(C) 2013 M. Alma Solis. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Solis AM, Cashatt ED, Scholtens BG (2013) New North American Chrysauginae (Pyralidae) described by E.D. Cashatt. ZooKeys 344: 55–71. doi: 10.3897/zookeys.344.5609
A Ph.D. dissertation completed by E.D. Cashatt in 1968 entitled “Revision of the Chrysauginae of North America” does not meet the criteria of publication so the new taxa described therein are not available per the International Code of Zoological Nomenclature. In order to validate the taxa proposed in that document we formally describe and illustrate the following: Arta brevivalvalis Cashatt, sp. n., Heliades lindae Cashatt, sp. n., Paragalasa Cashatt, gen. n., Paragalasa exospinalis Cashatt, sp. n., and Penthesilea sacculalis baboquivariensis Cashatt, subsp. n. We summarize other taxonomic actions proposed in the dissertation and those proposed by subsequent authors. We provide the current nomenclatural status with the literature citation of the paper in which the current status was proposed. A lectotype is designated for Clydonopteran tecomae. Adult holotypes and associated labels, and genitalia of paratypes are newly illustrated.
Chrysauginae, Pyralidae, North America, Campsis radicans
In this work we make available by publication the taxa described in the dissertation by
Nomenclatural acts and attributions relating to taxa in
| Taxon name | Action | Attribution |
|---|---|---|
| Salobrena Walker, 1863 | Revised status as genus | |
| Clydonopteron tecomae Riley, 1880 | Synonym of Pyralis sacculana | |
| Pyralis sacculana Bosc, [1800] | Combination in Clydonopteron Riley | |
| Basacallis Cashatt, 1984 | Genus description | |
| Artopsis Dyar, 1908 | Synonym of Parachma Walker | |
| Artopsis borregalis Dyar, 1908 | New revised status of Parachma ochracealis | Present paper |
| Polloccia Dyar, 1910 | Synonym of Acallis Ragonot | |
| Polloccia alticolalis Dyar, 1910 | Combination in Acallis Ragonot | |
| Balidarcha Dyar, 1914 | Synonym of Anemosella Dyar | |
| Balidarcha cuis Dyar, 1914 | Synonym of Anemosella viridalis B. & McD. | |
| Balidarcha cuis Dyar, 1914 | Combination in Anemosella Dyar | |
| Xantippides Dyar, 1908 | Synonym of Arta Grote | |
| Xantippides descansalis Dyar, 1908 | Synonym of Arta epicoenalis Ragonot | |
| Xantippides descansalis | Combination in Arta Grote | |
| Acallis centralis Dyar, 1910 | New synonym of Acallis gripalis Hulst | Present paper |
| Anemosella polingalis B. & B., 1926 | New synonym of Anemosella basalis Dyar | Present paper |
| Xantippe beatifica Dyar, 1921 | New synonym of Arta epicoenalis Ragonot | Present paper |
| Xantippe beatifica Dyar, 1921 | New combination in Arta Grote | Present paper |
| Xantippe uranides Dyar, 1921 | New synonym of Heliades mulleolella Hulst | Present paper |
| Xantippe uranides Dyar, 1921 | New combination in Heliades Ragonot | Present paper |
| Heliades huachucalis Haimbach, 1915 | New revised status as species | Present paper |
| Negalasa rubralis B. & McD., 1913 | Revised status as species | |
| Arta brevivalvalis | New species | Present paper |
| Heliades lindae | New species | Present paper |
| Paragalasa | New genus | Present paper |
| Paragalasa exospinalis | New species | Present paper |
| Penthesilea sacculalis sacculalis | New revised status as subspecies | Present paper |
| Penthesilea sacculalis baboquivariensis | New subspecies | Present paper |
In his abstract,
In the dissertation
Negalasa rubralis Barnes & McDunnough, 1913 was treated as a subspecies of Negalasa fumalis by
http://zoobank.org/BC60CF21-AF03-4025-B272-251827BA2B87
http://species-id.net/wiki/Arta_brevivalvalis
Figs 1, 5–7Head. Labial palpus reddish-brown laterad, inner surface ochreous; frons and vertex light reddish-brown to purplish; occiput tan to ochreous.
Thorax. Upper surface reddish-brown to tan, under surface darker. Forewing reddish- to purplish-brown with ochreous antemedial and postmedial lines; antemedial line irregular and extending obliquely from two-thirds costa to nearly one-half hind margin; postmedial line irregular and directed slightly inward near costa, extending from three-fifths costa to near anal angle; distance between the two lines greater at costa than at hind margin; fringe ochreous; under surface brown, purplish-red near costa and outer margin. Hind wing grayish-brown with ochreous fringe; underside purplish-red near costa and outer margin, a short postmedial line from costa fading inward. Legs purplish-brown with mid femur, midtibia, and inner surface of hind leg ochreous.
