(C) 2013 Daniela M. Takiya. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Takiya DM, Dietrich CH, Viraktamath CA (2013) The unusual Afrotropical and Oriental leafhopper subfamily Signoretiinae (Hemiptera, Cicadellidae): taxonomic notes, new distributional records, and description of two new Signoretia species. In: Popov A, Grozeva S, Simov N, Tasheva E (Eds) Advances in Hemipterology. ZooKeys 319: 303–323. doi: 10.3897/zookeys.319.4326
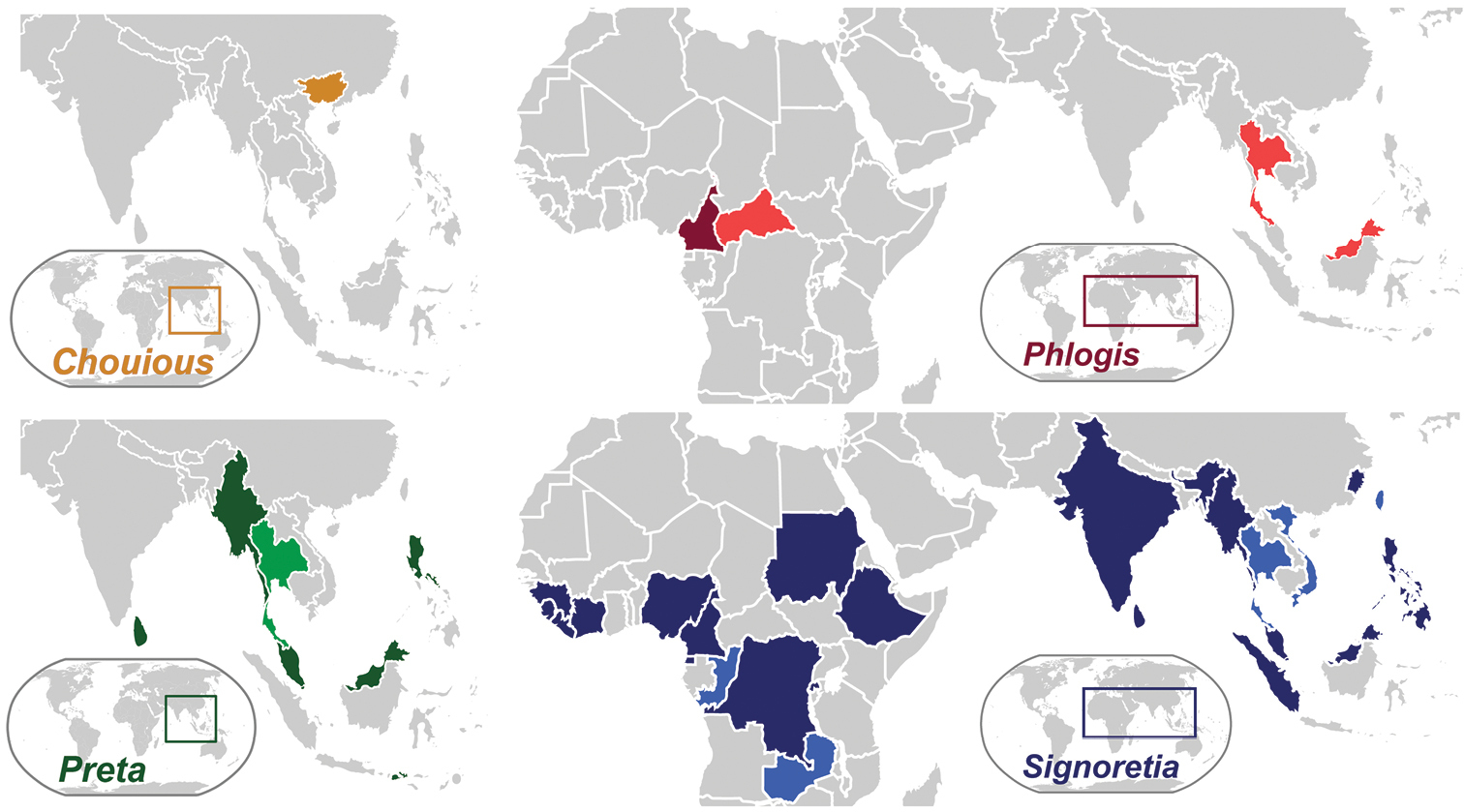
The leafhopper subfamily Signoretiinae is redescribed and includes two tribes: Signoretiini Baker and Phlogisini Linnavuori. Redescriptions of included tribes, diagnoses and a taxonomic key to genera are provided. New records for genera of Signoretiinae are as follows: Phlogis in Central African Republic, Malaysia and Thailand; Preta in Thailand; and Signoretia in the Republic of the Congo, Zambia, Thailand, Vietnam, and Taiwan (China). Signoretia pacifica is newly recorded from Cameroon. In addition, detailed illustrations of the male genitalia of the previously described species, Chouious tianzeus, Preta gratiosa, and Signoretia yangli are provided; the male genitalia of Signoretia malaya are described for the first time; and two new species of Signoretia are described, Signoretia delicata sp. n. from the Philippinesand Signoretia kintendela sp. n. from the Republic of the Congo.
Taxonomy, distribution, morphology, new species
Signoretiinae is a small, poorly known subfamily of leafhoppers apparently restricted to tropical forests in the Afrotropical and Oriental regions. The group is represented by few specimens in collections and is easily distinguished from nearly all cicadellids by the deeply punctured and enlarged pronotum (see subfamily remarks); other striking morphological features include: face strongly convex with cibarial muscle scars prominent; forewing outer anteapical cell present (vein s present); and hind femur macrosetal formula 2+0+0. Some of these morphological features have led to difficulties in placing the included genera in the Cicadellidae higher classification scheme.
