(C) 2013 Catherine S. McFadden. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: McFadden CS, van Ofwegen LP (2013) Molecular phylogenetic evidence supports a new family of octocorals and a new genus of Alcyoniidae (Octocorallia, Alcyonacea). ZooKeys 346: 59–83. doi: 10.3897/zookeys.346.6270
Molecular phylogenetic evidence indicates that the octocoral family Alcyoniidae is highly polyphyletic, with genera distributed across Octocorallia in more than 10 separate clades. Most alcyoniid taxa belong to the large and poorly resolved Holaxonia–Alcyoniina clade of octocorals, but members of at least four genera of Alcyoniidae fall outside of that group. As a first step towards revision of the family, we describe a new genus, Parasphaerasclera gen. n., and family, Parasphaerascleridae fam. n., of Alcyonacea to accommodate species of Eleutherobia Pütter, 1900 and Alcyonium Linnaeus, 1758 that have digitiform to digitate or lobate growth forms, completely lack sclerites in the polyps, and have radiates or spheroidal sclerites in the colony surface and interior. Parasphaerascleridae fam. n. constitutes a well-supported clade that is phylogenetically distinct from all other octocoral taxa. We also describe a new genus of Alcyoniidae, Sphaerasclera gen. n., for a species of Eleutherobia with a unique capitate growth form. Sphaerasclera gen. n. is a member of the Anthomastus–Corallium clade of octocorals, but is morphologically and genetically distinct from Anthomastus Verrill, 1878 and Paraminabea Williams & Alderslade, 1999, two similar but dimorphic genera of Alcyoniidae that are its sister taxa. In addition, we have re-assigned two species of Eleutherobia that have clavate to capitate growth forms, polyp sclerites arranged to form a collaret and points, and spindles in the colony interior to Alcyonium, a move that is supported by both morphological and molecular phylogenetic evidence.
Alcyonium, Eleutherobia, Parasphaerascleridae, Parasphaerasclera, Sphaerasclera, Indo-Pacific, taxonomy
The anthozoan sub-class Octocorallia comprises a clade of approximately 350 genera and 3400 species of soft corals, gorgonians and sea pens that are found throughout marine environments worldwide (
Among the many families of octocorals that appear from molecular phylogenetic analyses to be polyphyletic, the soft coral family Alcyoniidae stands out as one of the most phylogenetically heterogeneous (
Several genera of Alcyoniidae fall entirely outside of Holaxonia–Alcyoniina, and belong instead to two small clades located near the base of Octocorallia (
Specimens of Eleutherobia and Alcyonium suitable for molecular analyses were collected in South Africa in 2008 and Palau in 2005 and 2010 (Table 1). Following collection using SCUBA, colonies were preserved in 70% EtOH; tissue sub-samples to be used for molecular analyses were preserved in >90% EtOH. Specimens of Eleutherobia flammicerebra Williams, 2003 were collected by dredge from New Caledonia during the 2008 Terrasses cruise (R/V Alis), conducted as part of the MNHN/IRD Tropical Deep-Sea Benthos cruises, 2003–2012 (
Specimens of Alcyonium, Parasphaerasclera gen. n. and Sphaerasclera gen. n. included in molecular phylogenetic and morphological analyses. For GenBank accession numbers see Appendix. For abbreviations, see Methods section.
| Genus & Species | Authority | Museum & Cat. No. | Collection Location | Year |
|---|---|---|---|---|
| Alcyonium | Linnaeus, 1758 | |||
| Alcyonium bocagei | (Saville Kent, 1870) | RMNH Coel. 39672 | Portugal, Sagres | 1994 |
| Alcyonium coralloides | (Pallas, 1766) | RMNH Coel. 39678 | France, Marseilles | 1994 |
| Alcyonium digitatum | Linnaeus, 1758 | RMNH Coel. 39671 | Isle of Man | 1991 |
| Alcyonium glomeratum | (Hassall, 1841) | RMNH Coel. 39668 | France, Iles des Glenans | 1994 |
| Alcyonium haddoni | Wright & Studer, 1889 | ZSM 20061191 | Chile, Canal Pitt Chico | 2006 |
| Alcyonium hibernicum | Renouf, 1931 | RMNH Coel. 39661 | Isle of Man | 1991 |
| Alcyonium palmatum | Pallas, 1766 | RMNH Coel. 39685 | NE Spain | 1996 |
| Alcyonium varum | McFadden & Ofwegen, nom. n. | ZSM 20061195 | Chile, Paso del Abismo | 2006 |
| Alcyonium sidereum | Verrill, 1922 | USA, Massachusetts | 1989 | |
| Alcyonium variabile | (Thomson, 1921) | RMNH Coel. 40800 | South Africa, Algoa Bay | 1998 |
| Alcyonium variabile | (Thomson, 1921) | RMNH Coel. 41530 | South Africa, Algoa Bay | 2008 |
| Alcyonium variabile | (Thomson, 1921) | RMNH Coel. 41531 | South Africa, Algoa Bay | 2008 |
| Parasphaerasclera gen. n. | ||||
| Parasphaerasclera aurea | (Benayahu & Schleyer, 1995) | RMNH Coel. 40205 | South Africa, Park Rynie | 2008 |
| Parasphaerasclera aurea | (Benayahu & Schleyer, 1995) | RMNH Coel. 40799 | South Africa, Park Rynie | 2008 |
| Parasphaerasclera aurea | (Benayahu & Schleyer, 1995) | RMNH Coel. 41535 | South Africa, Aliwal Shoal | 2008 |
| Parasphaerasclera aff. grayi | (Thomson & Dean, 1931) | NTM C14092 | Palau, Babeldaob | 2005 |
| Parasphaerasclera aff. grayi | (Thomson & Dean, 1931) | RMNH Coel. 40920 | Palau, Pelelieu | 2010 |
| Parasphaerasclera rotifera | (Thomson, 1910) | UF3890 | South Africa, East London | 1999 |
| Parasphaerasclera valdiviae | (Kükenthal, 1906) | RMNH Coel. 40206 | South Africa, Algoa Bay | 2008 |
| Parasphaerasclera valdiviae | (Kükenthal, 1906) | RMNH Coel. 41532 | South Africa, Algoa Bay | 2008 |
| Parasphaerasclera valdiviae | (Kükenthal, 1906) | RMNH Coel. 41534 | South Africa, Algoa Bay | 2008 |
| Sphaerasclera gen. n. | ||||
| Sphaerasclera flammicerebra | (Williams, 2003) | MNHN-IK-2012-12004 | New Caledonia | 2008 |
| Sphaerasclera flammicerebra | (Williams, 2003) | ZMUC-ANT-000256 | Mauritius | 1929 |
Sclerites were obtained by dissolving tissues from the upper and lower regions of a colony in 10% sodium hypochlorite (household bleach). Sclerites were rinsed well with deionized water, dried, and mounted on stubs for SEM. They were imaged using a JEOL JSM-6480LV scanning electron microscope operated at 10 kV.