Abdomen. Ochreous dorsad, reddish-brown ventrad, terminal fringe ochreous.
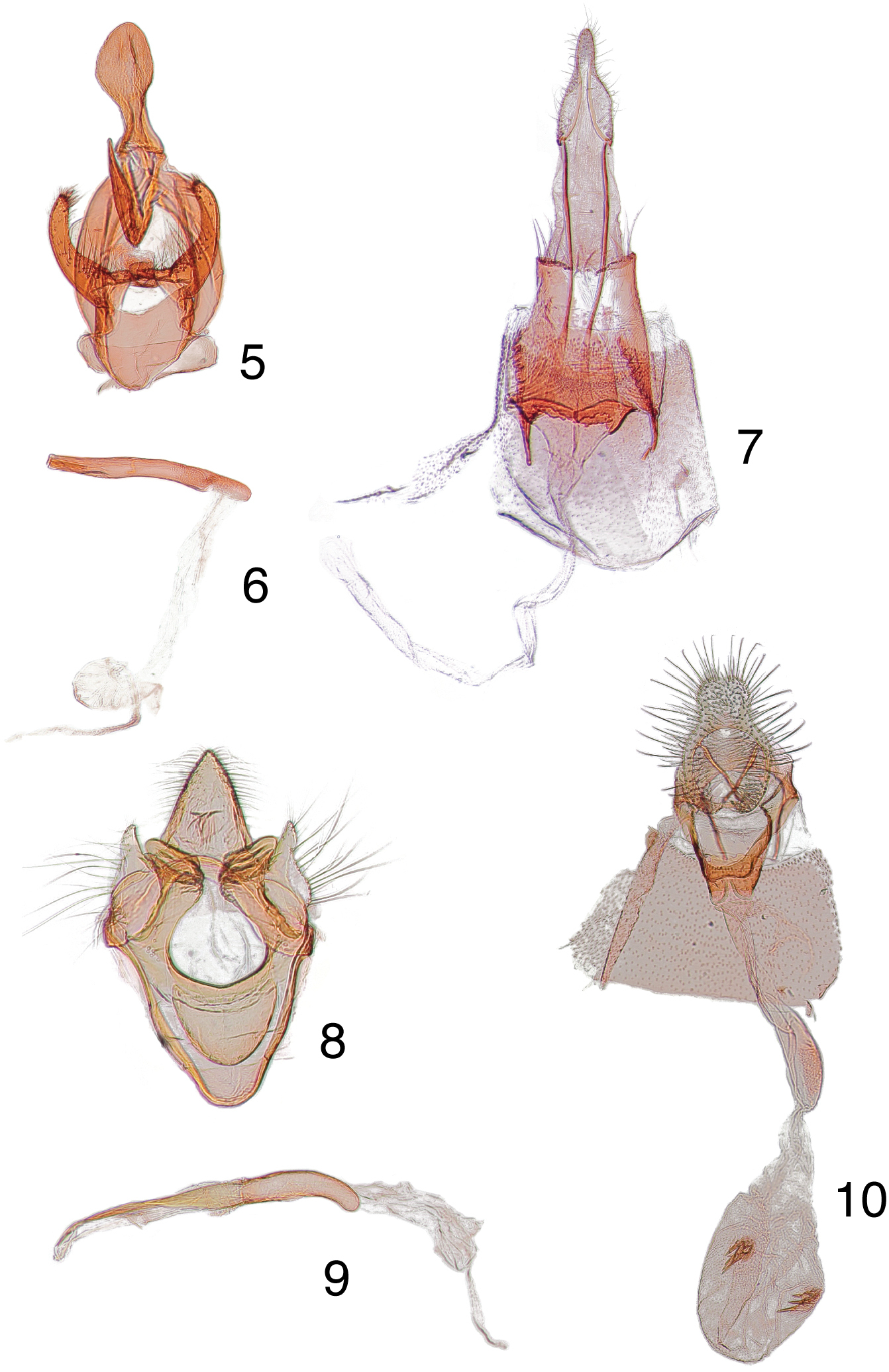
Male genitalia. Uncus broad and shovel-shaped; tegumen narrow, pedunculus unmodified; vinculum broad, saccus not narrowly produced anteriad as in Arta statalis, but rounded; juxta acutely hooked dorsad near base with apex directed slightly dorsad; valva as in Arta statalis except shorter with a broader base, apex unidentate; phallus long and slender with apex flattened without a coecum or cornutus.
Female genitalia. Ovipositor moderately enlongate, papillae anales small and unilobate; posterior apophysis extremely short; anterior apophysis short as in Arta statalis; ostium bursae wide; lamella antevaginalis broad and V-shaped, opening near anterior margin of eighth sternite; bursa copulatrix simple with inception of ductus seminalis below antrum; without a signum.
