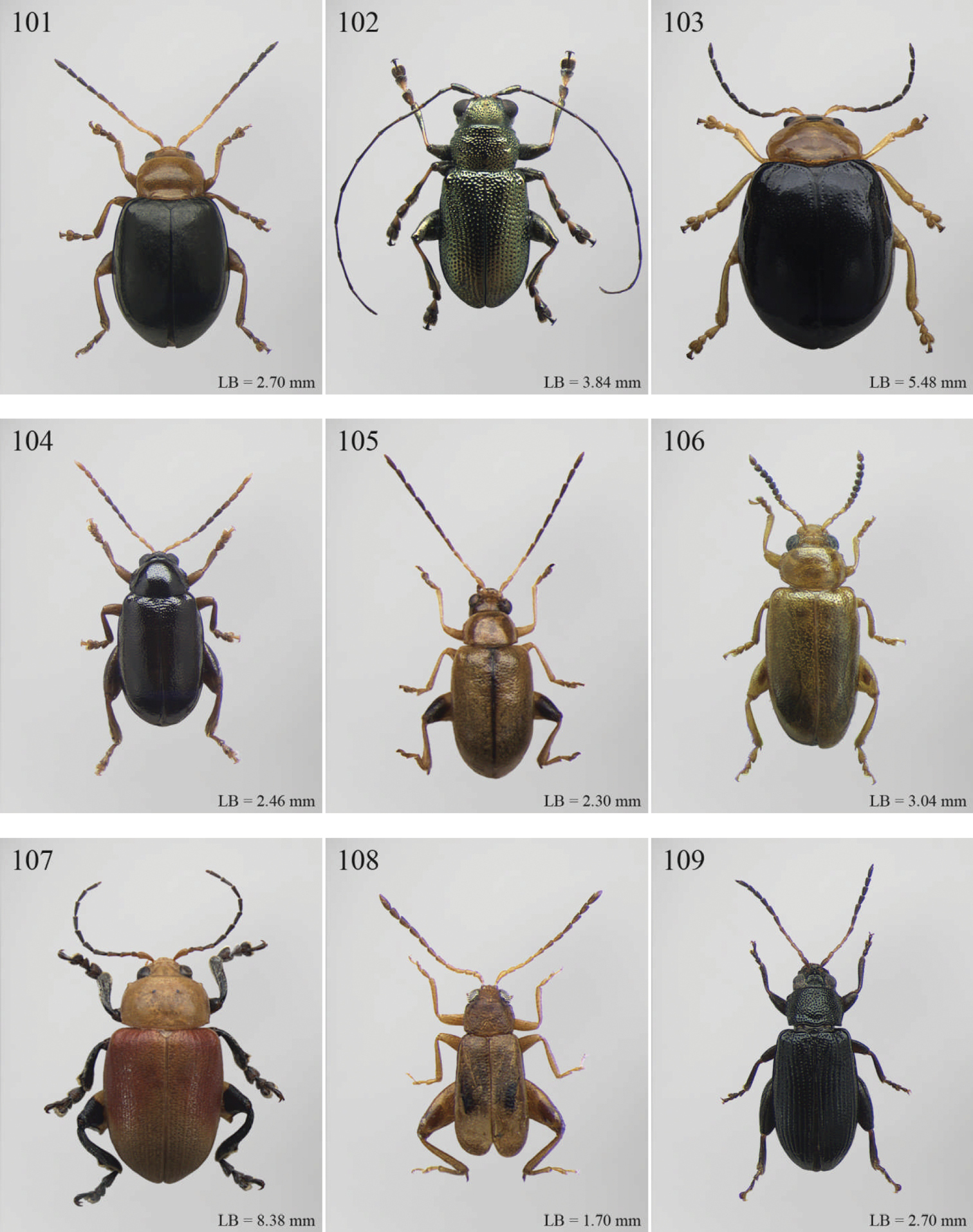
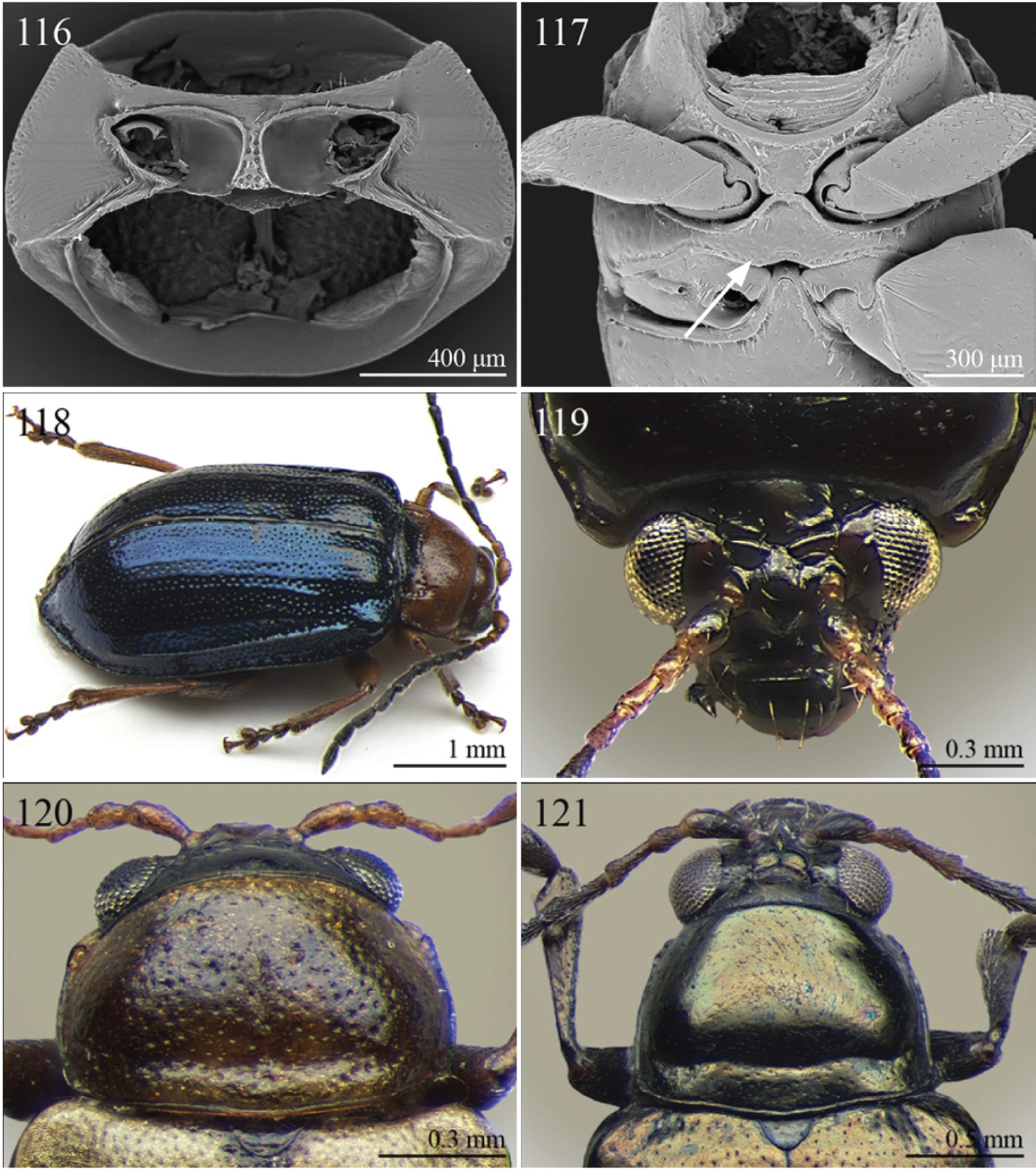
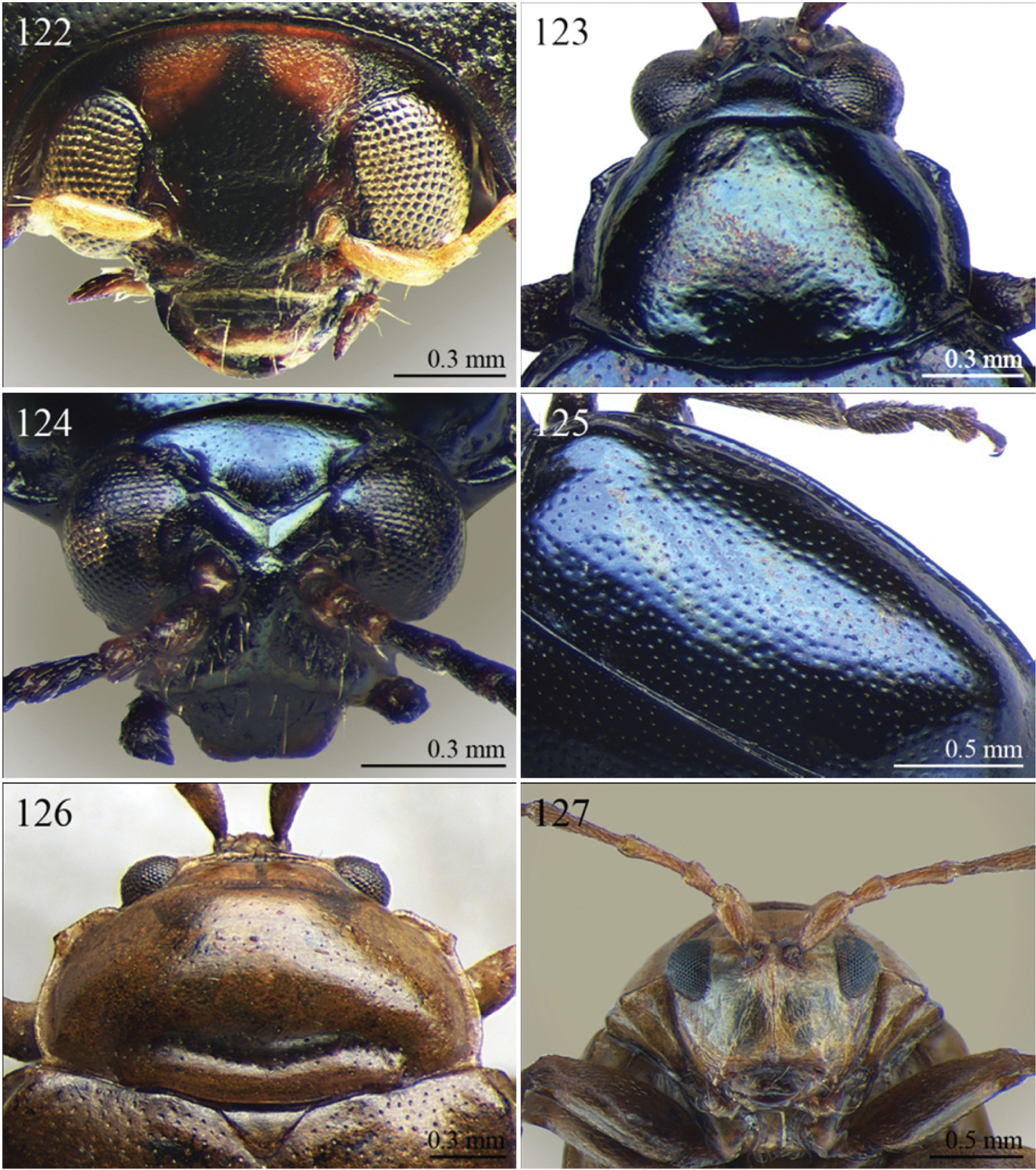
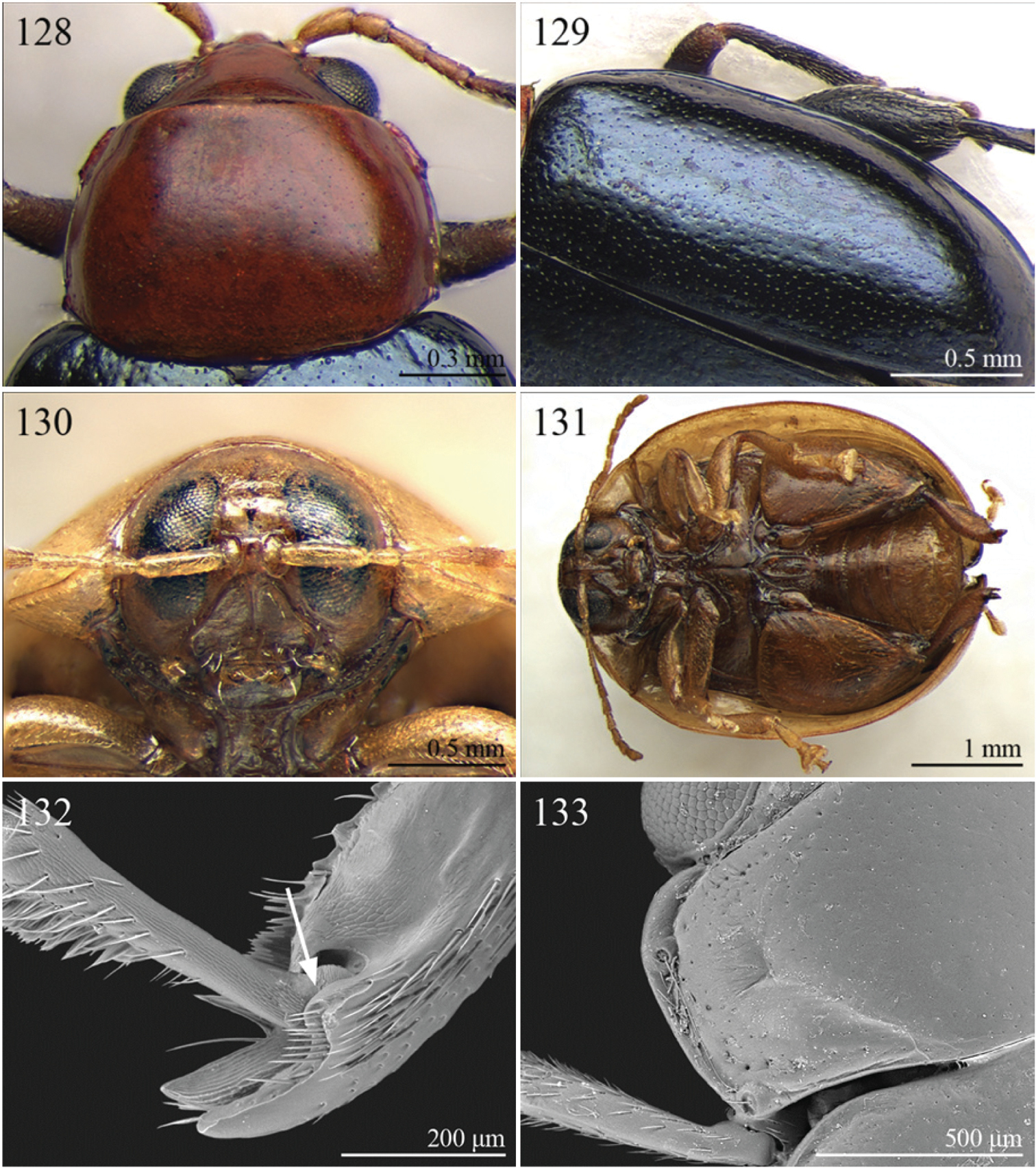
(C) 2012 Maurizio Biondi. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A revision of the Alticini genera from the Afrotropical region is reported. The paper includes the following for the flea beetle fauna occurring in Sub-Saharan Africa and Madagascar: a key to their identification; habitus photos of all the genera; microscope and scanning electron micrographs of many diagnostic morphological characters; and an updated annotated catalogue with biogeographical notes that include new distributional data. The following new synonymies are proposed: Aphthona Chevrolat, 1836 = Ethiopia Scherer, 1972 syn. n.; Sanckia Duvivier, 1891 = Eugonotes Jacoby, 1897 syn. n.; Eurylegna Weise, 1910a = Eurylegniella Scherer, 1972 syn. n.; Kimongona Bechyné, 1959a = Mesocrepis Scherer, 1963 syn. n.; Diphaulacosoma Jacoby, 1892a = Neoderina Bechyné, 1952 syn. n.; Sesquiphaera Bechyné, 1958a = Paropsiderma Bechyné, 1958a syn. n.; Podagrica Chevrolat, 1836 = Podagricina Csiki in
Taxonomy, Afrotropical region, Chrysomelidae, Galerucinae, Alticini, flea beetle genera, identification key, catalogue, synonymies, new combinations, status promotus
The Chrysomelidae is one of the largest phytophagous insect families and includes approximately 37, 000 to 40, 000 species (
We recently published an annotated catalogue of the Afrotropical flea beetle genera, based largely on data from the literature (
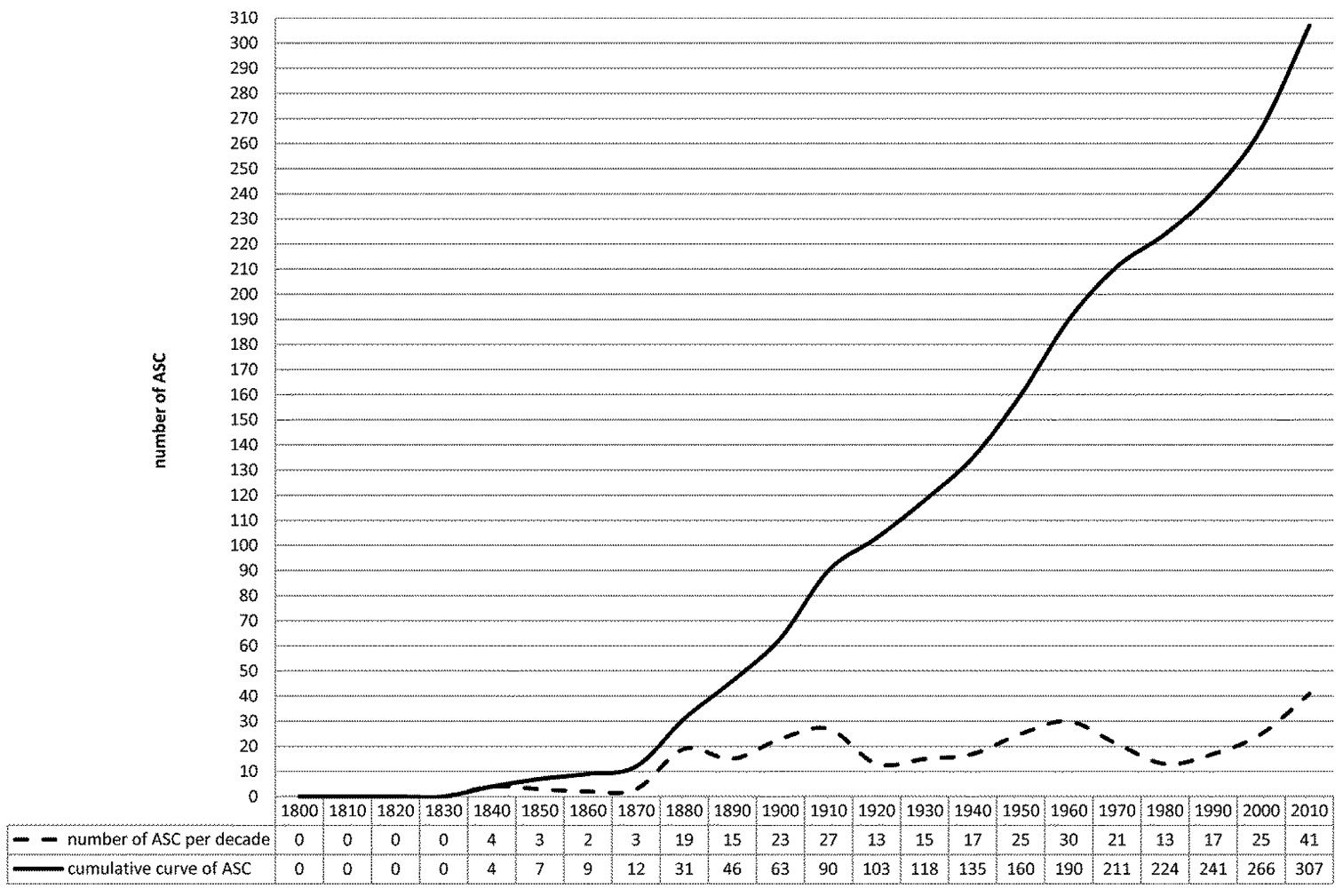
A decrease in the number of publications on the Afrotropical flea beetle fauna followed, until a revival in 1930−1940, initiated by the English coleopterist Gilbert Ernest Bryant (1878−1965) and the French chrysomelid specialist Victor Laboissière (1875−1942). Jan Bechyné (1920−1973) and Gerhard Scherer (1929-2012), specialists on the Alticinae, then published many monographs (see References) on the flea beetle fauna of Sub-Saharan Africa and, to a lesser extent, Madagascar. They described many new genera and species between 1950 and 1970. More recently, contributions on the Afrotropical flea beetle fauna were published by Gerhard Scherer, Maurizio Biondi, Paola D’Alessandro, Manfred Döberl, Serge Doguet, and Elizabeth Grobbelaar (see References).
Chronology of publications on the Afrotropical flea beetle fauna. ASC: Afrotropical Scientific Contributions (update from
The catalogue is arranged alphabetically by generic names. Names in bold refer to flea beetle genera primarily occurring in the Afrotropical region; those in square brackets refer to: synonymies; genera incorrectly reported in the Afrotropical region; in some cases genera transferred to Galerucini; or genus-group names that are unavailable. The rules of the
In addition to the author and date of publication, each genus-group name is accompanied by: a) synonymies, exclusively those for the Afrotropical region; b) bibliographic references, including the original description, other important taxonomic contributions, and distribution data; c) type species, including the method of species assignment; d) geographic distribution in the Afrotropical region (cf.
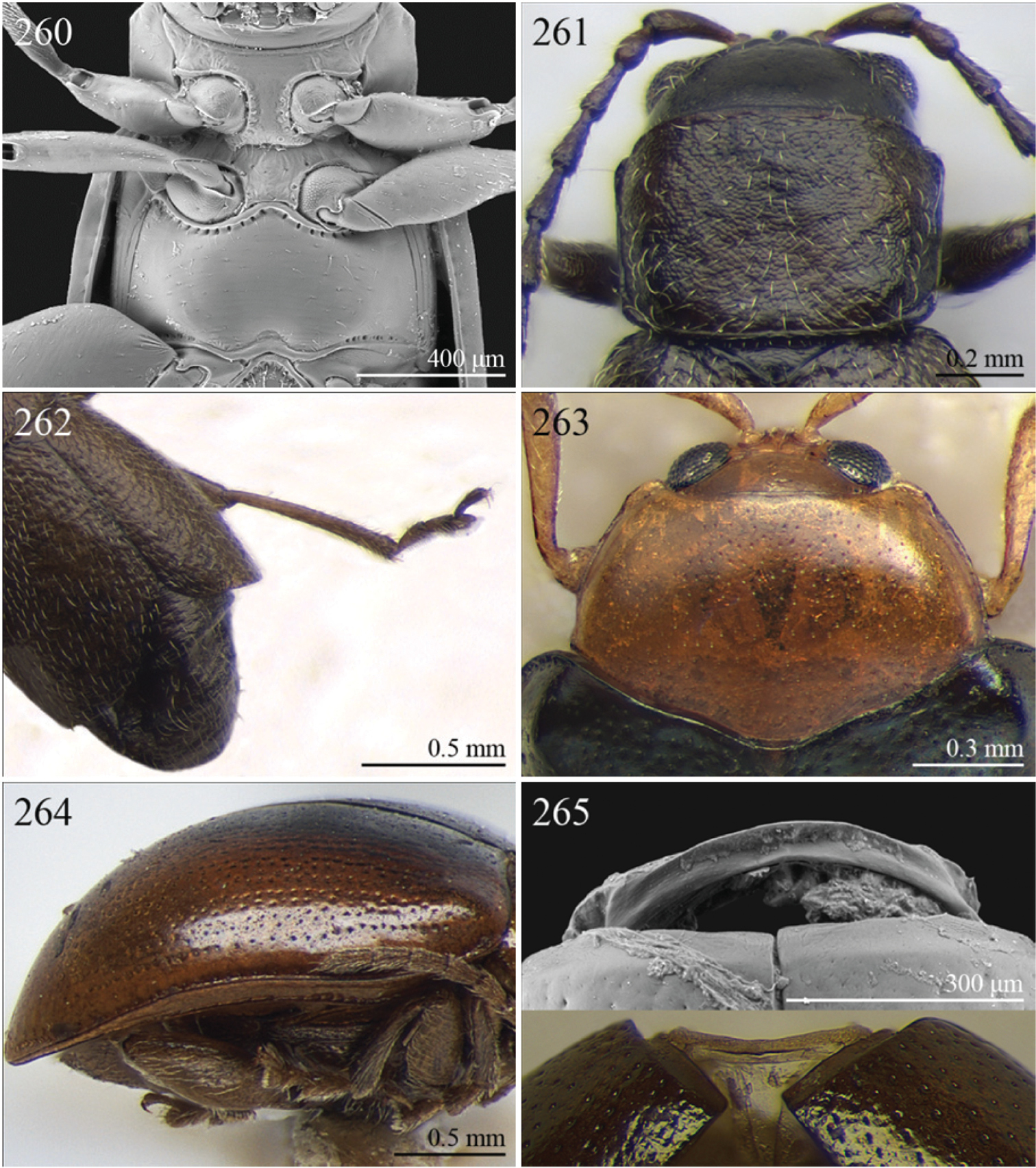
Specimens were examined and dissected using WILD MZ12.5 and LEICA M205C binocular microscopes. Photomicrographs were taken using a Leica DFC500 camera and the Auto-Montage Pro 2006 software (license number: 15224*syn2459*153a2112_maurizio_266836). Scanning electron micrographs were taken using a PHILIPS SEM XL30 CP and HITACHI TM-1000. Morphometric measures were taken using the image analysis software Image-Pro Insight 8.0 (license number: 03080000-5385).
The type material examined during this study is preserved in the following institutions: BAQ: collection of M. Biondi, University of L’Aquila, Italy; BMNH: The Natural History Museum, London, United Kingdom; ISNB: Institut Royal des Sciences Naturelles de Belgique, Brussels, Belgium; MNHN: Muséum National d’Histoire Naturelle, Paris, France; MCSN: Museo Civico di Storia Naturale ‘Giacomo Doria’, Genova, Italy; MRAC: Musée Royal de l’Afrique Centrale, Tervuren, Belgium; MUAF: Mendel University of Agriculture and Forestry, Brno, Czech Republic; MZHF: Finnish Museum of Natural History, University of Helsinki, Finland; MZLU: Lund University, Sweden; NHMB: Naturhistorisches Museum, Basel, Switzerland; NHRS: Naturhistoriska Riksmuseet, Stockholm, Sweden; NMPC: Entomologické Oddělení Národního Muzea, Praha-Kunratice, Czech Republic; SANC: South African National Collection, ARC-Plant Protection Research Institute, Pretoria, South Africa; SMNS: Staatliches Museum für Naturkunde, Stuttgart, Germany; TMSA: Ditsong: National Museum of Natural History (formerly Transvaal Museum), Pretoria, South Africa; ZMHB: Museum für Naturkunde der Humboldt-Universität, Berlin, Germany; ZSM: Zoologische Staatssammlung, Munich, Germany.
