Citation: Karasawa S, Goto K (2014) Burmoniscus kitadaitoensis Nunomura, 2009 (Crustacea, Isopoda, Oniscidea) from southern Japan, a junior synonym of B. meeusei (Holthuis, 1947). ZooKeys 386: 21–28. doi: 10.3897/zookeys.386.6727
Re-examination of the holotype of Burmoniscus kitadaitoensis Nunomura, 2009 from Kitadaitojima Island, southern Japan reveals that this species is a junior synonym of B. meeusei (Holthuis, 1947). Partial regions of mitochondrial COI, 12S and 16S rRNA genes, and nuclear 18S and 28S rRNA genes were detected for species identification in the future.
Kitadaitojima Island, mitochondrial DNA, nuclear DNA, Philosciidae, terrestrial isopods
Burmoniscus Collinge, 1914 can be dominant in terrestrial isopod communities in subtropical forests of East Asia (
Burmoniscus meeusei (Holthuis, 1947) was first described as Chaetophiloscia meeusei Holthuis, 1947, based on specimens found from a greenhouse at the Royal Botanic Gardens, Kew, United Kingdom. Later,
The aim of this study is to examine the holotype of Burmoniscus kitadaitoensis and clarify its taxonomic status. Moreover, partial sequences of the mitochondrial COI, 12S and 16S rRNA genes, and nuclear 18S and 28S rRNA genes are detected for DNA markers of species identification.
The holotype of Burmoniscus kitadaitoensis was deposited in Toyama Science Museum (male, TOYA-Cr 14899). We examined the holotype, but the specimen was dissected and in bad condition. However, we were able to observe some parts as follows: male pleopod 1 endo- and exopodites, pleopod 2 endo- and exopodites, male pereiopods 1 and 7, genital papilla, epimera of pereionite 7, and pleotelson. Four specimens were also collected from Kitadaitojima Island (type locality) and Amamioshima Island and were used for measurements of the co-ordinate of the noduli laterales and molecular analysis. The voucher specimens are deposited in the collection of Kitakyushu Museum of Natural History and Human History (KMNH-IvR), Kitakyushu, Fukuoka Prefecture, Japan.
The male pleopods 1 and 2, genital papilla, and male pereiopods 1 and 7 of the holotype, and the position of noduli laterales of specimens collected from Kitadaitojima Island were examined using a Nikon Eclipse E400 microscope (magnification of 40–400×). The epimera of pereionite 7 and pleotelson of the holotype were examined using an Olympus SZH-10 microscope (magnification of 7–64×). A color image was produced from multi-focused montage images using a digital microscope VHX-2000 (KEYENCE Corporation).
The partial sequences of mitochondrial cytochrome oxidase subunit I (COI), mitochondrial 12S and 16S ribosomal RNA (rRNA) genes, and nuclear 18S and 28S rRNA genes were determined for identifying this species in the future. DNA extraction and PCR amplification are described in
PCR primers used in this study.
| Genes | Primer | Sequences (5' to 3') | Source |
|---|---|---|---|
| Forward | |||
| COI | LCO1490 | GGTCAACAAATCATAAAGATATTGG | |
| 12S | 12Sai | AAACTAGGATTAGATACCCTATTAT | |
| 16S | 16Sar-int-sf | GCCGCAGTATHCTRACTGTGCT | |
| 18S | 18Sforward | TACCTGGTTGATCCTGCCAG | |
| 28S | D3A | GACCCGTCTTGAAACACGGA | |
| Reverse | |||
| COI | HCO2198 | TAAACTTCAGGGTGACCAAAAAATCA | |
| 12S | 12Sbi | AAGAGCGACGGGCGATGTGT | |
| 16S | 16Sbr | CCGGTCTGAACTCAGATCACGT | |
| 18S | 18S614r | TCCAAC TACGAGCTTTTTAACC | |
| 28S | D3B | TCGGAAGGAACCAGCTACTA | |
Locality, DDBJ accession numbers and voucher specimens of Burmoniscus meeusei.