Extraction of DNA from ethanol-preserved tissue samples, PCR amplification, and sequencing of the mtMutS (msh1) and COI genes followed the protocols published in
The separate maximum likelihood analyses of the 28S rDNA and mitochondrial gene alignments generated phylogenies that were congruent with one another (with the sole exception of some internal relationships within Pennatulacea), but were poorly resolved overall. The partitioned analyses of the combined mt + 28S dataset had much higher support values for many of the deeper nodes within the tree, and it is these analyses that we present (Fig. 1) and discuss here. As has been demonstrated previously based on analyses of similar datasets (
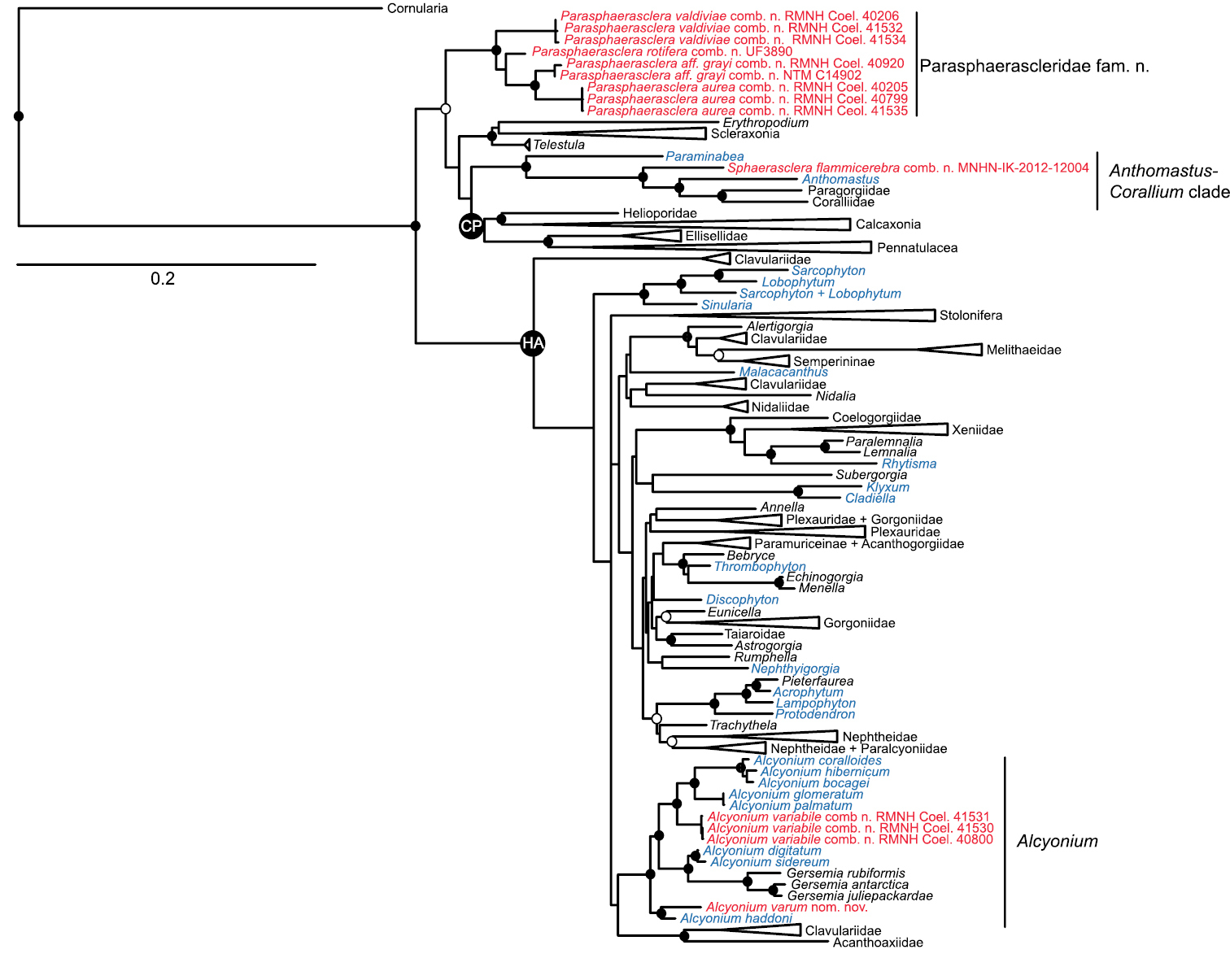
Maximum likelihood tree of Octocorallia based on combined, partitioned analysis of mtMutS, COI and 28S rDNA sequences. Taxa belonging to family Alcyoniidae are shown in blue; new combinations proposed herein are shown in red. Solid circles at nodes indicate strong support from both maximum likelihood (bootstrap values >70%) and Bayesian inference (posterior probability > 0.95); open circles indicate moderate support (bootstrap values >50%, Bayesian pp > 0.95). Strongly supported clades that include no alcyoniid taxa have been collapsed to triangles to facilitate readability. HA: Holaxonia–Alcyoniina clade; CP: Calcaxonia–Pennatulacea clade. Hexacorallian taxa used as outgroups are not shown. For a list of all reference taxa and sequences included in the analysis see Appendix.
Members of family Alcyoniidae were distributed throughout Holaxonia–Alcyoniina in nine distinct clades (Fig. 1). Some of these clades included only alcyoniid genera, while others supported close relationships among alcyoniids and genera classified in different families. There was insufficient resolution of the deeper nodes within Holaxonia–Alcyoniina to infer the phylogenetic relationships of any of these nine clades to one another. In addition, four genera of Alcyoniidae fell entirely outside of Holaxonia–Alcyoniina (Fig. 1). These included Paraminabea and Anthomastus, both of which belonged to the Anthomastus–Corallium clade, as well as several species of Eleutherobia and Alcyonium that formed a distinct, well-supported clade whose relationship to the Calcaxonia–Pennatulacea, Anthomastus–Corallium and Scleraxonia–Telestula clades was poorly resolved. This clade included Eleutherobia aurea Benayahu & Schleyer, 1995, Eleutherobia rotifera (Thomson, 1910), Eleutherobia aff. grayi (Thomson & Dean, 1931), and Alcyonium valdiviae Kükenthal, 1906. Two of the species of Eleutherobia we sequenced were not included in this clade: Eleutherobia variabile (Thomson, 1921) fell into a clade with northern hemisphere members of the genus Alcyonium, while Eleutherobia flammicerebra Williams, 2003 belonged to the Anthomastus–Corallium clade, phylogenetically distinct from both Paraminabea and Anthomastus (Fig. 1).
Alcyonium has long served as a repository for species that lack characters to support their placement in other more narrowly circumscribed genera. Over time the diagnosis of the genus has been broadened to include almost every possible colony growth form observed within Alcyoniidae (
Phylogenetic evidence suggests that genus Alcyonium should be further restricted to species in which the polyp sclerites are arranged as a collaret and points and the sclerites of the coenenchyme are in two distinct layers, a surface layer consisting of predominantly radiates or clubs, and an inner layer of spindles or rods (
Bellonella studeri Thomson, 1910, a species with a clavate to capitate growth form similar to that of Alcyonium variabile comb. n., was reassigned to Eleutherobia by
Our molecular phylogenetic analyses included a specimen of Alcyonium roseum Ofwegen, Häussermann & Försterra, 2007, a species recently described from Chile. We note that that name is pre-occupied by Alcyonium roseum (Tixier-Durivault, 1954), and hereby designate the Chilean species Alcyoniumvarum nom. nov. Etymology: from the Latin varus, crooked or bow-legged, denoting the shape of the sclerites in the polyps (
http://zoobank.org/3DF0B00F-CB14-4B4C-A28A-0AF019EA35E4
http://species-id.net/wiki/Sphaerasclera
Figs 2–3Eleutherobia flammicerebra Williams, 2003, by original designation.