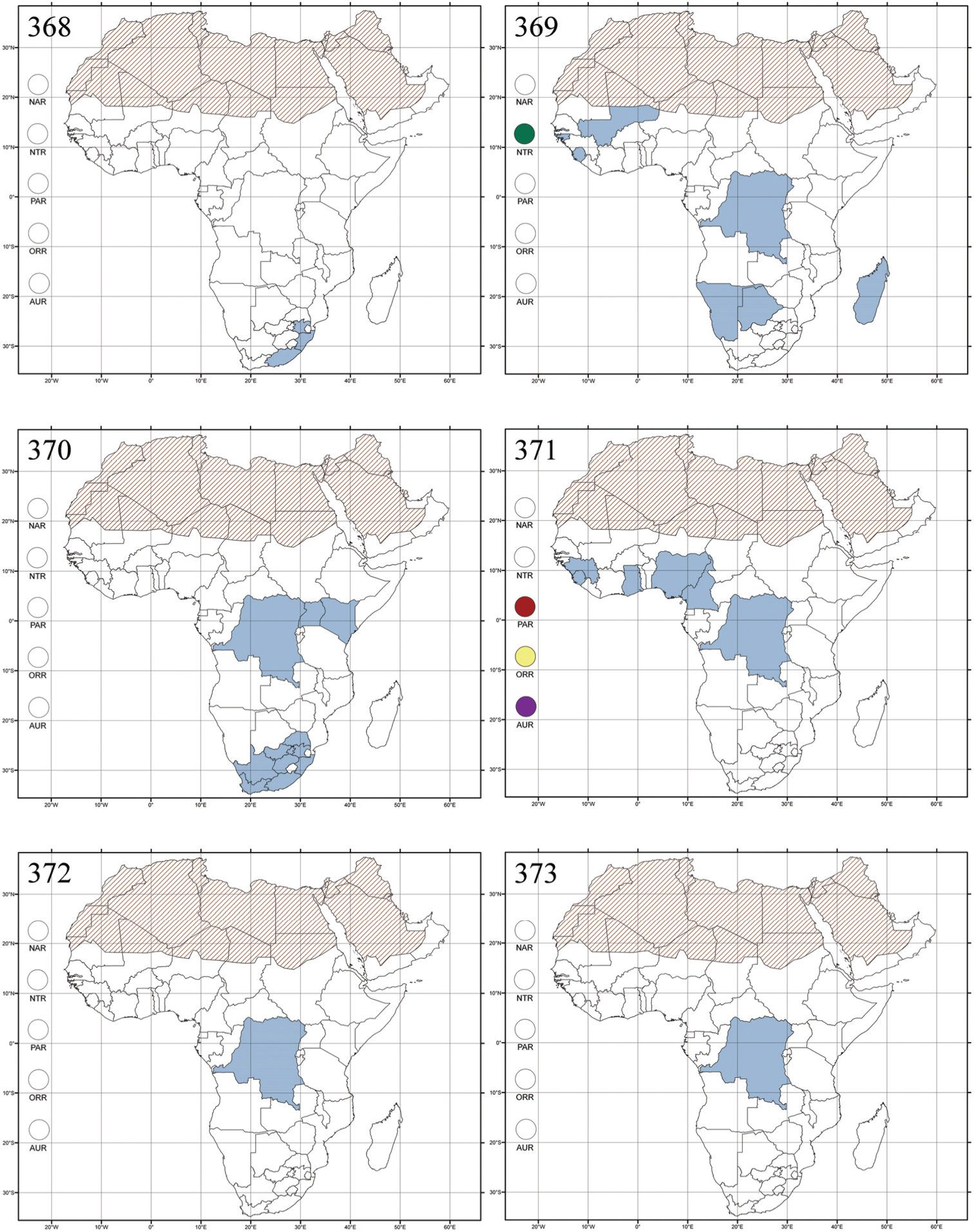
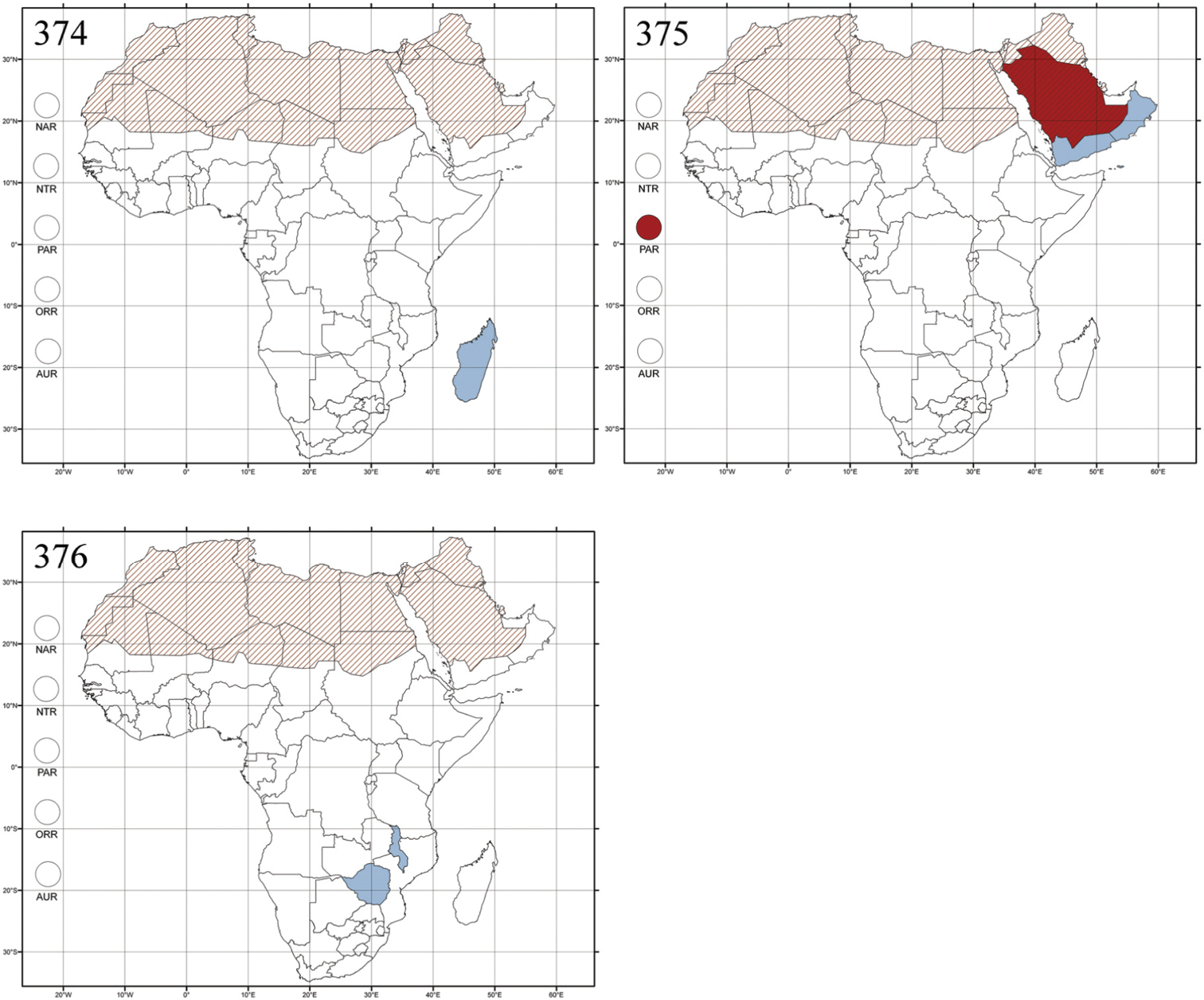
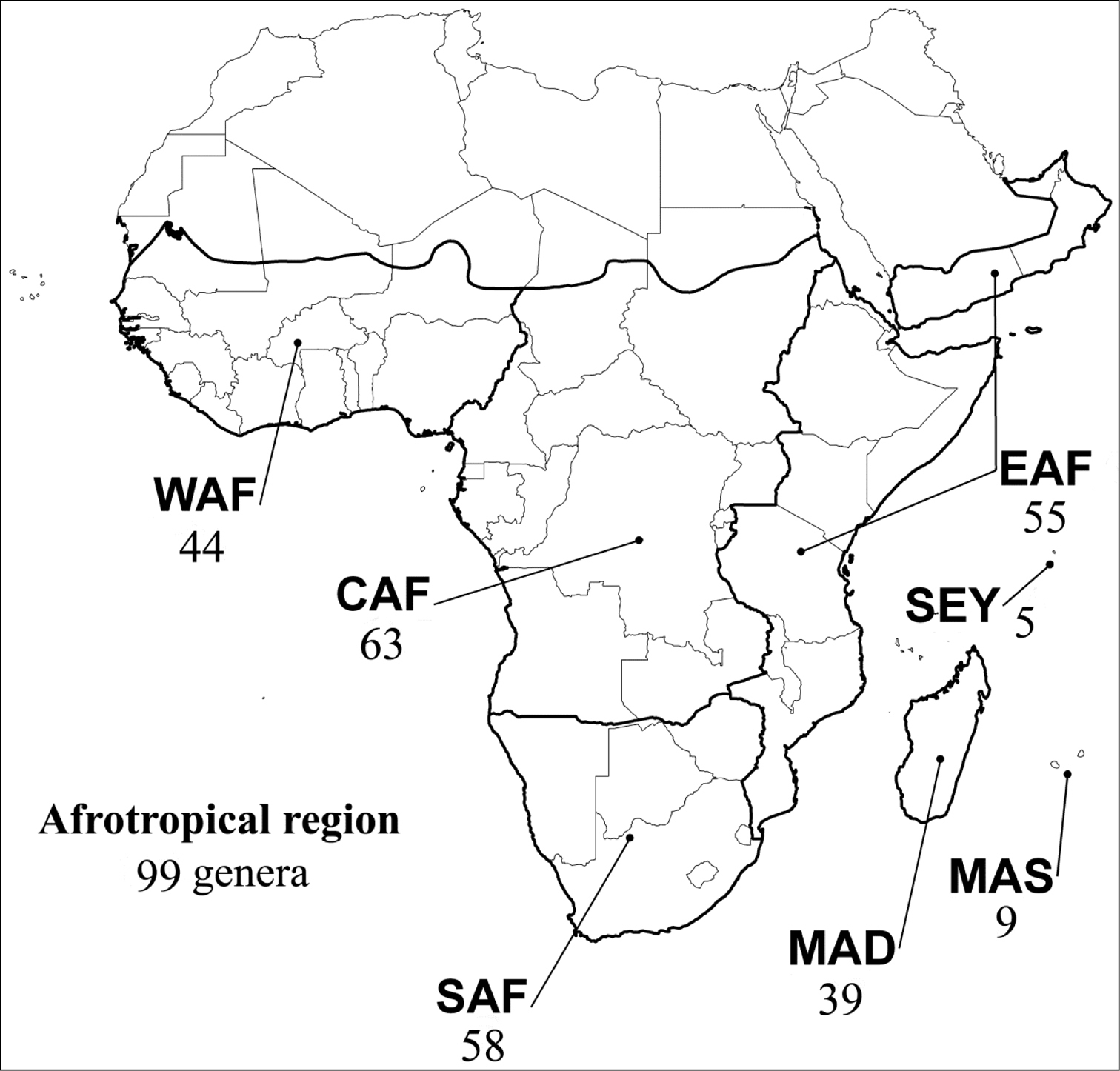
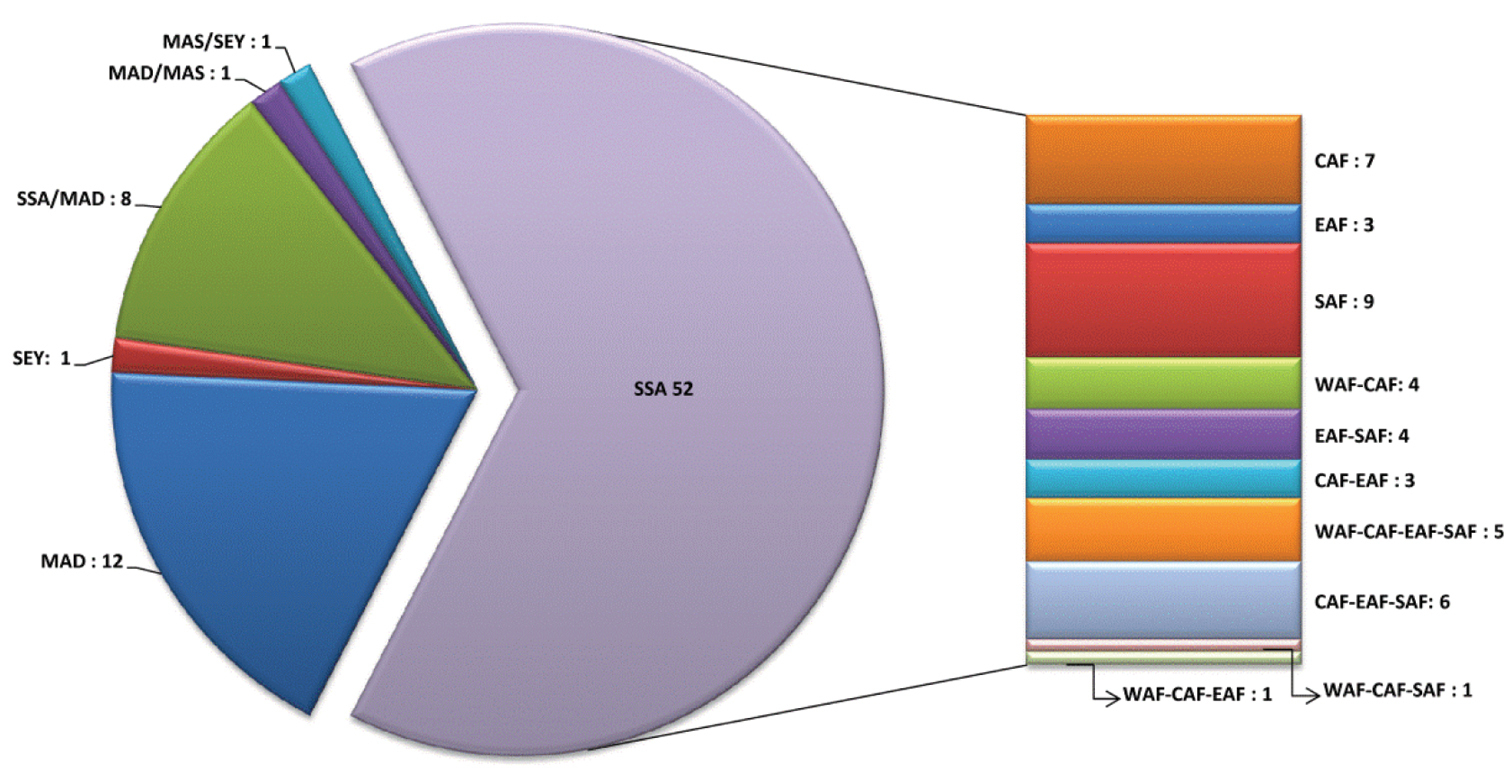
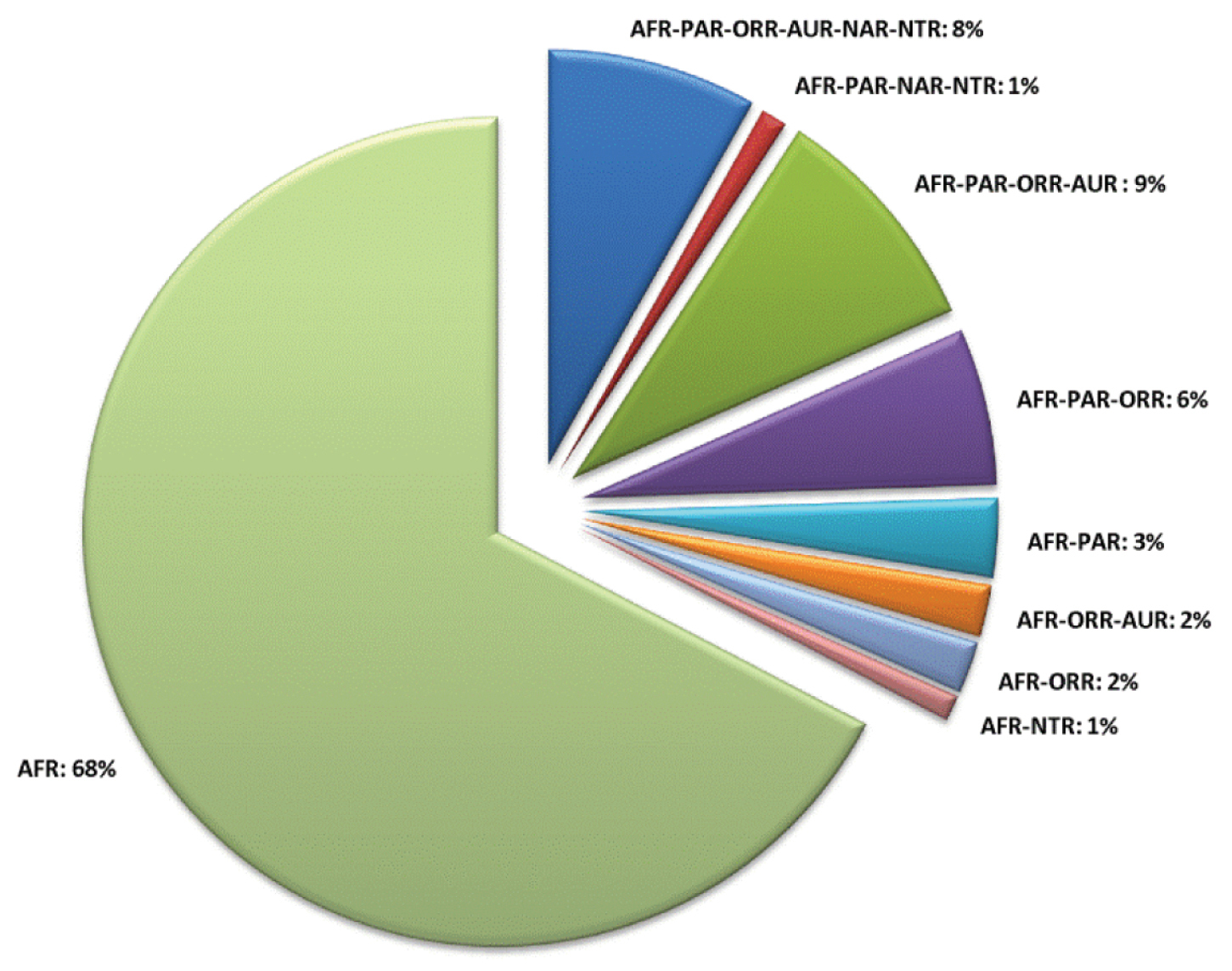
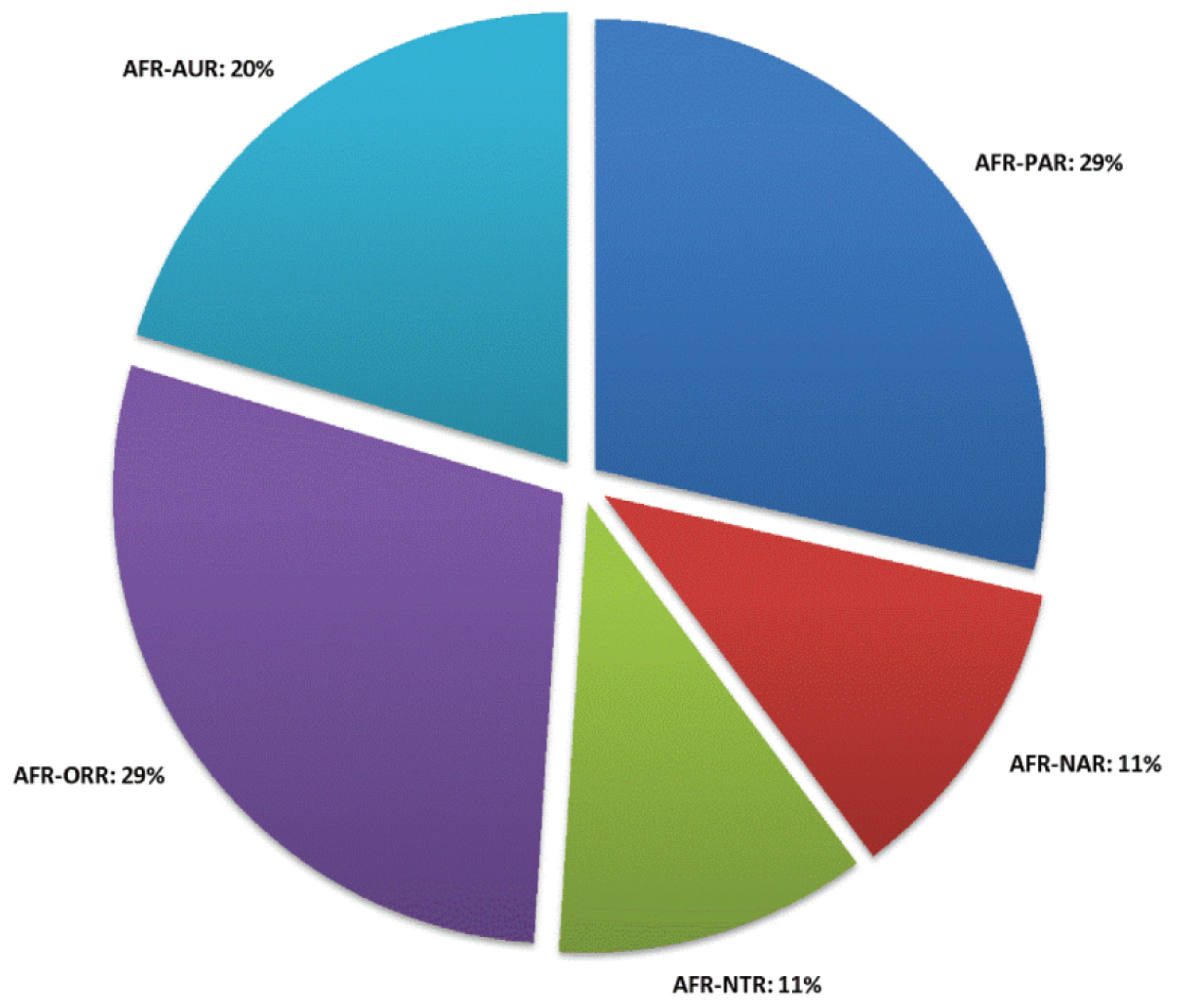
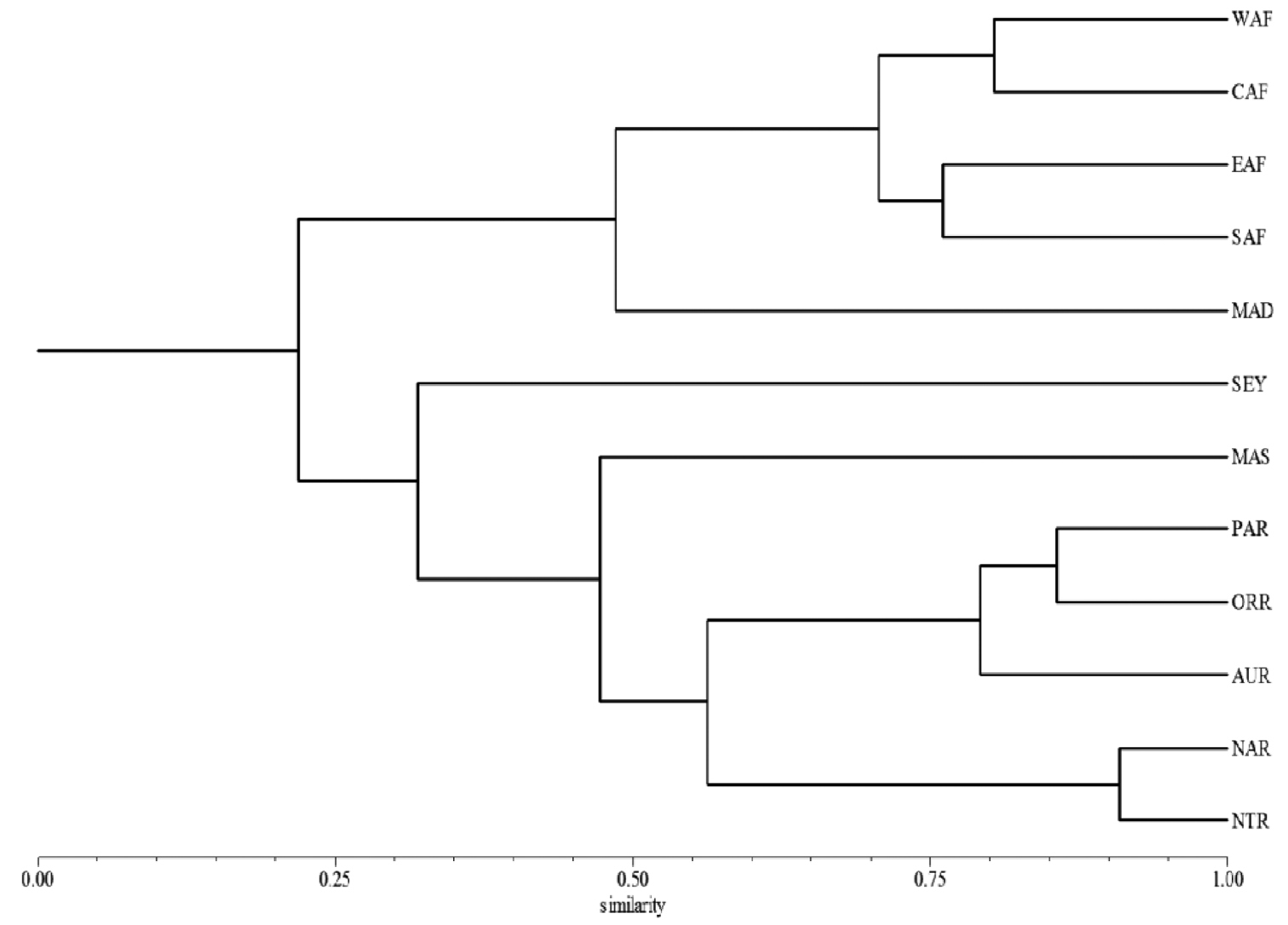
Abbreviations.Morphology - LAN: length of antennae; LB: total length of body; LE: length of elytra; LHT: length of hind tibia; LHTS: length of hind tibial spur; LP: length of pronotum; WE: width of elytra; WP: width of pronotum. Regions - AFR: Afrotropical; AUR: Australian; CAF: Central Afrotropical; EAF: Eastern Afrotropical; ORR: Oriental; MAD: Madagascar; MAS: Mascarene Islands; NAR: Nearctic; NTR: Neotropical; PAR: Palaearctic; SAF: Southern Afrotropical; SEY: Seychelles Islands; SSA: Sub-Saharan Africa; WAF: Western Afrotropical. (?) record to be confirmed; (!) new record; (i) introduced.
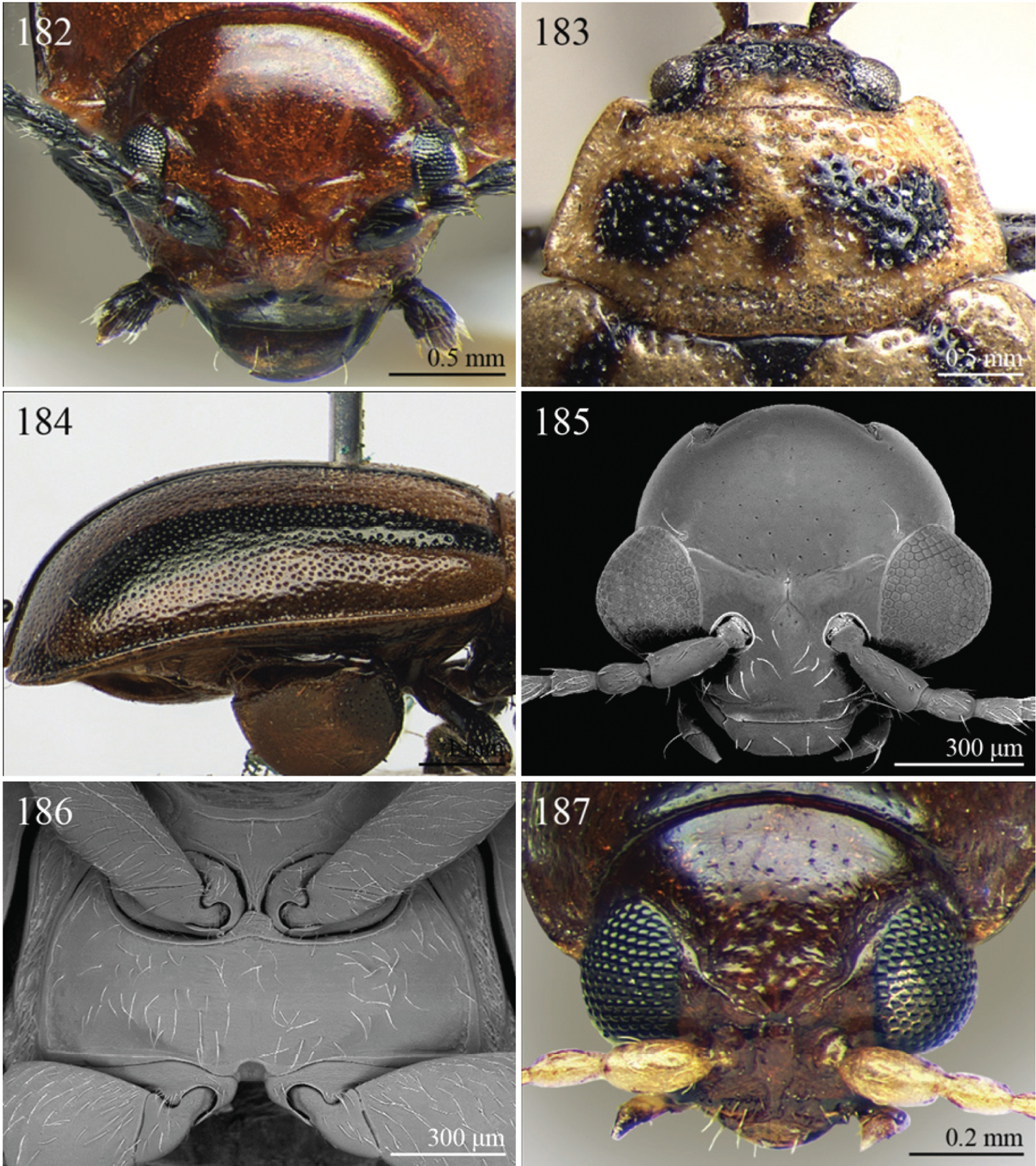
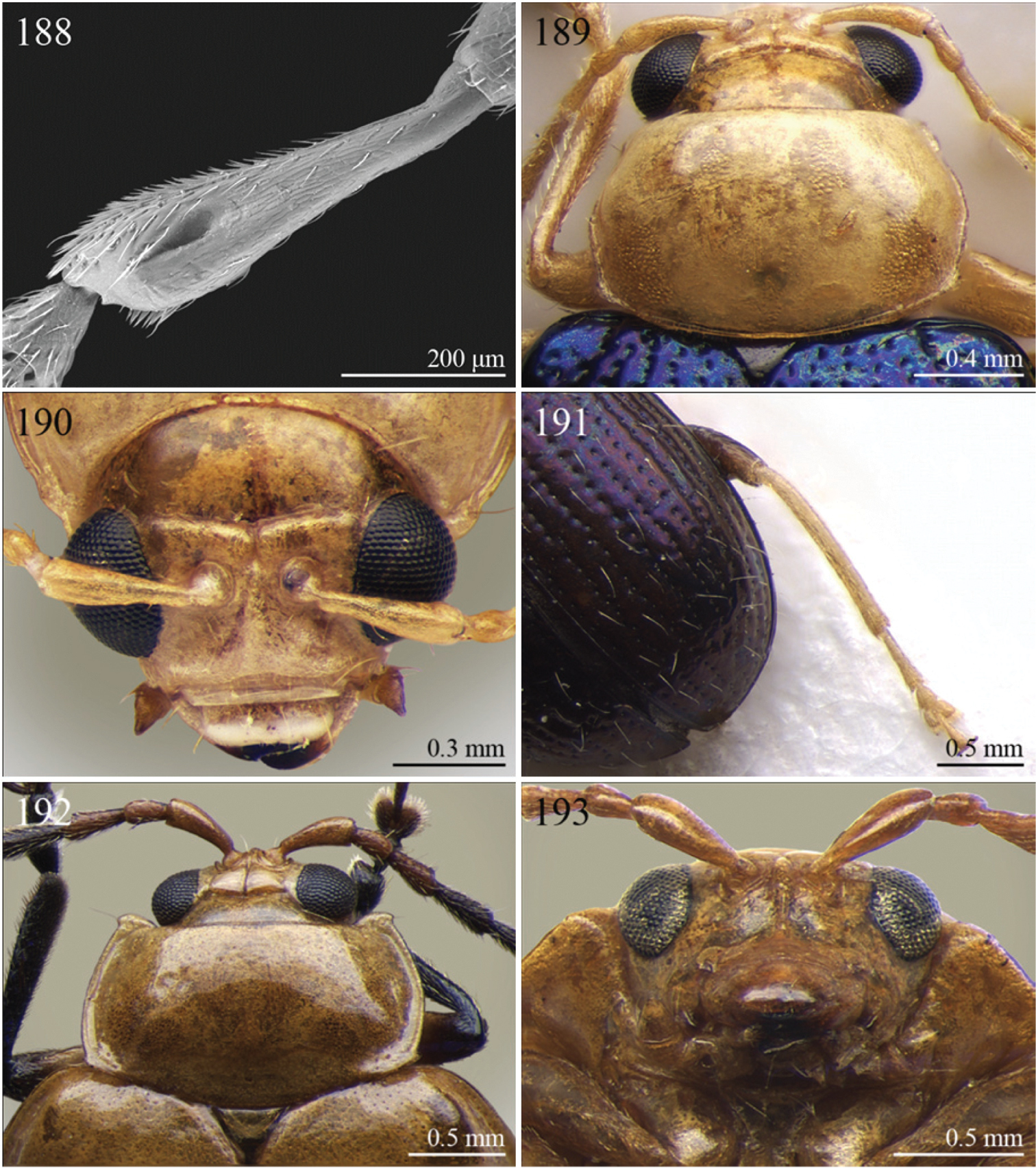
A new key for the identification of the Afrotropical flea beetle genera is proposed. In comparison with the key previously proposed by
| 1 | Antennae with 10 antennomeres | Group A |
| – | Antennae with 11 antennomeres. | 2 |
| 2 | Apical tarsomere of metatarsus distinctly swollen (Figs 200, 237) | Group B |
| – | Apical tarsomere of metatarsus not swollen (Figs 144, 149, 166, 169) | 3 |
| 3 | Dorsal margin of middle and hind tibiae with distinct ciliate dentate emargination, acute or subrounded apically (Figs 144, 148, 154, 163, 258) | Group C |
| – | Dorsal margin of middle and hind tibiae without distinct ciliate dentate emargination | 4 |
| 4 | Pronotum with a distinct but poorly defined median depression near each lateral margin with surface more strongly punctate (Fig. 135) | Group D |
| – | Pronotum uniformly or sparsely punctate, but never with more strongly punctate median depression near lateral margin | 5 |
| 5 | Pronotum with ante-basal transverse sulcus (Figs 120, 121, 126, 206, 209, 212, 225, 246) | Group E |
| – | Pronotum without ante-basal transverse sulcus | 6 |
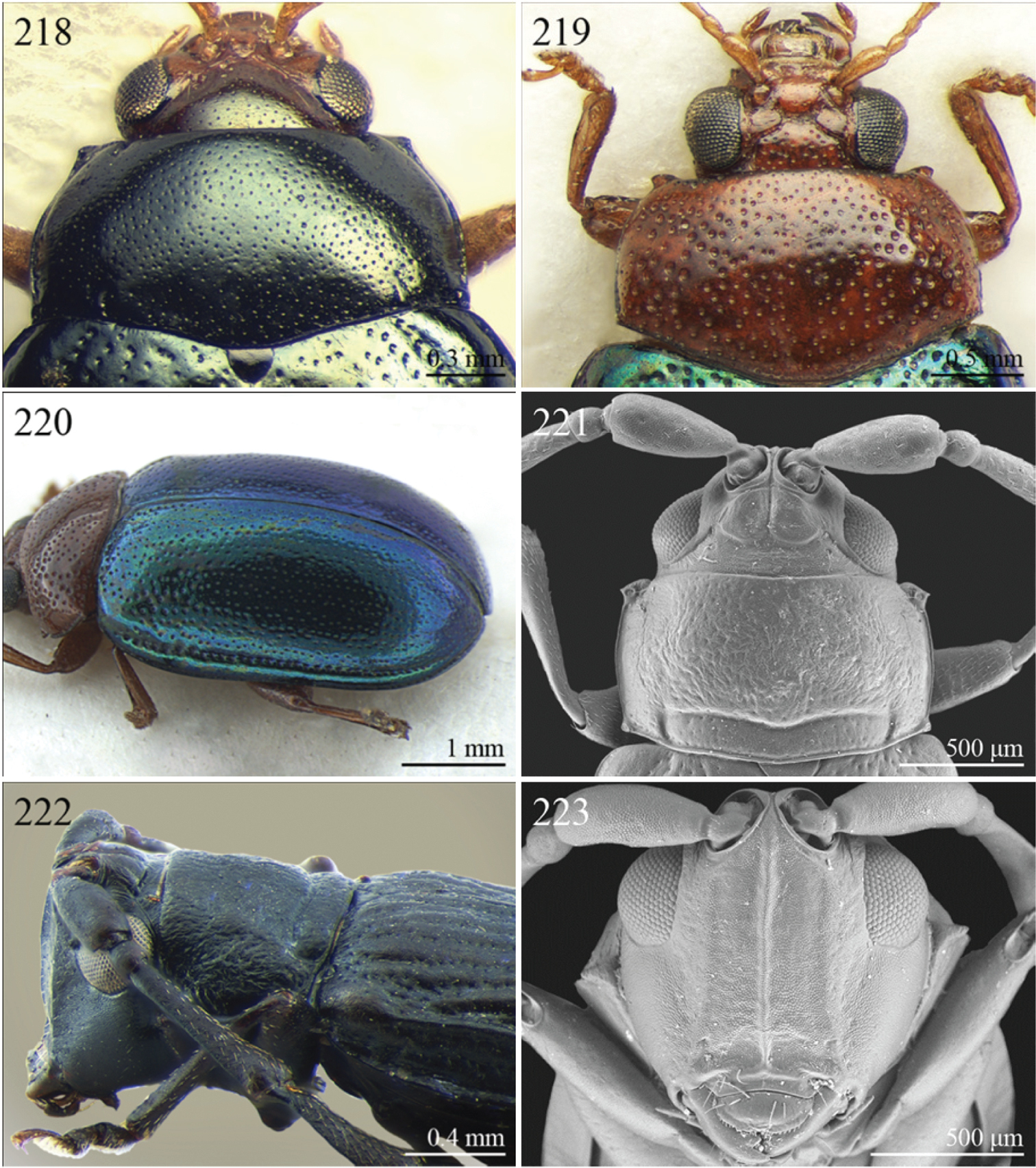
| 6 | Pronotal base with two short sublateral longitudinal striae (Figs 85–86) [sometimes also with two distinct longitudinal grooves on anterior pronotal margin (Figs 217–218)] | Group F |
| – | Pronotal base without short sublateral longitudinal striae | 7 |
| 7 | Pronotum with distinct sublateral mesal callosity bounded by more or less deeply impressed diagonal sulcus laterally (Figs 133, 153) | Group G |
| – | Pronotum without sublateral callosity | Group H |
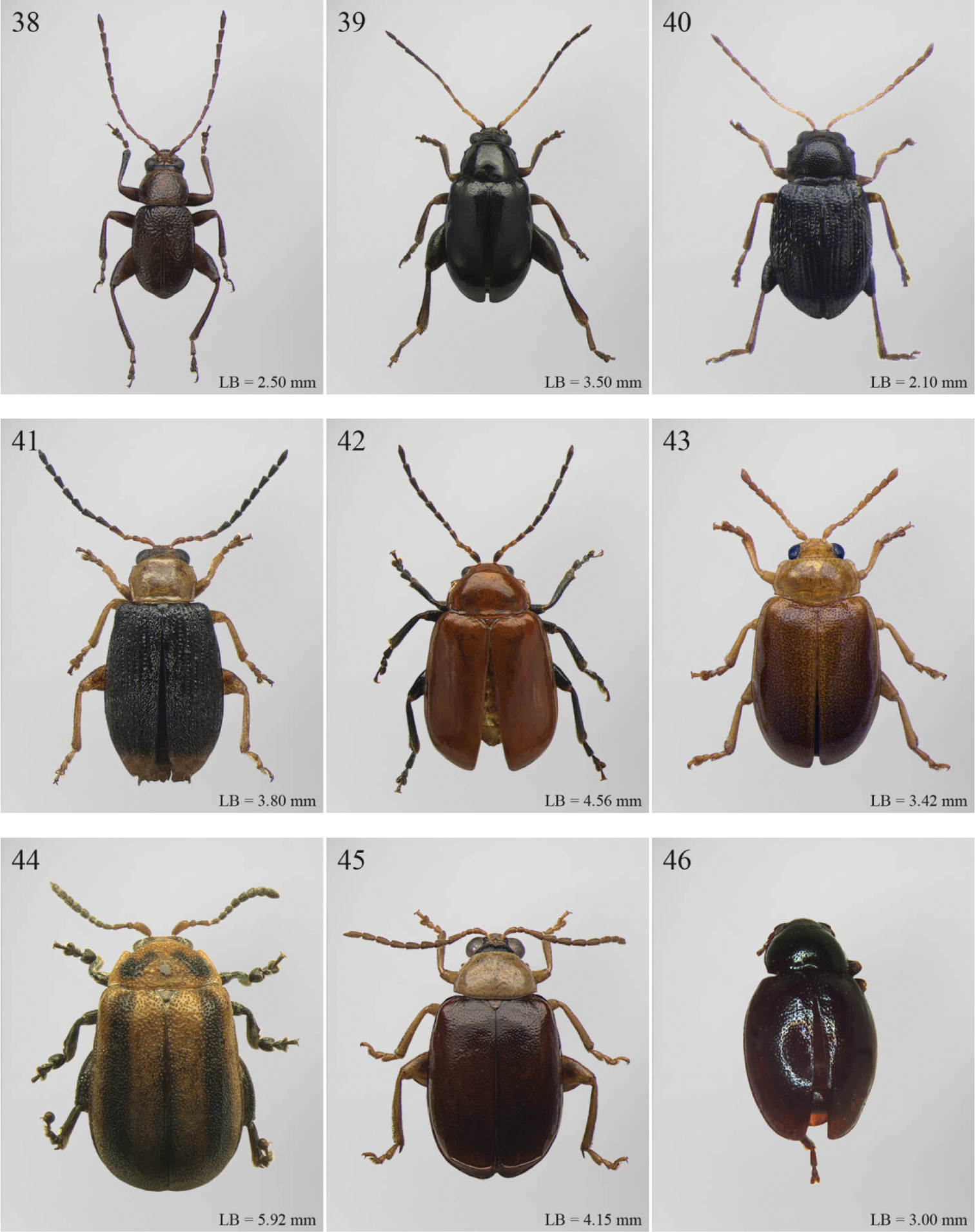
| 1 | Elytral punctation arranged in regular rows (Fig. 91). Metatarsus preapically inserted on tibia (Fig. 248). Procoxal cavities closed posteriorly | Psylliodes Berthold, 1827 (Fig. 91) |
| – | Elytral punctation confused (Fig. 32). Metatarsus apically inserted on tibia. Procoxal cavities open posteriorly | Decaria Weise, 1895 (Fig. 32) |
| 1 | Elytral punctation arranged in regular rows (Fig. 109). Procoxal cavities closed posteriorly | Zomba Bryant, 1922 (Fig. 109) |
| – | Elytral punctation confused. Procoxal cavities open posteriorly | 2 |
| 2 | Elytral epipleura vertically orientated in distal 2/3s, not visible in lateral view (Fig. 238) | 3 |
| – | Elytral epipleura horizontally or obliquely downward orientated in distal 2/3s, visible in lateral view (Figs 184, 229, 233) | 4 |
| 3 | Pronotum with anterior angles distinctly produced towards anterior and distinctly thickened; posterior angles rounded (Fig. 236). Hind tibiae often curved towards inside (Fig. 237). First metatarsomere wide and subtriangular (Fig. 237). Apical tarsomere of metatarsus moderately swollen (Fig. 237) | Physomandroya Bechyné, 1959 (Fig. 83) |
| – | Pronotum with anterior angles not thickened, dentiform apically, indistinctly produced forwards anterior; posterior angles dentiform apically (Fig. 199). Hind tibiae straight (Fig. 200). First metatarsomere narrow, subcylindrical (Fig. 200). Apical tarsomere of metatarsus distinctly swollen (Fig. 200) | Hyphasis Harold, 1877 (Fig. 54) |
| 4 | Vertex of head distinctly and densely punctate (Fig. 239). Frontal tubercles wide, subtriangular, well defined, and closely associated lengthwise (Fig. 239) | Physonychis Clark, 1860 (Fig. 84) |
| – | Vertex of head smooth or indistinctly and sparsely punctate (Figs 234–235). Frontal tubercles small, generally poorly defined (Figs 234–235) | 5 |
| 5 | Frons distinctly sharp-edged distally in lateral view or clearly produced anteriorly, forming a smooth wide surface, often with evident laminate extensions (Figs 234–235) | Physoma Clark, 1863 (Fig. 82) |
| – | Frons arcuate distally in lateral view, never with eversions or laminate extensions | 6 |
| 6 | Pronotum distinctly rounded laterally, generally at least 2x wider than long (WP/LP > 2.00); pronotal base depressed, sometimes with distinct transverse ante-basal sulcus, more distinctly impressed near posterior angles of pronotum (Fig. 78) | Philopona Weise, 1903 (Fig. 78) |
| – | Pronotum almost straight or very slightly rounded laterally, less than 2x wider than long (WP/LP ≤ 2.00); pronotal base generally not depressed, never with distinct transverse ante-basal sulcus (Figs 183, 232) | 7 |
| 7 | Elytral epipleura obliquely downward orientated in apical 2/3s, easily visible in lateral view (Fig. 184). Elytra subparallel or widening slightly laterally towards posterior, distinctly bordered and finely channeled laterally (Fig. 44). Pronotum generally more transverse (WP/LP > 1.90), indistinctly bordered laterally, with not uniformly distributed punctation (Fig. 183). Dorsal integument always with strongly impressed punctation (Figs 44, 183, 184). Elytra pale brown with longitudinal black stripes (Fig. 44). Body smaller (generally LB ≤ 7.50 mm) | Eutornus Clark, 1860 (Fig. 44) |
| – | Elytral epipleura horinzontally orientated in apical 2/3s, little visible in lateral view (Fig. 233). Elytra widening distinctly laterally towards posterior, narrowly bordered laterally (Fig. 81). Pronotum generally less transverse (WP/LP ≤ 1.90), finely bordered laterally, with uniformly distributed punctation (Fig. 232). Dorsal integument generally from finely to moderately punctate (Figs 81, 233). Elytra unicolor, never with longitudinal black stripes (Fig. 81). Body larger (generally LB > 7.50 mm) | Physodactyla Chapuis, 1875 (Fig. 81) |
| 1 | First metatarsomere distinctly shorter than second (Fig. 267). Elytral punctation arranged in 11 rows (+1 long sutural row), partially irregular only in sutural area (Fig. 102). Prothorax subcylindrical. Eyes roundish, distinctly protuberant (Fig. 266) | Terpnochlorus Fairmaire, 1904 (Fig. 102) |
| – | First metatarsomere as long as second or longer (Figs 117, 121, 124, 163). Elytral punctation confused or arranged in 9 single or double rows (+1 short sutural row). Prothorax distinctly depressed dorsally. Eyes generally subelliptical, not distinctly protuberant (Figs 146, 150, 158) | 2 |
| 2 | First metatarsomere narrow, subcylindrical or subrectangular (Figs 144, 154, 258). Claws simple or subappendiculate (Figs 144, 154). Body smaller (generally LB < 4.00 mm) | 3 |
| – | First metatarsomere wide, subtriangular (Figs 148, 149). Claws generally appendiculate (Fig. 162). Body larger (generally LB ≥ 4.00 mm) | 7 |
| 3 | Hind tibiae with dorsal margin distinctly bidentate (Figs. 144, 258) | 4 |
| – | Hind tibiae with dorsal margin unidentate (Fig. 154) [in Chaetocnema schlaeflii (Stierlin, 1866) and Chaetocnema major (Jacquelin du Val, 1852), the dorsal tibial margin may appear bidentate, but Seychellaltica is easily distinguishable from these two species mainly by having the frontal sulci very short, not visible around eyes (Fig. 252), and the first pro- and mesotarsomeres distinctly asymmetrical (Fig. 254); while Biodontocnema is easily distinguishable by having a wider socket on the hind tibia with inner margin dentiform (Figs 142–144); weakly developed metasternum (Fig. 145); antennomeres 6–10 distinctly shorter, as long as wide (Figs 22); and first metatarsomere that is laterally compressed (Fig. 142); in addition, Biodontocnema has shorter antennae that do not reach half of the elytral length and distinctly smaller size (LB > 3.00 mm; LB < 2.50 mm) (Fig. 22)] | 5 |
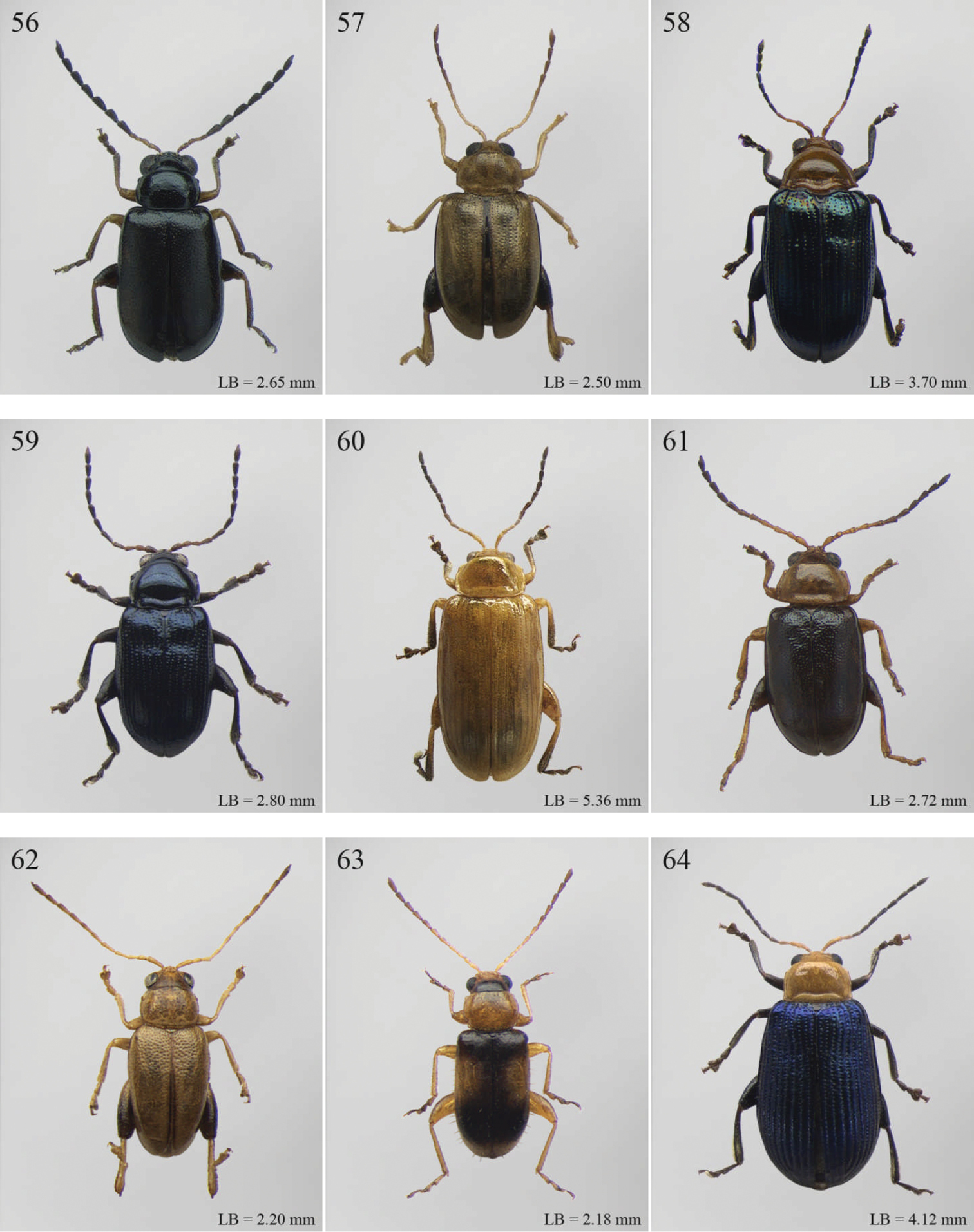
| 4 | Frontal sulci very short, not visible around eyes (Fig. 257). Antennae longer, reaching half of elytral length (Fig. 97). Antennomeres 6–10 distinctly longer than wide (Fig. 97). Body elongate, with pronotum distinctly transverse (generally WP/LP > 1.57); humeral calli distinct (Fig. 97). Ratio of metasternal width/metasternal length ≤ 2.50 (Fig. 260). First metatarsomere at least twice as long as second and third together, not laterally compressed (Fig. 258). First pro- and mesotarsomeres distinctly asymmetrical (Fig. 259). Hind tibial socket narrow without dentiform inner margin (Fig. 258) | Seychellaltica Biondi, 2002 (Fig. 97) |
| – | Frontal sulci elongate, distinctly visible around eyes (Fig. 141). Antennae shorter, only reaching base of elytra (Fig. 22). Antennomeres 6–10 about as long as wide. Body oval, with pronotum slightly transverse (generally WP/LP ≤ 1.57); humeral calli absent (Fig. 22). Ratio of metasternal width/metasternal length > 2.50 (Fig. 145). First metatarsomere shorter than second and third together, and laterally compressed (Figs 142–144). First pro- and mesotarsomeres symmetrical. Hind tibial socket wide with distinctly dentiform inner margin (Figs 142–143) | Biodontocnema Biondi, 2000 stat. prom. (Fig. 22) |
| 5 | Interocular space with at least a distinct transverse carina (Fig. 150). Distal margin of frons distinctly incised medially (Fig. 150). Interantennal space at least twice as wide as length of first antennomere (Fig. 150) | Carcharodis Weise, 1910 (Fig. 25) |
| – | Interocular space without transverse carinae. Distal margin of frons medially not incised. Interantennal space less than twice as wide as of first antennomere length (Fig. 159) | 6 |
| 6 | Prosternum distinctly convex anteriorly (Fig. 160), most of mouthparts fitting into this convexity when head is in resting position, except labrum and mandibles, which act as a ‘cover’ (Fig. 159); maxillae and labium sunken (Fig. 159). Pronotal punctation variable, displaying punctures of different sizes (Fig. 158) | Collartaltica Bechyné, 1959 (Fig. 31) |
| – | Prosternum moderately convex anteriorly, mouthparts do not fit into this convexity when head is in resting position; maxillae and labium exposed and not sunken. Pronotal punctation uniform | Chaetocnema Stephens, 1831 (Fig. 28) |
| 7 | Procoxal cavities closed posteriorly. Antennomere 4 about as long as antennomere 3, or shorter (Figs 147, 273, 274). Elytral punctation generally arranged in regular rows, more rarely partially in irregular double rows or confused (Figs 23, 107). Antennae shorter, not reaching half elytral length (Figs 23, 107) | 8 |
| – | Procoxal cavities open posteriorly. Antennomere 4 at least double length of antennomere 3 (Figs 161, 240, 241). Elytral punctation always confused, densely and uniformly impressed (Figs 33, 87). Antennae longer, reaching half elytral length (Figs 33, 87) | 9 |
| 8 | Pronotum with distinct apical, median or basal impressions, always with two sublateral series of large and deeply impressed punctures from anterior margin to middle of pronotum (subgenus Blepharidina Bechyné) (Fig. 146); pronotal punctation not uniformly distributed (Fig. 146). Hind tibiae broadly channeled dorsally (Fig. 148). Frontal sulci deeply impressed (subgenus Blepharidina Bechyné) (Fig. 147). Eyes generally very elongate longitudinally (Fig. 147). Elytral punctation from moderately to distinctly impressed, generally arranged in regular rows (Figs 23, 148) (in Afrotropical region only Blepharida geminata Bryant, 1944 shows elytral punctation arranged in regular partially double rows). Posterior margin of hind femora indistinctly or moderately emarginated | Blepharida Chevrolat, 1836 (Fig. 23) |
| – | Pronotum without distinct impressions; pronotal punctation uniformly distributed (Figs 107, 273). Hind tibiae indistinctly channeled dorsally (Fig. 275). Frontal sulci moderately impressed (Fig. 274). Eyes less elongate longitudinally, sometimes roundish (Fig. 274). Elytral punctation moderately impressed, arranged in double partially regular rows (Figs 107, 275) or mostly confused. Posterior margin of hind femora often distinctly emarginated (Fig. 275) | Xanthophysca Fairmaire, 1901 (Fig. 107) |
| 9 | Antennae filiform with antennomeres 4–10 filiform or slightly enlarged (Fig. 161) | Diamphidia Gerstaecker, 1855 (Fig. 33) |
| – | Antennae with antennomeres 4–10 pectinate or flabellate in male and serrate in female (Figs 240–241) | Polyclada Chevrolat, 1836 (Fig. 87) |
| 1 | Pronotum with two very short but distinct longitudinal sublateral impressions basally; transverse sulcus absent ante-basally (Fig. 103). Elytral punctation confused, finely but distinctly and densely impressed, mainly in basal half (Fig. 103). Body larger (LB > 3.90 mm), subsphaerical (Fig. 103) | Toxaria Weise, 1903 (Fig. 103) |
| – | Pronotum with transverse sulcus ante-basally, sometimes very slightly impressed. Elytral punctation generally very slightly or indistinctly impressed (Fig. 101), sometimes subseriate (Fig. 18). Body smaller (LB ≤ 3.90 mm), oval or elliptical (Fig. 18, 101) | 2 |
| 2 | Pronotum comparatively smaller (LE/LP > 2.80), slightly rounded laterally; transverse sulcus always distinctly impressed ante-basally, bounded by two distinct longitudinal striae laterally (Fig. 101) | Stuckenbergiana Scherer, 1963 (Fig. 101) |
| – | Pronotum comparatively larger (LE/LP ≤ 2.80), more distinctly rounded laterally; transverse sulcus very slightly impressed ante-basally, not bounded laterally and sometimes only visible medially (Fig. 18) | Bechuana Scherer, 1970 (Fig. 18) |
| 1 | Dorsal integument distinctly pubescent (Fig. 40, 41) | 2 |
| – | Dorsal integument apparently glabrous. Elytra sometimes very sparsely pubescent in Lampedona and Lypnea | 3 |
| 2 | Pronotum as wide as elytra basally, subparallel or convergent towards anterior laterally; sulcus more or less deeply impressed ante-basally, bounded by two short longitudinal striae laterally (Fig. 40). Frontal tubercles very small, elongate and narrow (Fig. 180). Antennomere 4 distinctly shorter than antennomeres 2–3 together. Body less elongate (LB/WE < 2.00) (Fig. 41). Elytra not modified apically in male | Epitrix Foudras, 1860 (Fig. 40) |
| – | Pronotum narrower than elytra basally, divergent towards anterior laterally; transverse sulcus finely impressed ante-basally, not bounded by longitudinal striae laterally (Fig. 41). Frontal tubercles larger, subrectangular or subtriangular, often elongate towards upper ocular margin (Fig. 181). Antennomere 4 as long as antennomeres 2–3 together. Body more elongate (LB/WE ≥ 2.00) (Fig. 41). Elytra generally with modified structures apically in male (Fig. 41) | Eriotica Harold, 1877 (Fig. 41) |
| 3 | Hind tibia with two apical spurs (Fig. 216) | Myrcina Chapuis, 1875 (Fig. 69) |
| – | Hind tibia with only one apical spur | 4 |
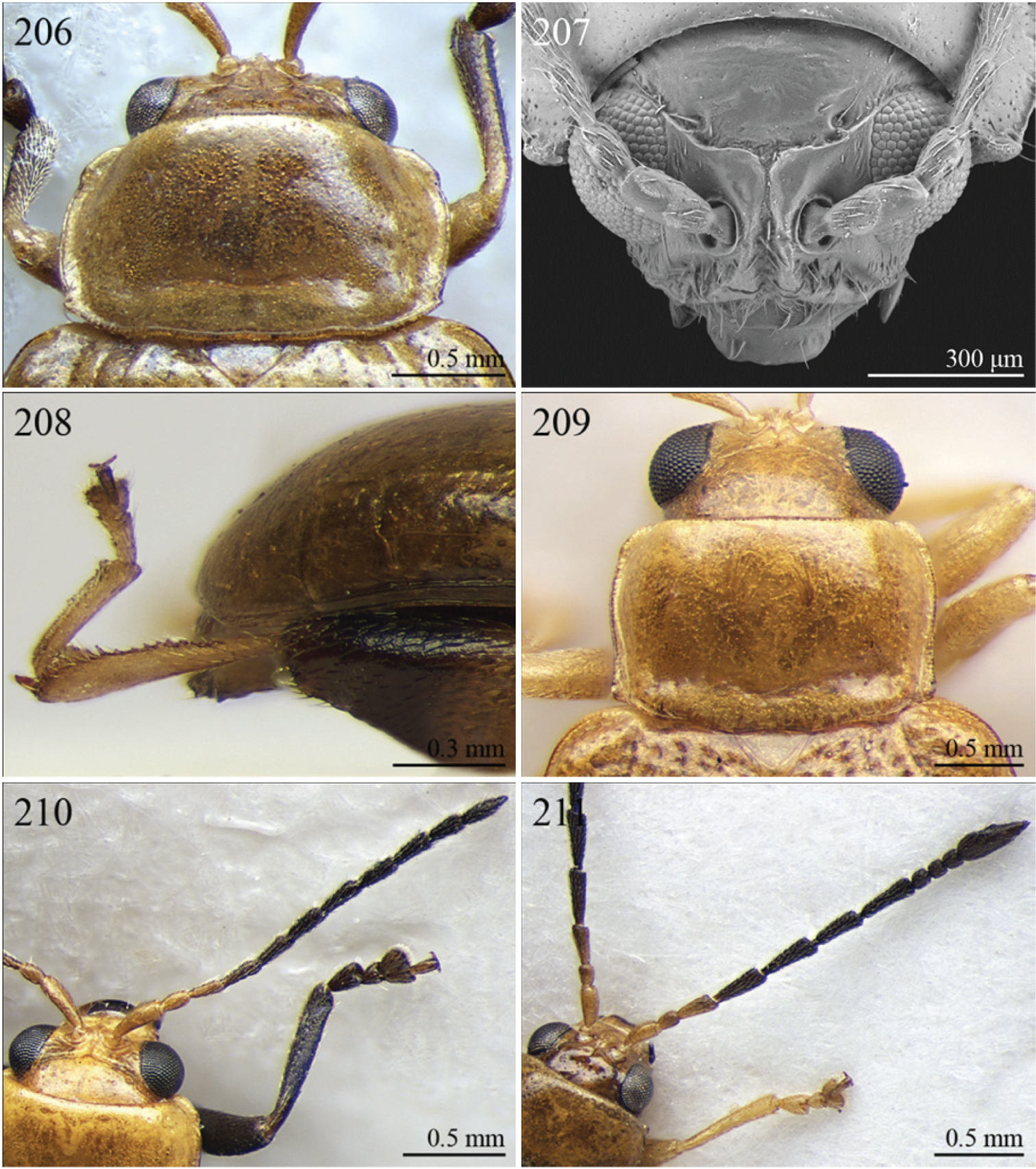
| 4 | Pronotum with transverse sulcus bounded laterally by two short longitudinal striae ante-basally or touching basal margin (Figs 126, 170, 209, 225, 246) | 5 |
| – | Pronotum with transverse sulcus not bounded by longitudinal striae laterally and ante-basally; sulcus interrupted laterally, or touching lateral margin or posterior angles (Figs 120, 121, 212) | 20 |
| 5 | Antennomere 3 distinctly longer than antennomere 1 (Figs 12, 73). Frons distinctly elongate (Figs 127, 222, 223). Genae about 1.5x length of vertical ocular diameter (Figs 127, 223). Antennae comparatively elongate (LB/LAN < 1.20) (Figs 12, 73) | 6 |
