 9 September 1966.
Eugorgia beebei (MCZ 36106) paratype. A entire colony B detail of branches; SEM sclerites.
Baja California sur, México.
Ascending colony sparse growing, branching irregularly pinnate, and multiplanar, subdividing up to 11 times, some pseudo-anastomosis present. Prominent polyp-mounds up to 0.70 mm tall, dome-shaped, arranged irregularly, and closely placed on branchlets, and very distant on thick branches. Colony and sclerites white. Spindles and disc-spindles up to 0.14 mm in length, double discs up to 0.07 mm long, and 0.05 mm wide. Anthocodial rods absent.
Holotype 24 cm tall, and 20 cm wide, ascending, sparse growing, (Fig. 1A). Branching irregularly pinnate, and multiplanar, several pseudo-anastomosis occurs in branchlets and branches (Fig. 1A–B). Main stem 4 mm diameter at base, slightly compressed, and short, about 80 mm long arising from a fragment of holdfast, 0.6 mm diameter. Main stem gives off several branches and stumps. The three main branches, 3.0–4.0 mm in diameter, emerging at angles of 45–90°and producing secondary branches subdividing and giving off thin branchlets, up to 2.5 mm diameter, including polyp-mounds. Branchlets irregularly arranged, separated 5–16 mm, and giving off 2 or 3 lateral, secondary branchlets, of same thickness and arrangement. Colony branching up to 11 times. Unbranched terminal twigs blunt, and reaching up to 50 mm long (Figs 1A–B). Polyp-mounds prominent, up to 0.7 mm height and 1 mm in diameter, dome-shaped, with slit-like apertures, arranged irregularly, close together along the branchlets, and very distantly distributed or absent along the thick branches (Fig. 1B). Holdfast devoid of polyps. Colony white (Fig. 1A–B). Sclerites of coenenchyme white, mostly double discs (Fig. 1C). Spindles and disc-spindles, up to 0.14 mm long and 0.04 mm wide, with 4 or 5 whorls of warty tubercles, the ends mostly blunt (Fig. 3A). Double discs up to 0.07 mm long, and 0.05 mm wide (Fig. 3B). Crosses about 0.08x0.06 mm, scarce on samples (Fig. 3C). No anthocodial sclerites present in the samples.
Eugorgia beebei (CASIZ 75783) holotype, SEM coenenchymal sclerites. A spindles and disc-spindles B double discs C cross.
Paratype MCZ 36106 reaches up to 34 cm tall, and 31 cm wide, the main stem 0.7 mm diameter, slightly compressed, and short, about 1.0 cm long arising from an oval holdfast 3.2 cm diameter, and 0.2 cm thick (Fig. 2A–B). Sclerites as in the holotype (Fig. 2C). The other examined specimens are smaller, but very consistent in all aspects with the holotype.
The morphology of the colony, i.e., irregular-pinnate branching and prominent polyps, immediately segregates the new species from the ampla-group, and suggest a similarity with daniana-, rubens- and siedenburgae-groups. Eugorgia beebei and Eugorgia siedenburgae differ from the species in the daniana-group, including Eugorgia mutabilis sp. n. (described below), firstly, in the colony growth, which is sparse and ascending in Eugorgia beebei sp. n. but bushy and profuse in Eugorgia siedenburgae, not flabellate as it is in the daniana-group species. Secondly, it differs in the branching patterns because branchlets in the daniana-group form flat pinnate fronds with pinnae projecting in the same plane. That is not the case in Eugorgia beebei and Eugorgia siedenburgae where the secondary branchlets stick out in several, irregular planes.
Eugorgia siedenburgae and Eugorgia rubens form monospecific groups, they differ especially in the colony growth. The rubens-group have pink, sparse and laterally branched colonies, and the siedenburgae-group, have bushy, bicolored colonies (
Eugorgia beebei and Eugorgia siedenburgae are very similar in sclerite content (Table 1), but they are different especially in the growth form and in the color. The conspicuous bushy colony immediately distinguished it from Eugorgia beebei; additionally, Eugorgia beebei has thicker branches and branchlets than Eugorgia siedenburgae; the polyp mounds are pointed and higher in the latter, and are more rounded in Eugorgia beebei. Branchlets in Eugorgia beebei are longer than in Eugorgia siedenburgae (Table 1).
