(C) 2012 Samad Ashrafi. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Meloidoderita salina sp. n. is described and illustrated from the halophytic plant Atriplex portulacoides L. (sea purslane) growing in a micro-tidal salt marsh in the Mont-Saint-Michel Bay in France. This new species is the first member of Meloidoderita Poghossian, 1966 collected from a saline environment, and is characterized by the following features: sedentary mature females having a small swollen body with a clear posterior protuberance; slightly dorsally curved stylet, 19.9 µm long, with posteriorly sloping knobs; neck region irregular in shape and twisted; well developed secretory-excretory (S–E) pore, with markedly sclerotized S-E duct running posteriorly; prominent uterus bordered by a thick hyaline wall and filled with eggs. The adult female transforms into a cystoid. Eggs are deposited in both egg-mass and cystoid. Cystoids of Meloidoderita salina sp. n. display a unique sub-cuticular hexagonal beaded pattern.
Male without stylet, pharyngeal region degenerated, S-E duct prominent, deirids small, developed testis 97.5 µm long, spicules 18.4 µm long, cloacal opening ventrally protruded, small phasmids posterior to cloaca opening and situated at 5.9 (3.2–7.7) µm from tail end, and conical tail ending in a rounded terminus marked with one (rarely two) ventrally positioned mucro. Additionally, some young malesof the new species were observed enveloped in the last J2 cuticle. Second-stage juvenile body 470 µm long, with a 16.4 µm long stylet, prominent rounded knobs set off from the shaft, hemizonid anterior and adjacent to S-E pore, small deirids located just above S-E pore level, genital primordium located at 68–77% of body length, phasmids small and located at about 19 µm from tail tip, and tail 38.7 µm long, tapering to finely pointed terminus with a finger-like projection. Phylogenetic analyses based on the nearly full length small subunit ribosomal DNA sequences of Meloidoderita salina sp. n. revealed a close relationship of the new species with Sphaeronema alni Turkina & Chizhov, 1986 and placed these two species sister to the rest of Criconematina.
Atriplex portulacoides, cystoid, halophyte, hexagonal, morphology, morphometrics, nematode, new species, sea purslane, SEM, SSU rDNA, taxonomy
Since
Meloidoderita kirjanovae has been recorded parasitizing on Mentha spp. (mint and water mint) and Utrica dioica L. (common nettle) (
The second species of Meloidoderita, Meloidoderita safrica, was described by
During a nematode survey conducted in Mont-Saint-Michel Bay in France, a Meloidoderita population was isolated from soil and roots of the halophyte Atriplex (= Halimione) portulacoides (L.) Aellen. This nematode was infecting roots of sea purslane (Atriplex portulacoides) growing in a muddy soil salt marsh region. Preliminary morphological and molecular analyses (G. Karssen, unpublished) indicated that the population differed from all three known described species of Meloidoderita and represented a new species. This was the first Meloidoderita species collected from a salt marsh environment.
The main objectives of the present study were to:i) describe a new species of Meloidoderita isolated from soil and roots samples of Atriplex portulacoides from a salt marsh region in France and provide a detailed morphological description based on LM and SEM; ii) characterize Meloidoderita species by means of small subunit rDNA sequencing; iii) determine the phylogenetic position of Meloidoderita within the suborder Criconematina.
Soil and root samples were isolated from Atriplex portulacoides grown in muddy soil of a costal tidal salt marsh environment in “Le Vivier- sur- Mer” at 48°36'32"N and 1°47'00"W at Mont-Saint-Michel Bay in France.
The Mont-Saint-Michel Bay (MSMB) is a costal embayment and macro-tidal environment located on the English Channel (Southern gulf of Normandy) between the Cotentin Peninsula and the Brittany coast, in the northwestern coast of France (
The Mont-Saint-Michel Bay is a specific ecosystem on a small geographic scale. Despite the presence of numerous ecological studies that have been applied since 1979 in MSMB, nematodes have been mostly neglected (
To obtain a homogenized sample of the cohesive muddy soil, we gently mixed samples in a kneading machine for 15 min. Afterwards, nematodes including juveniles, males, cystoids, and eggs, were extracted from soil samples by means of a magnesium sulphate centrifugal flotation technique (
Females were collected with two different methods: i) centrifugal flotation method (
The Meloidoderita populations and a Sphaeronema Raski & Sher, 1952 population used for comparison are listed in Table 1.
Host and origin of the populations of three Meloidoderita species and one Sphaeronema species which were compared with the population of Meloidoderita salina sp. n.
| Species | Host | Origin |
|---|---|---|
| Meloidoderita kirjanovae (Poghossian, 1966) Kirjanova & Poghossian (1973) | Mentha longifolia (L.) Huds. | Megri region, Armenia |
|
Meloidoderita kirjanovae characterized by |
Mentha longifolia | Mediterranean region |
|
Meloidoderita kirjanovae characterized by |
Mentha longifolia | Armenia |
|
Meloidoderita kirjanovae characterized by |
Mentha aquatic L. | Laceno Lake at Avellino, southern Italy |
| Meloidoderita safrica Van den Berg & Spaull, 1982 | Saccharum hybrid (Sugar cane) | Mposa area of Natal, South Africa |
| Meloidoderita polygoni Golden & Handoo, 1984 | Polygonum hydropiperoides Michx. | Beltsville, Maryland, USA |
| Sphaeronema alni Turkina & Chizhov, 1986 (topotype population) | Alnus incana (L.) Moench, A. glutinosa L., Betula pubescens Ehrh. | Russia |
Specimens for light microscopy (LM) were fixed in heated (70°C) TAF (2 ml triethanolamine, 7ml formaldehyde and 91 ml distilled water (
Measurements and drawings were performed on a light microscope Olympus BH-2 equipped with Nomarski Differential Interference Contrast (DIC).
Specimens were drawn with a drawing tube, scanned and modified using Photoshop software version CS 5.1.
Light micrographs of specimens were taken with a Leica DC 300 F camera attached to a Zeiss Axio Imager M1 microscope. The original descriptions of closely related species (Table 1) were used for morphological and morphometrical comparison.
For SEM observation nematodes were fixed in 3% glutaraldehyde buffered with 0.05M phosphate buffer (pH 6.8) for 1.5 h and post-fixed with 2% osmium tetroxide for 2h at 22°C. The specimens were dehydrated in a seven-graded ethanol series of 15-25-35-50-70-95 and 100% (
Single nematodes (five individuals in total) were transferred to a 0.2 ml Eppendorf vial containing 25 µl of sterile water. An equal volume of lysis buffer containing 0.2 M NaCl, 0.2 M Tris-HCl (pH 8.0), 1% (vol/vol) β-mercaptoethanol, and 800 µg/ml of proteinase K was added. Lysis took place in a Thermomixer (Eppendorf, Hamburg, Germany) at 65°C and 750 rpm for 2 h followed by a 5 min incubation at 100°C (to inactive proteinase). Lysate was immediately used or stored at –20°C. SSU rDNA was amplified as two partially overlapping fragments using three universal and one nematode-specific primer (1912R). The latter was included to avoid amplification of non-target eukaryotic SSU rDNA. For the first fragments, either the primer 988F (5'-ctc aaa gat taa gcc atg c-3') or the primer 1096F (5'-ggt aat tct gga gct aat ac-3') was used in combination with the primer 1912R (5'-ttt acg gtc aga act agg g-3'). The second fragment was amplified with primers 1813F (5'-ctg cgt gag agg tga aat-3') and 2646R (5' -gct acc ttg tta cga ctt tt-3'). PCR was performed in a final volume of 25 µl containing 3 µl of 100 times-diluted crude DNA extract, 0.1 µM of each PCR primer and a ready-To-Go PCR bead (GE Healthcare, Little Chalfont, UK). The following PCR program was used: 94°C for 5 min; 5× (94°C, 30 s; 45°C, 30 s; 72°C, 70 s) followed by 35× (94°C, 30 s; 54°C, 30 s; 72°C, 70 s), and 72°C for 5 min. Gel-purified amplification products (Marligen, Ijamsville, MD) were cloned into a TOPO-TA vector (Invitrogen, Carlsbad, CA) and sent off for sequencing using standard procedures (
SSU rDNA-obtained sequences were aligned using the ClustalW algorithm as implemented in the program BioEdit 7.0.1 (
The phylogenetic tree was constructed using Bayesian inference (MrBayes 3.1.2 (
The second phylogenetic tree was constructed with a fast maximum likelihood method. The SSU rDNA alignment was analysed at a distant server (CIPRES, http://www.phylo.org) running the program, RAxML-VI-HPC v.4.0.0 using the same GTR model. One hundred bootstrap replicates were performed.
