(C) 2013 Juan-Manuel Nieto Nafría. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Nieto Nafría JM, Mier Durante MP, Remaudière G (2013) The genus Aphidura (Hemiptera, Aphididae) in the collection of the Muséum national d’Histoire naturelle of Paris, with six new species. ZooKeys 318: 1–33. doi: 10.3897/zookeys.318.5693
Specimens were studied of 65 samples of the genus Aphidura (Aphididae, Aphidinae, Macrosiphini) from the collection of the Muséum national d’Histoire naturelle (Paris). The possible synonymies of three pairs of species are discussed. New aphid host plant relationships are reported for Aphidura bozhkoae, Aphidura delmasi, Aphidura ornata, Aphidura pannonica and Aphidura picta; this last species is recorded for first time from Afghanistan. The record of Aphidura pujoli from Pakistan is refuted. The fundatrices, oviparous females and males of Aphidura delmasi are described. Six new species are established: Aphidura gallica sp. n. and Aphidura amphorosiphon sp. n. from specimens caught on species of Silene (Caryophyllaceae) from France and Iran, respectively, Aphidura pakistanensis sp. n., Aphidura graeca sp. n. and Aphidura urmiensis sp. n. from specimens caught on species of Dianthus, Gypsophila and Spergula (Caryophyllaceae) from Pakistan, Greece and Iran, respectively, and Aphidura iranensis sp. n. from specimens caught on Prunus sp. from Iran. Modifications are made to the keys by Blackman and Eastop to aphids living on Dianthus, Gypsophyla, Silene, Spergula and Prinsepia and Prunus (Rosaceae). An identification key to apterous viviparous females of species of Aphidura is also provided.
New taxa, descriptions, synonymies, key of species, host plants, distributions
In the early 1980s G. Remaudière and D. Hille Ris Lambers studied some samples of Aphidura (Hemiptera, Aphididae, Aphidinae, Macrosiphini) belonging to the Remaudière collection, which was at that time at the Institut Pasteur in Paris, but later moved to the Muséum national d’Histoire naturelle. They made preliminary works to describe several new species of this genus, but the work was interrupted and a manuscript draft was never prepared due to the illness and death in April 1984 of Hille Ris Lambers. Some of the slides studied by Hille Ris Lambers were sent with the rest of his collection to the British Museum (Natural History), currently the Natural History Museum, in London. Some years later Remaudière did establish one new species in this genus (
All those samples, together with the rest of the specimens of Aphidura of the above-mentioned collection, have now been studied, and the results are presented in this paper.
The genus Aphidura was morphologically well defined by
Similar mesosternal processes are also present in several species of Brachycaudus van der Goot, 1913, mainly belonging to subgenus Acaudus van der Goot, 1913, and in the sole species of Zinia, Zinia veronicae Shaposhnikov, 1950. These species of Brachycaudus have a helmet-shaped cauda and wide, rounded spiracular apertures, and Zinia veronicae has a rounded cauda, reniform spiracular apertures that are placed in the posterior half of the spiracular sclerites and, in addition the dorsal cuticle is densely spinulose (
Aphidura currently includes 16 to 18 species and 1 subspecies (
Two species of Aphidura live on Rosaceae species and other species live on Caryophyllaceae species, mainly belonging to genus Silene. Aphidura pujoli is monoecious holocyclic on Caryophyllaceae; it is possible that the life cycle of other species of Aphidura is also monoecious holocyclic, though it is also possible that some species host-alternate between species of Rosaceae and Caryophyllaceae (
The genus exhibits a Mediterranean-Pontian-Turanian distribution with extensions to neighbouring areas and exceptionally – Aphidura mordvilkoi – to the Russian Far East. The current known distribution of each species is shown in the species identification key at the end of this paper, Aphidura picta being the species with the widest distribution (
Aphidura specimens of the aphid collection of the Muséum national d’Histoire naturelle of Paris, mounted in microscopic slides, belonging to 65 samples (Table 1) have been studied.
Aphids were identified, or their previous identifications were checked, by reference to the original descriptions (
Studied samples.
| Aphidura species Host plant | Country | Locality | Date | Coll. | Sample |
|---|---|---|---|---|---|
| Aphidura acanthophylli | |||||
| Acanthophyllum sp. | Iran | Sharh-e Babak [NW 50 km] (Kerman) | 4-IX-1972 | R. | i3749 |
| Aphidura amphorosiphon sp. n. | |||||
| Dianthus sp. | Iran | without locality | sans date | D. | i1440 |
| Silene sp. | Iran | Kuh-e Dinar (Kohgiluyed and Boyer-Ahmad) | 14-IX-1955 | R. | i1118a |
| Caryophyllaceae | Iran | Chalus [N 40 km Amol road] (Mazenderan) | 3-V-1963 | R. | i2417 |
| Aphidura bozhkoae | |||||
| Prunus spinosa | Iran | Karadj (Alborz) | 8-V-1955 | R. | i196 |
| Prunus ?prostrata | Iran | Bojnurd [E 10 km] (North Khorasan) | 21-V-1966 | R. | i2961 |
| Prunus ?prostrata | Iran | Kuh-e Choret [90 km. Bojnurd] (North Khorasan) | 25-V-1966 | R. | i3028 |
| Prunus sp. | Iran | ? | ? | ? | i4347 |
| Iran | Shiraz (Fars) | ?-V-1974 | C. | i4092 | |
| Aphidura delmasi | |||||
| Silene italica | France | Gémenos (Bouches-du-Rhône) | 13-VI-1967 | R. | 6455 |
| Silene italica | France | Lantosque (Alpes-Maritimes) | 24-X-1968 | R. | 7591 |
| Silene italica | France | Saint-Guilhem-le-Désert (Hérault) | 17-IV-1966 | R. | 5751 |
| Silene italica | France | Saint-Guilhem-le-Désert (Hérault) | 21-VII-1966 | L. | 5752 |
| Silene italica | France | Saint-Guilhem-le-Désert (Hérault) | 30-IX-1966 | L. | 5753 |
| Silene italica | France | Pont du Gard (Gard) | 19-III-1969 | R.&L. | 7728 |
| Silene italica | France | Utelle (Alpes-Maritimes) | 11-V-1969 | R.&L. | 7876 |
| Silene italia | France | Utelle (Alpes-Maritimes) | 13-VI-1988 | R. | 15798 |
| Silene ?viscosa | France | Finistret (Pyrénées Orientales) | 9-VI-1983 | R. | 14459 |
| Silene sp. | Greece | Lagadie [East] (Akadia) | 3-VII-1964 | R. | 03087 |
| Silene sp. | France | Lantosque (Alpes-Maritimes) | 28-II-1970 | R. | 9258 |
| Silene sp. | France | La-Garde-Freinet (Var) | 26-III-1970 | R. | 9357 |
| Silene sp. | France | Saint-Jean la-Rivière (Alpes-Maritimes) | 16-IX-1969 | R. | 8690 |
| vagrant | France | Utelle (Alpes-Maritimes) | 7-XI-1989 | R. | 16079 b |
| Aphidura gallica sp. n. | |||||
| Silene gallica | France | Banyuls-sur-Mer (Pyrénées-Orientales) | 11-VII-1957 | R. | 4241 |
| Silene paradoxa | France | Defilé de l’Inzecca (Haute-Corse) | 4-VI-1979 | L. | 17925 |
| Aphidura graeca sp. n. | |||||
| Gypsophila sp. | Greece | Veria [to Kastania] (Imanthia) | 18-VI-1964 | R. | 03026 |
| Aphidura gypsophilae | |||||
| Gypsophila paniculata | Slovakia | Chotín (Nitra) | 25-VI-1984 | H. | 015379 |
| Aphidura iranensis sp. n. | |||||
| Prunus sp. | Iran | Khoy [N 30 km] (West Azerbaijan) | 7-VIII-1955 | R. | i982 |
| Aphidura mordvilkoi | |||||
| Princepia sinensis | Russia | ? (Prymorsky Krai) | 20-VI-1967 | Sh. | 016559 |
| Princepia sinensis | Russia | ? (Prymorsky Krai) | 5-VI-1980 | Pa. | 014789 |
| Aphidura ornata | |||||
| Silene inaperta | France | Ste Catherine de Vars (Hautes-Alpes) | 1-VII-1990 | R.&M.V. | 16454 |
| Silene italica | France | Avène (Hérault) | 1-V-1967 | L. | 18054 |
| Silene nutans | Switzerland | Cassarate (Ticino) | 25-V-1950 | H.R.L. | 02946 |
| Silene nutans | Switzerland | Cassarate (Ticino) | 25-V-1950 | H.R.L. | 016758 |
| Silene saxifraga | France | La-Roche-de-Rame [S] (Hautes-Alpes) | 22-VI-1969 | R.&L. | 8010 |
| Aphidura ornatella | |||||
| Silene sp. | Pakistan | Matiltan (Khyber Pakhtunkhwa) | 14-VIII-1991 | N-E. | 014109 |
| trap | Pakistan | Kalam Khyber Pakhtunkhwa | ?-?-1987 | N-E. | |
| trap | Pakistan | Matiltan (Khyber Pakhtunkhwa) | 23-VII-1987 | N-E. | |
| trap | Pakistan | Matiltan (Khyber Pakhtunkhwa) | 30-VII-1987 | N-E. | |
| Aphidura pakistanensis sp. n. | |||||
| Dianthus sp.- | Pakistan | Kalam (Khyber Pakhtunkhwa) | 17-VIII-1981 | N-E. | 014072 |
| Aphidura pannonica | |||||
| Gypsophila paniculata | Hungary | Ágasegyháza (Bács-Kiskun) | 10-VI-1968 | Sz. | 014156 |
| Silene otites | Hungary | Budapest [Sas-hegy] (Pest) | 21-VI-1964 | Sz. | 014156 |
| Silene otites | Slovakia | Chotín (Nitra) | 25-VI-1984 | H. | 015380 |
| Silene otites | Slovakia | Somotor (Košice) | 27-VI-1962 | Pi. | 010615 |
| Aphidura picta | |||||
| Dianthus barbatus | Pakistan | Quetta (Baluchistan) | 14-V-1991 | N-E. | 013878 |
| Dianthus crinutus | Pakistan | Skardu (Gilgit–Baltistan) | 2-VII-1991 | N-E. | 013965 |
| Dianthus ?barbatus | Iran | Isfahan (Isfahan) | 25-IV-1978 | R. | i4222 |
| Dianthus sp. | Afghanistan | Kabul (Kabul) | 26-VI-1972 | 04565 | |
| Dianthus sp. | Iran | Karadj (Alborz) | ?-XI-1948 | D. | i81a |
| Dianthus sp. [cult.] | Turkey | Ankara (Ankara) | 8-X-1950 | T. | 011930 |
| Silene conoida | Iran | Laleeh zar (Kerman) | 26-VI-1955 | R. | i648 |
| Silene fruticosa | Italy | Castelmola (Messina) | 9-VI-1979 | B. | 012841 |
| Silene glauca | Spain | Callosa de Ensiarrá (Alicante) | 29-V-985 | G.F. | 012841 |
| Silene italica | France | Col Turini (Alpes-Maritimes) | 15-X-1969 | R. | 8672 |
| Silene italica | France | Lantosque (Alpes-Maritimes) | 24-X-1968 | R. | 7592 |
| Silene sp. | Iran | Karadj (Alborz) | 19-V-1955 | R. | i282c |
| Aphidura pujoli | |||||
| Dianthus caryophyllus | France | Defilé de l’Inzecca (Haute-Corse) | 4-VI-1970 | L. | 18055 |
| Dianthus caryophyllus | France | Defilé de l’Inzecca (Haute-Corse) | 4-VI-1970 | L. | 18056 |
| Dianthus caryophyllus | Italy | Ercolano [previously Resina] (Napoli) | 17-VIII-1936 | Ro. | 02947 |
| Dianthus sp. | Spain | Arenas de Cabrales (Asturias) | 7-VI-1981 | R.&N.N. | 012841 |
| Dianthus sp. | France | ? | ? | ? | 5670 |
| trap | France | Montpellier (Hérault) | 16-VII-1996 | ? | 17749 |
| trap | France | Valence (Charente) | 1-VII-1996 | ? | 17755 |
| Aphidura urmiensis sp. n. | |||||
| Spergularia marina | Iran | Charimboulaki, Lac Urmia (West Azerbaijan) | 9-VIII-955 | R. | i1004 |
| Spergularia marina | Iran | Shahi island, Lac Urmia (East Azerbaijan) | 5-VIII-1955 | R. | i962 |
NOTES:
In the “Locality” column, supplementary information and upper administrative unit (such as county, department, province, regional unit, etc.) are respectively given in square brackets and in parentheses.