Signoretiinae sensu lato are variable for some morphological characters generally used to define taxa at the subfamily-level in Cicadellidae. For example, the position of the ocelli differs between the two included tribes: in Signoretiini they are found on the crown margin close to the eyes; in Phlogisini they are found on the crown, far from the margin. Similarly, Phlogisinihave hind tibiae with macrosetae in row PD, and forewings with crossveins at the bases of the inner and median anteapical cells; while Signoretiini lack these features. Finally, Phlogisini have a distinct maxillary suture and Signoretiini have a complete longitudinal carina on the frontoclypeus, traits fairly uncommon in leafhoppers. Due to all above-mentioned morphological differences found in these taxa, Phlogisini and Signoretiini are herein treated as valid tribes within Signoretiinae.
Signoretiini, as treated herein (Fig. 1), includes Preta Distant with two species restricted to the Oriental region and Signoretia, occurring both in the Afrotropical and Oriental regions, with 27 species. Phlogisini includes the monotypic genera Phlogis Linnavuori from Africa and Chouious Yang from China. Although the African Signoretiini were revised by
Current distribution of signoretiine genera, Chouious, Phlogis, Preta, and Signoretia. Countries marked with a lighter shade are new records given herein.
Morphological terminology follows
Habitus images were taken with a Digital Lab XLT system by Microptics using a Nikon D1x digital SLR camera and genitalia images were taken with a Q Imaging Micropublisher 3.3 digital camera mounted on an Olympus BX41 compound microscope. Multiple images were combined using the CombineZP software (
http://species-id.net/wiki/Signoretiinae
Figs 1–42Medium-sized, cylindrical leafhoppers (Figs 2–14). Head (Figs 2–14) broader than pronotum; ocelli visible in dorsal aspect; frontoclypeus expanded with prominent transverse muscle scars; lateral frontal sutures extended ventromesad of ocelli; antennal ledges well developed; antennae subequal to or longer than width of head; anteclypeus convex and tapered from base to apex; lorum short, narrow, well separated from genal margin, partly bordering frontoclypeus; gena slightly emarginate below eyes, exposing proepisternum; rostrum tapered, surpassing front trochanters.
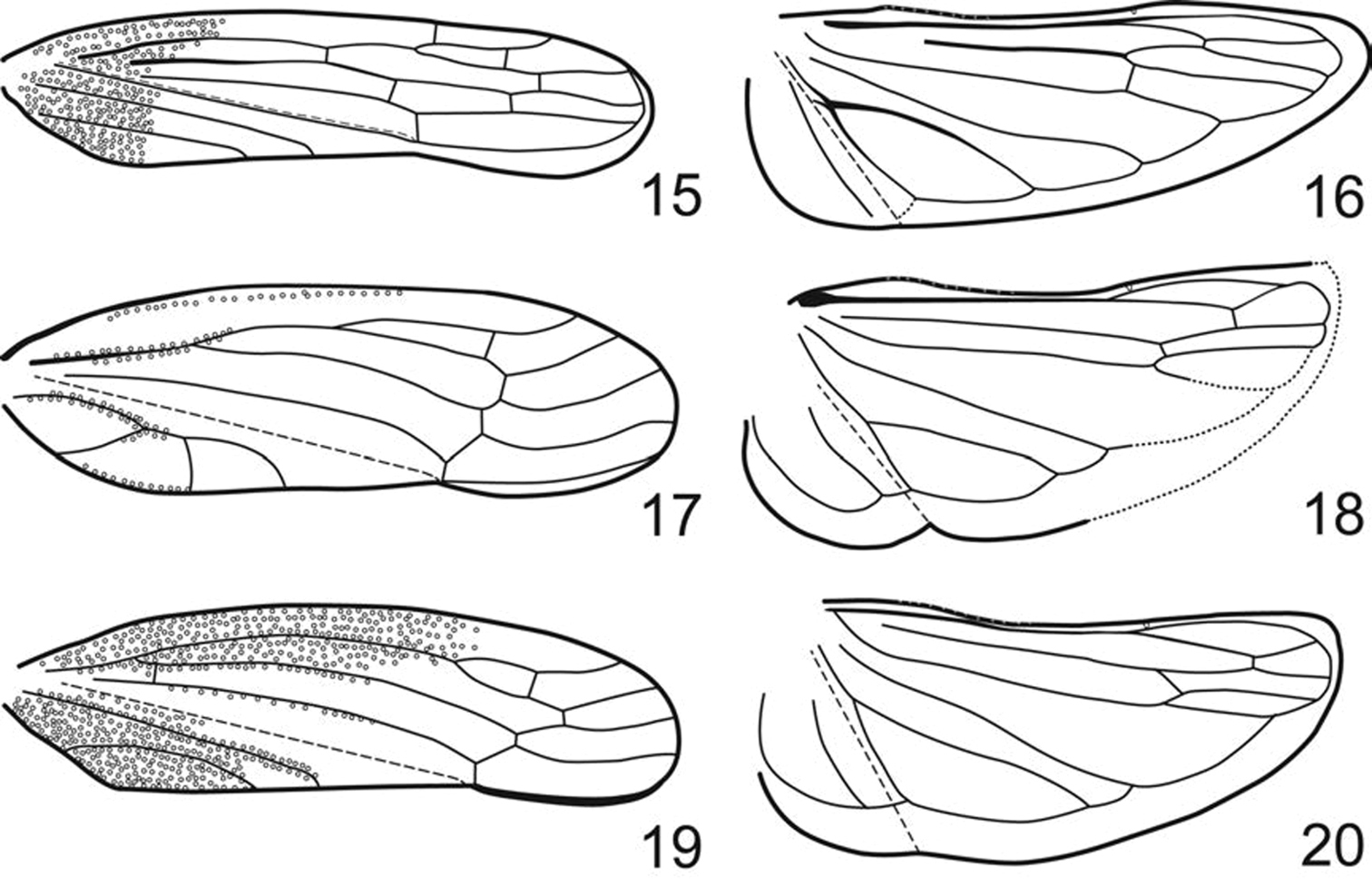
Pronotum (Figs 2–5, 7–11, 13, 14) greatly enlarged, strongly convex, distinctly punctate, weakly produced anterad, extended posterad to scutellar suture. Forewings (Figs 15, 17, 19) macropterous with venation distinct and opaque sclerotization, if present, limited to basal third; vein R with two (R1 not visible as separate vein) or three branches; crossvein s present (outer anteapical cell closed); inner apical cell elongate, parallel-sided, extended to wing apex. Hind wings (Figs 16, 18, 20) with venation complete; submarginal vein well separated from wing margin. Forelegs with femur with AM1 weakly developed or absent, intercalary row and distal half of AV well differentiated, each with several setae arranged in single row; tibia cylindrical, AD and PD undifferentiated. Hind legs with femur with macrosetal formula 2+0; tibia with macrosetae of dorsal rows reduced in size and number; tarsomere I without dorsoapical pair of macrosetae; pecten with 2 platellae.
