(C) 2012 Sergio Roig-Juñent. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Migadopini are a small tribe of Carabidae with 47 species that occur in South America, Australia, and New Zealand, in the sub-Antarctic areas. In South America, most of the genera inhabit areas related to sub-Antartic Nothofagus forest except two monogeneric genera, the Ecuadorian genus Aquilex Moret and the Pampean genus Rhytidognathus Chaudoir. These two genera are geographically isolated from the remaining five South American genera. New material of Rhytidognathus from the northeast of Buenos Aires province and from Entre Ríos province permits establishing that the previous records of Rhytidognathus ovalis (Dejean) for Argentina were erroneous and that it belongs to a new species. Based on external morphological characters and from male and female genitalia we describe Rhytidognathus platensis as a new species. In this contribution we provide illustrations, keys, habitat characteristics and some biogeographic considerations on the distribution of Rhytidognathus.
Migadopini, Rhytidognathus, New species, Male and female genitalia, Distribution
The Migadopini are a small tribe of Carabidae, with 16 genera and 47 species. This tribe was considered related to the Holarctic tribes Elaphrini and Loricerini (
The species of Migadopini are distributed over fragments of the austral Gondwana, called Paleantarctic by
The number of species per genus is low. Of the 16 genera, eight are monospecific, four have two species and the most diverse in number of species is Taenarthrus Broun with 12 species (
Migadopines constitute a characteristic element of the sub-Antarctic biota, and except some frequent species such as the South American Migadops latus (Guérin-Ménéville) the others are scarce in natural history collections, with just a few specimens of several species known. This is the case for the genus Rhythidognathus Chaudoir of which only 12 specimens are known: the holotype of Rhythidognathus ovalis (Dejean), nine more specimens from Uruguay, and two from Argentina. Of these last two specimens, one is lost, and we only have the account by
