(C) 2013 Shipher Wu. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Wu S, Chang W (2013) Review of the Parasa undulata (Cai, 1983) species group with the first conifer-feeding larva for Limacodidae and descriptions of two new species from China and Taiwan (Lepidoptera, Limacodidae). ZooKeys 345: 29–46. doi: 10.3897/zookeys.345.6179
Although the caterpillars are well-known for the stings and magnificent coloration, the systematics of Limacodidae is historically neglected and chaotic due to the difficulty in matching the larval with adult stages as well as the very conservative and convergent adult morphology. One of the biggest taxonomic problems surrounds a collective group from Southeastern Asia, termed the “green limacodid moths”, which harbours at least 90 species placed in the genus Parasa Walker, 1859 and 14 “subunits”. The P. undulata group was previously composed of 3 species from China and Taiwan, and characterized only by wing pattern. This species group is extensively studied herein with two new species described, i.e. P. viridiflamma sp. n. (Taiwan) and P. minwangi sp. n. (S. China), and discovery of female genitalia of three species, presenting new phylogenetic insights in this potentially paraphyletic genus. In addition, one limacodid larva was found to be feeding exclusively on Picea (Pinaceae) in Taiwan. Its identity, Parasa pygmy Solovyev, 2010 in P. undulata group, is confirmed through matching its COI sequence to the adult. This discovery is also biologically significant because the previous known host breadth of Parasa was of polyphagy on various angiosperm plant families. This case, therefore, represents the first record of conifer-feeding behavior in this family as well as the first of specialized herbivory in the genus. Meanwhile, the background match between Picea leaves and larval coloration is shared with other Picea-feeding insects. This phenomenon is worth of further investigation in the aspect of convergent evolution of crypsis associated with a particular plant.
Conifer-feeder, Limacodidae, Parasa, new species, Picea, Pinaceae, Taiwan
(1) Systematic problems surrounding the genus Parasa Moore, 1859 and its relatives.
The southeastern Asian limacodid moths comprise about 90 species of “green limacodids”, that are green at least on some parts of wings and thorax (
Though a subgrouping of Parasa into 14 subunits was proposed by
(2) Discovery of conifer-feeding habits with unique larval morphology and two additional new species in the Parasa undulata species group.