Male genital capsule (Figs 21–24, 29–42) with valve articulated or fused laterally to pygofer; pygofer without distinct membranous clefts near base; segment X very large, well sclerotized, with or without processes; subgenital plates digitiform, broadest at base, usually with numerous fine setae dorsally but only rarely with well differentiated macrosetae; connective Y-shaped; style sigmoid; with or without sclerotized dorsal connective or other sclerotized processes between anal tube and aedeagus usually present.
Female ovipositor (Figs 26, 27) elongate, variable in shape and dentition.
Habitus photographs. 2, 3 Chouious tianzeus, dorsal and lateral 4–6 Phlogis sp. from Malaysia, dorsal and details of head lateral and face 7 Phlogis sp. from Thailand, lateral 8, 9 Preta gratiosa, dorsal and lateral 10–12 Signoretia delicata sp. n., detail of head, dorsal, lateral, and frontal 13, 14 Signoretia kintendela sp. n., dorsal and lateral.
Fore- and hindwing. 15, 16 Phlogis sp. from Thailand. 17, 18 Preta gratiosa. 19, 20 Signoretia aureola.
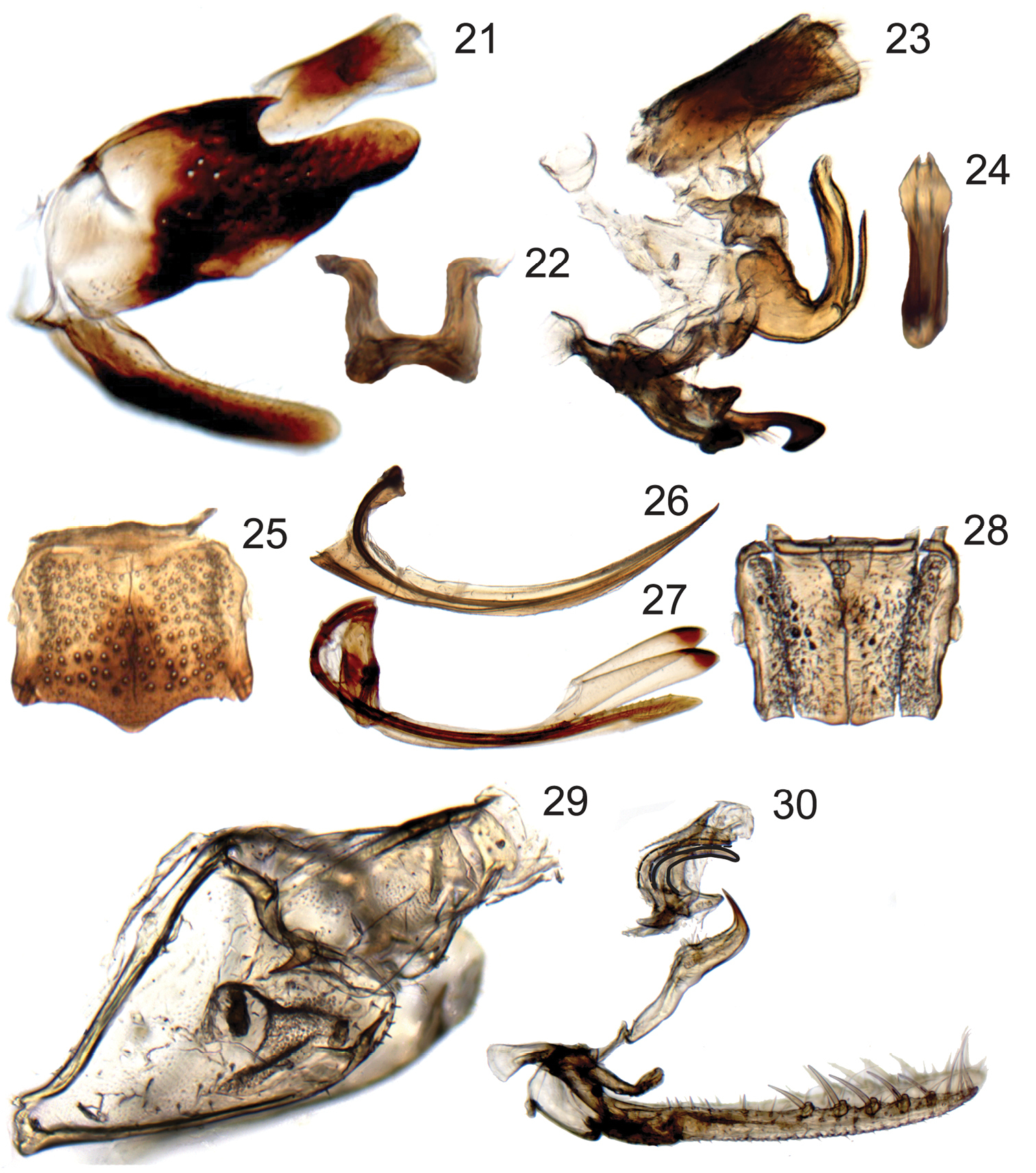
Terminalia of Chouious, Phlogis and Preta. 21–24 Chouious tianzeus, male genitalia 21 genital capsule (without anal tube), lateral view 22 dorsal connective, caudal view 23 connective, styles, aedeagus, dorsal connective, and anal tube, lateral view 24 aedeagus caudal view. 25–27 female terminalia of Phlogis sp. from Malaysia 25 sternite VII, ventral view 26 first valvula of ovipositor, lateral view 27 second valvifers, second valvulae and gonoplacs of ovipositor, lateral view 28 Phlogis sp. from Thailand, sternite VII, ventral view 29, 30 Preta gratiosa 29 pygofer and segment X of anal tube, lateral view 30 subgenital plates, connective, styles, and aedeagus, lateral view.
