(C) 2012 Jivanildo Pinheiro Miranda. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
We are presenting a list of the reptile species from Lençóis Maranhenses National Park (LMNP), Maranhão, Brazil, obtained during 235 days of field work. The study area is located in the contact zone between three major Neotropical ecosystems: Amazonia, Caatinga, and Cerrado. The PNLM encompasses the largest dune fields in Brazil, wide shrubby areas (restingas), lakes, mangroves, and many freshwater lagoons. We have recorded 42 species of reptiles in the area: 24 snakes, 12 lizards, two worm lizards, three turtles, and one alligator. About 81 % of the recorded species occurred only in restinga areas. Our data highlights the uniqueness of the PNLM in the context of the biomes that surround it and shows the importance of efforts to improve the conservation of reptiles living in the restinga, which currently comprise only about 20 % of the total area protected by the park, but which are the mesohabitat containing most of the reptile species in the Lençóis Maranhenses complex of habitats.
ResumoNo presente estudo apresentamos uma lista das espécies de répteis presentes no Parque Nacional dos Lençóis Maranhenses (LMNP), Maranhão, Brazil. A área do Parque Nacional dos Lençóis Maranhenses está localizada em um complexo ecótono na zona de contato entre a Amazônia, a Caatinga e o Cerrado. Além desta localização singular, a área inclui o maior campo de dunas do Brasil, extensas restingas, lagos, manguezais e uma grande quantidade de lagoas de água doce, formadas nos vales do campo de dunas. Registramos 42 espécies de répteis: 24 serpentes, 12 lagartos, duas cobras-cegas, três quelônios e um jacaré. Destas, cerca de 81 % foram encontrados apenas nos ambientes de restinga. Os resultados apresentados aqui enfatizam a singularidade do PNLM no contexto dos biomas que o cercam e ressaltam a importância de ações para incrementar a conservação das áreas de restinga, as quais, atualmente, constituem apenas 20 % da área total protegida pelo parque, mas constituem o mesohábitat onde ocorre a maioria das espécies de répteis que vivem no complexo de ambientes que compõe os Lençóis Maranhenses.
Richness, ecotone, lizards, snakes, turtles, worm lizards, dunes, restingas
Brazil is a megadiverse country, including six biomes (
The Lençóis Maranhenses National Park (LMNP) is located in the Northeastern coast of Brazil (central coordinates: 02°31'02"S, 43°01'54"W, SAD69). The area of the park (about 155, 000 hectares) is composed of sand dunes, freshwater lagoons, restingas (local name for herbaceous and shrubby vegetation), lakes, mangroves, and 70 km of beach. The dunefields arose from the varieties of sediments due to retrogradations from sedimentary deposits (Barreiras formation of Tertiary age), the correspondent widening of the continental shelf, successive marine transgressions since Pleistocene, and inputs of fluvial sediments from the main rivers in that region (
The climate in LMNP is warm (mean annual temperature: 28.5°C) with relatively little temperature variation throughout the year (about 1.1 °C, in average) and an annual rainfall between 1, 600 and 2, 400 millimeters (
We have distinguished the following seven mesohabitats in the LMNP: 1) Sand dunes “Morrarias” (vernacular expression): are sand dunes with no stabilizing vegetation, which is the most frequent and dynamic mesohabitat in the park (Figure 1A). The constant movement of sand dunes influences all other mesohabitats. The transportation of sand by wind constantly buries the vegetation in the area bordering the dunes (Figure 1B). Additionally, the migration of contiguous dunes spill water from one lagoon to the next closest one, resulting in a high interchange of water among lagoons. During the rainy season, however, the migration of dunes is slower because of the moisture that avoids sand transference (
Mesohabitats found at Lençóis Maranhenses National Park, Maranhão State, Northeastern Brazil: A Sand dunes “Morrarias” B Remains of restinga vegetation buried by sand transportation C Freshwater lagoon D “Vargem” E Restinga mosaics in the boundary of sand dunes, including a lagoon F Restinga mosaics far away from the sand dunes. Photos by J. P. Miranda.