Recently, a single limacodid larva was discovered on the conifer tree Taiwan Spruce (Picea morrisonicola Hayata, 1908, Pinaceae), at mid-elevation (ca. 2600 m) of the central mountain range of Taiwan. This presents unusual ground maculation and an external appearance similar to the stomatal band of conifer leaves that is described and discussed in detail below.
Since this larva failed to pupate successfully after the prepupal stage in an indoor rearing environment, we sequenced its mitochondrial COI for comparison to that of two other limacodid moths. These two moths, Parasa pygmy Solovyev, 2010 and Parasa martini Solovyev, 2010, only occur in mid to high elevation montane regions of Taiwan. These data deposited in Genbank (KF595045, KF595046, KF595047) reveal zero divergence between the collected larva and the adult of Parasa pygmy, but ca. 6.3% p-distance divergence to Parasa martini, thus confirming the identity of the first known conifer-feeding limacodid (although not unique among zygaenoids, e.g. Psycharium Herrich-Schäffer, 1856, Somabrachyidae feeds on Pinus:
The studied specimens were examined in or borrowed from the following institutions and private collections:
BMNH The Natural History Museum, London
CCMF Collection of Chien-Ming Fu, Taichung
CVAK Collection of Valentin A. Kalinin, Moscow
ESRI Taiwan Endemic Species Research Institute, Nantou
NMNS National Museum of Natural Science, Taichung
NSMT National Museum of Nature and Science, Tsukuba
SCAU Entomological Department, South China Agricultural University, Guangzhou
TFRI Insect collection of Taiwan Forestry Research Institute, Taipei
Genitalia were prepared following the general method described e.g. by
The terminology of wing patterns and genital structures follows that of
Genomic DNA was extracted from fragments of adult legs and part of larval tissues using an ALS Tissue Genomic DNA Extraction Kit (Kaohsiung, Taiwan). A partial COI sequence was amplified by a polymerase chain reaction (PCR) with a set of universal primers (LCO1490 and HC02198) (
Parasa undulata species group
Parasa viridiflamma sp. n. (Taiwan)
Parasa undulata (Cai, 1983) (central and southern China)
Parasa pygmy Solovyev, 2010 (Taiwan)
Parasa minwangi sp. n. (S. China)
Parasa martini Solovyev, 2010 (Taiwan)
The definition and diagnosis of the Parasa undulata species group given in
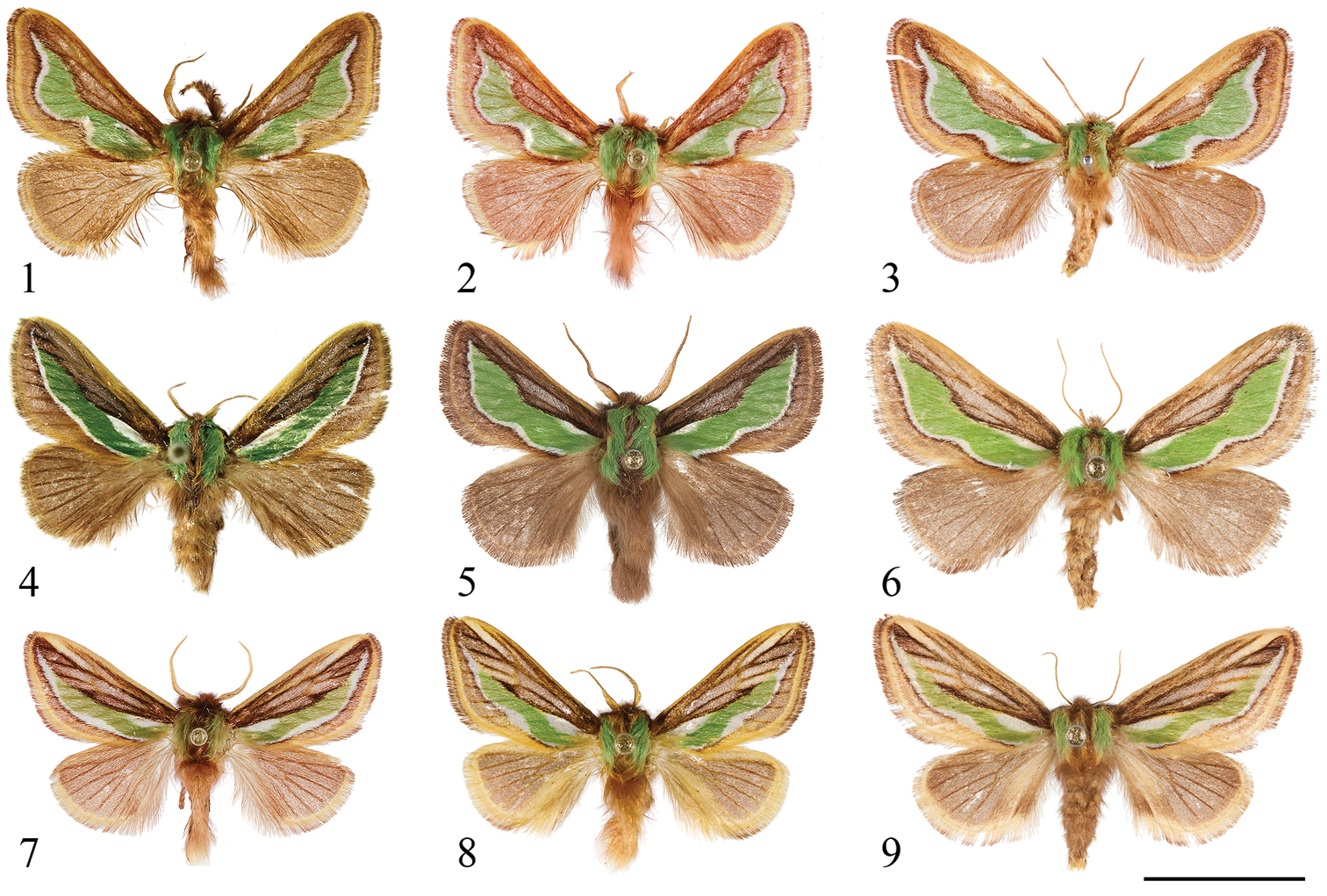
Forewing with median green patch surrounded by two white longitudinal stripes, i.e. a short basal stripe and another long one along outer margin of the patch (see Figs 1–9). Notes. The combined pattern of forewings of the resting moths is similar to the needle leaves and stomatal band of several conifer genera, e.g. Pinus, Tsuga and Abies (Pinaceae), thus revealing a potentially adult adaptation to such a resting environment (see Figs 26, 27).