urn:lsid:zoobank.org:act:02A22EB6-85D4-4783-98AB-A6FA894EEAAD
http://species-id.net/wiki/Meloidoderita_salina
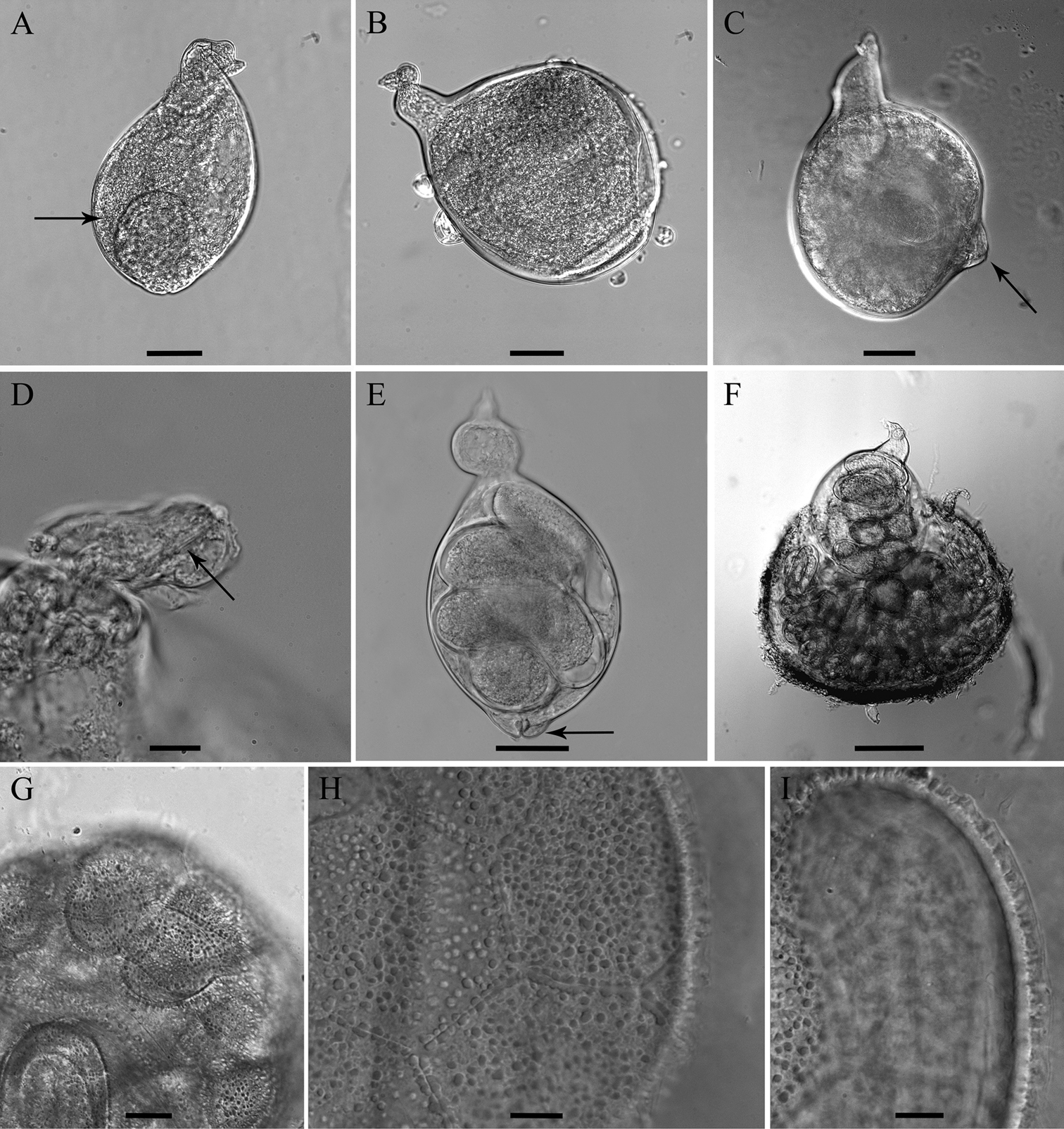
Figs 1–8; Table 2Females, males and second-stage juveniles: See Table 2. Embryonated eggs (n= 44): Length: 102.5 ± 5.0 (94.4–112) µm; diam.: 41.7 ± 1.9 (38.4–46.4) µm; length/width ratio: 2.5 ± 0.2 (2.1–2.9). Cystoids (n=18): Length: 224 ± 34.5 (176–336) µm; Width: 187.5 ± 33.1 (145.6–280) µm; length/width ratio: 1.2 ± 0.1 (1.0–1.7).
Female. Body swollen with a small posterior protuberance, pearly white to light brown, oval to pear-shaped. Neck region distinct, irregular shaped, usually twisted, 49 to 82 µm in length (Figs 2, 8). Body cuticle thick, without annulation. Head continuous with body, without annules. Cephalic framework weakly developed, lip region flattened. Stylet well developed, with posteriorly sloping oval-shaped knobs; stylet cone longer than shaft, slightly curved dorsally, shaft cylindrical (Fig. 2C). Dorsal gland orifice (DGO) close to basal knobs; vestibule extension visible. Secretory-excretory (S-E) pore well developed with clear cuticular lobes, located posterior to the neck, about 35 (20–56)% from anterior end of body; S-E duct markedly sclerotized, running posteriorly. Pharyngeal lumen from stylet to valve of metacorpus prominent. Metacorpus usually oval-shaped, situated at the posterior part of neck region, with distinct sclerotized valve apparatus, distance from middle of metacorpus to anterior end about 58 ± 10 µm long. Posterior gland bulb extending into anterior portion of swollen body cavity. Reproductive system extending towards pharyngeal region, monodelphic, spermatheca not observed; vulva with noticeable protruding lips, positioned usually at the posterior extremity of the body, rarely subterminal. Vulval lips forming thickened and muscular area around vulval slit (vulval area). Anus faint, opening pore-like, difficult to observe by LM, located at the base of dorsal vulval lip, apparently not functional (Figs 5E, 8C). Uterus swollen, prominent, bordered by a thick hyaline wall, becoming enlarged and filled with eggs, transforming into a cystoid within the female cuticle.