| – | Antennomere 3 shorter than antennomere 1 (Fig. 210). Frons short. Genae distinctly shorter than vertical ocular diameter. Antennae comparatively short (LB/LAN ≥ 1.20) | 7 |
| 6 | Elytral punctation arranged in 9 regular rows, partially confused in sutural area; interstriae distinctly convex on disc, and subcarinate laterally (Figs 73, 222). Anterior angles of pronotum not bevelled, distinctly dentiform apically (Fig. 221). Antennae extraordinarily elongate in male (LB/LAN < 0.90), with antennomeres 3–6 distinctly enlarged; antennae in female not enlarged and little shorter than the body (Fig. 73). Body elongate and slightly convex (Figs 73, 222) | Ntaolaltica Biondi & D’Alessandro, in press (Fig. 73) |
| – | Elytral punctation entirely confused (Fig. 12). Anterior angles of pronotum bevelled, moderately dentiform apically (Fig. 126). Antennae filiform in both sexes (LB/LAN ≥ 0.90), without enlarged antennomeres or only moderately enlarged only in male (Fig. 12). Body thickset and distinctly convex (Fig. 12) | Antanemora Bechyné, 1964 (Fig. 12) |
| 7 | Pronotum with ante-basal transverse sulcus not bounded by longitudinal striae laterally, and touching basal margin (Figs 170, 246) | 8 |
| – | Pronotum with ante-basal transverse sulcus bounded by two short longitudinal striae laterally (Fig. 225) | 13 |
| 8 | Elytral punctation arranged in regular, or sometimes partially irregular, single or double rows | 9 |
| – | Elytral punctation entirely confused | 10 |
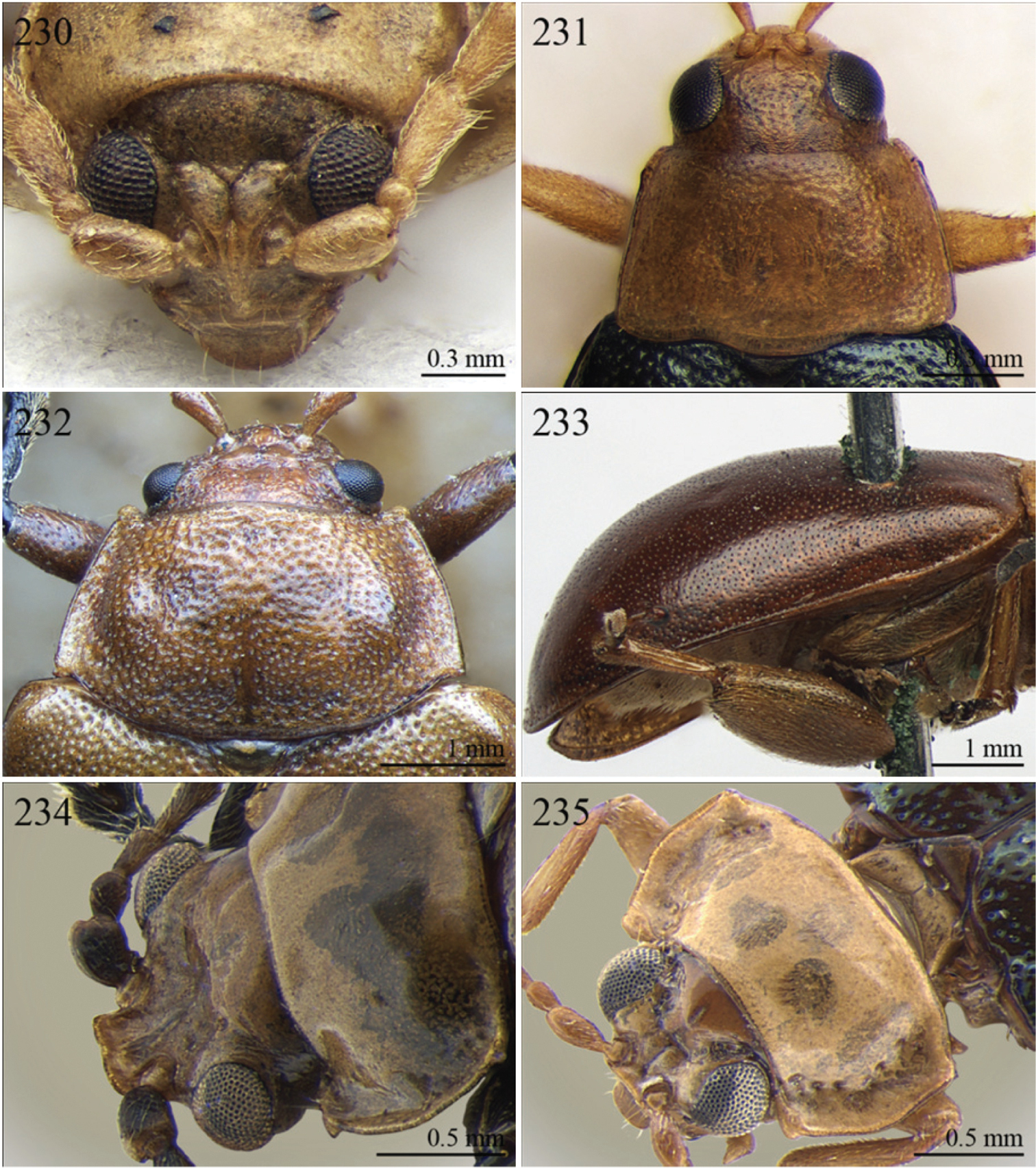
| 9 | Antennae in female robust, generally with antennomere 2 as long as antennomere 3; antennomeres 9–11 in male distinctly enlarged, about as wide as long (Fig. 90). Elytral punctation indistinctly impressed, sometimes hardly visible amongst interstrial punctuation (Fig. 247). Pronotum distinctly rounded and widely bordered laterally (Fig. 246) | Pseudophygasia Biondi & D’Alessandro, in press (Fig. 90) |
| – | Antennae filiform with antennomere 2 distinctly shorter than antennomere 3; antennomeres 9–11 elongate (Fig. 210). Elytral punctation distinctly impressed (Fig. 64). Pronotum slightly rounded and finely bordered laterally (Fig. 209) | Lypnea Baly, 1876a (Fig. 64) |
| 10 | Pronotum distinctly arcuate laterally, distinctly narrower basally; posterior angles distinctly laterally produced at apex (Fig. 170). Frontal tubercles rounded, distinctly raised (Figs 170–171). Antennae more robust, often with distinctly enlarged distal antennomeres (Fig. 171) | Diphaulacosoma Jacoby, 1892a (Fig. 36) |
| – | Pronotum moderately rounded, slightly narrower basally; posterior angles very indistinctly produced laterally, finely dentiform at apex. Frontal tubercles generally subtriangular, flat or slightly raised. Antennae filiform, never with distinctly enlarged antennomeres | 11 |
| 11 | Frontal tubercles extended distally between antennae, forming two parallel, acute apically longitudinal carinae which surround clypeus distally (Fig. 207). Body smaller (generally LB < 2.60 mm). Pronotum with distinctly impressed transverse sulcus ante-basally (Fig. 61) | Lepialtica Scherer, 1962 (Fig. 61) |
| – | Frontal tubercles not extended distally between antennae; frontal carina simple. Body larger (generally LB ≥ 2.60 mm). Pronotum with indistinctly impressed transverse sulcus ante-basally | 12 |
| 12 | Elytra widely bordered laterally (Fig. 77). Frontal carina narrow and acute apically (Fig. 228). Interantennal space narrow, about as wide as 1/3 of length of first antennomere (Fig. 228) | Perichilona Weise, 1919 (Fig. 77) |
| – | Elytra narrowly bordered laterally (Fig. 42). Frontal carina wider and rounded apically (Fig. 182). Interantennal space wider, about as wide as length of first antennomere (Fig. 182) | Eurylegna Weise, 1910 (Fig. 42) |
| [= Eurylegniella Scherer, 1972 syn. n. (Fig. 43)] | ||
| 13 | Elytral punctation arranged in simple or double rows, sometimes partially irregular | 14 |
| – | Elytral punctation entirely confused | 17 |
| 14 | Elytral punctation arranged in double partially irregular rows (Fig. 118). Elytra generally with a distinct longitudinal carina laterally (Fig. 118) | Afrocrepis Bechyné, 1954 (Fig. 4) |
| – | Elytral punctation arranged in single regular rows. Elytra never with longitudinal carinae laterally | 15 |
| 15 | Anterior tibiae distinctly enlarged distally, with a deep longitudinal depression in distal half (Fig. 188). Interocular space distinctly punctate (Fig. 187) | Guinerestia Scherer, 1959 (Fig. 47) |
| – | Anterior tibiae normally shaped. Interocular space impunctate or very sparsely punctate (Figs 119, 205) | 16 |
| 16 | Procoxal cavities open posteriorly. Pronotal sulcus curved towards base medially, bounded by two short longitudinal striae laterally (Figs 58, 59). Elytra with distinct basal calli (Figs 58, 59). Frontal carina narrow, acute apically (Fig. 205). Frontal tubercles next to each other (Fig. 205) | Kimongona Bechyné, 1959 (Fig. 58) |
| [= Mesocrepis Scherer, 1963 syn. n. (Fig. 59)] | ||
| – | Procoxal cavities closed posteriorly. Pronotal sulcus straight, bounded by two longer longitudinal striae laterally (Fig. 5). Elytra without basal calli (Fig. 5). Frontal carina wide, subrounded apically (Fig. 119). Frontal tubercles distant from each other (Fig. 119) | Afrorestia Bechyné, 1959 (Fig. 5) |
| 17 | Antennae comparatively short (LB/LAN ≥ 2.20), with antennomeres 6–11 as long as wide (Fig. 10) | Anaxerta Fairmaire, 1902 (Fig. 10) |
| – | Antennae comparatively long (LB/LAN < 2.20), with antennomeres 6–11 distinctly longer than wide | 18 |
| 18 | Procoxal cavities closed posteriorly. Pronotum moderately rounded laterally; posterior angles not dentiform apically (Fig. 70). Antennae often with alternate groups of black and yellowish antennomeres (Fig. 70) | Neodera Duvivier, 1891 (Fig. 70) |
| – | Procoxal cavities open posteriorly. Pronotum distinctly rounded laterally; posterior angles distinctly dentiform apically. Antennae never with alternate groups of black and yellowish antennomeres | 19 |
| 19 | Antennomere 2 about as long as antennomere 3 or slightly shorter (Fig. 225). Frontal tubercles indistinctly defined posteriorly, and slightly raised (Fig. 226) . Pronotum narrowly bordered laterally Fig. 225) | Orthocrepis Weise, 1888 (Fig. 75) |
| – | Antennomere 2 distinctly shorter than antennomere 3 (Fig. 230). Frontal tubercles distinctly defined posteriorly, and distinctly raised (Fig. 230). Pronotum widely bordered laterally (Fig. 79) | Phygasia Chevrolat, 1836 (Fig. 79) |
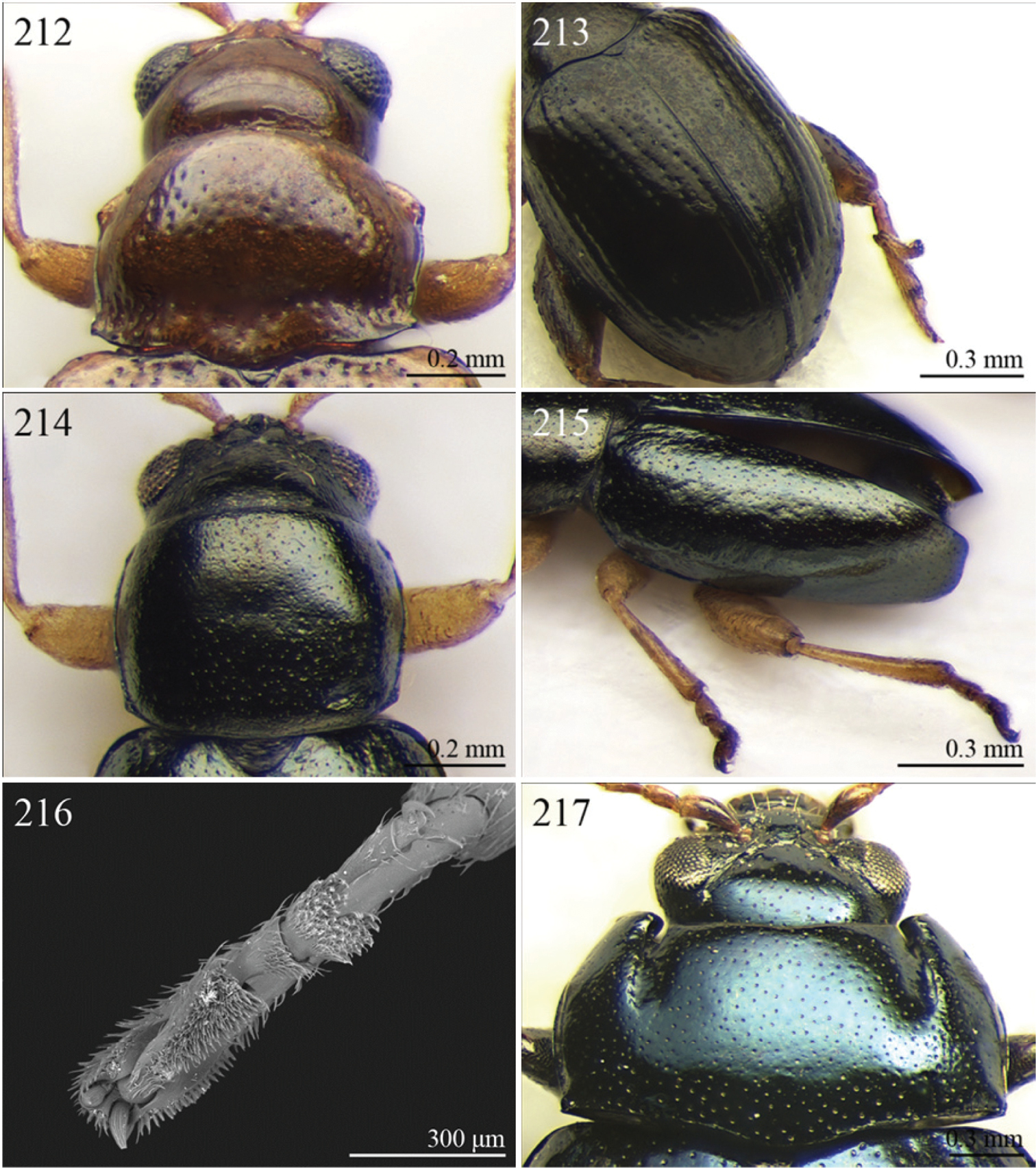
| 20 | Elytral punctation entirely confused [see also Guilielmia at couplet 24] | 21 |
| – | Elytral punctation arranged in simple or double rows, more or less irregular only in Guilielmia | 23 |
| 21 | Procoxal cavities closed posteriorly (157). Anterior and middle femora enlarged, particularly in male (Fig. 157) | Chirodica Germar, 1834 (see also Group H couplet 28) (Fig. 30) |
| – | Procoxal cavities open posteriorly. Anterior and middle femora not enlarged | 22 |
| 22 | Pronotum with distinctly impressed transverse sulcus ante-basally, often touching lateral margins of pronotum (Fig. 121); anterior angles generally rounded apically. Interantennal space narrower than length of first antennomere (Fig. 121). Frontal carina narrow and acute (Fig. 121). Pronotal punctation generally indistinctly impressed (Fig. 121). Dorsal integument usually blue or green, always with distinct metallic luster (Fig. 7) | Altica Geoffroy, 1762 (Fig. 7) |
| – | Pronotum with slightly impressed transverse sulcus ante-basally, never touching lateral margins of pronotum (Fig. 1); anterior angles obliquely bevelled apically . Interantennal space distinctly wider than length of first antennomere (Fig. 120). Frontal carina wide and flat. Pronotal punctation distinctly impressed (Fig. 120). Dorsal integument brownish with partially blackened elytra, without metallic luster (Fig. 6) | Alocypha Weise, 1911 (Fig. 6) |
| 23 | Humeral calli absent. Ante-basal pronotal sulcus barely visible (Figs 26, 46) | 24 |
| – | Humeral calli distinct. Ante-basal pronotal sulcus always distinctly impressed, medially at least (Figs 172, 206, 212) | 25 |
| 24 | Elytral punctation arranged in 9 regular rows. Dorsal integument from yellowish to brownish | Celisaltica Biondi, 2001a (Fig. 26) |
| – | Elytral punctation more or less irregular. Dorsal integument dark | Guilielmia Weise, 1924 (Fig. 46) |
| 25 | Antennomere 2 as long as antennomere 1 and at least twice as long as antennomere 3 (Fig. 172). Procoxal cavities closed posteriorly | Djallonia Bechyné, 1955 (Fig. 37) |
| – | Antennomere 2 distinctly shorter than antennomere 1 and about as long as antennomere 3. Procoxal cavities open posteriorly | 26 |
| 26 | Antennomere 1 at least 2.5 times longer than antennomere 2. Pronotal base not, or very slightly, sinuous (Fig. 206). Body larger (LB ≥ 4.50 mm). Elytra comparatively elongate (LE/WE > 1.60) (Fig. 60) | Lampedona Weise, 1907 (Fig. 60) |
| – | Antennomere 1 about 1.5 times longer than antennomere 2. Pronotal base distinctly sinuous (Figs 204, 212). Body smaller (LB < 4.50 mm). Elytra comparatively short (LE/WE ≤ 1.60) (Figs 57, 66) | 27 |
| 27 | Frontal tubercles distinctly defined posteriorly. Pronotum with distinctly impressed transverse sulcus ante-basally, touching lateral margins of pronotum; posterior angles distinctly dentiform apically (Fig. 212). Dorsal integument with distinctly impressed punctation (Fig. 66). First metatarsomere longer than second and third together. Elytra with prominent basal calli (Fig. 66) | Manobia Jacoby, 1885 (Fig. 66) |
| – | Frontal tubercles not distinctly defined posteriorly. Pronotum with a moderately or indistinctly impressed transverse sulcus ante-basally, not touching the lateral margins of pronotum; posterior angles subrounded apically, never distinctly dentiform (Fig. 204). Dorsal integument with slightly or moderately impressed punctation (Fig. 57). First metatarsomere shorter than second and third together. Elytra with basal calli barely visible (Fig. 57). | Kenialtica Bechyné, 1960 (Fig. 57) |
| 1 | Anterior margin of pronotum without distinct longitudinal impressions, with very short incisions at most. Pronotum slightly narrower than elytra basally. Body generally less convex and more elongate (Figs 85–86) | Podagrica Chevrolat, 1836 (Fig. 85) |
| [= Podagricina Csiki in |
||
| – | Anterior margin of pronotum with two longitudinal groove-like impressions, often deeply impressed (Figs 217–218) and sometimes reaching middle of pronotum. Pronotum as wide as elytra basally (Figs 71, 217, 218). Body thickset and distinctly convex (Fig. 71). | Nisotra Baly, 1864 (Fig. 71) |
| 1 | Body more elongate, less convex, with comparatively elongate elytra (LE/WE < 1.25) (Fig. 16). Elytral margin in dorsal view well visible in its all length. Pronotum with straight sublateral sulci, basally obliquely slanted and distally never touching the lateral margin of pronotum (Fig. 133). | Argopistoides Jacoby, 1892b (Fig. 16) |
| – | Body subsphaerical, distinctly convex, with comparatively short elytra (LE/WE ≥ 1.25) (Fig. 27). Elytral margin in dorsal view generally not visible or visible only basally. Pronotum with sinuous sublateral sulci, basally starting sub-parallel to lateral margin of pronotum and distally sometimes touching it (Fig. 153) | Chabria Jacoby, 1887 (Fig. 27) |
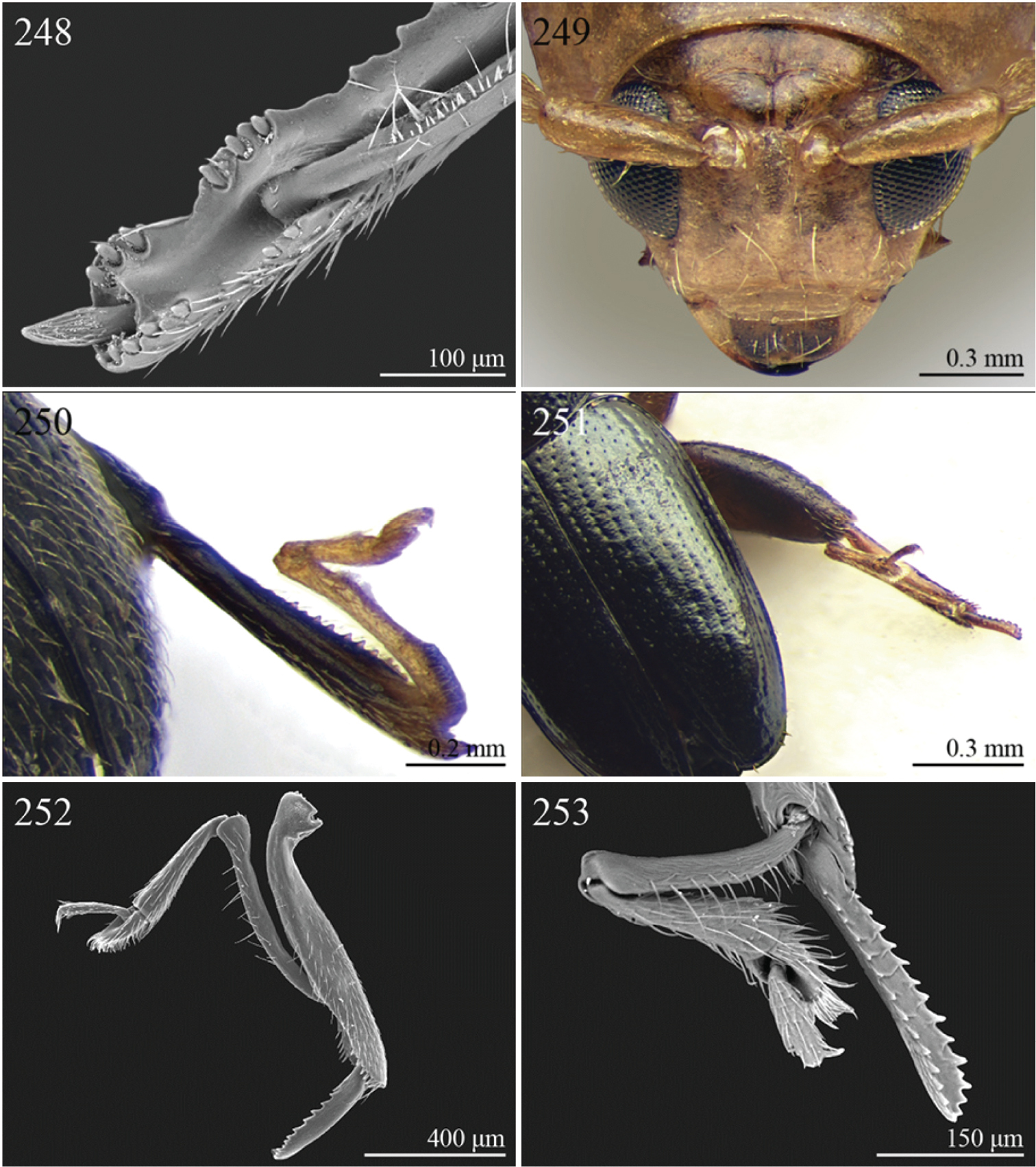
| 1 | Apical spur of hind tibiae distinctly serrate (Fig. 252, 253, 277) | 2 |
| – | Apical spur of hind tibiae differently shaped but never serrate | 3 |
| 2 | Elytral punctation distinctly impressed, arranged in regular rows (Fig. 251). First metatarsomere longer than 2/3s length of hind tibia and dorsally or preapically inserted on hind tibia (Fig. 252). Apical spur of hind tibiae distinctly elongate (LHT/LHTS ≤ 8.00) (Figs 252–253) | Serraphula Jacoby, 1897 (Fig. 94) |
| – | Elytral punctation slightly impressed, poorly visible and only partially arranged in regular rows (Fig. 276). First metatarsomere at most as long as half of hind tibial length and apically inserted on hind tibia (Fig. 276). Apical spur of hind tibiae very short (LHT/LHTS > 8.00) (Figs 276–277) | Yemenaltica Scherer, 1985 (Fig. 108) |
| 3 | Metatarsus dorsally inserted at about half of tibial length (Fig. 213) | Metroserrapha Bechyné, 1958a (Fig. 67) |
| – | Metatarsus apically inserted on tibia | 4 |
| 4 | Apical spur of hind tibiae distally bifid or trifid (Figs 179, 270) | 5 |
| – | Apical spur of hind tibiae simple [in Trachytetra hind apical spur thickset, subtruncate, sometimes apparently bifid (Fig. 269); but this genus is easily distinguishable from Dunbrodya and Dibolia by having distinctly defined frontal tubercles; and frons that is elongate distally in lateral view] | 8 |
| 5 | Apical spur of hind tibiae obtusely pointed apically (Fig. 270). Antennae about as long as body (Fig. 105). Habitus similar to Longitarsus | Tritonaphthona Bechyné, 1960 (Fig. 105) |
| – | Apical spur of hind tibiae bifid apically (Fig. 179). Antennae generally short, at most reaching half of elytra | 6 |
| 6 | First metatarsomere about 1/3 of length of hind tibia (Fig. 178). Pronotum less transverse, lateral margins converging slightly towards anterior (WP/LP ≤ 1.70) (Fig. 176). Frons with distinct longitudinal carina medially (Fig. 177). Body elongate and slightly convex (Fig. 39). Habitus similar to Aphthona | Dunbrodya Jacoby, 1906 (Fig. 39) |
| – | First metatarsomere about 1/5 of length of hind tibia (Fig. 166). Pronotum distinctly transverse, laterally margins converging distinctly towards anterior (WP/LP > 1.70) (Fig. 164). Frons without or with very short longitudinal carina medially (Figs 165, 227). Body thickset and distinctly convex (Figs 34, 76) | 7 |
| 7 | Eyes small, widely separated dorsally, interocular distance at least 3 times wider than length of second antennomere (Fig. 165) | Dibolia Latreille, 1829 (Fig. 34) |
| – | Eyes very large, closely associated dorsally; interocular space as wide as length of second antennomere (Fig. 227) | Paradibolia Baly, 1875 (Fig. 76) |
| 8 | Elytra distinctly reduced, obliquely truncate apically (Fig. 262). Pronotum subtrapezoidal, with maximum width at anterior angles; lateral margins straight (Fig. 261) | Sjostedtinia Weise, 1910 (Fig. 98) |
| – | Elytra not reduced, generally rounded apically. Pronotum subrectangular or subtrapezoidal with maximum width generally at middle or basally; lateral margins more or less rounded | 9 |
| 9 | Elytral surface distinctly and uniformly pubescent | 10 |
| – | Elytral surface apparently glabrous, rarely very sparsely setose towards apex | 14 |
| 10 | First metatarsomere about half length of tibia (Fig. 250). Elytral punctation arranged in regular rows (Figs 92–93) | Sanckia Duvivier, 1891 (Fig. 92) |
| [= Eugonotes Jacoby, 1897 syn. n. (Fig. 93)] | ||
| – | First metatarsomere distinctly shorter than half length of tibia. Elytral punctation confused | 11 |
| 11 | Pronotum pubescent | Hespera Weise, 1889 (Fig. 51) |
| – | Pronotum glabrous | 12 |
| 12 | Elytral punctation confused, densely and finely impressed (Figs 53, 198). Elytral surface with short dense pubescence (Fig. 198). Frontal tubercles not defined posteriorly. Antennae short, not reaching middle of elytra (Fig. 53). First metatarsomere shorter than length of second and third together (Fig. 198). Elytral margins widely bordered laterally (Fig. 198) | Homichloda Weise, 1902 (Fig. 53) |
| – | Elytral punctation arranged in regular or partially irregular rows, distinctly impressed (Figs 49, 52). Elytral surface with longer sparse pubescence (Fig. 191). Frontal tubercles well defined posteriorly (Figs 190, 196, 197). Antennae longer, reaching beyond middle of elytra. First metatarsomere longer than second and third together (Fig. 191). Elytral margins narrowly bordered laterally (Fig. 191) | 13 |