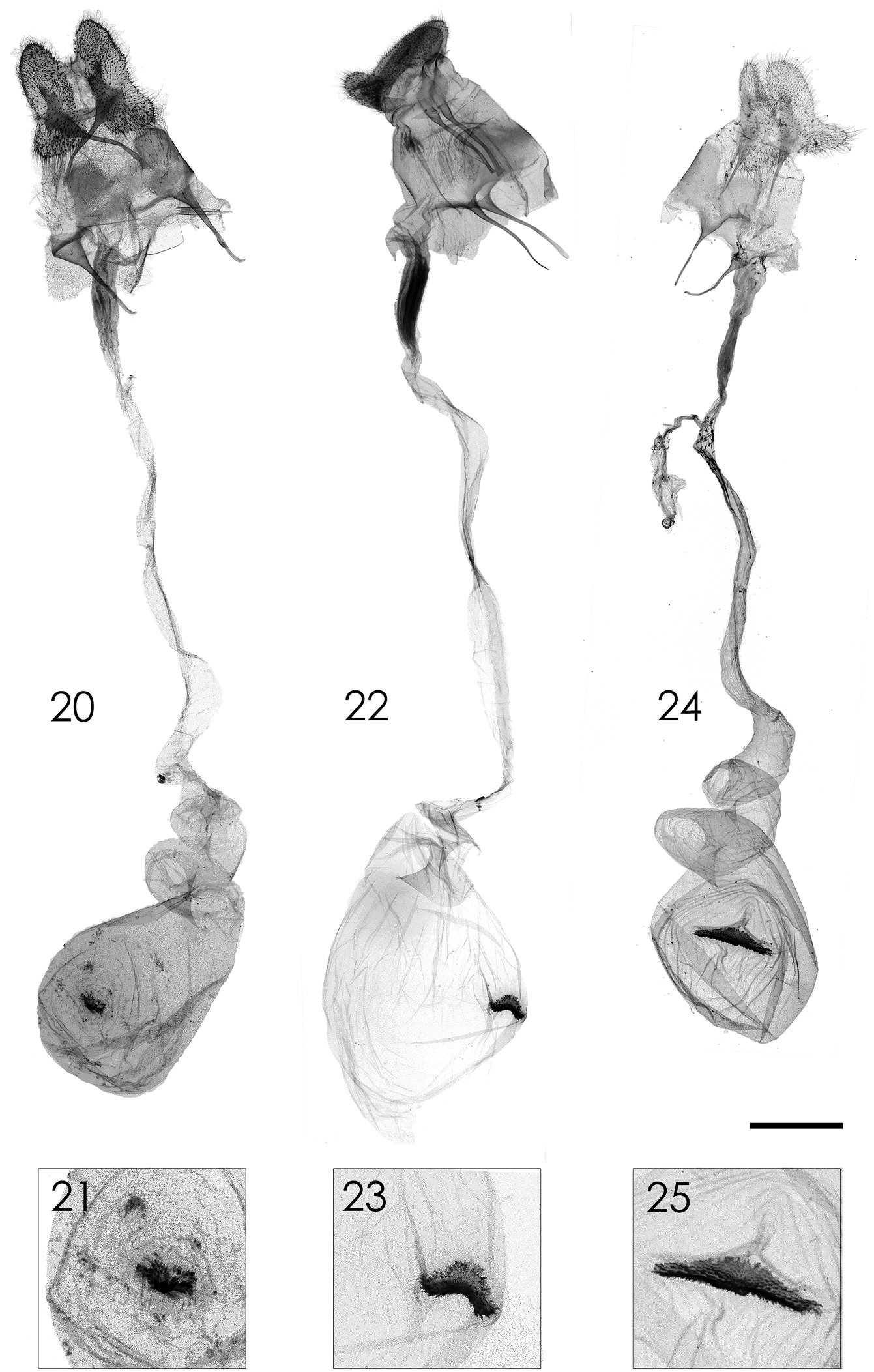
Corpus bursae with only one transverse signum (see Figs 20–25), rather than two in other species groups of the genus Parasa.
Mature larva, at least that of Parasa pygmy, with green ground coloration, white longitudinal stripes and without dorsal abdominal scolus structures. See description part of immature stage of Parasa pygmy in detail.
| 1 | Forewing green patch wide, extended over approximately half of discal area | 2 |
| – | Forewing green patch narrow, covering less than half of discal area; a pale ochreous stripe arising between vein R3 and R4 | 4 |
| 2 | Outer margin of green patch distinctly sinuous | 3 |
| – | Outer margin of green patch smoothly curved | Parasa undulata |
| 3 | Outer margin of green patch deeply incised between cubitals and anal vein | Parasa viridiflamma sp. n. |
| – | Outer margin of green patch slightly incised between cubitals and anal vein | Parasa pygmy |
| 4 | Forewing white stripes wide, terminal and anal areas of forewing with wider ochreous band; anal field of hindwing ochreous | Parasa martini |
| – | Forewing stripes slender, terminal and anal areas of forewing with narrower ochreous band; anal field of hindwing brown, without ochreous coloration | Parasa minwangi sp. n. |
http://zoobank.org/0F20787E-FA13-480F-87C4-9DBC99F06263
http://species-id.net/wiki/Parasa_viridiflamma
Figs 1–3, 10, 11, 20, 21Holotype: ♂, TAIWAN, Hualien County, Tayuling, 2550 m, 25-VI-2008, leg. H. H. Lin (coll. ESRI); paratypes: 3♂, Taichung County, Tashuehshan Mts., Anmashan, 2230 m, 14-16.VI.1989, leg. M. Owada; 1♂, same collecting data, slide NSMT-SW131; 1♀, Taichung County, Anmashan, 2300 m, 30-VII-1997, leg. T. Mano, slide NSMT-SW132 (coll. NSMT); 1♂, Taichung County, Anmashan, 2600 m, 23-V-1998, leg. C. M. Fu; 1♂, Taitung County, Yenping, 31-VII-1992, leg. Shiau & Yang (coll. NMNS); 1♂, Nantou County, Renluen, 1400 m, 21-VIII-1991, leg. Y. B. Fan, slide TFRI00061358; 1♂, Nantou County, Tatajia, 2610 m, 6-VII-2011, leg. S. Wu & W. C. Chang (coll. TFRI).