In the “Coll” column the names of collectors have been abbreviated as follows: B., Barbagallo (S.); C., Chodjaï (M.); D., Davatchi (A.); G.F., González Funes (M.P.); H.R.L, Hille Ris Lambers (D.); H., Holman (J.); L., Leclant (F.); M.V. Muñoz Viveros (A.L.); N.N., Nieto Nafría (J.M.); N-E, Naumann-Etienne (K.); Pa., Pashchenko (N.S.); P., Pintera (A.); R., Remaudière (G.); Ro., Roberti (D.) Sh., Shaposhnikov (G.C.); Sz., Szelegiewicz (H.); and T., Tuatay (N.).
The numbers in the “Sample” column are the numbers of the Remaudière samples.
Morphological measurements were made according to
In the modifications to the identification keys by
Apterous viviparous aphids can be identified as Aphidura by the presence of a pair of mesosternal mammariform processes, as mentioned above, and also by the following characters: (1) frons w-shaped with rugose or scabrous lateral tubercles not much higher than the broad median tubercle; (2) cephalic dorsum not ornamented or with spinules, which may be more-or-less scattered or in groups; (3) clypeus and mandibular and maxillar lamina more-or-less pigmented like cephalic dorsum and rostrum; (4) antennae not longer than body length; (5) secondary sensoria absent; (6) antennal segment I and II scabrous or rugose, segment III with scattered scales, and IV–VI more-or-less imbricated; (7) rostrum extending backward beyond middle coxae or reaching hind coxae; (8) ultimate rostral segment triangular with straight margins, usually darker than the previous segments; (9) legs with coxae and trochanters pale, femora entirely pale or with a darker distal part; tibiae pale in general with a distal portion smoky, exceptionally entirely pale, and tarsi brown; (10) first segment of tarsi with 2–4 setae; (11) abdominal spiracular apertures reniform, placed in the middle of small spiracular sclerites; (12) intersegmental sclerites well defined and usually pigmented, and embodied in the segmental sclerites if these are present; (13) thorax and abdomen often with a dorsal pattern of sclerotisation that is very variable between species, and can also vary within them (see below terminological usage); (14) siphunculi usually with a distinct preapical incision and flange, but variable in shape (see below for details and terminological usage); (15) cauda triangular to tongue-shaped; (16) spinules present, more-or-less conspicuously and densely, on mesosternal mammariform processes, postsiphuncular sclerites, spiracular sclerites 7, and abdominal terga 7 and 8; (17) antennal and dorsal setae short or very short, with blunt, frayed or (rarely) incrassated apex; (18) dorsal setae not placed on tubercles, except sometimes in Aphidura acanthophylli; (19) ventral setae longer than respective dorsal and pointed; and (20) setae on dorsal faces of femora and on proximal parts of tibiae with blunt or frayed apex, other setae on legs pointed.
The alate viviparous females have no mesosternal mammariform processes, and differ from apterae by having: (a) longer and more pigmented antennae, (b) round, double-rimmed secondary sensoria scattered along the ventral face of antennal segment III, and rarely on segment IV, (c) pigmentation of legs more extensive and darker; (d) dorsal abdomen often with more sclerotisation than in apterae, but again this varies greatly both between and within species; (e) spinules also present in the marginal sclerites.
Regarding the thoracic and abdominal dorsal sclerotisation, the term “spinopleural patch” is utilized here for a continuous sclerotisation of spinal and marginal areas of two or more segments (Figs 2B, D, 4A, 6A), and the term “discal plate” is utilized for the continuous and extensive sclerotisation of spinal, pleural and marginal areas of three or more segments (Figs 1A, C, 2A, C).
The siphunculi of Aphidura species are variable in shape: (a) cylindrical, subcylindrical (delicately tapering to the apex) or conspicuously tapering from base to apex, straight or curved outward (Figs 1D, 2A, 4B, 6A); (b) slightly swollen —«cylindrical with very tapering apex, below their middle very little attenuated, so that they might be considered as very slightly clavate» (
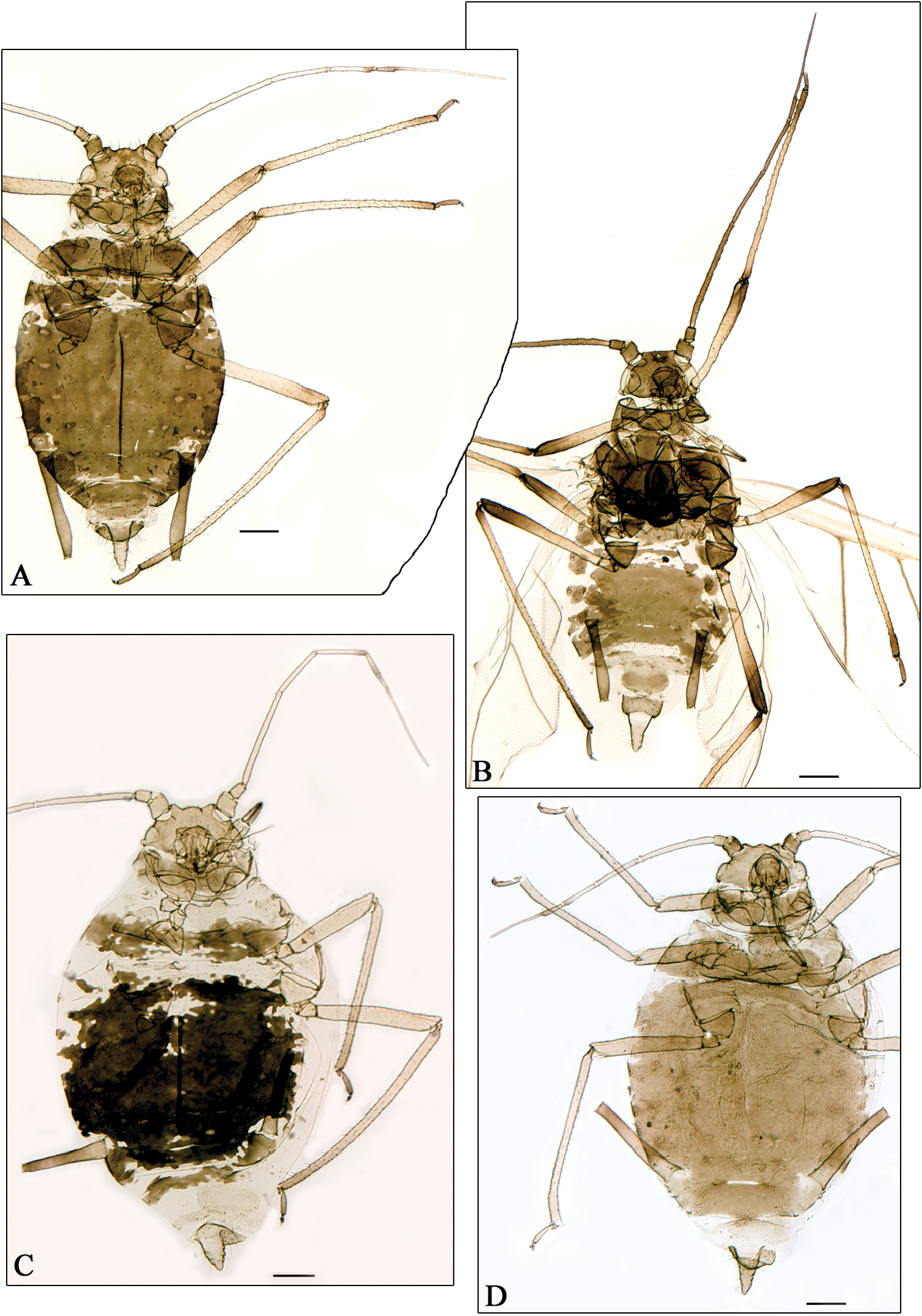
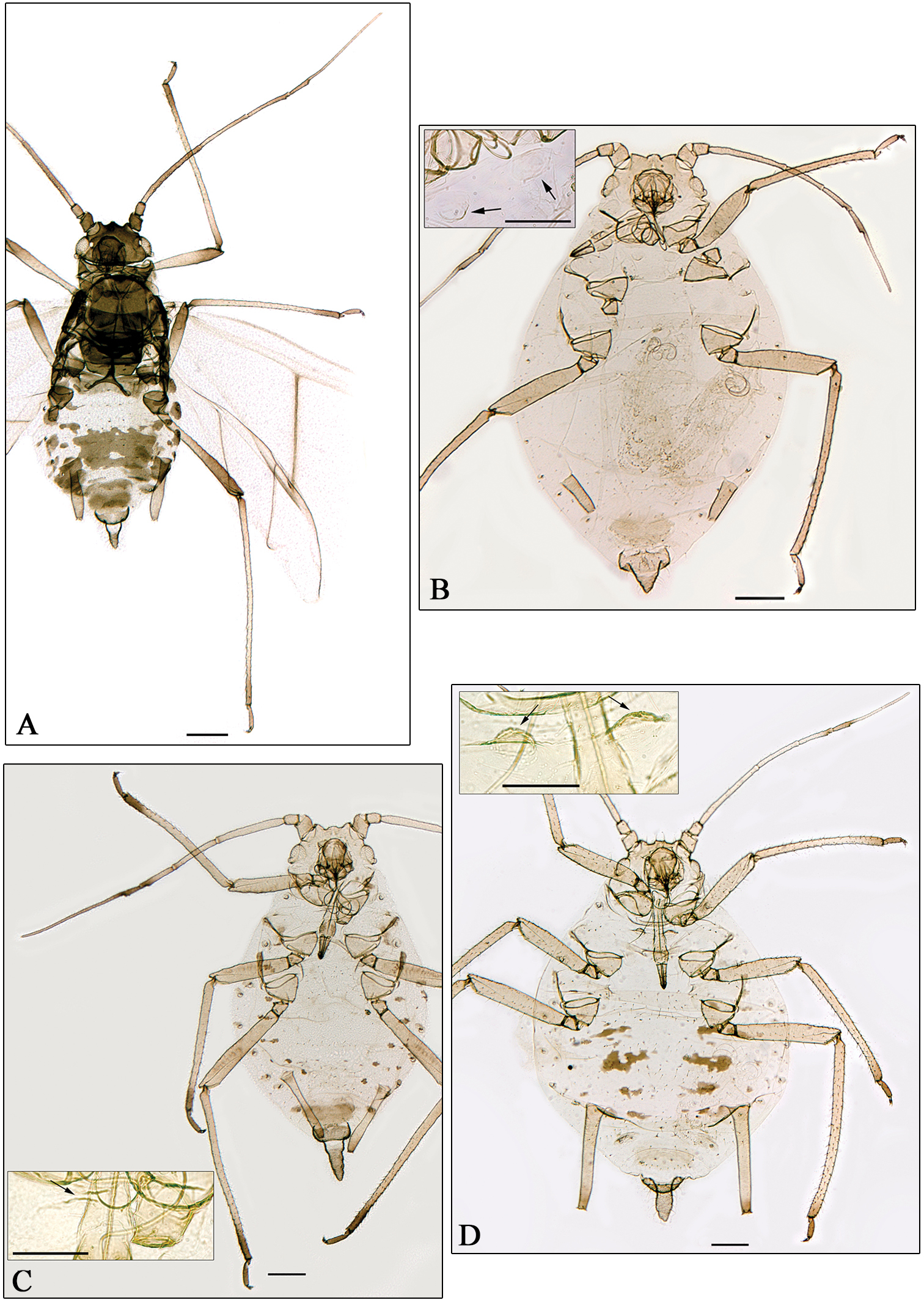
A–B Aphidura ornatella C Aphidura picta D Aphidura mordvilkoi A, C–D apterous viviparous female B alate viviparous female. Scale bars 0.2 mm.