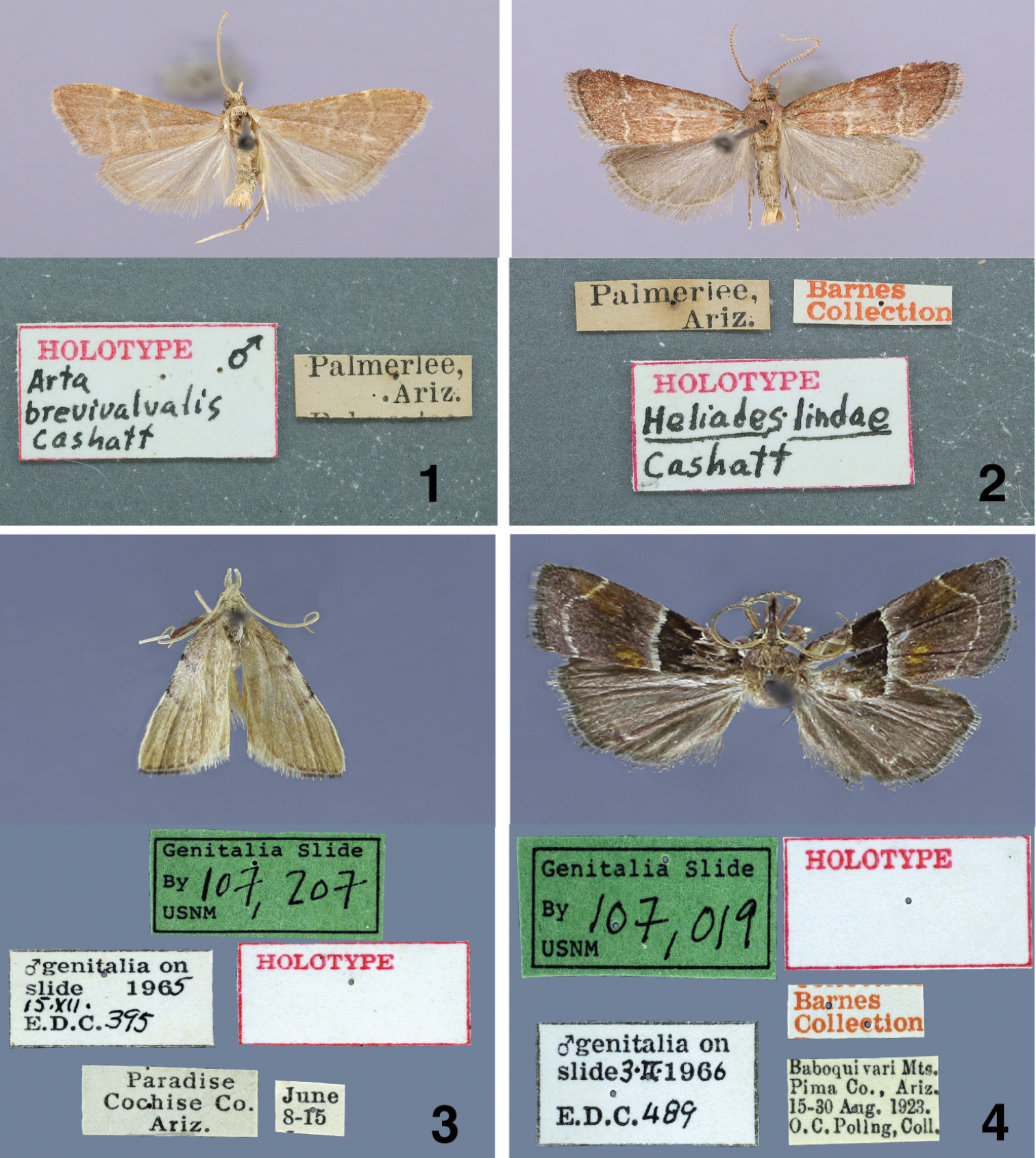
The type specimens are located as noted below. The male holotype is from Palmerlee, Arizona (no other data given) and is labeled as the holotype. Fifty-three male and twenty-two female paratypes from UNITED STATES: ARIZONA are labeled as follows: two females, Catalina Mts., no date given, Oslar Coll. (USNM); two males and two females, Catalina Mts., June 10, 1903, Oslar, Coll. (USNM); one female, Huachuca Mts., (USNM); one male, Madera Canyon, Santa Rita Mts., Aug. 18, 1953, Robert J. Ford (CNC); one male and one female, Madera Canyon, Santa Rita Mts., Aug. 19, 1953. Robert J. Ford (CNC); one male, Madera Canyon, Santa Rita Mts., Aug. 9, 1953 (CNC); one male, Palmerlee, Sept. 8–15 (USNM); twenty-five males and nine females, Palmerlee, no date given (USNM); seven males and four females, Ramsey Canyon, Huachuca Mts., Sept. 1-2, 1927, J. C. Bradley Coll. (CU); ten males and two females, White Mts., elevation 7200 ft., Aug. 1–15, 1925, Poling Coll. (USNM); one male and one female, White Mts., elevation 7200–11500 ft., Aug. 10–30, 1925, O. C. Poling (USNM); three males, White Mts., Apache Co., near McNary P. O., Sept. 15–30, 1925, O. C. Poling.