| 13 | Pronotal punctation distinctly impressed on surface with distinct transverse and longitudinal carinae, sulci and/or protuberances (Figs 196–197). Frontal tubercles medially separated by deep longitudinal sulcus (Figs 196–197) | Hildebrandtina Weise, 1910 (Fig. 52) |
| – | Pronotal punctation indistinctly impressed on surface without distinct impressions or protuberances Fig. 189). Frontal tubercles medially separated by fine longitudinal sulcus (Fig. 190) | Halticotropis Fairmaire, 1886 (Fig. 49) |
| 14 | Body slightly elongate, often subsphaerical (LB/WE < 1.70). Pronotum more transverse (generally WP/LP > 1.80) | 15 |
| – | Body distinctly elongate, never subsphaerical (LB/WE ≥ 1.70). Pronotum less transverse (generally WP/LP ≤ 1.70 ) | 24 |
| 15 | Pronotum with anterior setigerous pore near middle of lateral margin (Fig. 201). Antennomere 3 as long as, or longer than, antennomeres 4–5 together (Fig. 202) | Jacobyana Maulik, 1926 (Fig. 55) |
| – | Pronotum with anterior setigerous pores near anterior angles. Antennomere 3 distinctly shorter than antennomeres 4–5 together | 16 |
| 16 | Body smaller (LB < 1.60 mm). Elytral punctation with scutellar stria long, reaching apical declivity of elytra (Fig. 138). Apical spur of hind tibia very small and slender | Bezdekaltica Döberl, 2012 (Fig. 20) |
| – | Body larger (LB ≥ 1.60 mm). Elytral punctation with scutellar stria short, not reaching middle of elytra. Apical spur of hind tibia robust | 17 |
| 17 | Pronotum with lateral margins diverging from base towards middle, then converging slightly towards anterior; maximum pronotal width in anterior third; anterior angles distinctly dentiform apically (Fig. 219). Pronotal punctation very distinctly impressed especially laterally (Fig. 219). Elytra with submarginal stria of distinctly and deeply impressed punctures laterally, delimiting wide and distinctly raised lateral band (Fig. 220) | Notomela Jacoby, 1899 (Fig. 72) |
| – | Pronotum with lateral margins converging distinctly towards anterior; maximum pronotal width at base; anterior angles not distinctly dentiform apically. Pronotal punctation from finely to moderately impressed. Elytra lacking submarginal stria with distinctly impressed punctures and distinctly raised lateral band | 18 |
| 18 | Frontal tubercles and frontal carina absent (Fig. 122). Interantennal space at least as wide as transverse ocular diameter (Fig. 122). Elytral interstriae always densely punctuated | Amphimela Chapuis, 1875 (Fig. 8) |
| [= Sphaerophysa Baly, 1876b syn. n. (Fig. 9)] | ||
| – | Frontal tubercles distinctly defined. Frontal carina narrow, often raised. Interantennal space narrower than transverse ocular diameter. Elytral interstriae generally not densely punctuated | 19 |
| 19 | Pronotal base regularly rounded, not sinuate | 20 |
| – | Pronotal base bisinuate | 22 |
| 20 | Elytral punctation regularly striate. Scutellum not visible (Fig. 265). Body smaller (LB < 2.30 mm), distinctly convex (Fig. 100) | Stegnaspea Baly, 1877 (Fig. 100) |
| – | Elytral punctation confused and finely impressed. Scutellum distinctly visible. Body larger (LB ≥ 2.30 mm), moderately convex | 21 |
| 21 | Frontal carina not prolonged towards clypeus (Fig. 244). Frons distinctly raised distally in lateral view. Elytra with distinct basal calli (Fig. 89). Pronotum with anterior angles distinctly thickened, projecting distinctly towards anterior (Fig. 243). Body more convex (Fig. 89). Hind tibiae narrowly and less deeply channeled dorsally (Fig. 245) | Pseudadorium Fairmaire, 1885 (Fig. 89) |
| – | Frontal carina prolonged towards clypeus (Fig. 193). Frons not raised distally in lateral view. Elytra without distinct basal calli (Fig. 50). Pronotal anterior angles slightly or moderately thickened, not projecting towards anterior, but sometimes dentiform laterally (Fig. 192). Body less convex (Fig. 50). Hind tibiae broadly and more deeply channeled dorsally (Fig. 194) | Hemipyxis Chevrolat, 1836 (Fig. 50) |
| 22 | Hind tibiae with distinct preapical tooth on inside (Fig. 132). Hind femora at least as wide as length of hind tibia (Fig. 131). Eyes elongate, closely associated dorsally, separated by less than transverse ocular diameter (Fig. 130). First abdominal ventrite medially with two distinct longitudinal ridges forward convergent (Fig. 131) | Argopistes Motschulsky, 1860 (Fig. 15) |
| – | Hind tibiae lacking distinct preapical tooth on inside. Hind femora narrower than length of hind tibia. Eyes rounded, widely separated dorsally, separated by transverse ocular diameter at least. First abdominal ventrite without distinct longitudinal ridges | 23 |
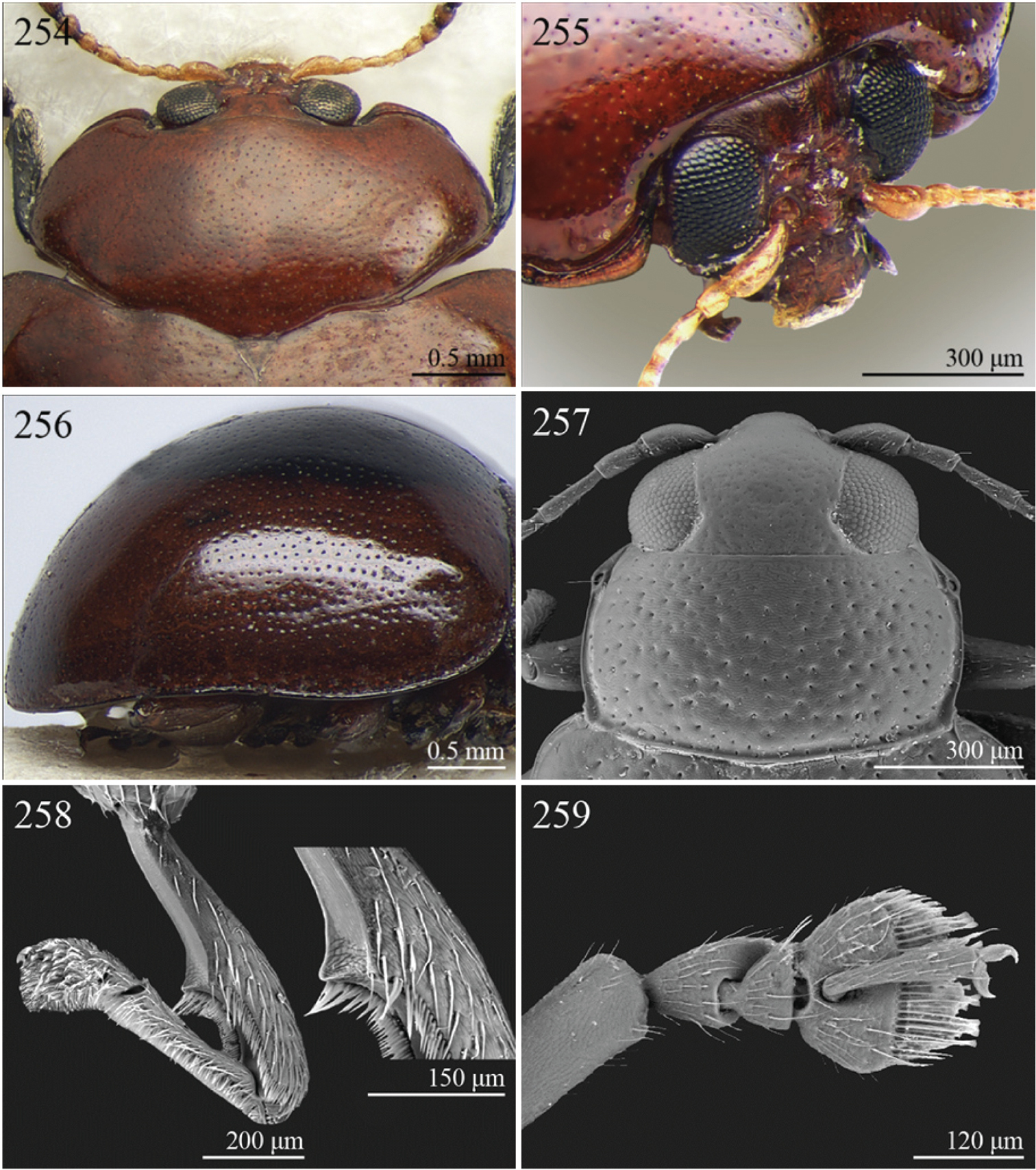
| 23 | Pronotum wider (WP/LP ≥ 2.30), distinctly bisinuate basally; anterior angles projecting distinctly towards anterior; apically widely rounded and completely bordered by thickened margin (Figs 254–255). Elytral epipleura obliquely upward orientated, generally not visible in lateral view (Fig. 256). Elytral punctation confused, finely impressed (Figs 95–96). Body subsphaerical (Figs 95–96) | Sesquiphaera Bechyné, 1958 (Fig. 95) |
| [= Paropsiderma Bechyné, 1958 syn. n. (Fig. 96)] | ||
| – | Pronotum narrower (WP/LP < 2.30), moderately bisinuate basally; anterior angles projecting slightly towards anterior; apically not completely bordered by thickened margin, limited to lateral edge of pronotum (Fig. 263). Elytral epipleura horizontally or slightly obliquely downward orientated, well visible in lateral view (Fig. 264). Elytral punctation often more or less seriate, punctures more distinctly impressed. Body generally short and oval, rarely subsphaerical (Fig. 99) | Sphaeroderma Stephens, 1831 (Fig. 99) |
| 24 | Apical spur of hind tibiae robust, often very short, generally absent on anterior and middle tibiae. Antennomere 2 about as long as antennomere 4 (only in some Chirodica antennomere 4 distinctly longer than antennomere 2 but anterior and middle femora enlarged and procoxal cavities closed posteriorly). Elytra apparently not pubescent. Body slightly elongate | 25 |
| – | Apical spur very slender and present on all tibiae; hind apical spur always elongate but sometimes very short on anterior and middle tibiae. Antennomere 2 much shorter than antennomere 4. Elytra or apical part of elytra very sparsely pubescent (Fig. 174). Body distinctly elongate. Habitus similar to Galerucini | 37 |
| 25 | First metatarsomere as long or longer than half tibial length (Figs 137, 140, 208) | 26 |
| – | First metatarsomere shorter than half tibial length | 28 |
| 26 | Elytral punctation confused | Longitarsus Berthold, 1827 (Fig. 62) |
| – | Elytral punctation regularly seriate | 27 |
| 27 | First metatarsomere about same length as tibia (Fig. 137). Interantennal space about as wide as transverse ocular diameter; frontal carina apically rounded, moderately raised (Fig. 136). Apical spur of hind tibiae long (LHT/LHTS ≤ 10.00) (Fig. 137). Dorsal integument from yellowish to pale brown (Fig. 19) | Bechynella Biondi & D’Alessandro, 2010b (Fig. 19) |
| – | First metatarsomere about half length of tibia (Fig. 140). Interantennal space distinctly narrower than transverse ocular diameter; frontal carina apically acute and raised (Fig. 139). Apical spur of hind tibiae shorter (LHT/LHTS > 10.00). Dorsal integument generally darker with more or less distinct metallic reflections (Figs 21, 140) | Bikasha Maulik, 1931 (also see couplet 34) (Fig. 21) |
| 28 | Procoxal cavities closed posteriorly (Fig. 157). Anterior and middle femora distinctly enlarged, particularly in male (Fig. 157). Pronotum sometimes with a very small transverse sulcus ante-basally. Second maxillary palpomere about as wide as first (Fig. 156) | Chirodica Germar, 1834 (also see Group E couplet 21) (Fig. 30) |
| – | Procoxal cavities open posteriorly. Anterior and middle femora not enlarged. Pronotum never with a transverse sulcus ante-basally. Second maxillary palpomere generally wider than first | 29 |
| 29 | Antennomeres 7–11 or 8–11 more enlarged than those remaining (Figs 2, 3, 111) | 30 |
| – | Antennomeres 7–11 similar in width to those remaining | 31 |
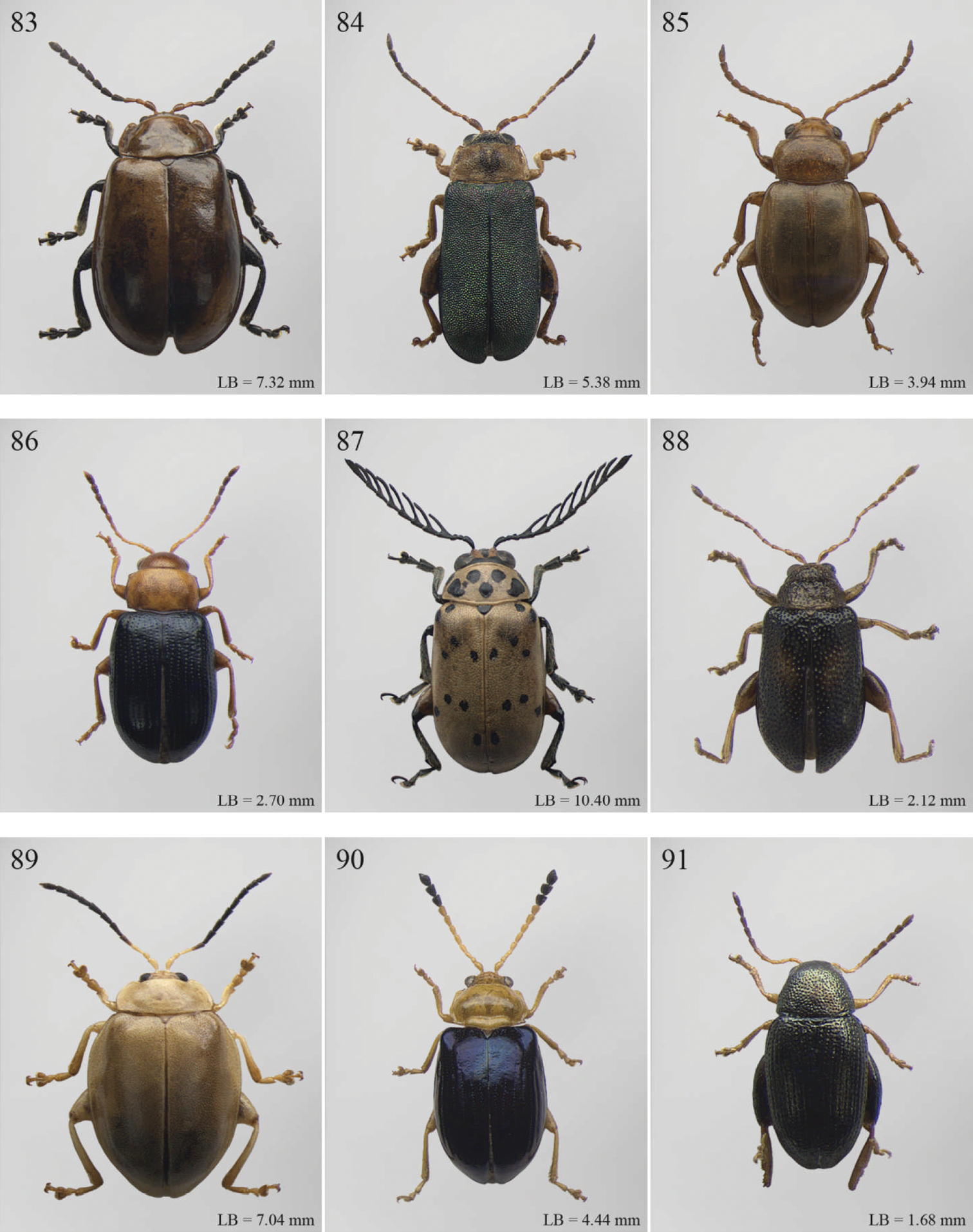
| 30 | Humeral calli absent (Fig. 3). Pronotum distinctly convex, very narrowly bordered laterally; anterior angles rounded or slightly bevelled; posterior angles rounded (Fig. 114). Metasternum shorter than length of mid-coxal cavity (Fig. 117). Middle and hind tibiae distinctly enlarged from base to apex, particularly in male; middle tibiae with distinct triangular hollow ventrally in male (Fig. 115) | Afroaltica Biondi & D’Alessandro, 2007 (Fig. 3) |
| – | Humeral calli distinct (Figs 2, 110). Pronotum moderately convex, distinctly bordered laterally; anterior angles distinctly dentiform and widely bevelled; posterior angles acute, often dentiform (Fig. 110). Metasternum longer than length of mid-coxal cavity (Fig. 112). Middle and hind tibiae moderately enlarged from base to apex; middle tibiae without distinct hollow ventrally in male | Abrarius Fairmaire, 1902 (Fig. 2) |
| 31 | Frontal carina narrow and distinctly raised with few large superficial punctures, and a narrow longitudinal groove distally between antennae (Fig. 242). Elytral and often also pronotal punctation, exceptionally dense and strongly impressed (Fig. 88) | Pratima Maulik, 1931 (Fig. 88) |
| – | Frontal carina wide, flat or convex but never grooved longitudinally. Elytral punctation from indistinctly to moderately impressed | 32 |
| 32 | Anterior angles of pronotum not obliquely bevelled (Fig. 214). Humeral calli absent (Figs 68, 215). Elytral apex subtruncate (Fig. 215) | Montiaphthona Scherer, 1961 (Fig. 68) |
| – | Anterior angles of pronotum obliquely bevelled. Humeral calli generally visible. Elytral apex generally rounded | 33 |
| 33 | Frontal tubercles absent or indistinctly defined (Fig. 231). Anterior angles of pronotum not widely and obliquely bevelled (Fig. 231). Elytral punctation confused. Body flatter and more elongate (Fig. 80) | Phyllotreta Chevrolat, 1836 (Fig. 80) |
| – | Frontal tubercles distinctly defined. Anterior angles of pronotum widely and obliquely bevelled. Elytral punctation sometimes partially regularly striate. Body more convex and less elongate | 34 |
| 34 | Apical spur of hind tibiae thickset, subtruncate apically, often apparently bifid (Fig. 269). Frons elongate distally in lateral view (Fig. 268). Elytral punctation confused | Trachytetra Sharp, 1886 (Fig. 104) |
| – | Apical spur of hind tibiae slender, acute apically, never apparently bifid. Frons short and regularly arcuate distally in lateral view. Elytral punctation confused, seriate or subseriate | 35 |
| 35 | Elytra with poorly defined but distinct basal calli (Fig. 140). Humeral calli bounded posteriorly by distinct, often deeply impressed, depression (Fig. 140). Frontal tubercles always well defined (Fig. 140). Elytral punctation seriate or subseriate, always distinctly impressed, and never confused (Fig. 140) | Bikasha Maulik, 1931 (see also couplet 27) (Fig. 21) |
| – | Elytra lacking basal calli. Humeral calli bounded posteriorly by more or less flat area. Frontal tubercles often not well defined. Elytral punctation generally confused, rarely seriate or subseriate; in this case punctation is slightly impressed and frontal tubercles are generally not distinct | 36 |
| 36 | Pronotum subtrapezoidal, straight or very slightly rounded and widely bordered laterally; anterior angles generally thickened, distinctly dentiform apically; posterior angles with distinct, laterally produced, tubercle at apex (Fig. 123). Pronotal base finely but distinctly sinuate (Fig. 123). Frontal tubercles elongate, V-shaped (Fig. 124). Elytra more widely bordered laterally, always with distinctly impressed punctation (Fig. 125). Dorsal integuments blue with distinct metallic reflections (Figs 11, 125). Spermatheca with coiled ductus | Angulaphthona Bechyné, 1960 (Fig. 11) |
| – | Pronotum subrectangular, more distinctly rounded and finely bordered laterally; anterior angles not thickened, not dentiform apically; posterior angles subrounded apically or slightly dentiform, without distinct tubercles (Fig. 128). Pronotal base generally not sinuate (Fig. 128). Frontal tubercles subtriangular or roundish, rarely elongate. Elytra narrowly bordered laterally, generally with slightly or moderately impressed punctation (Fig. 129). Dorsal integument varies in colour. Spermatheca with uncoiled ductus, very rarely coiled | Aphthona Chevrolat, 1836 (Fig. 13) |
| [= Ethiopia Scherer, 1972 syn. n. (Fig. 14)] | ||
| 37 | Pronotum with distinct oblique or transverse sublateral impressions medially (Figs 17, 29, 74) | 38 |
| – | Pronotum without impressions | 40 |
| 38 | Antennomeres 3 and 4 about same length, each about three times longer than antennomere 2; antennomeres 6–11 shorter and subequal in length (Fig. 134) | Bangalaltica Bechyné, 1960 (Fig. 17) |
| – | Antennomere 3 very much shorter than antennomere 4 (Figs 155, 224) | 39 |
| 39 | Antennomere 1 as long as antennomeres 2–4 together (Fig. 155). Antennae in male with antennomeres 2–3 very strongly reduced and 6–7 distinctly enlarged (Fig. 155). Pronotum distinctly more transverse (WP/LP > 1.65) (Fig. 29) | Chaillucola Bechyné, 1968 (Fig. 29) |
| – | Antennomere 1 much shorter than antennomeres 2–4 together (Fig. 224). Antennae similar in both sexes. Pronotum distinctly less transverse (WP/LP ≤ 1.65) | Nzerekorena Bechyné, 1955 (Fig. 74) |
| 40 | First metatarsomere distinctly compressed laterally (Figs 169, 272) | 41 |
| – | First metatarsomere not compressed laterally | 42 |
| 41 | Antennomeres 4–11 subglobose; antennomere 3 about twice as long as antennomere 4 (Fig. 271). Hind femora without processes or projections | Upembaltica Bechyné, 1960 (Fig. 106) |
| – | Antennomeres 4–11 not subglobose; antennomere 3 as long as antennomere 4 (Fig. 167). Hind femora of male with a distinct subtriangular, dentiform process, situated medially on ventral side (Fig. 168) | Dimonikaea Bechyné, 1968 (Fig. 35) |
| 42 | Antennomere 4 about as long as antennomere 3, but considerably shorter than antennomere 5; antennomere 5 about as long as antennomeres 2–4 together (Fig. 203). Female unknown | Kanonga Bechyné, 1960 (Fig. 56) |
| – | Antennomere 4 longer than antennomere 3 or as long as antennomere 5 | 43 |
| 43 | Antennomeres 8–10 very small, subglobose, each distinctly shorter than antennomeres 4–7 (Fig. 211) | Malvernia Jacoby, 1899 (Fig. 65) |
| – | Antennomeres differently shaped. In Gabonia, males often with antennae with distinctly modified segments, but never as in Malvernia | 44 |
| 44 | Metasternum shorter than length of mid-coxal cavity (Fig. 175). Legs very elongate, particularly hind femora and tibiae (Fig. 38). Antennae normally longer than body, particularly in male (Fig. 38). Elytra comparatively short (LE/LP ≤ 2.60); humeral calli absent (Fig. 38) . Wings vestigial | Drakensbergianella Biondi & D’Alessandro, 2003 (Fig. 38) |
| – | Metasternum at least 1.5x longer than length of mid-coxal cavity (Fig. 186). Legs not elongate (Figs 45, 63). Antennae very rarely longer than body (Figs 45, 63). Elytra comparatively elongate [LE/LP > 2.60 (normally > 3.00)]; humeral calli present. Wings well developed | Luperomorpha Weise, 1887 (Fig. 63) |
| [Gabonia Jacoby, 1893 (see Notes on page 48) (Fig. 45)] |
http://species-id.net/wiki/Abrarius
Figs 2, 110–112, 278Abrarius: Abrarius cribrosus Fairmaire, 1902: 261 (Madagascar: Plateau de l’Ankara), designation by monotypy; Entymosina: Entymosina parvula Weise, 1910b: 439 (Madagascar: Nossibé), by present designation.