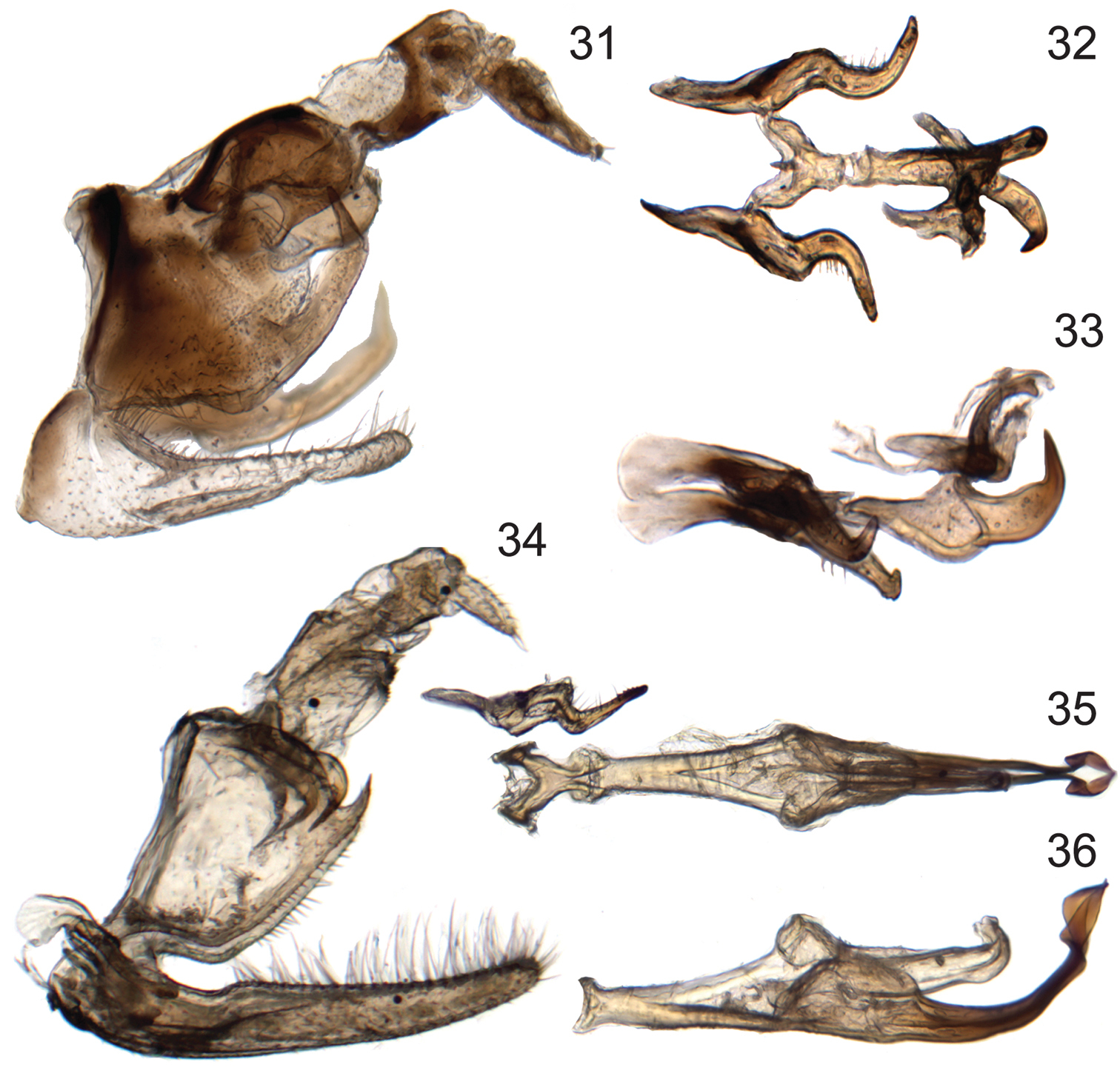
Male genitalia of new species of Signoretia. 31–33 Signoretia delicata sp. n. 31 genital capsule, lateral view 32 connective, styles, and aedeagus, dorsal view 33 connective, styles, and aedeagus, lateral view 34–36 Signoretia kintendela sp. n. 34 genital capsule, lateral view 35 connective, style, and aedeagus, dorsal view 36 aedeagus, ventrolateral view.