Soft corals with a capitate growth form, with a distinct, spherical polyparium raised on a sterile stalk. Polyps monomorphic. Anthocodial sclerites absent. Sclerites of colony surface and interior are large, tuberculate spheroids and smaller radiates. Sclerites permanently colored. Azooxanthellate.
From the Latin/Greek sphaera- meaning a sphere or ball and Greek sclero-, hard, denoting the large, spheroidal sclerites that characterize this genus. Gender: fem.
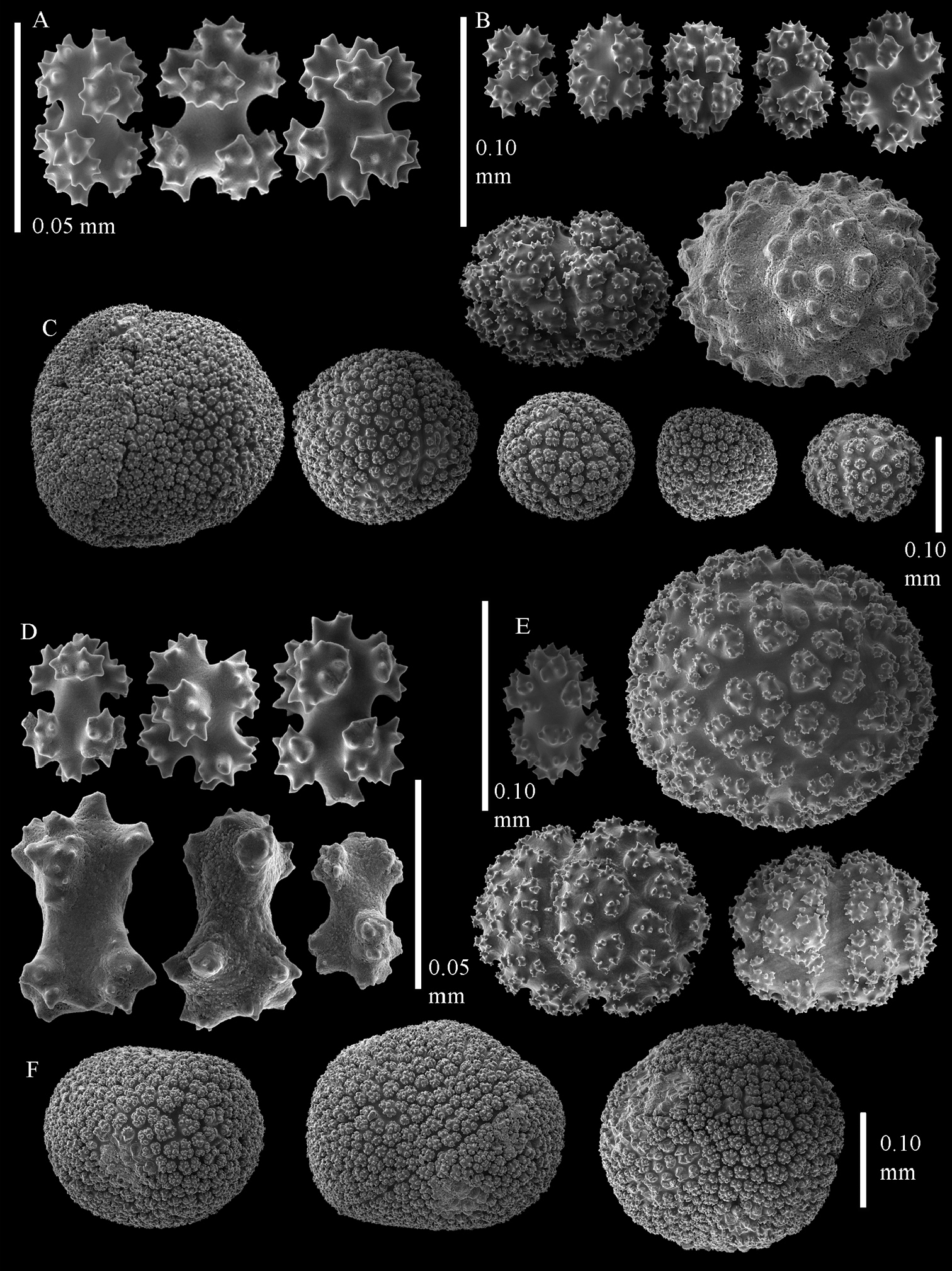
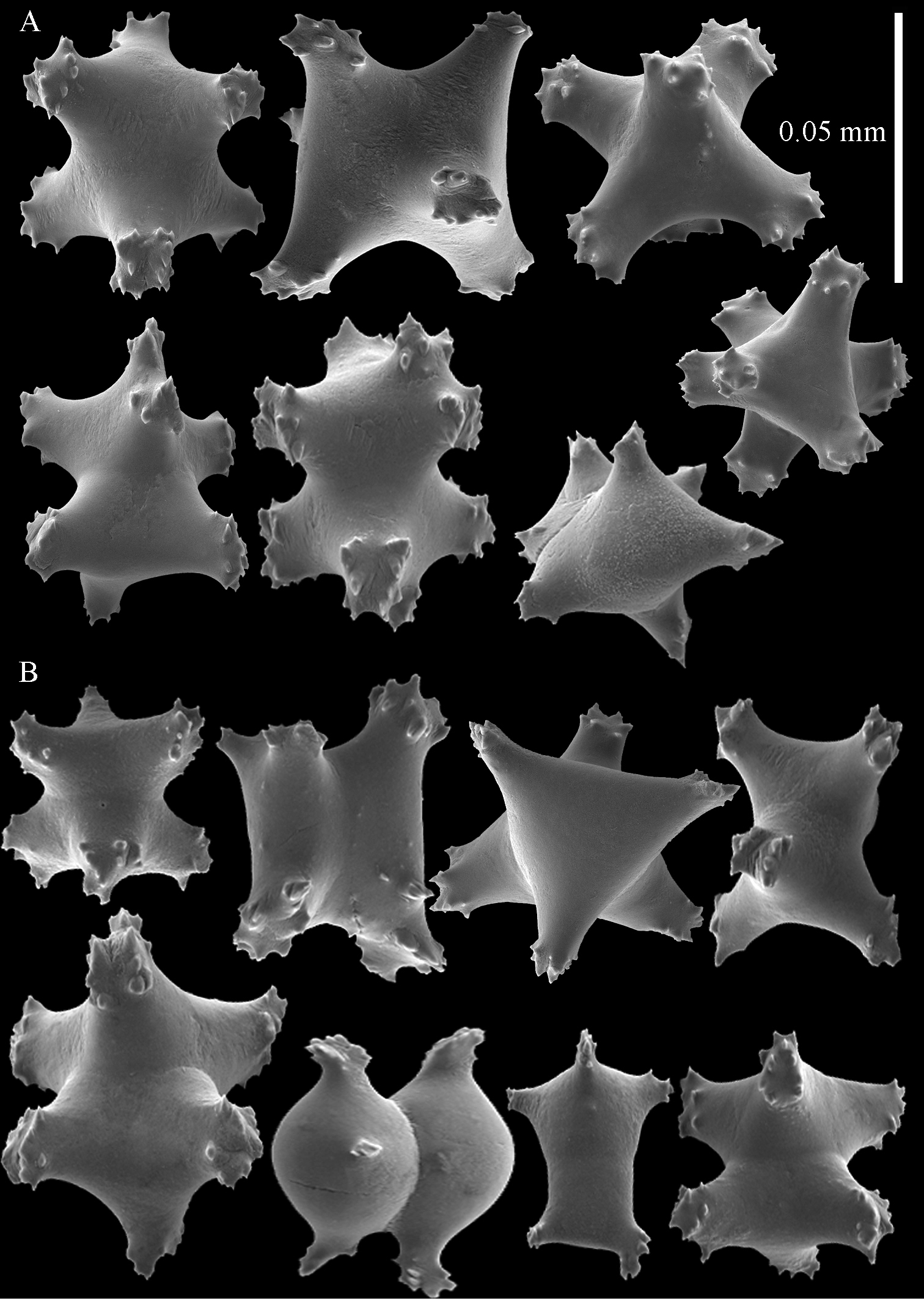
Sclerites of Sphaerasclera flammicerebra comb. n. MNHN-IK-2012-12004. A–C Surface layer of polyparium A Small radiates (0.05 mm scale bar) B Larger radiates and two small spheroids C Large tuberculate spheroids D–F Interior of polyparium D Small radiates (0.05 mm scale bar) E Larger radiate and three small spheroids F Large tuberculate spheroids.
