(C) 2013 Zhong-hua Fan. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Fan Z-h, Liu G-q (2013) The genus Cahara Ghauri, 1978 of China (Hemiptera, Heteroptera, Pentatomidae, Halyini) with descriptions of two new species. In: Popov A, Grozeva S, Simov N, Tasheva E (Eds) Advances in Hemipterology. ZooKeys 319: 37–50. doi: 10.3897/zookeys.319.4275
Cahara Ghauri from China with three species is reviewed here. Two of them, Cahara incisura sp. n. and Cahara nodula sp. n. are described here. Key to the three Chinese species, habitus photographs and illustrations of genitalia are also provided. All examined materials including the types of three species mentioned are deposited in the Institute of Entomology, Nankai University, Tianjin, China (NKUM).
Hemiptera, Heteroptera, Pentatomidae, Cahara, China, new species
Here we do not discuss the status of Cahara in Halyini, since both the monophyly and definition of Halyini are doubted.
Male genitalia were illustrated after treatment with warm 2% NaOH solution for about 30–50 min, while female genitalia were illustrated directly. Photographs of both dorsal and ventral habitus were made using a Nikon SMZ1000. All measurements are in millimeters. All the studied specimens including the examined types are deposited in the Institute of Entomology, Nankai University, Tianjin, China (NKUM). The terminology of aedeagus follows
http://species-id.net/wiki/Cahara
Dalpada brevivitta Walker, 1867 by original designation.
| 1 | Lateral margin of each mandibular plate with an angular process before eye (Figs 3a–b); ventral margin of male pygophore with two mesial processes originating from one stem (Fig. 29) | Cahara tibetana Zheng & Liu, 1986 |
| – | Lateral margin of each mandibular plate without any angular process before eye (Figs 1a–b, 2a–c); ventral margin of male pygophore without above processes | 2 |
| 2 | Apex of clypeus broad, mandibular plates not convergent at the apex (Figs 2a–c); humeral angles distinctly elevated (Fig. 5); rostrum passing beyond the middle of the 4th sternum; ventral margin of male pygophore without process (Fig. 21) | Cahara nodula sp. n. |
| – | Apex of clypeus narrow, mandibular plates convergent at the apex (Figs 1a–b); humeral angles not elevated (Fig. 4); rostrum reaching the middle of 3rd sternum; ventral margin of male pygophore with two lateral separated processes (Fig. 13) | Cahara incisura sp. n. |
urn:lsid:zoobank.org:act:0ABF44CA-EE92-47D2-8864-68409E204BEA
http://species-id.net/wiki/Cahara incisura
Figs 1a–1b, 4, 7–8, 13–20Holotype male, pinned, CHINA: Sichuan Province: Mianning County, Liangshan Prefecture, 29. VIII. 2008, Kai DANG leg. Paratypes: all pinned, CHINA: Sichuan Province: 1 female, same data as holotype; 1 male, with genitalia in a separate microvial, same data as holotype.
