(C) 2012 Malkie Spodek. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Thefirst-instar nymph and the adult female of Kermes echinatus Balachowsky (Hemiptera, Coccoidea, Kermesidae) are described and illustrated. This species is compared with Kermes vermilio Planchon, a morphologically similar species known in the Palaeractic region.
Scale insect, Quercus, evergreen oaks , Kermesidae, morphology, red dye
The scale insect family Kermesidae (Hemiptera, Coccoidea) develops and feeds exclusively on Fagaceae trees (
Seven Kermesidae species, belonging to two genera, Kermes Boitard and Nidularia Targioni Tozzetti, have been described or recorded from Israel off Quercus sp. (
Kermes echinatus Balachowsky is one of six Kermes species found in Israel (
Some scale insects have been known as sources of red dye used in the textile, art and wine industries in the Mediterranean, Middle East and Central Asia regions (
The first-instar of both Kermes echinatus and Kermes vermilio are easily distinguishable from other Mediterranean and European Kermes species due to the presence of conical, spine-like marginal setae (
Between 2010 and 2012, we collected specimens of Kermes echinatus off the evergreen oak, Quercus calliprinos Webb, from forests in the Golan Heights, the Western, Upper and Lower Galilee regions and the Judean Mountains in Israel. The collection site at Timrat in the Lower Galilee is three km from Nahalal, the type locality of Kermes echinatus, and therefore we consider these specimens to be topotypic material. Some of the first-instar nymphs examined in this study emerged from females that were kept in sealed glass containers in the laboratory and other specimens were recovered from thin branches or from trunks of trees.
Specimens were processed and mounted on microscope slides according to the methods outlined by
Abbreviations of specimen depositories are as follows: BMNH - The British Museum (Natural History), London, U.K.; ICVI - Coccoidea Collection, Department of Entomology, Agricultural Research Organization, Bet Dagan, Israel; MNHN - Museum National d’ Histoire Naturelle, Paris, France.
http://species-id.net/wiki/Kermes_echinatus
This species was originally described from the first-instar nymph collected from Israel, Nahalal forest, off Quercus coccifera.
Adult female of Kermes echinatus. Israel: All material was collected off Quercus calliprinos by M. Spodek. At least twenty specimens were examined and all material is deposited in ICVI. Alonei Abba Reserve, 19.vi.2011, 26.vi.2011, 3.vi.2012 (MC:530, C:4999), MC:711); Eilon, 19.vi.2011, 22.vi.2011, 26.vi.2011, 3.vi.2012 (MC:533, MC:542, C:4998), MC:692); Nahal Dolev Reserve, 27.vi.2010, 17.vi.2011, 8.vi.2012, 15.vi.2012, 22.vi.2012 (MC:261, MC:699, MC:528, MC:695, MC:709); Hanita, 6.vi.2010 (MC:227); Mas’ada, 4.vii.2010 (MC:285); Nebi Hazuri, 4.vii.2010, 6.vii.2011 (MC:288, MC:556).
Israel: Syntype (ICVI C:3691, MNHN 1065-8), Nahalal Forest, Quercus coccifera 10.v.1950, Bytinski-Salz; All non-type material was collected off Quercus calliprinos by M. Spodek, unless otherwise stated; at least twenty specimens were examined and all material is deposited in ICVI. Alonei Abba Reserve, 15.vii.2010, 26.vi.2011, 15.vii.2012 (MC:289, MC:559, MC:719); Eilon, 26.ix.2010, 21.iv.2011, 17.vi.2011, 1.vii.2011, 22.vii.2012 (MC:306, MC:486, MC:499, MC:550, MC:718); Hanita, 13.iii.2011 (MC:457); Nahal Dolev, 22.viii.2010, 8.viii.2011, 1.vii.2012 (MC:293, MC:562, MC:717); Nebi Hazuri, 17.viii.2000, Y. Ben-Dov (C:3409), 4.vii.2010, 6.vii.2011, 17.vii.2011, (C:4818, C:5003, MC:561); Neve Zuf, 10.vii.2000, 18.vii.2003, Y. Ben-Dov (C:4752, C:4751); Timrat, 21.vii.2011, 25.iii.2012 (MC:563, MC:651).