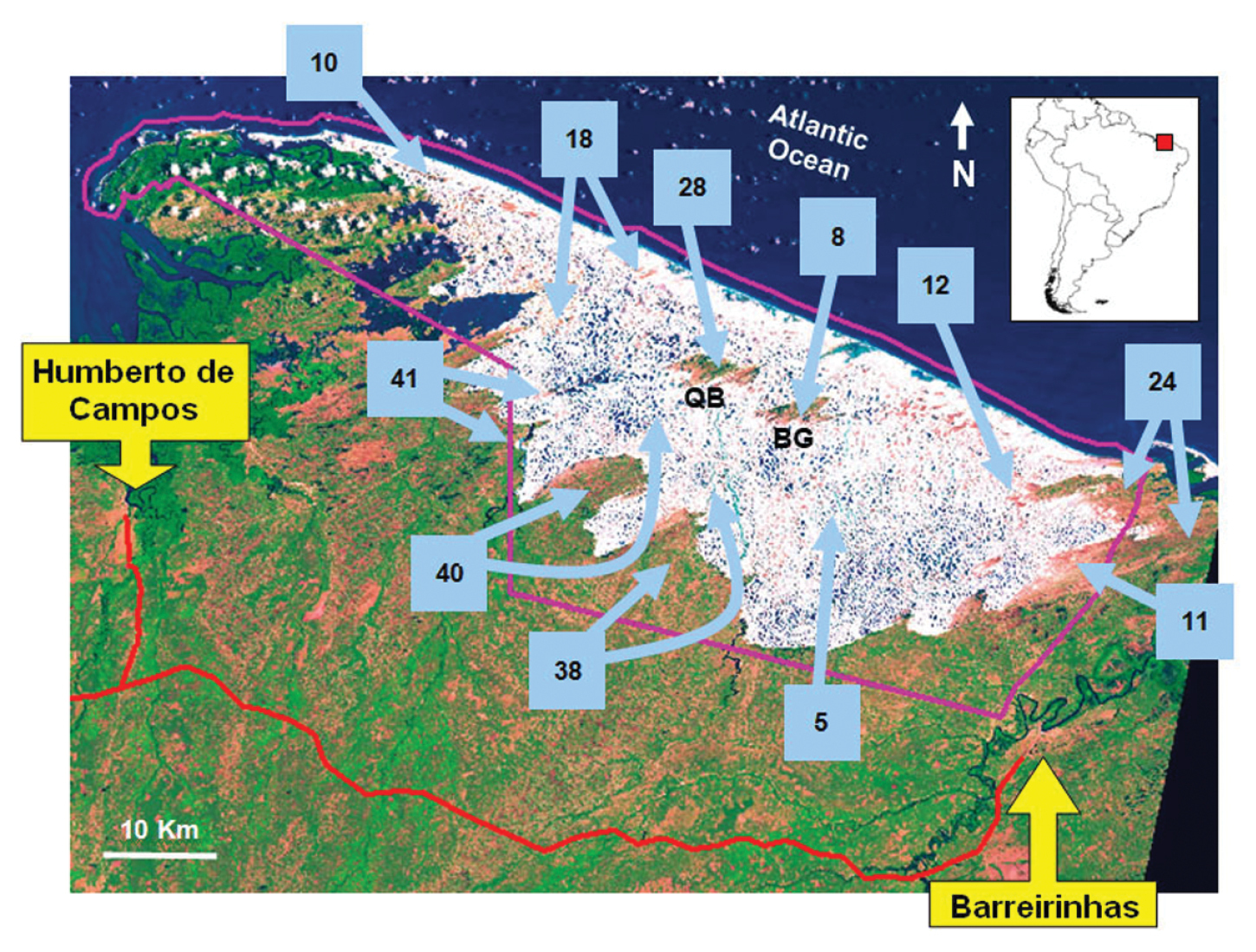
Satellite image Landsat showing sample sites (light blue arrows) in the region of the Lençóis Maranhenses National Park, Maranhão State, Northeastern Brazil. The amount of sampling days is specified inside light blue squares. Two arrows linked to the same squares and pointing to different places indicate that the sampling effort in the square is divided between these sites. White areas with blue spots are sand dunes and freshwater lagoons, respectively. The greenish areas flecked by the orange color, are restingas habitats (green represents shrubby areas and orange most opening areas). QB and BG indicate the position of the isolated restingas called Queimada dos Britos and Baixa Grande, respectively. The violet line indicates the territory of LMNP. The red line is the road that accesses the park (MA-402). The location of the provinces Barreirinhas and Humberto de Campos are provided in the yellow rectangles. The position of the park in South America is provided upper right. Satellite image modified after
The species survey of reptiles at LMNP was conducted from September, 2004 to April, 2006 (IBAMA permit number 02001.004089/03-50). In that period, we made 47 trips, totaling 235 days of field sampling. At each site (see Figure 2), we sampled about three hectares. We used the time-constrained sample method, which is performed by walking slowly and searching for specimens in all visually accessible microhabitats (
We constructed species-accumulation curves that were generated using the nonparametric binomial mixture model of
We recorded 42 reptile species in the LMNP: 12 species of lizards, belonging to 11 genera and eight families (Gekkonidae, Sphaerodactylidae, Mabuyidae, Gymnophthalmidae, Iguanidae, Polychrotidae, Teiidae and Tropiduridae); two species of worm lizards belonging to the genus Amphisbaena, in the family Amphisbaenidae; 24 species of snakes, belonging to 20 genera and four families (Boidae, Colubridae, Dipsadidae and Elapidae); three species of turtles, belonging to three genera and three families (Cheloniidae, Dermochelyidae, and Emydidae); and one species of alligator (Alligatoridae) (Table 1).