Ecological studies conducted in the area of La Plata (Buenos Aires, Argentina) yielded the discovery of new specimens of Rhytidognathus, and particularly the capture of males allowed establishing that the previously cited species of Rhytidognathus from Argentina (
The objective of the present contribution is to describe this new species, including new data on its habitat, and discuss some biogeographic considerations.
Material examined. The material is held in the following institutions: IADIZA: Instituto Argentino de Investigaciones de las Zonas Áridas (Mendoza, Argentina, Sergio Roig-Juñent); MACN: Museo Argentino de Ciencias Naturales “Bernandino Rivadavia” (Buenos Aires, Argentina, Arturo Roig-Alsina); MLP: Museo de La Plata (La Plata, Argentina, Analía Lanteri).
Dissection methods, measurements, and the terminology used follow previous revisions of Migadopini (
Predictive species distribution models were built using the MAXENT program version 3.4.1 (
Nebria ovalis Dejean, 1831, by monotypy.
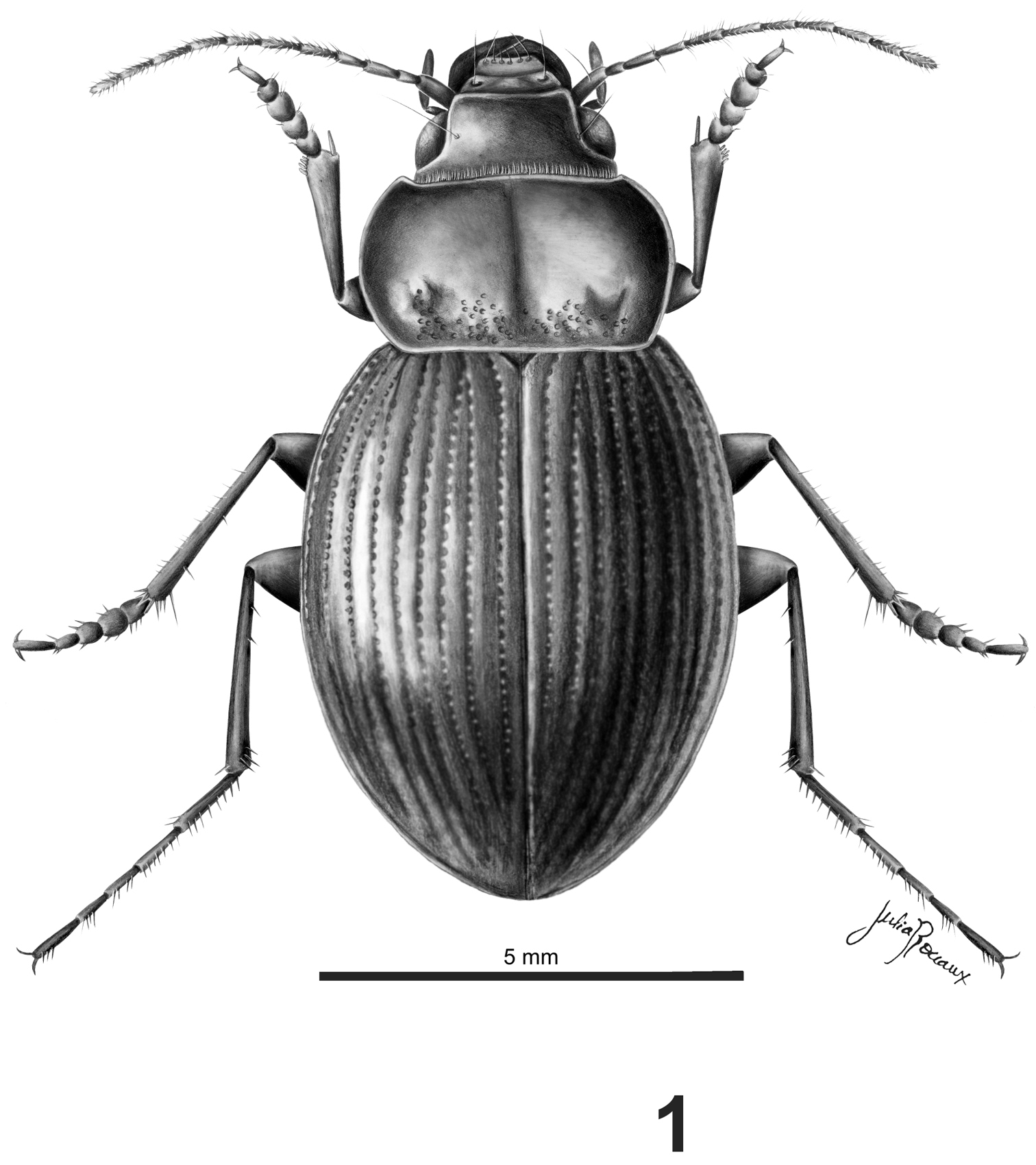
Habitus.Body shape rounded, depressed (Fig. 1)
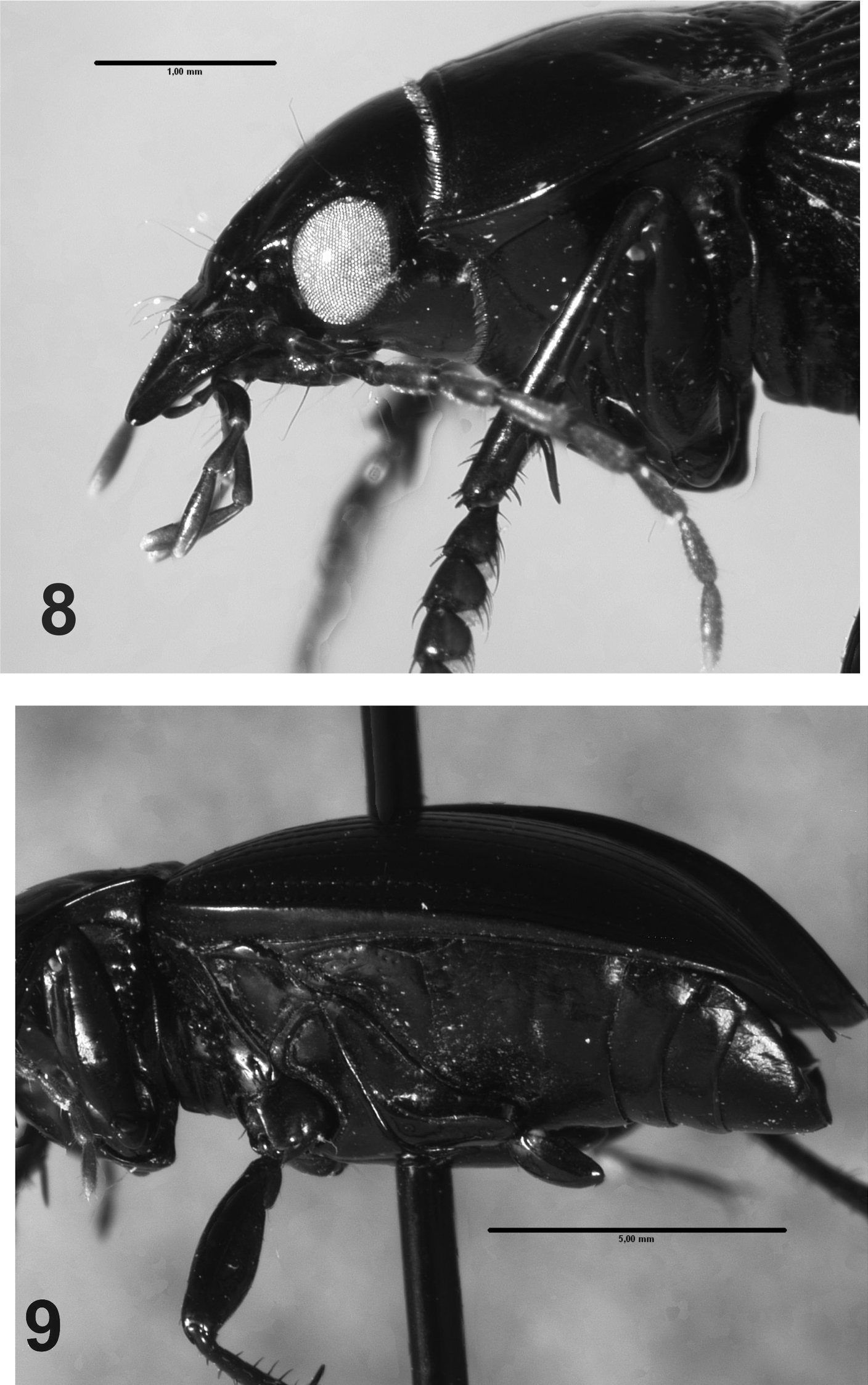
Head.Labrum short, transverse, bilobate at anterior margin; clypeus with two subparallel lateral sulci slightly developed, projected at the base of the frons (Figs 2, 5); mentum and submentum not fused, mentum with four setae, two lateral to the tooth, and two at the base; mentum-tooth bifid; glossa with a central carina, with two apical setae; glossa with two setae, paraglossae rounded, not projected; galea biarticulate, distal article as long as anterior one; mandibles with several dorsal transverse sulci; last maxillary and labial palpomeres long and truncate at apex; antennomeres three times as long as wide; antennae long, reaching the base of the elytra (Fig. 5); antennomeres fusiform, pubescent from the fifth antennomere (Fig. 8).
