(C) 2013 Sergey G. Ermilov. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Ermilov SG, Tolstikov AV, Mary N, Schatz H (2013) Oribatid mites (Acari, Oribatida) from riverine environments of some islands in Oceania. ZooKeys 318: 47–57. doi: 10.3897/zookeys.318.5971
A checklist of identified oribatid mite taxa from riverine freshwater environments from six islands in Polynesia (New Caledonia, Tahiti, Moorea, Rurutu, Tubuai, Raiatea) is presented; 18 species, 16 genera and eight families were recorded. Trhypochthoniellus longisetus (Berlese, 1904) and Trimalaconothrus albulus Hammer, 1972 prevailed on distribution. Fortuynia smiti sp. n. (Fortuyniidae) is described from New Caledonia. The new speciesis morphologically most similar to Fortuynia marina Hammen, 1960 from New Guinea, but it differs from the latter by the longer notogastral setae dm, lm, c2, p1, epimeral setae 3b and adanal setae ad1 and the presence of prodorsal lateral ridges.
Oribatida, riverine environment, checklist, new species, Fortuynia, Oceania
At present, the fauna of oribatid mites (Acari: Oribatida) of the Oceania islands (Australian region) is studied insufficiently (for example:
Our research is based on total oribatid mite material, which was collected by Nathalie Mary and Harry Smit from rivers of six islands of the Pacific region: New Caledonia (Melanesia), Tahiti, Moorea, Rurutu, Tubuai, Raiatea (all Polynesia). The primary purpose of this paper is to present a checklist of identified taxa.
In the course of taxonomic identification we found a new species, belonging to the genus Fortuynia Hammen, 1960 (Ameronothroidea, Fortuyniidae). The secondary purpose of the paper is to describe and illustrate this species under the name Fortuynia smiti sp. n. The genus Fortuynia is proposed by
The oribatid mite material was collected by Harry Smit and Nathalie Mary from several Pacific Islands. Smit’s oribatid mite material: all samples are water samples, made with a dip net. Mary’s oribatid mite material: all samples were taken with a surber net when sampled the benthos of the rivers and streams.
Melanesia: New Caledonia
01: Marais de la Rivière Blanche, Parc de la Rivière Bleue, 26.IX.2000, collected by H. Smit.
02: Koné Rivière, 10 km east of Koné, 01.X.2000, collected by H. Smit.
Polynesia – Society Islands: Tahiti
03: Papenoo River, 25.VI.2007, collected by N. Mary.
04: Vahiria River, 26.VI.2007, collected by N. Mary.
05: Vaitepiha River, 27.VI.2007, collected by N. Mary.
Polynesia – Society Islands: Moorea
06 Opunohu River, 24.VI.2007, collected by N. Mary.
07: Vaihana River, 07.VII.2007, collected by N. Mary.
08: Vaipapa River, 08.VII.2007, collected by N. Mary.
09: Paopao River, 09.VII.2007, collected by N. Mary.
Polynesia – Austral Islands: Rurutu
10: Vairee River, 30.VI.2007, collected by N. Mary.
11: Te Vaavai River, 01.VII.2007, collected by N. Mary.
12: Vaipapa River, 01.VII.2007, collected by N. Mary.
13: Peva Iti River, 02.VII.2007, collected by N. Mary.
Polynesia – Austral Islands: Tubuai
14: Vaitoaha River, 04.VII.2007, collected by N. Mary.
15: Matarahu River, 04.VII.2007, collected by N. Mary.
16: Hautara River, 05.VII.2007, collected by N. Mary.
17: Taahuaia River, 05.VII.2007, collected by N. Mary.
18: Vaiapu River, 05.VII.2007, collected by N. Mary.
Polynesia – Society Islands: Raiatea
19: Vaiatarau River, 11.VII.2007, collected by N. Mary.
20: Apoomau River, 11.VII.2007, collected by N. Mary.
21: Vaimariri River, 12.VII.2007, collected by N. Mary.