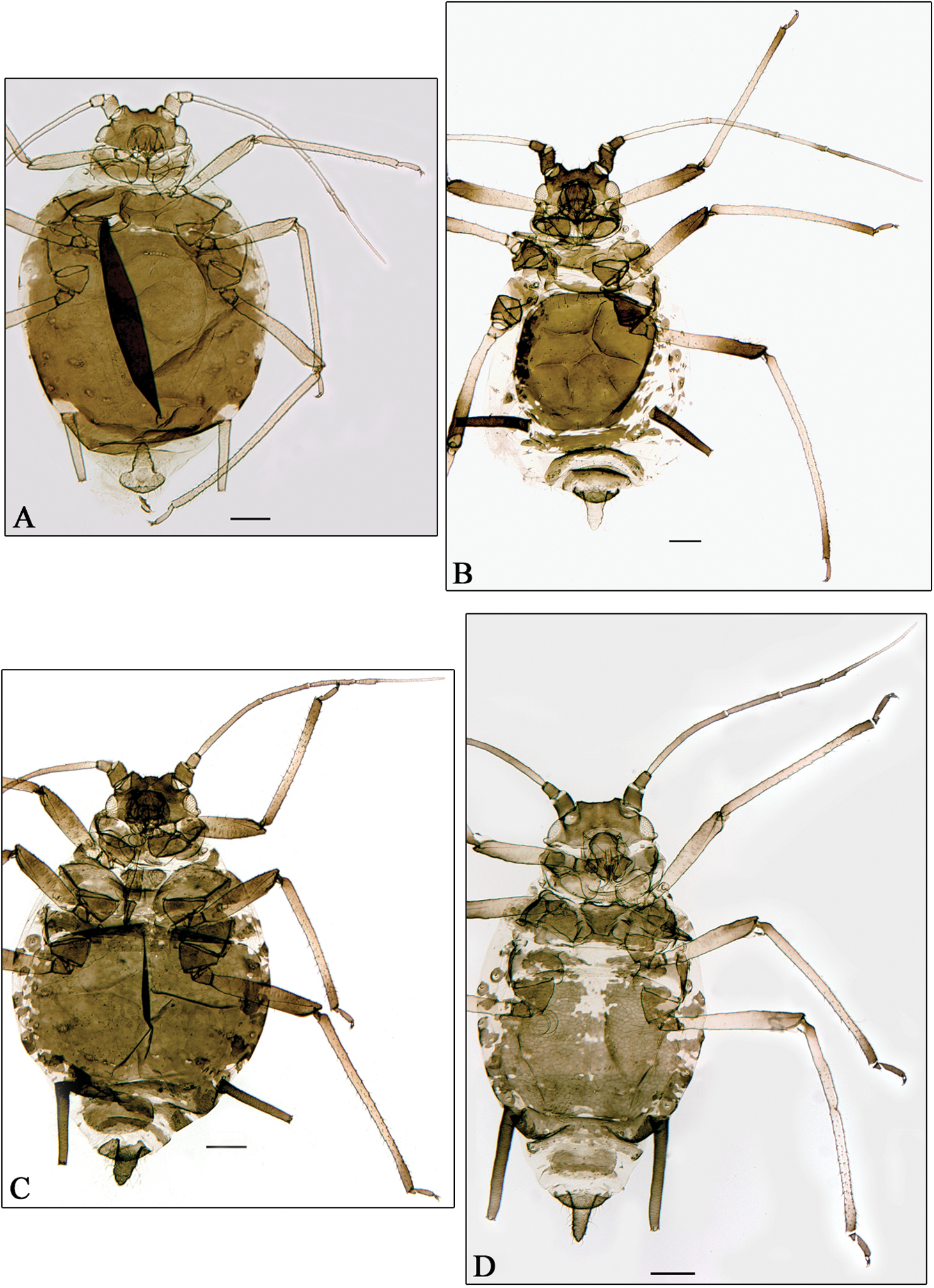
A Aphidura bozkhoae. B Aphidura delmasi. C Aphidura ornata. D Aphidura pannonica. A–D apterous viviparous female. Scale bars 0.2 mm.
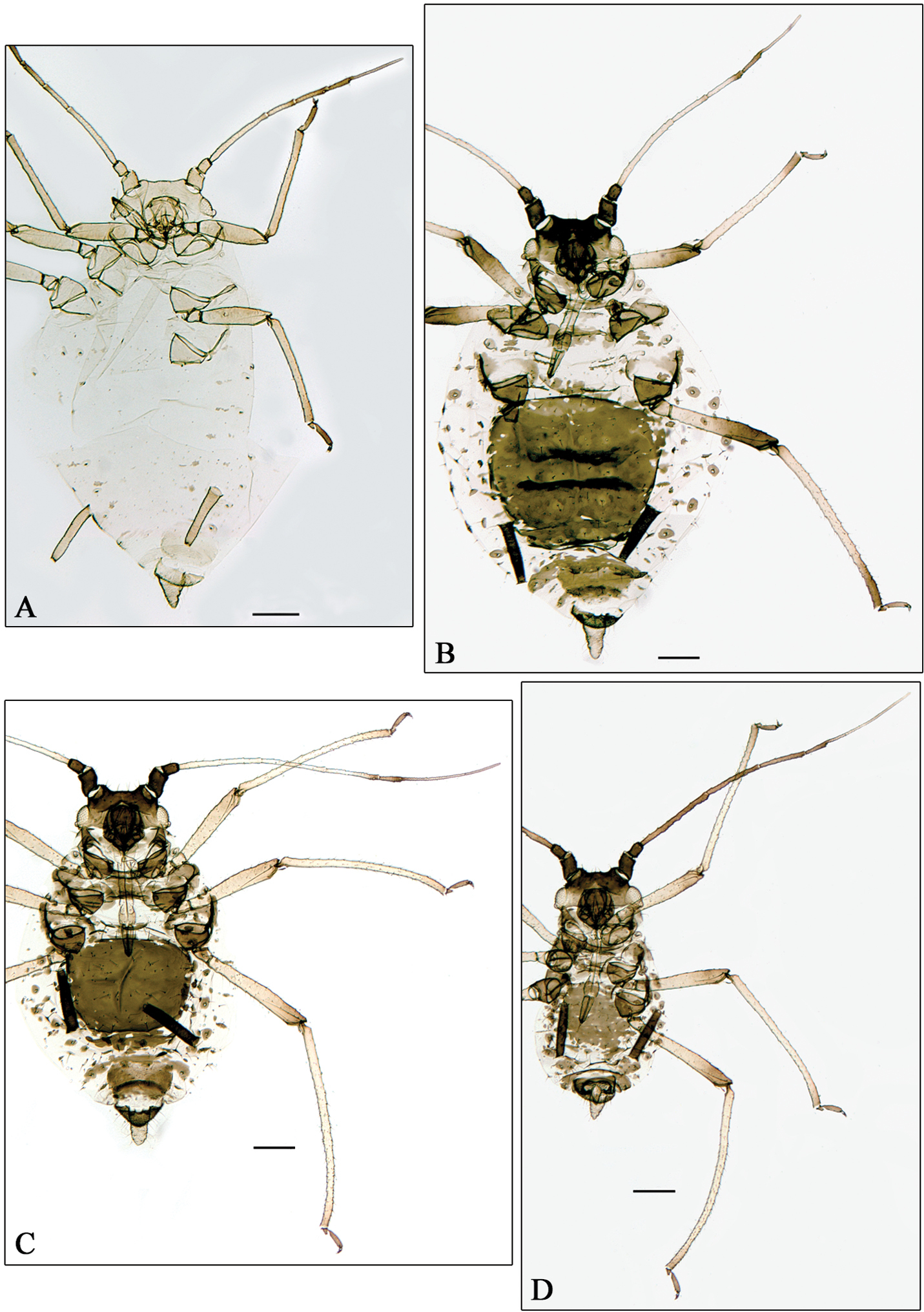
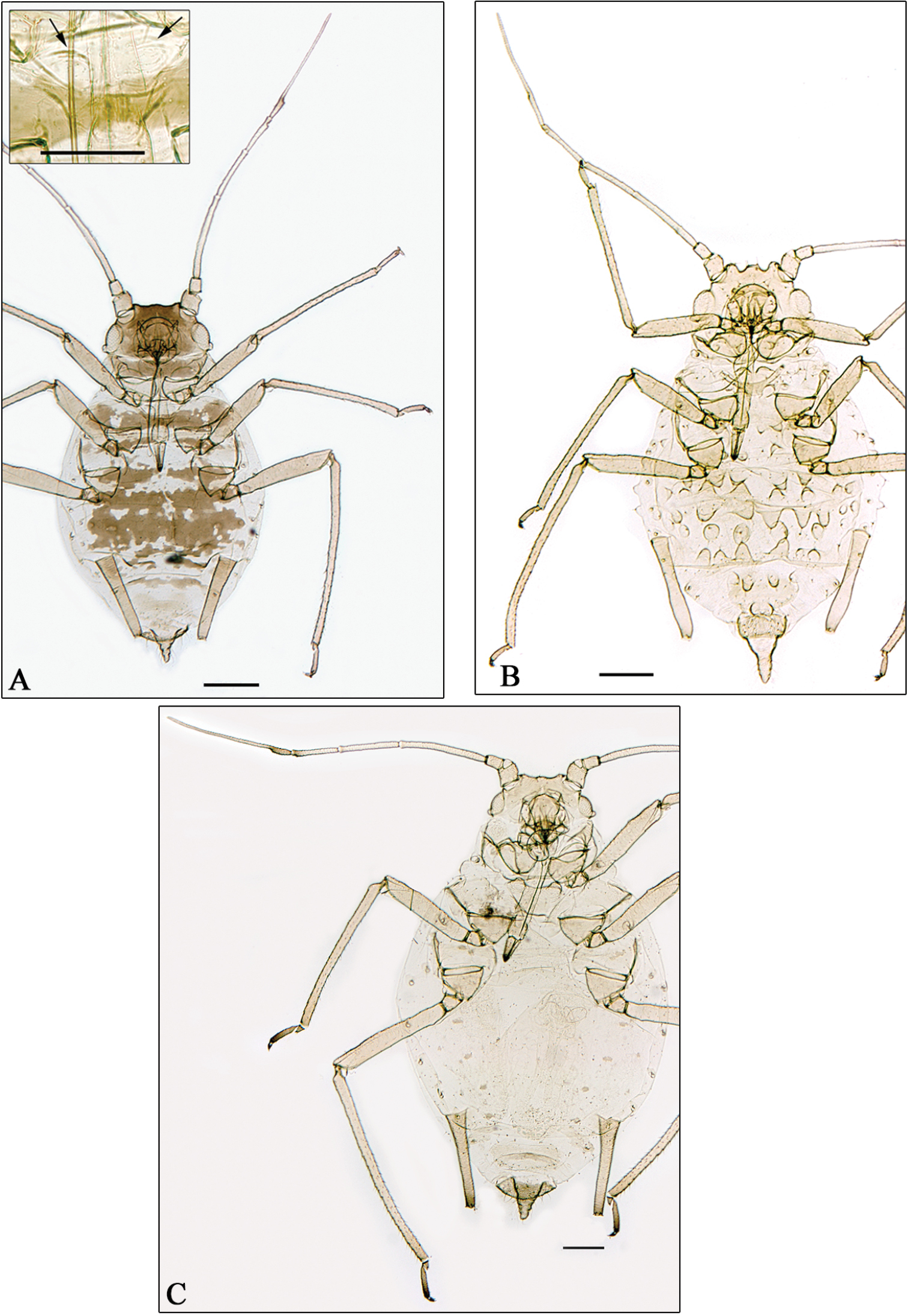
A Aphidura pujoli. B–D Aphidura delmasi B fundatrix C oviparous female D male. Scale bars 0.2 mm.