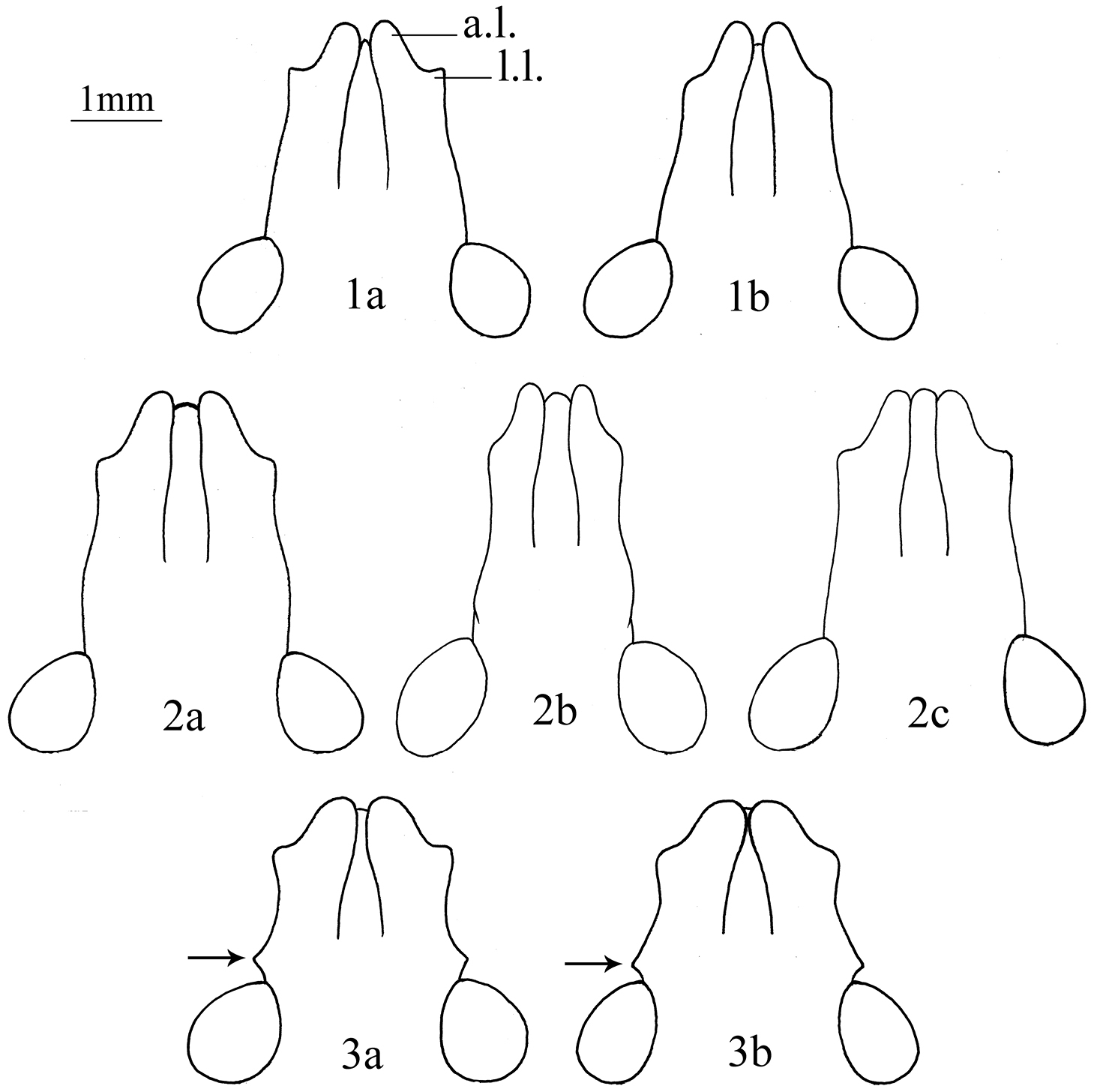
Rostrum reaching the middle of 3rd sternum, pronotal humeral angles not elevated upwards, apical meeting trend of mandibular plates are all similar to Cahara tibetana. But Cahara tibetana has a distinct angular process before each eye along the lateral margin of mandibular plate, mandibular plates about equal to or slightly longer than clypeus. While in this new species, mandibular plates are always longer than the clypeus, lateral margins of head sinuate and with no angular process before eye.
Body size Male, length 16.0mm, width between humeral angles 8.0mm. Female, length 17.0mm, width between humeral angles 8.5mm.
Color and puncturing. Dorsum fuscous, darkly and thickly punctured, with several obscure patches formed by dense punctures: four or five longitudinal strips on the pronotum, five on the scutellum (one short oblique strip near each arcuate callus behind the fovea of scutellar basal angle, one patch on central disk, two short longitudinal stripes at the level of the posterior apices of frena), two or three patches on each corium. Scutellar apex paler and punctures finer. Calli on the anterior disk of pronotum ochraceous with punctures in the middle. Humeral angles piceous, tips a little pale, with several transverse furrows and wrinkles on the dorsal base. Hemelytral membrane fuliginous, except apices of veins paler. Head beneath black, except buccula and one obscure strip behind each antenniferous tubercle ochraceous. Thoracical pleura thickly and darkly punctured, each episternum with an ochraeous, laevigate and arcuate fascia distally. Mesosternum black strips laterally. Legs ochraceous, with irregular brown spots, tibiae paler in the middle third and darker in the apical third, first two tarsal segment and apex of the third one white dorsally. Ventral abdomen smooth at center, punctures gradually getting denser laterally. Middle third of each laterotergite with a transverse brown impunctate stripe.