Reptile species recorded at Lençóis Maranhenses National Park, Maranhão State, Northeastern Brazil, respective environments of occurrence in the park, and figure numbers (when available). In the column mesohabitat, the numbers correspond to the following mesohabitats: 1) Sand dunes “Morrarias”; 2) Freshwater lagoons; 3) Vargem; 4) Restingas; 5) Innermost isolated restingas; 6) Rivers; 7) Beaches.
| Reptilia | Mesohabitat | Figure |
|---|---|---|
| OrderSAURIA | ||
| Family Sphaerodactylidae | ||
| Gonatodes humeralis (Guichenot, 1855) | 4 | 5A |
| Family Gekkonidae | ||
| Hemidactylus mabouia (Moreau de Jonnès, 1818) | 4, 5 | 5B |
| Family Gymnophthalmidae | ||
| Colobosaura modesta (Reinhardt and Lütken, 1862) | 4 | |
| Family Iguanidae | ||
| Iguana iguana (Linnaeus, 1758) | 4, 5 | 5C |
| Family Mabuyidae | ||
| Varzea bistriata (Spix, 1825) | 4, 5 | 5D |
| Brasiliscincus heathi (Schmidt and Inger, 1951) | 5E | |
| Family Polychrotidae | ||
| Polychrus acutirostris Spix, 1825 | 4 | 5F |
| Family Teiidae | ||
| Ameiva ameiva (Linnaeus, 1758) | 4, 5 | 5G |
| Ameivula ocellifera (Spix, 1825) | 1, 4, 5 | 5H |
| Kentropyx calcarata Spix, 1825 | 4 | 6A |
| Tupinambis teguixin (Linnaeus, 1758) | 1, 4, 5 | |
| Family Tropiduridae | ||
| Tropidurus hispidus (Spix, 1825) | 1, 4, 5 | 6B |