Sclerites of Sphaerasclera flammicerebra comb. n. ZMUC-ANT-000256. Sclerites of polyparium. A. Small radiates (left of 0.05 mm scale bar) B Larger radiates C Large tuberculate spheroids of colony surface and interior.
Unlike all other members of the Anthomastus-Corallium clade, Sphaerasclera gen. n. appears to have monomorphic rather than dimorphic polyps. As discussed by
Although Sphaerasclera gen. n. differs by having monomorphic polyps, it does share other morphological characters with Paraminabea and Anthomastus, the two genera with which it is most closely allied phylogenetically (Fig. 1). Anthomastus likewise includes species with capitate growth forms, but in that genus the autozooids have sclerites, and the sclerites in the surface and interior of the colony include rods and needles in addition to radiates (
Paraminabea and Anthomastus are both classified in family Alcyoniidae, and for convenience we have also assigned Sphaerasclera gen. n. to that family. All other genera of Alcyoniidae, however, belong to the Holaxonia–Alcyoniina clade of Octocorallia, far removed phylogenetically from the Anthomastus-Corallium clade (Fig. 1). In addition to Paraminabea, Anthomastus and Sphaerasclera gen. n., the Anthomastus-Corallium clade also includes all members of Coralliidae Lamouroux, 1812 and Paragorgiidae Kükenthal, 1916, two