(C) 2013 Jowita Drohojowska. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Drohojowska J, Kalandyk-Kołodziejczyk M, Simon E (2013) Thorax morphology of selected species of the genus Cacopsylla (Hemiptera, Psylloidea). In: Popov A, Grozeva S, Simov N, Tasheva E (Eds) Advances in Hemipterology. ZooKeys 319: 27–35. doi: 10.3897/zookeys.319.4218
The paper concerns with characteristics of a thorax morphological structure Cacopsylla Ossiannilsson, 1970 species, referring to an analysis of five species classified in the past in three subgenera. The structure of the sternites, tergites and pleurites of all the parts of the thorax was studied by a scanning microscope. Descriptions of particular elements building up thorax plates, their shape, size and links as well as a course of all the clefts and sulcus are provided. The study of thorax morphology of Cacopsylla species suggests that the thorax morphology is relatively homogenous within a genus.
Psylloidea, Cacopsylla, morphology, thorax
Jumping plant-lice or psyllids (Psylloidea) constitute a superfamily of Sternorrhyncha comprising over 3800 described species of small plant-sap feeding insects (
Studies of the adult morphology have concentrated mainly on the head, fore and hindwings as well as the terminalia (
The genus Cacopsylla Ossiannilsson, 1970 belongs to the subfamily Psyllinae (Psyllidae) and consists of approximately 170 described species associated with woody dicotyledonous plants as does the majority of species of this family and psyllids in general. The genus has a predominantly Holarctic distribution with a few species occurring in the Afrotropical, Oriental and Australian biogeographical regions (
Adult psyllids were collected with an entomological sweep-net and killed in vapours of potassium cyanide. After air drying and removing the wings and legs, the specimens were cleaned with 10% alcohol and then mounted on a stub for the analysis in a low vacuum electron scanning microscope S-3400N. The specimens were not gold coated. The following species were analysed: Cacopsylla peregrina (Foerster, 1848), syn. Cacopsylla (Cacopsylla) peregrina (Foerster, 1848); Cacopsylla sorbi (Linnaeus, 1767), syn. Cacopsylla (Cacopsylla) sorbi (Linnaeus, 1767); Cacopsylla ambigua (Foerster, 1848), syn. Cacopsylla (Hepatopsylla) ambigua (Foerster, 1848); Cacopsylla crataegi (Schrank, 1801), syn. Cacopsylla (Thamnopsylla) crataegi (Schrank, 1801); Cacopsylla melanoneura (Foerster, 1848), syn. Cacopsylla (Thamnopsylla) melanoneura (Foerster, 1848). Additional 24 Cacopsylla species deposited in the Department of Zoology, University of Silesia (Poland) were analysed using Nikon MZ1500 stereoscopic microscope.
Morphological terminology and the list of abbreviations used to describe the photographs is after
In all Cacopsylla species the head is strongly inclined from longitudinal body axis resulting in an arched dorsal outline of the thorax. The prothorax is the smallest thorax segment and undergoes strong modifications because the mouth parts are displaced posteriad. Mesothorax is the biggest part of the thorax related to a functional dominaton of forewings, which is further connected with the development of muscles that move them.