| OrderAMPHISBAENIA | ||
| Family Amphisbaenidae | ||
| Amphisbaena ibijara Rodrigues, Andrade & Lima, 2003 | 4 | |
| Amphisbaena vermicularis Wagler, 1824 | 4 | 6C |
| OrderSERPENTES | ||
| Family Boidae | ||
| Boa constrictor Linnaeus, 1758 | 4 | 6D |
| Eunectes murinus (Linnaeus, 1758) | 4, 5 | |
| Family Colubridae | ||
| Chironius flavolineatus (Jan, 1863) | 4 | |
| Drymarchon corais (Boie, 1827) | 4, 5 | 6E |
| Leptophis ahaetulla (Linnaeus, 1758) | 4, 5 | 6G |
| Mastigodryas bifossatus (Raddi, 1820) | 4, 5, 6 | |
| Oxybelis aeneus (Wagler, 1824) | 4 | 6H |
| Oxybelis fulgidus (Daudin, 1803) | 4 | 7A |
| Spilotes pullatus (Linnaeus, 1758) | 4 | |
| Tantilla melanocephala (Linnaeus, 1758) | 4 | |
| Family Dipsadidae | ||
| Helicops angulatus (Linnaeus, 1758) | 4, 5, 6 | 6F |
| Hydrodynastes gigas (Duméril, Bribon and Duméril, 1854) | 4, 6 | |
| Erythrolamprus poecilogyrus (Wied-Neuwied, 1825) | 1, 4, 5 | 7B |
| Erythrolamprus taeniogaster (Jan, 1866) | 4 | |
| Leptodeira annulata (Linnaeus, 1758) | 4 | |
| Lygophis meridionalis (Schenkel, 1902) | 4 | |
| Oxyrhopus trigeminus Duméril, Bibron and Duméril, 1854 | 4, 5 | 7C |
| Philodryas nattereri Steindachner, 1870 | 4 | 7D |
| Philodryas olfersii (Lichtenstein, 1823) | 4 | |