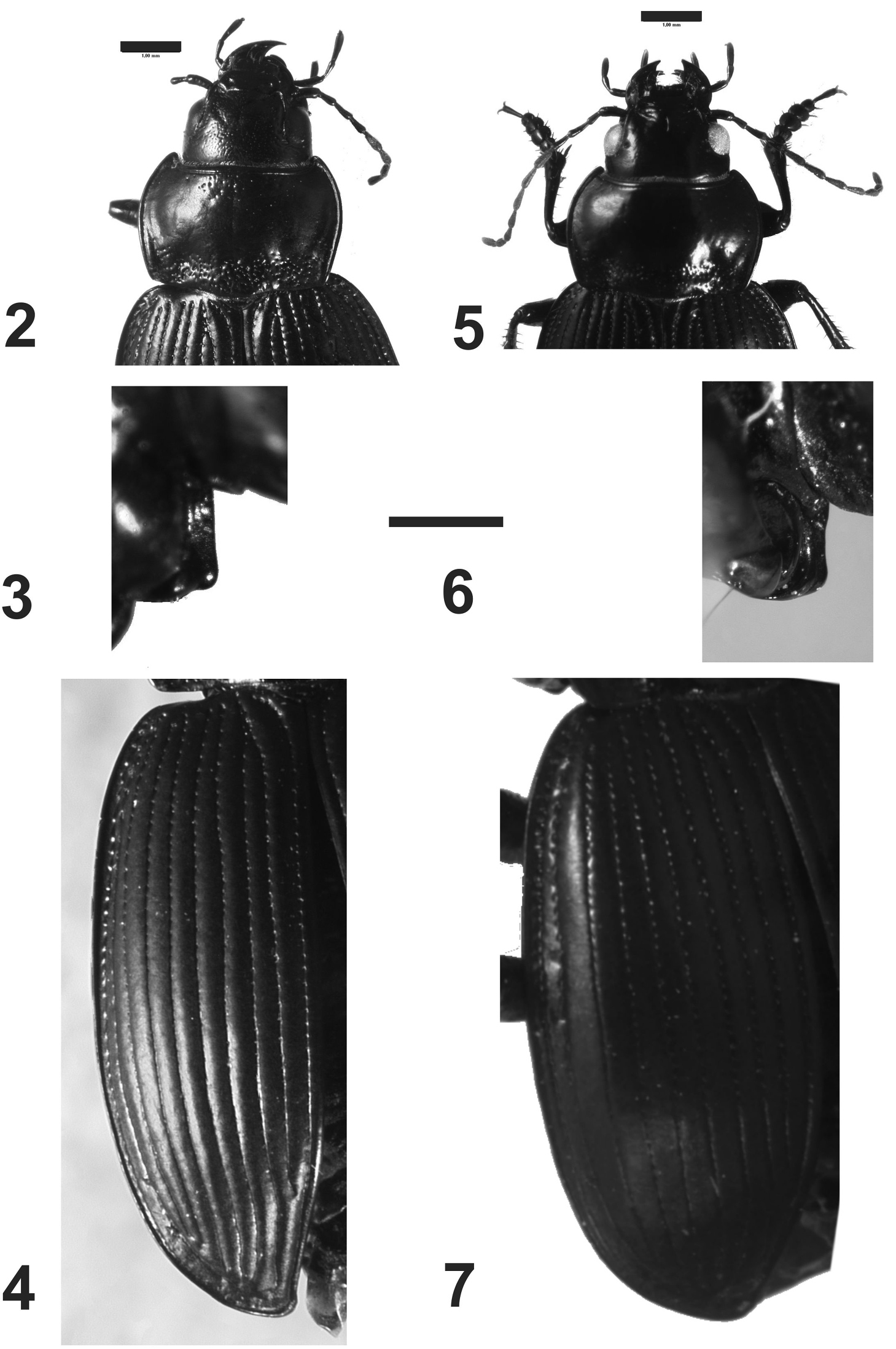
Prothorax.Pronotum wide, wider than head, with anterior angles projected forward (Figs 2, 5); median line slightly delimited; base of pronotum with strong punctures (Figs 2, 5); pronotum without setae on lateral margin; lateral margin rounded, without sinuosity, base bisinuate; prosternal apophysis with a longitudinal sulcus at apex, and a small protuberance or carina; prosternal apophysis projected posteriorly, but short, not touching the mesosternum, border of apophysis straight (Fig. 3) or concave (Fig. 6).