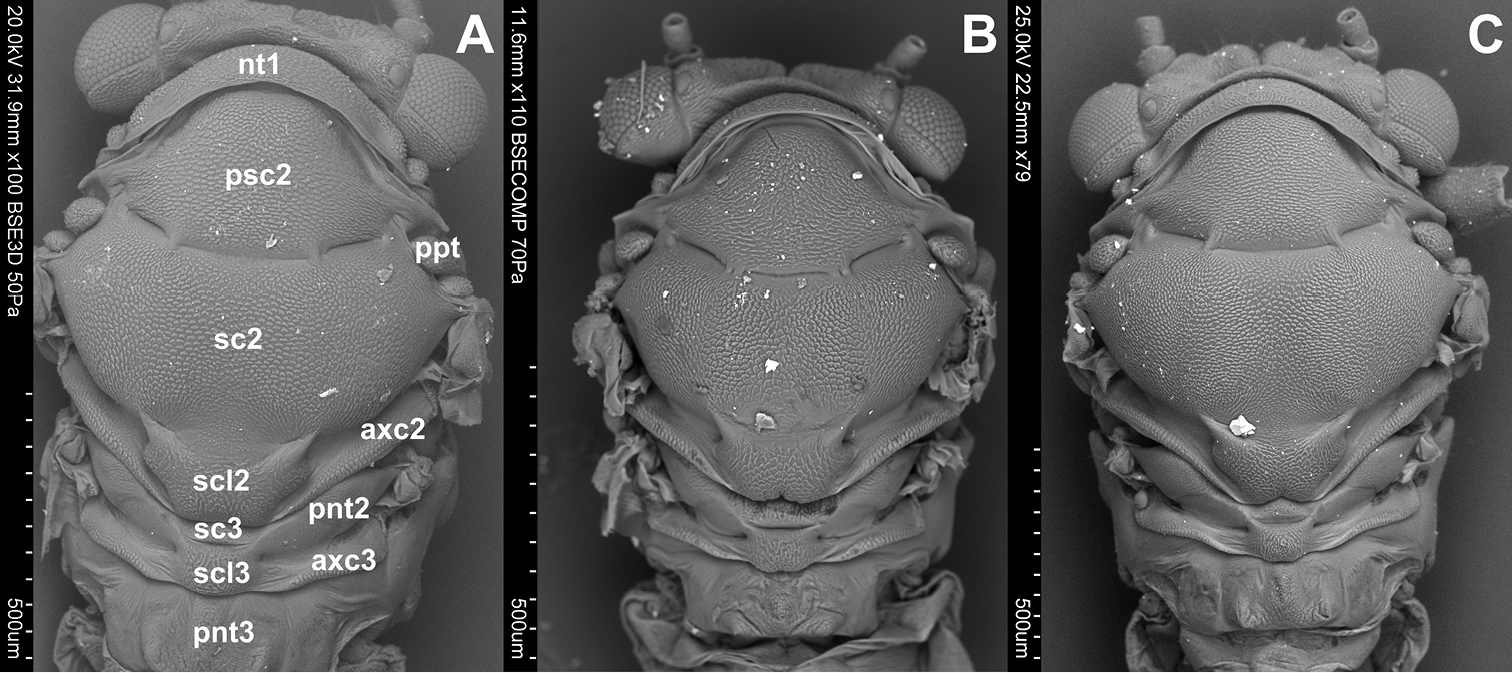
Dorsum (Figs 1A, B, C). The dorsum of the prothorax consists of one sclerite, the pronotum (nt 1), which is narrow and arcuate medially. Anterior and posterior margins of pronotum are distinct. The pronotum is narrower than the head including eyes but wider than vertex. Laterally the pronotum is as long as the pronotum along midline. The dorsum of the mesothorax is divided into the large mesonotum and the small, transverse, weakly raised mesopostnotum. The mesonotum is divided into three sclerites: mesopraescutum (psc2), mesoscutum (sc2) and mesoscutellum (scl2), which are separated from each other by a distinct sulcus. The mesopraescutum (psc2) is a medium sized sclerite, it’s fore margin is slightly arched and covered by the hind margin of the pronotum. The hind margin of the mesopraescutum is vaguely semicircular. The mesopraescutum and mesoscutum are linked together because the sulcus which separates them is not complete. In the middle there are two symmetrical joints except for Cacopsylla ambiqua. The mesoscuctum (sc2), the biggest sclerite of the mesonotum, is U-shaped with elongated processes reaching the parapteron. The mesoscutellum (scl2) is small and trapezoidal. The hind part of the mesoscutellum is strongly incised in the middle in Hepatopsylla and some Thamnopsylla species. The mesopostnotum, situated behind mesonotum, is not visible dorsally as it is covered by the mesoscutellum (scl2), laterally it forms a narrow ridge running diagonally towards the mesoepimeral sclerite. The metathorax consists dorsally of the metanotum and metapostnotum. The metanotum consists of two plates, the metascutum (sc3) and the metascutellum (scl3). The metascutum (sc3) is a small sclerite, while the metascutellum (scl3) is bigger and clearly raised in relation to the metascutum. The metapostnotum (pnt3) is relatively large and trapezoidal. The metapostnotum is completely fused with the first abdominal tergite.