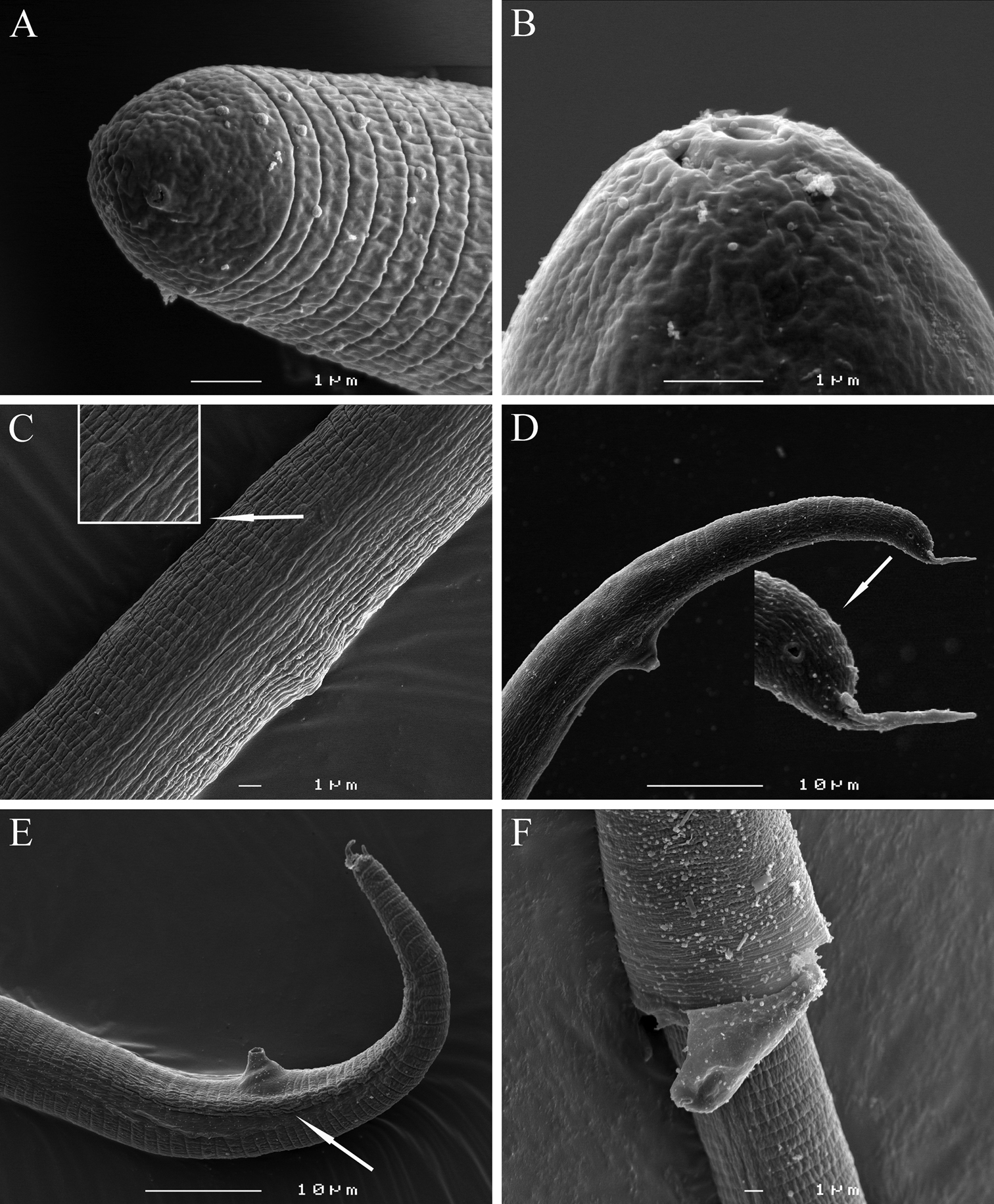
Male.Body slender, vermiform, tapering at both ends but more posteriorly, usually slightly curved ventrally at tail region. Cuticle marked by fine annulations, about 0.9 µm wide. Young males usually still enveloped in the last cuticle of second-stage juveniles (Fig. 4D). Lateral field beginning with 2 weak lines, roughly between head end and S-E pore level, and continuing with four weak lines behind S-E pore level. Head continuous with body, rounded-conoid, without annules and separated lips, distinct but weak cephalic framework present; amphidial apertures slit-like, angled, adjacent to oral opening surrounded by a small elevated oral disc (Fig. 7B). Pharyngeal region degenerated except for the posterior bulb, no stylet observed. S-E pore well developed, adjacent to hemizonid. S-E duct strongly sclerotized anteriorly (Fig. 4E). Deirids small, located just above S-E pore level (Fig. 7C). Monorchic, outstretched, testis well developed, with small vas deferens about6 µm long. Spicules paired, equal, not fused, arcuate, with rounded manubrium. Gubernaculum slightly curved. Cloacal tube about 2 µm long. Bursa-like structure visible by SEM (Fig. 7E). Phasmids small, posterior to cloacal opening. Tail conical, tapering to rounded terminus, marked with one or rarely two mucrones; if two are present, ventral mucro usually smaller; terminal mucro positioned ventrally, length 0.6‒3.2 µm (Fig. 1K–N).
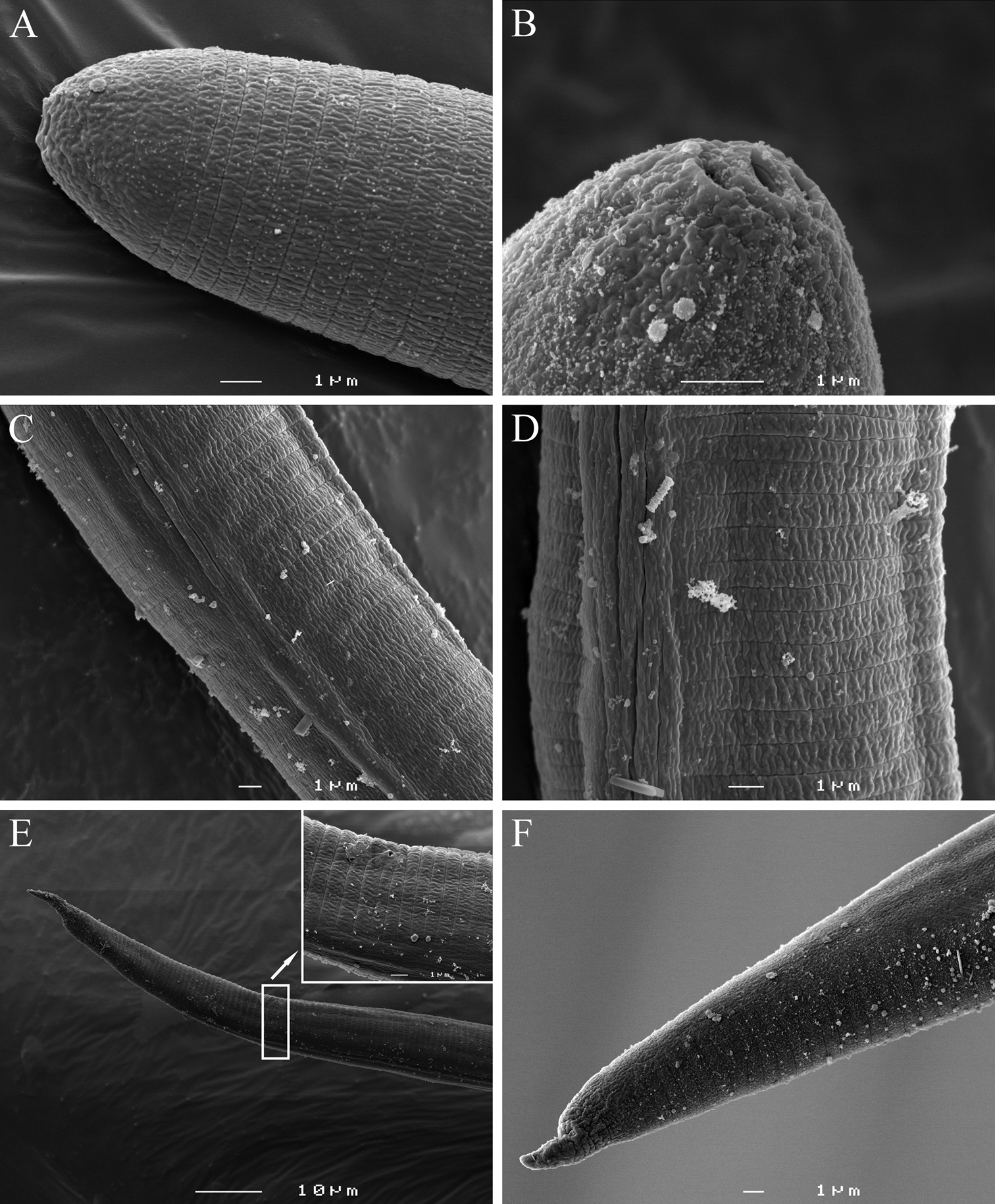
Second-stage juvenile. Body slender, vermiform, tapering at both ends but more so posteriorly, slightly ventrally curved at tail region; cuticle with fine annulations, annules about 1 µm wide. Lateral field with two visible outer lines in some specimens; in SEM, lateral field starts with three lines about 30 µm from head at neck region, four lines at 20%, and five lines at 33% of body length. Head continuous with body, rounded-conoid with slightly elevated concave oral disc, with distinct but relatively weak cephalic framework, without annules; two open slit-like amphidial apertures adjacent to slightly elevated concave oral disc surrounding the oral aperture, as visible by SEM (Fig. 6A). Lips not visible as distinct structures. Stylet well developed; cone tapering towards fine point; shaft straight; knobs rounded, prominent, sloping slightly posteriorly, set off from shaft (Fig. 1D). DGO close to stylet base. Metacorpus slightly elongated, with weak valves. S-E pore posterior and adjacent to hemizonid, located at isthmus level; hemizonid 2–3 annules long (Fig. 3D). Isthmus slender, distinct. Pharyngeal glands slightly overlapping intestine ventrolaterally. Deirids small, located just above S-E pore level. Genital primordium located posteriorly at 68‒77% of body length. Anus small, weakly developed, obscure by LM, pore-like (Fig. 6E). Phasmids small, difficult to observe by LM, located at about 19 µm from tail tip. Tail conical, slightly curved ventrally, tapering to finely pointed terminus, with finger-like projection. Hyaline tail part clearly delimitated anteriorly (Fig. 3G–I).