Unknown.
It is difficult to separate Arta brevivalvalis from Arta statalis and Arta epicoenalis on the basis of maculation. The ochreous fringe is sometimes a useful diagnostic character but is not reliable. The distance between the antemedial and postmedial lines is variable.
An examination of the genitalia is necessary for accurate identification. The flattened and spade-shaped uncus of Arta brevivalvalis easily separates this species from Arta statalis and Arta olivalis that have a narrow and more cylindrical shape. The valva of Arta olivalis is long and slim. The uncus of Arta epicoenalis is flattened, but not constricted at the base as in this species. The ostium bursae of Arta brevivalvalis is broad compared to that of Arta statalis, and the anterior apophyses are extremely short. The anterior apophyses of Arta olivalis and Arta epicoenalis are absent.
Male holotypes of adults and labels. 1 Arta brevivalvalis 2 Heliades lindae 3 Paragalasa exospinalis 4 Penthesilea sacculalis baboquivariensis.
Male, female genitalia. 5 Arta brevivalvalis paratype male, USA, Arizona, Palmerlee, [no collection date on label], EDC 981, USNM 1044736 phallus, data same as previous 7 paratype female, USA, Arizona, Palmerlee, [no collection date on label], EDC 982, USNM 104474 8 Heliades lindae paratype male, USA, Arizona, Palmerlee, [no collection date on label], EDC 80, USNM 105993 9 phallus, data same as previous 10 paratype female, USA, Arizona, Palmerlee, [no collection date on label], EDC 84, USNM 104482.
http://zoobank.org/F91B3D1C-706B-4341-80C4-10AA5117497D
http://species-id.net/wiki/Heliades_lindae
Figs 2, 8–10Alar expanse. 15 to 17 mm.
Head. Labial palpus dark reddish-brown with fuscous on under surface; frons, vertex, occiput, and antenna brownish-red.
Thorax. Upper surface brownish-red; under surface fuscous. Forewing brownish-red with white dentate antemedial and postmedial lines; antemedial line extending from about two-fifths costa to nearly two-fifths inner margin, postmedial line extending from three-fourths costa to just proximad of anal angle; terminal line fuscous; fringe gray with a dark medial line; under surface grayish-brown with apex brownish-red. Hind wing light grayish-brown; fringe gray with a dark medial line, under surface gray with apex reddish-brown. Legs fuscous with midtibia and tarsus white.
Abdomen. Upper surface concolorous with hind wings; terminal fringe ochreous.
Male genitalia. Uncus long and aculeate, setose dorsad; tegumen narrow dorsad; vinculum broad with a well-developed saccus, but more broadly rounded; gnathos reduced to a slender arm articulating at base of uncus; valva with sacculus small and papilliform, setose; valva heavily sclerotized and plate-shaped with apex truncate, ankylosed with flat truncate tips of arms produced by juxta; juxta shield-shaped and ankylosed with inner margin of vinculum; phallus long and slender, coecum well-developed.
Female genitalia. Ovipositor extremely short; apex of papillae anales bilobate and broad; eighth segment extremely short; anterior apophysis about one-half length of posterior apophysis; opening of ostium bursae at anterior of eighth sternite, small and sclerotized; anterior margin of sinus vaginalis bilobate and more broadly joined to the anterior margin of the eighth sternite; inception of ductus seminalis below antrum; ductus bursae weakly sclerotized and constricted near junction of corpus bursae; signum a pair of spines.
All the type specimens are in the USNM. The male holotype is from Palmerlee, Arizona (no other data given) and is labeled as the holotype. Twenty-three male and nineteen female paratypes from UNITED STATES: ARIZONA are labeled as follows: one female, Baboquivari Mts., Pima Co., 1-15 Sept. 1923, O. C. Poling; one male, Chiricahua Mts., July 4, H. G. Hubbard; one male, Fort Grant, July 20, H. G. Hubbard; one female, Hereford, no date, C. R. Biedermann; one male, Huachuca Mts., no date; one female, Huachuca Mts., Aug. 8-15; one male, Madera Canyon, Santa Rita Mts., Aug. 19, 1953, Robert J. Ford; one male, Nogales, July 15, 1903, Oslar; one female, Oracle, July 28, 1924, E. P. Van Duzee; nine males, four females, Palmerlee, no date given; one female, Palmerlee, Cochise Co., Aug. 1–7; one male, one female, Paradise, Cochise Co., no date; four males, five females, Paradise, Cochise Co., July; two females, Paradise, Cochise Co., Aug.; one female, Paradise, Cochise Co., Aug. 1–7; one male, S.W.R.R., 5 mi. W. Portal, Cochise Co., 5400 ft., July 9, 1956, Cazier and Ordway; one female, Santa Catalina Mts., no date given; one male, White Mts., El. 7000 ft., July 15–22, 1925, O. C. Poling.
