(C) 2012 Munetoshi Maruyama. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Termitotrox cupido sp. n. is described from Cambodia and represents the first discovery of Termitotrox Reichensperger, 1915 from the Indo-Chinese subregion of the Oriental region. The type series was collected from fungus garden cells of Hypotermes makhamensis Ahmad, 1965 (Isoptera, Termitidae, Macrotermitinae). Hypotermes Holmgren, 1917 was previously an unknown host of Termitotrox species. The new species is readily distinguished from all known congeners by having wing-shaped trichomes on the elytra and is most probably the world’s smallest scarab, at 1.2 mm in length.
Termitophily, Termitotroginae, Termitotrogini, new species, Indo-Chinese subregion, smallest scarab
The scarab genus Termitotrox Reichensperger, 1915 is composed of ten blind, flightless species from the Ethiopian region (eight species) and the Indian subregion of the Oriental region (two species) (
When
In August 2012, I visited Angkor Wat, Siem Reap, Cambodia, a typical tropical monsoon forest, for five days and worked a total of 15 hours collecting termitophiles associated with fungus-growing termites. I examined fungus gardens of the genera Macrotermes Holmgren, 1910 (four species), Odontotermes (three species), Microtermes Wasmann, 1902 (one species) and Hypotermes (one species). I found 25 Termitotrox beetles from fungus garden cells of Hypotermes makhamensis Ahmad, 1965 by examining more than 80 fungus gardens. The beetles were put in a killing tube (35 ml) with tissue paper and ethyl acetate; two hours later they were removed from the tube and kept in 80% ethanol. Twenty two specimens were dried and mounted for morphological observation, and remaining three are kept in 99% ethanol for future DNA extraction. Specimen photographs were taken using a Canon EOS 60D with a Canon MP-E 65 mm 1-5× macro lens and mounted using a software CombineZM. Images of living beetle were taken by the same camera set in the field by Dr. Takashi Komatsu. Terminology of the species description follows
See
urn:lsid:zoobank.org:act:03F2CD83-2D9D-4F22-8CFE-0CF88264AA2C
Holotype female, north of Preah Khan, Siem Reap, Cambodia, 19 VIII 2012, M. Maruyama (KUM). Paratypes, 6 males, 10 females, same data as holotype (KUM, NHM); 4 males, 1 females, 3 unsexed, same data but 21 VIII 2012 (CMN, KUM, UNSM).
Cupido is the god of desire and love in Roman mythology and is often illustrated as a small, winged boy. The new species is named in reference to the wing-shaped trichomes on the elytra and the remarkably small body size. Noun in apposition.
This species is probably related to Termitotrox minutus (Arrow, 1920) because of its small body size and shape of elytra but easily distinguished from it by the spherical elytra, the presence of the trichomes on the elytra and the smaller body.
General colour uniformly reddish brown, slightly matt; length 1.21 mm. Head. Surface generally evenly convex, with only a slight callosity at clypeofrontal transition. Lateral margin of head entirely, finely marginate. Clypeal outline evenly rounded over entire length. Clypeofrons reddish brown, glabrous, distinctly, moderately punctate; vertex with deep groove medially, and 6 or 7 pairs of sharply defined, elongate primary punctures. Clypeofrontal border at (vague) suture straight; genal tip obtusely angular (in dorsal view); genal surface depressed. Antennal club yellowish brown. Prothorax. Prothorax reddish brown, narrower than elytra, sides (in dorsal view) evenly rounded over anterior half. Emargination at center apex not margined, anterolateral lobe rounded, edge slightly projecting downward (forming side of anterolateral propectoral cavity). Pronotal sides steeply declivous. Posterolateral section of pronotum rounded. Basolateral areas concave, with 1 feeble ridge around base; asymmetrical, left part with a