All specimens were studied in lactic acid, mounted in temporary cavity slides for the duration of the study, and then stored in 70% alcohol in vials. Body measurements are presented in micrometers. The body length was measured in lateral view, from the tip of the rostrum to the posterior edge of the ventral plate. Notogastral width refers to the maximum width in dorsal aspect. Lengths of body setae were measured in lateral aspect. Formula for leg setation are given in parentheses according to the sequence trochanter–femur–genu–tibia–tarsus (famulus included). Formula for leg solenidia are given in square brackets according to the sequence genu–tibia–tarsus. Terminology used in this paper mostly follows that summarized by
We recorded 18 species belonging to 16 genera and eight families. Ceratozetes hamobatoides Hammer, 1967 is a new record for Oceania (previously known from New Zealand), all other taxa were recorded in Oceania previously. Trhypochthoniellus longisetus (Berlese, 1904) and Trimalaconothrus albulus Hammer, 1972 prevailed on distribution (found in 19 localities on six and five islands, respectively). Also, Hydrozetes lemnae (Coggi, 1897) is registered from 11 localities (on five islands), Scheloribates praeincisus (Berlese, 1910) in six localities (on three islands). The majority of species (13 from 18) were found in 1–3 localities (Table 1). Comparing a previous investigation of oribatid mites in freshwater on Pacific islands (
List and distributions of identified taxa from the Oceania islands (distribution data from
| Taxa | Distributions of species | NC | Ta | Mo | Ru | Tu | Ra | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | ||
| Lohmanniidae | ||||||||||||||||||||||
| Ozacarus tahitiensis (Hammer, 1972) | Oceania – Polynesia (Tahiti, Moorea) | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Trhypochthoniidae | ||||||||||||||||||||||
| Archegozetes magnus (Sellnick, 1925) | Afrotropic, Neotropic, Indo-Malay, Australasia (New Guinea), Oceania – Polynesia (Tonga, Eua, Moorea) | - | - | - | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Trhypochthoniellus longisetus (Berlese, 1904) | Holarctic, Afrotropic, Neotropic, Indo-Malay, Australasia (Australia, New Zealand), Oceania – Melanesia (New Caledonia), Polynesia (Hawaii, Samoa, Tahiti, Moorea, Rurutu, Tubuai, Raiatea) | - | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + |
| Malaconothridae | ||||||||||||||||||||||
| Trimalaconothrus albulus Hammer, 1972 | Indo-Malay (Taiwan). Oceania – Polynesia (Tahiti) | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Nanhermanniidae | ||||||||||||||||||||||
| Masthermannia sp. | – | - | - | - | - | + | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | + |
| Hermanniidae | ||||||||||||||||||||||
| Phyllhermannia pacifica (Hammer, 1972) | Oceania – Polynesia (Tongatapu, Eua, Tahiti, Rangiroa, Tubuai) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - |
| Hydrozetidae | ||||||||||||||||||||||
| Hydrozetes lemnae (Coggi, 1897) | Palaearctic, Afrotropic, Neotropic (also Galapagos islands), Indo-Malay (Indonesia, Philippines), Australasia (Australia, New Zealand), Oceania – Melanesia (New Caledonia), Polynesia (Fiji, Upolu, Tahiti, Moorea, Rurutu, Tubuai, Raiatea, Easter Island) | + | - | + | + | + | + | - | + | + | - | - | - | - | - | - | + | + | + | - | - | + |