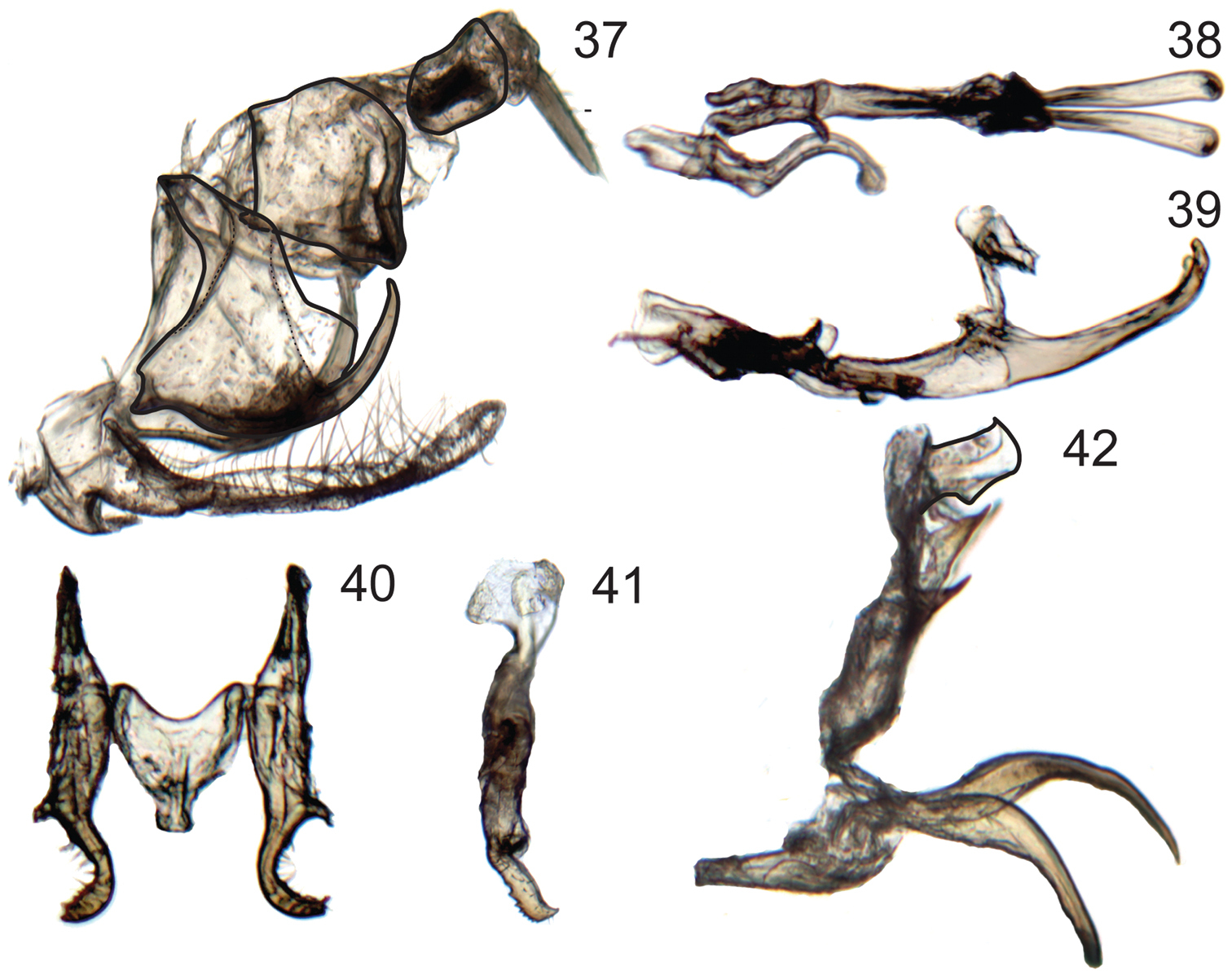
Male genitalia of Signoretia. 37–39 Signoretia malaya. 37 genital capsule, lateral view 38 connective, styles, and aedeagus, dorsal view 39 connective, styles, and aedeagus, lateral view 40–42 Signoretia yangli 40 connective and styles, dorsal view 41 style, lateral view 42 aedeagus, lateral view.
Afrotropical and Oriental.
With the exception of the proconiine sharpshooter genus Tretogonia Melichar, 1926 and the recently described dikraneurine (Typhlocybinae) genus Sweta Viraktamath & Dietrich 2011, Signoretiinae are the only leafhoppers with fully developed wings that have the pronotum extended to the scutellar suture.
Nothing is known about the ecology or feeding behavior of Signoretiinae, although the strongly convex or inflated face suggests that they preferably feed on xylem sap.
| 1 | Dorsal coloration black (Figs 2–7); crown and frontoclypeus without carinae (Figs 2–7); ocelli on crown, each equidistant to adjacent anterior eye angle and other ocellus (Figs 2, 4); forewing with 3 closed anteapical cells (Fig. 15); hind wing submarginal vein not extended onto jugum (Fig. 16); hind tibia with macrosetae in row PD; male pygofer without posteroventral process (Fig. 21); valve fused laterally to pygofer (Fig. 21) | Phlogisini, 2 |
| 1’ | Dorsal coloration usually white or yellow (Fig. 8–14); crown with transverse basal carina between ocelli (Figs 8, 10, 13); ocelli on crown-face transition, distinctly closer to adjacent eye angle than to median line of crown (Figs 8, 10, 13); frontoclypeus with complete median longitudinal carina (Figs 9, 12, 14); forewing with only outer anteapical cell closed, inner and median anteapical cells open at base (Figs 17, 19); hind wing submarginal vein extended onto jugum (Fig. 18, 20); hind tibia without macrosetae in row PD; male pygofer with posteroventral process (Figs 31, 34, 37); valve not fused laterally to pygofer, articulated by membranous connection (Fig. 31) | Signoretiini, 3 |
| 2 | Head in profile with lower part of clypeus distinctly produced and angulate, forming shelf over anteclypeus (Fig. 3); frontoclypeus with transverse carina ventrally and median longitudinal carina dorsally (Fig. 3); gena with conspicuous long, pale setae (Fig. 3) | Chouious |
| 2’ | Head in profile evenly rounded, with lower part of frontoclypeus continuing contour of anteclypeus (Figs 6, 7); face without carinae (Fig. 6); gena without conspicuous long, pale setae (Figs 5, 6) | Phlogis |
| 3 | Pronotum with complete paired longitudinal carinae (Fig. 8); forewings with claval veins fused for one-third of their distance (Fig. 17) | Preta |
| 3’ | Pronotum with or without incomplete paired longitudinal carinae at basal third (Figs 10, 13); forewings with claval veins separate (Fig. 19) | Signoretia |
Dorsal coloration dark brown to black (Figs 2–7). Head (Figs 2–7) with crown convex, punctate, carinae indistinct or absent, margins not elevated; ocelli on crown distant from anterior margin, approximately equidistant from eyes and midline; with distinct depression laterad of each ocellus; transition from crown to face rounded; antennal ledges not prominent, evenly rounded; frontoclypeus without median longitudinal carina; anteclypeus with apex emarginated; maxillary suture present.
Pronotum (Figs 2–5, 7) evenly convex, without carinae or deep depressions. Forewings (Fig. 15) with or without R1 and crossveins r-m1 and m-cu2 present. Hind wings with crossvein m-cu perpendicular to CuA; submarginal vein not extended onto jugum. Forefemora with intercalary row strongly arcuate. Hind tibiae with row PD with macrosetae and row PV with some setae blunt-tipped.