Adult female of Kermes vermilio.France: Corsica, Quercus ilex, 7.vi.1999, J. Casevitz-Weal (2 specimens, ICVI C:3277); Le Vert Lasalle, Quercus coccifera, 7.v.2007, D. Cardon (3 specimens, ICVI C:4257); Italy: Portofino, Quercus ilex, 27.v.1971, D. Matile-Ferrero (2 specimens, MNHN 4594-2), Pistoia, Quercus ilex, 13.viii.1986, A. Belcari (2 specimens, MNHN 10732-1), Bitonto (Bari), Quercus ilex, 25.vii.2012, F. Porcelli (10 specimens, ICVI C-5132); Spain: Mieras (Gerona), Quercus coccifera, 7.v.1987, A. Verhecken (1 specimen, MNHN 11526-1).
France: Le Vert Lasalle, Quercus coccifera, 24.vi.2007, D. Cardon (47 specimens, ICVI C:4272); Italy: Pistoia, Quercus ilex, 13.viii.1986, A. Belcari (6 specimens, MNHN 10732-3), Bitonto (Bari), Quercus ilex, 28.viii.2012, F. Porcelli (20 specimens, ICVI C-5133); Spain: Mieras (Gerona), Quercus coccifera, 7.v.1987, A. Verhecken (1 specimen, MNHN 11526-2).
General appearance. Young, pre-reproductive adult: Oval, soft and slightly convex; dorsum brownish-grey with 4 or 5 black longitudinal and 6–9 black transverse lines formed of dots and lines; 2.5–3.2 mm long and 2–3 mm wide (Fig. 1). Fully-mature reproductive female highly convex; dorsum brownish-grey with black, longitudinal and transverse lines; body tapering posteriorly (Figs 2, 3). Post-reproductive female oval and moderately convex, 2.9–4.4 mm long, 2.7–5.1 mm wide and 3.2–4.8 mm high; dorsum sclerotized; red with 6–9 black, transverse black lines represented as reticulated folds (Fig. 4).
Kermes echinatus Balachowsky young adult female, general appearance.
Kermes echinatus Balachowsky mature reproductive female, general appearance.
Kermes echinatus Balachowsky gravid females on tree trunk, general appearance.
Kermes echinatus Balachowsky female with emerging first-instar nymphs.
Slide-mounted adult female. 2–3 mm long and 2–2.8 mm wide (Fig. 5).
Kermes echinatus Balachowsky adult female.
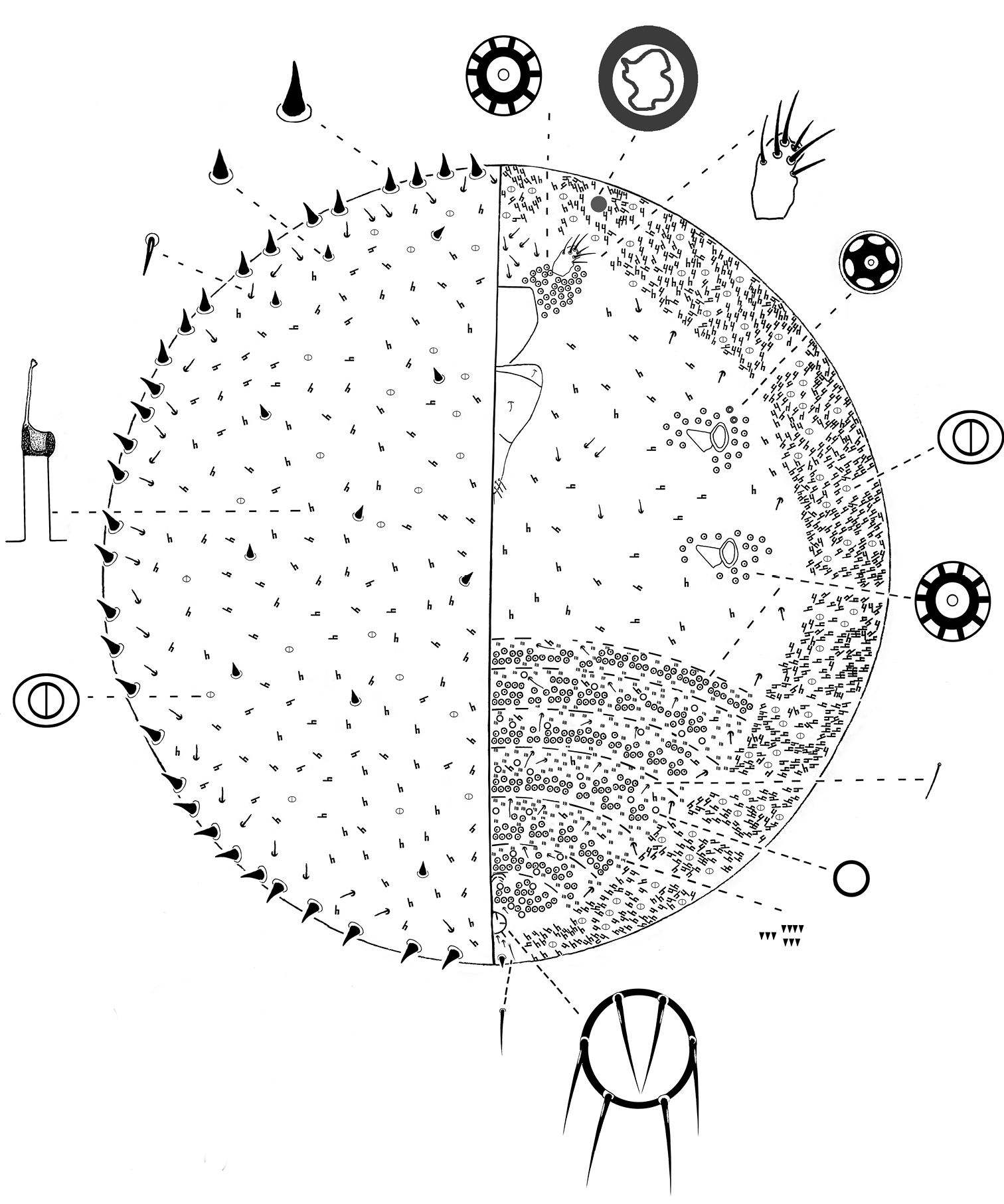
Margin. Marginal setae conical, 12–13 µm long and 10–11 µm wide at base; arranged in a single row of 30–38 setae on each margin.
Dorsum. Dorsal setae hair-like, 7–9 µm long, in submarginal band from cephalic tip of body to posterior end of body, about 28–33 on each side. Conical setae, similar in shape to marginal setae, randomly placed on dorsum, with 7–11 setae on each side; each seta 10–13 µm long and 7–10 wide at base. Bilocular pores oval with a sclerotized rim, each 3 µm long and 2 µm wide; present throughout. Tubular ducts diffused throughout dorsum; each with outer ductule 12–17 µm long, inner ductule 10–15 µm long and with a sclerotized cup 5 µm diameter.