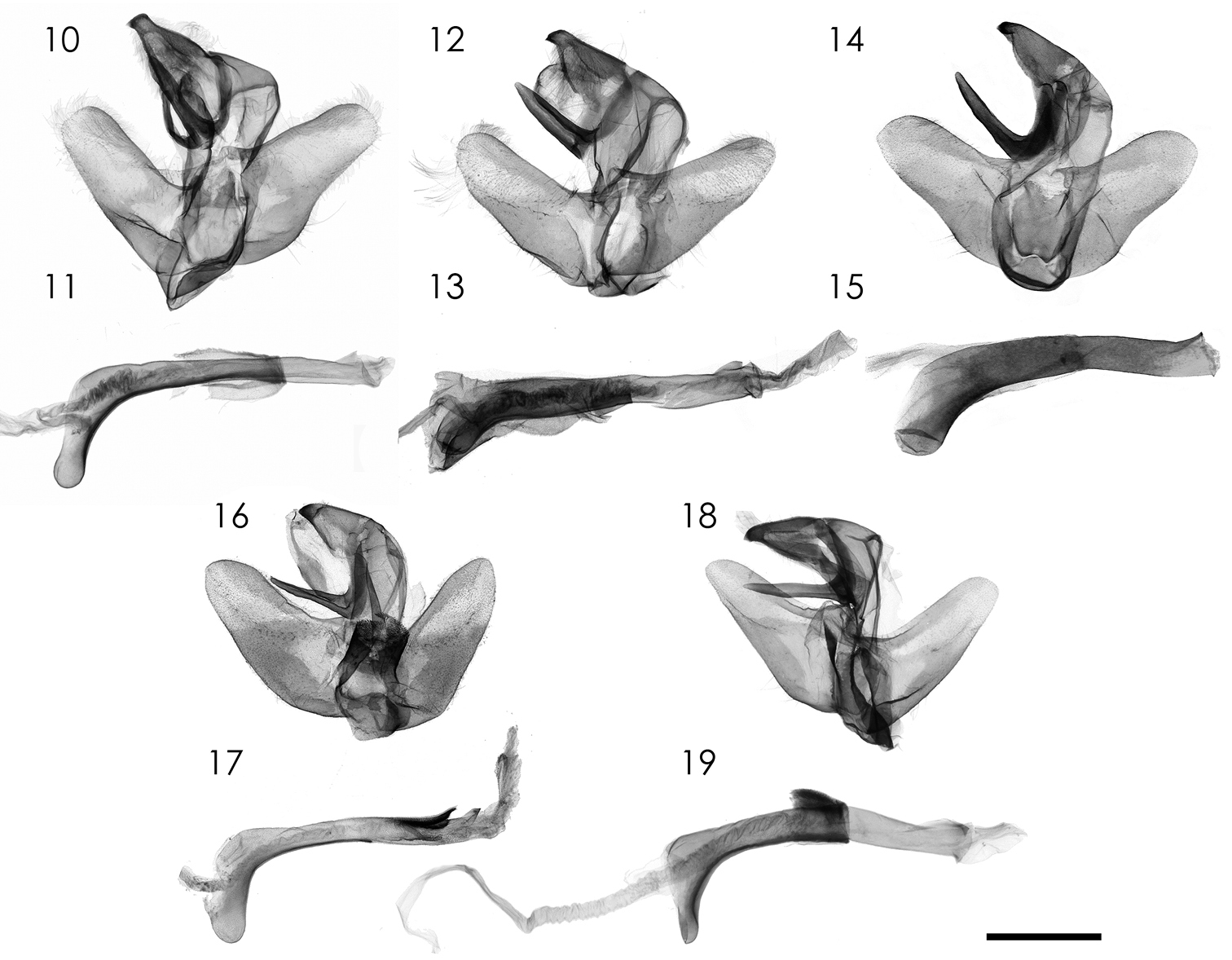
The new species is externally similar to Parasa undulata from central and southern China and Parasa pygmy from Taiwan but it can be easily distinguished by the forewing green patch strongly incised between cubitals and anal veins. In the male genitalia the basal part of aedeagus (coecum) is long, strongly extending toward ventral side in Parasa viridiflamma. Females of all three Taiwanese species of the Parasa undulata group are recorded in the present study, they can be distinguished by the shape of the single signum, that of Parasa viridiflamma is short, irregular in shape, that of Parasa pygmy is saddle-shaped and that of Parasa martini is straight and long in transverse axis.
Adult (Figs 1–3).