Structure. Head. Mandibular plates longer than clypeus, apices with meeting trend but still separated, forming an incision before clypeus. Lateral lobes of mandibular plates are found angulate in the male holotype and the female paratype (Fig. 1a), but obtuse in the male paratype (Fig. 1b). Buccula with anterior angle not produced, gradually evanescent posteriorly. Antennae ochraceous, darker to the end, antennomere I paler except each lateral side, base of antennomere IV and basal third of antennomere V stramineous, IV> V>III >II>I in length. Rostrum reaching the middle of 3rd sternum, apex of 1st segment equal to the posterior end of buccula.
Thorax. Pronotum with anterior margin slightly convex in the middle, anterior angle produced laterad, anterolateral margins concave, crenulate along the anterior half, crenulation getting weaker posteriorly. Humeral angles horn-like, apices obtuse, slightly produced and not elevated upwards. Hemelytral membrane longer than the abdominal end. Peritreme groove shaped according to
Abdomen. Connexiva exposed broadly, posterior angles sharp and produced. Mesial groove on ventral side not distinct.
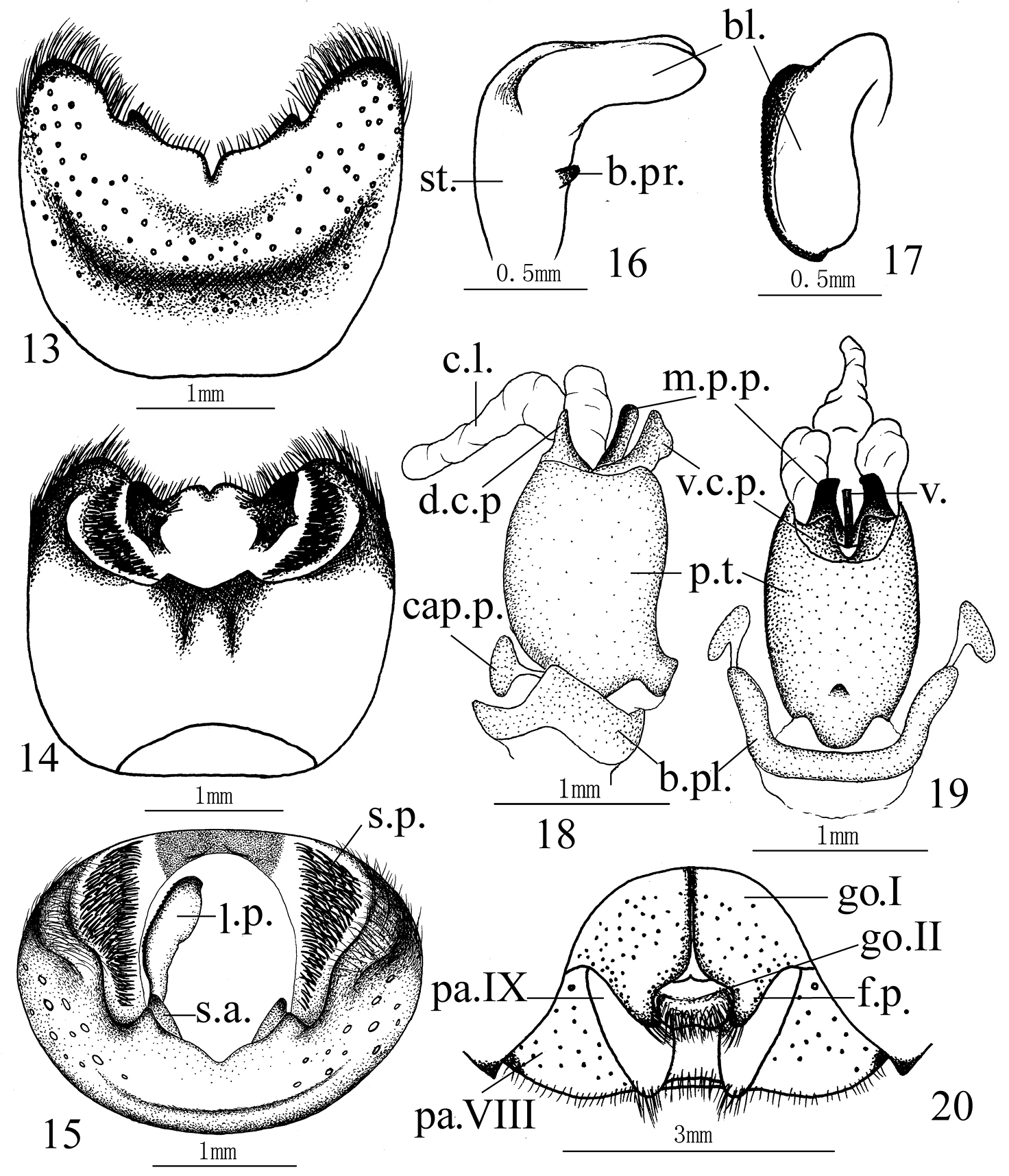
Male genitalia. Ventral rim of pygophore with two separated processes on both lateral sides. Suspensory apodeme and infoldings of lateral rims developed. Paramere L–shaped, stem with a short basal process, apex of blade obtuse without any distinct process. Phallotheca cylindric, with a mesial process on the base of ventral side. Aedeagus with a pair of dorsal conjunctival processes, sclerotized and fingerlike, a trifurcate membraneous conjunctival lobe, a pair of ventral conjunctival processes, slightly sclerotized. Median penial plates oblong and narrow, about as long as the protrudent vesica.