Venter. Eyes circular, 20–25 µm diameter, each placed anterolaterally to each antenna. Legs absent. Antenna each 1-segmented; 26–35 µm long, 20–31 µm wide; eachbearing 5–8 fleshy setae; each antennae is surrounded by a group of 40–45 multilocular pores; each pore 7–8 µm diameter with 10 loculi. Clypeolabral shield 235–250 µm long, 212–225 µm wide. Labium 3-segmented, triangular, 160–175µm long, 110–135 µm wide; labial setae as follow: basal segment with 2 setae, 5–8 µm long; medial segment with 2 setae, 12–20 µm long; apical segment with 4 setae; 6 apical setae, 10–12 µm long and 2 subapical seta, 7–8 µm long. Mesothoracic and metathoracic spiracles subequal in size; peritreme 50–68 µm long and 30–37 µm wide; pores with 10 loculi and 8 µm wide in a group of 15–22 locular pores laterad to each spiracle; also with 2 pores with 6 loculi, each 6µm diameter, laterad to each anterior peritreme. Tubular ducts present in a complete, dense submarginal band about 11 ducts wide and also sparsely throughout rest of venter; each duct with outer ductule 10–16 µm long; inner end of outer ductule with a sclerotized cup, 4–5 µm diameter, and inner ductule 11–15 µm long. Multilocular pores each diameter 10 µm with 10–12 loculi, arranged in 2–3 transverse rows on each abdominal segment; with a total of 114–120 pores on each segment; also with a group of 52–56 pores just posterior to vulva. Bilocular pores each3 µm long and 2 µm wide, interspersed between tubular ducts in submarginal band. Simple pores 2 µm diameter with a sclerotized rim, interspersed between multilocular pores on abdomen. Ventral setae 7–12 µm long, distributed as follows: about 12 setae just anterior to clypeus between antennae; about 8 setae on median and submedian areas of thorax; about 11 setae mesad to each submarginal band of tubular ducts, in a line from antennae to anal ring; 6 or 8setae, present in a band along each abdominal segment; plus 2 setae 20–25 µm long, placed medially on each abdominal segment. Microspines each 1–2 µm long, in groups of 3–5, in 3–8 rows on each abdominal segment. Anal ring ventral, forming a complete sclerotized circle; diameter 42–60 µm; cells absent; with 6 setae, each 25–40 µm long. Other ventral setae 1 pair of setae, each 10–12 µm long, present just anterior to anal ring; 2 pairs of setae, each 10–12 µm long, present posteriorly to anal ring; 1 pair of stout conical setae (similar in shape to marginal spinose setae but shorter), each 10–12 µm long and wide, present on venter slightly above posterior margin; and 1 pair of apical setae, each 33–35 µm long.