Pterothorax:mesoepisternum with deep punctures (Fig. 9); metaepisternum with a row of punctures and two apical sulci (Fig. 9); elytra twice as wide as than pronotum, without shoulders (Figs 4, 7), with borders rounded, elytra increasing in width to the apex, the widest part on apical third (Fig. 1); elytral epipleura more than twice wider at base than at apex, decreasing in width from base to apex; scutellar stria complete; striae with punctures, deep on the basal third, shallower on the second third and on apical third imperceptible, striae well delimited and deep all along their length (Fig. 4); setae only on ninth interval, with six or seven setae. Apterous.
Legs. Protarsomeres 1-4 and mesotarsomeres 1-3 of male with adhesive setae, wider than in females. Protrochanters with one seta present. Protarsomeres 2 and 3 of male wider than long; metatarsomeres long.
Abdominal sterna.Sterna III-V constituting more than two thirds of the length of abdomen; sulcus of separation of sterna III-IV and IV-V not reaching the center; female sterna VIII without apical sulcus, with two apical setae. Sternite III and IV with deep basal punctures.
The genus Rhytidognathus shares with Pseudomigadops Jeannelthe characteristic of having the elytral striae punctured and differs from it by having the articles of maxillary and labial palpi elongated and thin, as well as by having the mandibles carined dorsally. This last character is exclusive to the genus within the tribe.
Key for differentiating the species of Rhytidognathus
| 1 | Elytra oval, completely black; labrum black; elytral striae deep, interstriae convex (Fig. 4); superior border of eyes straight; prosternum with a median apical prolongation that projects dorsally (Fig. 3) | Rhytidognathus ovalis |
| – | Elytra more rounded, with interstria 8 reddish; eytral striae marked but not deep, interstriae flat (Fig. 7); labrum with lateral borders yellowish; upper border of eye rounded (Fig. 8); prosternum with a slight swelling in the apical region (Fig. 6) | Rhytidognathus platensis |
http://species-id.net/wiki/Rhytidognathus_ovalis
Male and female, Cerro Colorado Uruguay, Florida(MLP); male Banda Oriental (IADIZA).
Head with deep punctures in front, as well as at the base and apex of pronotum; elytra black, concolor; labrum concolor; legs black or dark red, tarsi reddish; apex of median lobe rounded.
Body shape oval. Length: 12–13 mm; coloration: black; with antennae light colored, reddish, and legs testaceous or dark reddish. Elytra black, concolor.
Head.Head with deep punctures in front, eyes slightly protruding, sub-quadrangular. Maxillary palpi black or dark red.
Prothorax. Wider than long, maximum width at middle (Fig. 2); dorsal surface with deep punctures at base and apex (Fig. 2); lateral margins narrow, curved; central longitudinal sulcus slightly developed; posterior transverse foveae impressed, with deep punctures (Fig. 2); prosternum with punctures; prosternal apophysis prolonged into a carina, which extends straight toward the dorsal region (Fig. 3).
Pterothorax:Elytra. Humeral angles rounded (Fig. 4); striae well impressed, and deeply foveate on basal third (Fig. 4), being less marked toward the apex; six to seven setae only in the ninth interval.