Dorsal side. A Cacopsylla peregrina B Cacopsylla crataegi C Cacopsylla ambigua
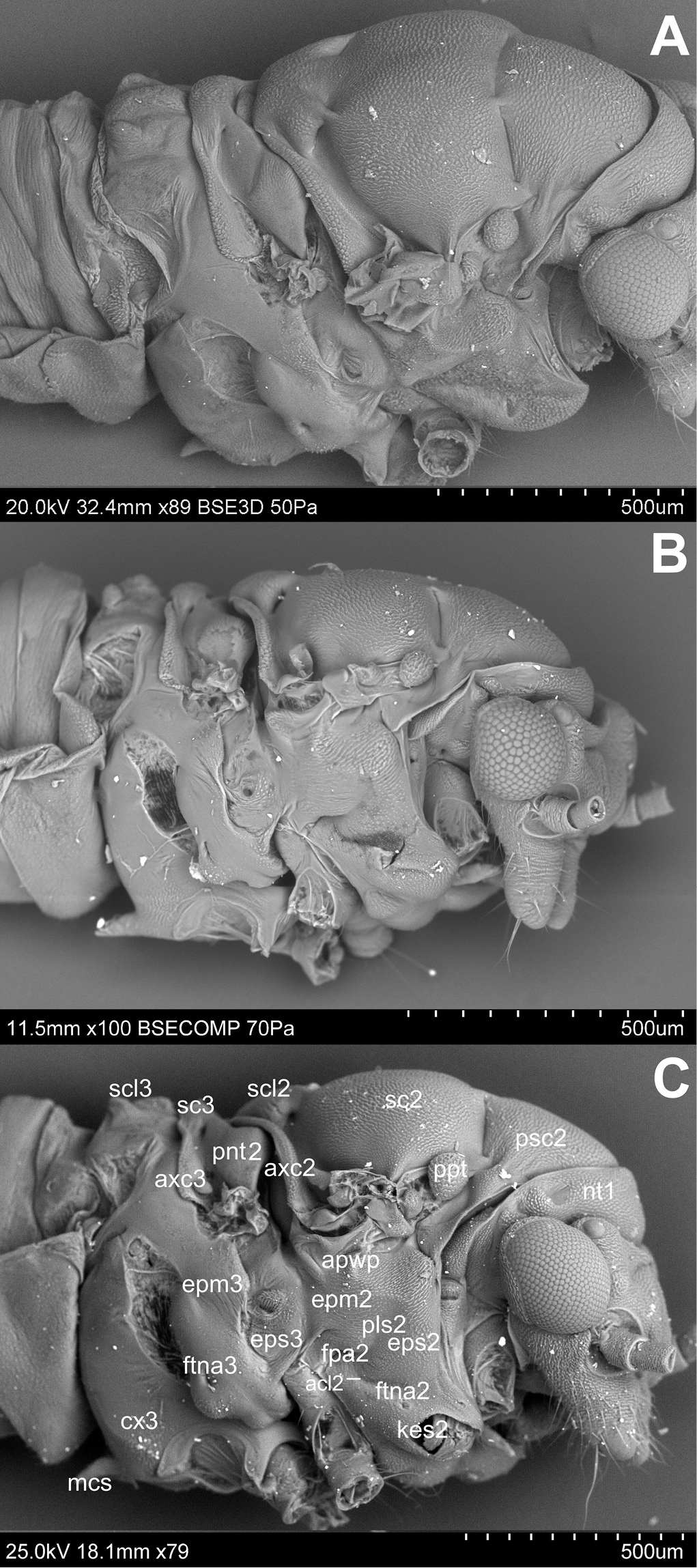
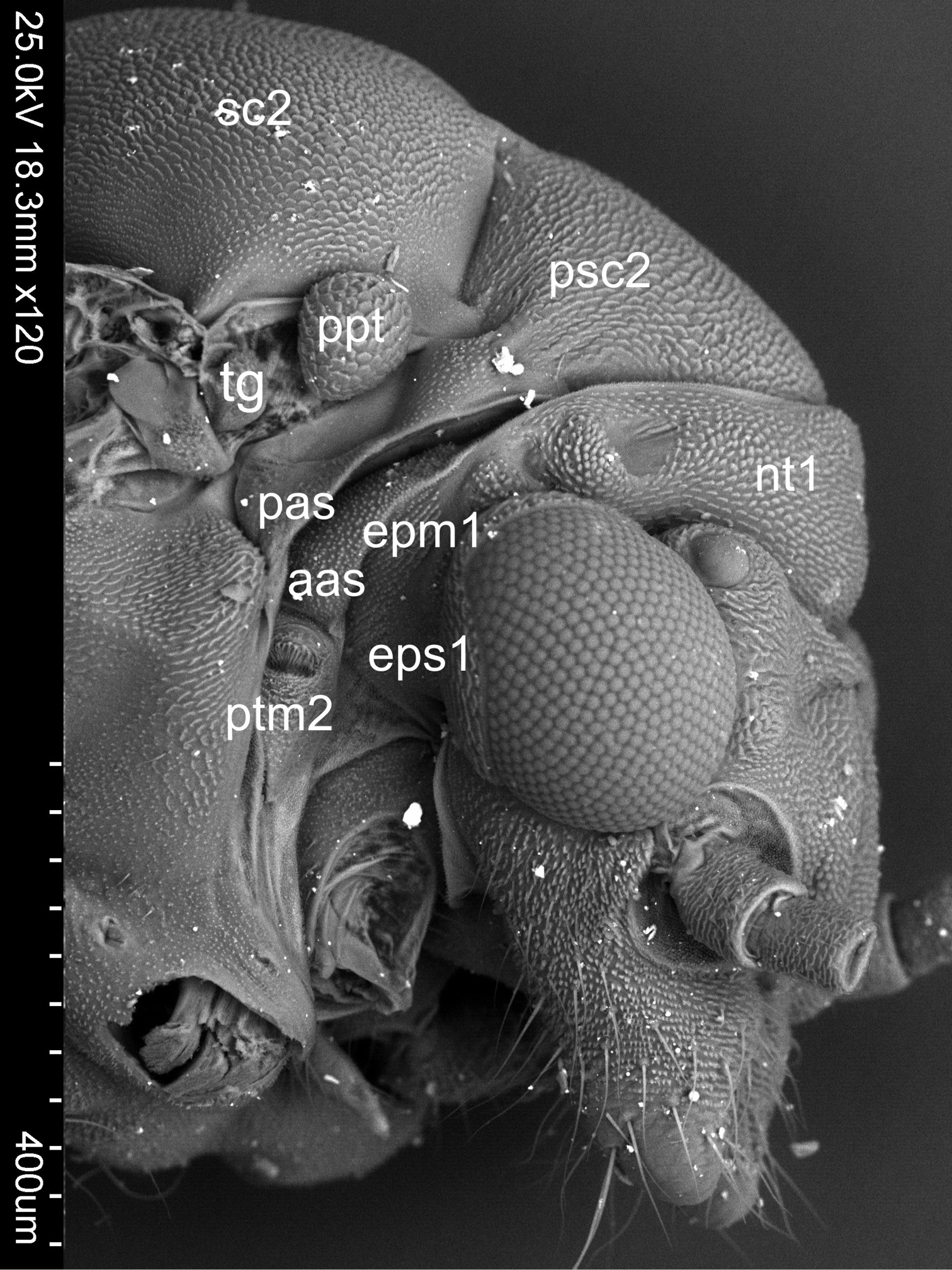
Pleura (Figs 2A, B, C). Laterally the pronotum is constituted by two easily visible pleurites (Fig. 4), the proepisternum (eps1) in front and the proepimeron (epm1) at the rear. Proepisternum and proepimeron are of the same size. They are always clearly separated from each other by the propleural sulcus, while their border with prothorax is not always visible. The propleural sulcus (pls1) runs from the basal coxal appendix up to the hind corner of the pronotum. The sulcus is straight without any curves. Between the pleurites of pro- and mesothorax, there are three tiny, well visible sclerites of arguable origin, which are called frontal and hind additional sclerites (aas and pas), and peritrema (ptm2) which surrounds the mesothoracic spiracle (Fig. 4). The mesothoracic pleurae consist of two large sclerites (Fig. 2), the anterior mesepisternum (eps2) and posterior mesepimeron (epm2). The shape of the mesepisternum (eps2) depends on the position of the pleural sulcus (pls2) and anapleural cleft (acl2). The mesothoracic pleural sulcus (pls2) is well visible and runs from the coxal condyle and approaches the middle of pleuron but does not reach the wing base. Shape and position of the mesopleural sulcus are variable within Cacopsylla. It is relatively straight in Cacopsylla peregrina, slighty curved in the distal part in Cacopsylla melanoneura or S-shaped and strongly curved in Cacopsylla crataegi and Cacopsylla ambigua. It forms a shallow furrow pleural with small, shallow fossa (ftna2) in Cacopsylla melanoneura or a distinct furrow with deep fossa in Cacopsylla ambigua. It is very long, reaching the end of the mesepisternum in Cacopsylla crataegi but considerably shorter in the other species. The anapleural cleft (acl2) is straight and almost horizontal, the transepimeral sulcus is very short and distinct, it is never straight. The anterior pleural wing process (apwp) is thin and elastic. Parapteron (ppt) and tegula (tg) have the same oval shape and the katepisternal complex (kes2) is rounded and subtle striped. The metapleurites are well developed. The metapleural sulcus (pls3) is visible and reduced at a short distance ventrad. This sulcus does not separate metaepimeron and metaepisternum entirely. The metapleuron is strongly modified by a vertical prolongation of the coxal meron. The latter enforces on metaepimeron and metaepisternum a development into a long, thickened arch stretching above the metacoxa. The metathoracic stigma (stg3), trochantin (trn3) and external fossa of the trochantinal apodeme (ftna3) are well visible.