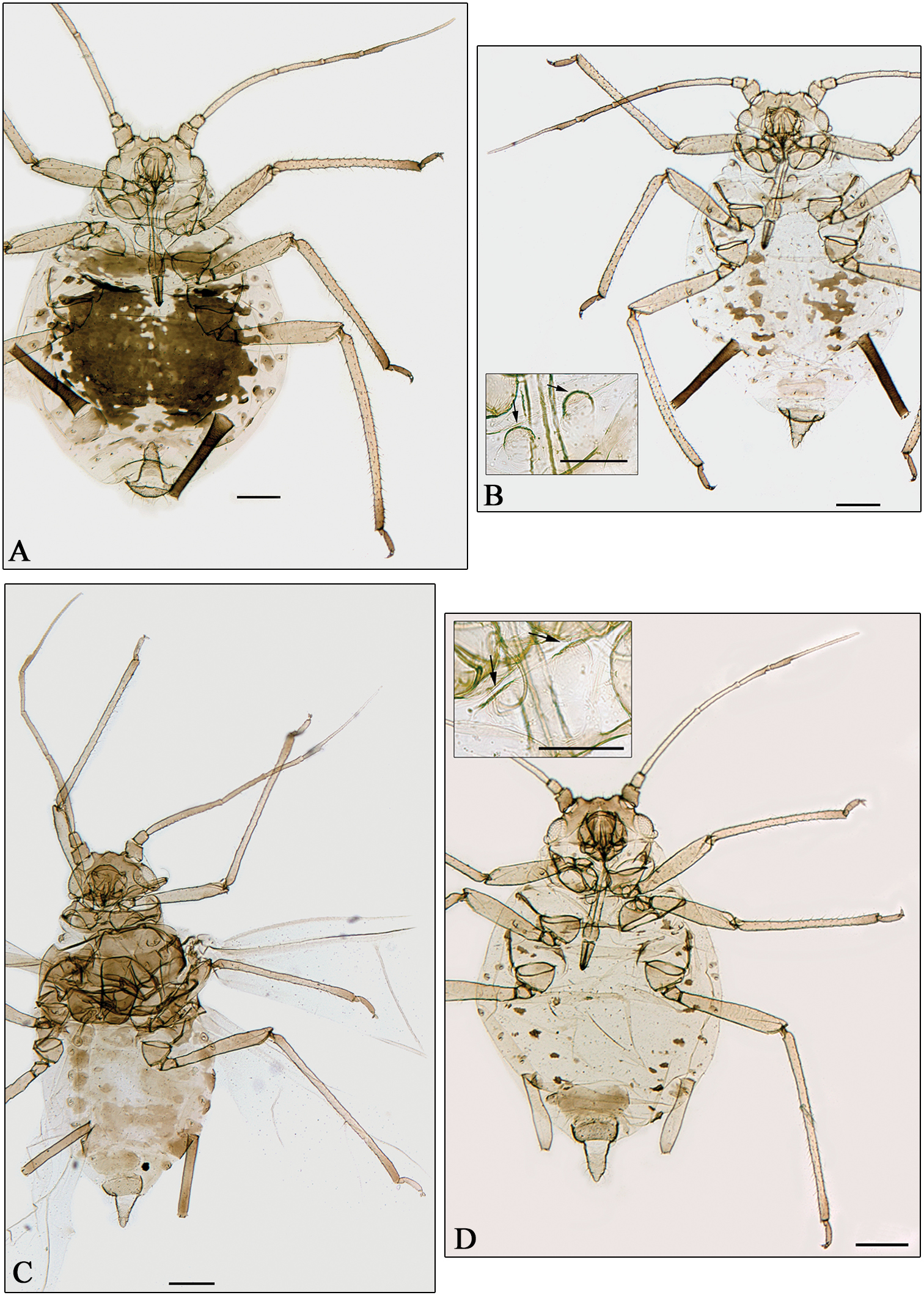
A–C Aphidura gallica sp. n. D Aphidura amphorosiphon sp. n. A–B, D apterous viviparous female C alate viviparous femaleA pigmented formB unpigmented form B, D boxes mesosternum with mammariform processes. General scale bars 0.2 mm, boxes scale bar 0.01 mm.
A Aphidura amphorosiphon sp. n. B Aphidura pakistanensis sp. n. C Aphidura graeca sp. n. D Aphidura urmiensis sp. n. A alatae viviparous female B–D apterous viviparous female B–D boxesmesosternum with mammariform processes. General scale bars 0.2 mm, boxes scale bar 0.01 mm.
A Aphidura iranensis sp. n. B Aphidura acanthophylli C Aphidura gypsophilae A–C apterous viviparous female A box mesosternum with mammariform processes. General scale bars 0.2 mm, box scale bar 0.01 mm.
Possible synonymies of three pairs of Aphidura species are discussed: Aphidura bharatia and Aphidura ornatella, Aphidura mingens and Aphidura picta, and finally Aphidura prinsepiae and Aphidura mordvilkoi.
Characters of studied apterous and alate vivipara (Figs 1A, B; Table 2 for six alatae from Pakistan; only one alate of this species was previously known [
Metric and meristic features of Aphidura ornatella, and Aphidura delmasi; n, number of measured specimens.
| Aphidura ornatella | Aphidura delmasi | Aphidura delmasi | Aphidura delmasi | |
|---|---|---|---|---|
| Al. viv. femal. | Fundatrices | Ovip. femal. | Males | |
| n = 6 | n = 4 | n = 4 | n = 4 | |
| Body [mm] | 1.637–2.100 | 1.700–2.200 | 1.625–1.775 | 1.175–1.425 |
| Antenna [mm] | 1.565–2.060 | 1.115–1.500 | 1.660–1.825 | 1.555–1.725 |
| Antenna / Body [times] | 0.96–1.11 | 0.65–0.68 | 1.01–1.12 | 1.20–1.44 |
| Ant. segm. III [mm] | 0.40–0.58 | 0.28 | 0.44 | 0.40–0.41 |
| Ant. segm. IV [mm] | 0.24–0.36 | 0.16–0.24 | 0.29–0.37 | 0.27–0.33 |
| Ant. segm. V [mm] | 0.21–0.29 | 0.15–0.23 | 0.25–0.26 | 0.21–0.25 |
| Ant. segm. VI base [mm] | 0.11–0.13 | 0.11–0.13 | 0.12–0.13 | 0.11–0.12 |
| Ant. segm. VI processus terminalis [mm] | 0.50–0.67 | 0.24–0.29 | 0.44–0.48 | 0.40–0.50 |
| Ant. segm. VI processus terminalis/ Ant. segm. III [times] | 1.09–1.24 | 0.62–0.93 | 1.1–1.20 | 1.00–1.22 |
| Ant. segm. VI processus terminalis/ base [times] | 4.62–5.36 | 1.96–2.48 | 3.62–4.04 | 3.48–4.30 |
| Secondary sensoria, Ant. segm. III [number] | 21–28 | 0 | 0 | 0 |
| Ultimate rostral segm. [mm] | 0.12–0.15 | 0.13–0.15 | 0.15–0.17 | 0.14 |
| Ultimate rostral segm. / its basal width [times] | 2.09–3.00 | 2.00–2.25 | 2.50–2.82 | 2.00–2.80 |
| Ultimate rostral segm. / Ant. segm. VI base [times] | 1.08–1.16 | 1.12–1.29 | 1.15–1.35 | 1.17–1.33 |
| Hind tarsus, 2nd segm. [mm] | 0.11–0.14 | 0.09–0.10 | 0.01–0.11 | 0.08–0.09 |
| Hind tarsus, 2nd segm. / Ultimate rostral segm. [times] | 0.93–1.04 | 0.67–0.70 | 0.65–0.71 | 0.57–0.67 |
| Abdominal Marginal papillae [number] | 0 | 0 | 0 | 0 |
| Siphunculus [mm] | 0.38–0.42 | 0.23–0.31 | 0.29–0.32 | 24–0.26 |
| Siphunculus / Body [times] | 0.19–0.23 | 0.13–0.15 | 0.17–0.20 | 0.17–0.21 |
| Siphunculus / Ant. segm. III [times] | 0.68–0.95 | 0.71–0.83 | 0.72–0.80 | 0.59–0.66 |
| Siphunculus / its basal width [times] | 5.07–6.25 | 3.22–4.43 | 4.41–4.75 | 4.00–4.64 |
| Siphuncular widths, maximal / basal [times] | 0.80–1.25 | 0.72–0.86 | 0.83–0.92 | 0.80–0.91 |
| Siphuncular widths, maximal / minimal [times] | 1.47–2.33 | 1 | 1 | 1 |
| Cauda [mm] | 0.13–0.19 | 0.15–0.22 | 0.18–0.19 | 0.10–0.15 |
| Cauda / Siphunculus [times] | 0.34–0.51 | 0.67–0.74 | 0.58–0.63 | 0.37–0.60 |
| Cauda / its basal width [times] | 1.05–1.41 | 1.20–1.43 | 1.29–1.42 | 0.68–1.33 |
| Setae on … | ||||
| … Frons [μm] | 21–28 | 35–50 | 45–55 | 35–45 |
| … Frons / b. d. Ant. segm. III [times] | 1.1–1.8 | 1.6–2.5 | 2.0–2.8 | 1.8–2.3 |
| … Ant. segm. III [μm] | 12–20 | 17–23 | 22–25 | 17–23 |
| … Ant. segm III / b. d. Ant. segm. III [times] | 0.7–1.1 | 0.9–1.0 | 1.0–1.3 | 0.9–1.3 |
| … Ultimate rostral segm. [number] | 14–17 | 5–8 | 8–12 | 9–12 |
| … Abdominal segm. 8 [μm] | 25–35 | 35–45 | 45–50 | 38–40 |
| … Abdominal segm. 8 / b. d. Ant. segm. III [times] | 1.3–2.0 | 1.6–2.3 | 2.0–2.4 | 2.0–2.1 |
| … Abdominal segm. 8 [number] | 4–5 | 4–7 | 4–5 | 3–6 |
| … Genital plate, discal [number] | 2–4 | 2–8 | 7–10 | – |
| … Genital plate, marginal [number] | 10–16 | 12–13 | 17–21 | – |
| … Cauda [number] | 6–8 | 6–8 | 8–10 | 69 |
NOTE. Used abbreviations: Al., Alate; Ant., Antennal; b. d., basal diameter; femal., females; Ovip., Oviparous; segm., segment; viv., viviparous.
The species pair Aphidura mingens and Aphidura picta provides a similar situation: they are considered synonymous names by
In our opinion the synonymy can stand, because V. F. Eastop studied a wide number of specimens from diverse provenances (host plants, localities and dates), including types (R. L. Blackman, pers.com.), and also because of our observations, or at least it should be maintained in the sense that there is only one variable species involved. Nevertheless the valid name for this species could be Aphidura mingens if the holotype of Aphidura picta could be shown to be a fundatrix of Aphidura ornata, in which case Aphidura picta would be a synonym of that species.
Collection data for the following first records are shown in Table 1.
Aphidura bozhkoae (Fig. 2A) is recorded for the first time on Prunus spinosa and on Prunus prostrata; it was previously recorded from several other species habitually placed in Prunus, although some of them can be classified in Cerasus or in Aflatunia.
Aphidura delmasi (Fig. 2B) is recorded for the first time on Silene viscosa; it has previously been recorded on other species of Silene.
Aphidura ornata (Fig. 2C) is recorded for the first time on Silene inaperta, Silene nutans and Silene saxifraga; it has been recorded previously on four other species of Silene.
Aphidura pannonica (Fig. 2D) is recorded for the first time on Gypsophila paniculata; this aphid has been previously recorded from several species of Silene.
Aphidura picta (Fig. 1C) is recorded for the first time (i) on Silene glauca and (ii) from Afghanistan. This aphid has been recorded on several species of Silene, and also of Dianthus; and it was known from Iran, Tajikistan and Pakistan, and other Asiatic and European countries.
The identification made by G. Remaudière, of four apterous viviparous females belonging to his sample 014072 from Pakistan, as Aphidura pujoli is not correct; in fact these specimens belong to a new species, Aphidura pakistanensis. In consequence the record of Aphidura pujoli from Pakistan by Naumann-Etienne and Remaudière (1976) is incorrect, and Aphidura pujoli (Fig. 3A) remains restricted to Europe, having been recorded from Portugal, Spain, France (including Corsica), Switzerland, Italy (including Sicily), and Ukraine.
Collecting data in Table 1: fundatrix, sample 7876; oviparous females and males, sample 7591.
