(C) 2013 Andja Vučetić. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Vučetić A, Vukov T, Jovičić I, Petrović-Obradović O (2013) Monitoring of aphid flight activities in seed potato crops in Serbia. In: Popov A, Grozeva S, Simov N, Tasheva E (Eds) Advances in Hemipterology. ZooKeys 319: 333–346. doi: 10.3897/zookeys.319.4315
Aphid flight activities in seed potato fields have been studied by the yellow water traps. It is a good method for monitoring aphids as vectors of viruses, but this study also showed it is a suitable method for insect-diversity research. During the four-year studies, over 11.500 specimens were collected and a total of 107 different taxa of aphids were identified. The most abundant species were polyphagous species, such as: Acyrthosiphon pisum (Haris), Aphis fabae Scopoli, Aphis gossypii Gloverand Brachycaudus helichrysi (Kaltenbach). The results of the studies show that diversity of aphids in different regions of Serbia is similar regardless of the altitude and the diversity of terrain. At most sites it ranged from 2 to 3. The highest value was recorded in Begeč, locality in northern part of Serbia, in year 2008, and it was 2.92. The maximum values of the Shannon-Weaver diversity index at all sites were recorded in the first weeks of the monitoring of aphid flight activities. Morisita-Horn similarity index shows no significant differences between sites regardless of altitudes. The sites are grouped by year, not by similarity of relief. In spite of these results, the Chi-square analysis showed highly significant difference in vector frequencies among seasons and sites with more pronounced differences for PVY. As a consequence of differences in vector frequencies, the vector pressure index in some regions was different also. The number of vectors and vector pressure index vary depending on the altitude of localities. At localities at altitudes under 1000 m, they were high. The highest index was at Kotraža, locality in central part of Serbia, in 2007, when PVY index exceeded the value of 180, while for PLRV it was 60. At high altitudes on mountain Golija, above 1100 m, the number of aphids was low, as well as the vector pressure index which indicates that these regions are suitable for producing virus-free seed potato.
Aphids, potato, Shannon-Weaver index diversity, Morisita-Horn similarity index, vectors of viruses
Aphids (Aphididae, Hemiptera) are the most efficient vectors of plant pathogenic viruses therefore they cause serious problems in potato growing. Production of healthy seed potatoes is possible in conditions of reduced number of aphids and their ability to come into contact with the plant and transfer the virus (
After infection of leaves, the virus is translocated into the tubers. In some countries, earlier sowing and haulm destruction is carried out at critical period of virus infection (