| Psomophis joberti (Sauvage, 1884) | 4 | 7E |
| Taeniophallus occipitalis (Jan, 1863) | 4 | |
| Thamnodynastes hypoconia (Cope, 1860) | 4 | 7F |
| Xenodon merremii (Wagler, 1854) | 4 | 7G |
| Family Elapidae | ||
| Micrurus ibiboboca (Merrem, 1820) | 4, 5 | |
| OrderTESTUDINES | ||
| Family Cheloniidae | ||
| Chelonia mydas (Linnaeus, 1758) | 7 | |
| Family Dermochelyidae | ||
| Dermochelys coriacea (Vandelli, 1761) | 7 | |
| Family Emydidae | ||
| Trachemys adiutrix Vanzolini, 1995 | 1, 2, 3 | 7H |
| OrderCROCODYLIA | ||
| Family Alligatoridae | ||
| Caiman crocodilus (Linnaeus, 1758) | 6 | |
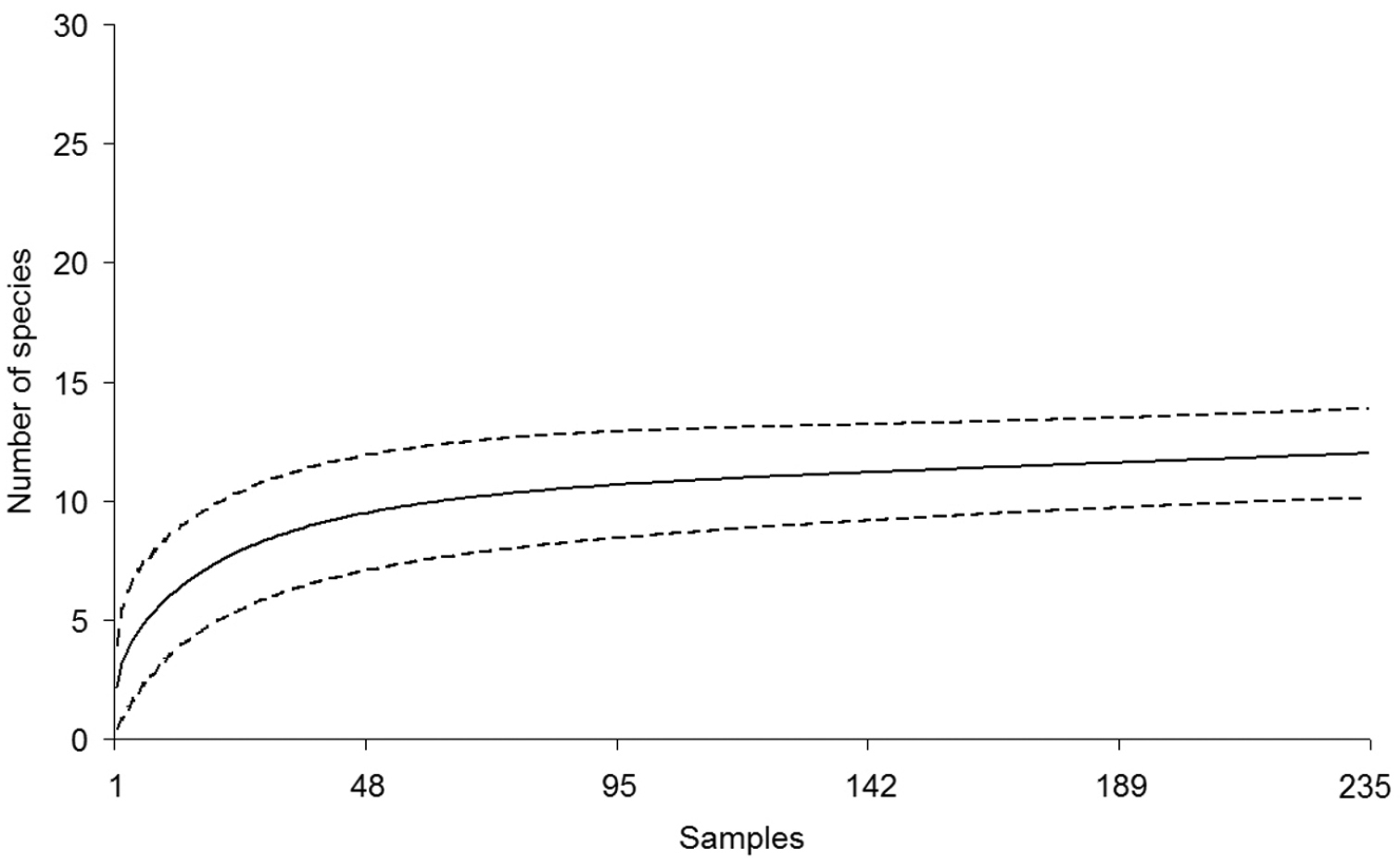
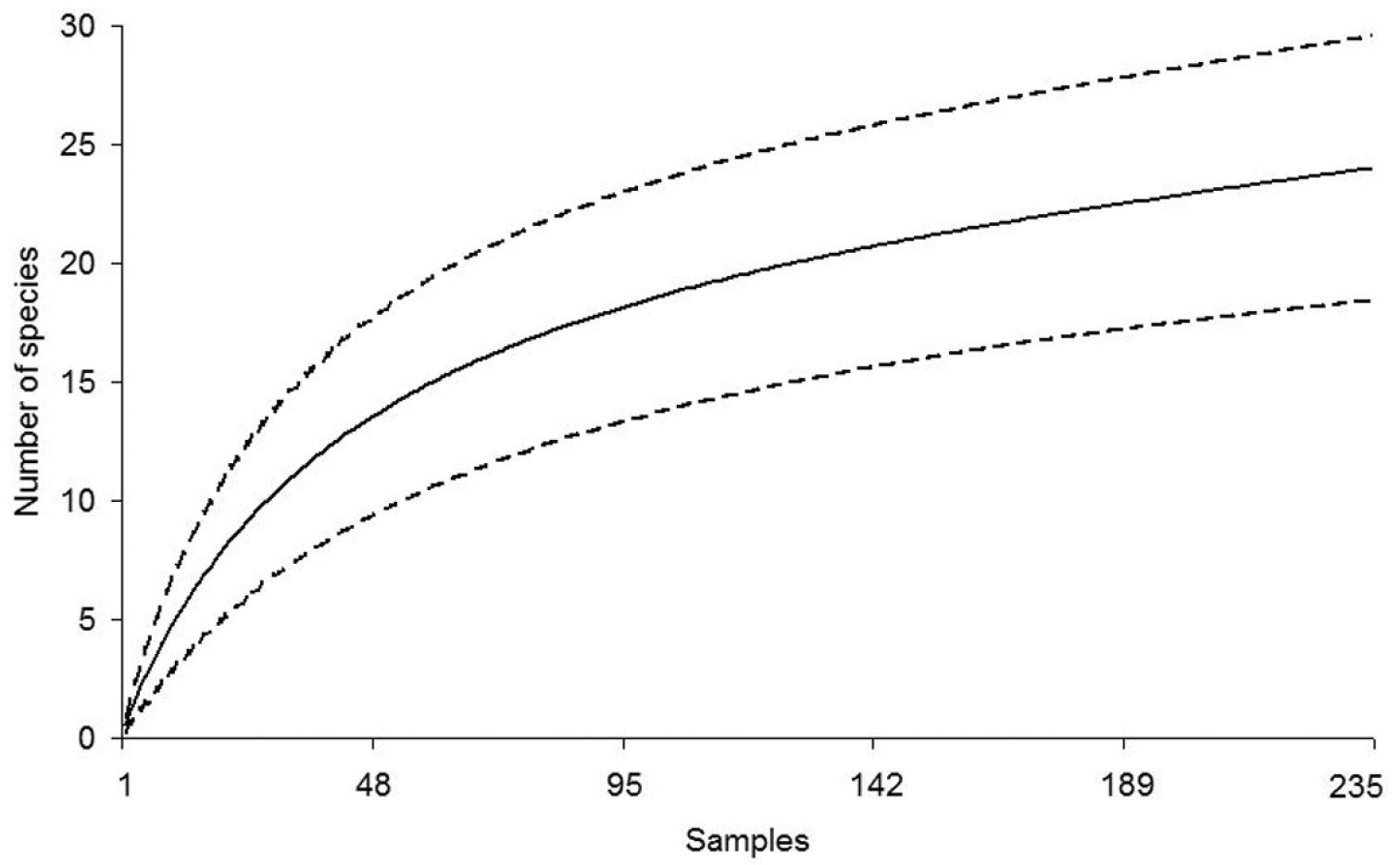
The species-accumulation curves for snakes and lizards have different slopes and confidence intervals according to the reptile group studied. Nevertheless, both curves predicted more species than currently recorded. Richness for each reptile group was quite close to the predicted values, especially for the lizards (Figures 3 and 4). The first order Jackknife estimator predicted that 13 to 15 species of lizards [N(J1) = 13, 99 ± 1, 40], and 28 to 34 species of snakes [N(J1) = 30, 97 ± 2, 95] might be recorded in LMNP. Overall, most reptile species that we recorded at LMNP were found in the restingas (Table 1).