| Locality | DDBJ sccession no. | Voucher specimens | ||||
|---|---|---|---|---|---|---|
| COI | 12S | 16S | 18S | 28S | ||
| Minami, Kitadaito Village, Kitadaitojima Island, Okinawa Prefecture, Japan | AB889795 | AB889798 | AB889801 | AB889804 | AB889807 | KMNH-IvR 500720 |
| Daitogu, Kitadaito Village, Kitadaitojima Island, Okinawa Prefecutre, Japan | AB889794 | AB889797 | AB889800 | AB889803 | AB889806 | KMNH-IvR 500722 |
| Yamato Village, Amamioshima Island, Kagoshima Prefecture, Japan | AB889796 | AB889799 | AB889802 | AB889805 | AB889808 | KMNH-IvR 500723 |
http://species-id.net/wiki/Burmoniscus_meeusei
Figs 1–3Holotype of Burmoniscus kitadaitoensis, TOYA-Cr-14899, male, dissected, near Daitogu, Kitadaitojima Island, Okinawa Prefecture, Japan, 25th November 2006, Noboru Nunomura leg; non types, 2 male, KMNH-IvR 500720 and 500721, 25.9314°N, 131.3094°E, Minami, Kitadaito Village, Kitadaitojima Island, Okinawa Prefecture, Japan, 30th June 2012, Takeshi Goto leg.; non type, 1 male, KMNH-IvR 500722, 25.9444°N, 131.3021°E, Daitogu, Kitadaito Village, Kitadaitojima Island, Okinawa Prefecture, Japan, 30th June 2012, Takeshi Goto leg.; non type, 1 male, KMNH-IvR 500723, 28.3560°N, 129.3935°E, Yamato Village, Amamioshima Island, Kagoshima Prefecture, Japan, 12th September 2012, Shigenori Karasawa leg.
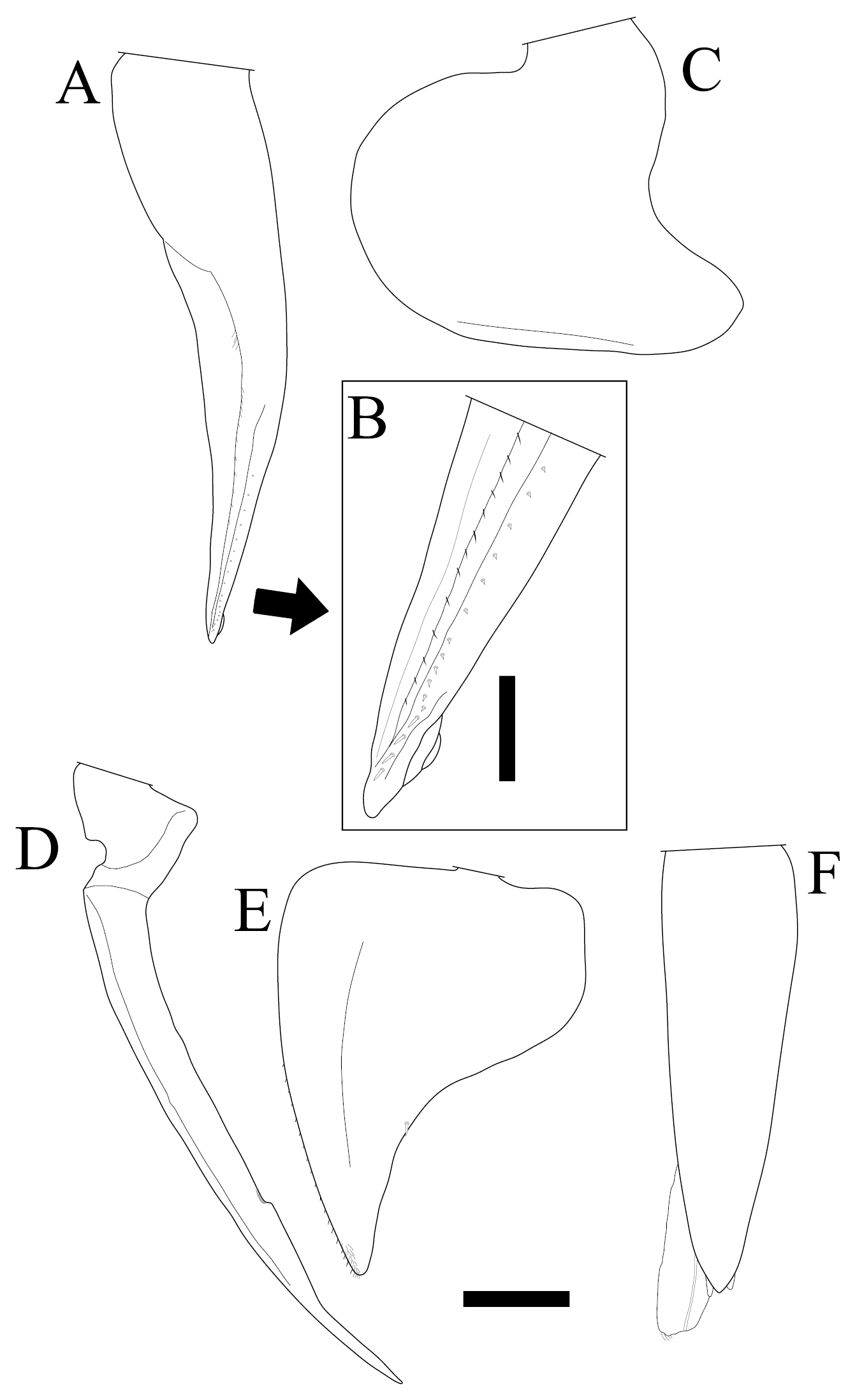
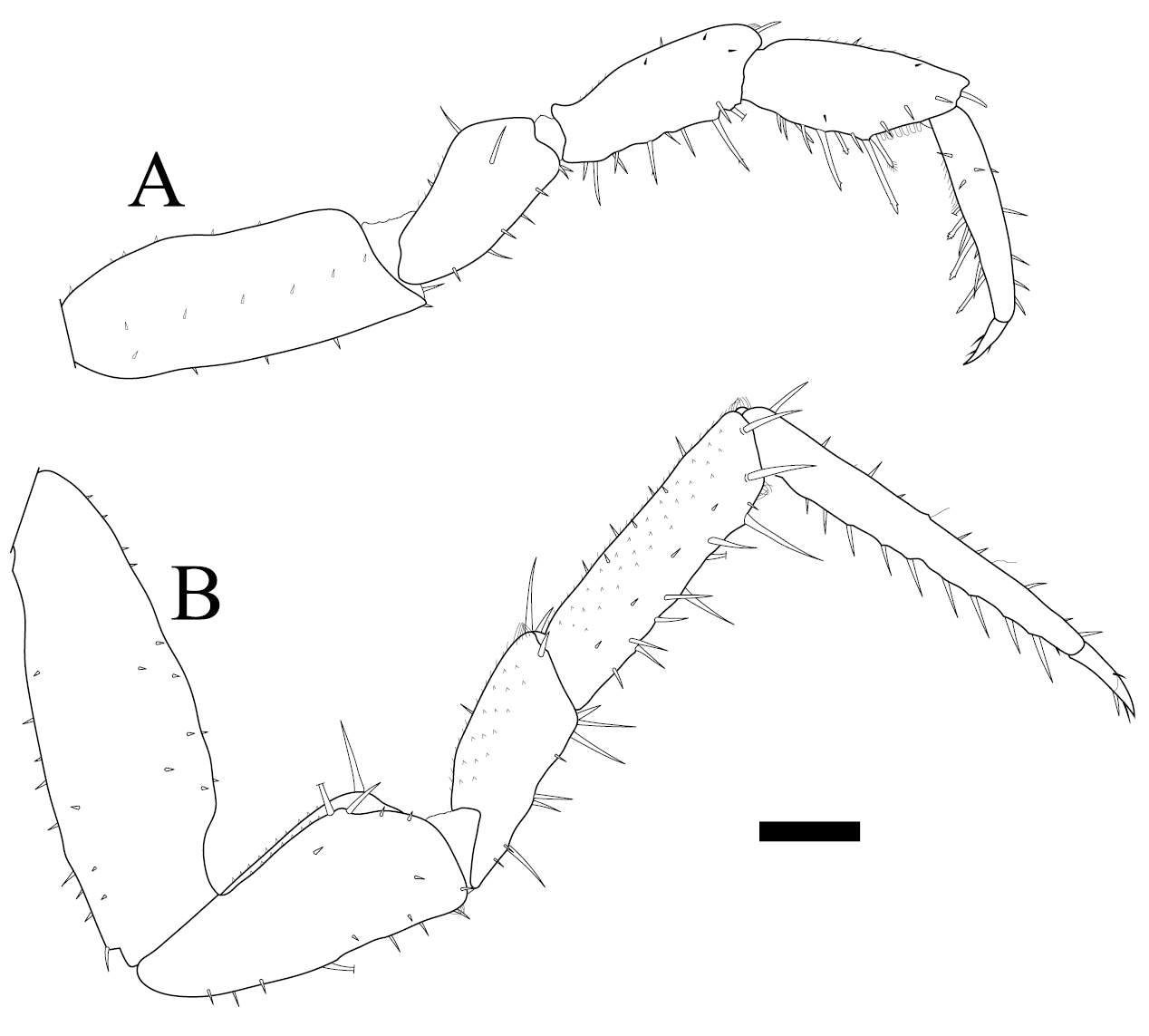
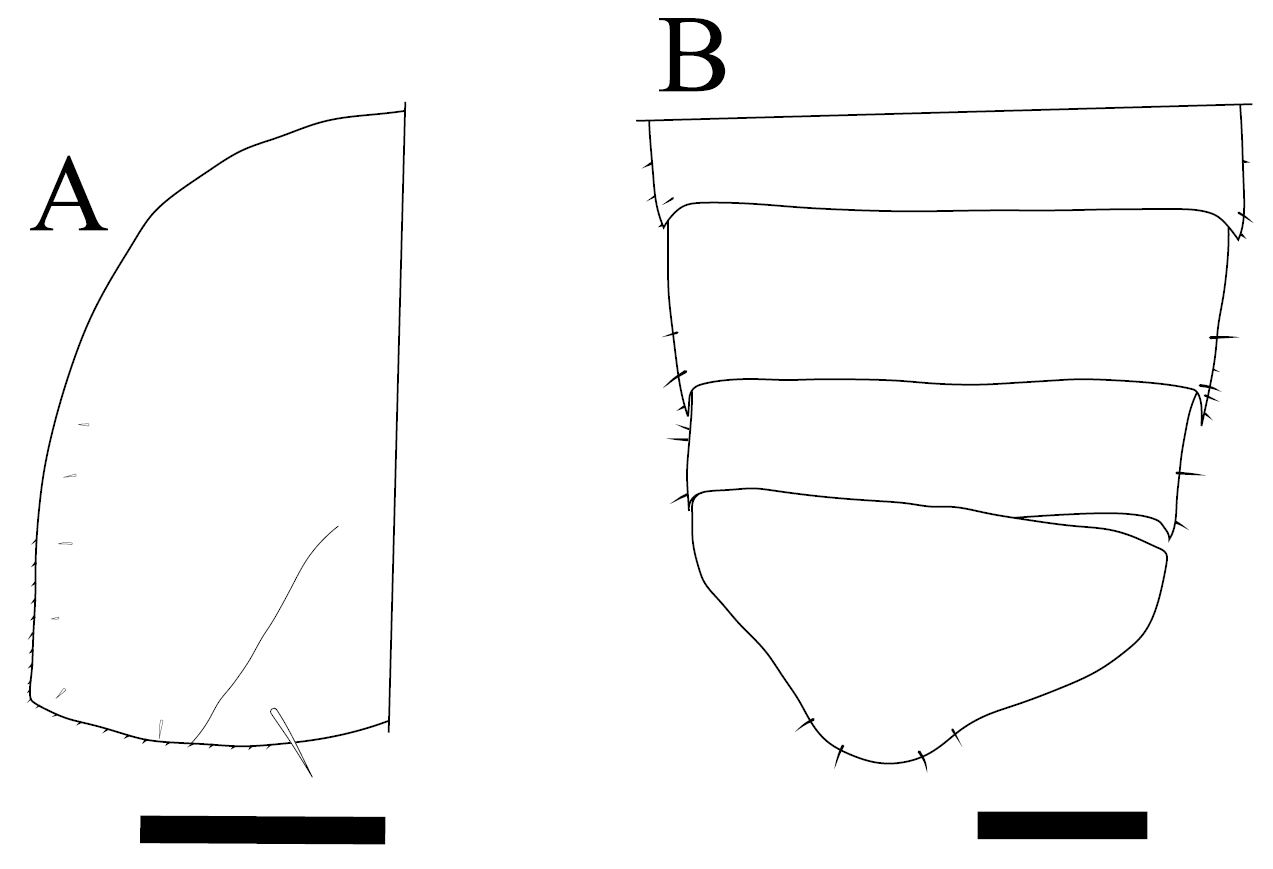