Fundatrix. From 4 specimens (Fig. 3B). Very similar to the fundatrigenous aptera described by
Oviparous female. From 4 specimens (Fig. 3C). Very similar to the fundatrigenous aptera, with paler antennae, yellowish legs (only tarsi are smoky). Hind tibiae not swollen, with 20–30 scent plates. Metric and meristic features in Table 2.
Male. From 4 specimens (Fig. 3D). Apterous. Also very similar to the fundatrigenous aptera, but smaller, with paler legs (only tarsi are smoky) and longer antennae. Aedeagus and parameres brown. Metric and meristic features in Table 2.
Six new species are established: Aphidura gallica and Aphidura amphorosiphon, which live on species of Silene, Aphidura pakistanensis, Aphidura graeca and Aphidura urmiensis, which live on other caryophyllaceous plants (respectively species of Dianthus, Gypsophila and Spergula), and Aphidura iranensis, which lives on Prunus.
urn:lsid:zoobank.org:act:7C59AAC8-D440-4EAC-B376-047DA69F053A
(Figs 4A, B). Colour in life unknown. Head yellowish brown with rugosity near the eyes. Antennal segments I-IV as pale as cephalic dorsum, and V and VI darker. Dorsal pigmentation and sclerotisation very variable. In several specimens, holotype included prothorax with complete but pale band, metathorax with brown spinopleural transverse band and setiferous marginal sclerites, abdominal segments 1-6 with an extensive dark spinopleural patch, partially fragmented in midline and with an irregular lateral margin partly incorporating the marginal sclerites; abdominal segments 7 and 8 with bands paler than patch. In other specimens, dorsum mainly membranous, with pale brown to brown pleural sclerites on abdominal segments 1-6, which are irregular in shape and sometimes joined between segments, and several very small and pale marginal setiferous sclerites. Other specimens have an intermediate degree of sclerotisation and pigmentation. Mesosternal mammariform processes yellowish, thin and tall. Siphunculi cylindrical, dark, and densely covered with denticulate scales. Cauda triangular (sometimes with a slight constriction) with pointed apex, and not darker than legs. Anal and genital plates as pale as cauda. Metric and meristic features in Table 3.
Metric and meristic features of Aphidura gallica sp. n. and Aphidura amphorosiphon sp. n.; n, number of measured specimens.
| Aphidura gallica | Aphidura gallica | Aphidura amphoro-siphon | Aphidura amphoro-siphon | Aphidura amphoro-siphon | |
|---|---|---|---|---|---|
| Apt. viv. femal. | Al. viv. femal. | Ap. viv. femal. | Al. viv. femal. | Male | |
| n = 30 | n = 2 | n = 12 | n = 3 | n = 1 | |
| Body [mm] | 1.475–2.325 | 1.600–1.725 | 1.275–1.638 | 1.100–1.525 | 1.275 |
| Antenna [mm] | 1.070–1.735 | 1.343–1.343 | 0.978–1.170 | 1.265–1.615 | 1.340–1.380 |
| Antenna / Body [times] | 0.66–0.93 | 0.84–0.84 | 0.71–0.89 | 1.05–1.15 | 1.05–1.08 |
| Ant. segm. III [mm] | 0.32–0.49 | 0.23–0.36 | 0.26–0.35 | 0.35–0.40 | 0.38–0.38 |
| Ant. segm. IV [mm] | 0.16–0.36 | 0.12–0.22 | 0.14–0.20 | 0.20–0.28 | 0.22–0.23 |
| Ant. segm. V [mm] | 0.14–0.26 | 0.11–0.18 | 0.13–0.18 | 0.16–0.23 | 0.17–0.19 |
| Ant. segm. VI base [mm] | 0.08–0.13 | 0.10–0.10 | 0.09–0.11 | 0.10–0.13 | 0.10–0.11 |
| Ant. segm. VI processus terminali mm] | 0.25–0.44 | 0.37–0.37 | 0.25–0.31 | 0.32–0.48 | 0.37–0.38 |
| Ant. segm. VI processus terminalis/ Ant. segm. III [times] | 0.66–0.99 | 1.04–1.04 | 0.77–1.07 | 0.85–1.22 | 1.00 |
| Ant. segm. VI processus terminalis/ base [times] | 2.88–4.08 | 3.89–3.89 | 2.57–3.08 | 3.37–4.04 | 3.57–3.70 |
| Secondary sensoria, Ant. segm. III [number] | 0 | 16–34 | 0 | 16–21 | 63–72 |
| Secondary sensoria, Ant. segm. IV [number] | 0 | 0 | 0 | 0 | 29–32 |
| Secondary sensoria, Ant. segm. V [number] | 0 | 0 | 0 | 0 | 11–15 |
| Ultimate rostral segm. [mm] | 0.11–0.15 | 0.10–0.13 | 0.12–0.14 | 0.10–0.13 | 0.10 |
| Ultimate rostral segm. / its basal width [times] | 2.00–3.00 | 2.00–2.60 | 2.18–2.80 | 2.88–2.88 | 2.67 |
| Ultimate rostral segm. / Ant. segm. VI base [times] | 1.10–1.87 | 1.37–1.37 | 1.12–1.42 | 0.96–1.05 | 0.95–1.00 |
| Hind femur [mm] | 0.39–0.60 | 0.39–0.50 | 0.35–0.46 | 0.38–0.45 | 0.42–0.43 |
| Hind tibia [mm] | 0.72–1.13 | 0.81–1.02 | 0.61–0.83 | 0.80–1.00 | 0.82–0.85 |
| Hind tibia / Body [times] | 0.43–0.59 | 0.51–0.59 | 0.45-0.55 | 0.58–0.73 | 0.64–0.67 |
| Hind tarsus, 2nd segm. [mm] | 0.10–0.12 | 0.11–0.11 | 0.08–0.10 | 0.10–0.11 | 0.09–0.10 |
| Hind tarsus, 2nd segm. / Ultimate rostral segm. [times] | 0.70–0.95 | 0.81–1.05 | 0.67–0.83 | 0.83–0.95 | 0.90–0.95 |
| Abdominal marginal papillae [number] | 0 | 0 | 0 | 0 | 0 |
| Siphunculus [mm] | 0.35–0.50 | 0.35–0.42 | 0.27–0.33 | 0.25–0.30 | 0.24 |
| Siphundulus / Body [times] | 0.20–0.29 | 0.22–0.24 | 0.18–0.24 | 0.18–0.24 | 0.19 |
| Siphunculus / Ant. segm. III [times] | 0.87–1.24 | 0.99–1.84 | 0.90–1.04 | 0.65–0.77 | 0.64–0.65 |
| Siphunculus / its basal width [times] | 4.00–5.57 | 7.00–7.00 | 3.60–5.80 | 3.85–5.89 | 5.33 |
| Siphuncular widths, maximal / basal [times] | 0.50–0.86 | 0.80–0.80 | 0.83–1.20 | 0.81–1.11 | 1.33 |
| Siphuncular widths, maximal / minimal [times] | 1.00–1.00 | 1.00–1.00 | 1.25–1.79 | 1.47–2.00 | 2.18 |
| Siphuncular minimal width / Hind tibia, diameter at middle [times] | 1.05–1.57 | 1.33–1.69 | 1.00–1.57 | 1.00-0.50 | 0.61 |
| Cauda [mm] | 0.15–0.24 | 0.15–0.19 | 0.14–0.19 | 0.09–0.15 | 0.09 |
| Cauda / Siphunculus [times] | 0.36–0.50 | 0.41–0.46 | 0.45–0.62 | 0.34–0.50 | 0.38 |
| Cauda / its basal width [times] | 1.07–1.32 | 1.12–1.36 | 1.10–1.46 | 0.86–1.45 | 0.78 |
| Setae on … | |||||
| … Frons [μm] | 26–45 | 23–23 | 17–38 | 15–21 | 10 |
| … Frons / b. d. Ant. segm. III [times] | 1.2–2.3 | 1.6–1.6 | 1.0–1.9 | 0.8–1.2 | 0.5 |
| … Vertex [μm] | 23–35 | 18–18 | 10–23 | 15–20 | 13 |
| … Vertex / b. d. Ant. segm. III [times] | 1.00–1.75 | 1.3–1.3 | 0.6–1.3 | 0.8–1.1 | 0.7 |
| … Ant. segm. III [μm] | 13–25 | 15–20 | 7–13 | 7–10 | 5 |
| … Ant. segm III / T. Ant. segm. III [times] | 0.6–1.3 | 0.7–1.5 | 0.5–0.7 | 0.4–0.6 | 0.3 |
| … Ultimate rostral segm. [number] | 10–16 | 11–11 | 11–17 | 9–13 | 12 |
| … Hind femur, dorsal [μm] | 13–25 | 13–20 | 7–15 | 10–15 | 10 |
| … Hind femur, ventral [μm] | 23–45 | 25–28 | 20–30 | 17–23 | 15 |
| … Hind tibia, dorsal, at middle [μm] | 25–38 | 25–28 | 20–30 | 20–23 | 20 |
| … Hind tibia, dorsal / Tibial diameter (at middle) [times] | 0.5–1.0 | 0.8–0.9 | 0.6–1.0 | 0.8–0.9 | 0.6 |
| … Hind tarsus, first segm. [number] | 2–3 | 2–3 | 2–3 | 2–3 | 2–3 |
| … Abdominal segm. 2–4 [μm] | 13–23 | 16–20 | 7–13 | 10–13 | 7 |
| … Abdominal segm. 2–4 / T. Ant. segm. III [times] | 0.6–1.1 | 0.7–1.5 | 0.4–0.8 | 0.5–0.6 | 0.4 |
| … Abdominal segm. 8 [μm] | 23–38 | 23–28 | 22–33 | 18–25 | 23 |
| … Abdominal segm. 8 / T. Ant. segm. III [times] | 1.1–2.0 | 1.2–1.6 | 1.0–2.0 | 1.0–1.3 | 1.1 |
| … Abdominal segm. 8 [number] | 4–5 | 4–4 | 4–4 | 4–4 | 4 |
| … Ventro-abdominal [μm] | 25–50 | 28–33 | 20–35 | 25–33 | 23 |
| … Genital plate, discal [number] | 2–4 | 2–2 | 2–2 | 2–2 | — |
| … Genital plate, marginal [number] | 10–17 | 10–12 | 10–16 | 8–10 | — |
| … Cauda [number] | 6–9 | 8–8 | 6–7 | 6–6 | 6 |
NOTE. Used abbreviations: Al., Alate; Ant., Antennal; Apt., Apterous; b. d., basal diameter; femal., females; n, number of measured specimens; segm., segment; viv., viviparous.
(Fig. 4C). Head brown, as dark as thorax. Abdominal segments 1 and 2 with spinal sclerites; segments 4-5 with spinopleural patch, sometimes partially joined with the spinopleural bands on 3 and 6; segments 1-6 with marginal sclerites; segment 7 with a band paler than previously mentioned sclerites; segment 8 with pale setiferous sclerites, sometimes coalesced together. Metric and meristic features in Table 3.
Holotype: Apterous viviparous female (specimen 2), on Silene gallica, Banyuls-sur-Mer (Pyrénées Orientales), France, 11-VII-1957, Remaudière leg. (sample 4241). Paratypes: 39 apterous and 9 alate viviparous females with the same data that the holotype; plus 49 apterous viviparae on Silene paradoxa, Défilé de l’Inzecca (Haute Corse), 4-VI-1970, F. Leclant leg. (sample 4660) [Remaudière sample 17925].
The specific name of the new species, gallica, is an adjective that refers to the Galia, France in times of the Roman Empire, in feminine; it is also coincident with the specific name of the host plant of the holotype.
See the discussion of the following new species.
urn:lsid:zoobank.org:act:812E7A5A-0BE0-4424-8661-38A7D0CC268C
(Fig. 4D). Colour in life unknown. Head yellowish brown to brown. Antennal segments II-III or II-V pigmented like cephalic dorsum, and I and IV-VI or only VI darker than others. Mesosternal mammariform processes rounded, low and pale. Several specimens (holotype included) are pale in general with dark brown intersegmental sclerites, brown postsiphuncular and spiracular sclerites, pale brown setiferous sclerites on abdominal segments 6–8, sometimes coalescing together into transverse bands. The most pigmented specimen has a transverse spinopleural band on prothorax, fragmented bands on mesothorax and abdominal segments 1, 6 and 7, fragmented spinopleural patches on abdominal segments 2–5, and setiferous sclerites on metathorax and abdominal segments 1 and 8. Siphunculi markedly swollen, with stem nearly smooth, and pale or with a smoky apical portion. Cauda triangular, sometimes with a slight constriction near the base, and as pale as the greater part of siphunculi and legs. Genital and anal plates as pale as cauda. Metric and meristic features in Table 3.