Lateral side A Cacopsylla peregrina B Cacopsylla crataegi C Cacopsylla ambigua
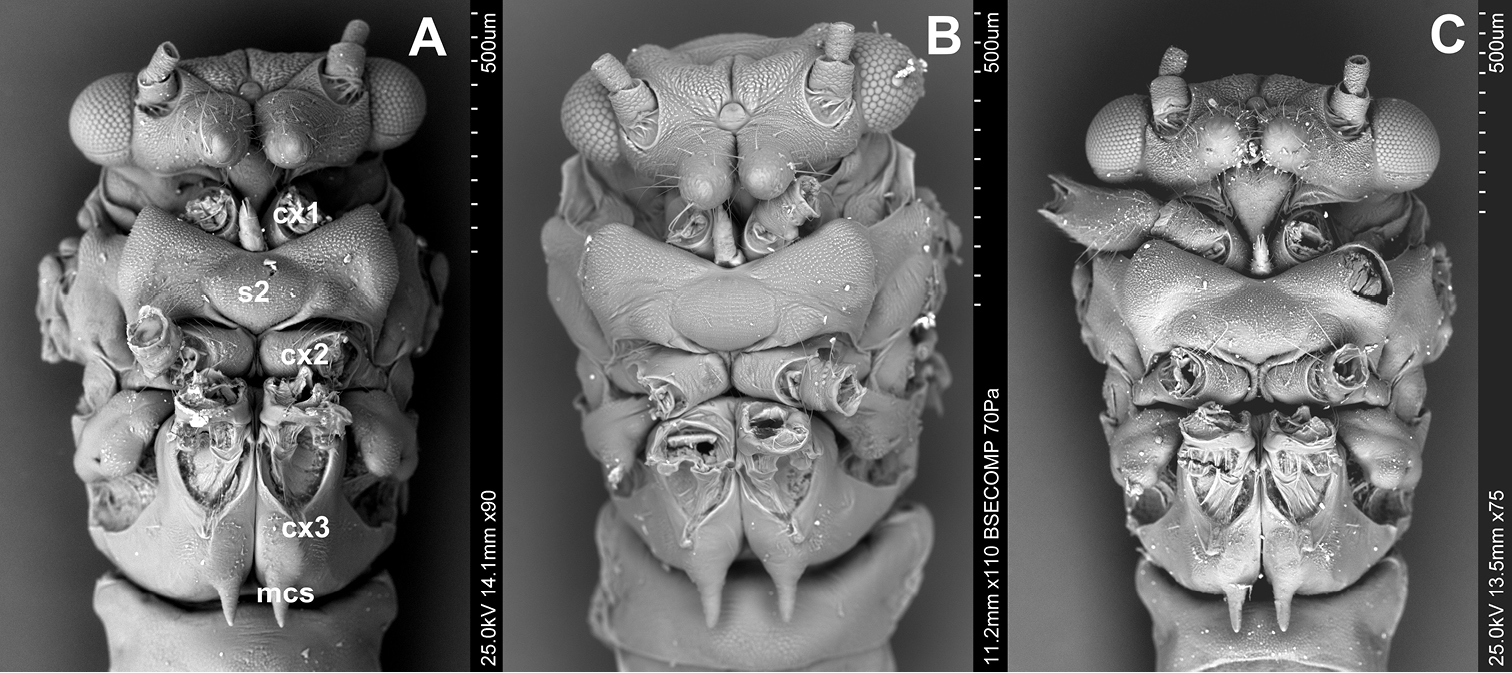
Venter (Figs 3A, B, C). The prothoracic sternum of psyllids is significantly reduced and covered by the rostrum. The mesothoracic venter is constituted by a well-developed sclerite, the mesosternum (s2), connected with mesocoxa. The metathoracic venter is narrow, in front it takes the form of a thickened plate, surrounding metacoxae (cx3) (Figs 3A, B).
Ventral side A Cacopsylla peregrina B Cacopsylla crataegi C Cacopsylla ambigua.
Sclerites between pleurites of pro- and mesothorax of Cacopsylla crataegi
The analysis of the thorax morphology of selected Cacopsylla species did not indicate any characters defining the subgenera described by
An additional feature common for all Cacopsylla species is the straight rather than undulating propleural sulcus stretching from the basal coxal appendix to hind corner of the pronotum, and not in the middle, like for example in Craspedolepta species or the fore corner of pronotum like in Livia species (
This and earlier studies (
The study of thorax morphology of Cacopsylla species shows that the only difference between the male and the female is in the size of the latter. Shapes and proportions between particular elements of the thorax are the same in the representatives of both sexes. The results confirm those of previous studies (
Special thanks go to Magdalena Kowalewska-Groszkowska and Malwina Roszkowska (Department of Scanning Microscopy, Polish Academy of Science) for taking microscopic pictures. Daniel Burckhardt (Naturhistorisches Museum, Basel, Switzerland) is thanked for critical reading of the manuscript and useful comments.