tubercule near base. Apical lobe asymmetrical, with right side near apex roundly emarginate. Pronotal surface glabrous. Costae densely punctate, broader intercostal sulci with distinct, longitudinal wrinkles. Discal depression deep; surface, apart from some local micropunctation, smooth. Pronotal pattern of longitudinal costae as follows: Median costa indistinct around apical 1/5; basomedian section narrow, surface deplanate, shallowly concave. Central depression posterolaterally delimited by depressed area of paramedian costa. Paramedian costa anteriorly broad, distinct, continuing to about half of pronotal length. Sublateral costa narrow, distinct, tapering posteriad to about half of pronotal length, reaching paramedian costa. Lateral costa anteriorly broad, distinct, extending from anterolateral lobe caudad, tapering to base of pronotum. Marginal costa posteriorly broad, ending at depressed basolateral area. Elytra.Semi-spherical, reddish brown, matt, with 6 interstrial costae and intervening striae, and with trichomes at base of costae 2–6 to form wing-shaped patches. Humeral and apical elytral umbones absent; apicosutural edge nearly rectangular, slightly protruding. Epipleuron wide. Elytral striae distinct, deeply impressed, with transverse, weak costae from base to apex to form quadrate cells; striae 1 and 2 reaching basal half. Discal interstrial costae broadly trapezoidal (in cross-section), surface with dense, scattered punctures. Elytral pattern of interstrial costae as follows: costa 1 (next to suture) narrow, shiny, almost rectilinear; costa 2 shiny, tapering in front, stopping at basal half. Costa 3 complete, slightly narrowed at middle. Costae 4–6 complete, strongly developed, Costa 7, 8 and 9 apparently fused together. Anterolateral part of propectus deeply excavate. Preprosternal apophysis distinct, with several setae. Remainder of propectus glabrous, dark reddish brown. Posterolateral area of propectus with some ridges and grooves. Postprosternal surface with small, shallow, median impression. Transverse mesometasternal groove between posterior edges of mesocoxae distinct. Mesothorax. Mesosternum with a pair of identical, question-mark shaped grooves bordering the mesocoxae ; mesosternal surface reddish brown, glabrous, flattened; anterior surface densely micropunctate. Metathorax. Metasternum evenly convex, glabrous, and with fine perimarginal groove all around; reddish brown, infuscate laterally. Abdomen. Venter with 5 visible sternites, all reddish brown, matt, without grooves, sparsely micropunctate. Pygidium reddish brown, glabrous, base broadly margined; surface generally convex; surface lacking distinct microsculpture, sparsely micropunctate. Legs. Procoxa protuberant. Profemur brown, underside glabrous, sparsely micropunctate; outline broadly elliptic, emarginate distally. Protibia pale brown, broad, with short setae, microsculpture weak; shape strongly complanate, with 2 external denticles, no basal serration; apex straight, transverse, with distinct apico-internal spine; internal side strongly dilated from slender base. Protarsus twice longer than width of tibial apex, slender, yellowish; segment 1 inserted in fine groove, as long as segments 2–4 combined. Mesocoxae reddish brown, widely separated, slightly divergent anteriad. Mesofemora brown, broadly elliptic in outline, distally emarginate, surface moderately micropunctate, glabrous. Mesotibiae reddish brown, with several setae, broad, dilated near base, nearly parallel-sided from basal half to apex, edges entire; tibial apex deeply emarginate, with pair of acuminate apico-internal spurs, external one long, slightly curved, internal one short, straight; upper side of mesotibiae with fine longitudinal ridge near outer edge, underside with fine sinuate ridge from base to apico-internal section; with long setae around apical 2/5. Metatibiae similar to mesotibiae, but gently dilated apicad, with apex shallowly emarginate. Meso- and metatarsi brown, compacted-complanate, segments 1–4 short. Length of inner apical spur of metatibia 1/4 of metatibia, reaching base of tarsal segment 5.