We found the paratype in the MCZ (36106), labelled in Elisabeth Deichmann’s handwriting (Ardis Johnston, pers. comm.) as a variety of Eugorgia rubens, however, she certainly had not published anything on this genus, thus the variety or the species was never established. The specimen was part of an MCZ public exhibition, the only data we have are the locality. It is probable that this specimen examined by Deichmann came from the Zaca expedition of 1937 and 1938. We do not consider Eugorgia beebei as a variety of Eugorgia rubens because they differ in the traits that have been shown to be informative to separate species in the genus: color, branching pattern, colony growth and sclerite content (see Table 1), as mentioned above.
This new species of Eugorgia is named for explorer/naturalist William Beebe (1877–1962) who studied the marine fauna at numerous locations along the west coast of Central America from Mexico to Columbia during the Templeton Crocker Zaca expedition between 1937 and 1938. Beebe subsequently wrote the book, Book of Bays, which chronicles the five month expedition (
Presently known from Piura, Perú and Baja California, but it is very likely that it exist along all along the geographic range. The depth range is 50–60 m, it is possible the range could extends deeper, but not as deep as reported for paratype USNM 56879, which is probably a mistake, as was remarked by F. M. Bayer (former USNM curator).
http://zoobank.org/B552B4F0-50F4-4E16-BC00-5A3CB2C565C5
http://species-id.net/wiki/Eugorgia_mutabilis
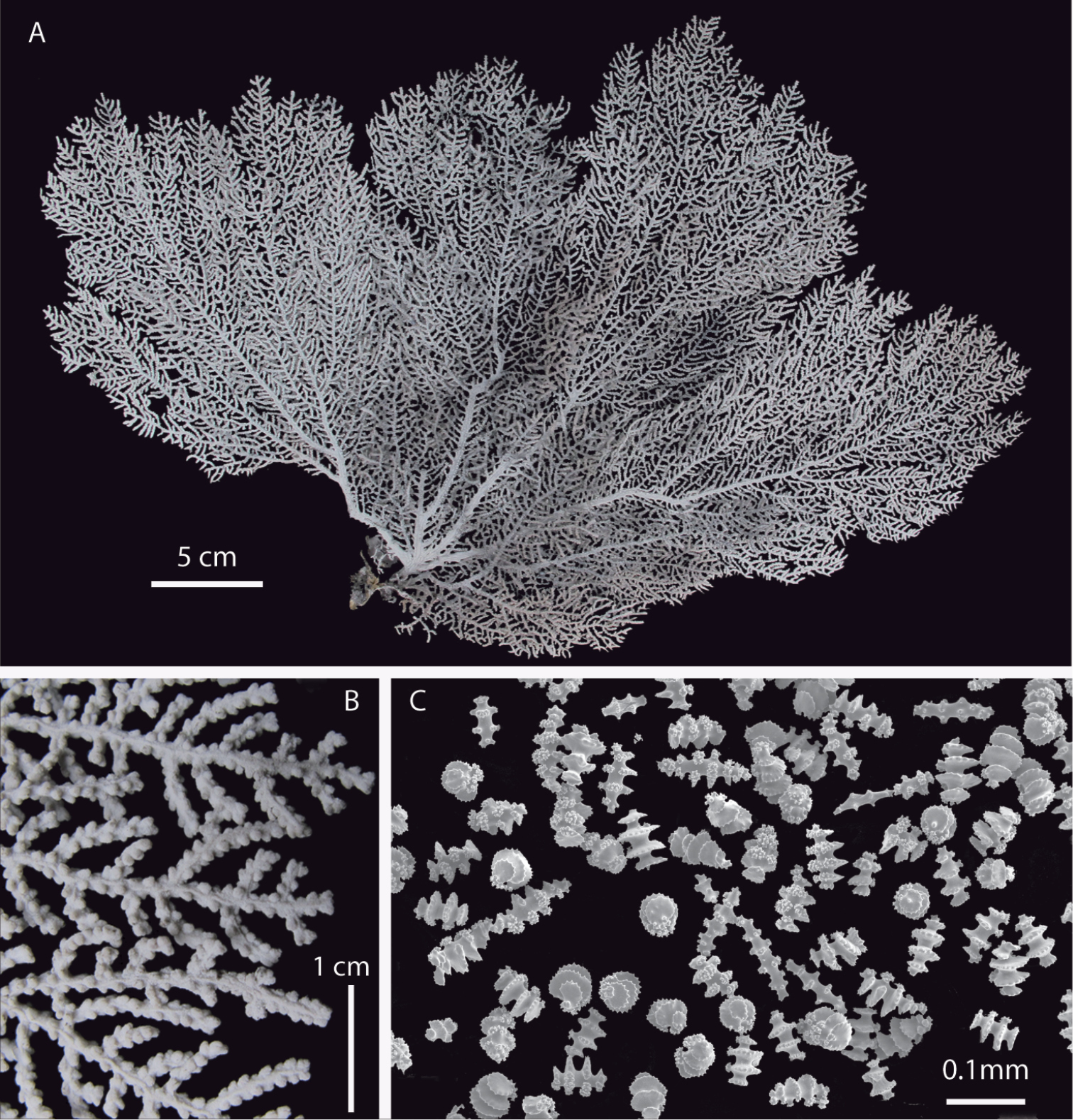