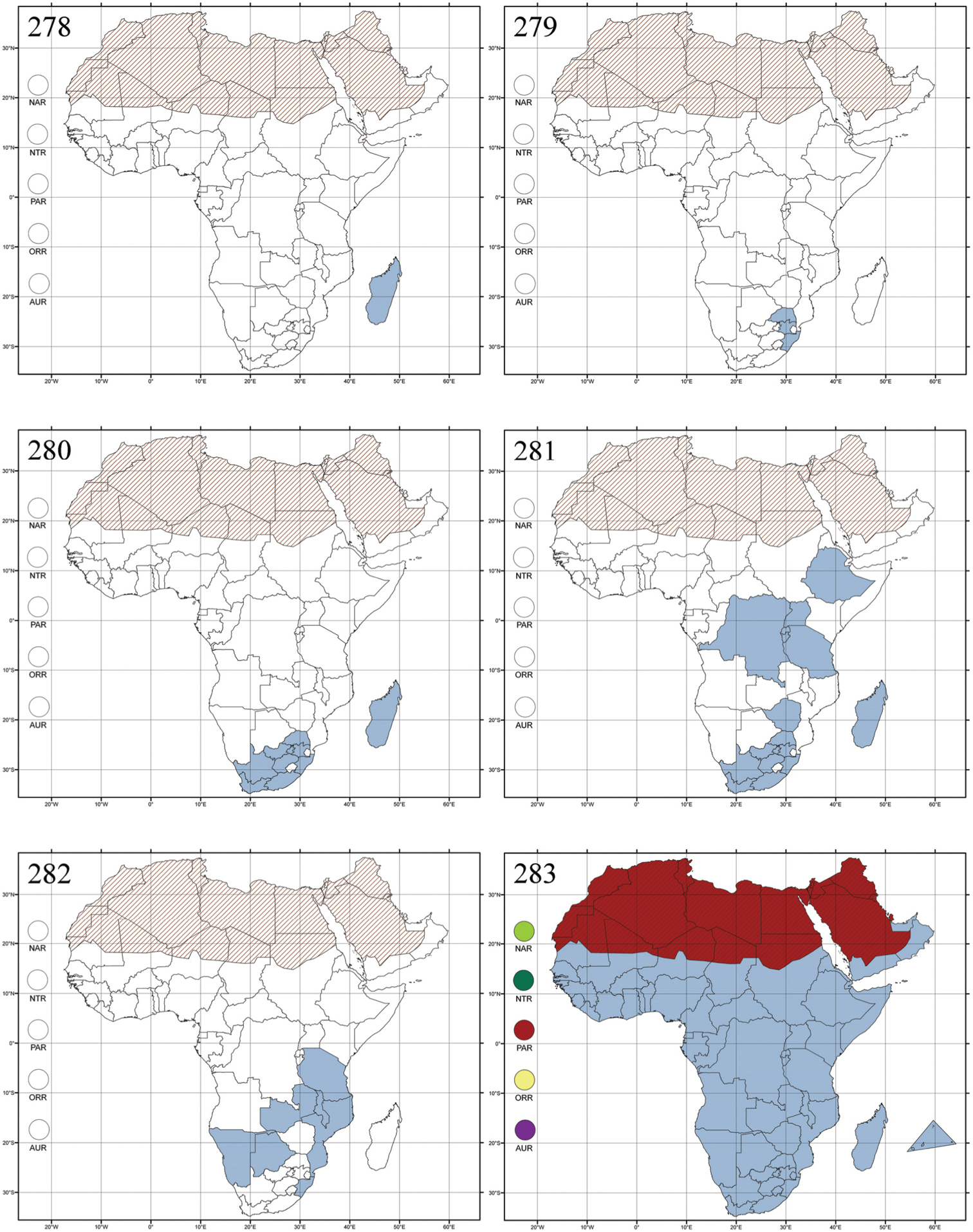
Madagascar (Fig. 278).
No information.
Endemic to Madagascar and comprises about ten known species. The Neotropical genus Gioia Bechyné (1955d: 77) is very similar to Abrarius, and may well be a synonym.
Afroaltica subaptera Biondi & D’Alessandro, 2007: 100 (Republic of South Africa, KwaZulu-Natal, Karkloof area), by original designation.
Republic of South Africa (KwaZulu-Natal, Limpopo, and Mpumalanga) (Fig. 279).
Afroaltica subaptera was collected in an open field on Poaceae (also known as Gramineae) (
Two species have been described.
[Afroalytus Scherer, 1961]
= Manobia Jacoby, 1885
Crepidodera carinipennis Jacoby, 1903a: 12 (KwaZulu-Natal, Malvern), by original designation.
Madagascar (!) [Ambalamanankana (NHMB); Perinet (NHMB); Andohahele (BAQ)] and the Republic of South Africa (Fig. 280).
No information.
Three species are known. Crepidodera betiokyensis Bechyné (1954a: 46) from Madagascar, erroneously attributed to this genus by
Crepidodera laeviuscula Csiki in
Burundi, Democratic Republic of the Congo, Ethiopia, Madagascar, Republic of South Africa, Rwanda, Tanzania, Uganda, and Zimbabwe (Fig. 281).
Some species of this genus have been collected from plants in the family Apiaceae in South Africa (personal data).
About twenty described species. Crepidodera betiokyensis Bechyné (1954a: 46) from Madagascar, erroneously attributed to Afrocrepis Bechyné by
[Allomorpha Jacoby, 1892b]
= Hespera Weise, 1889
Alocypha litura Weise, 1911: 171 (East Africa: Lindi) [(= Aphthona bimaculata Jacoby, 1903a: 11) (KwaZulu-Natal)], designation by monotypy.
Botswana, Malawi, Mozambique, Namibia, Republic of South Africa (KwaZulu-Natal), Tanzania and Zambia (Fig. 282).
Alocypha bimaculata is a harmful pest of Sesame (Sesamum indicum L.) (Pedaliaceae) crops, particularly in Tanzania (
Only one species is known.
http://species-id.net/wiki/Altica
Figs 7, 121, 283Altica: Chrysomela oleracea Linnaeus, 1758: 372 (Europe), by subsequent designation by
All zoogeographical regions (Fig. 283).
Polyphagous. This genus has been found associated with herbaceous plants, shrubs and trees belonging to several plant families (cf.
About fifty species are known from Madagascar and Sub-Saharan Africa.
http://species-id.net/wiki/Amphimela
Figs 8, 9, 122, 284Amphimela: Amphimela mouhoti Chapuis, 1875: 36 (Indonesia), designation by monotypy; Cercyonia: Cercyonia variabilis Weise, 1901: 303 (Tanzania: Bagamojo, Kunow), designation by monotypy; Diboloides: Diboloides bicolor Jacoby, 1897: 553 (Mashonaland), designation by monotypy; Dibolosoma: Dibolosoma quadripustulatum Jacoby, 1897: 560 (Madagascar: Diego-Suarez), designation by monotypy; Halticella: Halticella flavopustulata Jacoby, 1899b: 357 (Natal, Frere), designation by monotypy; Halticova: Halticova rufoguttata Fairmaire, 1898: 428 (Madagascar), designation by monotypy; Sphaerophysa: Sphaerophysa clavicornis Baly, 1876b: 582 (Madagascar), designation by monotypy.
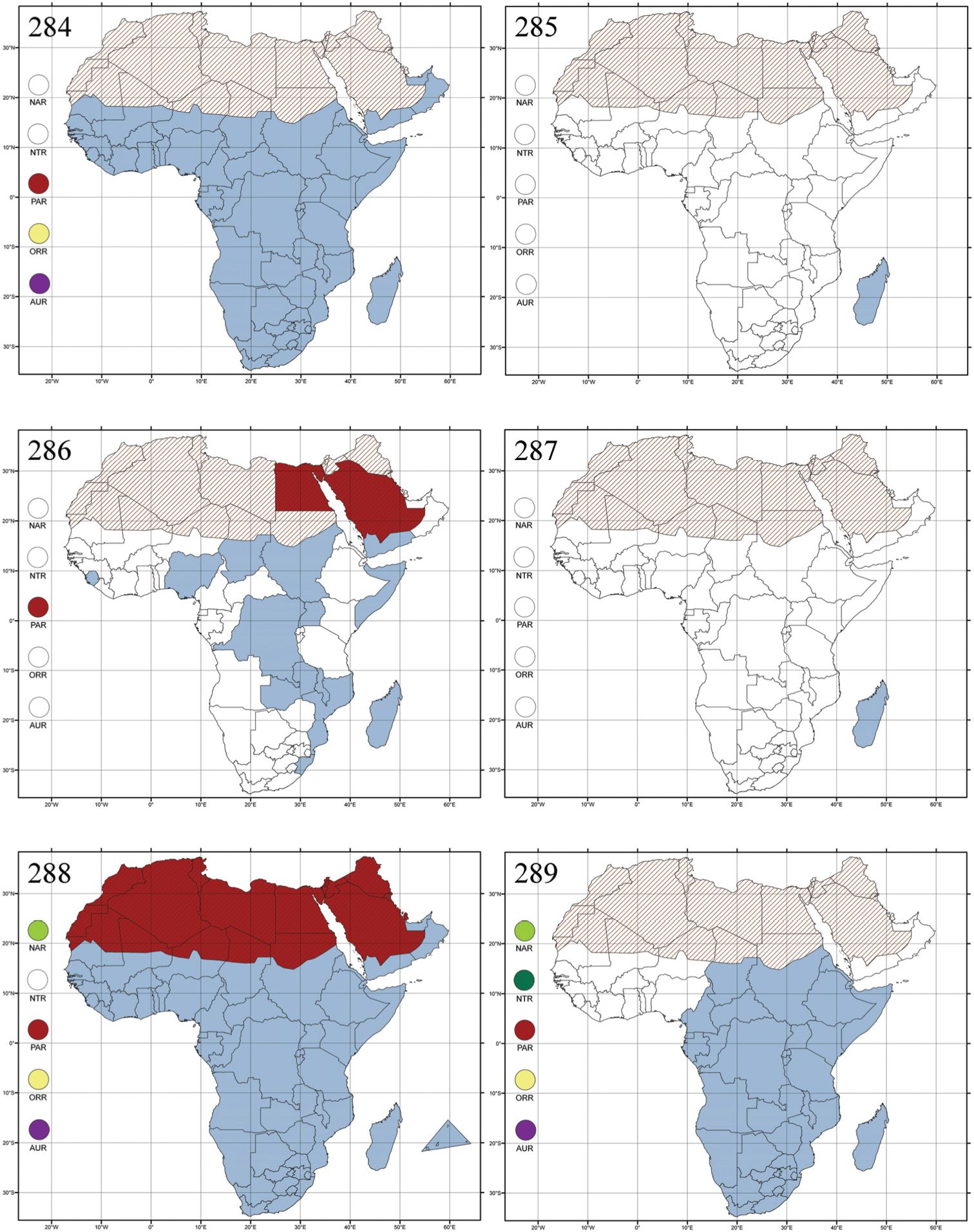
Afrotropical (including Madagascar), Australian, Eastern Palaearctic and Oriental regions (Fig. 284).
Amphimela bryanti (Csiki in
About thirty-five species have been described in the Afrotropical region.
Anaxerta castanea Fairmaire, 1902: 268 (Madagascar: Ankarahitra), designation by monotypy.
Madagascar (Fig. 285).
No information.
A single species has been described.
Aphthona heteromorpha Bechyné, 1955c: 62 (Madagascar: Bas Mangoky), by original designation.
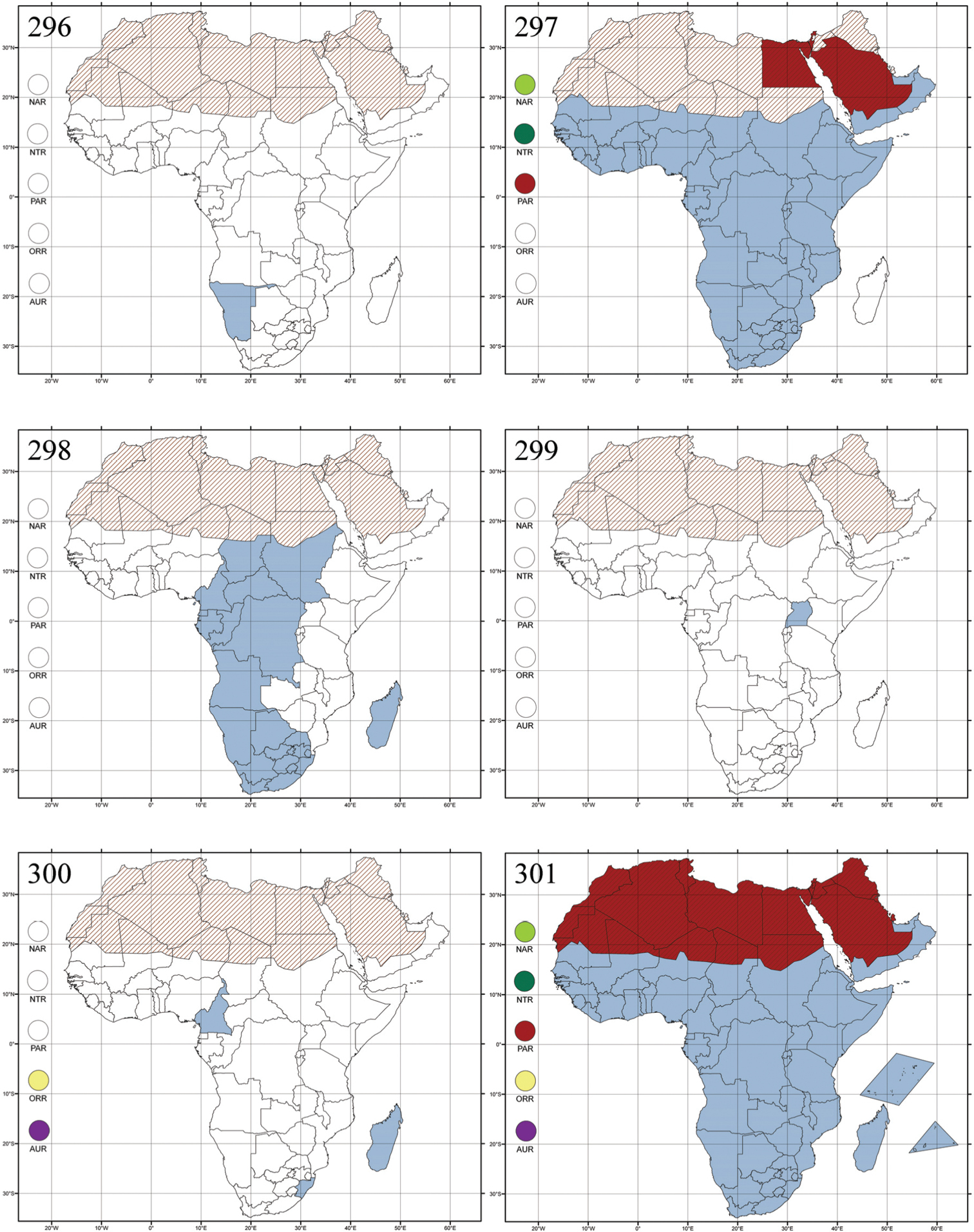
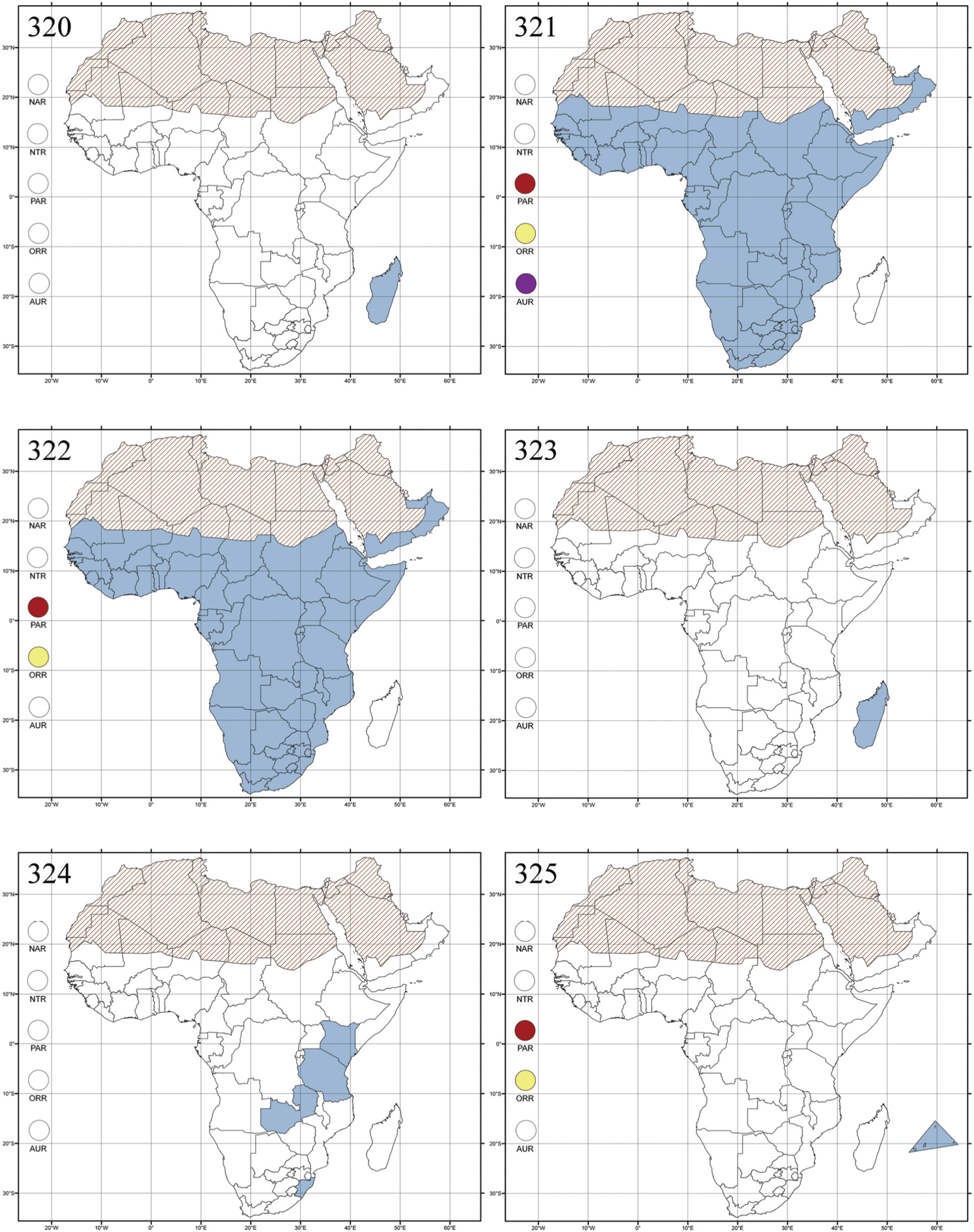
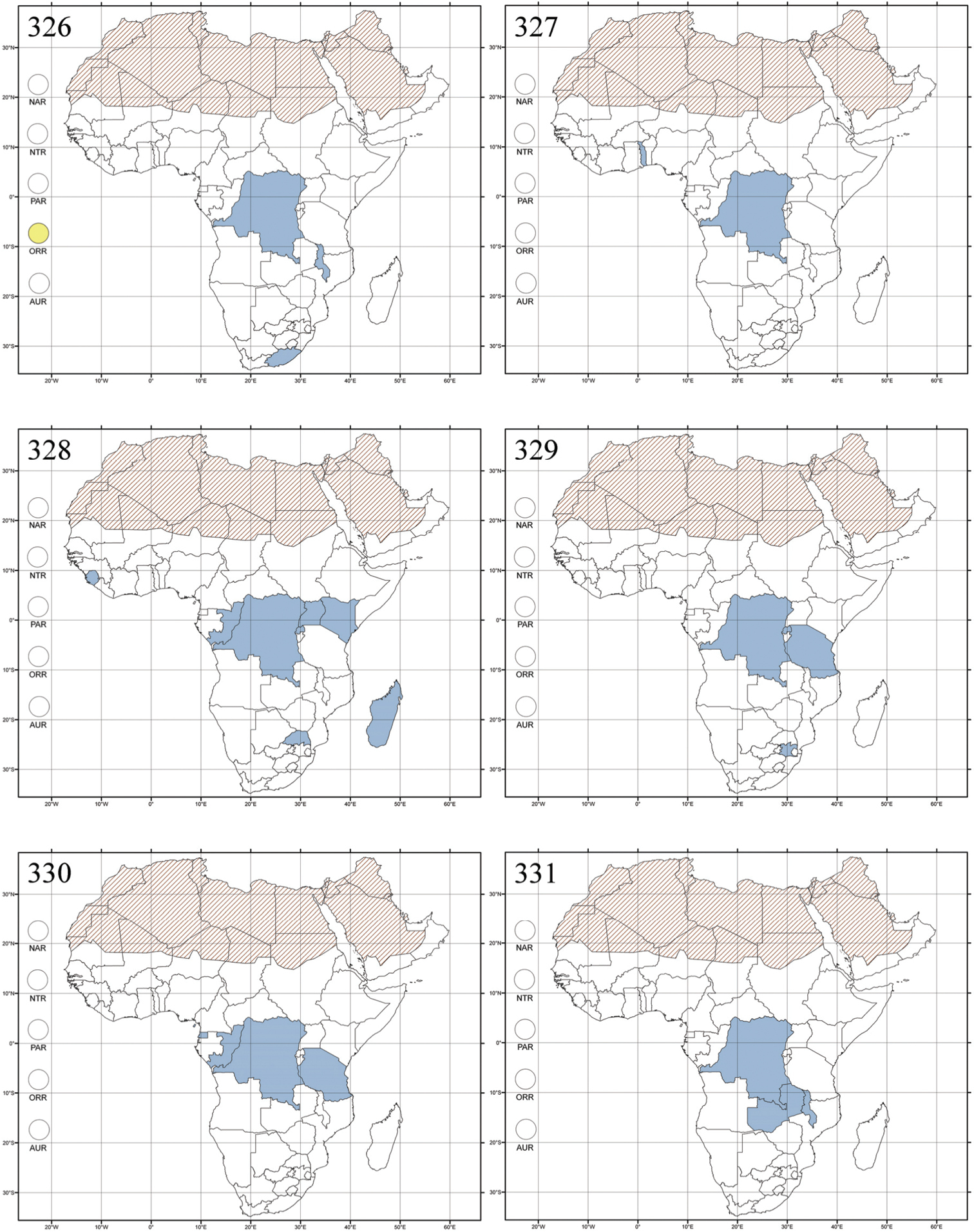
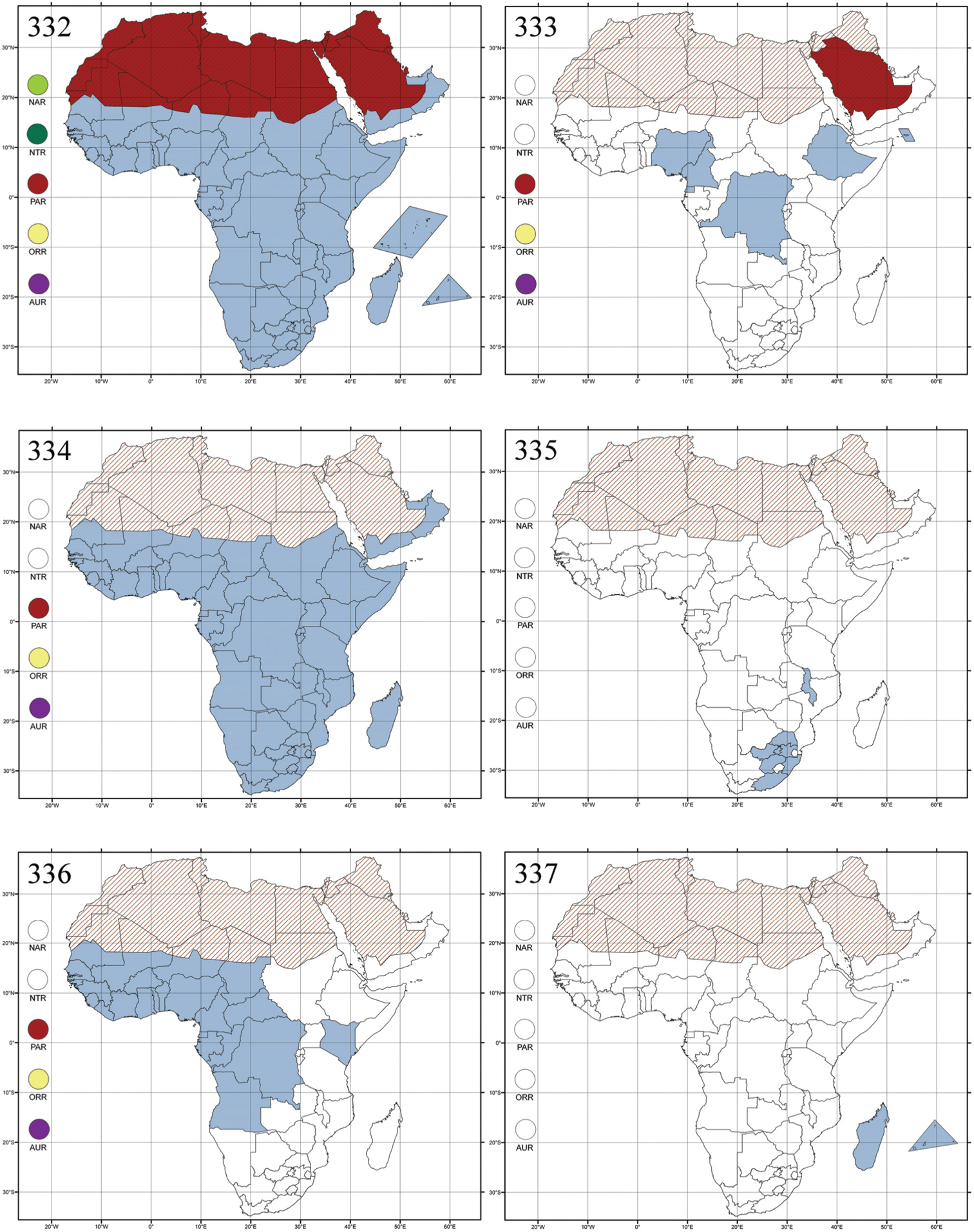
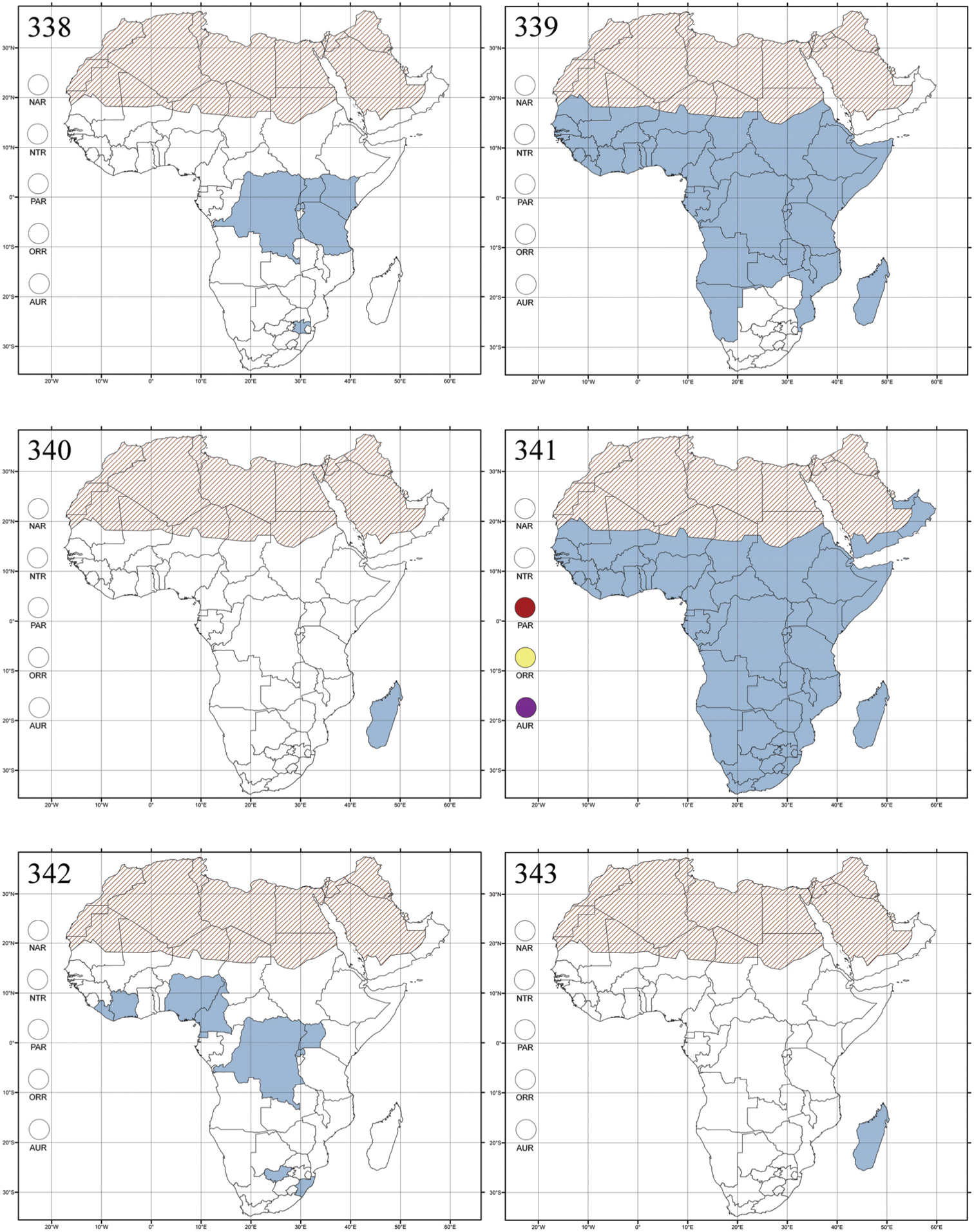
Egypt, Tchad, Sudan, Somaliland, Sierra Leone, Nigeria, Democratic Republic of Congo, Uganda, Zambia (!) [50 km W Kasama (BAQ)], Malawi (!) [Dedza (BAQ)], Mozambique, Republic of South Africa (!) [KwaZulu-Natal: Durban (SANC)], Madagascar, and Arabian Peninsula (Saudi Arabia and North Yemen) (Fig. 286).