The aim of these studies was to determine the biodiversity of aphids and similarity in aphid composition between different regions of Serbia. Also, the aim of these studies was to determine differences in vectors frequency among different sites in Serbia and to calculate the pressure of vectors for the two most important potato viruses (Potato Virus Y – PVY and Potato Leafroll Virus – PLRV), and thus determine which areas are suitable for the cultivation of healthy seed potatoes.
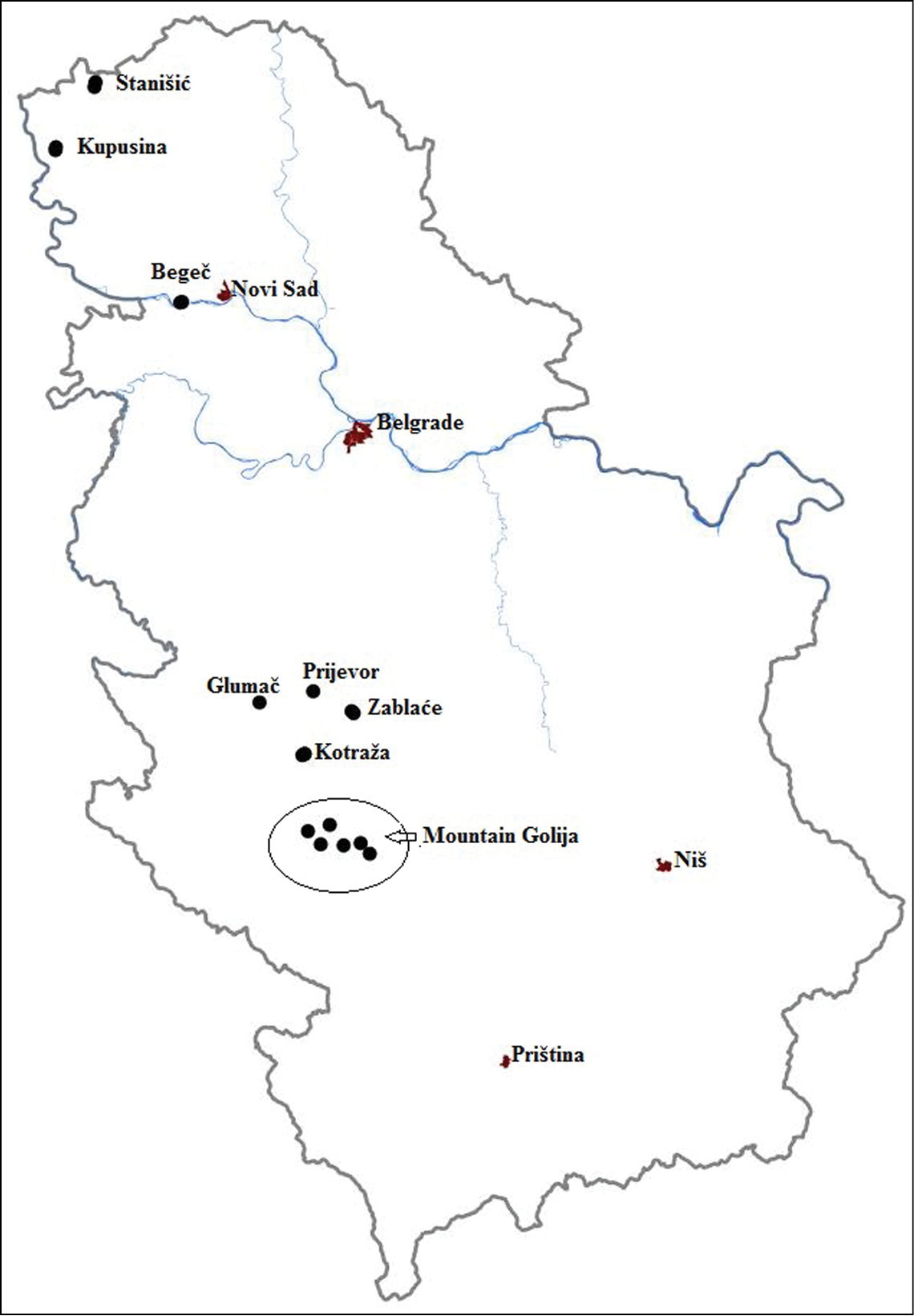
Aphid flight activity was studied in different areas of Serbia in twenty sites for four years (2007–2010). These 20 sites belong to the three major potato growing areas in Serbia. The first area is in northern part of Serbia under the altitudes of 80 m (localities: Begeč, Stanišić, Kupusina). The second one is in central part at altitudes of 400 – 900 m (localities: Kotraža, Zablaće, Prijevor, Glumač), and the third one is in southern part at altitudes above 1100 m (localities on mountain Golija) (Fig. 1). Monitoring of aphid flight activity was conducted by using yellow water traps. Yellow water traps were placed in potato crops (4traps/1ha) immediately after the emergence of potato. Traps have been raised gradually to be visible for aphids during the growth of the crop. Samples were taken once per week until drying of the above-ground mass. Aphids were identified using a stereoscopic microscope (Bio-optica, Italy, Type: 1000) and keys for identification of alatae aphids (
Map of Serbia with monitored aphid flight activities sites. Coordinates of localities: Begeč 2007 (45°13'26"N, 19°36'53"E), Begeč 2008 (45°13'34"N, 19°37'23"E), Glumač 2008 (43°52'27"N, 20°1'1"E), Golija 1 2007 (43°27'45"N, 20°20'58"E), Golija 2 2007 (43°21'58"N, 20°32'12"E), Golija 1 2008 (43°26'25"N, 20°14'56"E), Golija 2 2008 (43°23'36"N, 20°24'53"E), Golija 1 2009 (43°24'6"N, 20°29'38"E), Golija 2 2009 (43°23'48"N, 20°18'31"E), Kotraža 2007 (43°42'7"N, 20°13'49"E), Kotraža 2008 (43°42'17"N, 20°13'34"E), Kotraža 2010 (43°41'49"N, 20°13'9"E), Kupusina 2008 (45°44'24"N, 19°0'6"E), Kupusina 2009 (45°43'49"N, 18°59'58"E), Stanišić 2008 (45°56'48"N, 19°10'51"E), Stanišić 2009 (45°57'7"N, 19°9'10"E), Stanišić 2010 (45°57'46"N, 19°11'6"E), Prijevor (43°54'49"N, 20°16'2"E), Zablaće 2 2009 (43°50'51"N, 20°26'51"E), Zablaće 1 2010 (43°50'27"N, 20°27'25"E).
Shannon–Weaver index was used for the analysis of biodiversity (
During the four-year studies, over 11.500 specimens were collected and a total of 107 different taxa of aphids were identified. Seventy five different taxa were identified to the species level, thirty two to genus level. Thirty six heteroecious species and thirty nine monoecious species were identified (Table 1). According to the data
Identified aphid taxa (PVY vectors of PVY, PLRV vectors of PLRV).