Figs 4–6Holotype. UCR 2297, ethanol preserved, Burbujas, between Los Potreros and Playa Arenitas, Puerto Jiménez, Golfo Dulce, Costa Rica, 11 m, O. Breedy, 9 May 2013. Paratypes. UCR 2298, same data as the holotype. UCR 2272, 2276, fragments, ethanol preserved, Burbujas, 12 m, C. Sánchez, May 2012. UCR 2299, fragment, ethanol preserved, Roca Matapalo, Cabo Matapalo, Golfo Dulce, 20 m, O. Breedy, 6 February 2009. STRI 408, dry, Isla Seca Grande, Gulf of Chiriquí, Panamá, 20 m, H. Guzman, 26 August 2002. STRI 444, dry, Isla Jicarita, Gulf of Chiriquí, 20 m, H. Guzman, 29 August 2002. STRI 511, dry, Isla Ladrones, Gulf of Chiriquí, 15 m, H. Guzman, 14 April 2003. STRI 1073, Santa Cruz, Coiba Island, Panamá, 15 m, H. Guzman, 27 April 2007. STRI 1076, Twin Peaks, Coiba Island, 15 m, H. Guzman, 27 April 2007. STRI 1168, fragment, ethanol preserved, Bajo Hacha, Coiba Island, 20 m, O. Breedy, 16 April 2009. STRI 1122, ethanol preserved, La Blanca, Oxaca, Mexico, 46 m, R. Abeytia, 29 August 2004.
Eugorgia mutabilis (UCR 2297) holotype. A entire colony B detail of branches C SEM sclerites.
Eugorgia mutabilis (UCR 2297) holotype. SEM coenenchymal sclerites. A, B spindles and disc-spindles C double discs.
Puerto Jiménez, Golfo Dulce, Costa Rica, 11 m.
Broad, stout, flabellate colony, main branches sinuous, branching irregularly pinnate, subdividing 5–7 times, no anastomosis present. Prominent polyp-mounds closely spaced and irregularly distributed around branches and branchlets (Figs 4, 6A-C). Colony white, pale pink when alive (Fig. 6A–C), dark grayish when dry or ethanol preserved. Change in color after collection very conspicuous. Longitudinal grooves evident along branches and branchlets. Sclerites white. Spindles and disc-spindles up to 0.15 mm long, double discs mostly 0.05–0.08 mm long. Sclerite discs sharp, serrated and prominent. No anthocodial rods found.