Angulaphthona heteromorpha collected on cotton plants, Gossypium sp. (Malvaceae) (
Five species are known from the Afrotropical region.
http://species-id.net/wiki/Antanemora
Figs 12, 126–127, 287.Lactica carbonaria Bechyné, 1948a: 7 (Madagascar: Environs de Rogez; Ankazoabo), by original designation.
Madagascar (Fig. 287).
No information.
There are about twenty known species (personal data).
http://species-id.net/wiki/Aphthona
Figs 13, 14, 128–129, 288Aphthona: Altica cyparissiae Koch, 1803: 80 (Europe), by subsequent designation by
Afrotropical (including Madagascar), Australian, Nearctic, Oriental and Palaearctic regions (Fig. 288). All the species from the Neotropical region described as Aphthona should be attributed to different genera (cf.
Genus mainly associated with Euphorbiaceae, but also with Geraniaceae, Cistaceae, Rosaceae, Linaceae, Iridaceae, Malvaceae and Lythraceae (cf.
About one hundred species are described from Madagascar and Sub-Saharan Africa. There are no important diagnostic characters distinguishing Ethiopia Scherer from Aphthona. Therefore, the following new synonymy is proposed: Aphthona Chevrolat, 1836 = Ethiopia Scherer, 1972 syn. n. Type material examined: Ethiopia tricolor Scherer, holotype ♂ and paratype ♀, “Ethiopia, Agheresalam, 7.6.1963, Linnavuori” (MZHF).
Argopistes biplagiata Motschulsky, 1860: 236 (Siberia), designation by monotypy.
Central, Eastern and Southern Africa, and Madagascar; Australian, Eastern Palaearctic, Nearctic, Northern Neotropical and Oriental regions (Fig. 289).
Many species of this genus are associated with Oleaceae in Sub-Saharan Africa, especially with Olive trees [Olea europaea var. africana (Mill.)], on which the larvae are leaf miners and adults defoliators (cf.
About fifteen species recorded from Madagascar and Sub-Saharan Africa (personal data).
http://species-id.net/wiki/Argopistoides
Figs 16, 133, 290Argopistoides septempunctata Jacoby, 1892b: 932 [Burma (=Myanmar): Carin Chebà], designation by monotypy; Torodera: Torodera octomaculata Weise, 1902a: 164 (Kwai), by subsequent designation by Scherer (1987:67).
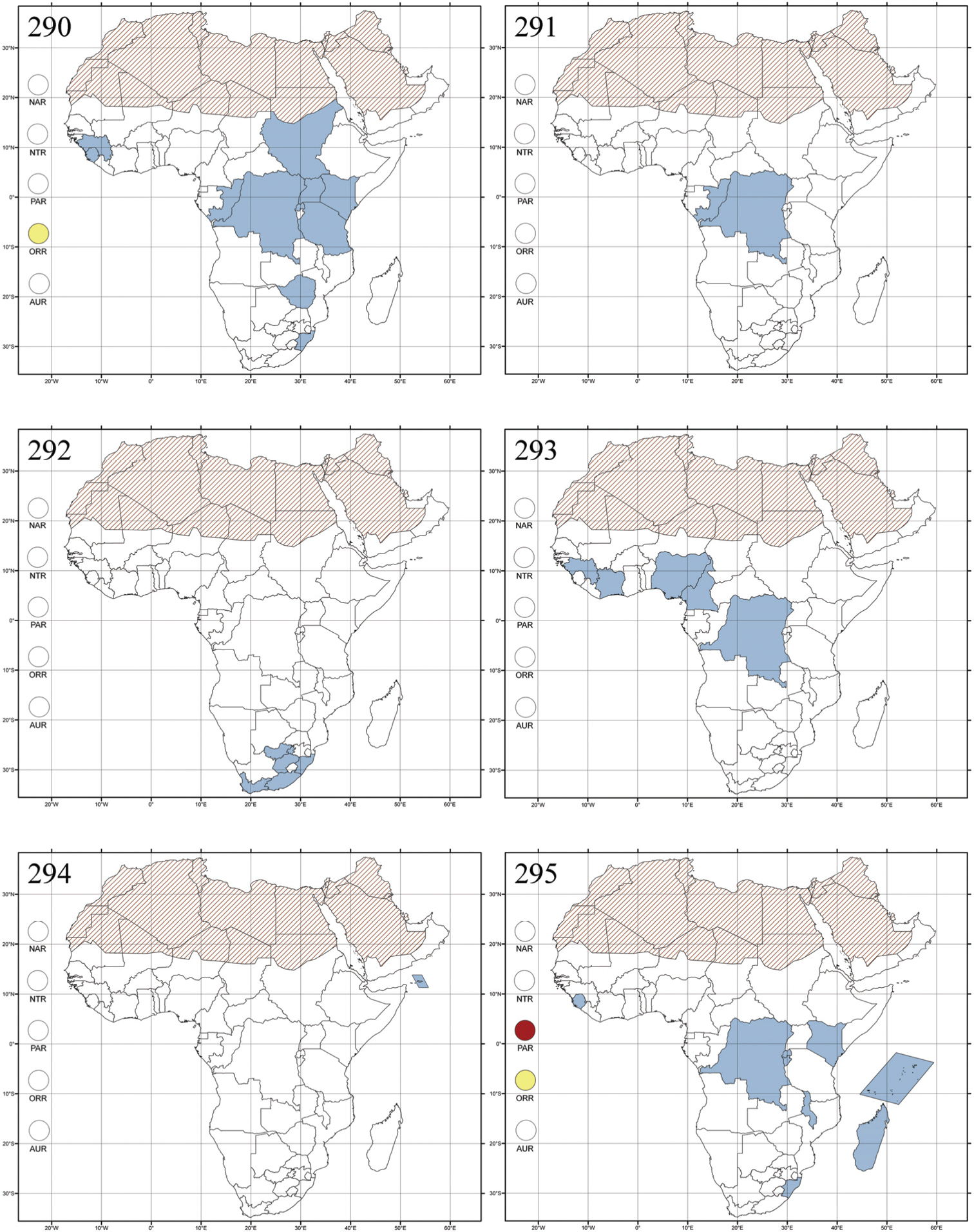
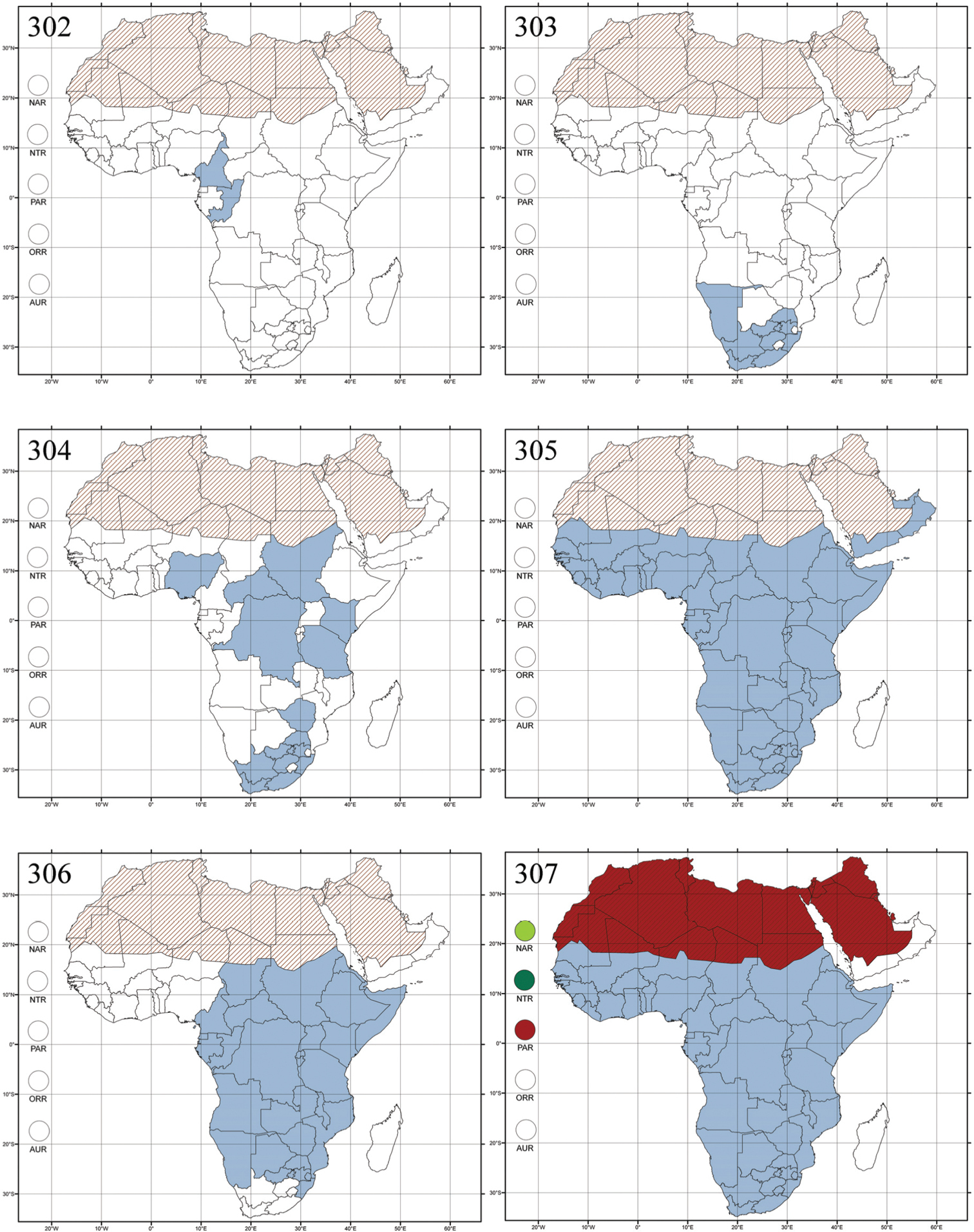
Republic of the Congo, Democratic Republic of the Congo, Guinea, Kenya, Rwanda (!) [Nyungwe National Park, Kamiranzovu (BAQ)], Republic of South Africa (KwaZulu-Natal), Sierra Leone, Sudan, Tanzania, Uganda, Zimbabwe, and Oriental region (Fig. 290).
Genus reported from Poaceae (also known as Gramineae) (Oryza) in Kenya [cf.
The Afrotropical region has four described species.
[Argopus Fischer, 1824]
Not present in the Afrotropical region.
References.
Notes. Argopus maculiceps Boheman (1859: 200) was transferred to the genus Toxaria Weise by
[Argosomus Wollaston, 1867]
= Sphaeroderma Stephens, 1831
[Aridohespera Selman, 1963]
= Eriotica Harold, 1877a
[Asiorestia Jacobson, 1925]
Not present in the Afrotropical region.
References.
Notes.
[Asphaera Chevrolat, 1843]
Not present in the Afrotropical region.
References.
Notes. Three species wrongly attributed to this Neotropical genus were transferred to the genera Physomandroya Bechyné and Hemipyxis Chevrolat by
Bangalaltica antennalis Bechyné, 1960a: 9 (Democratic Republic of the Congo: Bangala; Lubutu-Kituri), designation by monotypy.
Democratic Republic of the Congo and Republic of the Congo (Fig. 291).
No information.
One species is known.
[Balanomorpha Chevrolat, 1836]
= Mantura Stephens, 1831
Bechuana nigripes Scherer, 1970: 302 (Free State: Boshof; North-West Province: Vryburg), by original designation.
Republic of South Africa (Eastern Cape Province, Free State, Gauteng, KwaZulu-Natal, North-West Province and Western Cape Provinces) (Fig. 292).
No information.
There are two known species. Ochrosis natalensis Jacoby (1906: 17) was attributed to this genus by
http://species-id.net/wiki/Bechynella
Figs 19, 136–137, 293Serraphula bohumilae Bechyné, 1955b: 517 (French Guinea: Dalaba), by original designation.
Cameroon, Democratic Republic of the Congo, Guinea, Ivory Coast and Nigeria (Fig. 293).
No information.
The three species attributed to this genus were described by
Bezdekaltica socotranaDöberl, 2012: 435 (Socotra Island), by original designation.
Yemen (Socotra Island) (Fig. 294).
Collected in November in Dracaena (Dracenaceae) Forest.
Only one species has been described.
Bikasha tenuipunctata Maulik, 1931: 257 (Seychelles), by original designation.
Burundi (!) [Kibira National Park (BAQ)], Democratic Republic of the Congo (!) [Kivu, Mbingi (BAQ)], Kenya (!) [Mt. Kenya (BAQ); Kikuyu (BAQ)], Madagascar (!) [NW of Ranomafana (ZMHB); Andasibe (BAQ)], Malawi (!) [Dedza (BAQ)], Republic of South Africa (!) (KwaZulu-Natal, 17 km NE of Empangeni (MZLU)], Rwanda (!) [Nyungwe National Park, Pindura (BAQ)]; Seychelles, Sierra Leone (!) [Bumbuna (BAQ); S of Freetown (MZLU)], Eastern Palaearctic and Oriental regions (Fig. 295).
In Seychelles, Bikasha tenuipunctata Maulik and Bikasha fortipunctata Maulik (1931: 258) were collected in forest, and Bikasha minor Maulik (1931: 259) in wet coastal meadows.
About ten species are known from the Afrotropical region (personal data).
http://species-id.net/wiki/Biodontocnema
Figs 22, 141–145, 296Biodontocnema brunnea Biondi, 2000: 348 (Namibia, Kaross), designation by monotypy.
Namibia (Fig. 296).
Biodontocnema brunnea is the only species in this genus, and it is associated with moist habitats (
One species has been described. This genus was erroneously synonymized with Chaetocnema Stephens by
http://species-id.net/wiki/Blepharida
Figs 23, 146–149, 297Blepharida: Chrysomela rhois Forster, 1771: 21 (North America), by subsequent designation by
Afrotropical (excluding Madagascar), Nearctic, Neotropical and Southern Palaearctic (Egypt, Israel, and Saudi Arabia) regions (Fig. 297).
The Afrotropical species of Blepharida are generally associated with shrubs of Rhus (Anacardiaceae) (
Sub-Saharan Africa has about thirty-five described species. Two species from Madagascar previously attributed to this genus, Blepharida multiguttata Duvivier (1891: 242) and Blepharida insignis Brancsik (1897: 130), are transferred to Xanthophysca Fairmaire. The nominotypical subgenus of Blepharida is mainly widespread in Nearctic and Neotropical regions, whereas the subgenus Blepharidina Bechyné is restricted to the Afrotropical region (
[Blepharidina Bechyné, 1968]
subgenus of Blepharida Chevrolat, 1836
[Blepharidula Weise, 1916]
= Blepharida Chevrolat, 1836
References.
Notes. An unnecessary new name proposed by
[Brinckaltica Bechyné, 1959b]
= Chaetocnema Stephens, 1831
[Buphonella Jacoby, 1903a]
Fig. 24
The genus Buphonella(Fig. 24) is here transferred to the tribe Galerucini.
References.
Type species.Apophylia murina Gerstaecker, 1871: 83 (Zanzibar), by subsequent designation by
Distribution. Central, Eastern andSouthernAfrica.
Ecology. Associated with Poaceae (also known as Gramineae). Buphonella murina (Gerstaecker) and Buphonella nigroviolacea (Allard, 1889b: LXXI, LXXIV) can damage maize (cf.
Notes. Four species have been described.
[Calotheca Heyden, 1887]
= Blepharida Chevrolat, 1836
Chaetocnema rugiceps Baly, 1877: 308 (Madagascar), by subsequent designation by
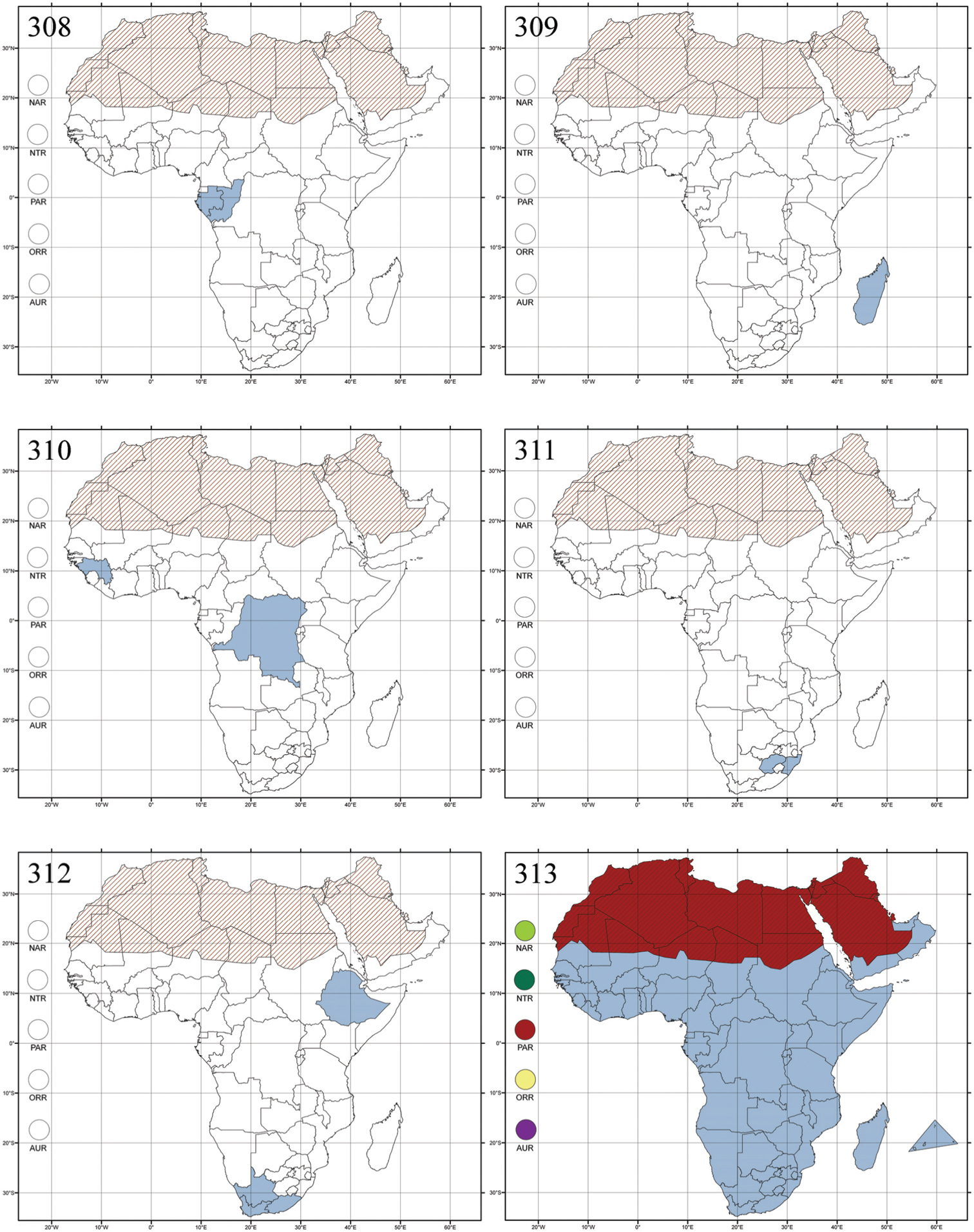
Central and Southern Africa, and Madagascar (Fig. 298).
Species of this genus live in moist habitats and are probably associated with plants of the family Cyperaceae (personal data).
There are seven known species.
Celisaltica ruwenzorica Biondi, 2001a: 644 (Ruwenzori), by original designation.
Uganda (Fig. 299).
This is the only species in this genus. It lives at high altitudes (3200–4000 m) on the Ruwenzori Massif, and is associated with the Ericetum (Ericaceae) plant community (
One species.
[Cercyonia Weise, 1901]
= Amphimela Chapuis, 1875
Chabria nigroplagiata Jacoby, 1887: 92 (Sri Lanka: Bogawantalawa), by subsequent designation by Maulik (1926: 312).
Cameroon (probably introduced); Madagascar, and Republic of South Africa (KwaZulu-Natal) (probably introduced), Australian and Oriental regions; (Fig. 300).
There is no information for the Afrotropical region. Chabria species were collected on Melastoma (Melastomataceae) in Malaysia (
Four species are known from Afrotropical region, one from Sub-Saharan Africa, probably introduced, and three from Madagascar.
http://species-id.net/wiki/Chaetocnema
Figs 28, 154, 301Chaetocnema: Chrysomela concinna Marsham, 1802 (Europe), by subsequent designation by
All zoogeographical regions (Fig. 301).
This genus is mainly associated with plants in the families Chenopodiaceae, Cyperaceae, Juncaceae, Poaceae (also known as Gramineae), and Polygonaceae (cf.
Over one hundred species are known from Madagascar and Sub-Saharan Africa.
Chaillucola formicicornis
Cameroon (!) [N’Kongsamba (MCSN)] and Republic of Congo (Fig. 302).
No information.
A single species has been described.
[Chaloenus Westwood, 1862]
Not present in the Afrotropical region.
References.
Notes. Terpnochlorus perrieri Fairmaire, 1904 (= Chaloenus viridis Bryant, 1927); this species was incorrectly attributed to the Oriental genus Chaloenus(Bechyné, 1955b).
Chirodica chalcoptera Germar, 1834: 16 (Cape of Good Hope), designation by monotypy.
Namibia and the Republic of South Africa (Fig. 303).
This genus is strictly associated with the plant family Proteaceae (mainly Protea spp. and Leucadendron spp.) (
Eight species have been described.
[Cladocera Hope, 1840]
= Polyclada Chevrolat, 1836
[Cladotelia Kolbe, 1894]
= Polyclada Chevrolat, 1836
Collartaltica cryptostoma Bechyné, 1959a: 27 (Democratic Republic of the Congo: Faradje, Tomati), designation by monotypy.
Central African Republic, Democratic Republic of the Congo, Kenya, Nigeria, Republic of South Africa, Sudan, Tanzania and Zimbabwe (Fig. 304).
This genus is associated with Poaceae (also known as Gramineae) in moist meadows and forest clearings (
There are six known species.
[Crepidodera Chevrolat, 1836]
Not present in the Afrotropical region.
References.
Notes. Afrotropical species initially attributed to this genus (cf.
[Cyrtoma Clark, 1863]
Nomen nudum.
Notes.
Decaria: Decaria tricolor Weise, 1895: 344 (Sierra Leone, Bang-Haas), designation by monotypy; Embolimus: Embolimus pauli Weise, 1902b: 304 (Kwai), designation by monotypy.
Afrotropical region (excluding Madagascar) (Fig. 305).
Decaria is associated mainly with plans from the genera Heliotropium (Boraginaceae), Cola (Sterculiaceae) and Ocimum (Lamiaceae) (cf.
About twenty species have been described.
[Decarthrocera Laboissière, 1937]
References.
This genus was transferred to the subfamily Galerucinae (currently tribe Galerucini) by
[Derocrepis Weise, 1886]
Not present in the Afrotropical region.
References.
Notes. Species initially attributed to this Palaearctic genus were transferred to the genera Afrocrepis Bechyné and Afrorestia Bechyné.
Diamphidia femoralis Gerstaecker, 1855: 638 (Sena and Port Natal), designation by monotypy.
Central, Eastern, and Southern-Eastern Africa (Fig. 306).
A genus associated with shrubs and trees of Commiphora (Burseraceae) (cf.
Seventeen species are known.
http://species-id.net/wiki/Dibolia
Figs 34, 164–166, 307Dibolia: Haltica occultans Koch, 1803: 22 (Europe), by subsequent designation by
Sub-Saharan Africa (absent in Madagascar); Nearctic, Neotropical and Palaearctic regions (Fig. 307).
A genus associated mainly with plants in the family Lamiaceae, but also with plants in the Scrophulariceae, Asteraceae and Apicaeae (cf.
About twenty species in Sub-Saharan Africa.
[Diboloides Jacoby, 1897]
= Amphimela Chapuis, 1875
[Dibolosoma Jacoby, 1897]
= Amphimela Chapuis, 1875
Dimonikaea descarpentriesi Bechyné, 1968: 1712 (Republic of Congo: Dimonika), by original designation.
Republic of Congo and Gabon (!) [Makokou (NMHN)] (Fig. 308).
No information.
A single species is known.
http://species-id.net/wiki/Diphaulacosoma
Figs 36, 170–171, 309Diphaulacosoma: Diphaulacosoma laevipenne Jacoby, 1892a: 574−575 (Madagascar), designation by monotypy; Neoderina: Neodera (Neoderina) crassicornis Bechyné, 1952: 251 (Madagascar, Ambohitsitondrona), designation by monotypy.
Madagascar (Fig. 309).
No information.
Four species (personal data). No diagnostic characters distinguish Neoderina Bechyné from Diphaulacosoma. Therefore, the following new synonymy is proposed: Diphaulacosoma Jacoby, 1892a = Neoderina Bechyné, 1952 syn. n. Material examined: Neodera (Neoderina) crassicornis Bechyné, “Madagascar, Ambohitsitondrona, x-xii.47, Michel leg.”, “typus” ♂ and “cotypus” ♀ (MNHN).
Djallonia maindra Bechyné, 1955b: 534 (French Guinea: Dalaba), designation by monotypy.
Democratic Republic of the Congo and Guinea (Fig. 310).
No information.
Only one species is known.
http://species-id.net/wiki/Drakensbergianella
Figs 38, 173–175, 311Drakensbergianella rudebecki Biondi and D’Alessandro, 2003: 100 (Republic of South Africa, Drakensberg), designation by monotypy.
Distribution. Republic of South Africa (Free State and KwaZulu-Natal) (Fig. 311).
Drakensbergianella rudebecki is the only species in this genus. It lives in alpine meadows (> 2, 000 m) on the Drakensberg and was collected on the inflorescences of Senecio and Helichrysum (Asteraceae) (
A single species has been described.
Dunbrodya nitida Jacoby, 1906: 20 (Cape Colony), designation by monotypy.
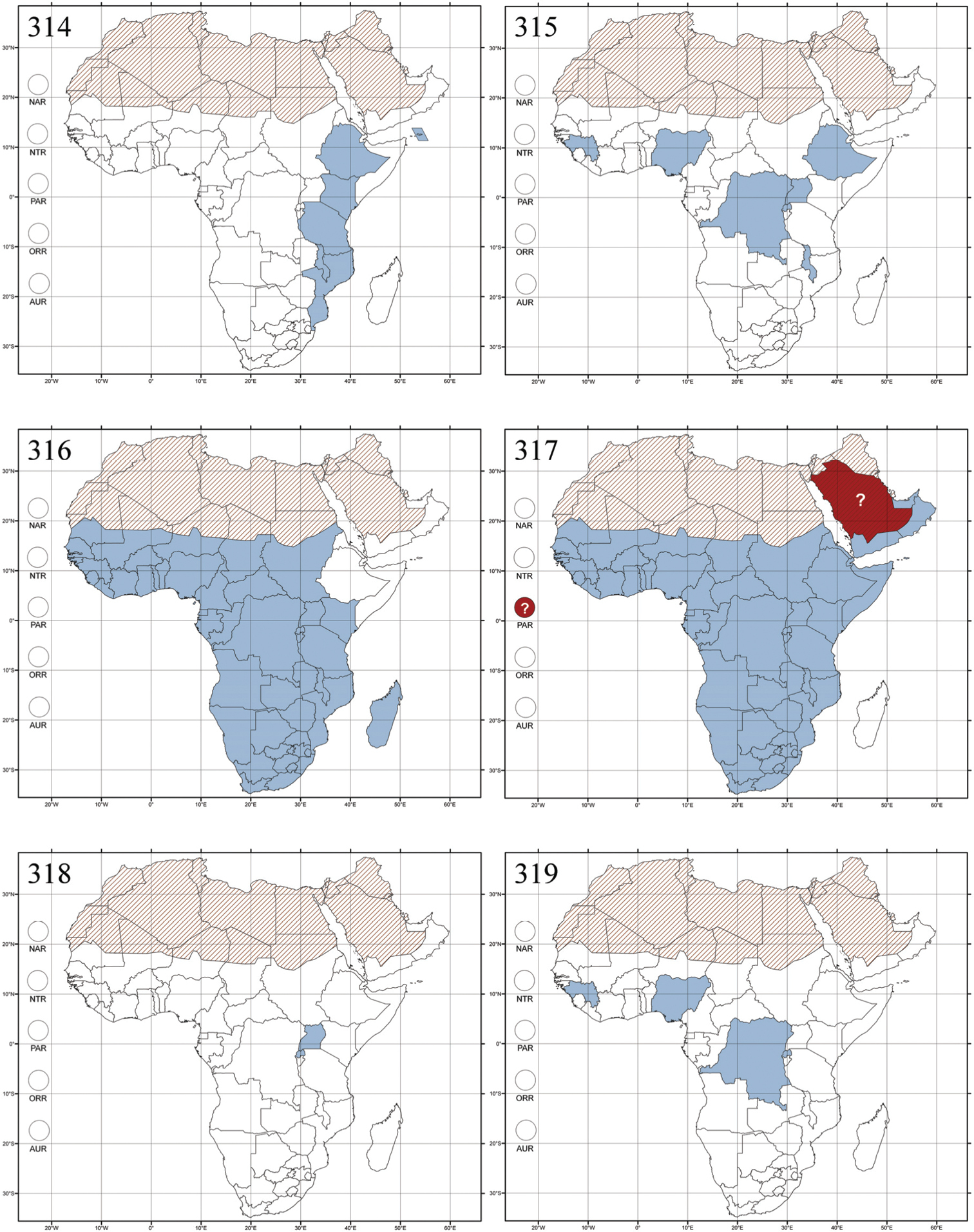
Ethiopia (!) [Sidamo (BAQ)] and the Republic of South Africa [Eastern (!): Kirkwood (SANC), Grahamstown (SANC), Uitenhage (BAQ); Northern (!): Port Nolloth (SANC); and Western Cape Provinces] (Fig. 312).
