(C) 2013 Vikas Suman. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Suman V, Kaur H (2013) First report on C-banding, fluorochrome staining and NOR location in holocentric chromosomes of Elasmolomus (Aphanus) sordidus Fabricius, 1787 (Heteroptera, Rhyparochromidae). In: Popov A, Grozeva S, Simov N, Tasheva E (Eds) Advances in Hemipterology. ZooKeys 319: 283–291. doi: 10.3897/zookeys.319.4265
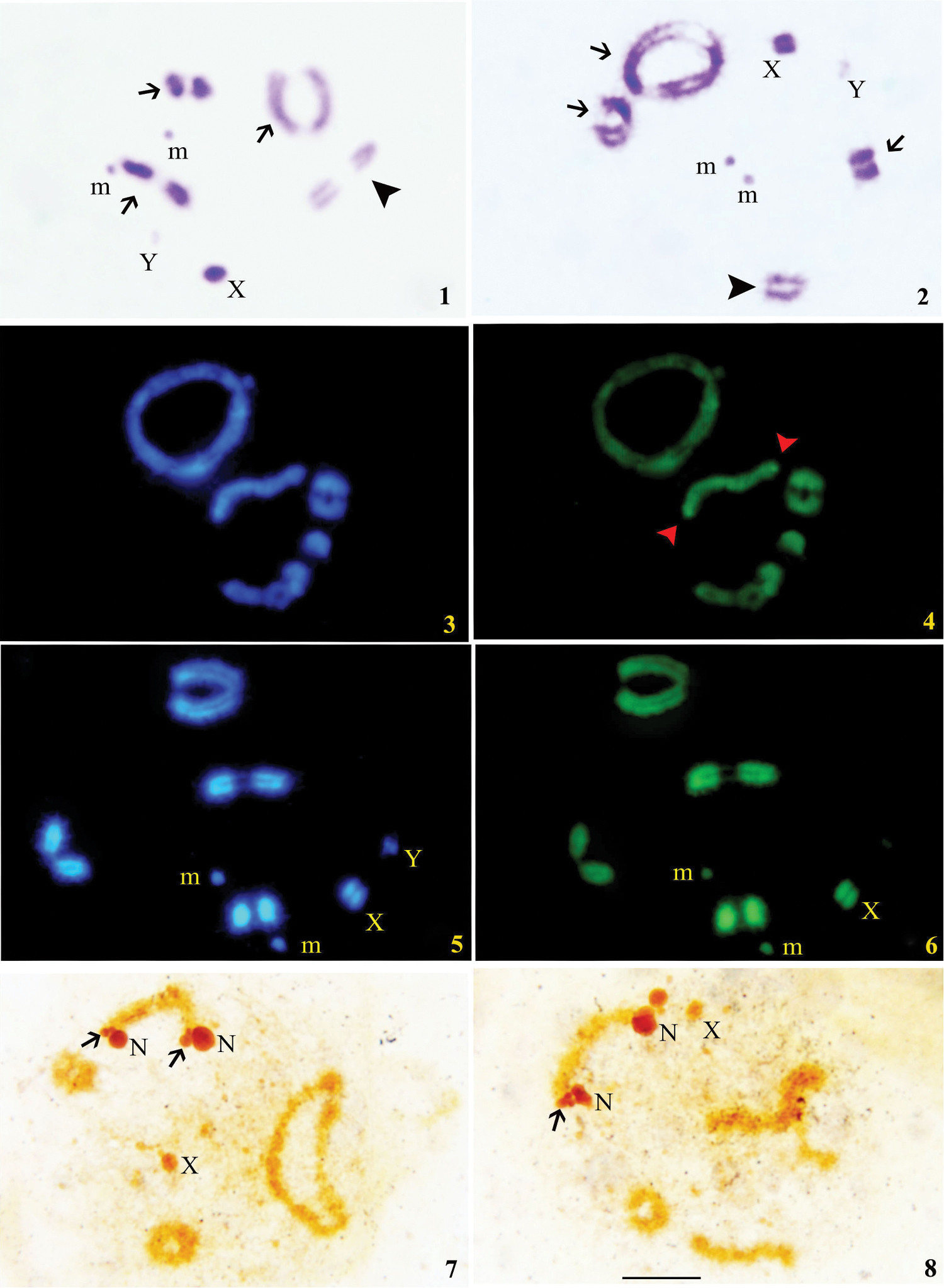
In spite of various cytogenetic works on suborder Heteroptera, the chromosome organization, function and its evolution in this group is far from being fully understood. Cytologically, the family Rhyparochromidae constitutes a heterogeneous group differing in chromosome numbers. This family possesses XY sex mechanism in the majority of the species with few exceptions. In the present work, multiple banding techniques viz., C-banding, base-specific fluorochromes (DAPI/CMA3) and silver nitrate staining have been used to cytologically characterize the chromosomes of the seed plant pest Elasmolomus (Aphanus) sordidus Fabricius, 1787 having 2n=12=8A+2m+XY. One pair of the autosomes was large while three others were of almost equal size. At diplotene, C-banding technique revealed, that three autosomal bivalents show terminal constitutive heterochromatic bands while one medium sized bivalent was euchromatic. Microchromosomes (m-chromosomes) were positively heteropycnotic. After DAPI and CMA3 staining, all the autosomal bivalents showed equal fluorescence, except CMA3 positive signals, observed at both telomeric heterochromatic regions of one medium sized autosomal bivalent. Silver nitrate staining further revealed that this chromosome pair carries Nucleolar Organizer Regions (NORs) at the location of CMA3 positive signals. The X chromosome showed a thick C-band, positive to both DAPI /CMA3 while Y, otherwise C-negative, was weakly positive to DAPI and negative to CMA3, m-chromosomes were DAPI bright and CMA3 dull.