Eugorgia mutabilis, in situ colonies. A, B Burbujas, Puerto Jiménez, Golfo Dulce, Costa Rica, 12 m deep (photographs by C. Sánchez) C Montaña Rusa, Islas Contreras, Panamá, 32 m deep.
Holotype 30 cm tall, and 47 cm wide; colony broad, flabellate, very flexible. Branching irregularly pinnate. Main stem 6 mm diameter, laterally flattened, and short, 14 mm long. Holdfast oval, 40 mm diameter without polyps. Main stem subdividing in 5 sinuous main branches. Main branches slightly flattened on plane of colony, 3–4 mm in diameter emerging at angles of about 45°, bifurcating and diverging producing five flat pinnate fronds of long pinnate branchlets (Fig. 4A–B). Pinnae short, up to 8 mm long, and 1.5–2 mm diameter including polyp-mounds, close together 1–3 mm apart. Branching up to 7 times. Unbranched terminal twigs blunt, and reach up to 8 mm long (Fig. 4A–B). Longitudinal grooves distinct along branches and pinnate branchlets, evident in living and preserved specimens. Polyps white (Fig. 4B). Polyp-mounds prominent, up to 0.7 mm height and 0.8 mm in diameter, arranged mostly in lateral rows along the branchlets and separated by the longitudinal grooves, and more sparsely and irregularly distributed along the thicker branches (Fig. 4B). Colony white to pale pink when alive, gray to dark grayish in ethanol/dry preservation (Fig. 4A). Change in color very conspicuous possibly by liberation of black pigments after collection. Sclerites of coenenchyme white. Sclerite discs conspicuous mostly sharp, serrated and prominent (Fig. 4C, 5A–B). Disc-spindles 0.08–0.12 mm long, and up to 0.06 wide with 4–5 whorls of discs (Fig. 5B); spindles and disc-spindles, longer and thinner up to 0.15 mm long and 0.05 mm wide, with 5–7 whorls of warty tubercles, the ends acute, blunt, or both (Fig. 5A). Double discs up to 0.08 mm long, and 0.05 mm wide with prominent discs (Fig. 5B), some almost complete (Fig. 5B). No crosses, capstans or anthocodial sclerites present in samples.
The specimens present some variation in sclerite color, white sclerites being dominant, but some pale yellow hues could be observed in the samples. In all other aspects they agree with the holotype, including the change of color from bright white alive or recently collected to grayish when fixed. It is interesting that after collection, the specimens discharge a black pigment that turns the water black or the alcohol, and the colony becomes gray. The specimens from Mexico represent the deeper record; they have been observed down to 50 m, meaning that the range of depth extends from 40 to 50 m as it is for Eugorgia rubens, Eugorgia siedenburgae, and Eugorgia beebei, but the morphology of the colony and sclerites remain the same described for Eugorgia mutabilis. Current flow and depth are some of the environmental factors that could influence interspecific variability in octocorals (
The species was mentioned before as a variety of Eugorgia daniana: ‘a white variety has been observed in shoals in Costa Rica and Mexico occurring together with the red form’ (
The new species is found on rocky substrates, in general with other species of gorgonians, including Eugorgia daniana, but in some places, it is the only Eugorgia present (Fig. 6A–C). Other gorgonians found normally inhabiting the same localities are Pacifigorgia irene, Pacifigorgia stenobrochis, Leptogorgia alba, and Carijoa riseii, which were very abundant in the type locality. A variety of associated invertebrates were found on the holotype and paratype UCR 2298, including ophiuroids, Ophiotrhix sp., and crustaceans, shrimps, Periclimenaeus sp. and abundant crabs, Orthochela sp.
The specific epithet is from Latin, mutablilis, changeable, in allusion to the change in color after collecting.