Unknown.
It gives me pleasure to name this species in honor of my wife, Linda. The coloration of Heliades lindae is similar to Heliades huachucalis except the former is lighter and more reddish. The antemedial and postmedial lines of Heliades huachucalis are white, but unlike Heliades lindae the lines are margined with fuscous.
http://zoobank.org/E96A1722-5429-4294-9CCC-8A2727C56859
http://species-id.net/wiki/Paragalasa
Figs 3, 11–13Paragalasa exospinalis, Cashatt, new species.
Head. Labial palpus porrect, length approximately equal to head width; maxillary palpus vestigial, two segmented, pilifers moderately developed; proboscis well developed; frons rounded with a tuft produced obliquely; vertex and occiput roughly scaled; ocellus immediately posteriad to base of antenna; chaetosema a row of fine setae along ocular sutura posteriad to ocellus.
Thorax. Forewing long and narrow, costa slightly incurved near middle, apex sub-lanceolate, outer margin rounded; sexually dimorphic: male with a small glandular vesicle at base of costa, discal cell shorter than in female, R1 not reaching costa, posterior angle obtuse; female without a glandular vesicle, R1 intercepting the costa, posterior angle of discal cell acute; both sexes with Sc long, R1 arising from just before end of discal cell; R2 stalked short with R3, R4, and R5, stem arising from anterior angle of discal cell; R3 stalked with R4 and R5; R4 and R5 coincident; M1 separate, arising from anterior angle of discal cell; male with M2 separate, M3 end Cu1 stalked short; Cu2 separate, arising from posterior angle of discal cell; female M2 and M3 stalked short, Cu1 and Cu2 widely separated; 2A and 3A separate at base, anastomosed briefly a short distance from base; retinaculum normally developed. Hind wing frenulum normal; Sc and Rs anastomosed beyond end of discal cell; M1 separate from anterior angle of discal cell; M2 and M3 short stalked from posterior angle of discal cell; posterior angle of discal cell extremely long and slender; Cu1 and Cu2 widely separated. Legs long, midtibia with two scale tufts.
Abdomen. Long and slender, without scale tufts.
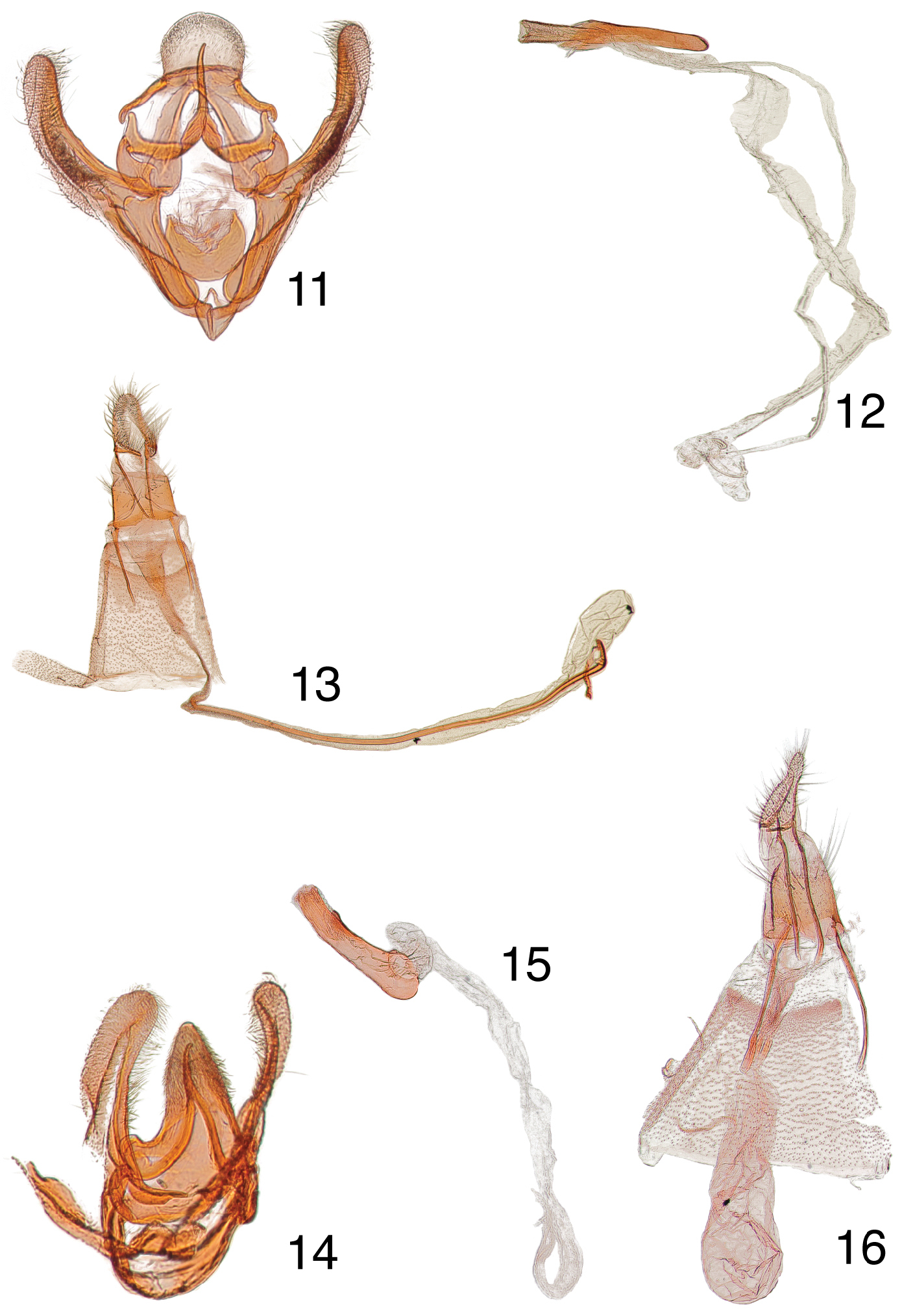
Mala genitalia. Uncus moderately broad with apex rounded, slender arms from base modified to articulate with gnathos; tegumen narrow dorsad; pedunculus strongly modified for articulation with gnathal arms; vinculum moderately broad with saccus slightly produced; gnathos slender and aculeate, apex hooked dorsad; valva with sacculus distinct from valva, ventral margin of sacculus rounded; transtilla weak and incomplete; juxta with dorsal margin V-shaped; phallus small, coecum long, apex with microspines, cornutus with spines short and spur-shaped.