Accumulation curve for lizards recorded at the region of the Lençóis Maranhenses National Park (LMNP), Maranhão State, Northeastern Brazil (solid line). Dashed lines are confidence intervals at 95 %. The total number of sampling days is 235. A sample is equal to the search effort of two people looking for reptile species from 09:00 to 15:00 h and from 19:00 to 23:00 h.
Accumulation curve for snakes recorded at the region of the Lençóis Maranhenses National Park (LMNP), Maranhão State, Northeastern Brazil (solid line). Dashed lines are confidence intervals at 95 %. The total number of sampling days is 235. A sample is equal to the search effort of two people looking for reptile species from 19:00 to 23:00 h and from 09:00 to 15:00 h.
In the management plans of the LMNP, there is no list of Herpetofauna’s species (
In LMNP there are only three species of lizards: (Tropidurus hispidus, Ameivula ocellifera, and Tupinambis teguixin), one snake (Erythrolamprus poecilogyrus), and Trachemys adiutrix which were recorded in sand dune areas. Additionally, two sea turtles were recorded at the coastal area of the park (Chelonia mydas and Dermochelys coriacea). Thus, about 81 % (34 species) of reptile species recorded at LMNP were only found in restingas. In addition, the management plans of the LMNP emphasize the innermost isolated restingas for their actions in conservation, as opposed to restinga areas located at the southern LMNP (
During our field work, we observed that the restingas in the south area of the park (both inside the park and in the buffer zones) have been strongly disturbed by the clandestine openings of paths created to transport tourists to the dunes and lagoons in the park, using off-road vehicles. This problem is more severe during the rainy season (from January from June) when paths become muddy quickly, and new paths are continuously opening. Restinga are extremely sensitive to clearing because the poor soils hinder habitat recomposition (
On the beach and sand dune areas, we recorded three species of turtles which are included in the IUCN Red List of Threatened Species (
In the sand dunes at LMNP, which is an extremely open area, the ground temperature can easily exceed 70° C during the warmest period of the day (JPM Pers. Obs.). This particular characteristic of sand dune areas reinforces the importance of shelters (dead branches and patches of vegetation) and burrows for the species that live there. For lizards, shelters and burrows are important for thermoregulation, as this is one of the few options to decrease exposure to the sun (
The only exotic invasive reptile species at LMNP was the gecko Hemidactylus mabouia, which was found on different occasions in natural habitats and microhabitats within the study area. This lizard, which is native from Africa, is one of the five invasive reptile species presently known to occur in Brazil (
Our data highlight the singularity of the LMNP in the context of the biomes that surround it, and also demonstrate the importance of actions to improve conservation of reptiles that live in both sand dunes and restingas in LMNP. Currently, restingas comprise only about 20% of the total area protected by the park, but most reptile species live in restinga mesohabitats. Thus, we suggest the addition of restinga areas adjacent to the park (buffer zones) to be incorporated into the national park, which is a fully protected conservation unit in the Brazilian system of conservation units. This would be the most effective way to protect the biodiversity of reptiles in the restinga areas in that region. Moreover, regarding the sand dunes areas; we suggest an improvement in the security at the LMNP to prevent the illegal use of off-road vehicles inside the park territory, the promotion of actions to monitor the activities of sea turtles at the coast of LMNP, and the implementation of an effective strategy to protect the Brazilian slider turtle. This strategy could be in the form of awareness campaigns, or even the promotion of training courses (e. g. tourist guides, waiters, cooks, or hotel maids) for those living in the park region. This would place local people into the tourism business, which would not only improve their economical capacity, but also reduce their need to use Trachemys adiutrix as a food item.