| Monoecious species | Heteroecious species | Genus |
|---|---|---|
| Acyrthosiphon cyparissiae (Koch) | Anoecia corni (F.) | Acyrthosiphon spp. |
| Acyrthosiphon malvae (Mosley) | Aphis fabae ScopoliPVY /PLRV | Amphorophora spp. |
| Acyrthosiphon pisum (Haris)PVY | Aphis gossipii GloverPLRV | Anoecia spp. |
| Amphorophora rubi (Kaltenbach) | Aphis nasturtii Kaltenbach | Aphis spp. |
| Aphis craccivora Koch | Aphis spiraecola Patch | Brachycaudus spp. |
| Aphis idaei van der Goot | Aphis sambuci L. | Capitophorus spp. |
| Aphis pomi De Geer | Aulacorthum solani (Kaltenbach)PVY/PLRV | Cavariella spp. |
| Atheroides serrulatus Haliday | Brachycaudus cardui (L.) | Chaitophorus spp. |
| Brevycorinae brassicae (L.) | Brachycaudus helichrysi (Kaltenbach)PVY | Cinara spp. |
| Callipterinela calliptera (Hartig) | Capitophorus eleagni (del Guercio) | Dysaphis spp. |
| Callipterinela tuberculata (von Heyden) | Cavariella theobaldi (Gillete and Bragg) | Eriosoma spp. |
| Chaitophorus populialbe (Boyer de Fonscolombe) | Cryptomyzus galeopsidis (Kaltenbach) | Euceraphis s pp. |
| Cinara tujafilina (del Guercio) | Cryptomyzus ribis (L.) | Forda spp. |
| Ctenocallis setosus (Kaltenbach) | Dysaphis plantaginea (Passerini) | Hyadaphis spp. |
| Drepanosiphum aceris Koch | Eriosoma ulmi (L.) | Hyperomyzus spp. |
| Eucalipterus tiliae (L.) | Forda marginata Koch | Macrosiphoniella spp. |
| Euceraphis betulae (Koch) | Hyadaphis foeniculi (Passerini) | Microlophium spp. |
| Eriosoma lanigerum (Hausmann) | Hyalopterus pruni complex | Myzocallis spp. |
| Hyadaphis polonica Szelegiewicz | Hyperomyzus lactuce (L.) | Myzus spp. |