Female genitalia. Paler in color, punctured on gonocoxites I and paratergites VIII, punctures on gonocoxites I finer. Mesial margins of gonocoxites I narrowly black, meeting each other along the basal halves, lateral margins of the fingerlike processes not vertical. Gonocoxite II with a transverse tumescent beam full of setae. Paratergits IX obtuse apically, slightly passing beyond the posterior margin of 8th sternum. Paratergites VIII not protrudent apically.
Heads in dorsal view. 1a–b. Cahara incisura sp. n. (a holotype, b paratype). 2a–c Cahara nodula sp. n.(a holotype, b paratype, c paratype) 3a–b Cahara tibetana (a holotype, b allotype). (a.l. apical lobe of mandibular plate, l.l. lateral lobe of mandibular plate).
Right humeral angles in cephalic view. 4 Cahara incisura sp. n. 5 Cahara nodula sp. n. 6 Cahara tibetana.
Cahara spp. 7–8 Cahara incisura sp. n., holotype 9–10 Cahara nodula sp. n., holotype 11–12 Cahara tibetana, allotype.
Cahara incisura sp. n. 13–15 Pygophore (13 ventral view, 14 dorsal view, 15 caudal view) 16–17 Paramere (16 lateral view, 17 caudal view) 18–19 Aedeagus (18 lateral view, 19 ventral view) 20 Female genitalia. (bl. blade, b.pl. basal plate, b.pr. basal process, cap.p. capitate process, c.l. conjunctival lobe, d.c.p. dorsal conjunctival process, f.p. fingerlike process, go.I gonocoxite I, go.II gonocoxite II, l.p. left paramere, m.p.p. median penial plate, pa.VIII paratergite VIII, pa.IX paratergite IX, p.t. phallotheca, s.a. suspensory apodeme, s.p. setal patch, st. stem, v. vesica, v.c.p. ventral conjunctival process)
The species name, incisura, refers to the longer mandibular plates that always leave an incision before clypeus. It’s feminine.