(Fig. 5A). Head brown, as pigmented as pro- and pterothorax and darker than antennae, tarsi and distal portions of femora and tibiae. Abdominal segments 3–5 with a spinopleural patch, and 7–8 with transverse bands. Siphunculi as dark as pigmented parts of femora. Other qualitative features as in apterae. Metric and meristic features in Table 3.
Winged. Qualitatively very similar to alate viviparous females; with dark parameres. Metric and meristic features in Table 3.
Holotype: Apterous viviparous female (specimen 5), on Silene sp. Kuh-e Dinar (Kohgiluyed and Boyer-Ahmad), Iran, 14-IX-1955, Remaudière leg. (sample i1118a). Paratypes: 15 apterous, 2 viviparous females and 1 male with the same data that the holotype; plus 1 apterous viviparae and 2 alate viviparae on an unidentified species of Caryophyllaceae, Chalus [road to Amol] (Mazenderan), Iran, 3-V-1963, Remaudière leg. (sample i2417).
The specific name is a neutral noun in apposition, formed for the Greek words “amphora” and “siphon”, which respectively mean flask and siphon, like in the genus Amphorosiphon.
The distinctive features of Aphidura amphorosiphon sp. n. and Aphidura gallica sp. n. are summarized in the identification key to apterae of Aphidura in the general discussion, and in the following modification to the key to aphids on Silene (
| 27 | Anterior part of mesosternum without mammariform processes [rest of the proposition without modification] | Volutaphis schusteri |
| – | Anterior part of mesosternum with a pair of mammariform processes [rest of the proposition without modification] | 35 |
| 35 | SIPH markedly clavate (distal maximum width habitually at least 1.2 times basal minimum) | 36 |
| – | SIPH cylindrical, subcylindrical, tapering from base to apex (sometimes outward curved), or slightly clavate (distal maximum width at most 1.2 times basal minimum width) | 38 |
| 36 | Tergum with an extensive almost solid black shield extending over metanotum and ABD TERG 1–6, usually incorporating marginal sclerites | Aphidura ornatella |
| – | Tergum pale or with variable sclerotisation, sometimes extensive but with large windows spinally and marginally, not forming a solid black shield | 37 |
| 37 | ANT PT/BASE 2.55–3.1. RIV+V 1.2–1.5 times HT II. SIPH light with smoky apex. Cauda 1.1–1.5 times its basal width, with 6–7 hairs. Abdomen variably sclerotised and pigmented | Aphidura amphorosiphon |
| – | ANT PT/BASE 3.2–3, 9. RIV+V 1–1.2 times HT II. SIPH uniformly pigmented. Cauda 1.1–1.2 times its basal width, with 7–11 hairs. ABD TERG 1–6 with a dark central patch and marginal sclerites | Aphidura nomadica |
| 38 | SIPH pale or dusky, slightly clavate, 1.5–1.8 times cauda, which is short triangular. Tergum without sclerotisation, completely pale | Aphidura pujoli |
| – | SIPH brown to black at least in part, sometimes slightly clavate, 1.9–2.8 times cauda. Tergum with variable sclerotisation and pigmentation, rarely complete pale | 39 |
| 39 | ABD TERG 2–3 with longest hairs 35–55 μm long, 1.5–2.0 times ANT BD III. ANT I long, 1.3–1.5 times its maximal width. Dorsal abdomen with a large central oval sclerite on ABD TERG (1)2–5 | Aphidura delmasi |
| – | ABD TERG 2–3 with longest hairs 4–25 μm long, 0.2–1.1 times ANT BD III. ANT I short, 1.1 times its maximal width at most. Dorsal abdomen with variable sclerotisation and pigmentation, but rarely with a central oval sclerite on ABD TERG 2–5 | 40 |
| 40 | Tergum with an extensive almost solid black shield extending over metanotum and ABD TERG 1–6, usually incorporating marginal sclerites. Cauda dark broad triangular, longer than 2 times its basal width and usually shorter than 0.5 times SIPH, and with 10–16 hairs | Aphidura ornata |
| – | Tergum pale or with variable sclerotisation, sometimes extensive but with large windows spinally and marginally, not forming a solid black shield. Cauda variable in shape, proportions and colour | 41 |
| 41 | Cauda tongue-shaped, 1.4–1.8 times its basal width | 42 |
| – | Cauda triangular, although sometimes with a slight constriction, 1.05–1.4 times its basal width | 43 |
| 42 | ANT PT/BASE 4.0–5.7. Hairs on ANT III and ABD TERG 2–3 minute, maximally 4–7μm long, 0.15–0.3 times BD III. SIPH 2.2–2.8 times cauda | Aphidura pannonica |
| – | ANT PT/BASE 2.5–4.0. Hairs on ANT III and ABD TERG 2–3 maximally 8–22μm, 0.4–1.0 times BD III. SIPH 1.9–2.5 times cauda | Aphidura picta |
| 43 | RIV+V 0.9–1.0 times HT II, with 8–10 accessory hairs. Cauda 1.3–1.4 times its basal width. Hairs on ABD TERG 2–3 8–11 μm long, 0.3–0.5 times BD III | Aphidura massagetica |
| – | RIV+V 1.05–1.45 times HT II, with 10–16 accessory hairs. Cauda 1.05–1.35 times its basal width. Hairs on ABD TERG 2–3 13–23 μm long, 0.6-1 times BD III | Aphidura gallica |
urn:lsid:zoobank.org:act:D3B0B038-D4A8-41E8-803A-0D2CFE97A8CE
(Fig. 5B). Colour in life unknown. Antennae, rostrum, legs, siphunculi, genital plate and cauda yellowish. Frontal tubercles low. Mesosternal mammariform processes low, rough and pale, sometimes inconspicuous. Dorsum of thorax and abdomen without segmental sclerites; intersegmental and spiracular sclerites inconspicuous. The characteristic spinules on postsiphuncular area and tergum of abdominal segments 7 and 8 are dispersed and delicate. Siphunculi short, slightly swollen and densely covered with scales. Cauda short triangular, with broad basis. Metric and meristic features in Table 4.
Metric and meristic features of apterous viviparous females of Aphidura pakistanensis sp. n., Aphidura graeca sp. n., Aphidura urmiensis sp. n., and Aphidura iranensis sp. n.; n, number of measured specimens.
| Aphidura pakistanensis | Aphidura graeca | Aphidura urmiensis | Aphidura iranensis | |
|---|---|---|---|---|
| n = 4 | n = 1 | n = 20 | n = 6 | |
| Body [mm] | 1.725–1.850 | 1.838 | 1.900–2.125 | 1.100–1.300 |
| Antenna [mm] | 1.053–1.655 | 1.670 | 1.333–1.755 | 1.005–1.210 |
| Antenna / Body [times] | 0.59–0.89 | 0.91 | 0.67–0.84 | 0.80–1.08 |
| Ant. segm. III [mm] | 0.32–0.49 | 0.37–0.40 | 0.39–0.52 | 0.30–4.33 |
| Ant. segm. IV [mm] | 0.15–0.26 | 0.26–0.26 | 0.21–0.34 | 0.16–0.20 |
| Ant. segm. V [mm] | 0.14–0.20 | 0.19–0.2 | 0.15–0.21 | 0.14–0.16 |
| Ant. segm. VI base [mm] | 0.11–0.14 | 0.14 | 0.09–0.12 | 0.08–0.10 |
| Ant. segm. VI [mm] | 0.22–0.31 | 0.56 | 0.30–0.44 | 0.31–0.35 |
| Ant. segm. VI processus terminalis/ Ant. segm. III [times] | 0.63–0.80 | 1.51 | 0.65–0.95 | 0.96–1.13 |
| Ant. segm. VI processus terminalis/ base [times] | 2.00–2.39 | 4.00 | 2.78–4.00 | 3.18–4.53 |
| Ultimate rostral segm. [mm] | 0.10–0.12 | 0.13 | 0.13–0.15 | 0.10–0.11 |
| Ultimate rostral segm. / its basal width [times] | 1.10–2.00 | 1.86 | 2.36–2.64 | 2.20–2.50 |
| Ultimate rostral segm. / Ant. segm. VI base [times] | 0.85–1.00 | 0.93 | 1.17–1.61 | 1.08–1.26 |
| Hind femur [mm] | 0.39–0.52 | 0.55–0.54 | 0.48–0.59 | 0.33–0.37 |
| Hind tibia [mm] | 0.73–0.95 | 0.92–0.92 | 0.86–1.05 | 0.63–0.70 |
| Hind tibia / Body [times] | 0.41–0.51 | 0.50–0.50 | 0.44–0.52 | 0.49–0.62 |
| Hind tarsus, 2nd segm. [mm] | 0.11–0.13 | 0.14–0.13 | 0.10–0.12 | 0.09–0.10 |
| Hind tarsus, 2nd segm. / Ultimate rostral segm. [times] | 1.05–1.20 | 1.04–1.00 | 0.69–0.81 | 0.84–0.95 |
| Abdominal Marginal papillae [number] | 0 | 0 | 0 | (0)2–4 |
| Siphunculus [mm] | 0.17–0.20 | 0.39 | 0.47–0.58 | 0.26–0.31 |
| Siphundulus / Body [times] | 0.09–0.11 | 0.21 | 0.23–0.29 | 0.21–0.27 |
| Siphunculus / Ant. segm. III [times] | 0.41–0.56 | 1.04 | 1.11–1.36 | 0.79–1.02 |
| Siphunculus / its basal width [times] | 2.62–3.18 | 5.50 | 4.14–5.50 | 3.86–6.56 |
| Siphuncular widths, maximal / basal [times] | 0.69–0.82 | 0.79 | 0.44–0.67 | 0.61–0.89 |
| Siphuncular widths, maximal / minimal [times] | 1.00–1.06 | 1.38 | 1.00–1.00 | 1.06–1.13 |
| Siphuncular minimal width / Hind tibia, diameter at middle [times] | 1.42–1.50 | 0.94 | 1.24–1.65 | 1.25–1.89 |
| Cauda [mm] | 0.11–0.14 | 0.23 | 0.18–0.24 | 0.11–0.12 |
| Cauda / Siphunculus [times] | 0.55–0.82 | 0.60 | 0.33–0.45 | 0.37–0.40 |
| Cauda / its basal width [times] | 0.71-0.00 | 1.80 | 1.20–1.60 | 0.92–1.05 |
| Setae on … | ||||
| … Frons [μm] | 8–10 | 9 | 33–45 | 5–10 |
| … Frons / b. d. Ant. segm. III [times] | 0.38–0.44 | 0.39 | 1.40–2.12 | 0.25–0.67 |
| … Vertex [μm] | 8–10 | 8 | 25–40 | 8 |
| … Vertex / b. d. Ant. segm. III [times] | 0.38–0.57 | 0.3 | 1.1–1.9 | 0.4–0.5 |
| … Ant. segm. III [μm] | 8–10 | 10 | 14–21 | 5–8 |
| … Ant. segm III / b. d. Ant. segm. III [times] | 0.4–0.6 | 0.4 | 0.6–0.9 | 0.3–0.5 |
| … Ultimate rostral segm. [number] | 5–7 | 13 | 10–15 | 8–11 |
| … Hind femur, dorsal [μm] | 10–13 | 5 | 13–20 | 3–5 |
| … Hind femur, ventral [μm] | 13–18 | 13 | 25–40 | 8–10 |
| … Hind tibia, dorsal, at middle [μm] | 20–25 | 18 | 25–38 | 15–23 |
| … Hind tibia, dorsal / Tibial diameter (at middle) [times] | 0.7–0.8 | 0.41 | 0.6–0.9 | 0.6–0. 9 |
| … Hind tarsus, first segm. [number] | 2–3 | 2–3 | 2–3 | 2–3 |
| … Abdominal segm.s 2-4 [μm] | 10–10 | 3 | 15–23 | 4–8 |
| … Abdominal segm.s 2-4 / b. d. Ant. segm. III [times] | 0.4–0.6 | 0.11 | 0.7–1.1 | 0.2–0.4 |
| … Abdominal segm. 8 [μm] | 20–25 | 10 | 23–38 | 8–15 |
| … Abdominal segm. 8 / b. d. Ant. segm. III [times] | 1.0–1.4 | 0.44 | 1.0–1.8 | 0.4–0.9 |
| … Abdominal segm. 8 [number] | 4–5 | 2 | 3–5 | 4 |
| … Ventro-abdominal [μm] | 20–38 | 28 | 30–45 | 11–16 |
| … Genital plate, discal [number] | 2 | 2 | 2 | 2 |
| … Genital plate, marginal [number] | 10–14 | 13 | 9–18 | 7–10 |
| … Cauda [number] | 6–8 | 7 | 9–14 | 6–6 |
NOTE. Used abbreviations: Ant., Antennal; b. d., basal diameter; n, number of measured specimens; segm., segment.