Dorsal views of Parasa spp. from China and Taiwan. 1 Parasa viridiflamma sp. n., male, paratype, Taiwan 2 ditto, male, paratype, Taiwan 3 ditto, female, paratype, Taiwan 4 Parasa undulata (Cai, 1983), male, Guangxi Province, S. China 5 Parasa pygmy Solovyev, 2010, male, Taiwan 6 ditto, female, Taiwan 7 Parasa minwangi sp. n., male, holotype, Guangdong Province, S. China 8 Parasa martini Solovyev, 2010, male, Taiwan 9 ditto, female, Taiwan. Bar scale=10 mm. Specimens by courtesy of: NSMT (1–3, 8); CVAK (4); TFRI (5, 9); ESRI (6); SCAU (7). Photo by Shipher Wu (1–3, 5–9); Alexey Solovyev (4).
Measures. Wingspan 23–24 mm in male (n=7); 26 mm in female (n=1).
Head. Antennae bipectinate in male, rami longer at basal part and gradually shortening to absent at 5/6 from base; filiform in female. Eyes black, round. Frons, vertex, labial palpi fringed with long, chestnut hair-like scales, 3rd labial palpal segment short.
Thorax. Thoracic segments green with chestnut dorsal stripe. Forewing ground coloration chestnut with median large green patch delimited externally by white line which is in turn lined by brown border, all these pattern elements strongly incurved between cubitals and anal veins, less so towards termen; marginal scales ochreous. Hind wings chestnut, marginal scales ochreous.
Abdomen. Abdominal segments fringed with long chestnut hair-like scales.
Male genitalia (Figs 10, 11). Uncus robust, wide with hook-like apex. Gnathos. Gnathos large, sclerotized, apically narrowed; juxta plate-like with two lateral sides extending dorsally. Valva short, apex tongue-like. Aedeagus long, tubular, coecum strongly bent ventrally.
Male genitalia of Parasa spp. from China and Taiwan. 10, 11 Parasa viridiflamma sp. n., holotype, Taiwan 12, 13 Parasa pygmy Solovyev, 2010, Taiwan 14, 15 Parasa undulata (Cai, 1983), Guangxi Province, S. China 16, 17 Parasa minwangi sp. n., holotype, Guangdong Province, S. China 18, 19 Parasa martini Solovyev, 2010, Taiwan 10, 12, 14, 16, 18 Male genital apparatus 11, 13, 15, 17, 19 Aedeagi. Bar scale=1 mm. Specimens by courtesy of: ESRI (10, 11, 12, 13); CVAK (14, 15); SCAU (16, 17); TFRI (18, 19). Photo by Shipher Wu (10–13, 16–19); Alexey Solovyev (14, 15).
Female genitalia (Figs 20, 21). Apophyses elongated, length of anterior and posterior ones equal; ductus bursae long; corpus bursae small, about 3.5 times shorter than ductus bursae, signum small, irregular-shaped.
Female genitalia of Parasa spp. from Taiwan. 20, 21 Parasa viridiflamma sp. n., paratype 22–23 Parasa pygmy Solovyev, 2010 24–25 Parasa martini Solovyev, 2010 21, 23, 25 Magnified images of signa. Bar scale=1 mm. Specimens by courtesy of: NSMT (20–21); ESRI (22–23); TFRI (24–25). Photo by Shipher Wu.
This species is endemic to Taiwan. The adults occur in May and mid June to late August in mid-elevation mountain areas (1400–2610 m). The fresh individuals appear earlier in the season. Possibly univoltine. Hostplant unknown.
The new species is named through the combination of viridis (green) and flamma (flame), according to its flame-shaped green median patch on forewing.
http://species-id.net/wiki/Parasa_pygmy
Figs 5, 6, 12, 13, 22, 23, 26–33TAIWAN, 2♂, Chiai Hsien [Nantou County], Luhlin Lodge, [ca. 2600 m], 16-VIII-1990, leg. B. S. Chang (coll. NMNS); 3♂, Miaoli County, Guanwu, 2000 m, 27-IX-2010, S. Wu leg.; 1♂, Nantou County, Black Water Cottage, 2757 m, 7-IX-2012, S. Wu & W. C. Chang leg.; 1♂, Nantou County, Chen-gong Lodge, 2853 m, 10-IX-2012, leg. S. Wu & W. C. Chang; 7♂, Nantou County, Piluchi, 2000 m, 3-IX-1986, leg. Y. J. Chang; 4♂, same locality, 4-IX-1986, Y. J. Chang leg.; 18♂, same locality, 14-IX-1986, Y. J. Chang leg.; 5♂, same locality, 15-X-1987, Y. B. Fan (coll. TFRI); 1♂, Nantou County, Hohuanshan, 3006 m, 14-IX-2009, L. C. Shih leg., slide ESRI A12-20090914-037 (coll. ESRI); 1♂, Nantou County, Yuanfeng, 2700 m, 11-IX-2012, leg. S. Wu, slide TFRI00148804; 1♂, Nantou County, Xiaofengko, 3002 m, 13-VIII-2012, leg. S. Wu & W. C. Chang; 1♂, Ilan County, Jianchin, 1930 m, 8-X-2012, leg. S. Wu; 8♂, Hualien County, Guanyuan, 2400 m, 13-IX-2012, leg. S. Wu (coll. TFRI); 13♂, Hualien County, Kuanyan (=Guanyuan), 2370 m, 13-IX-2012, leg. M. Owada & S. Wu (coll. NSMT); 1 Mature larva, Hualien County, 820 Logging Trail, 2600 m, 26-V-2012, leg. S. Wu & W. C. Chang (coll. TFRI); 1♀, Hualien County, Jinma Tunnel, 2400 m, 23-IX-2009, leg. L. C. Shih, slide ESRI A09-20090923-127 (coll. ESRI); 1♀, Hualien County, Biluishenmu, 2150 m, 22-VIII-1991, leg. H. Y. Wang (coll. NMNS).