families of gorgonians that have historically been assigned to the sub-ordinal group Scleraxonia. Although it is clear from the phylogenetic evidence that the soft coral taxa that fall within this clade should be removed from Alcyoniidae, we defer their reassignment to another family pending an in-depth analysis of the morphological character states shared among the seemingly heterogeneous genera and families that are united within Anthomastus-Corallium.
Our records extend the known geographic distribution of Sphaerasclera flammicerebra comb. n. from Palau (
Parasphaerasclera McFadden & Ofwegen, gen. n.
Parasphaerasclera gen. n.
Soft corals with a digitiform, digitate or lobate growth form, usually with a sterile stalk although this may be indistinct. Polyps monomorphic. Permanent calyces absent, although retracted polyps may remain visible as small mounds on the polyparium surface. Anthocodial sclerites absent. Sclerites of colony surface and interior predominantly radiates and tuberculate spheroids, occasionally rods and crosses. Sclerites permanently colored. Azooxanthellate.
As diagnosed by
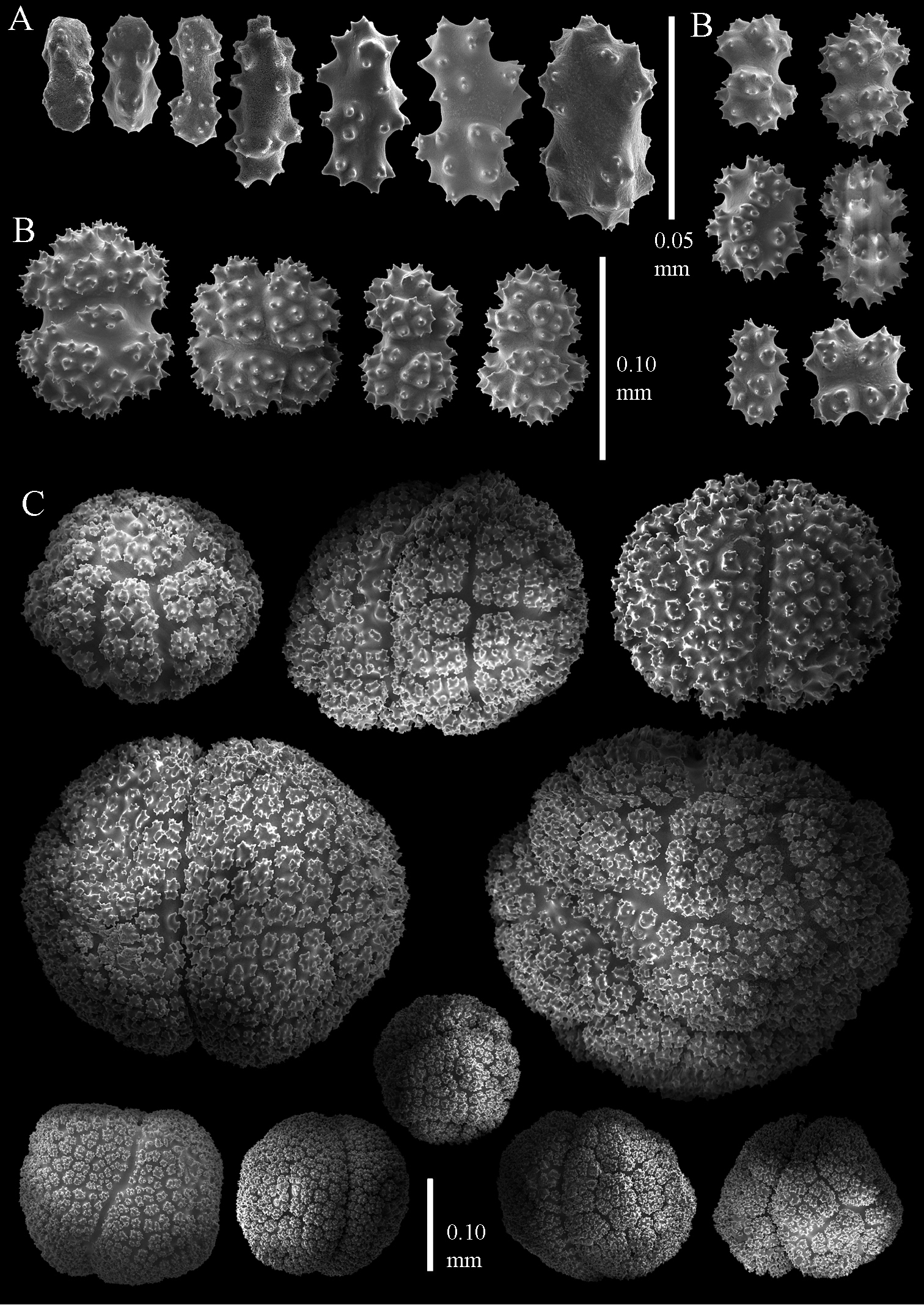
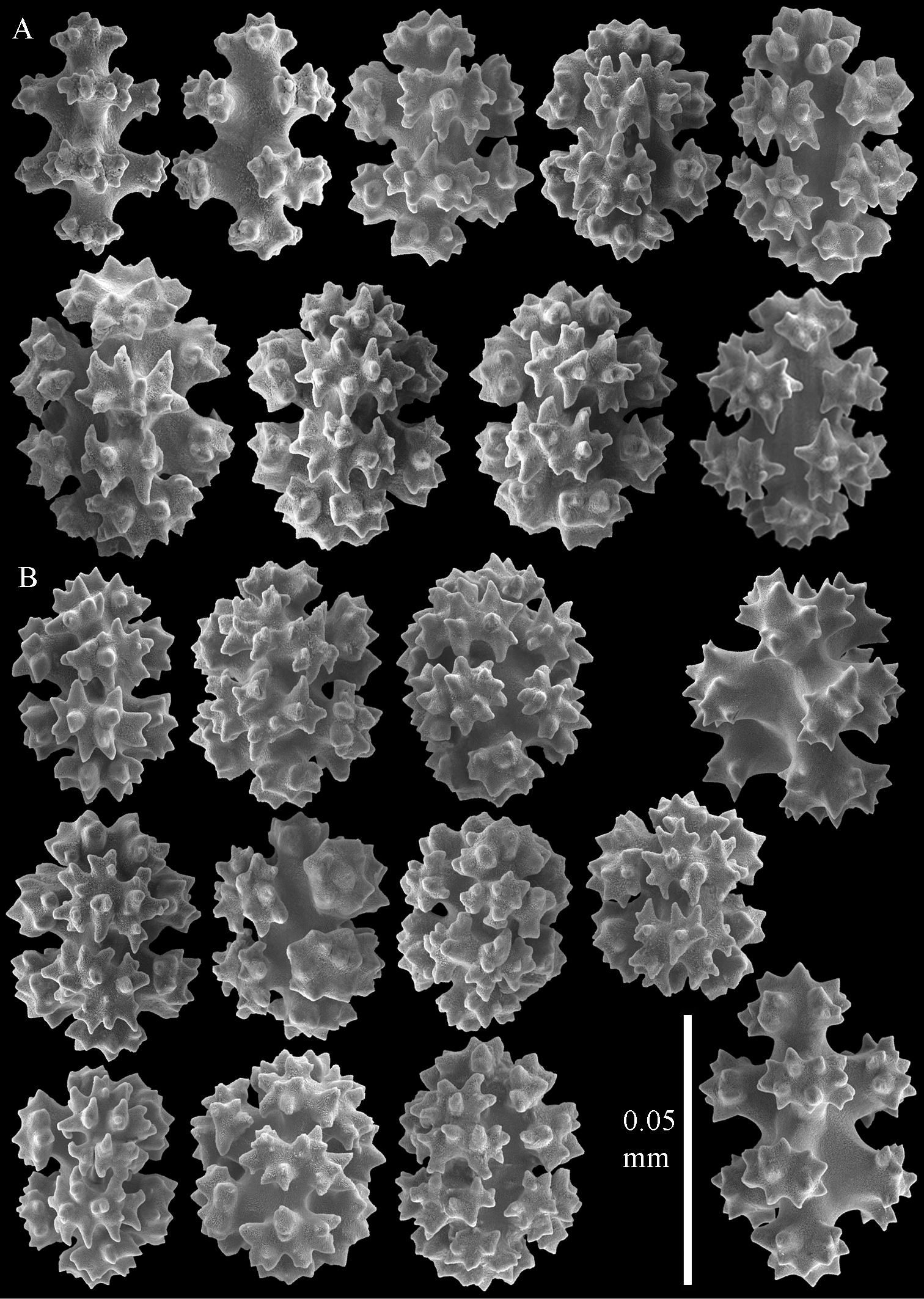
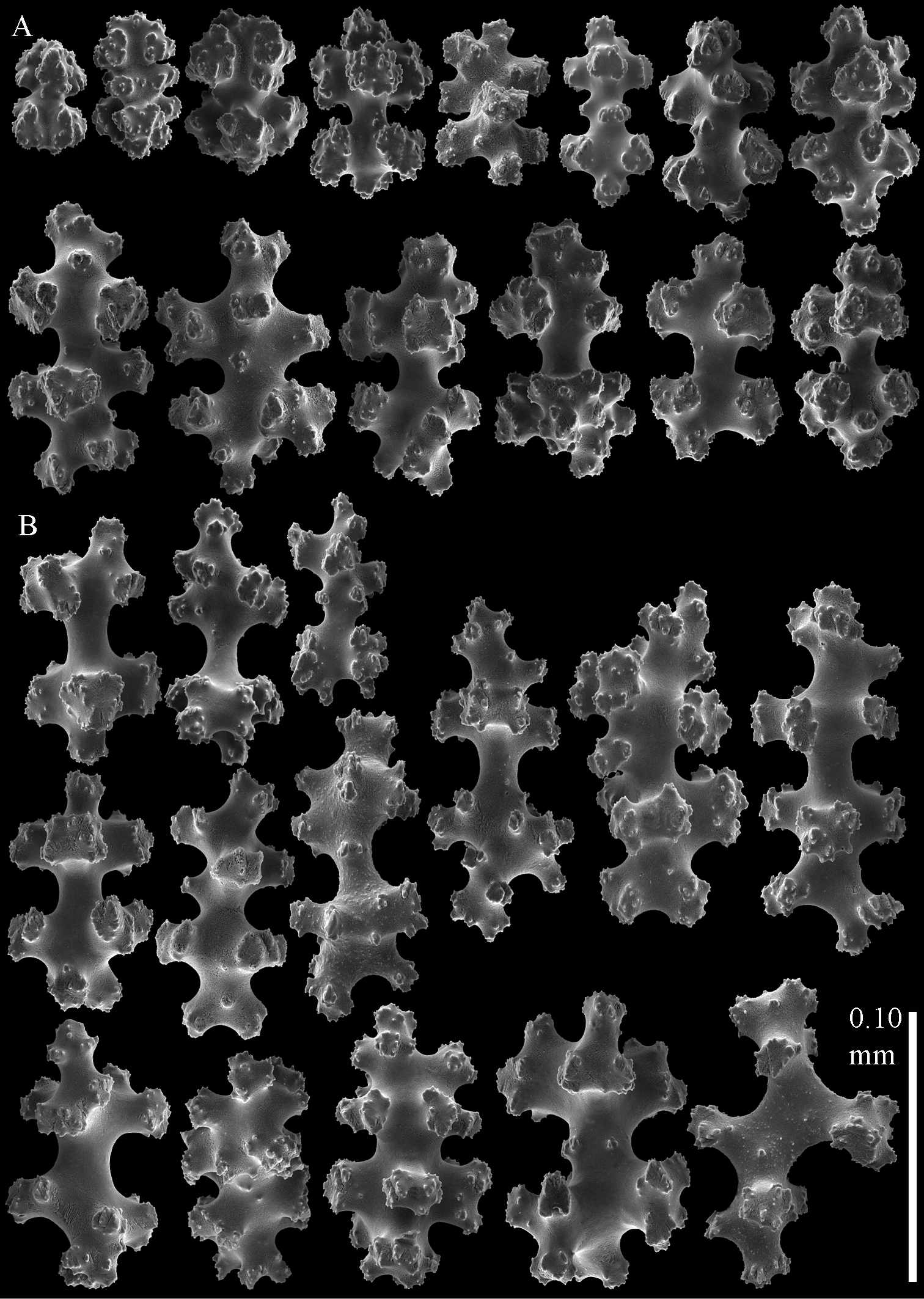
Sclerites of Parasphaerasclera aurea comb. n., RMNH Coel. 40779. A Surface of polyparium B Interior of polyparium.