| Lachnus roborus (L.) | Hyperomyzus pallidus Hille Ris Lambers | Ovatus spp. |
| Lipaphis erysimi (Kaltenbach) | Hyperomyzus picridis (Börner and Blunck) | Pemphigus spp. |
| Macrosiphum albifrons Essig | Macrosiphum euphorbiae (Thomas)PVY/PLRV | Periphyllus spp. |
| Macrosiphum funestrum (Macchiati) | Macrosiphum rosae (L.) | Protaphis spp. |
| Megoura viciae Buckton | Metopolophium dirhodum (Walker)PVY | Protrama spp. |
| Megourella purpurea Hille Ris Lambers | Myzus cerasi (Fabricus) | Rhopalosiphum spp. |
| Myzocallis castanicola Baker | Myzus persicae (Sulzer)PVY/PLRV | Semiaphis spp. |
| Myzocallis occidentalis Remaudie et Nieto Nafria | Nasonovia ribis-nigri (Mosley) | Sipha spp. |
| Myzodium modestum (Hottes) | Phorodon humuli (Schrank)PLRV | Subsaltusaphis spp. |
| Myzus ligustri (Mosley) | Rhopalomyzus poae (Gill) | Tetraneura spp. |
| Ovatus inulae (Walker) | Rhopalosiphoninus staphylleae (Koch)PLRV | Therioaphis spp. |
| Phyllaphis fagi (L.) | Rhopalosiphum maidis (Fitch) | Tuberculatus spp. |
| Pterocallis alni (de Geer) | Rhopalosiphum nimfaeae (L.) | Uroleucon spp. |
| Schizaphis graminum (Rondani) | Rhopalosiphum padi (L.)PVY | |
| Sipha elegans del Guercio | Sitobion fragariae (Walker) | |
| Sipha maydis Passerini | Smynthurodes betae Westwood | |
| Sitobion avenae (Fabricius) | Trichosiphonaphis polygonifoliae (Shinji) | |
| Therioaphis trifolii (Monell) | ||
| Tinocallis platani (Kaltenbach) | ||
| Wahlgreniella ossiannilssoni Hille Ris Lambers |
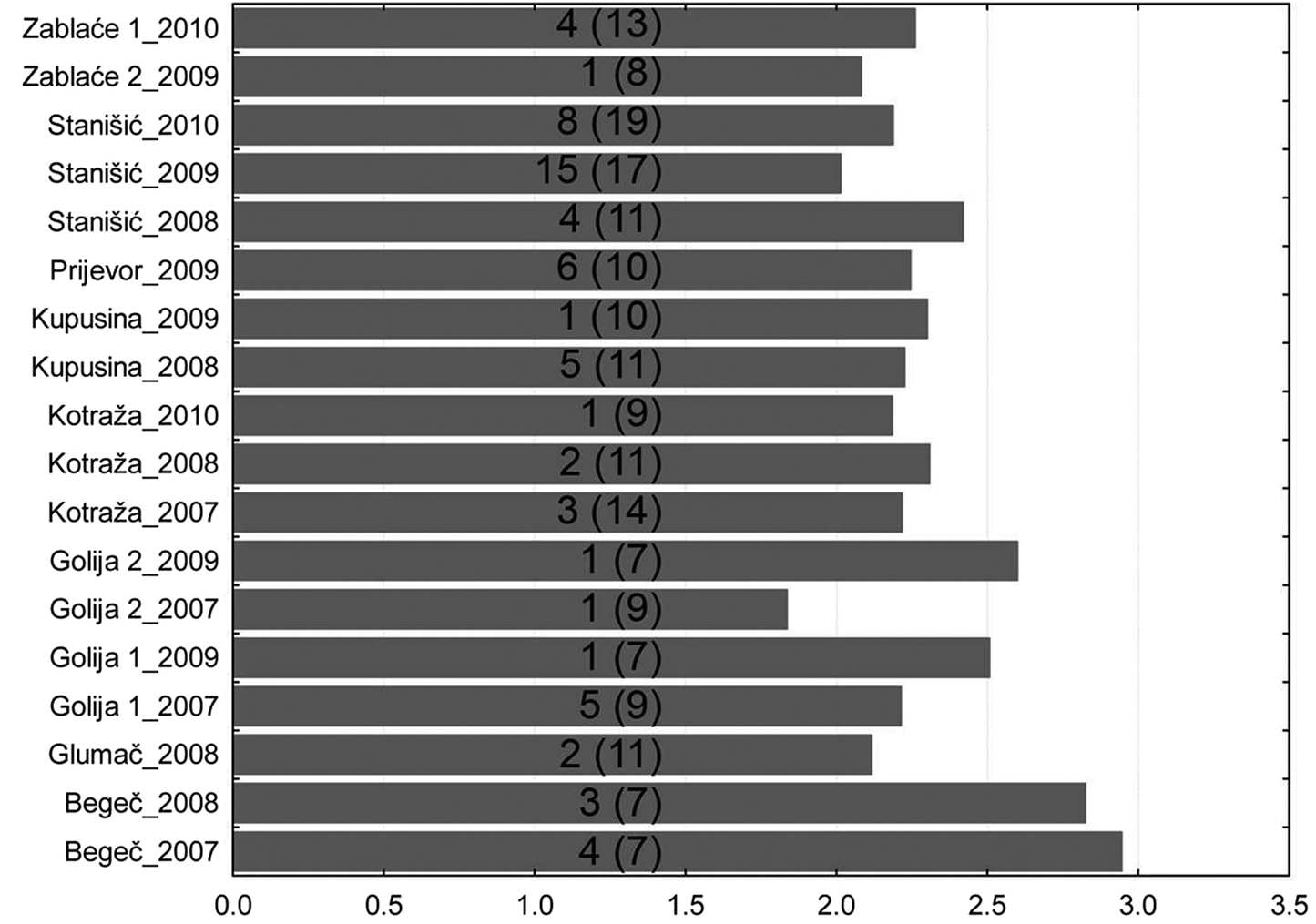