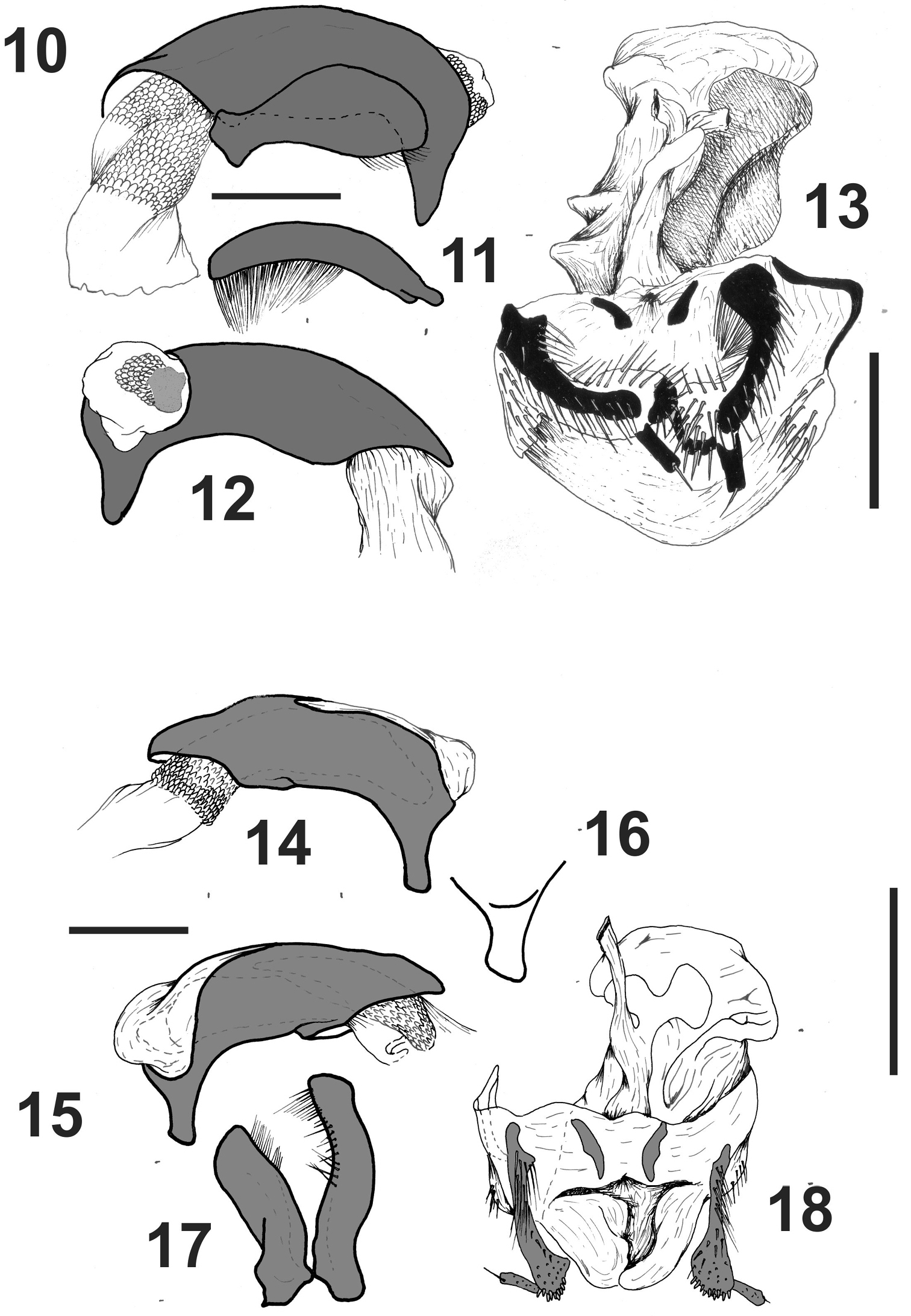
Male genitalia(Figs 10–12). Median lobe wide, with apex rounded (Figs 10-12), apical orifice small, opening laterally to the right with a sclerified plate. Basal orifice wide, closed dorsally (Fig. 10), without basal keel. Left paramere wide with apex rounded (Fig. 11), with setae on apical third (Fig. 11). Right paramere straight and thin, the same width all along its length, with several setae from middle to apex (Fig. 11).
Female genital track(Fig. 13). With gonopod VIII small. Gonopod IX dimerous, the base with two sclerified plates, the apex small and without setae, with subapical setose organ (Fig. 13). Bursa copulatrix big, without accessory glands. Spermatheca on the base of oviduct, digitiform. Bursa copulatrix with a well developed sclerite.
Intraspecific variation.
Uruguay: Montevideo: Montevideo (
Dorsal aspect of male Rhytidognathus platensis (Scale = 5 mm).
Rhytidognathus ovalis: 2 Head and pronotum, dorsal view (Scale = 1 mm) 3 Lateral view of prosternal apophysis (Scale = 1 mm) 4 Dorsal view of elytra 5 Head and pronotum, dorsal view (Scale = 1 mm) 6 Lateral view of prosternal apophysis (Scale = 1 mm) 7 Dorsal view of elytra.
Rhytidognathus platensis: 8 Lateral view of head showing the eyes (Scale = 1 mm) 9 Lateral view of meso-metathorax and abdomen (Scale = 5 mm).
urn:lsid:zoobank.org:act:89A5BF3B-FB4B-4B75-95DA-D86FD0F667C8
Holotype: male, Argentina: Buenos Aires, Los Olmos (MLP); Paratypes, same date, one male two females (MELP, IADIZA); Entre Ríos(MACN), one female.
Head with small punctures, on the borders; elytra black with interstria 8 reddish; labrum with the borders yellowish; interstriae flat; apex of median lobe sub-quadrangular.
Habitus as in Fig. 1. Length: 10.3 mm. Coloration: black; with antennae light colored, reddish, and legs testaceous, dark reddish. Labrum with borders yellowish; elytra black with interstria 8 reddish.
Head. Head with small punctures in front; eyes slightly protruding, rounded (Fig. 8). Maxillary palpi black or dark red.
Prothorax. Wider than long, maximum width at middle (Fig. 5); dorsal surface with punctures on the base (Figs 1, 5), apex with small or no punctures. Lateral margins narrow, curved; central longitudinal sulcus slightly developed; posterior transverse foveae slightly impressed. Posterior angles rounded. Prosternum without punctures or one or two on the apex. Prosternal projections not marginate, with a small apical tubercle, sinuate dorsally (Figs 6, 9).
Metathorax.Elytra with humeral angles rounded (Fig. 7); striae on basal third well impressed, and foveate, less impressed at apex. Ninth interval with six setae; elytral interval flat.
Male genitalia(Figs 14–17). Median lobe wide, with apex sub-quadrangular (Figs 14-16), apical orifice big, open dorsally and straight; basal orifice wide, closed dorsally (Fig. 14), without basal keel. Left paramere wide with apex rounded (Fig. 16), setae on apical third (Fig. 16). Right paramere thin, constricted in the middle, with setae from middle to apex (Fig. 16).