Female ovipositor (Figs 26, 27) slender and evenly curved throughout length; first valvulae (Fig. 26) with dorsal sculpturing imbricate along margin and strigate ventrally; second valvulae (Fig. 27) with dorsal teeth small, simple, somewhat irregularly distributed, restricted to distal half. [Female characters of tribe based on Phlogis]
Male terminalia (Figs 21–24) with pygofer with apical two-thirds distinctly more sclerotized than base, without posteroventral process; valve fused laterally to pygofer; subgenital plates not extended posteriorly as far as pygofer lobe apex; style without preapical teeth or denticuli; with distinct dorsal connective (separate sclerite connecting aedeagus to anal tube); anal tube segment X with (Phlogis) or without (Chouious) posterolateral processes.
Chouious tianzeus Yang, 1991.
Head (Figs 2, 3) coarsely punctate, lateral frontal sutures elevated and carinate; frontoclypeus with median longitudinal carina dorsally, ventral part produced, in contour forming shelf over anteclypeus, with distinct transverse carina, area above carina depressed medially; genae conspicuously pubescent.
Chouious was described based on one new species, Chouious tianzeus from south China (
China (Guangxi,
http://species-id.net/wiki/Chouious_tianzeus
Figs 2, 3, 21–24Forewings of the paratype studied lack crossvein s, but this should be an anomaly of this particular specimen.
China (
Male paratype, China, Guanxi, Bose Prov., Tialin Co., 28–30 V 1982, NWAF.
http://species-id.net/wiki/Phlogis
Figs 4–7, 15, 16, 25–28Phlogis mirabilis Linnavuori, 1979.
Head (Figs 4–7) distinctly but finely punctate; lateral frontal sutures distinct, but not carinate; frontoclypeus evenly convex, without median or transverse carinae, in profile continuing contour of anteclypeus; genae bare.
Phlogis was originally described based on the single female type specimen of Phlogis mirabilis from Cameroon (
Afrotropical: Cameroon (
http://species-id.net/wiki/Phlogis mirabilis
Previously, the only known specimen of Phlogis mirabilis was the female type from Cameroon. A male specimen from Central African Republic is tentatively considered as conspecific to the type-specimen, based on distribution, external morphology, and size (7.4 mm). The male genitalia of this specimen agree with those of Chouious in the characters described for Phlogisini. However, this male can be easily distinguished from Chouious because its: (1) pygofer lobe lacks the deep concavity on the dorsal margin, (2) aedeagus lacks ventral atrial processes, but has paired apical recurved processes; and (3) anal tube segment X has posterolateral paired processes.
Cameroon (
Central African Republic: male, Boukoko, 15 III 1969, Michel Boulard, MNHN.
The only other known specimens of Phlogis are two females from the Oriental region, Malaysia and Thailand, examined for this study. Both specimens studied herein (Figs 4–7) agree in most respects with Linnavuori’s original description of the genus, but differ in size (6.4 and 9.5 mm vs. 7.5 mm for Phlogis mirabilis) and in some details of body form, indicating that they represent two additional, presumably new species. However, we do not provide formal descriptions, given the meager material available at present. The specimen from Malaysia (Figs 4–6) closely resembles Phlogis mirabilis, but is longer (overall length 9.5mm vs. 7.5mm) and differs in the shape of the seventh sternite (Fig. 25). The specimen from Thailand is smaller (6.4mm) and has the shape of the seventh sternite (Fig. 28) very similar to that of Phlogis mirabilis (comparison based on photographs of the type-specimen).
Malaysia [new record]: female, Ranau, 500m, 22–25 I 1959, BMNH. Thailand [new record]: female, Petchaburi Kaeng Krachan National Park, km33 helipad, 12°50.177'N, 99°20.688'E 735m, Malaise trap, 18–25 V 2009, Sirichai, DNA voucher LH199, INHS.
Dorsal coloration pale yellow to white (Figs 8–14; except Signoretia greeni Distant, 1908). Head (Figs 8–17) with crown bearing prominent medial, lateral and posterior carinae, margins elevated, punctations indistinct; ocelli closely adjacent to eyes, laterad of submarginal carinae, directly above antennal ledges; transition from crown to face sharp, indicated by transverse carina; antennal ledges prominent, with anterior depression; frontoclypeus with complete median longitudinal carina; anteclypeus with apex truncate; maxillary suture absent.
Pronotum (Figs 8–11, 13–14) with pair of carinae or with median anterior depression. Forewings (Figs 17, 19) without R1 and crossveins r-m1 and m-cu2. Hind wings (Figs 18, 20) with crossvein m-cu oblique relative to CuA; submarginal vein extended onto jugum. Forefemora with intercalary row weakly arcuate. Hind tibiae with row PD without macrosetae and row PV without blunt-tipped setae.
Female ovipositor sigmoid, broadened near midlength; first valvulae with dorsal sculpturing strigate; second valvulae with dorsal teeth numerous, close-set, and bidentate; toothed area occupying more than half entire length of valvula.
Male terminalia (Figs 29–42) with pygofer with well-developed posteroventral process; valve articulated laterally to pygofer; subgenital plates extended posteriorly beyond pygofer lobe apex; style with preapical teeth or denticuli; dorsal connective absent; anal tube segment X with or without lobes and/or processes at base or more apically; aedeagus divided into ventral paraphyses-like structure articulated to connective consisting of basal preatrium and paired robust processes and dorsal shaft, dorsal and ventral parts may be loosely connected by membrane (all Oriental species) or completely fused to eachother (some African species).
http://species-id.net/wiki/Preta
Figs 8, 9, 17, 18, 29, 30Preta gratiosa (Melichar, 1903).
Head (Figs 8, 9) strongly and angulately produced. Pronotum (Figs 8, 9) with pair of well-developed submedial longitudinal carinae extended entire length. Forewings (Fig. 17) with claval veins fused for short distance near midlength.
Preta is restricted to the Oriental region and currently includes two species, Preta gratiosa and Preta luzonensis Baker, 1923, the latter known only from the Philippines. It can be easily distinguished from Signoretia by its complete paired longitudinal carinae on the pronotum (Fig. 8) and medially fused claval veins of forewings (Fig. 17), as indicated in the key.