Results from 20 different localities were used for the analysis of biodiversity using Shannon–Weaver index. The maximum values of the Shannon–Weaver index diversity at all sites were recorded in the first weeks of the monitoring of aphid flight activities (Fig. 2).
Maximum of Shannon–Weaver index per locality (number in brackets - number of weeks of monitoring aphid flight activities, number without brackets - week with maximum value of Shannon–Weaver index).
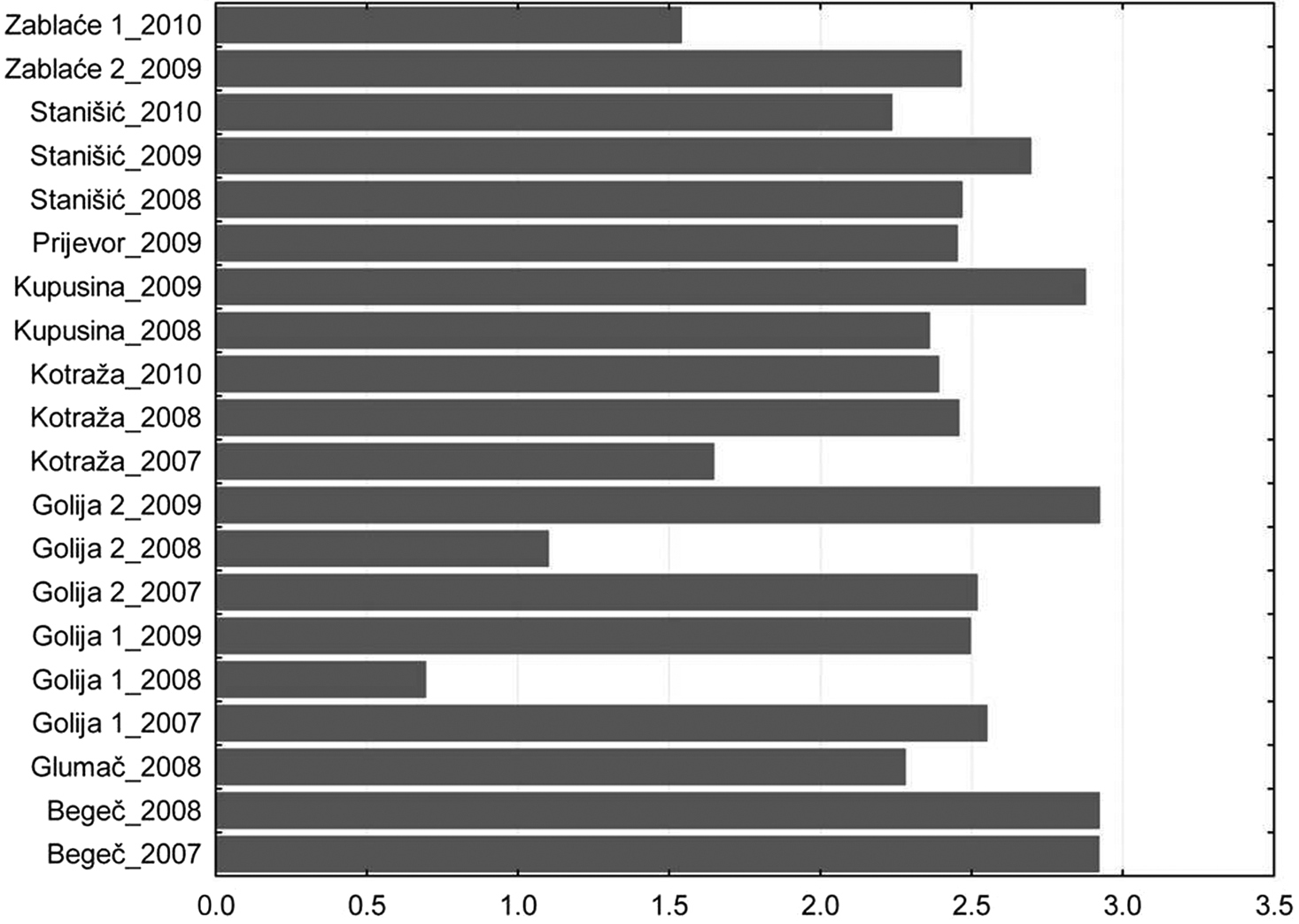
The results of studies show that diversity of aphids in different regions of Serbia is similar regardless of the altitude and the diversity of terrain. At most sites, it ranged from 2 to 3. The highest value was recorded in Begeč in 2008, where it was 2.92. The lowest values were on the mountain Golija in 2008, where on the locality at the lower altitudes was 0.69 and at the higher altitudes was 1.098. In that year, in two localities on this mountain, a total of 5 aphid specimens were caught (Fig. 3).
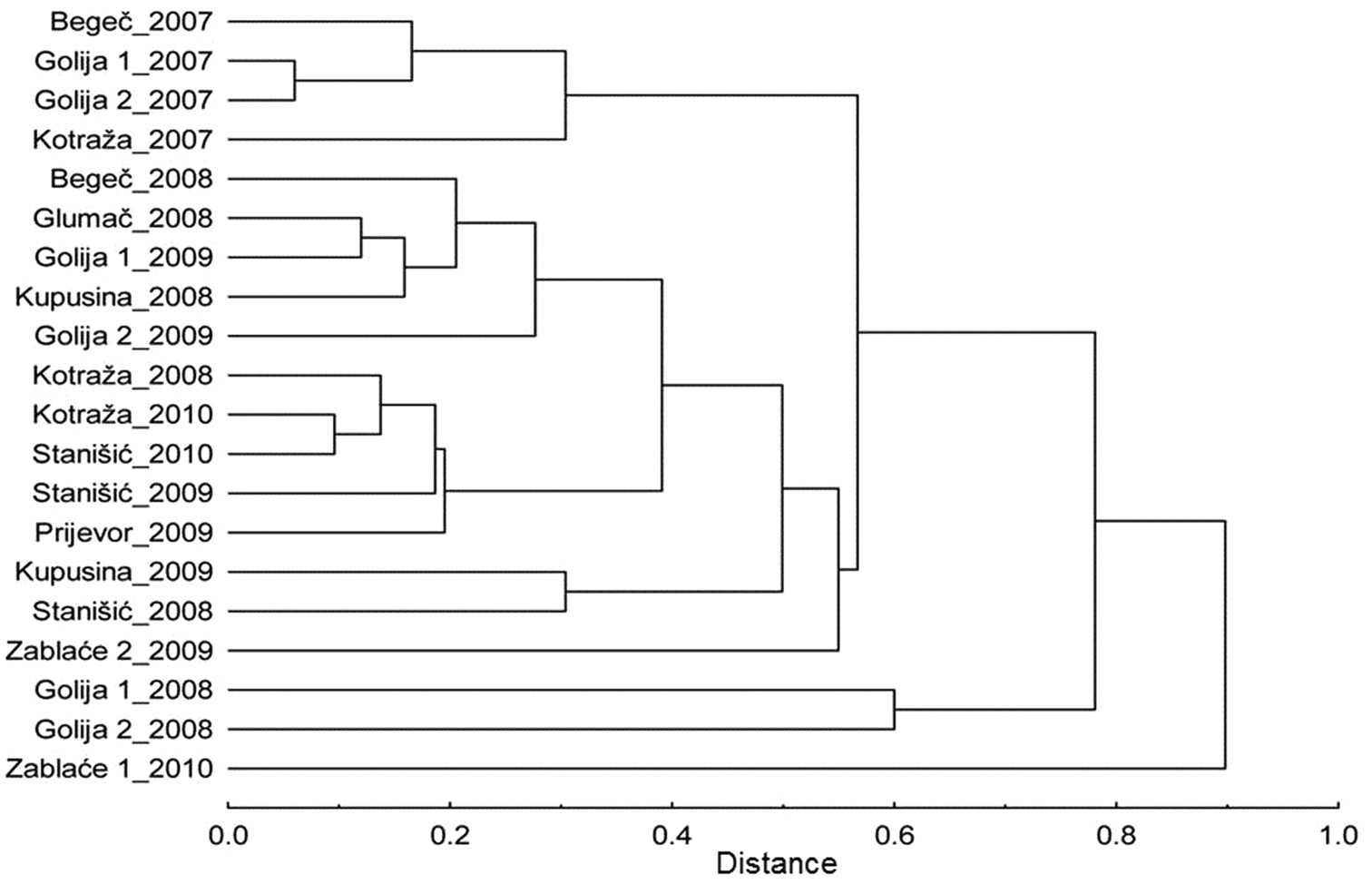
Also, Morisita–Horn similarity index shows no significant differences in aphid composition between sites regardless of altitudes. The cluster analysis on the basis of this index was carried out (Fig. 4). The sites are grouped by year, not by similarity of relief. Sites on the mountain Golija were clearly separated from the rest because of the low number of aphids in the year 2008. In the locality Zablaće, five aphid species were caught which were not recorded previously in other localities during these studies. These species are: Chaitophorus populialbae (Boyer de Fonscolombe), Myzocallis castanicola Baker, Myzocallis occidentalis Remaudie et Nieto Nafria, Protrama spp. and Sminthuroides betae Westwood.