Holotype: Apterous viviparous female (specimen 1), on Dianthus sp. Kalam (Khyber Pakhtunkhwa), Pakistan, 1800 m, 17-VIII-1991, Naumann-Etienne leg. [Remaudière’s sample 014072]. Paratypes: 3 apterous viviparous females with the same data that the holotype.
The specific name of the new species is an adjective that refers to Pakistan, in feminine.
Aphidura pakistanensis sp. n. is the third species of the genus living on Dianthus. Its distinctive features are summarized in the identification key to apterae of Aphidura in the general discussion, and in the following modification to the key to aphids on Dianthus (
| 7 | ABD TERG 1 and 7 without MTu. SIPH subcylindrical or slightly swollen). Anterior part of mesosternum with a pair of spinal mammariform processes | 7A |
| – | ABD TERG 1 and 7 with MTu. SIPH tapering from base to flange, with no trace of swelling. Anterior part of mesosternum without a pair of spinal mammariform processes | 9 |
| 7A | Cauda as long as its basal width or shorter. SIPH not longer than 0.20 mm and 0.6 times ANT III. Mesosternal processes small and pale, sometimes inconspicuous. Abdomen without dorsal pigmentation | Aphidura pakistanensis |
| – | Cauda longer than its basal width. SIPH longer than 0.26 mm and 0.60 times ANT III. Mesosternal processes pale or pigmented, always conspicuous. Abdomen pale or variably pigmented | 8 |
| 8 | [without modification] | Aphidura picta |
| – | [without modification] | Aphidura pujoli |
urn:lsid:zoobank.org:act:927C4017-2E8C-417E-BDBD-2A3262557025
(Fig. 5C). Colour in life unknown. Head pale yellow. Antennal segment I-IV and proximal half of V as pale as cephalic dorsum, distal part of V and VI yellow brown. Dorsum of thorax and abdomen membranous and pale, with yellowish brown spiracular and brown intersegmental sclerites. Mesosternal mammariform processes low, rugose and pale. Siphunculi gently and asymmetrically swollen, rugose and more-or-less pigmented like tibiae. Cauda tongue-shaped with broad apex, pigmented like siphunculi. Anal and genital plates as pale as cauda. Metric and meristic features in Table 4.
Holotype: Apterous viviparous female, on Gypsophila sp., Veria [road to Kastania] (Imanthia), Greece, 18-VI-1964, G. Remaudière leg. (sample 03026).
The specific name of the new species is an adjective that means inhabitant of Greece, in feminine.
Aphidura graeca sp. n. lives on Gypsophila, as does Aphidura gypsophilae, and also Aphidura pannonica, which has been above recorded on this plant-genus for first time. The distinctive features of Aphidura graeca are summarized in the identification key to apterae of Aphidura in the general discussion and in the following modification to the key to aphids on Gypsophila (
| 3 | Anterior part of mesosternum with a pair of mammariform processes, ornamented with spinules | 3A |
| – | Anterior part of mesosternum without a pair of mammariform processes | 4 |
| 3A | SIPH markedly clavate | 3B |
| – | SIPH not markedely clavate | 3C |
| 3B | ANT PT at least 1.40 times ANT III. Abdominal dorsum mostly membranous, and pale. SIPH pale | Aphidura graeca |
| – | ANT PT at most 1.20 times ANT III. Abdominal dorsum with pigmented patches and sclerites. SIPH pigmented | Aphidura naimanica |
| 3C | Head, prothorax (with a complete or fragmented transversal band) and SIPH brown. Abdominal spinopleural patch variably developed and pigmented and sometimes fragmented or (often in small specimens) absent | Aphidura pannonica |
| – | Head, prothorax and SIPH (sometimes brownish apicad) pale. Abdomen variable sclerotised and pigmented | 3C |
| 3D | ANT PT/BASE 3.4-4.4. R IV+V at least 1.0 times HT II | Aphidura togaica |
| – | ANT PT/BASE 5.0-5.5. R IV+V shorter than HT II | Aphidura gypsophilae |
urn:lsid:zoobank.org:act:41642023-E1EE-4E82-BD61-931EF3866450
(Fig. 5D). Colour in life unknown. Head yellowish brown to brown. Clypeus bigger than those of the other species of Aphidura. Antennae yellowish brown, with brown segment VI, distal 1/3 of V, and articulation between IV and V. Mesosternal mammariform processes well separated from one another, pale and round. Intersegmental sclerites small and dark brown; spiracular sclerites on segment 7 wider and darker than other abdominal spiracular sclerites; abdominal segments 3-6 with pleural and sometimes very small setiferous spinal sclerites, or with spinopleural sclerites; abdominal terga 7 and 8 pale. Siphunculi with narrow base, cylindrical (usually with slight outward curve) or slightly swollen, and as pale as tibiae. Cauda tongue-shaped, pale like genital and anal plate. Metric and meristic features in Table 4.
Holotype: Apterous viviparous female (specimen 5), on Spergula marina, Shahi island, Lake Urmia (East Azerbaijan), Iran, 5-VIII-1955, Remaudière leg. (sample i962). Paratypes: 42 apterous with the same data that the holotype; plus 6 apterous viviparae on Spergularia marina, Charimboulaki, Lake Urmia (West Azerbaijan), Iran, 9-VIII-1955, Remaudière leg. (sample i004a).
The specific name, urmiensis is an adjective that refers to lake Urmia, in feminine, from the name of the Catholic Chaldean Archdiocese of Urmia.
The distinctive features of Aphidura urmiensis sp. n., which lives on Spergula marina are summarized in the identification key to apterae of Aphidura in the general discussion and in the following modification to key to aphids on Spergula and Spergularia (
| 0 | Anterior part of mesosternum with a pair of spinal mammariform processes | Aphidura urmiensis |
| – | Anterior part of mesosternum without a pair of spinal mammariform processes | 1 |
urn:lsid:zoobank.org:act:B6A4D5D2-4826-4C5D-AF94-EFE0DCFF4A2C
(Fig. 6A). Colour in life unknown. Head brown. Vertex with spinules disposed in scattered groups. Prothorax and at least some of abdominal segment 2-4 with small marginal tubercles; abdominal segment 8 with 0-2, most frequently 1, small spinal tubercles. Mesosternal mammariform processes rounded and pale. Dorsal pigmentation and sclerotisation very variable. In several specimens (holotype included) prothorax with a complete band, mesothorax with a band with lateral windows, metathorax with two large spinopleural sclerites; abdominal segments 1-5 with several setiferous marginal sclerites, and a spinopleural patch, which has irregular edges and windows and may be coalesced with the metathoracic sclerites; abdominal segment 6 with small intersiphuncular and two postsiphuncular sclerites; segments 7 and 8 with brownish band; intersegmental sclerites are embodied in the above; spiracular sclerites inconspicuous. In less sclerotized and paler specimens the bands and patch are broken. Siphunculi slightly swollen, ornamented with denticulate scales, and paler than cephalic dorsum and dorsal thoracic-abdominal sclerotized areas. Cauda thin triangular, paler than siphunculi. Genital plate pale; anal plate coloured like cauda. Metric and meristic features in Table 4.
Holotype: Apterous viviparous female (specimen 1), on Prunus sp., Khoy [30 km North] (West Azerbaijan), Iran, 1700 m, 7-VIII-1955, G. Remaudière leg. (sample i982). Paratypes: 5 apterous viviparous females, with the same collecting data as holotype.
The specific name of the new species, iranensis, is an adjective that refers to Iran, in feminine.
Aphidura iranensis sp. n. is the second species of the genus living on species of Prunus. Its distinctive features are summarized in the identification key to apterae of Aphidura in the general discussion, and in the following modification to the key to aphids on Prunus (
| 7 | [without modification] | 8 |
| – | Head capsule with spiculose (sometimes delicate) or nodulose ornamentation | 30 |
| 32 | Anterior part of mesosternum with a pair of spinal mammariform processes, ornamented with spinules (Fig. 89B) | 32B |
| – | [without modification] | 33 |
| 32B | A continuous sclerotic and dark shield on (metanotum) ABD TERG 1-6(7), including marginal areas; and dorsum of other thoracic segments with sclerotic dark bands. ABD TERG 1-4 without marginal tubercles, and ABD TERG 8 without spinal tubercles | Aphidura bozhkoae |
| – | A continuous dorsal sclerotic shield absent; dorsum of thoracic segments with sclerotic bands, and ABD TERG 1-5(7) with spinal and pleural sclerites or patches, which may be coalescing. ABD TERG 2-4 frequently with marginal tubercles, and ABD TERG 8 frequently with spinal tubercles | Aphidura iranensis |
The features that distinguish the apterous viviparous females of the Aphidura species which share host plants have been described in the modifications to Blackman and Eastop’s keys to aphids on different plant genera (
The previously known and the new species together can be distinguished from each other using the following key to apterous viviparous females of species of Aphidura. In brackets are: (1) morphological characters that do not have correspondence in the other proposition of the disjunctive, but which are useful to confirm identification; (2) host plants, and distribution data; and (3) illustration reference. In the distribution of each species the countries are in geographical order from West to East, so that a quick general assessment of the distribution of each species can be made. The key uses data of species recently described from the respective original descriptions (
| 1 | Siphunculi markedly swollen (maximal swollen width at least 1.2 times minimal stem width) | 2 |
| – | Siphunculi of different form (cylindrical, subcylindrical, tapering or slightly swollen, see above “generic characters” section) | 9 |
| 2 | Most of dorsal setae placed on conical tubercles. [Dorsum without segmental pigmented sclerotisation. On Acanthophyllum sp.; Iran. Fig. 6B] | Aphidura acanthophylli |
| – | Dorsal setae not placed on tubercles | 3 |
| 3 | Mesosternal processes and cauda pale | 4 |
| – | Mesosternal processes and cauda more or less pigmented, light brown to brown | 6 |
| 4 | Siphunculi dark brown, 2.3–2.7 times cauda which has 7–11 setae. Abdominal dorsum with spino-pleural patch, postsiphuncular sclerites pigmented and marginal sclerites. [Ultimate rostral segment 1.0–1.2 times second segment of hind tarsi. Cauda 1.1–1.2 times its basal width. On Silene suffrutescens and Silene sp.; Kazakhstan. |
Aphidura nomadica |
| – | Siphunculi pale, sometimes with smoky apex, 1.6–2.2 times cauda, which has 6–7 setae. If a spino-pleural patch present then ultimate rostral segment is 1.2–1.5 times second segment of hind tarsi | 5 |
| 5 | Antennal segment VI processus terminalis at least 1.4 times antennal segment III and approximately 4 times antennal segment VI base. Longest dorsal setae on abdominal segment 2–4 approximately 3 μm. Cauda tongue-shaped. Dorsum pale with dark intersegmental sclerites. [On Gypsophila sp.; Grece. Fig. 5C] | Aphidura graeca sp. n. |
| – | Antennal segment VI processus terminalis at most 1.1 times antennal segment III and at most 3.1 times antennal segment VI base. Longest dorsal setae on abdominal segment 2–4 are 7–13 μm. Cauda triangular, sometimes slight constricted. Dorsum with variable sclerotisation and pigmentation, sometimes mostly pale. [On Silene sp., and an unidentified caryophyllaceous species; Iran. Fig. 5D] | Aphidura amphorosiphon sp. n. |