This species represents the insular sister species of Parasa undulata from China. It can be easily distinguished from Parasa undulata by its broader forewing medial green patch and its longer coecum. The comparison of the female genitalia is given under the diagnosis of the preceding species.
The female and mature larva are described for the first time.
Female (Fig. 6).
Measures. Wingspan 24–25 mm (n=3).
Head. Antennae filiform. Eyes black, round. Frons, vertex, labial palpi fringed with long, chestnut hair-like scales, 3rd labial palpal segment short.
Thorax. Thoracic segments green with chestnut dorsal stripe. Forewing ground coloration chestnut with large median green patch delimited externally by thin white line which is in turn lined by brown border; marginal scales ochreous. Hind wings chestnut, marginal scales ochreous.
Abdomen. Abdominal segments fringed with long chestnut hair-like scales.
Female genitalia (Figs 22–23). Apophyses elongated, length of anterior and posterior ones equal; ductus bursae long; corpus bursae small, about 3.5 times shorter than ductus bursae, signum saddle-shaped in transverse axis.
Immature stages.
Mature instar (Figs 28–33). Body spindle-like, length 20 mm when fully extended. Legs very small, largely reduced. Prolegs fully absent; adhesive, sucker-like regions on abdomen present. Head and body ground coloration green; a pair of prominent conical dorsal scoli arising from the dorsal part of mesothorax and on the 9th abdominal segment, respectively, the remaining parts smooth. 10 fresh red spots, circled by light blue ring, arranged longitudinally along mid-dorsum; two cream yellow subdorsal lines running parallel adjacent to the red spots; dorso-lateral, lateral and ventro-lateral lines wide; regions between subdorsal and dorso-lateral lines pale green; small subdorsal scoli, arising from mesothorax, metathorax and abdominal segment A2 to A8, orange, along on lateral lines and reduced as small scobinate patches; spiracles orange.
Photos of alive Parasa pygmy Solovyev, 2010 in Taiwan. 26, 27. Adult male on Abies kawakamii (Pinaceae) 26 Lateral side 27 Dorsal side 28–33 Mature instar on hostplant, Picea morrisonicola (Pinaceae) 28 Resting posture on ventral side of hostplant 29 ditto, dorsal view, denoted by red arrow 30 Magnified image 31–33 Larva feeding on leaf. Photo by Shipher Wu.
Two new COI sequences (identical) from adult male and mature larva, respectively, were deposited in the GenBank database (KF595046, KF595047).
Parasa pygmy is endemic to Taiwan. The adults occur from mid August to early October in mid to high elevation mountains of central Taiwan (~2000–3000 m), where they match the distribution range of the presently known hostplant, Picea morrisonicola Hayata (Pinaceae). The single mature larva was taken in late May, the leaf flushing period of Picea morrisonicola. This observation suggests a univoltine life cycle for Parasa pygmy and the overwintering stage is inferred to be the egg. The patterns of the adult and mature larva are similar to the needle leaves of Pinaceae, especially the hostplant species. This potential evolutionary adaptation is detailed in results and discussion.
http://species-id.net/wiki/Parasa_undulata
Figs 4, 14, 15CHINA, 1♂, Guangxi Province, Dayao Shan Mts. Jingxiu, 100 km SE Liuzhou, 24°07'N, 110°14'E, 1700 m, VII-2008, leg. V. Siniaev (coll. CVAK).
This species is closely related to Parasa pygmy, their comparison is given under the diagnosis of preceding species.
According to
http://zoobank.org/F5D21EB2-EB61-42D4-953C-48F8478E2EB5
http://species-id.net/wiki/Parasa_minwangi
Figs 7, 16, 17Holotype: ♂, CHINA, Guangdong Prov., Shaoguan, Nanling, 700-1200 m, 22-25-IV-2005, leg. K. Horie, slide NSMT-SW133 (coll. SCAU); paratypes: 4♂, Guangdong Prov., Shaoguan, Nanling, 600–1400 m, 11-18-V-2005 (coll. NSMT); 4♂, same collecting data (coll. SCAU); 1♂, same collecting locality, 21-28-VI-2008 (coll. NSMT); 2♂, same collecting locality, 1-6-VIII-2006 (coll. NSMT); 1♂, same collecting data (coll. SCAU); 1 male, same collecting locality, 31-VIII-1-IX-2003 (coll. NSMT); 1♂, same collecting data (coll. SCAU); 2♂, 5-11-IX-2005 (coll. NSMT); 2 males, same collecting data (coll. SCAU); 1 male, 26-27-IX-2003, leg. M. Wang et al. (coll. NSMT); 1 male, same collecting data (coll. SCAU), all leg. Wang et al.