Southwest China (Sichuan)
urn:lsid:zoobank.org:act:EFEF49E2-14C7-41AF-9901-FB3F60AAC9FE
http://species-id.net/wiki/Cahara_nodula
Figs 2a–2c, 5, 9–10, 21–28Holotype male, pinned, CHINA: Yunnan Province: Xiang Mount., 5. VIII. 1979, Huan–guang ZOU leg. Paratypes: all pinned, CHINA: Yunnan Province: 1 female, same place and collector as holotype, 2. VIII. 1979; 1 female, same place as holotype, 15. VIII. 1979, Zuo–pei LING leg.; 2 males (one with genitalia in a separate microvial), 1 female, Kunming, VII. 1957; 1 female, same data as above except date, IV. 1986; 1 female, same data as above except date, V. 1986; 1 female, same data as above except date, VII. 1986; 1 male, same data as above except date, 17. VI. 1988; 1 male, Anning City, 12. V. 1988, Yun–xu WANG leg.; 1 female, Wushan Town, Mile County, alt. 2000m, 20. V. 1979, Guang–qiang YIN leg.; 1 male, with genitalia in a separate microvial, Santai Village, Dayao County, 13. VI. 1978; 1 female, Dayao County, VIII. 1980; 1 female, Dechang County, VI. 1958; 1 female, Weishan County, 4. VI. 1978; CHINA: Guizhou Province: 2 males, Huaxi District, Guiyang City, 23. V. 1987; 1 female, Changming Town, alt. 1050m, 9. IX. 2000, Chuan–ren LI leg.; 1 female, Fanjing Mount., alt. 1300m, 1. VIII. 2001, Wei–bing ZHU leg.
Humeral angles nodular and elevated upwards (Fig. 5), rostrum longer to pass beyond the middle of the 4th sternum, mandibular plates without meeting trend apically, 1st rostral segment passing beyond the posterior end of buccula, ventral rim of pygophore without any distinct processes (see discussion part).
Male, length 16.0–18.0 mm, width between humeral angles 8.0–8.8 mm. Female, length 19.0–20.0 mm, width between humeral angles 9.0–10.0mm.
Color and puncturing Very similar to Cahara incisura, but with some differences: Punctures on dorsal head denser, while sparser and finer on the endocorium, pronotum with four or five longitudinal strips, laevigate parts of calli more distinct.
Stucture. Head. Mandibular plates about equal to clypeus or slightly longer than clypeus, apices porrect and having not convergent, both apical and lateral lobes obtuse distally, lateral margins before eye sinuate and without any distinct process. Apex of clypeus broadly exposed (Figs 2a–c). Antennae brown, antennomere I paler, with a longitudinal black strip laterally, apical two third of antennomere IV and apical half of antennomere V black, IV>III≥V>II>I in length. Buccula low, anterior angles pointed and protrudent, outer margins straight. Rostrum with 1st joint extending beyond the buccula, apex reaching to the middle of 4th sternum.
Thorax. Pronotum with anterior half depressed and posterior half tumescent, anterior margin broad, sinuate, slightly convex mesially, anterior angle small, angulate and produced laterad, anterolateral magins crenulate, humeral angles nodular, protrudent, elevated upwards. Scutellum longer than width, basal disk and longitudinal midline tumescent. Meso sternum flat with a mesial narrow carina. Peritreme similar to Cahara incisura. Hemelytral corium longer than scutellum, membrane extending beyond the abdominal end.
Abdomen. Connexiva exposed, posterior angles pointed and produced. Venter, from 3rd to 6th abdominal sternite, with a mesial shallow groove.