Female genitalia. Ovipositor moderately long; papillae anales apex unilobate; anterior apophysis slightly longer than posterior apophysis; lamella postvaginalis triangulate; ostium bursae membranous, antrum lightly sclerotized, inception of ductus seminalis just below antrum; ductus bursae extremely long; corpus bursae small and without a signum.
The venation and genitalia indicate a close relationship between this genus and Negalasa. The male Paragalasa has a small glandular vesicle at the base of the costa on the forewing, but is without a costal spur. The costa is straight. Negalasa and Galasa have a larger glandular vesicle, an incurved costal margin and a costal spur at the end of Sc. The uncus of Negalasa is more narrow and pointed, the tip of the valva is directed acutely mediad, and the phallus has a broadly rounded coecum and cornutus with long spines. The male genitalia of Paragalasa is similar to Galasa except the dorsal margin is V-shaped, there is no process on the sacculus, and the phallus is smaller with a long cylindrical coecum and a small cornutus. The female Paragalasa has the inception of the ductus seminalis just below the antrum. The ductus bursae is extremely long with a small corpus bursae. Negalasa has the inception of the ductus seminalis more sclerotized, nearly two-thirds length from ostium bursae, and a large corpus bursae. The female venation of Paragalasa and Negalasa is identical. The male forewing of Negalasa shows more specialized structures.
Male, female genitalia. 11 Paragalasa exospinalis holotype male, USA, Arizona, Cochise Co., Paradise, June 8-15 [no year given], EDC 395, USNM 107207 12 phallus, data same as previous 13 paratype female, USA, Arizona, Redington, [no collection date on label], EDC 398, USNM 107089 14 Penthesilea sacculalis baboquivariensis holotype male, USA, Arizona, Pima Co., Baboquivari Mts., 15–30 Aug 1923, O.C. Poling, Coll., Barnes Collection, EDC 489, USNM 107019 15 phallus, data same as previous 16 allotype female, USA, Arizona, Pima Co., Baboquivari Mts., [days crossed out] Aug 1924, O.C. Poling, Coll., Barnes Collection, EDC 138, USNM 100018.
http://zoobank.org/8B654928-5F29-4939-A494-E15FB480B90A
http://species-id.net/wiki/Paragalasa_exospinalis
Figs 3, 11–13Alar expanse. 19 to 22 mm.
Head. Labial palpus ochreous, darker laterad; frons, vertex, and occiput ochreous to tan; antenna ochreous.