General appearance. Dorsum and venter red, body oval and tapering posteriorly, 0.37–0.44 mm long and 0.14–0.2 mm wide. Each with a fringe of curly white wax on margins once first-instars settle on branch for feeding (Fig. 6).
Kermes echinatus Balachowsky first-instar nymph, general appearance.
Mounted specimen. Oval, 0.45–0.49 mm long and 0.20–0.25 mm wide (Fig. 7).
Kermes echinatus Balachowsky first-instar nymph.
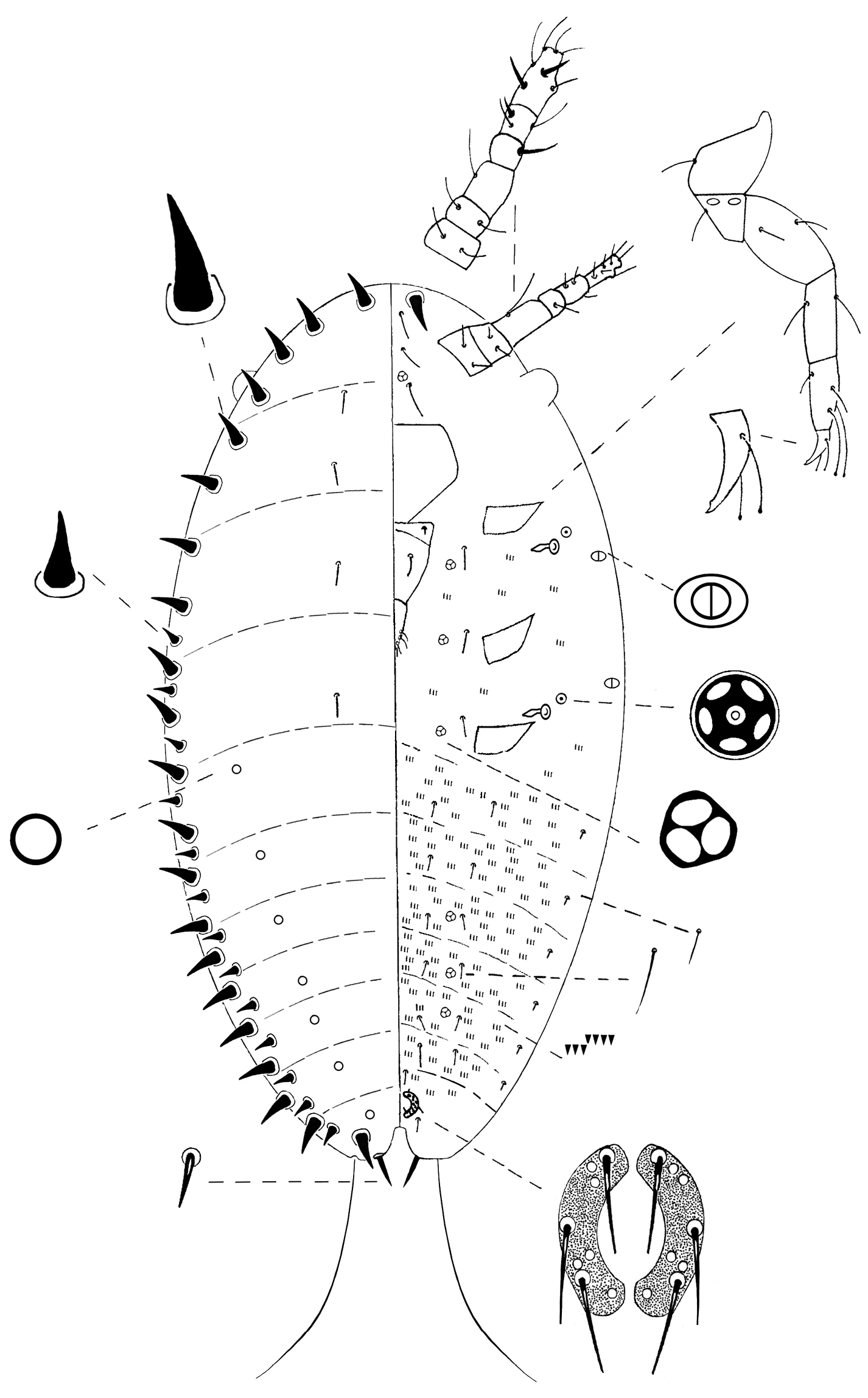
Margin. Marginal setae conical and slightly curved apically, each 10–13 µm long and 5 µm wide at base, in a complete line of 17–22 on each side, smaller conical setae, not-curved, each 5–8 µm long and 3–5 µm wide at base, in a submarginal row of 12–14 setae extending from mesothorax to anal lobe.