This species is closely related to the allopatric species Parasa martini from Taiwan. Externally its forewing white stripes are more slender. In the male genitalia the aedeagus has a more slender coecum and bears a separated, sclerotized dorsal process at the apex, the latter structure being absent in other species of the same group.
Adult (Fig. 7).
Measures. Wingspan 21–22 mm (n=21).
Head. Antennae bipectinate in male, rami longer at basal part and gradually shortening to absent at 5/6 from base; filiform in female. Eyes black, round. Frons, vertex, labial palpi fringed with long, chestnut hair-like scales, 3rd labial palpal segment short.
Thorax. Thoracic segments green with chestnut dorsal stripe. Forewing ground coloration chestnut with ochreous stripe situated between vein R4 and R5 and a large median green patch delimited by slender white lines and subsequent wide brown border; marginal scales ochreous. Hind wings chestnut, marginal scales pale chestnut fringed with ochreous.
Abdomen. Abdominal segments fringed with long chestnut hair-like scales.
Male genitalia (Figs 16, 17). Uncus robust, wide with hook-like apex. Gnathos large, sclerotized, apically narrowed; juxta sclerotized with two lateral sides extending dorsally. Valva short, apex tongue-like. Aedeagus long in straight distal part and down-curved basal part, respectively, and with a distal sclerotized dorsal process.
This species is recorded only from mid-elevation (600–1400 m) of Nanling mountain areas, S. China. The adults occur in April, May, June, August and September. Possibly bivoltine. Hostplant unknown.
This species is dedicated to Dr. Min Wang (SCAU), who represents the main collector of most of the type material of this new species in Nanling mountain areas, S. China.
http://species-id.net/wiki/Parasa_martini
Figs 8, 9, 18, 19, 24, 25Type material: Holotype. ♂, “TAIWAN, Taichung County., He-ping, Dayueshan National Forest Recreation Area, N24°15.315 E121°00.374, 28-V-2007, 2223 m, At MV light, leg. G. Martin & D.L.J. Quicke, BMNH (E) 2007-43”, ”BMNH (E) # 820958”, ”BMNH genital slide 1422” (coll. BMNH), paratypes: 2♂, same collecting data as holotype (coll. BMNH)
Other material: TAIWAN, 1♂, Miaoli County, Guanwu, 2000 m, 29-VI-2010, leg. S. Wu & W. C. Chang; 1♀, same collecting data, slide TFRI00143030 (coll. TFRI); 1♂, Taichung County, Anmashan, 2100 m, 19-VIII-1996, leg. C. M. Fu; 4♂, same collecting locality, 13-IX-1996, leg. C. M. Fu; 1♂1♀, same collecting locality, 30-VI-1997, leg. C. M. Fu (CCMF); 1♂, same collecting locality, 2200 m, 29-VII-1997, leg. C. M. Fu; 1♂, Baxianshan, 1000 m, 26-VII-1997, leg. C. M. Fu (coll. CCMF); 3♂1♀, Taichung County, Chingshan, 1400 m, 9-10-IX-1993, leg. W. T. Yang & M. L. Chan (coll. NMNS); 3♂, Taichung County, Wushihken, 950 m, 23-V-2012, leg. L. C. Shih (ESRI); 1♂, Nantou County, Piluchi, 2000 m, 12-VIII-1987, leg. Y. B. Fan, slide TFRI00061365; 1♂, Hualien County, Ci’en, 1950 m, 13-IX-2012, leg. S. Wu (coll. TFRI); 1♂, Hualien County, Cien, 2039 m, 20-VII-2009, leg. L. C. Shih (coll. ESRI); 1♂, Hualien County, Tsuen (=Ci’en), 2000 m, 13-IX-2012, leg. M. Owada & S. Wu (coll. NSMT).
This species is the allopatric sister-species of Parasa minwangi sp. n. from southern China. Their comparison is given under the diagnosis of the preceding species.
The female is described here for the first time.
Female (Fig. 9).
Measures. Wingspan 24–25 mm (n=3).
Head. Antennae filiform. Eyes black, round. Frons, vertex, labial palpi fringed with long, chestnut hair-like scales, 3rd labial palpal segment short.
Thorax. Thoracic segments green with chestnut dorsal stripe.
Forewing ground coloration chestnut with ochreous stripe situated between vein R4 and R5 and one median longitudinal green patch delimited by white lines and subsequent wide brown border; marginal scales ochreous. Hind wings chestnut, anal margin and marginal scales ochreous.