| 6 | Abdominal (or thoracic-abdominal) discal plate present, sometimes divided in transversal bands | 7 |
| – | Abdominal discal plate absent; a broken an irregularly edged spinopleural patch usually present, sometimes with bridges to marginal sclerites | 8 |
| 7 | Mesosternal processes wide and low. Longest dorsal setae on abdominal segment 2–4 are 10–11 μm. Discal plate sometimes divided in transversal bands. Siphunculus 1.6–2.0 times cauda, which has 7–11 setae. [On Melandrium album; Kazakhstan. |
Aphidura melandrii |
| – | Mesosternal processes more or less narrow and tall. Longest dorsal setae on abdominal segment 2–4 are 10–55 μm. Discal plate always complete. Siphunculus 1.6–2.6 times cauda, which has 5–8 setae. [On Saponaria sp., Silene commutata, Silene kuschakewiczii, Silene lithophila, Silene vulgaris, Silene wallichiana, Silene wolgensis and Silene sp.; Kazakhstan, Pakistan, Tajikistan, and India. Fig. 1A] | Aphidura ornatella |
| 8 | Siphunculus 1.7–2.7 times cauda. Longest frontal setae 22–28 μm and 1.0–1.4 times basal diameter of antennal segment III. [On Gypsophila altissima and Gypsophila paniculata; Kazakhstan. |
Aphidura naimanica |
| – | Siphunculus 1.5–1.7 times cauda. Longest frontal setae 35–40 μm and 1.6–1.8 times basal diameter of antennal segment III. [On Cerastium cerastoides; Kazakhstan. |
Aphidura alatavica |
| 9 | First segment of tarsi with 4 or less habitually with 3 setae. [Head and prothoracic transversal band as dark as thoracic-abdominal discal plate. Siphunculi cylindrical and straight. On Rosaceae species] | 10 |
| – | First segment of tarsi habitually with 3 setae, sometimes with 2; very infrequently with 4 | 11 |
| 10 | Antennal segment VI processus terminalis 2.2–2.7 times antennal segment VI base. Ultimate rostral segment with 2–5 accessory setae. Marginal tubercles usually present on abdominal segments 2–4. [On Prinsepia sinensis; Russia (Far Est, Primorsky Krai). Fig. 1D] | Aphidura mordvilkoi |
| – | Antennal segment VI processus terminalis 3.8–4.2 times antennal segment VI base. Ultimate rostral segment with 8–10 accessory setae. Abdominal marginal tubercles always absent. [On Prunus erythrocarpa, Prunus fruticosa, Prunus incana, Prunus spinosa, Prunus tianschanica, Prunus triloba, Prunus ulmifolia, Prunus verrucosa and Prunus sp.; Georgia, Kazakhstan, Iran, Uzbekistan, Tajikistan, and Kyrgyzstan. Fig. 2A] | Aphidura bozhkoae |
| 11 | Siphunculus slightly swollen with a maximal width close to 1.2 times minimal stem width and 1.6–2.0 times cauda, which is 1.5–1.8 times its basal width and has 7–11 setae; both as dark as head dorsum and thoracic and abdominal sclerotisation (a discal plate can be present). Longest dorsal setae on abdominal segment 2–4 are 10–11 μm and approximately 0.5 times basal diameter of antennal segment III. [On Melandrium album; Kazakhstan. |
Aphidura melandrii |
| – | Characters not in above combination | 12 |
| 12 | Siphunculus at most 1.95 times cauda (which is short triangular), pale or uniformly dusky and slight swollen. Dorsum of head and mesosternal processes pale. Segmental thoracic and abdominal sclerotisation and pigmentation absent | 13 |
| – | Siphunculus at least 1.90 times cauda, both diversely shaped and coloured. Dorsum of head and mesosternal processes pale or pigmented. Thoracic and abdominal segmental sclerotisation and pigmentation rare completely absent | 14 |
| 13 | Siphunculus at least 0.26 mm, 0.6–0.95 times antennal segment III, and 1.7–1.95 times cauda, which is longer than its basal width. Mesosternal processes conspicuous. [On Dianthus carthusianorum, Dianthus caryophyllus, Dianthus commutatus, Dianthus monspessulanus, Dianthus rupicola, Dianthus sp. and Silene borysthenica, Portugal, Spain, France, Switzerland, Italy and Ukraine. Fig. 3A] | Aphidura pujoli |
| – | Siphunculus shorter than 0.20 mm, 0.41–0.56 times antennal segment III, and 1.7–1.9 times cauda, which is not longer than its basal width. Mesosternal processes sometimes inconspicuous. [On Dianthus sp.; Pakistan. Fig. 5B] | Aphidura pakistanensis sp. n. |
| 14 | Antennal segment I at least 1.25 times its maximal width. Longest dorsal setae on abdominal segments 2–4 are 35–55 μm and 1.5–2.0 times basal diameter of antennal segment III. [Discal plate oval and dark. Siphunculi weakly ornamented, smooth distad. On Silene italica, Silene nutans¸ perhaps Silene viscosa, and Silene sp.; France, Italy, Greece. Fig. 2B] | Aphidura delmasi |
| – | Antennal segment I at most 1.1 times its maximal width. Longest dorsal setae on abdominal segments 2–4 at most 25 μm and 1.2 times basal diameter of antennal segment III | 15 |
| 15 | Abdomen usually with spinopleural patch and separate marginal sclerites; if a discal plate is present then it has irregular margins and frequently there are windows in spinal areas of the thoracic, if integrated, and anterior abdominal segments. Dorsal patch or plate smooth and reticulated. Siphunculi dark brown to black, subcylindrical and usually straight, 1.8–2.0 times cauda, which is broad triangular and has 10–16 setae. Ultimate rostral segment with 6–10 accessory setae. [On Silene inaperta, Silene italica, Silene nutans, Silene saxifraga, Silene otites, Silene vulgaris, Silene wolgensis and Silene sp.; France, Switzerland, Italy, Hungary, Romania, Ukraine and Russia. Fig. 2C] | Aphidura ornata |
| – | Characters not in above combination | 16 |
| 16 | Longest setae on abdominal segments 2–4 (dorsum) and antennal segment III 3–8 μm and 0.15–0.50 times basal diameter of antennal segment III | 17 |
| – | Longest setae on abdominal segments 2–4 (dorsum) and antennal segment III 8–25 μm and 0.15–0.50 times basal diameter of antennal segment III; if they are 8 μm long then marginal abdominal tubercles present or ultimate rostral segment shorter than second segment of hind tarsi | 18 |
| 17 | Siphunculi dark brown, head dorsum, mesosternal processes and cauda brown to dark brown. Ultimate rostral segment 1.15–1.25 times second segment of hind tarsi. Cauda 1.4–1.8 times its basal width. [On Gypsophila paniculata, Silene borysthenica, Silene moldavica, Silene otites, Silene wolgensis and Silene sp.; Slovakia, Hungary, Greece, Ukraine, and Moldova. Fig. 2D] | Aphidura pannonica |
| – | Siphunculi (with smoked apex, head dorsum, mesosternal processes and cauda pale. Ultimate rostral segment as long as second segment of hind tarsi. Cauda 1.0–1.1 times its basal width. [On Gypsophila perfoliata; Kazakhstan. |
Aphidura togaica |
| 18 | Marginal tubercles on abdominal segments 2–4 and usually at least 1 spinal tubercle on abdominal segment VIII. [Cauda triangular 0.92–1.05 times its basal width. On Prunus. Iran. Fig. 6A] | Aphidura iranensis sp. n. |
| – | Marginal and spinal abdominal tubercles absent | 19 |
| 19 | Siphunculi pale, usually as pale as most part of tibiae | 20 |
| – | Siphunculi pigmented, usually darker than most part of tibiae | 21 |
| 20 | Antennal segment VI processus terminalis 5.0–5.5 times antennal segment VI base. Cauda triangular or tongue-shaped with slight proximal constriction. Ultimate rostral segment shorter than second segment of hind tarsi. [On Gypsophila arenaria, Gypsophila paniculata, Gypsophila perfoliata, Gypsophila sp.; Slovakia, Hungary, Ukraine, Kazakhstan, Russia (Western Siberia). Fig. 6C] | Aphidura gypsophilae |
| – | Antennal segment VI processus terminalis 2.8–4.0 times antennal segment VI base. Cauda tongue-shaped. Ultimate rostral segment 1.23–1.45 times second segment of hind tarsi. [Clypeus swollen both forward and laterally. On Spergularia marina; Iran. Fig. 5D] | Aphidura urmiensis sp. n. |
| 21 | Cauda tongue-shaped, 1.40–1.80 times its basal width. Mesosternal processes more or less pigmented, usually darker than tibiae. [Thoracic and abdominal sclerotisation variable, usually a spinopleural abdominal patch with irregular edges and windows in several segments, including the posterior ones. Siphunculi pigmented, but usually pale than abdominal sclerotised dorsum. On Dianthus barbatus, Dianthus caryophyllus, Dianthus crinitus, Dianthus sp., Silene conoidea, Silene fruticosa, Silene italica, Silene otites, Silene paradoxa, Silene thymifolia, Silene vulgaris, and Silene sp. Spain, France, Italy, Slovenia, Hungary, Greece, Bulgaria, Turkey, Israel, Iran, Afghanistan, Pakistan, Tajikistan, and Russia (Asiatic part). Fig. 1C] | Aphidura picta |
| – | Cauda triangular, although sometimes with a slight proximal constriction, 1.05–1.40 times its basal width. Siphunculi and mesosternal processes as pale as tibiae | 22 |
| 22 | Ultimate rostral segment 0.90–1.00 times second segment of hind tarsus, with 8–10 accessory setae. Cauda approximately 1.30–1.40 times its basal width. Longest dorsal setae on abdominal segment 2–3 are 8–11 μm and 0.3–0.5 basal diameter of antennal segment III. [On Silene lithophila; Kazakhstan. |
Aphidura massagetica |
| – | Ultimate rostral segment 1.05–1.45 times second segment of hind tarsus, with 10–16 accessory setae. Cauda approximately 1.05–1.35 times its basal width. Longest dorsal setae on abdominal segment 2–3 are 13–23 μm and 0.6–1.0 basal diameter of antennal segment III. [On Silene gallica and Silene paradoxa; France. Figs 4A, B] | Aphidura gallica sp. n. |
Aphidura pujoli (from Blackman and Eastop op. cit.) and Aphidura delmasi (this paper) are monoecious holocyclic, and Aphidura amphorosiphon is very possibly holocyclic (this paper). The life cycle of the other species of the genus is unknown. It is possible that three types of life cycle currently exist in this genus, as in Brachycaudus van der Goot, 1913: (i) monoecious (and probably holocyclic) on a rosaceous species (e.g. Aphidura bozhkoae on Prunus spp. and Aphidura mordvilkoi on Prinsepia sinensis), (ii) monoecious on a caryophyllaceous species (and also probably holocyclic, e.g. Aphidura delmasi and Aphidura pujoli), and (iii) dioecious cycle with rosaceous species as primary host, and caryophyllaceous species as secondary host.
For us the more probable hypothesis is that all current species of Aphidura are monoecious, but that their common ancestor was dioecious, as in various other genera of Macrosiphini, and later the Aphidura branch diversified into two monoecious lineages, one Rosaceae-feeding and the other Caryophyllaceae-feeding. This is analogous to the South American species of Pentamyzus Hille Ris Lambers, 1966 which are all monoecious holocyclic, with several species living on Acaena (Rosaceae) and others on Alopecurus, Hordeum or Poa (Poaceae) (
Authors wish to thank the Muséum national d’Historie naturelle of Paris (France) for the facilities given for the study of the Aphidura slides of its collection. They acknowledge Dr. Roger Blackman for the critical reading of the manuscript draft, and for several pieces of information about the specimens conserved in the Natural History Museum in London. Also our thanks to Andrey V. Stekolshchikov (Zoological Institute, Russian Academy of Sciences, St. Petersburg) who provided us with the translation of the Kadyrbekov’s recently published paper, and to L. M. Fernández Blanco for taking photomicrographs and preparing figure plates.