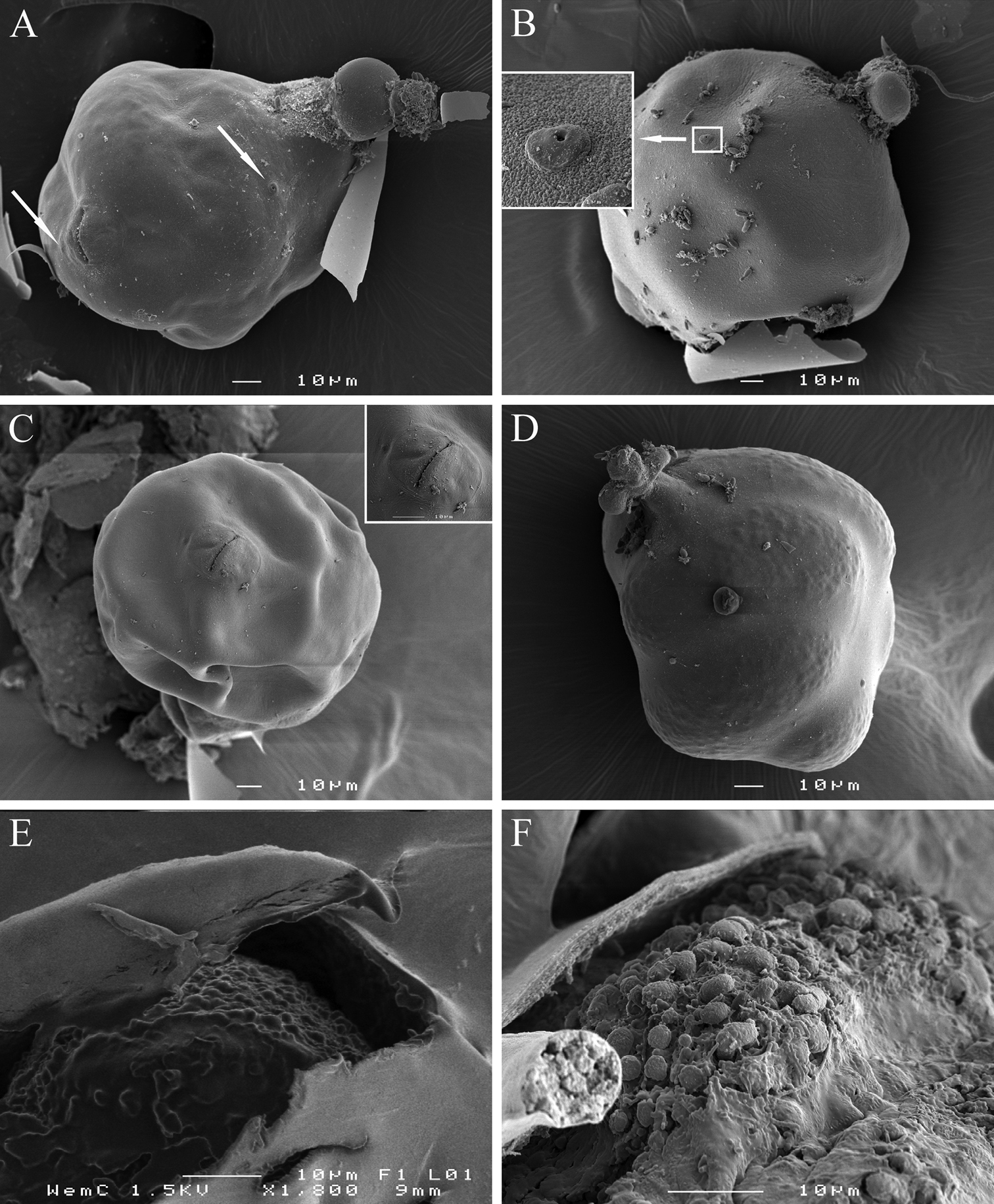
Cystoid. Irregularly spherical to oval, filled with embryonated and non-embryonated eggs. Colour ranging from light in young cystoids to brown in older cystoid bodies. Body wall thickness 5.3 ± 1.2 (3.2–8.3) µm, containing bead-like outgrowths, displaying a specific sub-cuticular hexagonal beaded pattern (Figs 5, 8).
Egg mass.Females and cystoids usually completely surrounded by a gelatinous matrix (egg-mass) measuring about 316 ± 71.0 µm in length and 275 ± 54.0 µm in diameter (Fig. 5F).
Eggs. Oblong, translucent, egg shell without any visible markings, enveloped in a gelatinous matrix or within a cystoid.
Collected from rhizosphere and roots of the salt marsh halophytic shrub Atriplex portulacoides L. (= Halimione portulacoides (L.) Aell.), the most abundant species in ungrazed European salt marshes (
Morphometrics of Meloidoderita salina sp. n. All measurements are in µm and in the form: mean ± SD (range).