Dunbrodya nitida was collected on an Asparagus sp. (Asparagaceae) (
Two species are known (personal data).
[Embolimus Weise, 1902b]
= Decaria Weise, 1895
[Entymosina Weise, 1910b]
= Abrarius Fairmaire, 1902
http://species-id.net/wiki/Epitrix
Figs 40, 180, 313Epitrix: Epitrix atropae Foudras, 1860: 55 (Europe), by subsequent designation by Maulik (1926: 130, 133); Euplecnema: Euplecnema nigrita Jacoby, 1906: 22 (Dunbrody, Cape Colony), designation by monotypy.
All zoogeographical regions (Fig. 313).
The genus Epitrix is mainly associated with plants in the family Solanaceae. Some species can be harmful to plants of economic importance (cf.
About a dozen species are known from Madagascar and Sub-Saharan Africa.
[Eremiella Weise, 1910a]
= Eurylegna Weise, 1910a
http://species-id.net/wiki/Eriotica
Figs 41, 181, 314Eriotica: Eriotica fuscipennis Harold, 1877a: 107 (Nyassa), designation by monotypy; Aridohespera: Aridohespera mateui Selman, 1963: 1157 (Terr. N. Tchad, Ouedi Saala, Mortcha), by original designation. Niphraea: Niphraea hirtipennis Baly, 1878a (Lake Nyassa), designation by monotypy.
Ethiopia, Kenya (!) [S of Garissa (BAQ); NW of Garsen (BAQ); Taita, Mwatate (BAQ)], Malawi, Mozambique, Tanzania and Socotra Island (Yemen) (Fig. 314).
No information.
Seven species are known.
[Escaleriella Weise, 1907a]
= Lypnea Baly, 1876a
[Ethiopia Scherer, 1972]
= Aphthona Chevrolat, 1836
[Eugonotes Jacoby, 1897]
= Sanckia Duvivier, 1891
[Euplecnema Jacoby, 1906]
= Epitrix Foudras, 1860
http://species-id.net/wiki/Eurylegna
Figs 42, 43, 182, 315Eurylegna: Eurylegna fulva Weise, 1910a: 228 (Kilimanjaro), designation by monotypy; Eremiella: Eremiella rubra Weise, 1910a: 229 (Kilimanjaro in Kiboscho, 3.000 m), designation by monotypy; Eurylegniella: Eurylegna guineensis Bechyné, 1955b: 528 (French Guinea: Mount Gangan; Dalaba; Nzérékoré; Mount Nimba), by original designation.
Nigeria, Guinea, Ethiopia, Democratic Republic of the Congo, Uganda (!) [Budongo Forest, Sonso (BAQ)], Rwanda, and Malawi (!) [Dedza (BAQ)] (Fig. 315).
No information.
Six species have been described. There are no important diagnostic characters distinguishing Eurylegniella Scherer from Eurylegna. The following synonymy is therefore proposed: Eurylegna Weise, 1910 = Eurylegniella Scherer, 1972 syn. n. Material examined: Eurylegniella guineensis (Bechyné) (det. G. Scherer), “Imperial College, Expdn. Ghana 1960, 24.8.60, Bobiri Forest, Kumasi, Ashanti”, 1 specimen (ZSM); “Congo Belge, P.N.G., Miss. H. De Saeger, Mt Embe, 20-iv-1952, H. De Saeger, 3347”, 1 specimen (MRAC).
[Eurylegniella Scherer, 1972]
= Eurylegna Weise, 1910a
[Eutheca Baly, 1878c]
= Blepharida Chevrolat, 1836
http://species-id.net/wiki/Eutornus
Figs 44, 183–184, 316Oedionychus (Eutornus) africanus Clark, 1860: 65, by original designation.
Madagascar and Sub-Saharan Africa (absent in the northern-eastern part of EAF) (Fig. 316).
No information.
About eight species are known, one of which is from Madagascar.
[Exorhina Weise, 1886]
= Chaetocnema Stephens, 1831
http://species-id.net/wiki/Gabonia
Figs 45, 185–186, 317Gabonia: Gabonia unicostata Jacoby, 1893: 101 (Gabon), designation by monotypy; Orneates: Orneates nigritus Jacoby, 1899b: 345 (Natal), designation by monotypy; Thrymnes: Thrymnes custos Weise, 1895: 339 (Ashante), by present designation.
Afrotropical region (excluding Madagascar) and Arabian Peninsula (?) (Fig. 317).
Polyphagous. This genus has been associated with several plant families (cf.
About one hundred and fifty species are known to occur in Sub-Saharan Africa. According to
[Gastrida Chapuis, 1879]
References. Chapuis, 1879: 20;
Notes. This genus was transferred to the subfamily Galerucinae (currently tribe Galerucini) by
[Graptodera Chevrolat, 1836]
= Altica Geoffroy, 1762
Guilielmia monticola Weise, 1924: 24 (Birunga, Mount Mukeno), designation by monotypy.
Uganda and Rwanda (Fig. 318).
The only species in this genus was collected at a high altitude (3100 m) (
A single species is known.
Guinerestia rubripes Scherer, 1959: 244 (Nigeria-Cameroon: Mamfe), by original designation.
Democratic Republic of the Congo, Guinea, Nigeria and Rwanda (Fig. 319).
No information.
Three species have been described.
[Haltica Illiger, 1801]
= Altica Geoffroy, 1762
[Halticella Jacoby, 1899b]
= Amphimela Chapuis, 1875
[Halticopsis Fairmaire, 1883a]
Fig. 48
The genus Halticopsis (Fig. 48) is here transferred to the tribe Galerucini.
References.
Type species.Halticopsis spissicornis Fairmaire, 1883b: 112 (Mountains of Abyssinia), designation by monotypy.
Distribution. Ethiopia.
Ecology. No information.
Notes. This Afrotropical genus, including a single species known from Ethiopia, is here transferred to the tribe Galerucini because of the absence of a metafemoral spring.
[Halticorthaea Csiki, 1940 in
= Amphimela Chapuis, 1875
Halticotropis multiplicata Fairmaire, 1886: 95 (Madagascar), designation by monotypy.
Madagascar (Fig. 320).
No information.
Two species have been described.
[Halticova Fairmaire, 1898]
= Amphimela Chapuis, 1875
[Haltitarsus Berthold, 1827]
= Dibolia Latreille, 1829
http://species-id.net/wiki/Hemipyxis
Figs 50, 192 –194, 321Hemipyxis: Haltica troglodytes Olivier, 1808: 700 (India), by subsequent designation by
Afrotropical (excluding Madagascar), Australian, Eastern Palaearctic, and Oriental regions (Fig. 321).
Polyphagous. This genus has been associated with herbaceous plants and shrubs belonging to many plant families (cf.
About thirty species are known from Sub-Saharan Africa. Six species of Hemipyxis, known from Madagascar, are here transferred to the genus Pseudadorium Fairmaire (see Notes in Pseudadorium).
[Hermaeophaga Foudras, 1859]
Not present in the Afrotropical region.
References. Foudras, 1859: 147.
Notes. Species originally described in this Palaearctic genus were subsequently transferred to Orthocrepis Weise by Scherer (1961: 267).
http://species-id.net/wiki/Hespera
Figs 51, 195, 322Hespera: Hespera sericea Weise, 1889: 639 (China), by original designation; Allomorpha: Allomorpha sericea Jacoby, 1892b: 934 [Burma (=Myanmar): Carin Chebà], designation by monotypy.
Afrotropical (excluding Madagascar), Eastern Palaeartic, and Oriental regions (Fig. 322).
The Afrotropical species of this genus are mainly associated with plants in the families Anacardiaceae and Ericaceae (cf.
About thirty species are known from Sub-Saharan Africa.
Hildebrandtina variegata Weise, 1910b: 465 (Madagascar), by original designation.
Madagascar (Fig. 323).
No information.
This genus, with about ten species known to occur in Madagascar, was transferred from the subfamily Galerucinae to the Alticinae (currently the tribe Alticini) by
http://species-id.net/wiki/Homichloda
Figs 53, 198, 324Homichloda: Homichloda pauli Weise, 1902a: 166 (Kwai), designation by monotypy; Weiseana: Weiseana barkeri Jacoby, 1903: 16 (Natal, Malvern), by original designation.
Kenya, Republic of South Africa (KwaZulu-Natal), Tanzania and Zambia (Fig. 324).
A genus that has been associated with a variety of Acacia species, trees in the family Fabaceae (
Three species have been described.
http://species-id.net/wiki/Hyphasis
Figs 54, 199 –200, 325. Hyphasis: Oedionychis magica Harold, 1877b: 434 (India), by original designation. Hyphasoma: Hyphasoma inconspicua Jacoby, 1903b: 111 (India), by subsequent designation of Maulik (1926: 156).
Mascarene Islands (probably introduced), Oriental region and South-Eastern part of the Palaearctic region (Fig. 325).
This genus is associated with plants in the families Verbenaceae and Lamiaceae (cf.
The Malagasy species initially attributed to this Oriental genus were previously transferred to Hyphasoma Jacoby (
Sphaerophysa piceicollis Jacoby, 1889c: 195 (Burma), by original designation.
Democratic Republic of the Congo, Malawi, Republic of South Africa (Eastern Cape Province), Zimbabwe (!) [Bulawayo, Shangani (BAQ)], and the Oriental region (Fig. 326).
No information for Afrotropical region.
Three species have been described.
[Jamesonia Jacoby, 1895]
= Gabonia Jacoby, 1893
Kanonga atra Bechyné, 1960b: 54 (Upemba National Park: Kanonga), by original designation.
Democratic Republic of the Congo and Togo (Fig. 327).
No information.
One species is known.
http://species-id.net/wiki/Kenialtica
Figs 57, 204, 328Kenialtica: Aphthona muhavura Bechyné, 1955a: 207 (Rwanda, East of Muhavura), by original designation; Mediafra: Aphthona muhavura Bechyné, 1955a: 207 (Rwanda, East of Muhavura), by original designation.
Democratic Republic of the Congo, Kenya, Madagascar, Republic of South Africa (Limpopo), Republic of the Congo, Rwanda, Sierra Leoneand Uganda (!) [Budongo Forest, Sonso (BAQ)] (Fig. 328).
No information.
Seven species have been described.
http://species-id.net/wiki/Kimongona
Figs 58, 59, 205, 329Kimongona: Kimongona callifera Bechyné, 1959a: 19 (Democratic Republic of the Congo: Mayumbe, Kimongo), by original designation. Mesocrepis: Mesocrepis lindemannae Scherer, 1963: 669 (Tanzania: Njombe), by original designation.
Democratic Republic of the Congo, Republic of South Africa (Mpumalanga), Rwanda, and Tanzania (Fig. 329).
No information.
Three species are known.There are no significant diagnostic characters distinguishing Mesocrepis Scherer from Kimongona. Therefore, the following new synonymy is proposed: Kimongona Bechyné, 1959 = Mesocrepis Scherer, 1963 syn. n.Type material examined: Mesocrepis lindemannae Scherer, “Tanganjika, Uwemba b. Njombe, 2000 m, 8–11.XI.1958, leg. C. Lindemann”, paratypes 1♂ and 1♀ (NHMB).
[Lactica Erichson, 1847]
Not present in the Afrotropical region.
References.
Notes. The Afrotropical species initially attributed to this Neotropical genus were transferred to Phygasia Chevrolat (
Lampedona tarsalis Weise, 1907a: 399 (Spanish Guinea), designation by monotypy.
Democratic Republic of the Congo, Equatorial Guinea, Republic of the Congo and Tanzania (Fig. 330).
No information.
There are three described species.
Lepialtica bicolor Scherer, 1962a: 31 (Garamba National Park), designation by monotypy.
Democratic Republic of the Congo, Malawi (!) [Mulanje Mts (BAQ); Kasungu(BAQ)] and Zambia (!) [35 km S of Kasama (BAQ)] (Fig. 331).
No information.
Four species are known (personal data).
[Livolia Jacoby, 1903]
References. Jacoby, 1903a: 16;
Notes. Genus transferred to the subfamily Galerucinae (currently tribe Galerucini) by
Chrysomela atricilla
All zoogeographical regions (Fig. 332).
This genus is polyphagous and has been associated with several plant families, particularly the Boraginaceae, Asteraceae, Lamiaceae and Scrophulariaceae (cf.
Over one hundred species known from Madagascar and Sub-Saharan Africa (personal data).
Luperomorpha trivialis Weise, 1887: 204 (Siberia: Raddefka; Chingan), by original designation.
Cameroon, Democratic Republic of the Congo, Equatorial Guinea, Ethiopia (!) [Oromia region (BAQ)], Nigeria, and Saudi Arabia, Socotra Island (Yemen) and the Australian, Eastern Palaearctic and Oriental regions (Fig. 333).
Polyphagous (cf.
Two species have been described for the Afrotropical region: Luperomorpha vittula (Weise, 1915) [described as Jamesonia Jacoby but then transferred to Luperomorpha by
http://species-id.net/wiki/Lypnea
Figs 64, 209–210, 334)Lypnea: Lypnea flava Baly, 1876a: 446 (New Guinea, Batchian), designation by monotypy; Escaleriella: Escaleriella marginata Weise, 1907: 398 [Spanish Guinea (= Equatorial Guinea)], by present designation; Poephila: Poephila lacessita Weise, 1895: 342 (Addah), designation by monotypy.
Afrotropical (including Madagascar), Australian, Eastern Palaearctic, and Oriental regions (Fig. 334).
Lypnea flaveola (Bryant, 1944) collected on Oncoba echinata Oliver (Flacourtiaceae) (
About ten species are known from Madagascar and Sub-Saharan Africa . Bechyné (1968: 1718) considered Escaleriella Weise and Lypnea Baly to be separate genera because of the difference in the shape of their elytral epipleura: expanded in Lypnea, but straight and narrow in Escaleriella.
[Macroorthocrepis Pic, 1921]
= Phygasia Chevrolat, 1836
Malvernia varicornis
Malawi and the Republic of South Africa (Eastern Cape Province, Free State, Gauteng, KwaZulu-Natal, Limpopo, Mpumalanga, and North-West Province) (Fig. 335).
Malvernia varicornis has been collected on the flowers of Burchellia bubalina (L. f.) Simms (Rubiaceae) (
Two known species.
http://species-id.net/wiki/Manobia
Figs 66, 212, 336Manobia: Manobia nigripennis Jacoby, 1885: 73 (Sumatra), by subsequent designation by Maulik (1926: 285, 407); Afroalytus: Afroalytus kivuensis Scherer, 1961: 269, 286 (Kivu: T. Kalehe, 2850 m), by original designation.
Central, Eastern [Kenya (!), Taita Hills, Ngangao (BAQ)] and Western Africa; Australian, Eastern Palaearctic, and Oriental regions (Fig. 336).
This genus is probably polyphagous and has been reported mainly from the following plant families: Epacridaceae, Urticaceae, Cyatheaceae, Asteraceae and Arecaceae (cf.
About fifteen species described from Sub-Saharan Africa.
[Mantura Stephens, 1831]
Not present in the Afrotropical region.
= Balanomorpha Chevrolat, 1836 (synonymy reported in
References.
Notes. Mantura quadriplagiata Jacoby (1895: 321) transferred to Podagrica Chevrolat by
[Mediafra Scherer, 1961]
= Kenialtica Bechyné, 1960a
[Mesocrepis Scherer, 1963]
= Kimongona Bechyné, 1959a
Metroserrapha prima Bechyné, 1958a: 86 (Mauritius Island), by original designation.
Madagascar and the Mascarene Islands (Fig. 337).
The species in this genus are probably polyphagous, and have been associated mainly with plants in the Ericaceae, Asteraceae and Polygonaceae (cf.
Seven species are known from the Mascarene Islands and about ten, as yet undescribed, from Madagascar (personal data).
[Monodaltica Bechyné, 1955b]
= Trachytetra Sharp, 1886
Montiaphthona monticola Scherer, 1961: 285 (Kivu: Mont Muhi), by original designation.
Democratic Republic of the Congo, Kenya, Republic of South Africa (!) [Mpumalanga, Mount Sheba (BAQ)], Rwanda, Tanzania and Uganda (Fig. 338)
The species of this genus generally live at altitudes above 2, 500 m in mixed bamboo forests.
Six species have been described.
[Musaka Bechyné, 1958a]
= Sphaeroderma Stephens, 1831
http://species-id.net/wiki/Myrcina
Figs 69, 216, 339Myrcina: Myrcina nigra Chapuis, 1875: 127 (Vieux-Calabar), by original designation; Myrcinella: Myrcina spectabilis Baly, 1878b: 232 (Madagascar), by subsequent designation by Bechyné (1964: 150); Xenaltica: Xenaltica murrayi Baly, 1875: 26 (Old Calabar) (= Myrcina nigra Chapuis, 1875: 127), by present designation.
Madagascar and Sub-Saharan Africa (absent in the southern part of SAF) (Fig. 339).
Some species of this genus were collected from Spathodea sp. (Bignoniaceae) in Eastern Africa (cf.
Twenty-three species are known.
[Myrcinella Jacoby, 1901]
= Myrcina Chapuis, 1875
Crepidodera picticornis Harold, 1877a: 107 (Madagascar), by present designation.
Madagascar. Weise (1923: 122) described Neodera australis from specimens from Australia (Queensland); however the generic placement of this species is not correct (C. Reid, 2010, pers. comm.) (Fig. 340).
No information.
About fifteen known species.The subgenusNeoderina
[Neoderina Bechyné, 1952]
= Diphaulacosoma Jacoby, 1892a
[Neumannia Weise, 1907b]
= Podagrica Chevrolat, 1836
[Niphraea Baly, 1878a]
= Eriotica Harold, 1877a
http://species-id.net/wiki/Nisotra
Figs 71, 217 –218, 341Nisotra: Haltica gemella Erichson, 1834: 275 (Philippines: Luzon), by subsequent designation by
Afrotropical (including Madagascar), Australian, Eastern Palaearctic and Oriental regions (Fig. 341).
Species in this genus are mainly associated with plants in the family Malvaceae (cf.
About seventy species of this genus are known to occur in Madagascar and Sub-Saharan Africa.
Notomela cyanipennis Jacoby, 1899b: 357 (Cameroon), designation by monotypy.
Cameroon, Democratic Republic of the Congo, Equatorial Guinea (Fernando Poo Island), Ivory Coast, Liberia, Nigeria, Republic of South Africa (North-West Province and KwaZulu-Natal), Rwanda and Uganda, (Fig. 342).
Notomela fulvicollis Bryant, 1931 was collected on Xanthoxylum capense (Thunb.) Harv. (Rutaceae) in South East Africa (
Four species have been described.
http://species-id.net/wiki/Ntaolaltica
Figs 73, 221–223, 343Biondi and D’Alessandro, in press.
Ntaolaltica antennata Biondi and D’Alessandro, in press (Madagascar: Analamerana, 50 km SE of Diégo-Suarez), by original designation.
Madagascar (Fig. 343).
No information.
A single species has been described.
Nzerekorena cerambycina Bechyné, 1955b: 507 (French Guinea: Nzérékoré; Liberia: Kaouyéké; Dahomey: Forèt de Ketou), by original designation.
Benin, Cameroon, Democratic Republic of the Congo, Guinea, Kenya, Liberia, Malawi (!) [Mulanje Mts. (BAQ)], Nigeria and Uganda (Fig. 344).
No information.
Nine species are known.
[Ochrosis Foudras, 1860]
Not present in the Afrotropical region.
References.
The species Ochrosis natalensis
[Oedionychis Latreille, 1829]
Not present in the Afrotropical region.
References.
Notes. Currently, four species that occur in southwestern Europe and North Africa are attributed to this genus (
[Orneates Jacoby, 1899b]
= Gabonia Jacoby, 1893
http://species-id.net/wiki/Orthocrepis
Figs 75, 225–226, 345Haltica ruficollis Lucas, 1849: 546 (Algeria), designation by monotypy.
Afrotropical (including Madagascar), Oriental and Palaearctic regions (Fig. 345).
The species in this genus are mainly associated with plants in the family Euphorbiaceae, but also with Leguminosae and Malvaceae (cf.
About twenty-five species have been recorded in Sub-Saharan Africa and sixteen from Madagascar.
Paradibolia indica Baly, 1875: 31 (India), designation by monotypy.
Cameroon, Democratic Republic of the Congo, Guinea, Namibia (!) [Fish River Canyon (ZMHB); Hereroland (ZMHB); Windhoek (BAQ); Waterberg National Park (BAQ)], Republic of South Africa, Sierra Leone, Australian and Oriental regions (Fig. 346).
Species in this genus are associated with plants from the family Lamiaceae in South Africa (personal data).
Three species are known.
[Paropsiderma Bechyné, 1958a]
= Sesquiphaera Bechyné, 1958a
Perichilona rufa Weise, 1919: 203 (Gaviro, Kwiro), by present designation.
Tanzania (Fig. 347).
No information.
Two species have been described.
http://species-id.net/wiki/Philopona
Figs 78, 229, 348Oedionychis (?) vernicata Gerstaecker, 1871: 84 (Zanzibar), by original designation.
Afrotropical (excluding Madagascar), Australian, Oriental and Southern-Eastern Palaearctic regions (Fig. 348).
Philopona usambarica Csiki (in
About twenty species known from Sub-Saharan Africa.
http://species-id.net/wiki/Phygasia
Figs 79, 230, 349Phygasia: Altica unicolor Olivier, 1808: 699 (India), by original designation; Macroorthocrepis: Macroorthocrepis pallidicolor Pic, 1921: 14 (Abyssinia), designation by monotypy.
Afrotropical (excluding Madagascar), Oriental and Palaearctic regions (Fig. 349).
Species in this genus are mainly associated with plants in the family Asclepiadaceae (cf.
About thirty-five species have been described in Sub-Saharan Africa. The Madagascan species previously attributed to this genus have been transferred to the genus Pseudophygasia Biondi and D’Alessandro, in press.
Chrysomela brassicae Fabricius, 1787: 78 (Europe), by subsequent designation by
All zoogeographical regions (Fig. 350).
A genus that has mainly been associated with plants in the families Cruciferae (= Brassicaceae), Resedaceae, and Capparidaceae (cf.
About forty species are known from the Arabian Peninsula, Madagascar and Sub-Saharan Africa.
http://species-id.net/wiki/Physodactyla
Figs 81, 232–233, 351Physonychis africana Chapuis, 1875: 89 (East Africa), by original designation.
Democratic Republic of the Congo, Kenya, Republic of South Africa (Mpumalanga and KwaZulu-Natal), Sudan and Tanzania (Fig. 351).
Physodactyla rubiginosa (
Six described species.
http://species-id.net/wiki/Physoma
Figs 82, 234–235, 352Physoma:Physoma tripartitum (Thomson, 1858) (Gabon)(= Physonychis rugicollis Clark, 1860 in litteris), by subsequent designation by
Central and Western Africa, and Madagascar (Fig. 352)
No information.
Two species known from Sub-Saharan Africa and about twenty from Madagascar.The genus-nameTropidophora Thomson is not available because it was ambiguously applied (ICZN, 1999: art. 12.2.5).
http://species-id.net/wiki/Physomandroya
Figs 83, 236–238, 353Physomandroya decorsei Bechyné, 1959c: 319 (Madagascar: Ambowombé), by original designation.
Madagascar (Fig. 353).
No information.
Seven described species.
Physonychis smaragdina Clark, 1860: 31 (Western Africa), by original designation.
Sub-Saharan Africa (absent in the southern-western part of SAF and Madagascar). Physonychis varicornis Duvivier, 1891 from Madagascar was transferred to the genus Physoma Clark by
No information.
About thirty known species.
[Plectroscelis Chevrolat, 1836]
= Chaetocnema Stephens, 1831
http://species-id.net/wiki/Podagrica
Figs 85, 86, 355Podagrica: Altica fuscipes Fabricius, 1775: 114 (Europe), by subsequent designation by Maulik, (1926: 273); Neumannia: Balanomorpha aethiopica Chapuis, 1879: 13 (Ethiopia), by present designation; Podagrixena: Podagrica decolorata Duvivier, 1892a: 60 (Democratic Republic of the Congo: Ibembo), by original designation.
Afrotropical [including Madagascar (!): Nossibé (ZMHB); Ranohira (ZMHB); Tamatave (ZMHB)], Oriental , and Palaearctic regions (Fig. 355).
The species in this genus are mainly associated with plants in the family Malvaceae (cf.
About fifty species are known from Sub-Saharan Africa, with one having been recorded from Madagascar. There are no significant diagnostic characters distinguishing Podagricina Csiki from Podagrica. Therefore, the following new synonymy is proposed: Podagrica Chevrolat, 1836 = Podagricina Csiki in Heikertinger and Csiki, 1940 syn. n. Type material examined: Balanomorpha aethiopica Chapuis, “Bogos, 1870, Keren, O. Beccari”, 4 syntypes (MCSN).
[Podagricina Csiki in
= Podagrica Chevrolat, 1836
[Podagrixena Bechyné, 1968]
= Podagrica Chevrolat, 1836
[Poephila Weise, 1895]
= Lypnea Baly, 1876a
[Poephilina Csiki in
= Lypnea Baly, 1876a
http://species-id.net/wiki/Polyclada
Figs 87, 240–241, 356Polyclada: Clythra pectinicornis Olivier, 1789: 31 (Africa), designation by monotypy.
Sub-Saharan Africa (absent in the south-western part of SAF and Madagascar), Saudi Arabia, and Yemen (Fig. 356).
This genus is generally associated with plants in the family Anarcadiaceae (cf.
Sixteen species have been described.
Pratima variabilis Maulik, 1931: 253 (Seychelles: Silhouette and Mahe), by original designation.
Indian Ocean (Seychelles and Mascarene Islands) (Fig. 357).
The species of this genus live in forests (300–700 m) (
There are eight known species.
[Prototrigona Chevrolat, 1837]
Nomen nudum.
Notes.
http://species-id.net/wiki/Pseudadorium
Figs 89, 243–245, 358Pseudadorium vernicatum Fairmaire, 1885: 239 (Madagascar), designation by monotypy.
Only found onMadagascar (Fig. 358).
No information.
About twenty described species. We define this genus in a broader sense than
[Pseudonisotra Bechyné, 1968]
= Nisotra Baly, 1864
http://species-id.net/wiki/Pseudophygasia
Figs 90, 247 –248, 359Biondi and D’Alessandro, in press;
Crepidodera analis Harold, 1877a: 107 (Madagascar), by original designation.
Only found on Madagascar (Fig. 359).
No information.
Nine described species. All the species previously attributed to the genus Phygasia Chevrolat from Madagascar have been moved to Pseudophygasia (Biondi and D’Alessandro, in press).
Chrysomela chrysocephala Linnaeus, 1758: 372 (Europe), by subsequent designation by Maulik (1926: 144).
Found inall the zoogeographical regions. In the Afrotropical region this genus is known from Ethiopia, Kenya, Republic of South Africa, Tanzania and Socotra Island (Yemen) (Fig. 360).
Known host plants for this genus fall in the plant families Cruciferae (= Brassicaeae), Solanaceae and Poaceae (also known as Gramineae) (cf.
There are seven described species.
Pydaristes attagenoides Harold, 1875: 447 (Africa), designation by monotypy.
“Africa”.
No information.
Only one species is known. Unfortunately, the location for the type material is unknown. The original description of Pydaristes appears to be identical or very close to that of Amphimela Chapuis. The synonymy proposed by
http://species-id.net/wiki/Sanckia
Figs 92, 93, 249–250, 361Sanckia: Sanckia johanna Duvivier, 1891: 316 (Madagascar: Antsianaka Forest), by original designation; Eugonotes: Eugonotes longicornis Jacoby, 1897: 559 (Madagascar: Diego-Suarez), designation by monotypy.
Burundi (!) [Bururi (ZSM)], Democratic Republic of the Congo, Ethiopia, Guinea, Kenya, Madagascar, Rwanda, Senegal, Uganda, and the Oriental region (Fig. 361).
No information.
About twenty species are known from the Afrotropical region, most of these are from Madagascar. There are no significant diagnostic characters distinguishing Eugonotes Jacoby from Sanckia. The following new synonymy is therefore proposed: Sanckia Duvivier, 1891 = Eugonotes Jacoby, 1897 syn. n. Type material examined: Eugonotes longicornis Jacoby, “Madagascar, Diego Suarez”, syntype ♀ (BMNH).
Serraphula aenea Jacoby, 1897: 557 (Mashonaland), designation by monotypy.
Republic of South Africa (Limpopo, Mpumalanga, Free State, KwaZulu-Natal, Eastern, and Western Cape Provinces) and Zimbabwe (Fig. 362).
Species in this genus are known to be associated with plants in the family Asteraceae (
Nineteen species have been described.
http://species-id.net/wiki/Sesquiphaera
Figs 95, 96, 254–256, 363Sesquiphaera: Sphaeroderma mashonanum Jacoby, 1900: 252 (Mashonaland: Salisbury), by original designation; Paropsiderma: Sphaeroderma anthrax Brancsik, 1910: 185 (Madagascar), designation by monotypy.
Democratic Republic of the Congo, Guinea, Guinea Bissau, Madagascar, Namibia, Republic of South Africa (Gauteng, Mpumalanga, and KwaZulu-Natal), Rwanda, Tanzania, and Zimbabwe (Fig. 363).
No information.
There are about ten described species. No significant diagnostic characters distinguish Paropsiderma Bechyné from Sesquiphaera. The following new synonymy is therefore proposed: Sesquiphaera Bechyné, 1958 = Paropsiderma Bechyné, 1958 syn. n. Material examined: Paropsiderma anthrax (Brancsik) (det. J. Bechyné), “Madagascar, Joffreville, 13.V.1953, F. Kaiser”, 1 specimen (NHMB).
Chaetocnema mahensis Maulik, 1931: 250 (Seychelles: Mahé), by original designation.
Indian Ocean (Seychelles) (Fig. 364).
The species in this genus are associated with indigenous forests in the Seychelles.
Four species have been described.
Sjostedtinia montivaga Weise, 1910a: 206 (Kilimanjaro: Kibocho), by original designation.
Kenya, Tanzania, and Uganda, (Fig. 365).
This genus lives at high altitudes on Kilimanjaro and Mount Elgon. Sjostedtinia montivaga has been collected from Lobelia deckeni (Asch.) Hemsl. (Lobeliaceae) (Weise, 1910a), and Sjostedtinia fordi Bryant, 1953 from a Senecio sp. (Asteraceae) in Uganda (Bryant, 1953), and from a Lobelia sp. in Kenya (S. Zoia 2009, pers. comm.).
Two species are known.
http://species-id.net/wiki/Sphaeroderma
Figs 99, 263–264, 366Sphaeroderma: Altica testacea Fabricius, 1775: 114 (Europe), by subsequent designation by Maulik, (1926: 316); Argosomus: Argosomus epilachnoides Wollaston, 1867: 152 (Cape Verde Islands: Brava), by subsequent designation by
Afrotropical (including Madagascar) and Australian, Oriental, and Palaearctic regions. The species of Sphaeroderma reported from the Neotropical and Nearctic regions should be attributed to different genera (cf.
Species in this genus are mainly associated with plants in the families Asteraceae and Ranunculaceae (cf.
Over fifty species have been recorded from Sub-Saharan African and about ten from Madagascar.
[Sphaerophysa Baly, 1876b]
= Amphimela Chapuis, 1875
Stegnaspea trimeni Baly, 1877: 182 (Cape of Good Hope), designation by monotypy.
Republic of South Africa (Western Cape Province) and Tristan da Cunha (Fig. 367).
Stegnaspea trimeni collected from Poaceae (also known as Gramineae) in meadows (D’Alessandro et al. 2012).
Six species are known.
Podagrica glabrata Jacoby, 1899b: 349 (KwaZulu-Natal: Umtenweni River; Eastern Cape Province: Port St. John), by original designation.
Republic of South Africa [Mpumalanga, KwaZulu-Natal, and Eastern Cape Province] (Fig. 368).
No information.
Only one species is known.
http://species-id.net/wiki/Terpnochlorus
Figs 102, 266–267, 369Terpnochlorus perrieri Fairmaire, 1904: 269 (Madagascar: Soalala), designation by monotypy.
Botswana, Democratic Republic of the Congo, Gambia, Guinea Bissau, Madagascar, Mali, Namibia, Sierra Leone, and South America (Venezuela and Mexico) (Fig. 369).
This genus lives in moist habitats generally associated with plans from the family Juncaceae (
Two species have been described from the Afrotropical region. Bechynè (1955b) synonymised Chaloenus viridis Bryant (
[Torodera Weise, 19082a]
= Argopistoides Jacoby, 1892b
Galleruca indica Fabricius, 1798: 98 (Western Cape), by original designation.
Democratic Republic of the Congo, Kenya, the Republic of South Africa, and Uganda (Fig. 370).
No information.
Five species are known.
http://species-id.net/wiki/Trachytetra
Figs 104, 268–269, 371Phyllotreta rugulosa Broun, 1880: 636 (New Zealand), by original designation; Monodaltica: Monodaltica guineensis Bechyné, 1955b: 510 (French Guinea), by original designation.