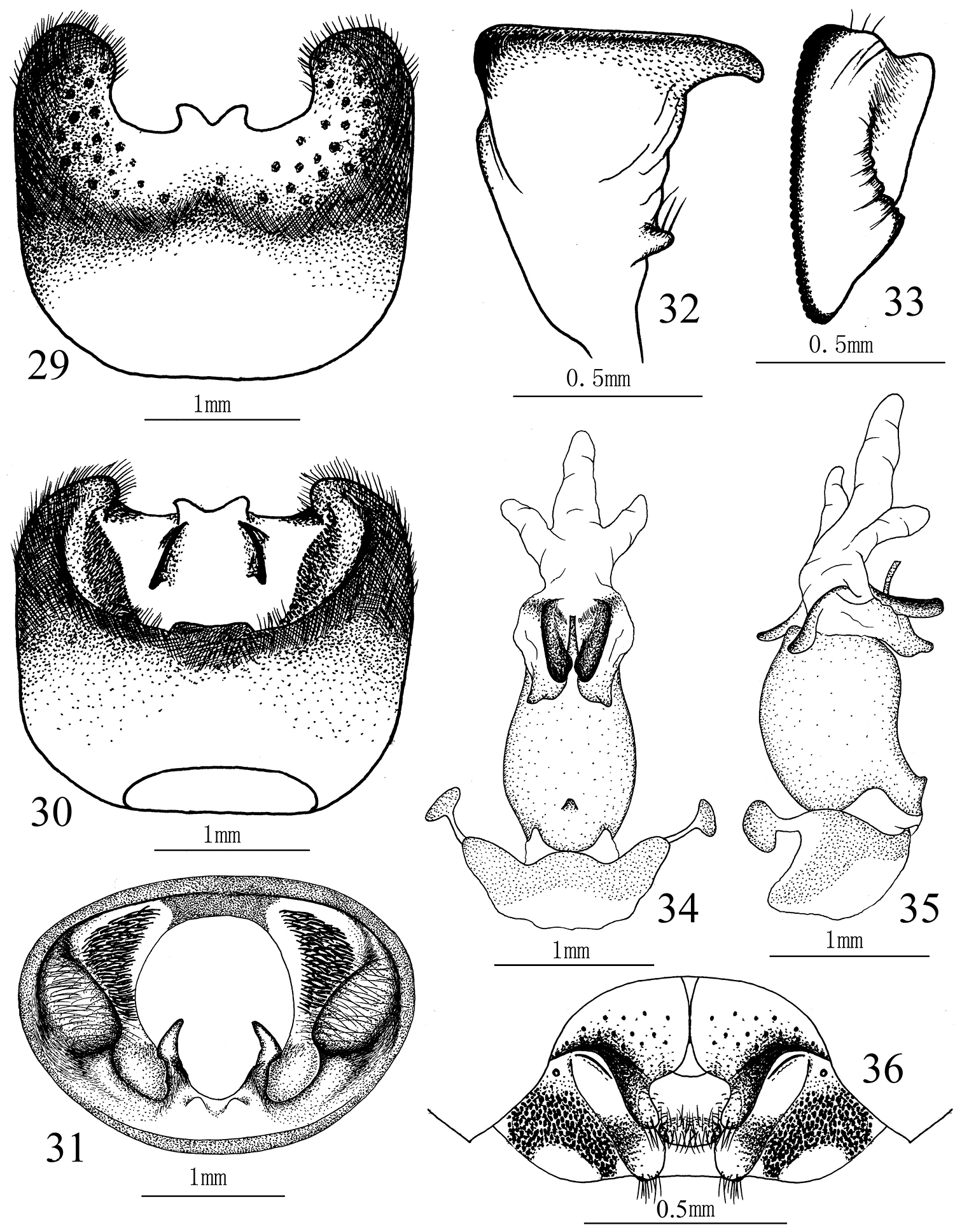
Male genitalia. Ventral rim of pygophore V–shaped excavated, sinuate along the margin but without distinct process. Suspensory apodeme and infoldings of lateral rims developed. Paramere L–shaped, stem broad with a small basal process, blade long with an apical process and a basal process, these two processes all directed caudad. Aedeagus with paired sclerotized dorsal conjunctival processes, a trifurcate membranous conjunctival lobe, and a pair of slightly sclerotized ventral conjunctival processes. Median penial plates oblong, apices obtusely angulate. Vesica slim, protrudent.
Female genitalia. Outer margins of gonocoxites I black, so are the apical halves of paratergites IX, pratergites VIII thickly punctured. Gonocoxites I strongly sinuate mesially, broadly and distinctly depressed in the middle of the lateral margins so the fingerlike processes bent dorsally and almost vertical. Apices of fingerlike processes reaching the apical third of paratergites IX. Gonocoxite II with a transverse tumescent beam. Paratergite IX base with a short oblique ridge, apex passing a little beyond the posterior margin of 8th sternum. Paratergites VIII obtuse distally.
Cahara nodula sp. n. 21–23 Pygophore (21 ventral view, 22 dorsal view, 23 caudal view) 24–25 Paramere (24 lateral view, 25 caudal view) 26–27 Aedeagus (26 ventral view, 27 lateral view). 28 Female genitalia.
The name, nodula, refers to the bulbous, distinct nodular humeral angles of pronotum. It’s feminine.