Female genital track(Fig. 18). With gonopod VIII small. Gonopod IX dimerous, the base with two sclerites, the apex small without setae, with apical setose organ (Fig. 18). Bursa copulatrix large, without accessory glands. Spermatheca on the base of oviduct, digitiform. Bursa copulatrix with a large sclerite.
The name of the new species is related to the area where it was collected, La Plata district, near the La Plata river in Buenos Aires Province, Argentina.
Argentina: Buenos Aires: San Isidro(
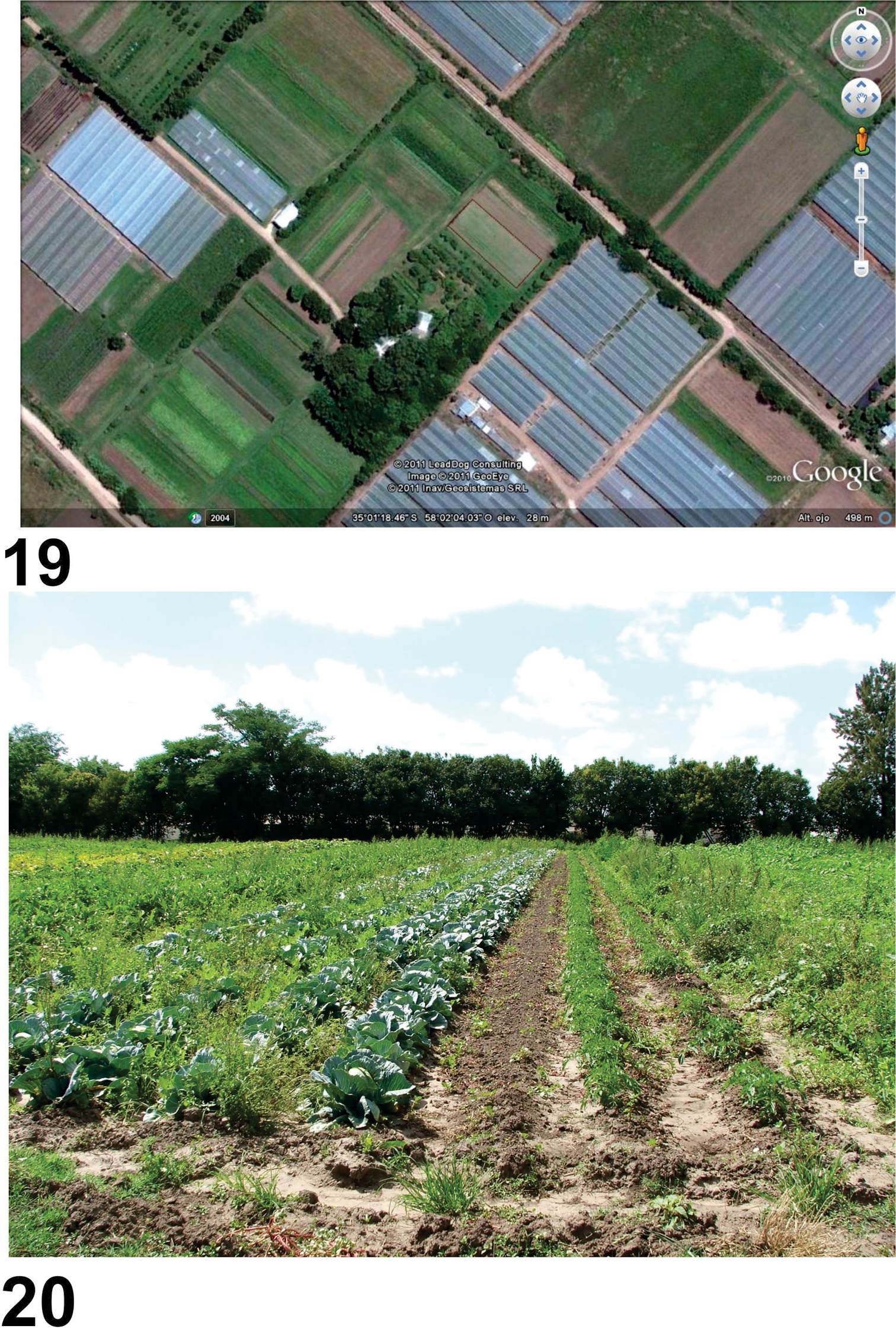
The new material was collected in the locality of Lisandro Olmos (La Plata, Buenos Aires) at “La Nueva Era” farm (35°01'18"S, 58°02'07"W) (Fig. 20), devoted to horticultural production under organic management (Fig. 21). The area has elevations of about 30 m, with soils derived from the Buenos Aires belt corresponding to grassland soils. It is surrounded by horticultural crops grown under cover and in the open, primarily tomato, pepper, leafy vegetables, celery, eggplant and small plots of corn, among others. Cut flower production in greenhouse conditions is also important in this area.