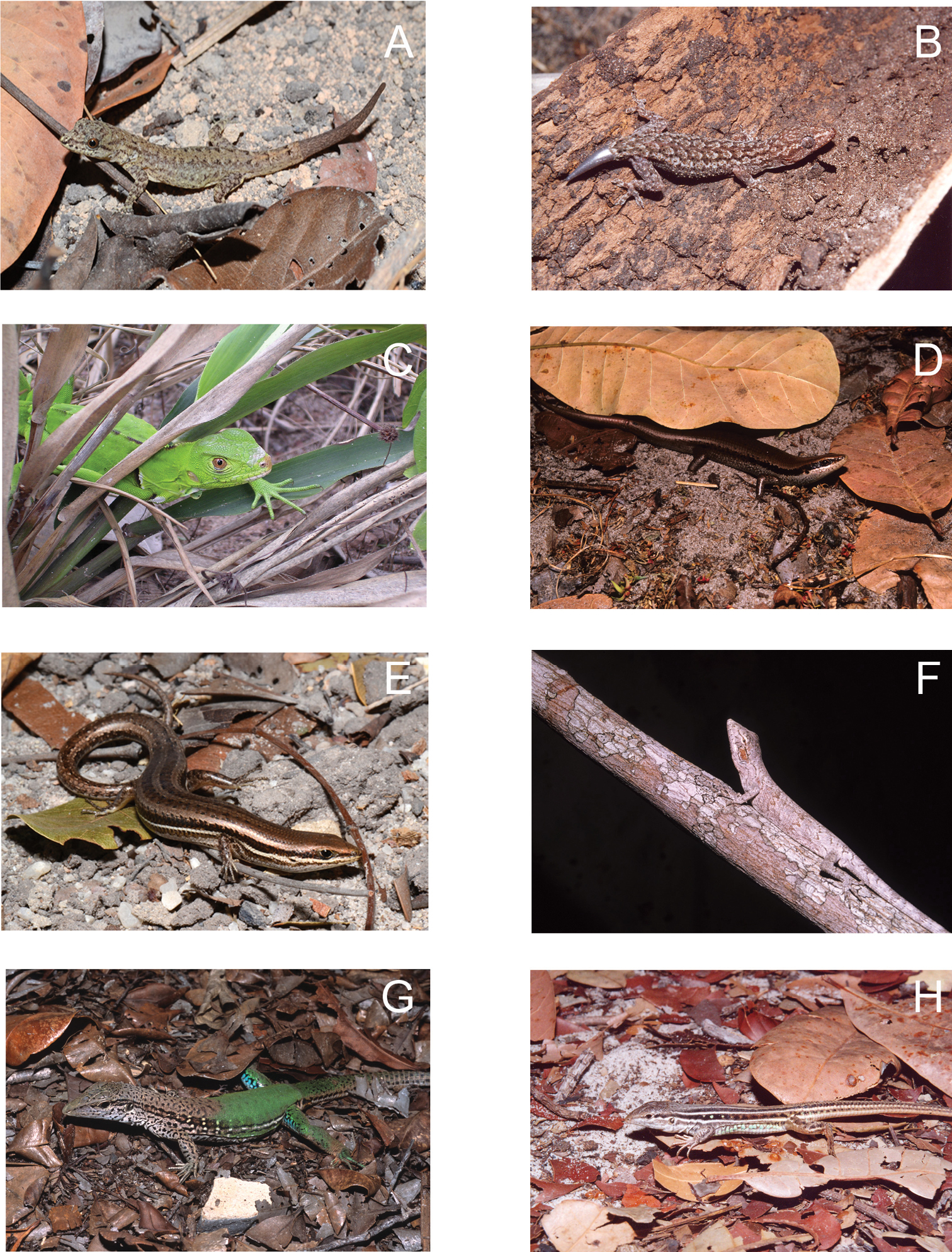
A Gonatodes humeralis (female) B Hemidactylus mabouia C Iguana iguana (juvenile) D Varzea bistriata E Brasiliscincus heathi F Polychrus acutirostris G Ameiva ameiva H Ameivula ocellifera from Lençóis Maranhenses National Park, Maranhão State, Northeastern Brazil. Photos by J. P. Miranda.