C-banding, DAPI, CMA3, NOR location
Heteroptera is a large cosmopolitan suborder comprising about 42, 300 known species (
In the present contribution, cytological characterization of Elasmolomus (Aphanus) sordidus, reported as Aphanus sordidus having chromosomal complement 2n=12=8A+2m+XY (
Adult males of Elasmolomus sordidus (9 specimens) were collected from fields of sesame and groundnut plants in Punjab (India). Insects were dissected to remove the gonads and air dried slides were prepared. Aged air dried slides were used for C-banding after
The chromosomal complement consisted of twelve elements. Of these, eight were autosomes, two were m-chromosomes, while two of different sizes were sex chromosomes, large X and small Y, respectively. The chromosomal complement was confirmed as 2n=12=8A+2m+XY.
At diplotene, three bivalents showed terminal C-bands while one was euchromatic. The X chromosome showed thick C-band covering almost two thirds of the chromosome while Y was C-negative; m-chromosomes were slightly C-positive (Figs 1, 2).
C-banding (1, 2) 1, 2 Diplotene stages showing distribution of C-bands. Arrows showing heterochromatic chromosomes while arrowhead showing single euchromatic chromosome. Sequence-specific banding (3–6) 3 Diplotene stage with DAPI 4 Diplotene stage with localized CMA3 signals on one autosomal bivalent (shown by arrows) 5 Late diplotene stage with DAPI 6 Late diplotene stage with CMA3. Silver banding (7, 8) 7, 8 Diplotene stages showing location of NORs (shown by arrows) and nucleolar bodies (N). Bar=0.01 mm.
All the four autosomal bivalents showed equal fluorescence with both DAPI and CMA3 (Figs 3, 4). However, one of the medium sized autosomal bivalents showed bright CMA3 signals at both ends, which correspond to NORs (Fig. 4). The X was positive to both DAPI/CMA3 while Y was weak to DAPI and negative to CMA3; m-chromosomes were DAPI bright and CMA3 dull (Figs 5, 6).
Elasmolomus sordidus is a pest of pod crops, mainly groundnut and sesame in India.
Terminal C-bands have been observed in three autosomal pairs of Elasmolomus sordidus. In Heteroptera, the terminal C-bands are of wide occurrence. This kind of C-band location has been reported in Antiteuchus mixtus (Fabricius, 1787) (Pentatomidae) by
One of the autosomal bivalent in Elasmolomus sordidus was found to be euchromatic. A similar condition is observed in Nezara icterica (Horvath, 1889) (Pentatomidae) by
The X chromosome is almost (2/3) completely C-positive and this condition has been earlier reported in Pentatomidae by
Microchromosomes were originally described by
The use of DNA binding fluorochromes having different base specificities allows a better characterization of heterochromatic regions in terms of their relative enrichment with AT or GC base pairs. In Heteroptera, still there is little information on heterochromatin base composition. The bright fluorescence after DAPI and CMA3 staining observed in Elasmolomus sordidus indicates that the constitutive heterochromatic regions possess interspersed AT and GC repeats. Similar observations have been made in Edessa meditabunda (Fabricius, 1974) and Edessa rufomarginata (De Geer, 1773) (Pentatomidae) by
After silver banding and fluorochrome staining, the localization of CMA3 positive bands in NOR regions on medium sized autosomal bivalent was revealed. It was confirmed that ribosomal genes are GC rich. This correspondence of CMA3 signals with NORs have also been reported for several true bug species at interstitial or terminal positions either on autosomes or sex chromosomes by
A common feature of the sex chromosomes of Heteroptera is that they demonstrate bright fluorescence after both DAPI and CMA3 during the meiotic prophase (
The silver impregnation stains not only the NORs but also the nucleolus at specific points of some chromosomes (
Till date, very few Rhyparochromid species have been analysed cytologically based on banding techniques. The present study was able to reveal some cytogenetic characters which were used as markers for better knowledge of chromosome organization and the identification of separate chromosomes in Elasmolomus sordidus. Much more information about true bug chromosomes could be obtained if new molecular cytogenetic techniques involving FISH (fluroscence in situ hybridization) mapping of chromosomes are used (
The authors thank the Department of Science and Technology, New Delhi, India for providing financial support (Grant Number 96915) to carry out the present study.