Southwest China (Guizhou, Yunnan)
http://species-id.net/wiki/Cahara_tibetana
Figs 3a–b, 6, 11–12, 29–36Holotype, male, pinned, with genitalia in a separate microvial, CHINA: Xizang Autonomous Region: Chayu County, alt. 1700m, 26. VI. 1978, Fa–sheng LI leg. Allotype, 1 female, pinned, same data as holotype.
CHINA: Xizang Autonomous Region: 1 female, Dongjiu Nature Reserve, 21. IX. 2007, Fu–min SHI leg.; 1 female, Motuo County, alt. 800m, VIII. 1984.
See diagnosis of Cahara incisura sp. n. Besides, in this species, two processes on the ventral rim of pygophore connected basally, suspensory apodeme longer than those in the other two species, stem of paramere broad with two lateral margins not parallel. Gonocoxites I depressed along the lateral margin and fingerlike processes bent dorsally like Cahara nodula, but the transverse tumescent beam of gonocoxite II shorter in this species.
1. “Juga longer than tylus”. There are variations at least in Cahara nodula sp. n. and Cahara tibetana, but it’s true that “juga” is never found shorter than “tylus”.
2. “Pygophore, ventral margin with more or less deep concavity with a pair of median lobes.” The mesial concavity is also present in many species of Dalpada. But the paired median processes play a vital role to identify the genus. It happens in most of the species of Cahara, but not in Cahara nodula sp. n.
3. “Female genitalia: … first valvifer produced posteriorly.” In our views, this finger-like process elongated from the posterior apex of gonocoxite I is the most effective diagnostic character to distinguish Cahara from the other genera of Pentatominae in which it’s rare. Izharocoris Afzel & Ahmad, 1981 (Pentatomidae: Halyini) is the other related genus that some of its species share this character. But it’s different for having paramere with both the inner and the outer processes (
4.
Those twelve Cahara spp. (nine species recorded by