Cameroon, Democratic Republic of the Congo, Ghana, Guinea, Nigeria, Sierra Leone, and Australian, Eastern Palaearctic, and Oriental regions (Fig. 371).
No information.
Five species have been described from Sub-Saharan Africa.
Aphthona longicornis Laboissière, 1942: 21 (Albert National Park), by original designation;
Democratic Republic of the Congo (Fig. 372).
No information.
One described species.
[Thrymnes Weise, 1895]
= Gabonia Jacoby, 1893
[Tropidophora Thomson, 1858]
= Physoma Clark, 1863
Upembaltica scolytina
Democratic Republic of the Congo (Fig. 373).
No information.
Only one species has been recorded.
[Weiseana Jacoby, 1906]
= Homichloda Weise, 1902
Xanthophysca perrieri Fairmaire, 1901: 242 (Madagascar: Saberbieville), designation by monotypy.
Madagascar (Fig. 374).
No information.
Five species have been described. These usually display different colour varieties. Two species from Madagascar, previously included in the genus Blepharida, are here attributed to this genus: Xanthophysca multiguttata (Duvivier, 1891: 242) comb. n. and Xanthophysca insignis (Brancsik, 1897: 130) comb. n.
[Xenaltica Baly, 1875]
= Myrcina Chapuis, 1875
Yemenaltica scorteccii Scherer, 1985: 86 (Yemen: El Kasaba), by original designation.
Arabian Peninsula and Socotra Island (Yemen) (Fig. 375).
No information.
Two species have been described.
Zomba gossypii Bryant, 1922a: 264 (Nyasaland: Luchenza; N.W. Rhodesia: Livingstone), designation by monotypy.
Malawi and Zimbabwe (Fig. 370).
The only species in this genus was collected from Cotton plants, Gossypium sp. (Malvaceae) (
Only one species is known. This genus is particularly interesting because it is the only representative of the tribe Monoplatini in the Afrotropical region. This tribe occurs almost exclusively in the Neotropical and southern part of the Nearctic regions. The only exceptions are the genera Zomba and Opisthopygme Blackburn (1896: 41); this last genus is occurring with two species in the Australian region.
There are 99 flea beetle genera known from the Afrotropical region. Of these, 83 occur in continental Sub-Saharan Africa, with the different regions each having a varying number of species: CAF: 63; EAF; 55; SAF: 58; and WAF: 44. Furthermore, 39 genera are known from Madagascar, 9 from the Mascarene Islands and 5 from the Seychelles Islands ((Table 1, Fig. 377). However, these numbers are still provisional as information concerning the Afrotropical flea beetle fauna is limited, particularly for Madagascar.
Our preliminary analysis indicates that this fauna is distinct and can be separated from the faunas of other zoogeographical regions. In Fig. 381, a dendrogram obtained from the cluster analysis [Coincidence index and Weighted Pair Group Method using Arithmetic averaging (WPGMA) (cf.
The Seychelles (SEY) and the Mascarene islands (MAS) are more closely associated with the other zoogeographical regions [(NAR-NTR)(PAR-ORR)AUR)] because of the occurrence of a high percentage of widespread genera that characterize the flea beetle fauna of these two archipeligoes. Moreover, faunistic similarity based on the widespread flea beetle genera also clusters the other zoogeographical regions together in two distinct groups, namely the Palaearctic-Oriental-Australian regions [(PAR-ORR)AUR] and Nearctic-Neotropical regions (NAR-NTR).
The geographic distribution of Afrotropical flea beetle genera is therefore well characterized and it has distinct Malagasy and Sub-Saharan African components (Fig. 381).
The percentage of Alticini genera endemic to the Afrotropical region is very high (71.0%), with the following distribution: Sub-Saharan Africa, 52 genera; Madagascar, 12; Seychelles Islands, 1; Sub-Saharan Africa-Madagascar, 4; Madagascar-Mascarene Islands, 1; Seychelles-Mascarene Islands, 1 (Fig. 378). Within the endemic Sub-Saharan Africa component, only 6 genera occur in all four subregions, while 25 genera occur only in one subregion (SAF: 11; CAF: 9; EAF: 5). There are no exclusively endemic flea beetle genera in WAF (Fig. 378).
The percentage of genera occurring in both the Afrotropical and another zoogeographical region is 32.0%, with the cosmopolitan component significant and well represented [8.0% of the total of 99] (Fig. 379). The Afrotropical region shares the highest percentage of genera with the Oriental (27.0%) and Palaearctic (27.0%) regions (Fig. 380). The co-presence of genera in both the Afrotropical and Oriental regions, often called Palaeotropical distribution, may be due to a possible Gondwanian origin., Sanckia is such an example, although the genus occurs mainly in Madagascar, species are also found in Sub-Saharan Africa and the southern part of the Oriental region. Other examples are the genera Argopistoides and Jacobyana which occur in Sub-Saharan Africa and the Oriental region and are absent from Madagascar; and Amphimela, Chabria, Nisotra, and Paradibolia, which occur in the Afrotropical, Oriental and the Australian regions. Other genera, such as Bikasha, Hemipyxis, Luperomorpha, Lypnea, Manobia, Philopona and Trachytetra occur not only in the Afrotropical (including Madagascar, although infrequently), Oriental and, generally Australian regions, but also in the eastern part of the Palaearctic region. More specifically, the Palaeartic region shares 27 flea beetle genera with the Afrotropical region (Fig. 380), including the unique Pan-African flea beetle genus, Angulaphthona, which occurs in Mediterranean Africa, Sub-Saharan Africa and Madagascar (cf.
As expected, the lowest percentage of genera occur in both the Afrotropical/Nearctic regions (10.0%) and Afrotropical/Neotropical regions (10.0%). All genera common to the Afrotropical, Nearctic and Neotropical regions are also found in all other zoogeographical regions with the exception of the genus Terpnochlorus, which only occurs in the Afrotropical region, Venezuela and Mexico (cf.
As reported in
The unique Afrotropical genus Zomba belonging to the tribe Monoplatini, which mainly occurs in the Neotropical region with a few species found in the Nearctic region. The genus Opisthopygme, also from the Monoplatini, is also present in Australia (see above).
Two new, as yet undescribed, flea beetle genera that occur in Madagascar and South Africa (Western Cape Province). Both these genera have clavate or subclavate antennae with 11 segments, are subsphaerical in shape and very small, characteristics they share with related genera in Central America, such as: Bubiscus Savini, Furth and Joly (2009: 53), a recently described Costa Rican genus (1 species); Normaltica
There are 39 flea beetle genera known from Madagascar, 13 of which are endemic. One of them, Metroserrapha Bechyné, also occurs in the Mascarene Islands (
Occurrence of Alticinae genera of the Chrysomelidae in different areas of the Afrotropical region and other zoogeographical areas (see text for abbreviations) (updated from
| Genus | WAF | CAF | EAF | SAF | MAD | SEY | MAS | PAR | NAR | NTR | ORR | AUR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abrarius | X | ? | ||||||||||
| Afroaltica | X | |||||||||||
| Afrocrepis | X | X | ||||||||||
| Afrorestia | X | X | X | X | ||||||||
| Alocypha | X | X | ||||||||||
| Altica | X | X | X | X | X | X | X | X | X | X | X | |
| Amphimela | X | X | X | X | X | X | X | X | ||||
| Anaxerta | X | |||||||||||
| Angulaphthona | X | X | X | X | X | X | ||||||
| Antanemora | X | |||||||||||
| Aphthona | X | X | X | X | X | X | X | X | X | X | ||
| Argopistes | X | X | X | X | X | X | X | X | X | X | ||
| Argopistoides | X | X | X | X | X | |||||||
| Bangalaltica | X | |||||||||||
| Bechuana | X | |||||||||||
| Bechynella | X | X | ||||||||||
| Bezdekaltica | X | |||||||||||
| Bikasha | X | X | X | X | X | X | X | X | ||||
| Biodontocnema | X | |||||||||||
| Blepharida | X | X | X | X | X | X | X | X | X | |||
| Carcharodis | X | X | X | |||||||||
| Celisaltica | X | |||||||||||
| Chabria | i? | i? | X | X | ||||||||
| Chaetocnema | X | X | X | X | X | X | X | X | X | X | X | X |
| Chaillucola | X | |||||||||||
| Chirodica | X | |||||||||||
| Collartaltica | X | X | X | X | ||||||||
| Decaria | X | X | X | X | ||||||||
| Diamphidia | X | X | X | |||||||||
| Dibolia | X | X | X | X | X | X | X | |||||
| Dimonikaea | X | |||||||||||
| Diphaulacosoma | X | |||||||||||
| Djallonia | X | X | ||||||||||
| Drakensbergianella | X | |||||||||||
| Dunbrodya | X | X | ||||||||||
| Epitrix | X | X | X | X | X | X | X | X | X | X | X | |
| Eriotica | X | |||||||||||
| Eurylegna | X | X | ||||||||||
| Eutornus | X | X | X | X | X | |||||||
| Gabonia | X | X | X | X | ||||||||
| Guilielmia | X | |||||||||||
| Guinerestia | X | X | ||||||||||
| Halticotropis | X | |||||||||||
| Hemipyxis | X | X | X | X | X | X | X | |||||
| Hespera | X | X | X | X | X | X | ||||||
| Hildenbrandtina | X | |||||||||||
| Homichloda | X | X | X | |||||||||
| Hyphasis | i? | X | X | |||||||||
| Jacobyana | X | X | X | X | ||||||||
| Kanonga | X | X | ||||||||||
| Kenialtica | X | X | X | X | X | |||||||
| Kimongona | X | X | X | |||||||||
| Lampedona | X | X | ||||||||||
| Lepialtica | X | X | ||||||||||
| Longitarsus | X | X | X | X | X | X | X | X | X | X | X | X |
| Luperomorpha | X | X | X | X | X | |||||||
| Lypnea | X | X | X | X | X | X | X | X | ||||
| Malvernia | X | X | ||||||||||
| Manobia | X | X | X | X | X | X | ||||||
| Metroserrapha | X | X | ||||||||||
| Montiaphthona | X | X | X | |||||||||
| Myrcina | X | X | X | X | X | |||||||
| Neodera | X | |||||||||||
| Nisotra | X | X | X | X | X | X | X | X | ||||
| Notomela | X | X | X | |||||||||
| Ntaolaltica | X | |||||||||||
| Nzerekorena | X | X | X | |||||||||
| Orthocrepis | X | X | X | X | X | X | X | |||||
| Paradibolia | X | X | X | X | X | |||||||
| Perichilona | X | |||||||||||
| Philopona | X | X | X | X | X | X | X | |||||
| Phygasia | X | X | X | X | X | X | ||||||
| Phyllotreta | X | X | X | X | X | X | X | X | X | X | ||
| Physodactyla | X | X | X | |||||||||
| Physoma | X | X | X | |||||||||
| Physomandroya | X | |||||||||||
| Physonychis | X | X | X | X | ||||||||
| Podagrica | X | X | X | X | X | X | X | |||||
| Polyclada | X | X | X | X | X | |||||||
| Pratima | X | X | ||||||||||
| Pseudadorium | X | |||||||||||
| Pseudophygasia | X | |||||||||||
| Psylliodes | X | X | X | X | X | X | X | |||||
| Sanckia | X | X | X | X | X | |||||||
| Serraphula | X | |||||||||||
| Sesquiphaera | X | X | X | X | X | |||||||
| Seychellaltica | X | |||||||||||
| Sjostedtinia | X | X | ||||||||||
| Sphaeroderma | X | X | X | X | X | X | X | X | X | X | X | |
| Stegnaspea | X | |||||||||||
| Stuckenbergiana | X | |||||||||||
| Terpnochlorus | X | X | X | X | X | |||||||
| Toxaria | X | X | X | |||||||||
| Trachytetra | X | X | X | X | X | |||||||
| Tritonaphthona | X | |||||||||||
| Upembaltica | X | |||||||||||
| Xanthophysca | X | |||||||||||
| Yemenaltica | X | X | ||||||||||
| Zomba | X | X |
Habitus. 2 Abrarius cribrosus Fairmaire 3 Afroaltica subaptera Biondi & D’Alessandro 4 Afrocrepis malvernensis (Jacoby) 5 Afrorestia peringueyi (Jacoby) 6 Alocypha bimaculata (Jacoby) 7 Altica madagascariensis (Allard) 8 Amphimela bryanti (Csiki) 9 Amphimela (ex Sphaerophysa Baly) heikertingeri (Bechyné) 10 Anaxerta castanea Fairmaire.
Habitus .11 Angulaphthona heteromorpha (Bechyné) 12 Antanemora ghesquierei (Bechyné) 13 Aphthona senegalensis Jacoby 14 Aphthona (ex Ethiopia Scherer) tricolor (Scherer) 15 Argopistes sexvittatus Bryant 16 Argopistoides africanus (Bryant) 17 Bangalaltica antennalis Bechyné 18. Bechuana nigripes Scherer 19 Bechynella pallens (Bechyné).
Habitus. 20 Bezdekaltica socotrana Döberl 21 Bikasha tenuipunctata Maulik 22 Biodontocnema brunnea Biondi 23 Blepharida ornata Baly 24 Buphonella nigroviolacea (Allard) (genus transferred to Galerucini) 25 Carcharodis rugiceps (Baly). 26 Celisaltica ruwenzorica Biondi 27 Chabria bezanozana Biondi & D’Alessandro 28 Chaetocnema purpurea Jacoby.
Habitus. 29 Chaillucola formicicornis Bechyné 30 Chirodica chalcoptera Germar 31 Collartaltica cryptostoma Bechyné 32 Decaria abdominalis Jacoby 33 Diamphidia nigroornata Stål 34 Dibolia bimaculata Jacoby 35 Dimonikaea descarpentriesi Bechyné 36 Diphaulacosoma laevipenne Jacoby 37 Djallonia maindra Bechyné.
Habitus. 38 Drakensbergianella rudebecki Biondi & D’Alessandro 39 Dunbrodya nitida Jacoby 40 Epitrix aethiopica Weise 41 Eriotica fuscipennis Harold 42 Eurylegna fulva Weise 43 Eurylegna (ex Eurylegniella Scherer) guineensis Bechyné 44 Eutornus rugicollis (Jacoby) 45 Gabonia unicostata Jacoby 46 Guilielmia monticola Weise.
Habitus. 47 Guinerestia rubra Scherer 48 Halticopsis spissicornis Fairmaire (genus transferred to Galerucini) 49 Halticotropis costipennis Bechyné 50 Hemipyxis africana (Allard) 51 Hespera africana Jacoby 52 Hildebrandtina similis Bechyné 53 Homichloda barkeri (Jacoby) 54 Hyphasis sita (Maulik) 55 Jacobyana bezdeki Biondi & D’Alessandro.
. Habitus. 56 Kanonga atra Bechyné 57 Kenialtica muhavura Bechyné 58 Kimongona callifera Bechyné 59 Kimongona (ex Mesocrepis Scherer) lindemannae (Scherer) 60 Lampedona testacea Bechyné 61 Lepialtica bicolor Scherer 62 Longitarsus africanus Jacoby 63 Luperomorpha biondii Döberl 64 Lypnea costatipennis ( Jacoby).
Habitus. 65 Malvernia varicornis Jacoby 66 Manobia africana Laboissière 67 Metroserrapha sp 68 Montiaphthona gedyei (Bryant) 69 Myrcina vandenplaasi Laboissière 70 Neodera fraterna Duvivier 71 Nisotra aruwimiana Weise 72 Notomela fulvicollis Bryant 73 Ntaolaltica antennata Biondi & D’Alessandro.
Habitus. 74 Nzerekorena filicornis Scherer & Boppré 75 Orthocrepis togoensis Weise 76 Paradibolia abdominalis (Jacoby) 77 Perichilona bicolor Weise 78 Philopona fulvicollis (Fabricius) 79 Phygasia rubripennis Weise 80 Phyllotreta puncticollis (Jacoby) 81 Physodactyla rubiginosa (Gerstaecker) 82 Physoma costuliferum Bechyné.
Habitus. 83 Physomandroya melanarthra (Fairmaire) 84 Physonychis violaceipennis (Baly) 85 Podagrica oneili (Jacoby) 86 Podagrica (ex Podagricina Csiki) aethiopica (Chapuis) 87 Polyclada bohemani (Baly) 88 Pratima variabilis Maulik 89 Pseudadorium amplum (Weise) 90 Pseudophygasia analis (Harold) 91 Psylliodes calcarata (Bryant).
Habitus. 92 Sanckia lebisi Bechyné 93 Sanckia (ex Eugonotes Jacoby) longicornis (Jacoby) 94 Serraphula elongata Jacoby 95 Sesquiphaera mashonana (Jacoby) 96 Sesquiphaera (ex Paropsiderma Bechyné) near vadoni (Bechyné) 97 Seychellaltica mahensis (Maulik) 98 Sjostedtinia montivaga Weise 99 Sphaeroderma femoratum Jacoby 100 Stegnaspea trimeni Baly.
Habitus. 101 Stuckenbergiana glabrata (Jacoby) 102 Terpnochlorus perrieri Fairmaire 103 Toxaria indica (Fabricius) 104 Trachytetra guineensis (Bechyné) 105 Tritonaphthona longicornis (Laboissière) 106 Upembaltica scolytina Bechyné 107 Xanthophysca perrieri Fairmaire 108 Yemenaltica scorteccii Scherer 109 Zomba gossypii Bryant.
Morphological characters. 110 Abrarius aethiops (Weise), head and pronotum in dorsal view 111 Ditto, head and right antenna in dorsal view 112 Abrarius cribrosus Fairmaire, ventral parts 113 Afroaltica parvula D’Alessandro & Biondi, head in frontal view 114 Afroaltica subaptera Biondi & D’Alessandro, head and pronotum in dorsal view 115 Ditto, triangular hollow on ventral side of middle tibiae in male.
Morphological characters. 116 Afroaltica subaptera Biondi & D’Alessandro, prosternum 117 Ditto, meso- and metasternum (white arrow) 118 Afrocrepis carinipennis (Jacoby), pronotum and elytra in sublateral view 119 Afrorestia jonesi (Bryant), head in frontal view 120 Alocypha bimaculata (Jacoby), head and pronotum in dorsal view 121 Altica nigrita Laboissière, head and pronotum in dorsal view.
Morphological characters. 122 Amphimela citri (Bryant), head in frontal view 123 Angulaphthona latipennis (Pic), head and pronotum in dorsal view 124 Ditto, head in frontal view 125 Ditto, elytra in dorsal view 126 Antanemora ghesquierei (Bechyné), head and pronotum in dorsal view 127 Ditto, head in frontal view.
Morphological characters. 128 Aphthona braunsi (Jacoby), head and pronotum in dorsal view 129 Ditto, elytra in dorsal view 130 Argopistes silvestrii Weise, head in frontal view 131 Ditto, ventral parts 132 Argopistes sexvittatus Bryant, apical part of hind tibia 133 Argopistoides africanus (Bryant), sublateral sulci on pronotum.
Morphological characters. 134 Bangalaltica antennalis Bechyné, head and right antenna in dorsal view 135 Bechuana nigripes Scherer, sublateral depression on pronotum 136 Bechynella pallens (Bechyné), head in frontal view 137 Ditto, hind tibia and metatarsus 138 Bezdekaltica socotrana Döberl, elytra in dorsal view 139 Bikasha fortipunctata Maulik, head in frontal view.
Morphological characters. 140 Bikasha fortipunctata Maulik, hind leg and elytra in lateral view 141 Biodontocnema brunnea Biondi, head in frontal view 142 Ditto, distal half of hind tibia and metatarsus 143 Ditto, hind tibial socket 144 Ditto, dentiform process (white arrow) in inner apical side of hind tibiae 145 Ditto, ventral parts.
Morphological characters. 146 Blepharida intermedia Jacoby, head and pronotum in dorsal view 147 Blepharida reticulata (Baly), head in frontal view 148 Blepharida ugandae Bryant, hind leg 149 Blepharida geminata Bryant, metatarsus 150 Carcharodis malvernensis Bechyné, head in frontal view 151 Celisaltica ruwenzorica Biondi, prosternum.
Morphological characters. 152 Celisaltica ruwenzorica Biondi, metasternum and abdomen 153 Chabria betsimisaraka Biondi & D’Alessandro, sublateral sulci on pronotum 154 Chaetocnema sudafricana Biondi & D’Alessandro, ciliate dentate emargination of hind tibiae 155 Chaillucola formicicornis Bechyné, head and right antenna in dorsal view in male 156 Chirodica similfulva Biondi, buccal parts 157 Chirodica chalcoptera Germar, prosternum and anterior femora in ventral view.
Morphological characters. 158 Collartaltica tenebrosa (Laboissière), head and pronotum in dorsal view 159 Ditto, head in ventral view 160 Ditto, prosternum 161 Diamphidia femoralis Gerstaecker, left antenna in dorsal view 162 Ditto, mesotarsus 163 Diamphidia nigroornata Stål, hind leg and elytral punctation.
Morphological characters. 164 Dibolia thoracica Jacoby, head and pronotum in dorsal view 165 Ditto, head in frontal view 166 Ditto, hind leg and elytral punctation 167 Dimonikaea descarpentriesi Bechyné, head and right antenna in dorsal view 168 Ditto, dentiform process on ventral side of hind femora in male 169 Ditto, metatarsus in lateral (left) and dorsal (right) view.
Morphological characters. 170 Diphaulacosoma laevipenne Jacoby, head and pronotum in dorsal view 171 Ditto, head and right antenna in dorsal view 172 Djallonia maindra Bechyné, head, pronotum and right antenna in dorsal view 173 Drakensbergianella rudebecki Biondi & D’Alessandro, head in frontal view 174 Ditto, elytral surface 175 Ditto, meso- and metasternum.
Morphological characters. 176 Dunbrodya nitida Jacoby, head and pronotum in dorsal view 177 Ditto, head in frontal view 178 Ditto, hind leg 179 Ditto, bifid apical spur of hind tibiae 180 Epitrix aethiopica Weise, head in frontal view 181 Eriotica fuscipennis Harold, head in frontal view.
Morphological characters. 182 Eurylegna rubra (Weise), head in frontal view 183 Eutornus dilatatus Bryant, head and pronotum in dorsal view 184 Eutornus taeniatus Bechyné, elytra in lateral view 185 Gabonia nigripennis (Jacoby), head in frontal view 186 Ditto, meso- and metasternum 187 Guinerestia rubra Scherer, head in frontal view.
Morphological characters. 188 Guinerestia rubra Scherer, longitudinal hollow in distal half of anterior tibiae 189 Halticotropis costipennis Bechyné, head and pronotum in dorsal view 190 Ditto, head in frontal view 191 Ditto, hind leg and elytral surface 192 Hemipyxis burgeoni (Laboissière), head and pronotum in dorsal view 193 Ditto, head in frontal view.
Morphological characters. 194 Hemipyxis obscuretestaceum (Thomson), hind tibia and metatarsus 195 Hespera maculicollis Jacoby, head and pronotum in dorsal view 196 Hildebrandtina obscura Bechyné, head and pronotum in dorsal view 197 Hildebrandtina similis Bechyné, head and pronotum in dorsal view 198 Homichloda barkeri (Jacoby), hind tibia and metatarsus and elytral surface 199 Hyphasis sita (Maulik), head and pronotum in dorsal view.
Morphological characters. 200 Hyphasis sita (Maulik), hind tibia and metatarsus 201 Jacobyana bezdeki Biondi & D’Alessandro, head and pronotum in dorsal view 202 Ditto, ventral parts 203 Kanonga atra Bechyné, head and right antenna in dorsal view 204 Kenialtica muhavura Bechyné, head and pronotum in dorsal view 205 Kimongona callifera Bechyné, head in frontal view.
Morphological characters. 206 Lampedona testacea Bechyné, head and pronotum in dorsal view 207 Lepialtica bicolor Scherer, head in frontal view 208 Longitarsus africanus Jacoby, hind leg in lateral view 209 Lypnea flaveola (Bryant), head and pronotum in dorsal view 210 Lypnea costatipennis (Jacoby), head and right antenna in dorsal view 211 Malvernia varicornis Jacoby, head and right antenna in dorsal view.
Morphological characters. 212 Manobia africana Laboissière, head and pronotum in dorsal view 213 Metroserrapha sp., hind leg and elytral punctation 214 Montiaphthona gedyei (Bryant), head and pronotum in dorsal view 215 Ditto, elytra in sublateral view 216 Myrcina vandenplaasi Laboissiére, double apical spur of hind tibia 217 Nisotra aruwimiana Weise, head and pronotum in dorsal view.
Morphological characters. 218 Nisotra sp., head and pronotum in dorsal view 219 Notomela viridipennis Bryant, head and pronotum in dorsal view 220 Ditto, elytra in lateral view 221 Ntaolaltica antennata Biondi & D’Alessandro, head and pronotum in dorsal view 222 Ditto, head and pronotum in lateral view 223 Ditto, head in frontal view.
Morphological characters. 224 Nzerekorena filicornis Scherer & Boppré, head and right antenna in dorsal view 225 Orthocrepis kibonotensis (Weise), head and pronotum in lateral view 226 Ditto, head in frontal view 227 Paradibolia abdominalis (Jacoby), head in frontal view 228 Perichilona bicolor Weise, head in frontal view 229 Philopona sulcicollis (Jacoby), elytra in lateral view.
Morphological characters. 230 Phygasia sulphuripennis Jacoby, head in frontal view 231 Phyllotreta natalensis Jacoby, head and pronotum in lateral view 232 Physodactyla rubiginosa (Gerstaecker), head and pronotum in lateral view 233 Physodactyla gerstaeckeri (Jacoby), elytra in lateral view 234 Physoma atripes (Fairmaire), head in sublateral view 235 Physoma costuliferum Bechyné, head in sublateral view.
Morphological characters. 236 Physomandroya melanarthra (Fairmaire), head and pronotum in dorsal view 237 Ditto, hind tibia and metatarsus 238 Physomandroya punctulifera Bechyné, elytra in lateral view 239 Physonychis viridipennis (Dalman), head in frontal view 240 Polyclada smythi Bryant, left antenna in male in dorsal view 241 Ditto, left antenna in female in dorsal view.
Morphological characters 242 Pratima vinsoni Bechyné, head in frontal view 243 Pseudadorium balyanum (Csiki), head and pronotum in dorsal view 244 Ditto, head in frontal view 245 Ditto, hind leg in sublateral view 246 Pseudophygasia analis (Harold), head and pronotum in dorsal view 247 Ditto, elytral punctation.
Morphological characters. 248 Psylliodes teresae Biondi, apical part of hind tibia and preapical insertion of metatarsus 249 Sanckia sp., head in frontal view 250 Sanckia longicornis (Jacoby), hind leg 251 Serraphula grobbelaariae Biondi & D’Alessandro, hind leg and elytral punctation 252 Serraphula elongata Jacoby, hind leg 253 Serraphula audisiana Biondi & D’Alessandro, serrate apical spur of hind tibiae.
Morphological characters. 254 Sesquiphaera mashonana (Jacoby), head and pronotum in dorsal view 255 Ditto, head and anterior margin of pronotum in subfrontal view 256 Sesquiphaera nigrosignata (Bryant), elytra in lateral view 257 Seychellaltica krishna (Maulik), head and pronotum in dorsal view 258 Ditto, hind tibia and metatarsus 259 Ditto, protarsus in dorsal view.
Morphological characters. 260 Seychellaltica mahensis (Maulik), pro-, meso- and metasternum 261 Sjostedtinia fordi Bryant, head and pronotum in dorsal view 262 Ditto, hind leg and elytra in dorsal view 263 Sphaeroderma nigripennis Bryant, head and pronotum in dorsal view 264 Sphaeroderma iyengari Bechyné, elytra in lateral view 265 Stegnaspea trimeni Baly, basal part of elytra.
Morphological characters. 266 Terpnochlorus perrieri Fairmaire, head in subfrontal view 267 Ditto, metatarsus in dorsal view 268 Trachytetra guineensis (Bechyné), head and pronotum in lateral view 269 Ditto, hind tibia, metatarsus and apical spur 270 Tritonaphthona longicornis (Laboissière), trifid apical spur of hind tibiae 271 Upembaltica scolytina Bechyné, head and right antenna in dorsal view.
Morphological characters. 272 Upembaltica scolytina Bechyné, metatarsus in lateral (left) and dorsal (right) view 273 Xanthophysca perrieri Fairmaire, head and pronotum in dorsal view 274 Ditto, head in subfrontal view 275 Ditto, hind leg and elytral punctation 276 Yemenaltica scorteccii Scherer, hind leg and elytral punctation 277 Yemenaltica furthi Döberl, serrate apical spur of hind tibiae.
Maps of distribution. 278 Abrarius Fairmaire 279 Afroaltica Biondi & D’Alessandro 280 Afrocrepis Bechyné 281 Afrorestia Bechyné 282 Alocypha Weise 283 Altica Geoffroy.
Maps of distribution. 284 Amphimela Chapuis 285 Anaxerta Fairmaire 286 Angulaphthona Bechyné 287 Antanemora Bechyné 288 Aphthona Chevrolat 289 Argopistes Motschulsky.
Maps of distribution. 290 Argopistoides Jacoby 291 Bangalaltica Bechyné 292 Bechuana Scherer 293 Bechynella Biondi & D’Alessandro 294 Bezdekaltica Döberl 295 Bikasha Maulik.
Maps of distribution. 296 Biodontocnema Biondi 297 Blepharida Chevrolat 298 Carcharodis Weise 299 Celisaltica Biondi 300 Chabria Jacoby 301 Chaetocnema Stephens.
Maps of distribution. 302 Chaillucola Bechyné 303 Chirodica Germar 304 Collartaltica Bechyné 305 Decaria Weise 306 Diamphidia Gerstaecker 307 Dibolia Latreille.
Maps of distribution. 308 Dimonikaea Bechyné 309 Diphaulacosoma Jacoby 310 Djallonia Bechyné 311 Drakensbergianella Biondi & D’Alessandro 312 Dunbrodya Jacoby 313 Epitrix Foudras.
Maps of distribution. 314 Eriotica Harold 315 Eurylegna Weise 316 Eutornus Clark 317 Gabonia Jacoby 318 Guilielmia Weise 319 Guinerestia Scherer.
Maps of distribution. 320 Halticotropis Fairmaire 321 Hemipyxis Chevrolat 322 Hespera Weise 323 Hildebrandtina Weise 324 Homichloda Weise 325 Hyphasis Harold.
Maps of distribution. 326 Jacobyana Maulik 327 Kanonga Bechyné 328 Kenialtica Bechyné 329 Kimongona Bechyné 330 Lampedona Weise 331 Lepialtica Scherer.
Maps of distribution. 332 Longitarsus Berthold 333 Luperomorpha Weise 334 Lypnea Baly 335 Malvernia Jacoby. 336 Manobia Jacoby 337 Metroserrapha Bechyné.
Maps of distribution. 338 Montiaphthona Scherer 339 Myrcina Chapuis 340 Neodera Duvivier 341 Nisotra Baly. 342 Notomela Jacoby 343 Ntaolaltica Biondi & D’Alessandro.
Maps of distribution. 344 Nzerekorena Bechyné 345 Orthocrepis Weise 346 Paradibolia Baly 347 Perichilona Weise 348 Philopona Weise 349 Phygasia Chevrolat.
Maps of distribution. 350 Phyllotreta Chevrolat 351 Physodactyla Chapuis 352 Physoma Clark 353 Physomandroya Bechyné 354 Physonychis Clark 355 Podagrica Chevrolat.
Maps of distribution. 356 Polyclada Chevrolat 357 Pratima Maulik 358 Pseudadorium Fairmaire 359 Pseudophygasia Biondi & D’Alessandro, in press 360 Psylliodes Berthold 361 Sanckia Duvivier.
Maps of distribution. 362 Serraphula Jacoby 363 Sesquiphaera Bechyné 364 Seychellaltica Biondi 365 Sjostedtinia Weise 366 Sphaeroderma Stephens 367 Stegnaspea Baly.
Maps of distribution. 368 Stuckenbergiana Scherer 369 Terpnochlorus Fairmaire 370 Toxaria Weise 371 Trachytetra Sharp 372 Tritonaphthona Bechyné 373 Upembaltica Bechyné.
Maps of distribution. 374 Xanthophysca Fairmaire 375 Yemenaltica Scherer 376 Zomba Bryant.
Number of flea beetle genera occuring in the various areas of the Afrotropical region (CAF: Central Afrotropical; EAF: Eastern Afrotropical; MAD: Madagascar; MAS: Mascarene Islands; SAF: Southern Afrotropical; SEY: Seychelles Islands; WAF: Western Afrotropical.) (updated from
Number of flea beetle genera endemic to the various areas of the Afrotropical region (CAF: Central Afrotropical; EAF: Eastern Afrotropical; MAD: Madagascar; MAS: Mascarene Islands; SAF: Southern Afrotropical; SEY: Seychelles Islands; SSA: Sub-Saharan Africa; WAF: Western Afrotropical.) (updated from
Distribution of Afrotropical flea beetle genera in the different zoogeographical regions (AFR: Afrotropical; AUR: Australian; ORR: Oriental; NAR: Nearctic; NTR: Neotropical; PAR: Palaearctic) (updated from
Percentage of flea beetle genera co-occuring in the Afrotropical and another zoogeographical region (AFR: Afrotropical; AUR: Australian; ORR: Oriental; NAR: Nearctic; NTR: Neotropical; PAR: Palaearctic) (updated from
Faunistic similarities of the different areas of the Afrotropical region with those of other zoogeographical regions [Coincidence index and WPGMA clustering method (Weighted Pair Group Method using Arithmetic averaging) (cf.
We are very grateful to all the colleagues who enabled us to study the valuable material preserved in their respective institutions. A special thank you to Elizabeth Grobbelaar (SANC) and David G. Furth (National Museum of Natural History, Smithsonian Institution, Washington, USA) for his valuable suggestions and linguistic improvements, and to Fabrizia Urbani (University of L’Aquila, Italy) for the construction of the maps of distribution. This contribution was partially granted by the Italian PRIN 20085YJMTC (Research Programmes of National Interest).