Sclerites of Parasphaerasclera aurea comb. n., RMNH Coel. 40779. A Surface of stalk B Interior of stalk.
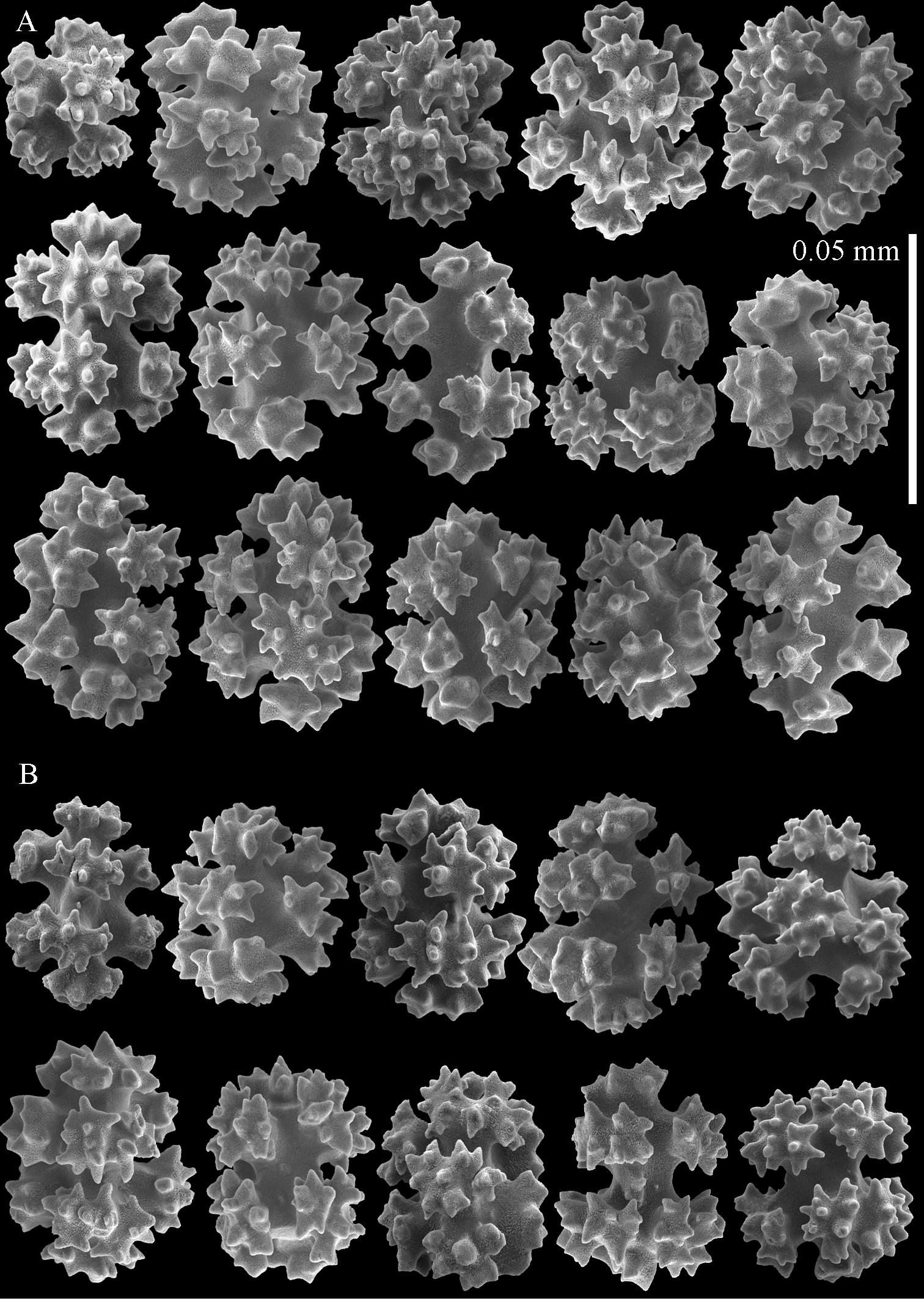
Sclerites of Parasphaerasclera valdiviae comb. n., RMNH Coel. 40206 A Surface layer of polyparium B Interior of polyparium.
Sclerites of Parasphaerasclera valdiviae comb. n., RMNH Coel. 40206 A Surface layer of stalk B Interior of stalk.