A Kentropyx calcarata B Tropidurus hispidus C Amphisbaena ibijara D Boa constrictor E Drymarchon corais F Helicops angulatus G Leptophis ahaetulla H Oxybelis aeneus from Lençóis Maranhenses National Park, Maranhão State, Northeastern Brazil. Photos by J. P. Miranda.
A Oxybelis fulgidus B Erythrolamprus poecilogyrus C Oxyrhopus trigeminus D Philodryas nattereri E Psomophis joberti F Thamnodynastes hypoconia G Xenodon merremii H Trachemys adiutrix (two individuals in a freshwater lagoon) from Lençóis Maranhenses National Park, Maranhão State, Northeastern Brazil. Photos by J. P. Miranda.
We thank Antonio Pereira, Maria Grossa, Arnaldo Oliveira Silva, Edmilson Godé, Adriano Kid Azambuja and Thiare Fortes for field assistance; Romário Ferreira de Matos, Raiana Cristina Araújo for help in laboratory, and Kristen Hammer for English review of the manuscript. JPM thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq for granting his PhD Scholarship and Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão – FAPEMA (INFRA 00563/10) for research grants. JCLC was supported by a scholarship from CNPq. CFDR received grants from CNPq (304791/2010-5 and 470265/2010-8) and from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ (Process E-26/102.404.2009) through Programa Cientistas do Nosso Estado. Valdir Germano, Francisco Franco, Daniel Fernandes and Miguel T. Rodrigues helped us with the identification of the specimens. This study was supported by Instituto Biomas and by a research grant from Fundação “O Boticário” de Proteção à Natureza (Process 0612/2004-1). Three anonymous reviewers provided valuable suggestions on the manuscript.
Voucher specimens of the present study were deposited in the Coleção Herpetológica “Claude d’Abbeville” (CHMA) at Universidade Federal do Maranhão, Chapadinha, Maranhão, Brazil.
Order SAURIA
Family Sphaerodactylidae
Gonatodes humeralis – CHMA 554.
Family Gekkonidae
Hemidactylus mabouia – CHMA 538, 542, 545, 555, 557, 561.
Family Mabuyidae
Varzea bistriata – CHMA 503.
Brasiliscincus heathi – CHMA 504, 505, 549.
Family Polychrotidae
Polychrus acutirostris – CHMA 551, 556.
Family Teiidae
Ameiva ameiva – CHMA 507, 509, 511, 535, 548, 569.
Ameivula ocellifera – CHMA 506, 508, 510, 515, 517, 520-524, 571, 574.
Kentropyx calcarata – CHMA 512.
Tupinambis teguixin – CHMA 567.
Family Tropiduridae
Tropidurus hispidus – CHMA 558-560, 562-564.
Order AMPHISBAENIA
Family Amphisbaenidae
Amphisbaena ibijara – CHMA 516, 518.
Amphisbaena vermicularis – CHMA 513, 519.
Order SERPENTES
Family Colubridae
Chironius flavolineatus – CHMA 537, 572.
Drymarchon corais – CHMA 576.
Mastigodryas bifossatus – CHMA 566.
Oxybelis aeneus – CHMA 541.
Spilotes pullatus – CHMA 570.
Tantilla melanocephala – CHMA 575.
Family Dipsadidae
Helicops angulatus – CHMA 529, 530, 573.
Hydrodinastes gigas – CHMA 568.
Erythrolamprus poecilogyrus – CHMA 536, 539, 543, 552.
Leptodeira annulata – CHMA 528
Oxyrhopus trigeminus – CHMA 525-527.
Philodryas nattereri – CHMA 534, 544.
Philodryas olfersii – CHMA 540.
Psomophis joberti – CHMA 500, 501, 553.
Taeniophallus occipitalis – CHMA 550.
Thamnodynastes hypoconia – CHMA 531-533.
Xenodon merremii – CHMA 546.
Family Elapidae
Micrurus ibiboboca – CHMA 547.
Order TESTUDINES
Family Emydidae
Trachemys adiutrix – CHMA 565.