Burmoniscus meeusei is characterized by the male pleopod 1 endopodite slender and gradually narrowing to the apex (Fig. 1A) with a small lobe on the inner margin close to the apex (Fig. 1B); the male pleopod 1 exopodite with triangular posterior point bent outward and inner margin evenly convex (Fig. 1C); the male pleopod 2 endopodite slender (Fig. 1D) and exopodite trapezoidal (Fig. 1E); genital papilla elongated and simple (Fig. 1F); male pereiopods 1 and 7 without particular modifications (Fig. 2A, B); the pereonite 7 with postero-lateral corners at obtuse angle (Fig. 3A); and the apex of pleotelson broadly rounded (Fig. 3B).
Burmoniscus kitadaitoensis, male, holotype, TOYA-Cr 14899. A, B Pleopod 1 endopodite C pleopod 1 exopodite D pleopod 2 endopodite E pleopod 2 exopodite F genital papilla. Scale bars: A, C–E 200 μm, B 50 μm.
Burmoniscus kitadaitoensis, male, holotype, TOYA-Cr 14899. A Pereiopod 1 B pereiopod 7. Scale bar: 200 μm.
Burmoniscus kitadaitoensis, male, holotype, TOYA-Cr 14899. A Left epimeron of pereonite 7 B telson. Scale bars: 300 μm.
On the holotype of Burmoniscus kitadaitoensis, a small lobe was found on the inner margin of the male pleopod 1 endopodite, although this character was not shown in the original description (Fig. 3P in
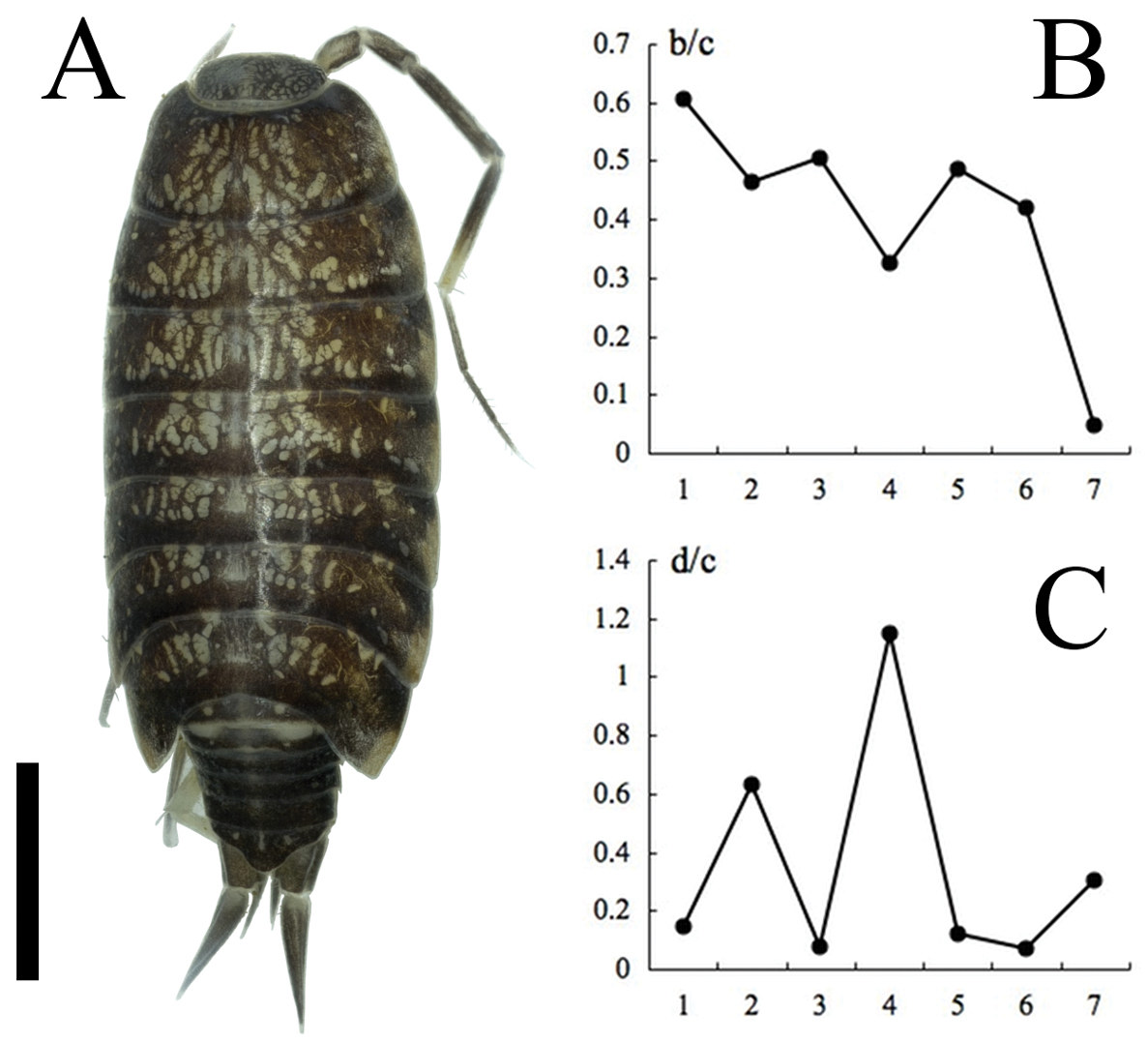
Burmoniscus meeusei, male, collected from Kitadaitojima Island, Japan. A Dorsal view of body, left antenna 2 broken, KMNH-IvR 500720 B co-ordinate of noduli laterales (b/c), KMNH-IvR 500721 C co-ordinate of noduli laterales (d/c), KMNH-IvR 500721. Scale bar: 1 mm.
Burmoniscus meeusei was previously reported from the United Kingdom (greenhouses), Hawaii, Brazil, Taiwan (
The COI, 12S rRNA, 16S rRNA, 18S rRNA and 28S rRNA alignments comprised 653, 354, 453, 675 and 635 bp, respectively. With the exception of the 12S rRNA gene, there is no difference in the four genes among the specimens collected from Kitadaitojima and Amamioshima Islands. Only one 12S rRNA gene base varied between the specimens collected from the two islands.
We thank Dr. Hisashi Negoro (Toyama Science Museum) and Mr. Noboru Nunomura (Kanazawa University, Institute of Nature and Environmental Technology) for loaning the holotype of Burmoniscus kitadaitoensis.