| Character | Female | Male Paratypes | J2 Paratypes | |
|---|---|---|---|---|
| Holotype | Paratypes | |||
| n | - | 43 | 21 | 27 |
| L | 286 | 260 ± 34 (186–358) | 469 ± 28 (416–522) | 471 ± 19 (419–496) |
| a | 1.8 | 1.3 ± 0.2 (0.9–1.8) | 40.0 ± 2.8 (35.0–45.0) | 30.4 ± 1.1 (28.2–32.5) |
| b | - | - | 4.1 ± 0.4 (3.3–4.8) | 3.7 ± 0.2 (3.4–4.3) |
| c | - | - | 12.9 ± 1.4 (11.1–15.9) | 12.2 ± 0.9 (9.9–13.9) |
| c´ | - | - | 3.9 ± 0.5 (2.5–4.6) | 4.2 ± 0.2 (4.0–4.3) |
| Greatest body diam. | 152 | 206 ± 37 (126–320) | 11.8 ± 0.8 (10.9–13.4) | 15.5 ± 0.5 (14.1–16.0) |
| Body diam. At excretory pore | - | - | 10.4 ± 1.1 (7.7–12.8) | 14.4 ± 0.5 (13.4–15.4) |
| Body diam. at anus or cloacal opening | - | - | 9.6 ± 0.9 (7.0–10.9) | 9.2 ± 0.6 (8.3–10.9) |
| Head region height | - | - | 2.2 ± 0.3 (1.9–2.6) | 4.0 ± 0.2 (3.8–4.5) |
| Head region diam. | - | - | 3.7 ± 0.4 (3.2–4.5) | 7.0 ± 0.4 (6.4–7.7) |
| Stylet length | 19.2 | 19.9 ± 0.7 (19.0–22.0) | - | 16.4 ± 0.5 (14.7–17.3) |
| Stylet cone | 12 | 11.6 ± 0.6 (10.5–12.8) | - | - |
| Stylet shaft | - | - | - | 5.1 ± 0.3 (4.5–5.8) |
| Stylet knob height | 2.6 | 3.0 ± 0.4 (2.6–4.0) | - | 2.6 ± 0.2 (1.9–3.2) |
| Stylet knob width | 3.2 | 3.7 ± 0.5 (3.2–5.0) | - | 3.7 ± 0.2 (3.2–3.8) |
| Ant. end to knobs base | - | - | - | 18.4 ± 0.4 (17.3–19.2) |
| DGO | 3.2 | 3.3 ± 0.5 (2.5–4.0) | - | 2.4 ± 0.4 (1.9–3.2) |
| Ant. end to metacorpus | 42.9 | - | - | 65 ± 1.2 (63–67) |
| Metacorpus valve length | 16.0 | 15.8 ± 0.9 (15.0–17.9) | - | - |
| Metacorpus valve width | 8.9 | 8.5 ± 0.8 (7.7–10.0) | - | - |
| Pharynx length | - | - | 115 ± 13 (90–138) | 126 ± 7 (111–144) |
| Ant. end to excretory pore | 74 | 92 ± 22.1 (55–125) | 82 ± 5.5 (74–96) | 87 ± 3.0 (77–93) |
| Ant. end to genital primordium | - | - | - | 340 ± 20 (305–371) |
| Genital promordium to posterior end | - | - | - | 131 ± 12 (105–150) |
| Genital primordium length | - | - | - | 13.0 ± 1.3 (9.6–15.4) |
| Genital primordium width | - | - | - | 6.8 ± 1.0 (4.5–9.0) |
| Tail length | - | - | 36.6 ± 3.8 (27.5–41.6) | 38.7 ± 2.5 (33.9–44.2) |
| Hyaline tail terminus | - | - | - | 8.1 ±1.0 (6.4–9.6) |
| Phasmid to posterior end | - | - | 5.9 ± 1.5 (3.2–7.7) | - |
| Spicule length | - | - | 18.4 ± 1.8 (15.4–21.1) | - |
| Gubernaculum length | - | - | 5.3 ± 0.5 (4.5- 6.4) | - |
| Testis | - | - | 98 ± 21.9 (62- 137) | - |
| Vulva slit length | 20.4 | 19.5 ± 1.4 (16.0–22.5) | - | - |
| Vulva-anus | 16.0 | 17.3 ± 2.6 (13.4–23.0) | - | - |
| Vulva area length | - | 41.0 ± 4.9 (32.0–54) | - | - |
| Vulva area diam. | - | 32.4 ± 3.7 (25.6–40.0) | - | - |
| Cuticle thickness | 3.2 | 5.0 ± 1.4 (2.5–7.7) | - | - |
| (Excretory pore/L)*100 | - | - | 17.5 ± 0.8 (16.2–18.9) | 18.6 ± 0.8 (17.1–20.6) |
| Genital primordium % of body length | - | - | - | 72.1 ± 2.6 (68.2–77.2) |
| Hyaline % of tail length | - | - | - | 21.0 ± 3.0 (15.1–26.3) |
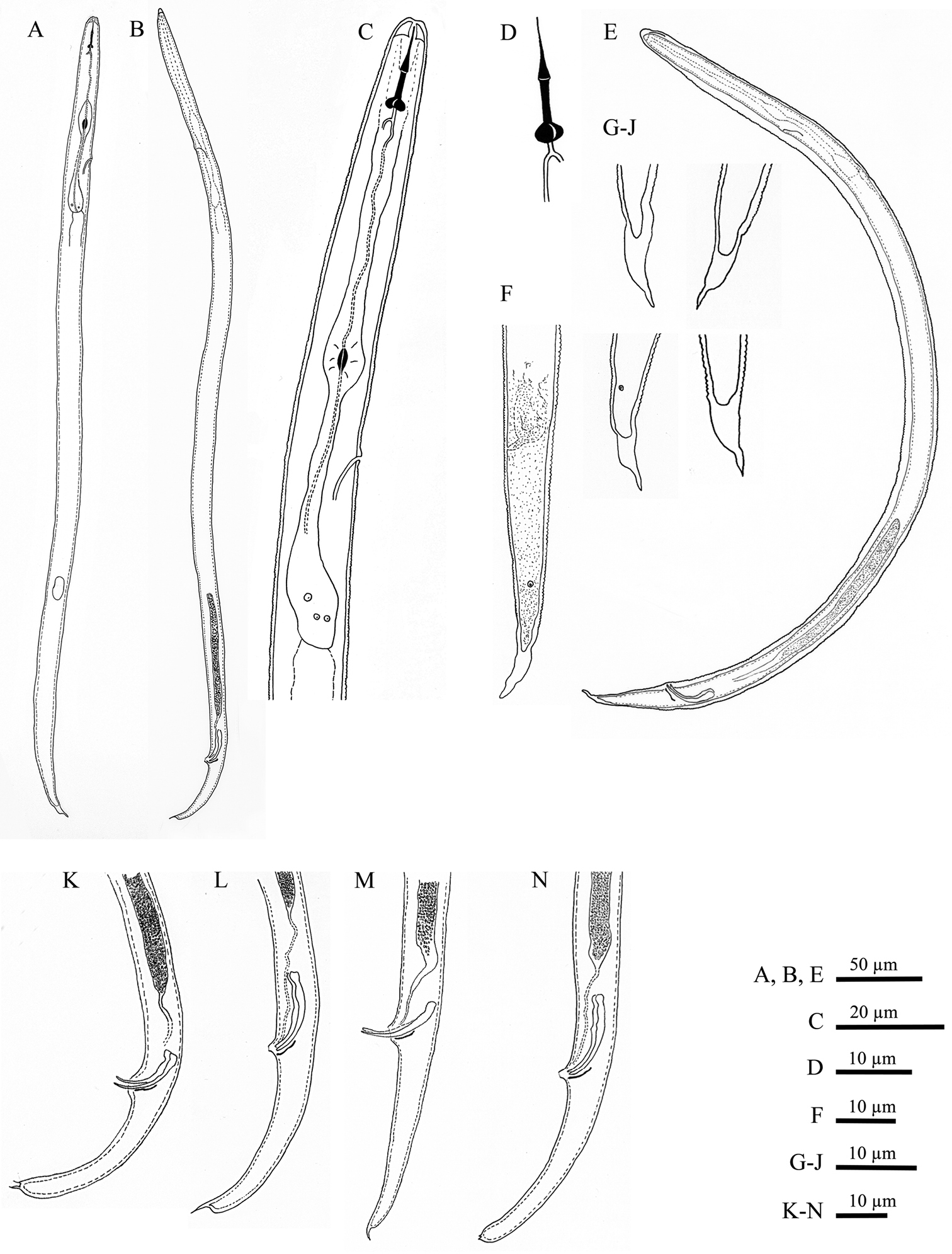
Meloidoderita salina sp. n. A Second-stage juvenile (J2) B Male C J2 anterior region D J2 stylet E Male within old J2 cuticle F J2 posterior region G–J J2 Tail tip K–N Male posterior region.