Eugorgia mutabilis belongs to the daniana-group with a characteristic flabellate colony composed of flat pinnate fronds, and irregular pinnate-branching pattern, and prominent polyp mounds. The white color of the colony and sclerites of Eugorgia mutabilis separates it from the rest of the group. However, the new species is similar to Eugorgia daniana in some features, e.g. maximum number of branches, branchlet distance, polyp distribution (see Table 1), but the sclerite composition is very different. The dominant sclerites in Eugorgia mutabilis have very sharp crested discs that are very consistent in all specimens revised from Mexico, Costa Rica and Panama, and along the depth range. These type of sclerites are distinct also from the ones in Eugorgia beebei, and in Eugorgia siedenburgae.
Records from Costa Rica, México and Panamá suggest a wide distribution, at least from Mexico to Panamá, but this has to be further explored. The deepest record in Panama is 35 m, in Costa Rica 25 m, and in Mexico 50 m. Thus, the occurrence of this species from 11 to 50 m deep also suggests a large bathymetric range of distribution.
There are not many morphological characters to differentiate species in octocorals, normally the combination of growth form, and size and color of colony and sclerites are the features used for identification. However, as it was acknowledged above, colony shape can vary within species in response to environmental conditions, light availability, wave exposure and currents (
Molecular studies have been taken to understand boundaries between species and interspecific or intraspecific phylogenetic relationships; however, a complete molecular phylogeny has not been achieved due to the lack of molecular markers with adequate resolution to distinguish species (or sometimes genera) (
Morphological phylogenetic studies in Eugorgia (
Four groups of Eugorgia have been proposed for the eastern Pacific, four species in the daniana-group, one in the rubens-group, onein the siedenburgae-group, and eight in the ampla-group.
Although, Eugorgia beebei sp. n. was first mentioned as a variety of Eugorgia rubens, we have demonstrated that it is a different species that does not even fit in the rubens-group, or in the other related groups (daniana-, siedenburgae-). Thus, a new group is here proposed, the beebei-group characterised by white colonies and sclerites, and with ascending, sparse colony growth.
The diagnostic characters of the daniana-group are herein modified adding the white color for colony and sclerites, to include Eugorgia mutabilis sp. n. in the group. It is important to mention that occurrence of complete double discs sclerites in these species-groups is scant, and the closest example to this type are the ones in the new species. Actually, after examined many specimens especially, in the daniana-group, the occurrence of neat complete double discs is not frequent. It seems a more common character of the ampla-group. The recently described Eugorgia ahorcadensis Soler-Hurtado & López-González, 2012 from Ecuador should be placed in the latter group. However, there is not enough evidence to separate this species from Eugorgia nobilis Verrill, 1868, from which it represents a morphological variety, basically with longer branches and darker sclerites. Therefore, herein we synonymize it with Eugorgia nobilis.
Presently, a total of 15 valid species are recorded for the eastern Pacific but this number should increase when more geographic areas and bathymetric ranges are explored. This research is a contribution to the knowledge of the eastern Pacific octocoral biodiversity.
We are grateful to anonymous reviewers for critical improvements of the manuscript. We thank Adam Baldinger (MCZ) and Stephen Cairns (USNM) for specimen loans, and Ardis Johnston (former collection manager MCZ) for providing information about the MCZ collection, and Rita Vargas (UCR), and Carlos Guevara (STRI) for laboratory and field work. We thank Rosalinda Abeytia for collecting paratype STRI 1122, and Celeste Sánchez for providing the submarine pictures in Figure 3. We thank Federico Bolaños, director of the Field Biology and Conservation in the Tropics Program (Universidad de Costa Rica/ University of Manitoba, Canada) for funding the 2013 collection trip to Golfo Dulce.
This project was partially funded by the Vicerrectoría de Investigación, Universidad de Costa Rica, and Smithsonian Tropical Research Institute.
Specimens from Costa Rica were collected under the MINAE 0890 permit, and from Panama under the permits: DAPVS-02-2007, DAPVS-01-2008, SE/A-71-12, SE/A-16-13 of the Panama Environmental Authority.