Dorsum. Derm membranous; intersegmental lines observable. Dorsal setae 8, each 6–8 µm long, placed in 2 submedian, longitudinal rows on thorax. Simple pores circular, 14, eachabout1 µm diameter, placed in 2 submarginal, longitudinal rows on abdomen.
Venter. Antennae each 6-segmented; total length 102–110 µm; with segment III and VI longest; setal distribution as follows: scape and pedicel each with 2 thin, hair-like setae; segment III with 1 long thin, hair-like seta; IV with 1 fleshy setae; V with 1 fleshy seta and 2 hair-like setae; apical segment with 2 fleshy setae and 5 hair-like setae. Legs well-developed; measurements of hind legs (length in µm); coxae 25–30, trochanter + femur 68–70, tibia 33–38, tarsus 45–50, claw 15–20; total leg length 187–200 µm; trochanter with 2 oval, sensory pores on each side, each pore 3 µm long and about 2 µm wide; setae present on all leg segments; tarsal digitules each 25–30 µm long and knobbed apically, extending beyond apex of claw;claw digitules knobbed apically, each 15–20 µm long; each claw with a single denticle near tip. Clypeolabral shield well-developed; 68–75 µm long and 63–75 µm wide. Labium 3-segmented, triangular, 75–83 µm long and 45–47 µm wide; labial setae as follows: basal segment with 1 setae, rarely 2 setae, 5–8 µm long, median segment with 2 hair-like setae on dorsal surface, 12–13 µm long, apical segment with 6 subapical setae, each 16–20 µm long and 2 apical setae 10–12 µm long. Spiracles subequal in size; each peritreme 3–5 µm diameter; apodeme crescent shaped, 13–15 µm long; each spiracle with 1 quinquelocular pore, 5 µm diameter, placed anterolaterally. Trilocular pores, each about 3 µm wide, distributed as follows: 2 pores between scape just anterior to clypeus; 1 mesad to each coxa, and 2 submedially on abdominal segments V–VII. Bilocular pores oval, 4 total, each 3 µm long and 2 µm wide, present between margin and each spiracle. Ventral setae interantennal setae 6, each 38–45 µm long, in an longitudinal line medially between scapes; also 2 conical setae, about 14–16 µm long and 5 µm wide at base, on anterior apex of head; 1 seta 10–11 µm long, mesad to each coxa associated with each trilocular pore; and 6 longitudinal lines of setae on abdomen; with 2 medial, 2 submedial and 2 submarginal seta per segment; medial and submedial setae each 10–15 µm long, and submarginal setae 5–6 µm long. Microspines each about 3 µm long, arranged in groups of 3 or 4 in 2 transverse rows on each abdominal segment and sparsely on thorax. Anal ring located ventrally; composed of 2 semi-circles; diameter 20–25 µm; each half circle with 4–6 cells and 3 pointed setae, each 13–18 µm long. Other setae with a pair of setae, each 15–18 µm long, anterior to anal ring, and a pair, each 15–20 µm long, latero-posteriorly to anal ring. Anal lobes slightly developed; inner margin of each lobe with 1 pointed seta, 10–13 µm long and 2–3 µm wide, and 1 very long, flagellate seta apically 220–275 µm long.