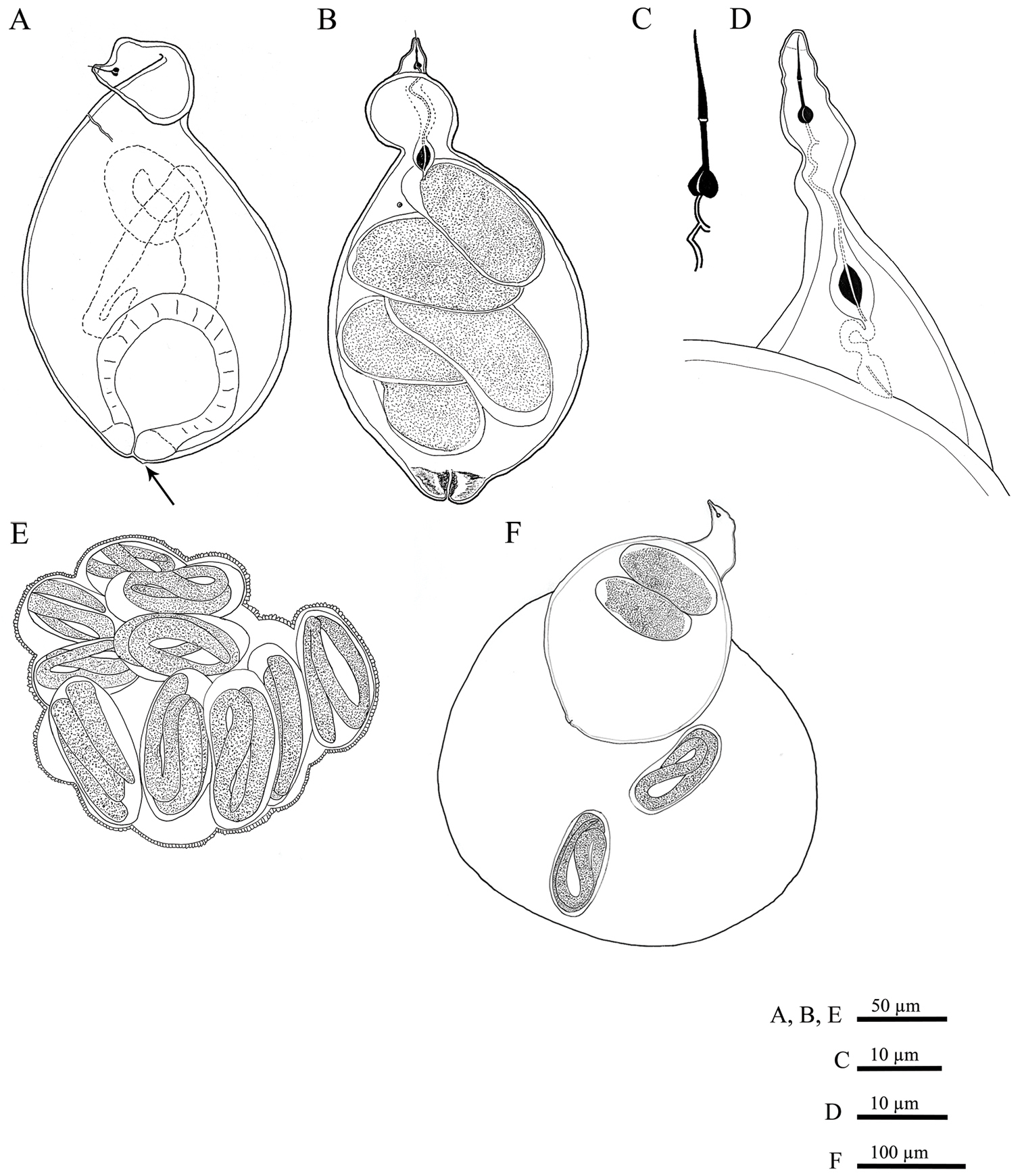
Meloidoderita salina sp. n. A, B Female body (arrow = anus) C Female stylet D Female neck region E Cystoid F Female with egg-mass.
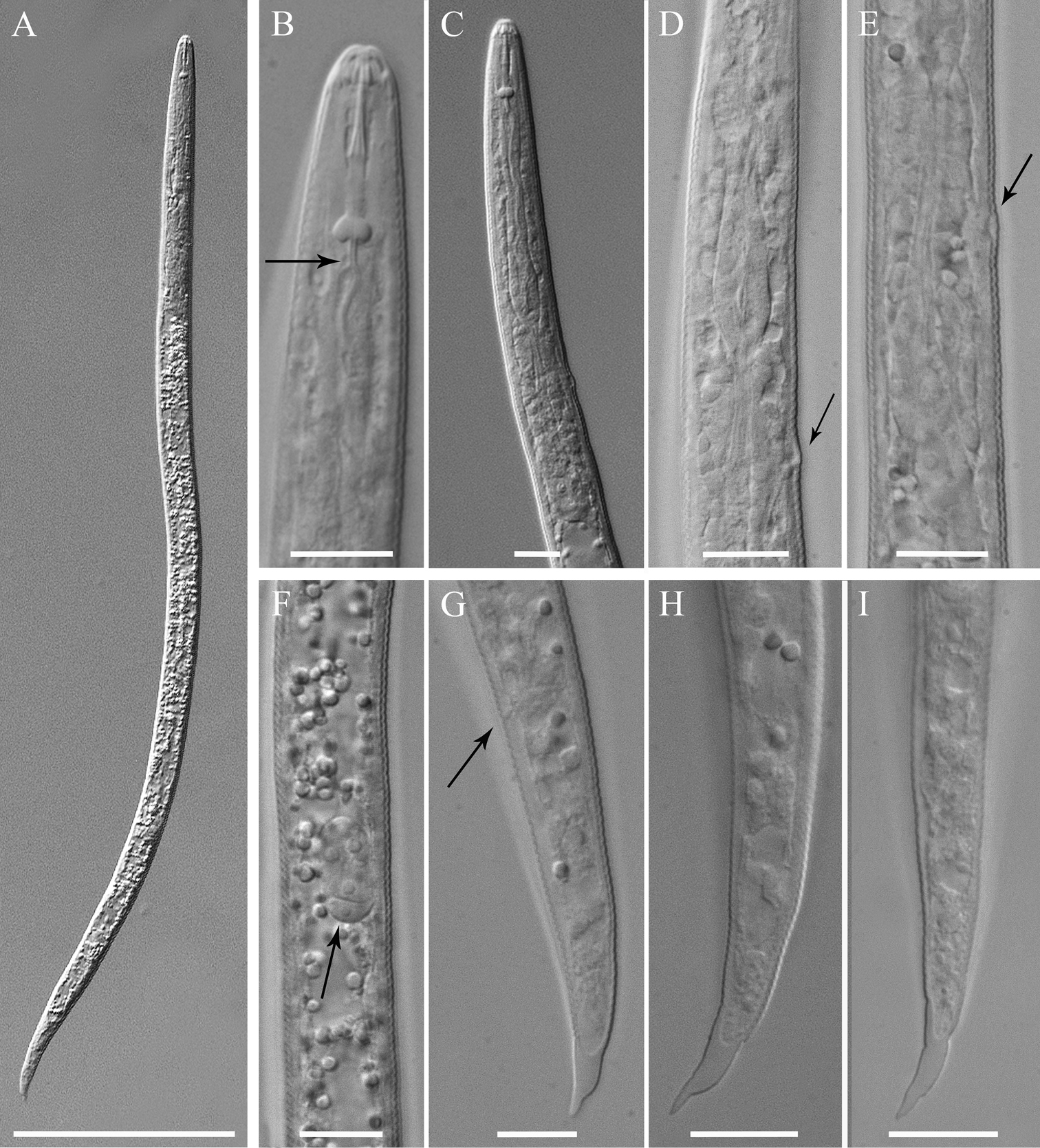
Meloidoderita salina sp. n. LM photographs of second-stage juveniles. A Entire body B, C Anterior body (arrow =DGO) D S-E duct adjacent to hemizonid (arrow = S-E duct) E Basal bulb (arrow = hemizonid) F Mid-body portion (arrow = primordium) G-I Tail (arrow = anus). Scale bars: A =100 µm B–I = 10 µm.