Abdomen. Abdominal segments fringed with long chestnut hair-like scales.
Female genitalia (Figs 24, 25). Apophyses elongated, length of anterior and posterior ones equal; ductus bursae long; corpus bursae small, about 3.5 times shorter than ductus bursae, signum transverse, long with medial part more expanded.
A new COI sequence was deposited in the GenBank database (KF595045).
This species is endemic to Taiwan. The adults occur from late May to late June, mid and late July then mid August to mid September in mid-elevation mountain areas with primary vegetation (ca. 950–2223 m). Possibly bivoltine. Hostplant unknown.
The present study reports on the first record of a conifer-feeding limacodid moth on the Taiwan Spruce (Picea morrisonicola) in Taiwan, describing the specialised morphology of the last larval instar. The larval identity is confirmed through COI sequence (636 bp) comparison between Parasa martini and Parasa pygmy. The sequences of adult and larval Parasa pygmy are identical but about 6.3% divergent to Parasa martini.
The conifer-feeder, Parasa pygmy (Taiwan), together with Parasa undulata (central and southern China), Parasa martini (Taiwan), and the newly described Parasa viridiflamma sp. n. and Parasa minwangi sp. n., form the Parasa undulata species group sensu
Previously known Parasa larvae are mostly regarded as being polyphagous, often as agricultural pests on broad-leaved trees (
Additionally, the larval habits of Parasa pygmy are also interesting. The observed larva always moves and feeds on the ventral side of needle leaf and branches, thus, its patterns and behavior can be regarded as a case of countershaded crypsis on Pinaceae to prevent predation by high mountain birds (lizard species occur more rarely in this high altitude biotope of Taiwan). Although it is cryptic, the prominent red spots of the mid-dorsal line of the mature larva, in contrast to its green ground coloration, act as a potentially aposematic signal (a similar pattern occurs in some Pinus feeding Bombycoidea, such as Lapara bombycoides Walker, 1856, Sphingidae). The combination of visual crypsis and aposematism was reported in previous studies, e.g.
In addition to the descriptions of new species and the discovery of conifer-feeding larval habits, the female of three representatives of the Parasa undulata species group in Taiwan are reported for the first time. Their genitalia are different to those of other Parasa species groups by the presence of only one signum rather than two. The number of signa is hitherto regarded as a significant character state to distinguish a broad sense pantropical Parasa from Madagascan Latoia. The latter has no signum.
Though the known immature and female characters of the Parasa undulata species group show remarkable differences compared to the other Parasa species groups, the characters of wing venation and male genitalia are typical for the genus. Therefore, we hesitate to treat this lineage as an independent taxonomic unit until the mentioned characters can be comprehensively analyzed in all green limacodid groups.
In addition, the species richness and distribution of the Parasa undulata species group is extensively reviewed in this study, comprising wide-distributed Parasa undulata and local-ranged Parasa minwangi sp. n. in Asian continental region (China) and three endemic species found in a mountainous island (Taiwan). Assuming that no more or a few undescribed species may be discovered in the mainland in future studies, their distribution patterns show a rather higher species diversification in a small biogeographic unit. Though
We would like to express our sincere thanks to Geoff Martin, Alessandro Giusti (BMNH), Utsugi Jinbo, Mamoru Owada (NSMT), Min Wang (SCAU), Valentin A. Kalinin (Moscow), Ming-Lung Chen, Mei-Lin Chan, Huei-Hong Liang (NMNS), Juag-Tai Chao, Shen-Shan Lu, Yun-Yin Yeh (TFRI), Hsu-Hong Lin and Li-Chen Shih (ESRI), who provided great help in the study of the collections of their institutes or private collections, to Ming-Tang Hsiao (Shei-Pa National Park Headquarters, Miaoli, SPNP), Mei-Chen Chuang (Taoyuan), Wei-Ting Wu (Taipei) and Wen-Hsin Wu (Taipei) who assisted specimen collecting or helped in many other ways; to Shen-Horn Yen (National Sun Yat-sen University, Kaohsiung, NSYSU) who always share valuable references and comments for our background knowledge, to Ming-Chung Chiu (National Taiwan University, Taipei, NTU) who assisted the molecular work, to Shiuh-Feng Shiao (NTU) who gave opportunity for the first author to operate the molecular work in the Insect Systematics Laboratory of NTU and examine Type materials in BMNH when conducting the project, International Cooperation Project of National Digital Contents and Technique: International Cooperation Project of Studies on Taiwanese Insect Type Specimens (NSC 101-2631-H-002-019), to all the staffs of High Altitude Experimental Station (Nantou), Dongpu Lodge (Nantou), Guanwu Visitor Center (Miaoli) and Meifeng Farm (Nantou) for their kindly association during our fieldwork. Last but not least, we sincerely thank David Lees (Department of Zoology, University of Cambridge) for manuscript reading, giving valuable comments and Alexey Solovyev (Ul’yanovsk State Pedagogical University, Ul’yanovsk) for providing latest Limacodidae information for compacting our study.