Total Shannon–Weaver index diversity per locality.
Dendrogram shows similarity between the sites, constructed on the basis of Morisita–Horn similarity index.
Important potato virus vectors such as: Acyrthosiphon pisum (Haris), Aphis fabae Scopoli, Aphis gossypii Glover, Aulacorthum solani (Kaltenbach), Brachycaudus helichrysi (Kaltenbach), Macrosiphum euphorbiae (Thomas), Metapolophium dirhodum (Walker), Myzus persicae (Sulzer) and Rhopalosiphum padi (L.) were found in almost all localities, but in different numbers. The most important vector of potato viruses Myzus persicae, was found in all localities, but in high number just in localities Begeč and Kotraža in 2007.
The Chi–square analysis showed highly significant difference in vector frequencies among seasons and sites, with more pronounced differences for PVY (Table 2).
Results from Chi–square analysis used to compare the different sites by the number of vectors for PVY and PLRV viruses.
| PVPLRV | Begec 2007 | Begec 2008 | Glumac 2008 | Golija 1 2007 | Golija 1 2009 | Golija 2 2007 | Golija 2 2009 | Kotraza 2007 | Kotraza 2008 | Kotraza 2010 | Kupusina 2008 | Kupusina 2009 | Prijevor 2009 | Stanisic 2008 | Stanisic 2009 | Stanisic 2010 | Zablace 1 2010 | Zablace 2 2009 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Begec 2007 | ||||||||||||||||||
| Begec 2008 | ||||||||||||||||||
| Glumac 2008 | ||||||||||||||||||
| Golija 1 2007 | ||||||||||||||||||
| Golija 1 2009 | ||||||||||||||||||
| Golija 2 2007 | ||||||||||||||||||
| Golija 2 2009 | ||||||||||||||||||
| Kotraza 2007 | ||||||||||||||||||
| Kotraza 2008 | ||||||||||||||||||
| Kotraza 2010 | ||||||||||||||||||
| Kupusina 2008 | ||||||||||||||||||
| Kupusina 2009 | ||||||||||||||||||
| Prijevor 2009 | ||||||||||||||||||
| Stanisic 2008 | ||||||||||||||||||
| Stanisic 2009 | ||||||||||||||||||
| Stanisic 2010 | ||||||||||||||||||
| Zablace 1 2010 | ||||||||||||||||||
| Zablace 2 2009 |
* - significant differences at a level of significance α = 0.05
** - significant differences at a level of significance α = 0.01
*** - significant differences at a level of significance α = 0.001
ns - not significant differences
Similarity Percentage analysis shown that similarities in presence of PVY vectors between localities in 2007 year was almost 60%. In that year the most common species was Brachycaudus helichrysi, which was dominant species in all localities, but in localities Begeč and Kotraža it was found in large number. In the locality Kotraža over 1500 specimens were caught during the monitoring period. In the next years, large number of the specimens of this species has not been repeated, and because of that there was low similarity percent between localities in this year and localities in the fallowing years. In 2008 and 2010 similarity percent between localities were 70%, but in 2009 it was 50%. In all those years the most common vector species were Aphis fabae and Acyrthosiphon pisum which were most responsible for high percent of similarities between localities. In all years the least number of aphids was recorded in localities in mounting Golija at altitudes above 1100m. There were recorded no significant differences between these localities and locality Prijevor because of low number of aphids in this locality and similar number of species Aphis fabae and Aulacorthum solani. Similarity between localities in presence of PLRV vectors was around 70% in 2008 and 2010. In 2007 it was 51% and in 2009 just 44%. In all years the most common vector species was Aphis fabae. The best average dissimilarity was recorded between localities Begeč in 2007 and 2008 and all others localities because of constantly high number of vectors in this area. Especially high percent was recorded between this locality and localities Golija 2 in all three years, and it was 60%.