According to
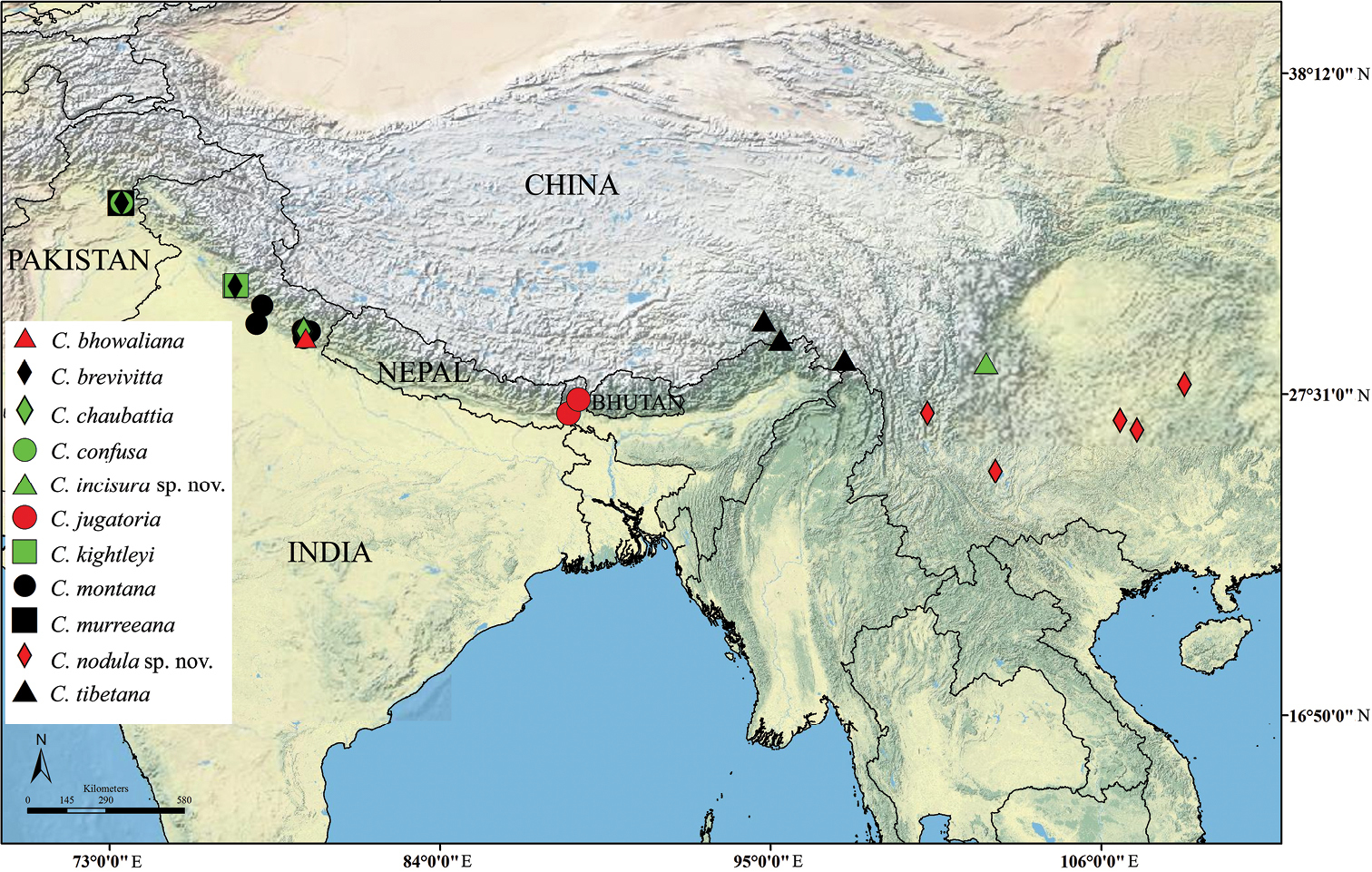
Distribution information of twelve Cahara species.
| Species | Locality | Geographic coordinates |
|---|---|---|
| Cahara bhowaliana | Bhowali, India | 29.3833°N, 79.5167°E |
| Cahara brevivitta | Simla, India | 31.1046°N, 77.1734°E |
| Murree, Pakistan | 33.9065°N, 73.3937°E | |
| *Koozagali, Pakistan | ? | |
| *Cahar (Bowring), India | ? | |
| Cahara chaubattia | Chaubattia, India | 29.6137°N, 79.4563°E |
| Cahara confusa | Murree, Pakistan | 33.9065°N, 73.3937°E |
| Cahara incisura sp. n. | Mianning, China | 28.5496°N, 102.1770°E |
| Cahara jugatoria | Kurseong, India | 26.8800°N, 88.2783°E |
| Gantok, Sikkim, India | 27.3389°N, 88.6065°E | |
| *Himalayas Terai, India | ? | |
| Cahara kightleyi | Simla, India | 31.1046°N, 77.1734°E |
| Mashobra, India | 31.1296°N, 77.2283°E | |
| Cahara metallica | *Hardwicke Bequest, ?? | ? |
| *??, Nepal | ? | |
| Cahara montana | Roorkee, India | 29.8543°N, 77.8880°E |
| Nainital, India | 29.3803°N, 79.4636°E | |
| Almora, India | 29.5984°N, 79.6615°E | |
| Ranikhet, India | 29.6434°N, 79.4322°E | |
| Mussoorie, India | 30.4553°N, 78.0741°E | |
| Cahara murreeana | Murree, Pakistan | 33.9065°N, 73.3937°E |
| Ghora gali, Pakistan | 33.8874°N, 73.3620°E | |
| Cahara nodula sp. n. | Xiang Mount., China | 26.8910°N, 100.2160°E |
| Anning City, China | 24.9594°N, 102.4821°E | |
| Huaxi, China | 26.3331°N, 107.1949°E | |
| Changming, China | 27.8407°N, 108.7735°E | |
| Fanjing Mount., China | 26.6474°N, 106.6301°E | |
| Cahara tibetana | Chayu, China | 28.6613°N, 97.4669°E |
| Dongjiu, China | 29.9601°N, 94.7792°E | |
| Motuo, China | 29.3253°N, 95.3332°E |
Geographical distribution of eleven Cahara spp.
This work was supported by the National Natural Science Foundation of China (grant numbers 31201738, J1210005, J0930005). The authors thank Zhen Ye for his help for the distribution map.