Samples were collected by pitfall traps set up in a 2000 m2-area cultivated with lettuce (Lactuca sativa), onion (Allium cepa), radish (Raphanus sativus), rocket (Diplotaxis sp.), cabbage (Brassica oleracea) and different types of weeds. This habitat has no native vegetation. Probably Rhytidognathus platensis inhabits the patches of semi-natural vegetation surrounding the crops. It has been proven that carabids move between cultivated and uncultivated patches (
On the shores of La Plata river in Buenos Aires province we found two natural habitats. One habitat is close to the river and includes: a) cliffs, with small forest of Celtis tala and other arboreal species, b) riparian shallows extending between the cliffs and the river and constituting a low plain that gets flooded, similar to the marshes of the Paraná river delta. The soil is clay and salty, and the vegetation is characterized by halophytic steppe with dominance of low grasses such as Distichlis spicata. The second habitat, the Pampean plain, lies above the cliffs. This lowland has a temperate climate, with an even year-round precipitation regime, soil type is loam, and the plants that dominate the landscape are herbs that compose the extensive Pampean grassland, a steppe. The typical original plant community comprises species of the genera Stipa and Piptochaetium. This landscape is accompanied on different sites by low shrubs of several species of Bacharis.
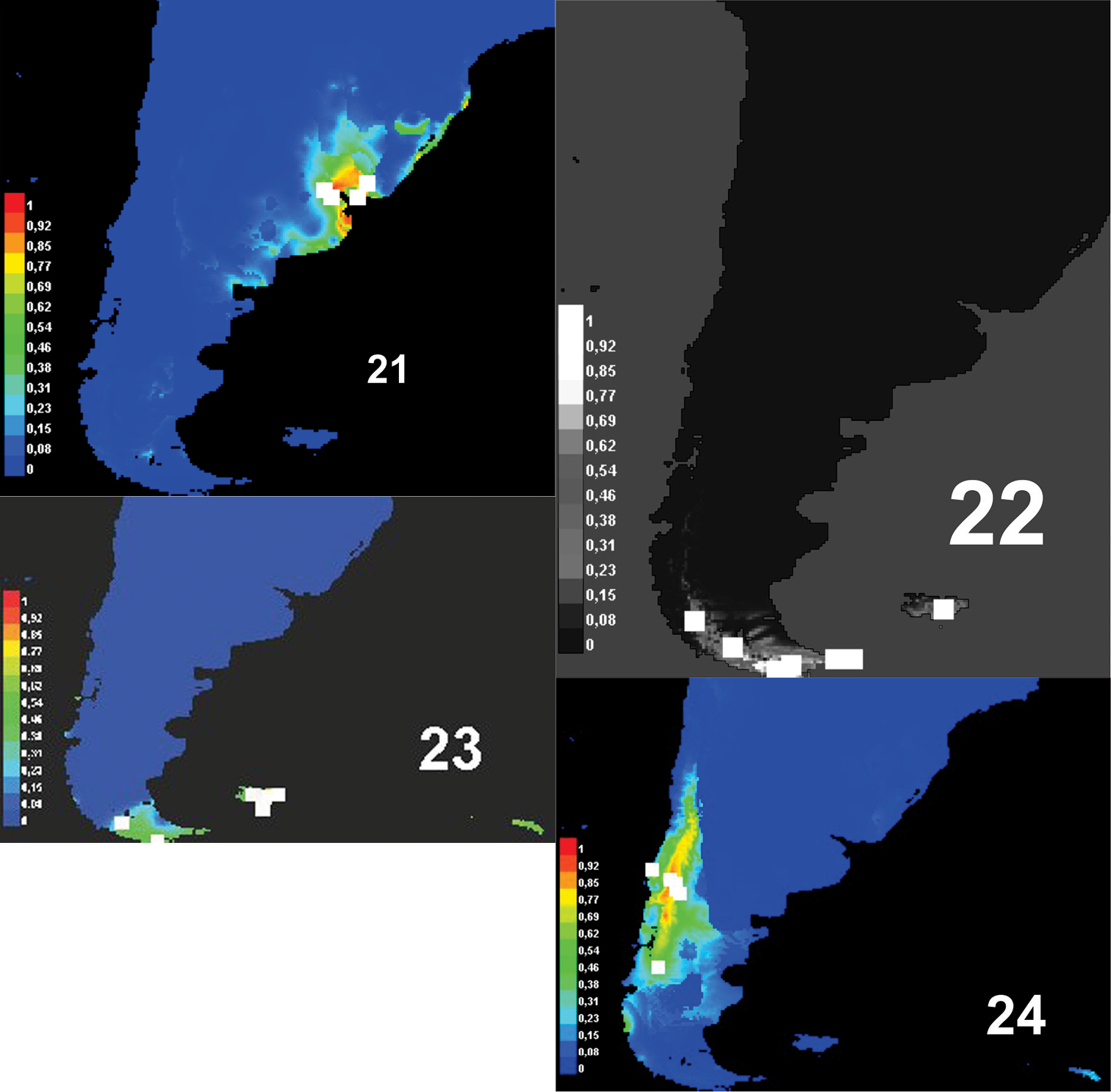
Predictive models of distribution show that the genus Rhytidontahus isrestricted to the coast and areas close to the La Plata river and the delta of the Paraná and Uruguay Rivers (Fig. 20), occupying shore habitats and the Pampean grassland near the shore. This Pampean plain has been strongly modified, allowing for great agricultural development with establishment of annual crops and pastures, leaving hardly any native vegetation in the region. The Pampean grassland and forest close to the La Plata river and to the high Paraná River differ in species and habitat conditions from the areas inhabited by nearly all sister groups of Rhytidognathus, the genera Lissopterus Waterhouse, Migadopidius Jeannel and Pseudomigadops. Migadopidius occupy temperate Nothofagus forests(Fig. 24, Table 1). Lissopterus and Pseudomigadops (Figs 22-23) occur in habitats closer to the shore, principally sub-Antarctic forest or moorlands (Figs 22–23, Table 1). The unique genus of the sister group inhabiting grassland is Pseudomigadops, in some part of Malvinas Islands. As we can see, Pseudomigadops inhabits coastal forest and grassland, like Rhytidognathus, but species composition in their habitats is far from being the same, as the former is of sub-Antarctic origin and the other of Neotropical origin (
Because of its particular distribution pattern and its phylogenetic relationships with other tribes, the Migadopini have been used to explain some very different biogeographic views, such as an austral origin and separation by vicariance (
Regarding the present distribution of the Migadopini in South America, it is restricted to three disjunct areas. The first is in the Ecuadorian Andes, where the genus Aquilex occurs at about 4300 m elevation at Páramo (
Considering the particular distribution of Rhytidognathus, the biogeographic analysis carried out by