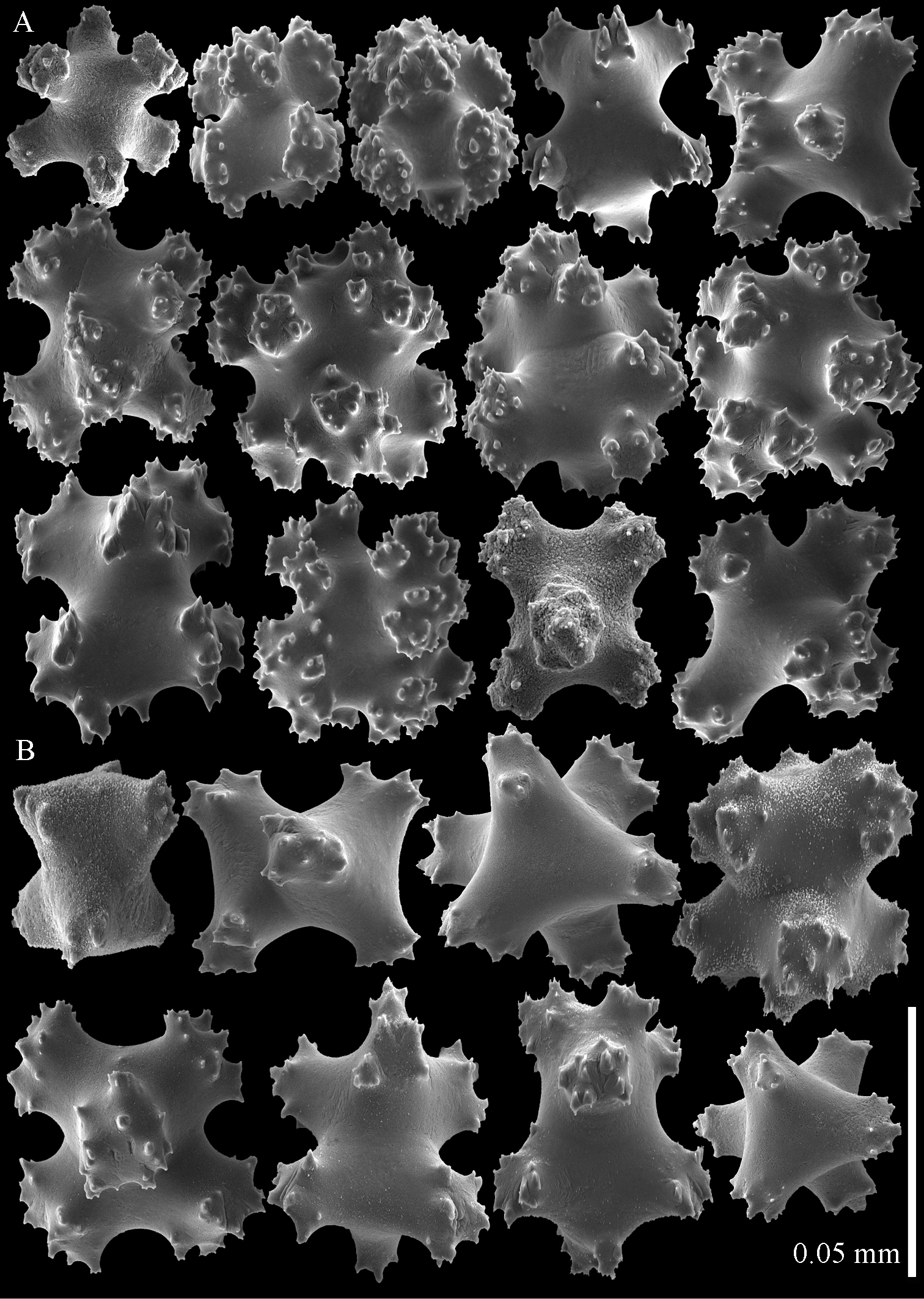
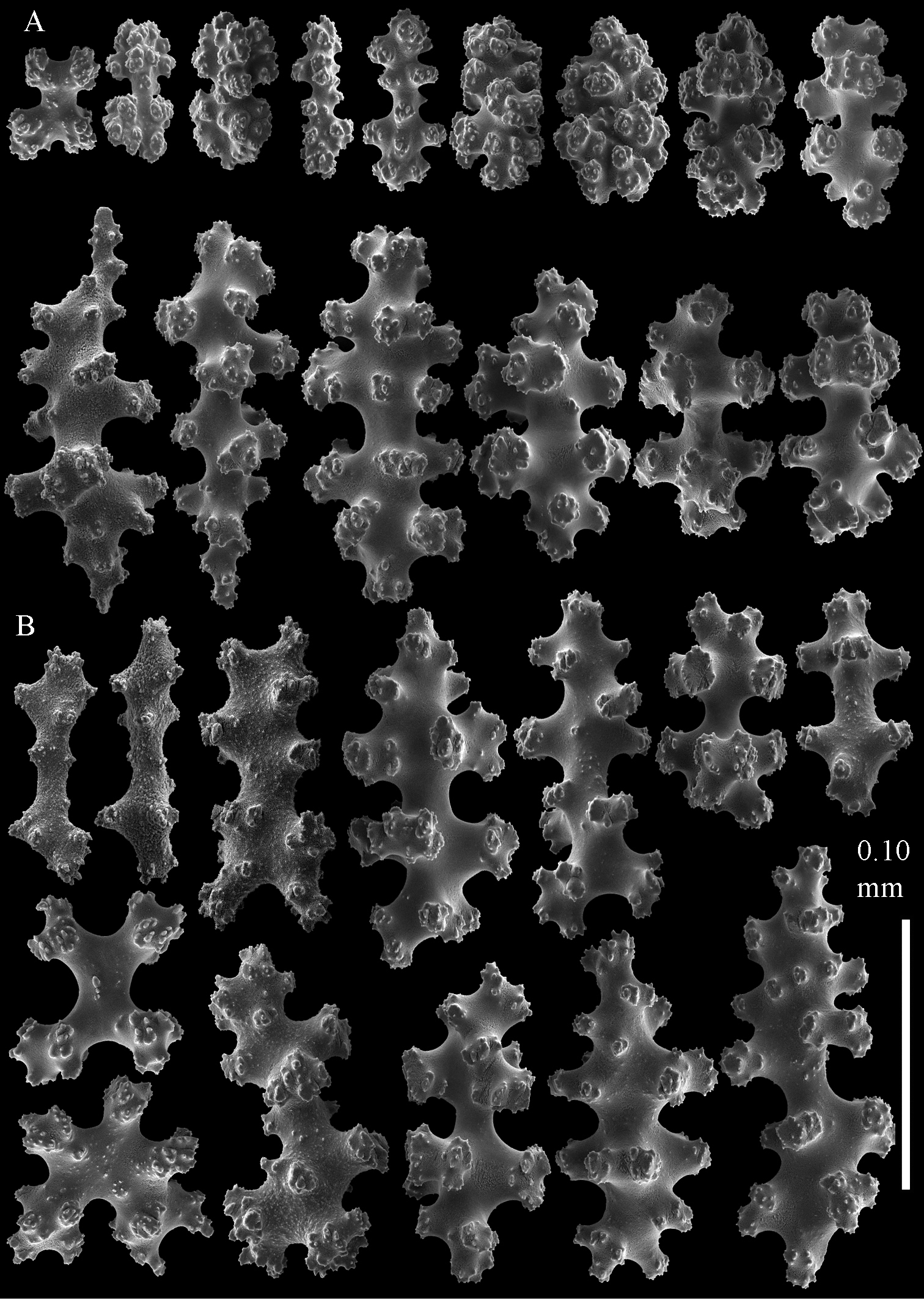
Sclerites of Parasphaerasclera aff. grayi comb. n. RMNH Coel. 40920 A Surface layer of polyparium B Interior of polyparium.
Sclerites of Parasphaerasclera aff. grayi comb. n. RMNH Coel. 40920 A Surface layer of stalk B Interior of stalk.
http://zoobank.org/F0625B3D-65FD-4B94-A535-2FFA46D84AD1
http://species-id.net/wiki/Parasphaerasclera
Figs 4–9Alcyonium rotiferum Thomson, 1910, by original designation.
As for the family.