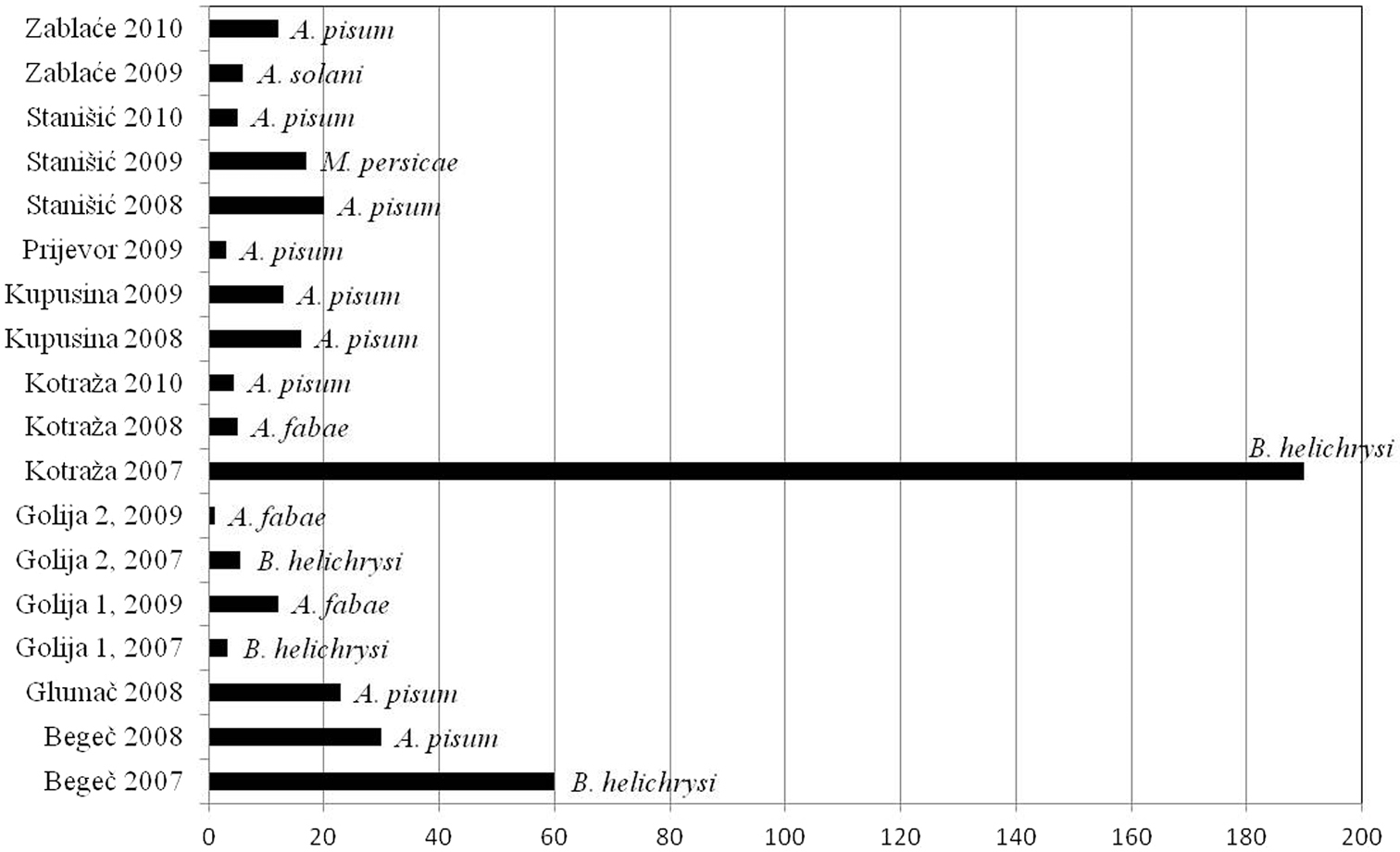
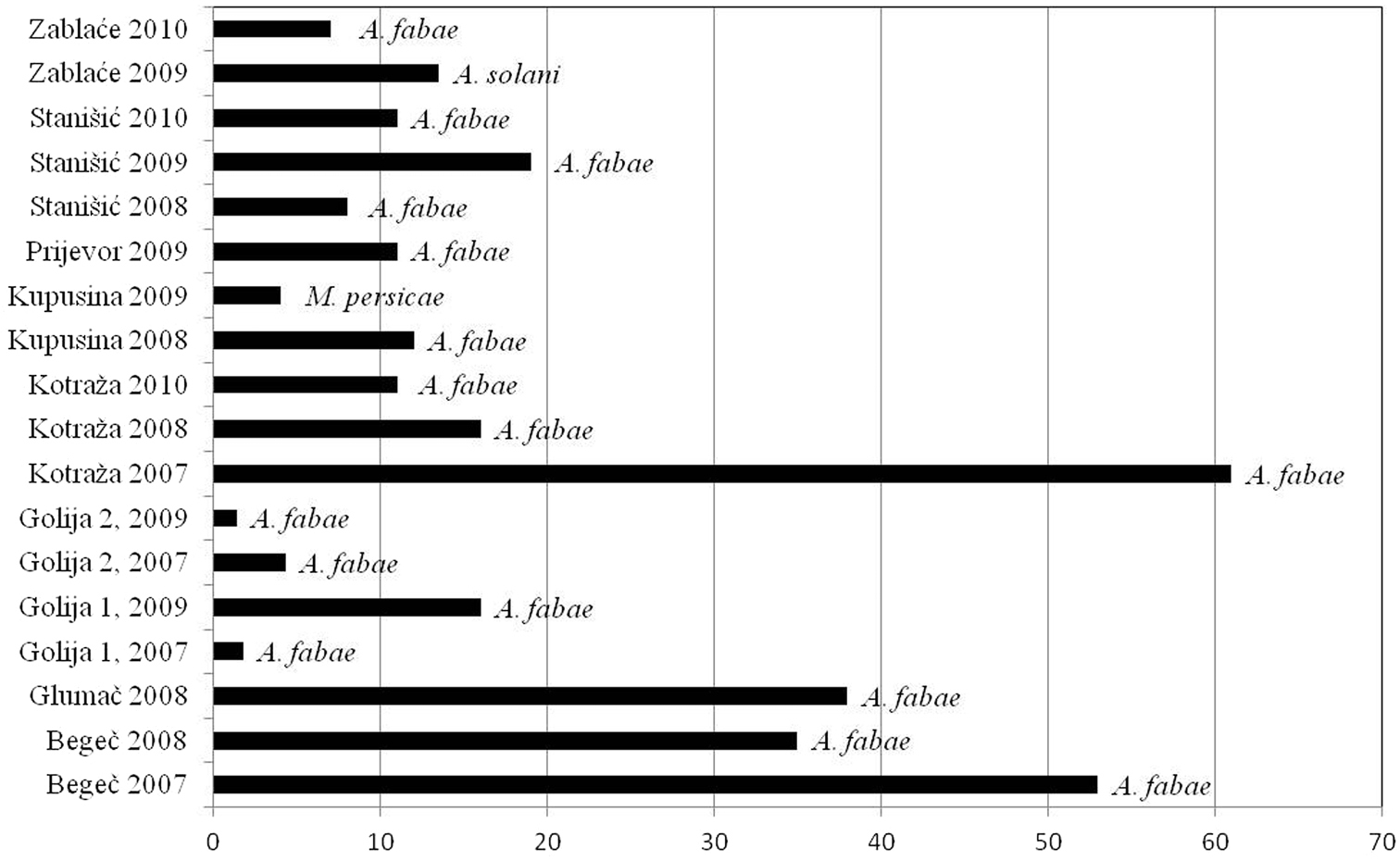
As a consequence of differences in diversity among sites and difference in vector frequencies, the vector pressure index in some regions was different also. The lowest vector number and the lowest vector pressure were observed in Golija mountain area during the study. In localities at above 1100m in Golija, vector pressure index newer exceeded 10. The highest value was recorded in locality Kotraža in 2007 (at altitudes of 850m), when pressure of vectors for PVY exceeded 180, and for PLRV exceeded 60. In the following years, these high values were not repeated. Pressure of vectors for both viruses was constantly high in locality Begeč. In other localities, at the lowest altitudes, Kupusina and Stanišić, values of pressure vectors were low, but reached their maximums early in the season (Figs 5, 6).
Vector pressure index for PVY and most important vector per locality.
Vector pressure index for PLRV and most important vector per locality.
In the studies of biodiversity, in order to obtain a result with a scientific value, it is necessary to standardize sampling method, i.e. adapt it to target organisms. Different types of traps are the best known and the most widely used methods of sampling (
The values of Shannon–Weaver diversity index varied during the growing season. Temperature changes and rain influence the abundance of aphids (
In spite of similar values of Shannon–Weaver diversity index among different localities, participation of the vectors in it is different. The Chi–square analysis showed highly significant difference in vector frequencies among seasons and sites, with more pronounced differences for PVY. As a consequence of differences in vector frequencies, the vector pressure index in some regions was different also. In areas at lower altitudes such as Begeč, a higher number of vector species was registered, as well as more individuals of each present species. Myzus persicae, the most important vector of viruses was found in most localities, but in high number only in localities Begeč and Kotraža, while at the localities above 1100 m it was registered very rarely and in a low number. In localities under 900m, potato sowing is usually done in April, while there is an intensive growth of potato in May when aphid flight is at maximum and virus infection risk is the highest. Potato is the most sensitive in the first development phases, until flowering (
Results of these studies show that only in localities at high altitudes, such as mountain Golija, it is possible to grow healthy, virus free seed potato. This research indicated that the potential of other mountainous regions of Serbia is also high and that Serbia has the capacity for production of quality seed potato. Also, this research may have relevance and application in other neighboring countries, too, because of similar relief, vegetation composition, composition of the fauna of aphids, and the possibility of crop production.
The research was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Project No. III 46008 – Development of integrated management of harmful organisms in plant production in order to overcome resistance and to improve food quality and safety).