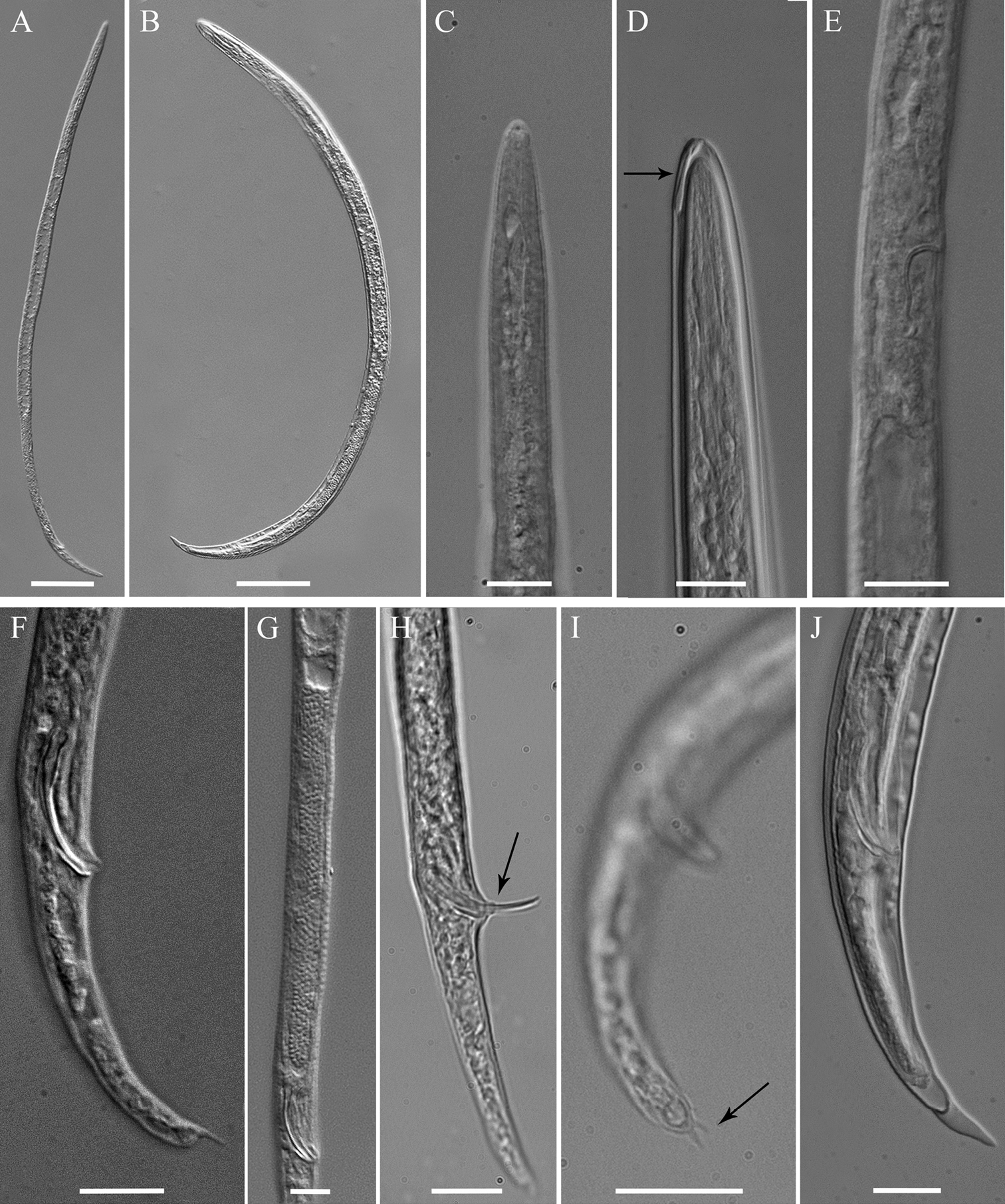
Meloidoderita salina sp. n. LM photographs of males. A Entire body B Male within the second-stage juvenile (J2) cuticle C Anterior body D Anterior body of male within the old cuticle of J2 (arrow = anterior portion of J2 stylet) E S-E duct F Posterior region G Testis H Spicule and cloacal tube (arrow) I Tail tip (arrow = mucron) J Posterior end of male within the old cuticle of J2. Scale bars: A, B = 50 µm C–J = 10 µm.
Meloidoderita salina sp. n. LM photographs of females. A, B Entire body (arrow = uterus) C Sub-terminal protruded vulva (arrow) D Head region (arrow = stylet) E Entire body (arrow = vulva) F Female surrounded by egg-mass G Cystoid H, I Hexagonal beaded pattern. Scale bars: F= 100 µm A–C, E = 50 µm D, G–I = 10 µm.
Holotype female (slide WT 3591) and paratypes (second-stage juveniles, females, cystoids and males) (slides WT 3592-WT 3595) deposited in the Wageningen Nematode Collection (WaNeCo), Wageningen, The Netherlands. Additional second-stage juvenile, female, cystoid and male paratypes deposited at each of the following collections: Biology Department, Gent University, Gent, Belgium; Central Science Laboratory (CSL), Sand Hutton, York, UK.
The specific epithet refers to salty soil (saline environment) and is derived from the Latin word sal or salis meaning “salt”.
Meloidoderita salina sp. n. is characterized by sedentary mature females having a small swollen body with a clear posterior protuberance, stylet 19.9 (19–22) µm long, stylet cone slightly curved dorsally and longer than shaft, with posteriorly sloping knobs, neck region irregular in shape and twisted, well developed S-E pore, prominent uterus bordered by a thick hyaline wall and filled with eggs. Meloidoderita salina sp. n. is further distinguished by the cystoid having a unique sub-cuticular hexagonal beaded pattern.
Male without stylet, pharyngeal region degenerated, S-E duct prominent, spicules 18.4 (15.3–21.1) µm long, deirids just above S-E pore level, small phasmids posteriorly to cloaca opening and situated at 5.9 (3.2–7.7) µm from tail end, conical tail ending in a rounded terminus with one (rarely two) ventrally positioned mucro.
Second-stage juvenile body is 470 (419–496) µm long, with a 16.4 (14.7–17.3) µm long developed stylet, prominent rounded knobs set off from the shaft, hemizonid anterior and adjacent to S-E pore, tail 38.7 (33.9–44.2) µm long tapering to a finely pointed terminus with a finger-like projection.
On the basis of morphology, the female of Meloidoderita salina sp. n. resembles other species of the genus (Meloidoderita kirjanovae, Meloidoderita safrica and Meloidoderita polygoni) in the shape of the neck region (twisted, irregular and variable in size), the shape of the vulva (protruded), and the shape of the uterus (prominent, with large cells and a thick wall). Males of the four species are similar in lack of a stylet, degenerated pharyngeal region, the shape of the spicules (arcuate), the shape of the cloacal opening (ventrally protruded), and the shape of the tail (slightly curved ventrally, ending in a terminal mucro). Second-stage juveniles have a continuous head region, weakly sclerotized cephalic framework, similar shape of the tail (conically tapering to a pointed terminus, often with a finger-like terminal mucro), obscure anus, and position of hemizonid (anterior and adjacent to S-E pore).
Meloidoderita salina sp. n. differs from the previously described species by a smaller female body, a longer J2 body, the male with a longer body length and (except Meloidoderita kirjanovae described by
The new speciesdiffers in other characters from Meloidoderita kirjanovae by females having a longer stylet length and a much shorter distance from anus to vulval slit. Male differs from those characterized by
Meloidoderita salina sp. n. differs from Meloidoderita safrica by the female having DGO closer to base of stylet (2.5–4.0 vs 8.1–22.1µm), shorter distance from vulval slit to anus (13.4–23.0 vs 22.4–24.3 µm), by the male having a shorter testis (62–137 vs 190–319 µm), and by the J2 having a longer distance from anterior end to base of pharynx (111–144 vs 51.8–75.4 µm).
It differs from Meloidoderita polygoni females having a longer stylet (19.0–22.0 vs 15.0–17.4 µm), shorter distance from vulval slit to the anus (13.4–23.0 vs 32.0–86 µm), and a shorter vulval slit (16.0–22.5 vs 22.0–34.0 µm), and by the male without stylet vs visible anterior stylet part, a shorter tail (27.5–41.6 vs 32.0–56).
The new species is morphologically close related to the genus Sphaeronema, particularly to Sphaeronema alni Turkina & Chizhov, 1986. According to their observed phylogenetic relationships, they form together a highly supported clade. The absence of a cystoid stage in Sphaeronema is the most import differences compared to Meloidoderita.Additionally Meloidoderita salina sp. n. differs from Sphaeronema alni by females having a head region continuous with body vs head cap set off from neck and the lip region lacking annulations vs 2 annuli. The second-stage juveniles has a tail conically tapering to a pointed terminus, often with a finger-like projection, whereas in Sphaeronema alni the tail tapers gradually to a finely rounded terminus.