Indonesia (Sumbawa, Jacobi 1941 apud Knight 2010), E. and W. Malaysia (
http://species-id.net/wiki/Preta_gratiosa
Figs 8, 9, 17, 18, 29, 30Identification based on illustrations of the external morphology and male genitalia of this species (
Indonesia (Sumbawa, Jacobi 1941 apud Knight 2010), E. and W. Malaysia (
Thailand: male, Phetchabun Khao Kho NP, mixed deciduous forest at Ta Phol River, 16°32.561'N, 101°2.479'E, 242m, Malaise trap, 5–12 XI 2006, Somchai Chachumnan & Saink Singhtong, INHS. Malaysia: specimen without abdomen, Kedah Province, Lang Kawi island, 25 V 1975, N. D. Penny, INHS.
http://species-id.net/wiki/Signoretia
Figs 10–14, 19, 20, 31–42Thamnotettix malaya Stål, 1855.
Head (Figs 10–14) weakly to strongly produced. Pronotum (Figs 10, 11, 13, 14) with longitudinal carinae absent or, if present, weakly developed and not extended entire length. Forewings (Fig. 19) with claval veins separate throughout length.
Signoretia currently includes 10 Oriental species and 15 Afrotropical species, in addition to the new species described herein. Members of Signoretia can be easily distinguished from Preta by the lack of paired complete longitudinal carinae on pronotum (Figs 10, 13) and separate claval veins on forewings (Fig. 19). Several nominal species do not have the male genitalia described and illustrated, specially the Oriental ones.
Distribution. Afrotropical: Cameroon (
http://species-id.net/wiki/Signoretia_aureola
Figs 19, 20Identification of specimens at hand is based on
Myanmar (
Thailand: male, Chiang Mai, Doi Chiang Dao WS Nature trail, 19°24.278'N, 098°55.311'E, 491m, Malaise trap, 7–14 X 2007, Songkran and Apichart, DNA voucher LH193, INHS.
urn:lsid:zoobank.org:act:B8DB41FA-7E49-4134-942D-EE3011CC62D6
http://species-id.net/wiki/Signoretia_delicata
Figs 10–12, 31–33Holotype, 6.0 mm
Crown (Figs 10, 11) very short, median length approximately half interocular and three-tenths of transocular width; median longitudinal carina obsolete. Male pygofer (Fig. 31) with caudal margin of lobe weakly sclerotized; ventral appendage robust, spiniform, produced posteriorly beyond pygofer lobe apex, abruptly narrowed and bent dorsad near apex. Valve triangular. Subgenital plates (Fig. 31) extending posteriorly beyond pygofer lobe apex by approximately one-third lobe length, with relatively few long, fine setae dorsally, concentrated near apex. Connective (Fig. 32) Y-shaped; with dorsal median keel and short, slender median anterior lobe. Style (Figs 32, 33) slender, tapering towards apex; apex directed dorsolaterally. Aedeagus (Figs 32, 33) with ventral paraphysis-like structure with pair of robust, tapered, recurved distal processes; dorsal part consisting of pair of parallel dorsolateral arms and median shaft, shaft somewhat depressed and strongly arcuate. Anal tube (Fig. 31) basal section with pair of basal processes short, blunt, extended anteromesad, distal ring weakly sclerotized, retracted into basal section.
Coloration. Stramineous to white (Figs 10–12). Crown (Fig. 10) with paired black markings basolaterally, connecting to paired black maculae at apex. Frontoclypeus (Fig. 12) with longitudinal carina black. Legs yellow, coxae and femora infused with fuscous.
The species epithet refers to the relatively small size of this species and its delicate habitus.
This species is described as new because it does not agree with any of the ten previously described Oriental species based on the following combination of characteristics: (1) stramineous dorsal coloration with two pairs of dark markings on crown; (2) median carina on crown absent; (3) each ocellus close to eye for distance of approximately its own diameter; (4) frontoclipeal longitudinal carina not continuing on clypellus; and (5) pronotum longer than wide and without paired incomplete longitudinal carinae on anterior portion, but with very faintly elevated median longitudinal carina. Exceptionally, the above-mentioned characteristics, will not separate Signoretia delicata sp. n. from Signoretia tagalica Baker, 1915 (another Philippine species described from Luzon and Banahao), with which shares other morphological characters, such as the less produced crown, making the frontoclypeus appear more inflated, and very short outer anteapical cell. Nevertheless, based on the original illustrations and description, Signoretia tagalica is larger (male is 6.5 mm) and has a much longer pronotum (more than 4 times the median length of crown) than the species described herein.
The short crown of this new species, shared with other described Signoretia, could be viewed as sufficient diagnostic characteristics to place this group in a new genus. Considering that at the moment only a small fraction of Oriental Signoretia have the male genitalia described, it would be premature to erect a new genus without reviewing all other described Oriental Signoretia.
Male holotype, “Mindanao: Davao;\ E. slope Mt. Apo, \ Camp Baclayan. Elev.\ 6500 ft. XI-11-1946”, “CMHN-Philippine\ Zool.Exp. (1946–47) \ H.Hoogstraal leg.”, FMNH.
http://species-id.net/wiki/Signoretia_errans
urn:lsid:zoobank.org:act:ECC53C40-DD5D-483B-988D-4D276DEB9F81
http://species-id.net/wiki/Signoretia_kintendela
Figs 13, 14, 34–36Holotype, 7.4 mm.
Crown (Fig. 13, 14) elongate, median length approximately six-tenths of interocular and half of transocular width; median longitudinal carina well developed. Male pygofer (Fig. 34) with ventral appendage short, spiniform, produced posteriorly beyond pygofer lobe apex. Valve short, rectangular. Subgenital plate (Fig. 34) extended posteriorly beyond pygofer lobe apex by approximately one-third lobe length, with numerous long, fine setae distributed evenly over entire length of dorsum. Connective (Fig. 35) Y-shaped; without dorsal median keel or median anterior lobe. Style (Fig. 28) slender, tapering towards apex; apex directed laterally, with several denticuli preapically. Aedeagus (Figs 35, 36) with ventral paraphysis-like structure with pair of elongate, recurved distal processes expanded preapically; dorsal part without sclerotized apodemes, consisting only of shaft, shaft strongly compressed, extended posterad, abruptly bent dorsad in distal third, distal part sinuate. Anal tube (Fig. 34) segment X with pair of posterolateral lobes each bearing small spine and several denticuli and at base with separately articulated robust process with pygofer, extended ventrad and terminating in anteriorly directed spine.