In analyzing the environmental features of each genus, we find that there could also have been environmental features involved in the split. Figures 21–24 show the potential distribution range of Rhytidognathus and that of its sister genera. For these four genera, we find three clearly separate areas, one is austral sub-Antarctic, another one comprises the cold-temperate forests, and the third one encompasses the Pampean steppe and riparian forests along the La Plata river. The Pampean region is the exception with respect to the other habitats where migadopines occur in South America, and to the remaining circum-Antarctic regions, because most are from cold-temperate or cold environments, such as the species of Loxomerus Chaudoir (Johnson 2010). Although the Pampean grassland is a temperate area, it has warm summers and the vegetation is Neotropical in origin, not austral.
In other cases, it has been put forward that there often is niche conservation, commonly observed in species of the same genus whose potential distributions show areas occupied by other species of the genus rather than by them. However, we see that a shift has occurred among these four genera regarding the environment occupied by some of them. We propose that the environment occupied by the ancestor of Rhytidognathus and the sister group could have been cold-temperate coastal or riparian habitats, either forest or grassland (present in Rhytidognathus and Pseudomigadops). An arid barrier formed during the Cenozoic between the Pampean and sub-Antarctic regions (
Habitat characterization and the major variables explaining the predictive model of distribution obtained by Maxent.
| Habitat | variables | |
|---|---|---|
| Rhytidognathus | Lowlands, 30-m altitude, in Pampean grasslands, and probably in riparian forests along the La Plata river and the Paraná river delta. | 67.3%: Isothermality:17.0% :Precipitation Seasonality (Coefficient of Variation)10.1 Mean Temperature of Wettest Quarter |
| Pseudomigadops | Lowlands, sea level to 10-meter altitude; in Malvinas grasslands (mainly of Poa flabellata) and Magellanic moorland (of Empetrum rubrum).In Navarino, southern Tierra del Fuego (near Beagle Channel), Isla de los Estados and Cape Horn Nothofagus betuloides forest on the coast and Magellanic moorland (Empetrum rubrum) (Niemela 1990) | 46.9% Max Temperature of Warmest Month14.7 % Mean Temperature of Driest Quarter11.8 % Mean Annual Temperature |
| Lissopterus | Lowlands, sea level to 5-meter altitude; in Malvinas grasslands (mainly of Poa flabellata) and Magellanic moorland (of Empetrum rubrum).In Navarino, southern Tierra del Fuego (near Beagle Channel), Isla de los Estados and Cape Horn Nothofagus betuloides forest on the coast and Magellanic moorland (Empetrum) (Niemela 1990).Sub-Antarctic maritime areas including off-shore and more remote islands (Erwin 2011) | 66.0% Max Temperature of Warmest Month11.9% Altitude6.1% Annual Temperature Range |
| Migadopidius | Nothofagus forest and Araucaria habitat; mixed forest (Araucaria araucana, Nothofagus dombeyi, Nothofagus antarctica and Nothofagus pumilio) (Dapoto et al. 2005) | 63.0% Mean Temperature of Wettest Quarter29.0% Precipitation of Coldest Quarter |
Rhytidognathus ovalis. 10 Median lobe and left paramere 11 Right paramere 12 Median lobe, right view 13 Female genital track, ventral view. Rhytidognathus platensis. 14 Median lobe, left view 15 Median lobe, right view 16 Apex of median lobe 17 parameres 18 female genital track, ventral view. Scale 1 mm.
Habitat of Rhytidognathus platenesis. 19 Aerial view of the collecting area 20 Area where the study was developed, showing the crops.
Potential distribution of: 21, Rhytidognathus 22 Pseudomigadops 23 Lissopterus and 24 Migadopidius. Known localities are in white, probabilities of occurrence are indicated in different shades of grey.
To Dr. Carrara for criticism of a previous version of the manuscript. To Nelly Horak for the English corrections. This contribution is part of CONICET PIP nro. 11220080101869“La región austral del Chaco, su evolución histórica a través de reconstrucciones de los patrones biogeográficos y evolutivos de los componentes de su artropodofauna”.