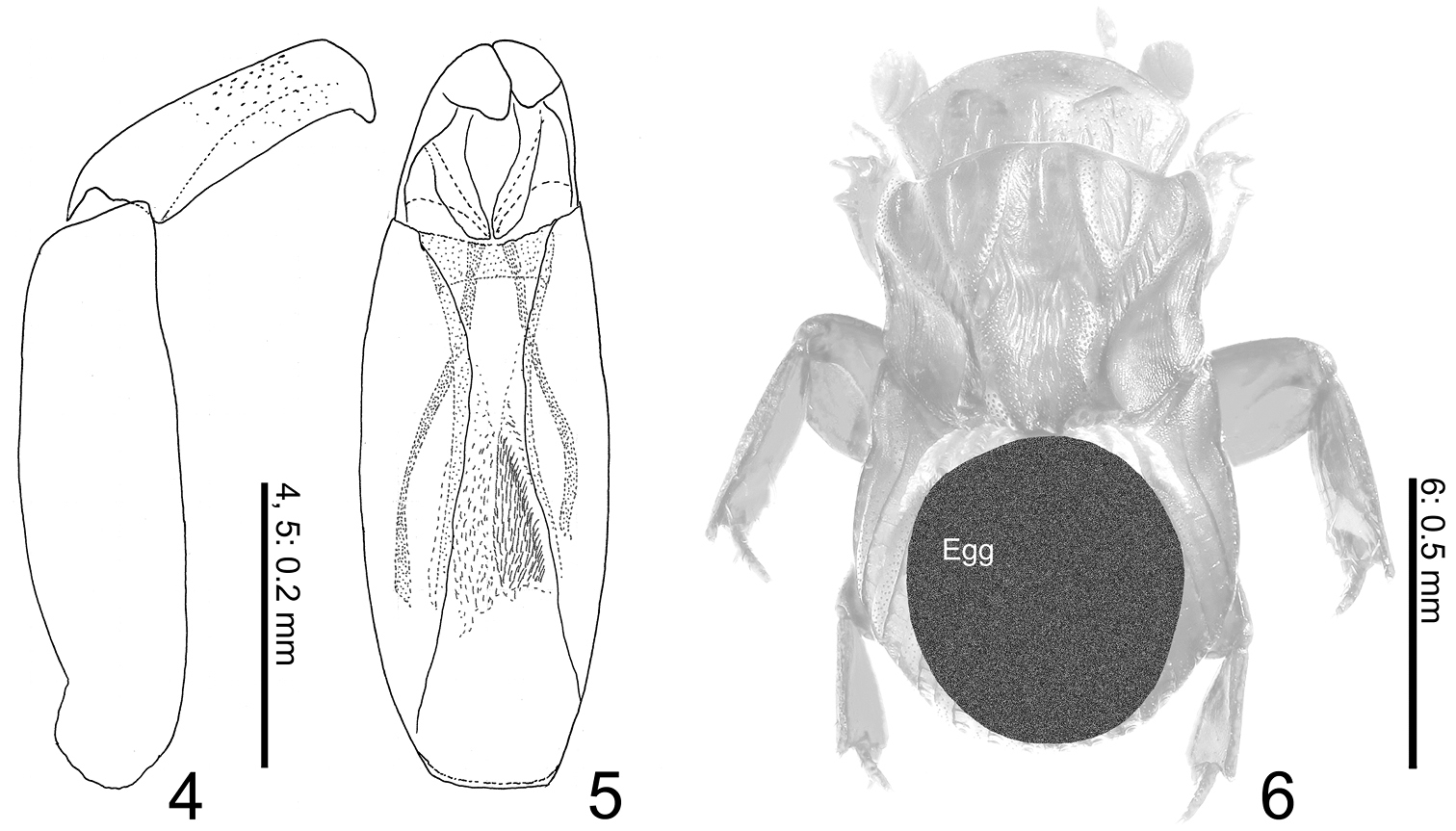
Aedeagus (Figs 4, 5) large, half as long as body length; paramere half as long as phallobase, gently narrowed apicad, curved near truncate apex.
Habitus of Termitotrox cupido sp. n. 1 Holotype, dorsal view 2 same, antero-lateral view 3 paratype, ventral view
Termitotrox cupido sp. n. 4 Aedeagus, lateral view 5 same, ventral view 6 mature egg (shaded circle) inside of female body.
Apical lobe of pronotum variable in shape, sometimes symmetrical, gently rounded at apex. Costa 7 of elytra sometimes indistinct.
Body length 1.13–1.22; maximum width of head 0.48–0.52; median dorsal length of pronotum 0.47–0.51, maximum width 0.54–0.59, sutural length of elytron 0.50–0.54, maximum width 0.62–0.67.
Hypotermes makhamensis (determined by Dr. Yoko Takematsu).
No significant sexual dimorphism is detected. Male aedeagus is large compared with its body size. Female ovary contained a single huge egg occupying the greater part of the abdomen and metathorax (Fig. 6).
Termitotrox cupido specimens were found only on the walls of the fungus garden cells of Hypotermes makhamensis (Figs 7, 8), unlike many other termitophiles associated with fungus-growing termites, which are usually found inside the fungus gardens themselves. In the same habitat (on the cell wall), undescribed species of Odontoxenia Schmitz, 1915, Clitelloxenia Kemner, 1932, Ridiculiphora Disney, 1997 (Diptera, Phoridae) and Discoxenus Wasmann, 1904 (Coleoptera, Staphylinidae) were found, but they were also found inside the fungus gardens.
Termitotrox cupido sp. n. walking on a fungus garden cell.
A schematic illustration of a fungus garden cell of Hypotermes makhamensis and places where Termitotrox cupido beetles were found (black stars).
When the fungus garden was removed from the cell, the beetles walked slowly on the cell wall to escape to a tunnel connected to the other fungus garden cells, by following the termites. No direct contact with the beetle by the termites was observed even though the beetle walked among highly dense columns of the termites. Since Termitotrox cupido is so small (≈ 1.2 mm) and the termites were rushing to escape the disturbance, further observations of its termite association were not possible.
No behavioural information of Termitotrox species is available, other than that reported here for Termitotrox cupido. In two species of Corythoderini, the other termitophilous scarab tribe associated with fungus growing termites, beetles were being carried by worker termites as they do for their own nymphs (
Termitotrox cupido is characterized by the large trichomes on the elytral costae, and these trichomes are not known among other Termitotrox species. Among inquilinous Aphodiinae trichomes on body surfaces have been observed in members of the tribes Corythoderini, Termitoderini, Stereomerini, and Rhyparini.
In the Oriental region the known distribution of Termitotrox species was restricted to the Indian subregion before the present finding of Termitotrox cupido in Cambodia in the Indo-Chinese subregion.
I thank Dr Yoko Takematsu (Yamaguchi University) for identification of the termite host; Dr Takashi Komatsu (Shinshu University) for taking the pictures of the living individual (Fig. 7); Mr Chamroeun Pisyth (Siem Reap) for assistance in the field; Dr Brett Ratcliffe (University of Nebraska State Museum) for critically reading the manuscript and for information on the smallest scarab; Dr. Alberto Ballerio (Brescia, Italy), Dr. Andrey Frolov (Zoological Institute of Russian Academy of Sciences) and Dr. Joseph Parker (Columbia University) for reviewing the manuscript.