From the Greek root para-, meaning beside or alongside of Sphaerasclera gen. n. These two genera share similar sclerite complements and forms, and both were previously considered to belong to Eleutherobia. Gender: fem.
All species of Eleutherobia that have digitiform to digitate or lobate growth forms, lack polyp sclerites, and have radiates or spheroids in the colony surface and interior are hereby reassigned to Parasphaerasclera gen. n. These include Parasphaerasclera albiflora (Utinomi, 1957) comb. n., Parasphaerasclera aurea (Benayahu & Schleyer, 1995) comb. n., Parasphaerasclera grayi (Thomson & Dean, 1931) comb. n., Parasphaerasclera nezdoliyi (Dautova & Savinkin, 2009) comb. n., Parasphaerasclera rotifera (Thomson, 1910) comb. n., and Parasphaerasclera zanahoria (Williams, 2000) comb. n. Although molecular data to support their inclusion in this clade are only available for Parasphaerasclera aurea, Parasphaerasclera aff. grayi and Parasphaerasclera rotifera, the other three species all share the diagnostic morphological characters of the family (
We also reassign two species of Alcyonium to Parasphaerasclera gen. n., Parasphaerasclera morifera (Tixier-Durivault, 1954) comb. n. and Parasphaerasclera valdiviae (Kükenthal, 1906) comb. n. The inclusion of Parasphaerasclera valdiviae comb. n. in Parasphaerasclera gen. n. is supported by both molecular phylogenetic (Fig. 1) and morphological evidence. Parasphaerasclera valdiviae comb. n. lacks polyp sclerites, the sclerites found in the surface of the colony are compact radiates and spheroids (Figs 6–7), and it shares with Parasphaerasclera rotifera comb. n. a growth form in which a conspicuous stalk gives rise to either branched or digitate lobes (
Parasphaerasclera morifera comb. n., another speciesfor which we lack molecular data, shares many morphological characters with other species of Parasphaerasclera gen. n.
It is possible that another South African species of Alcyonium, Alcyonium distinctum Williams, 1988, may also belong to this genus. Like other species of Parasphaerasclera gen. n. it lacks sclerites in the polyps, and the sclerites in the stalk surface are tuberculate spheroids and radiates (
A number of specimens of Parasphaerasclera gen. n. are known that differ somewhat from the descriptions of any of the nominal species listed above, and may represent additional, undescribed species of the genus. For example, there is considerable variation among those specimens of Parasphaerasclera grayi comb. n. that have been described and illustrated in the literature. Thomson and Dean’s original description (
Parasphaerasclera gen. n. is most similar morphologically to the alcyoniid genera Paraminabea and Sphaerasclera gen. n. All three genera lack sclerites in the polyps and have spheroids or radiates in the colony surface and interior. The polyps of Paraminabea, however, are dimorphic, while those of Parasphaerasclera gen. n. are monomorphic. The unique capitate growth form of Sphaerasclera gen. n. distinguishes it from all species of Parasphaerasclera gen. n., which are digitiform to digitate or lobate. Parasphaerasclera gen. n. is also easily distinguished from the alcyoniid genera Eleutherobia and Alcyonium sensu stricto, both of which have sclerites arranged to form a collaret and points in the polyps, and spindles or rods in the colony interior.
Following the taxonomic changes we have made here, eleven species remain in Eleutherobia. The morphological heterogeneity of these species and their similarities to some other genera suggest that further taxonomic revisions are likely to be necessary. Six of the remaining species of Eleutherobia have polyps with a distinct collaret and points of spindles; radiates, spindles and club-like sclerites in the colony surface; and spindles in the interior coenenchyme. These species likely belong to Alcyonium sensu stricto. Included among them is the type species of Eleutherobia, Eleutherobia rigida (=Eleutherobia japonica, Pütter, 1900), as well as Eleutherobia grandiflora (Kükenthal, 1906), Eleutherobia rubra (Brundin, 1896), Eleutherobia somaliensis Verseveldt & Bayer 1988, Eleutherobia splendens (Thomson & Dean, 1931), and Eleutherobia unicolor (Kükenthal, 1906). Another four species of Eleutherobia likewise have a collaret and points of spindles but have tuberculate radiates, spheroids and irregular forms in the colony surface, and spindle-like sclerites with narrow pointed ends and thick waists in the colony interior (
Until such time as the phylogenetic relationships among the species that remain in Eleutherobia can be determined, we modify the most recent diagnosis of the genus (
Alcyoniid soft corals, usually digitiform (conical to cylindrical), sometimes digitate to lobate or clavate; polyparium arising from a common unbranched stalk. Polyps monomorphic. Calyces absent, although retracted polyps may form low rounded or mound-like protuberances of the coenenchyme. Anthocodial sclerites present, arranged in points or collaret and points. Coenenchymal sclerites mostly derived from radiates, although spindles, barrels, tuberculate spheroids, rod-like forms or crosses sometimes present. Color permanent and contained in the sclerites. Azooxanthellate.
Molecular phylogenetic analyses of a number of species belonging to the alcyoniid genera Eleutherobia and Alcyonium have highlighted the heterogeneity of these two taxa as well as some overlap between them. As an initial effort to align the morphology-based taxonomic classification of species in these genera with molecular phylogenetic evidence of their evolutionary affinities we have reassigned two species of Eleutherobia with capitate growth forms, polyp sclerites arranged as a collaret and points, and spindles in the colony interior to Alcyonium; assigned a phylogenetically unique species with monomorphic polyps and a capitate growth form to a new genus of Alcyoniidae, Sphaerasclera gen. n.; and designated a new family, Parasphaerascleridae fam. n., and genus, Parasphaerasclera gen. n., to accommodate species with monomorphic polyps that lack sclerites in the polyps and have predominantly radiates and spheroids in the colony surface and interior. These are the first of many taxonomic revisions that will be required to reconcile the classification of genera currently assigned to Alcyoniidae with their phylogenetic relationships. Whereas the revisions we have made herein are well supported by both morphological and molecular phylogenetic evidence, considerably more evidence of both types will be necessary before we can begin to make taxonomic sense of the poorly resolved relationships among the many additional alcyoniid genera that belong to the Holaxonia–Alcyoniina clade of octocorals.
We thank S. Abdalla, C. Hunt, J. Roy, and K. Whitfield for assistance with DNA sequencing. B. Picton, E. Rodriguez, S. Parker-Nance, M. Janes, K. Maloney and the Coral Reef Research Foundation (P. and L. Colin) assisted with collections in South Africa and Palau. Specimens from New Caledonia were provided by the Muséum national d’Histoire naturelle (MNHN; Paris, France), and were collected by E. Pante during the “Terrasses” cruise, undertaken by the MNHN and the Institut de Recherche pour le Développement (IRD). The “Terrasses” cruise, organized by Sarah Samadi (IRD/MNHN), is part of the “Tropical Deep-Sea Benthos” research program (formerly MUSORTSOM), spearheaded by the MNHN and the IRD (
Taxa and sequences included in phylogenetic analysis. (doi: 10.3897/zookeys.346.6270.app) File format: Microsoft Excell file (xls).
Explanation note: Taxa and sequences included in phylogenetic analysis. Museum accession numbers of sequenced specimens given when known. NA: no accession.