Meloidoderita salina sp. n. SEM photographs of second-stage juveniles. A Lateral view of head region B Amphids C Lateral field at 30 µm from anterior end D Lateral field at 33% of body length E Posterior region (arrow = anus) F Lateral view of tail region.
Meloidoderita salina sp. n. SEM photographs ofmale. A, B Head region C Lateral field at S-E pore level (arrow = deirid) D Lateral view of tail region(arrow = phasmid) E Tail region (arrow = bursa-like structure) F Young male within the second-stage juvenile’s old cuticle.
Meloidoderita salina sp. n.SEM photographs of female and cystoid. A Female body (arrows = S-E pore, anus) B Female body (arrow = S-E pore surrounded by cuticular lobes) C Vulva and anus D Young cystoid with irregular shaped neck region and surface displaying a beaded pattern E Sub-cuticular beaded pattern F Detail of surface markings in cystoid.
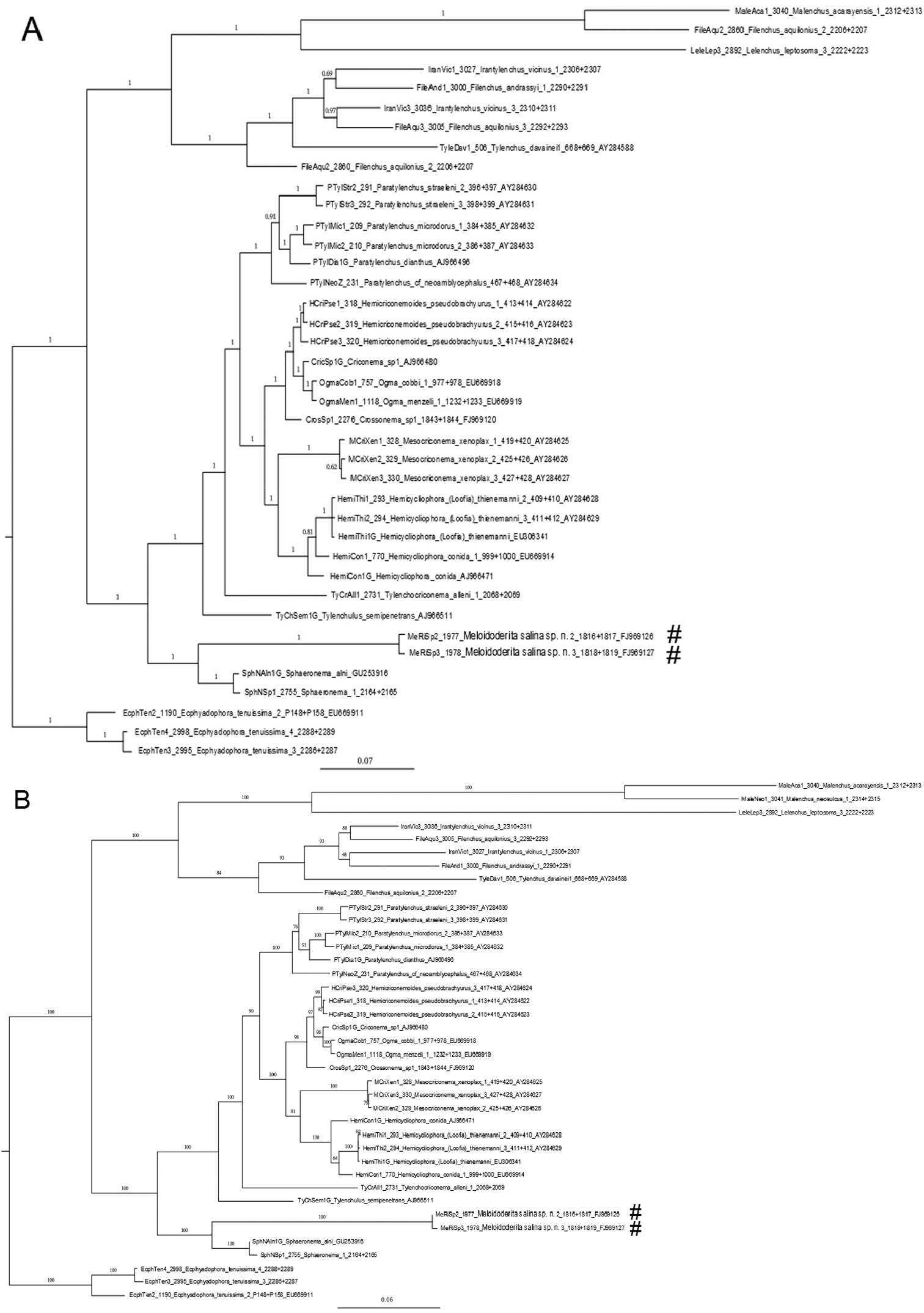
The nearly complete rDNA sequence length of SSU rDNA obtained for Meloidoderita salina sp. n. (GenBank FJ969126 and FJ969127) both spanned1728 bp. A local alignment (1883 aligned position) included 39 nearly full length SSU rDNA sequences from related taxa and representatives of the genus Ecphyadophora were selected as outgroup. The SSU rDNA sequence analysis and the gene tree represented by the Bayesian and RAxML trees (Fig. 9) revealed a robust sister relationship between the new species and Sphaeronema alni within the Criconematina, and the two combined were positioned at the basal part of the local tree. The phylogenetic position of the suborder Criconematina has been analyzed several times (
Phylogenetic relationships as inferred from nearly full length of SSU rDNA sequence using GTR + I + G model. Dataset obtained sequences were aligned with the ClustalW algorithm. Numbers near the nodes indicate posterior probabilities in the Bayesian tree (A) and ML tree (B) as implemented in the program BioEdit 7.0.1. Newly generated SSU rDNA sequences are labeled with a (#).
Meloidoderita salina sp. n. was described from a salt marsh area at Mont-Saint-Michel Bay in France, parasitizing the halophyte plant Atriplex portulacoides. On average, this area has a salinity of about 34–35g/L which usually increases after submersion by the tides. The presence of a well sclerotized S-E duct is a noticeable character, especially in adult males and matured females of Meloidoderita salina sp. n. which could be correlated with their saline environment and their halophytic host plant. The presence of a strongly sclerotized S-E duct has been also reported in the genus Halenchus N.A. Cobb in M.N. Cobb, 1933 as the only known marine Tylenchomorpha. The genus Halenchus with three species is exclusively marine parasitic nematode which produces galls on sea algae (
Cohen and Mordechai (1982), while studying the biology of Meloidoderita kirjanovae, observed several males attached to or enveloped by old second-stage juveniles cuticle. They reported that it “could obviously be identified as offspring of the particular female beneath the egg-mass, rather than having migrated from outside. Furthermore, often more than one molting cuticle was present at the same time, indicating that development of juveniles into adult males was a relatively short process and apparently did not necessitate feeding on the host tissues”. These enveloped males in second-stage juveniles cuticle have been reported by
In the classification scheme proposed by
Recently
Phylogenetic studies done by
Based on the distribution of the type host Atriplex portulacoides in tidal salt marshes in France, it may be expected that Meloidoderita salina sp. n. is more widely distributed in West-European salt marshes.
Human consumption is currently one of the most important aspects for cultivation of Atriplex spp. It has a salty taste when it is eaten raw or cooked, and is presently served in luxury restaurants. Atriplex portulacoides has an important role in primary production, and in the food web in salt marsh ecosystems (
It is interesting to report that during this study we found a unique sub-cuticular hexagonal beaded pattern in the cystoids of Meloidoderita salina sp. n. This specific pattern can be seen on the surface of the cystoid and displays symmetrical hexagons (Figs 5H, I, 8D–F). This pattern reported in this study is probably the first to be observed among all the identified species of nematodes so far.
Authors express their thanks to ing. Paul Mooyman (WU, NL) for his help with the phylogenetic analysis.