Upper surface pale reddish-brown, lower surface reddish-brown. Forewing pale reddish-ochreous; costa irrorated with fuscous, especially at base and at origin of antemedial and postmedial lines; antemedial line light reddish-brown, indistinct, extending from about one-third length of costa to about one-third length of inner margin; postmedial light reddish-brown and extending from about two-thirds length of costa sharply excurved to about two-thirds length of inner margin. Hind wing light pinkish to brownish-white with terminal line darker; fringe ochreous to brownish-ochreous. Legs ochreous, sprinkled with dark brown laterad, midtibia scale tufts fuscous.
Abdomen. Upper surface greyish-ochreous, fuscous laterad, lower surface ochreous.
Genitalia. As described for the genus.
All the type specimens in the USNM. The male holotype is from Paradise, Cochise Co., Arizona, June 8–15 and is labeled as the holotype. Twenty-six male and thirteen female paratypes from UNITED STATES: ARIZONA are labeled as follows: UNITED STATES: ARIZONA: eighteen males and two females, Baboquivari Mts., Pima Co., Ariz., elevation approximately 5000 ft., 15–30 June, 1923, O. C. Poling Coll.; four males, Brown’s Canyon, Baboquivari Mts., Pima Co., Ariz., elevation approximately 5000 ft., 1–15 June 1923, O. C. Poling Coll.; one female, Brown’s Canyon, Baboquivari Mts., Pima Co., Ariz., elevation approximately 5000 ft., 15–30 May 1923, O. C. Poling Coll.; two females, Huachuca Mts., Ariz., no date given; three males and three females, Palmerlee, Arizona, no date given; one male, Paradise, Cochise Co., Ariz., June 8–15; one male, Paradise. Cochise Co., Ariz., July; one male and two females, Redington, Ariz., no date given; one female, Santa Rita Mts., Ariz., June 11, 1898. B. A. Schwarz.
Unknown.
This species might be confused with Negalasa rubralis at first glance. Distinguishing characters are the fuscous antemedial and postmedial lines on the costa, generally lighter coloration, and the longer, more narrow forewings. The distinctness of the median band is variable.
http://species-id.net/wiki/Penthesilea
Figs 4, 14–16Penthesilea sacculalis Ragonot, by monotypy.
Head. Labial palpus decumbent; length of male palpus nearly equal to head width, length of female palpus longer than head width; maxillary palpus vestigial; proboscis moderately well-developed; frons rounded with vestiture extended obliquely; vertex smooth-scaled; occiput rough-scaled; eye large; ocellus separated from base of antenna by scales; without a chaetosema.
Thorax. Forewing broad and arched at base, apex broadly rounded, outer margin and anal angle broadly rounded; sexually dimorphic; male with a tympanic vesicle at base of costa, with a large hair-pencil gland as in Salobrena; female without a glandular vesicle; both sexes with Sc long, intercepting costa past one-half length; R1 and R2 separate; R3 and R4 stalked, R5 from stem; M1 from end of discal cell just below anterior angle; M2 and M3 separate and arising from posterior angle of discal cell; Cu1 and Cu2 separate and arising from below posterior angle of cell; 1A absent, 2A and 3A separate at base but briefly anastomosed a short distance distad; retinaculum of male loop-shaped and strongly developed with inner surface corrugated as in Salobrena, Clydonopteron, Satole and Tosale. Hind wing of male with frenulum stoutly developed with a short hook at base, female normal; Sc arched at base and anastomosed with Rs past end of discal cell; M1 arising from anterior angle of discal cell; M2 and M3 separate, arising from the posterior angle of discal cell; Cu1 and Cu2 separate, from before the posterior angle of the discal cell. Legs with scale tufts on mid and hind tibia.
Abdomen. Short and stout; male with a small lateral pleurite on the terminal segment bearing a tuft of scales as in Tosale and Salobrena.
Male genitalia. Uncus narrow, dorsally setose, aculeate with apex rounded, base with arms produced for articulation with gnathos, tips broadly rounded; vinculum narrow, saccus not produced anteriad; gnathos apex aculeate, gently curved dorsad, arms gradually expanded to broad articulation with modified pedunculus and base of uncus; valva narrow, tips directed slightly upward and mediad, sacculus without a clasping process; transtilla moderately developed and incomplete; juxta trapezoidal, dorsal margin concave; phallus slightly curved upward, proximal end slightly expanded, coecum small, without a cornutus.
Female genitalia. Ovipositor moderately short, apex of papillae anales unilobate; anterior apophysis slightly longer than posterior apophysis; lamella postvaginalis triangulate; anterior margin of eighth tergite rounded; ostium bursae membranous; a sclerotized constriction below antrum on ductus seminalis as in Tosale; inception of ductus seminalis at junction of ductus bursae and corpus bursae; corpus bursae without a signum.