Prior to this study, Kermes echinatus had only been reported off the evergreen oak, Quercus coccifera, from Nahalal forest, located in the Lower Galilee of Israel (
The present description of the first-instar nymph of Kermes echinatus agrees well with that of
In addition, we observed other distinguishing features between the two species and these are summarized in Table 1.
Comparison of some characters of the first-instar nymph of Kermes echinatus and Kermes vermilio.
| Character | Kermes echinatus | Kermes vermilio |
|---|---|---|
| Dorsal simple pores | present | absent |
| Dorsal bilocular pores | absent | present |
| Locular pores associated with prothoracic spiracles | 1 pore, 5 loculi | 1 pore, 5 loculi and 7 loculi |
| Arrangement of marginal setae | 2 rows | 2 rows |
| Type of conical marginal setae | 2 types | 1 type |
| Denticle on claw of legs | present | absent |
Comparison of some characters of the adult females of Kermes echinatus and Kermes vermilio.
| Character | Kermes echinatus | Kermes vermilio |
|---|---|---|
| Marginal and dorsal conical setae | present | present |
| Hair-like setae in submarginal band on dorsum | present | absent |
| Conical setae in submarginal band on venter | absent | present |
| Legs | absent | absent |
| Position of anal ring | ventral | ventral |
| 6 -locular pores associated with prothoracic spiracles | 2 pores present | absent |
| Setae on anal ring | present | absent |
| Cells on anal ring | absent | present |
| Simple pores on abdomen | present | absent |
| Multilocular pores posterior to vulva | present | absent |
The general appearance of young females and fully-grown reproductive females of Kermes echinatus differs from that of Kermes vermilio. The young female of Kermes echinatus is slightly convex, has a brownish-grey dorsum with 4 or 5 black longitudinal and 6–9 black transverse lines composed of dots and lines. The young female of Kermes vermilio is reddish without transverse and longitudinal lines. The fully-grown reproductive female of Kermes vermilio has been described as dark red or brown covered with a fine, white or pale grey mealy wax (
The morphological features of the adult female of Kermes echinatus and Kermes vermilio are similar and are summarized in Table 2. Some of the shared features are the following; (i) dorsal and marginal conical setae; (ii) absence of legs; (iii) presence of numerous multilocular pores on abdominal segments as well as surrounding the antennae and spiracles; (iv) one-segmented antennae with fleshy setae; and (v) the anal ring located ventrally in both species. The most distinguishing feature of Kermes echinatus is the anal ring which has six setae and no cells whereas the anal ring of Kermes vermilio has cells but no setae. Some other differences between the two species are that Kermes echinatus has less conical setaeon its margins and dorsum compared to Kermes vermilio. Kermes echinatus has 30–38 setae on each half margin compared to 73–133 in Kermes vermilio. Kermes echinatus has 7–11 dorsal setae compared to about 70 dorsal setae in Kermes vermilio. Ventral loculate pores are only found on the abdominal segments and surrounding the spiracles in Kermes echinatus in contrast to Kermes vermilio, where they extend onto the metathorax from the abdomen.
This paper describes the adult female of Kermes echinatus for the first time and redescribes the first-instar nymph. The general appearance and morphological features of Kermes echinatus and Kermes vermilio, two species that have been linked to sources of red dye in the Palaerarctic region, are compared. Distinguishing characters of the first-instar nymph and female of Kermes echinatus are presented. Kermes echinatus has only been recorded in Israel to-date and is one of seven species of Kermesidae occurring there.
This study was partly funded to the senior author by The Karen Kayemeth LeIsrael (Project # 131-1621-11) and the Israel Taxonomy Initiative. This manuscript is part of the PhD thesis for the senior author. We thank Daniele Matile-Ferrero, Imre Foldi, (MNHN), Jon Martin (BMNH) and Francesco Porcelli (Department of Entomology, University of Bari, Italy) who helped us obtain specimens and slide-mounted material for this study. We would also like to thank Zvi Mendel and Murad Ghanim (Volcani Center, Bet Dagan, Israel) for their support throughout this project. Special gratitude is expressed to Alex Protasov for his photographic skills and technical support (Volcani Center, Bet Dagan, Israel). Collection permits at Nature Reserves in Israel, were kindly provided by the Israel Nature and Parks Authority.