Stramineous to white (Figs 14, 15). Crown (Fig. 14) with paired black markings at base of crown and paired maculae at apex of crown. Etymology. The specific epithet, “kintendela”, means “cicada” in Kongo language (
Signoretia kintendela sp. n. shares with other members of the pacifica group (
Male holotype, “Rep. of Congo: Dept Pool;\ Iboubikro; Lesio-Loun Pk.\ 3°16.196'S, 15°38.267'E\ 330m, malaise trap, A138\ 23.x.2008, Braet & Sharkey”, INHS.
http://species-id.net/wiki/Signoretia_malaya
Figs 37–39Pygofer (Fig. 37) with caudal margin of lobe membranous; ventrocaudal process elongate evenly curved and tapered, spiniform, produced posteriorly beyond pygofer lobe apex. Subgenital plates (Fig. 37) extending posteriorly beyond pygofer lobe apex by approximately one-third of lobe length, dorsal surface with numerous long, fine setae evenly distributed throughout length and three macrosetae in longitudinal row near midlength. Connective (Fig. 38) H-shaped, arms subparallel; without dorsal median keel or median anterior lobe. Style (Fig. 38) slender and elongate; apex globose, directed laterally, with few small denticuli. Aedeagus (Figs 38, 39) with ventral paraphyses-like structure with pair of robust, tapered, recurved distal processes; dorsal part consisting of pair of round basolateral lobes and tubular shaft. Anal tube (Fig. 37) with segment X without basal processes and distal margin thickened, terminating ventrally in short lobe.
Identification based on Stål’s (1859) illustration and collecting locality. Male genitalia previously undescribed.
W. Malaysia (Stål 1855), Philippines (
Malaysia: male, Selangor, Kuala Lumpur, IX 1964, N. L. H. Krauss on Melastoma malabathricus, USNM.
http://species-id.net/wiki/Signoretia_pacifica
Identification based on
Cameroon [new record]; Democratic Republic of Congo, Guinea, Liberia (
Cameroon: male, Litteral, nr. Limbe on road to Bimbia Village, 03°58'192."N, 009°14'16.7"E, 15–30 III 2009, J. R. Cryan & G. J. Svenson, INHS.
http://species-id.net/wiki/Signoretia_yangi
Figs 40–42Identification based on illustrations in original description, although male specimens studied differ from original illustration in the shape of the connective. Additionally, distinctive features not mentioned or illustrated in the original publication include the: highly membranous, small and tubular aedeagal shaft; ventral aedeagal processes being separately articulated and densely clothed with microtrichia; and pair of slender ventral spines on segment X extending basad.
China: Fujian (
Taiwan: male, Nantou Hsien, Tungpu, 1200m, 18–21.x.1982, K. C. Chou & S. C. Lin, TARI; male, Nantou Hsien, Sungkang, 2100m, XI.1985, Malaise trap, K. S. Lin, TARI.
Malaysia: female, Sabah, 1km S. Kundasang, 1530m, 11 IX 1983, G. F. Hevel & W. E. Steiner, USNM. Nigeria: female, Kaduna, Kagoro forest, 29–30 VIII 1973, R. Linnavuori, AMNH [This specimen is similar in external morphology and coloration with the type specimen of Signoretia astraea imaged by the AMNH]. Philippines: Mindanao, Davao, Santa Cruz, Badiang, 2000ft, 10 XII 1946, M. Celeston, FMNH. Vietnam [new genus record]: female, Thua Thien-Hue: Bach Ma Natl. Pk., edge of stream, ca. 1km along Five Lakes trail 4–16 VI 2000 B. Hubley, Malaise trap, 1200m, subtropical evergreen forest, 16°11'20.1"N, 107°51'08.5"E, DNA voucher PR173, ROM. Zambia [new genus record]: female, Northwestern Province, ~15 km N Mwinilunga Lwakera National Forest, 11°34'28.2"S, 24°23'40.1"E, 1445m, 5 XI 2007, Hg-vapor light, J. N. Zahniser, INHS.
As mentioned in the introduction, despite sharing some features unique among Cicadellidae, the two recognized tribes of Signoretiinae show striking differences in major characters of the external morphology. Likewise, the male genitalia also show differences between the two groups. All described Signoretiini have the valve not fused laterally to the pygofer, a strongly developed ventrocaudal process on the pygofer lobe, and long subgenital plates. Males of Phlogisini have, on the other hand, the valve fused laterally to the pygofer, pygofer without processes, and shorter subgenital plates. Furthermore, the aedeagus is articulated with the anal tube by an additional sclerite, the dorsal connective (
We are indebted to the following curators or collection managers who granted access to their respective collection or loaned specimens to us: R. Schuh (AMNH), M. Webb (BMNH), V. Thompson and P. Parrillo (FMNH), T. Bourgoin and A. Soulier-Perkins (MNHN), Y. Zhang (NWAF), C. Darling (ROM), G. Hevel (USNM), and H.-T. Shih (TARI). Additionally, recently collected material from Cameroon was made available to us by J. Cryan (North Carolina Museum of Natural Sciences), Zambia by J. Zahniser (INHS), and from Republic of Congo and Thailand by M. Sharkey (University of Kentucky). M. Webb kindly reviewed preliminary versions of this manuscript and sent us images of Phlogis mirabilis type, currently on loan to him. Y. Cao (NWAF) helped us obtain and translate part of the Chinese bibliography. Travel to Illinois by the senior author was funded in part by a Conselho Nacional de Desenvolvimento Científico e Tecnológico grant (CNPq PROTAX proc. 562303/2010-3).