The genera Penthesilea and Tosale are closely related. The female genitalia have a membranous ostium bursae, a short sclerotized constriction on the ductus bursae, and the inception of the ductus seminalis at the junction of the ductus bursae and ostium bursae are common to both genera. Tosale differs in having the anterior margin of the eighth tergite heavily sclerotized. The male genitalia show more divergence. The uncus and valva of Penthesilea are more narrow than in Tosale and the saccus is not produced. Both genera have small lateral pleurites on the hind margin of the last abdominal segment of the male for support of lateral scale tufts. The venation indicates the Tosale is more specialized, with stalking of R2, R3, R4, and R5 in the forewing. The forewing of Penthesilea has R2 free with R3 and R4 stalked and R5 short-stalked. Both genera have M1 widely separately from the stem of R5.
http://species-id.net/wiki/Penthesilea_sacculalis_sacculalis
Alar expanse. 13 to 16 mm.
Head. Labial palpus dark brown with black; frons and vertex dark brown with white around the base of scape; occiput reddish-brown.
Thorax. Brown dorsad and ventrad. Forewing dark brown to fuscus; basal angle occasionally overscaled with reddish-brown, base darker than distal part; antemedial line white and slightly excurved; a yellow suffusion distad of white antemedial line; postmedial line white with a large brownish-orange suffusion near the costa, acutely excurved mediad; fringe fuscous. Hind wing dark brown to fuscous; Cu2 with a small white spot near outer margin, a small reddish-brown dash along Cu2 anteriad and posteriad to spot; fringe fuscous. Legs dark brown to fuscous; midtarsi white, hind tarsi white except first subsegment fuscous.
Abdomen. Brown overscaled with fuscous and reddish-brown, lateral tufts fuscous.
Genitalia. As described for genus.
One male holotype, with no locality data is in the Museum National D’Histoire Naturalle in Paris.
Six males and eleven females from the following localities:
UNITED STATES: FLORIDA: Coconut Grove (USNM); Lake Placid, Archbold Bio. Sta., May (USNM); Royal Palm State Park (USNM); Winer Park [Winter Park?] (AMNH). GEORGIA: Atlanta (USNM). LOUISIANA: Lafayette, June (AMNH). NORTH CAROLINA: Southern Pines, July, Aug. (USNM). TEXAS: Brownsville (USNM); San Benito, July, Sept. (USNM). VIRGINIA: Skyland, July (USNM).
Life history. Unknown.
http://species-id.net/wiki/Penthesilea_sacculalis_baboquivariensis
Figs 4, 14–16Alar expanse. 13 to 17 mm.
Head. Labial palpus dark pinkish-brown; frons, vertex, occiput, and antenna pinkish-brown.
Thorax. Upper and lower surfaces pinkish-brown. Forewing same as the nominate species except pinkish-brown. Hind wing pinkish-brown occasionally with a small white spot as in Penthesilea sacculalis, but with no dark scaling anteriad or posteriad. Legs dark pinkish-brown with mid and hind tarsi white.
Abdomen. Pinkish-brown with terminal scale tufts pinkish-brown.
Genitalia. As described for the genus.
The holotype, allotype, and forty-four paratypes are from the Baboquivari Mts., Pima Co., Arizona. The male holotype and female allotype are in the USNM. The male holotype is from Baboquivari Mts., Pima Co., Arizona, August 15-30, 1923, O. C. Poling, is labeled as the holotype, and the female allotype is labeled, August, 1924, O. C. Poling. The paratypes are labeled as follows: UNITED STATES: ARIZONA: two males and two females, elevation approximately 5000 ft., June 15-30, 1923 (USNM); one female, July 1–15, 1924, O. C. Poling (USNM); one female, July 15–30, 1924, O. C. Poling (USNM); two females, Aug. 1–15, 1924, O. C. Poling (USNM); one male and four females, Aug. 15–30, 1923, O. C. Poling (USNM); one male Aug., (USNM); three males and ten females, Sept. 1-15, 1923, 1924; O. C. Poling (USNM); one male and one female, Sept. 15–30, 1924, O. C. Poling (USNM); one male and four females, Oct. 1–15, 1923, O. C. Poling (USNM); one male and one female, Oct. 15–30, 1924, O. C. Poling (USNM); three males and two females, Sabino Canyon, Sept. 5-6, 1951, L. M. Martin (CNC); one male and one female, Sabino Canyon, Sept. 5, 1951, R. J. Ford (CNC).
Unknown.
This subspecies differs from the nominal species only by the pinkish-brown coloration.
We thank Gary Ouellette, Terry Nuhn and Mark Metz, SEL, USDA for technical support to accomplish this project. Chris Thompson, retired, and Mark Metz, SEL, USDA, provided invaluable support with respect to the nomenclatural rules and provided suggestions for the manuscript. Paul Goldstein (University of Maryland), John Brown (SEL, USDA) and pyraloidologists Matthias Nuss, Museum für Tierkunde, Dresden, Germany, and James Hayden, Florida State Collection of Arthropods, Gainesville, Florida, provided suggestions that greatly improved the clarity of the manuscript.