| Fortuyniidae | ||||||||||||||||||||||
| Fortuynia smiti sp. n. | Oceania – Melanesia (New Caledonia) | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Ceratozetidae | ||||||||||||||||||||||
| Ceratozetes hamobatoides Hammer, 1967 | Australasia (New Zealand), Oceania – Melanesia (New Caledonia) | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Humerobatidae | ||||||||||||||||||||||
| Humerobates rostrolamellatus Grandjean, 1936 | Holarctic, Afrotropic, Neotropic, Indo-Malay (Okinawa), Oceania – Polynesia (Eua, Moorea, Hawaii) | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Scheloribatidae | ||||||||||||||||||||||
| Nasozetes stunkardi Sengbusch, 1957 | Indo-Malay (Philippines), Oceania – Micronesia (Guam), Polynesia (Fiji, Raiatea) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - |
| Tuberemaeus indentatus (Hammer, 1973) | Oceania - Polynesia (Upolu, Raiatea) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - |
| Scheloribates praeincisus (Berlese, 1910) | Neotropic (Panama, Brazil, Galapagos islands) Indo-Malay (India, Indonesia, Philippines). Oceania – Polynesia (Fiji, Tonga, Eua, Upolu, Tahiti, Moorea, Borabora, Rangiroa, Rurutu, Raiatea) | - | - | - | - | - | - | - | + | - | + | - | - | + | - | - | - | - | - | + | + | + |
| Scheloribates tubuaiensis Sellnick, 1959 | Palaearctic (Caucasus), Oceania – Polynesia (Tongatapu, Upolu, Moorea, Tubuai, Rurutu) | - | - | - | - | - | - | + | + | + | + | - | - | - | - | - | + | - | - | - | - | - |
| Oripodidae | ||||||||||||||||||||||
| Benoibates marginatus (Hammer, 1973) | Oceania – Polynesia (Eua, Raiatea) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + |
| Haplozetidae | ||||||||||||||||||||||
| Protoribates bipilus (Hammer, 1972) | Oceania – Polynesia (Tongatapu. Tahiti, Rurutu, Tubuai) | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | + | - | - | - | - |
| Galumnidae | ||||||||||||||||||||||
| Galumna euaensis Hammer, 1973 | Oceania – Polynesia (Eua, Moorea, Raiatea) | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - | + | - |
| Galumna valida Aoki, 1994 | Oceania – Micronesia (Marianas: Maug islands), Polynesia (Moorea) | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - | - | - |
urn:lsid:zoobank.org:act:7400FB31-6262-4525-A1A5-AD92C8696501
http://species-id.net/wiki/Fortuynia_smiti
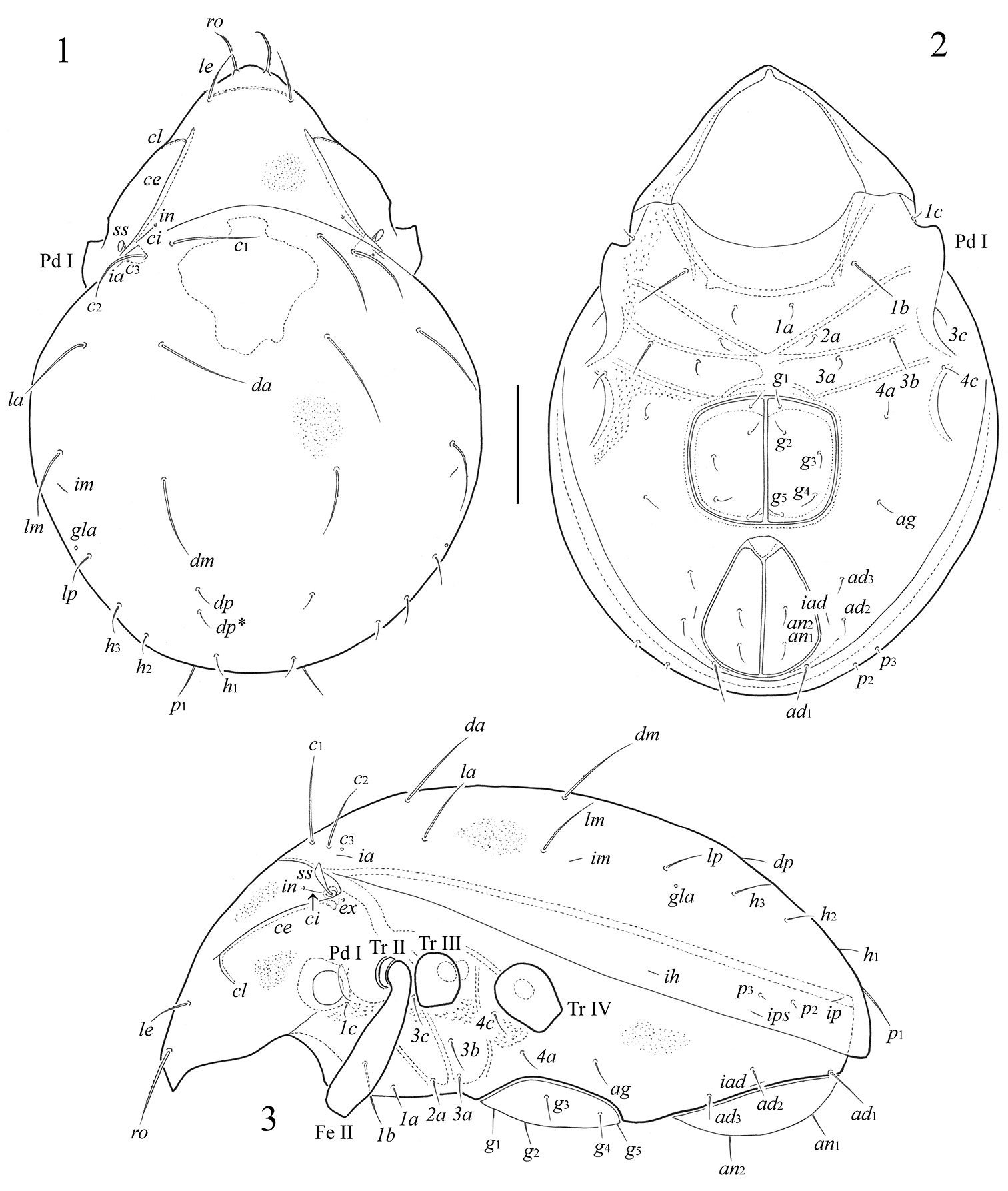
Figs 1 – 13Body size 564–614 × 381–431. Body surface microfoveolate. Lamellar lines, internal and lateral ridges developed. Rostral setae weakly thickened, with short cilia; lamellar setae thin, slightly barbed. Interlamellar and exobothridial setae minute. Sensilli short, clavate, smooth. Notogaster with 14 pairs of setae and one pair of setal alveoli (c3). Length of setae c1, da > c2, dm, la, lm > p1 > lp, h3 > dp, h1, h2 > p1, p2. Adanal setae ad1 longer than other adanal setae.
Male. Measurements. Body length 581 (holotype, male), 564–614 (six paratypes, all males); body width 398 (holotype), 381–431 (six paratypes, all males).
Integument. Body color brown to yellow-brownish. Body surface microfoveolate (clearly visible under high magnification, ×1000). Lateral podosomal regions with tuberculate cerotegument (diameter of tubercles up to 6).
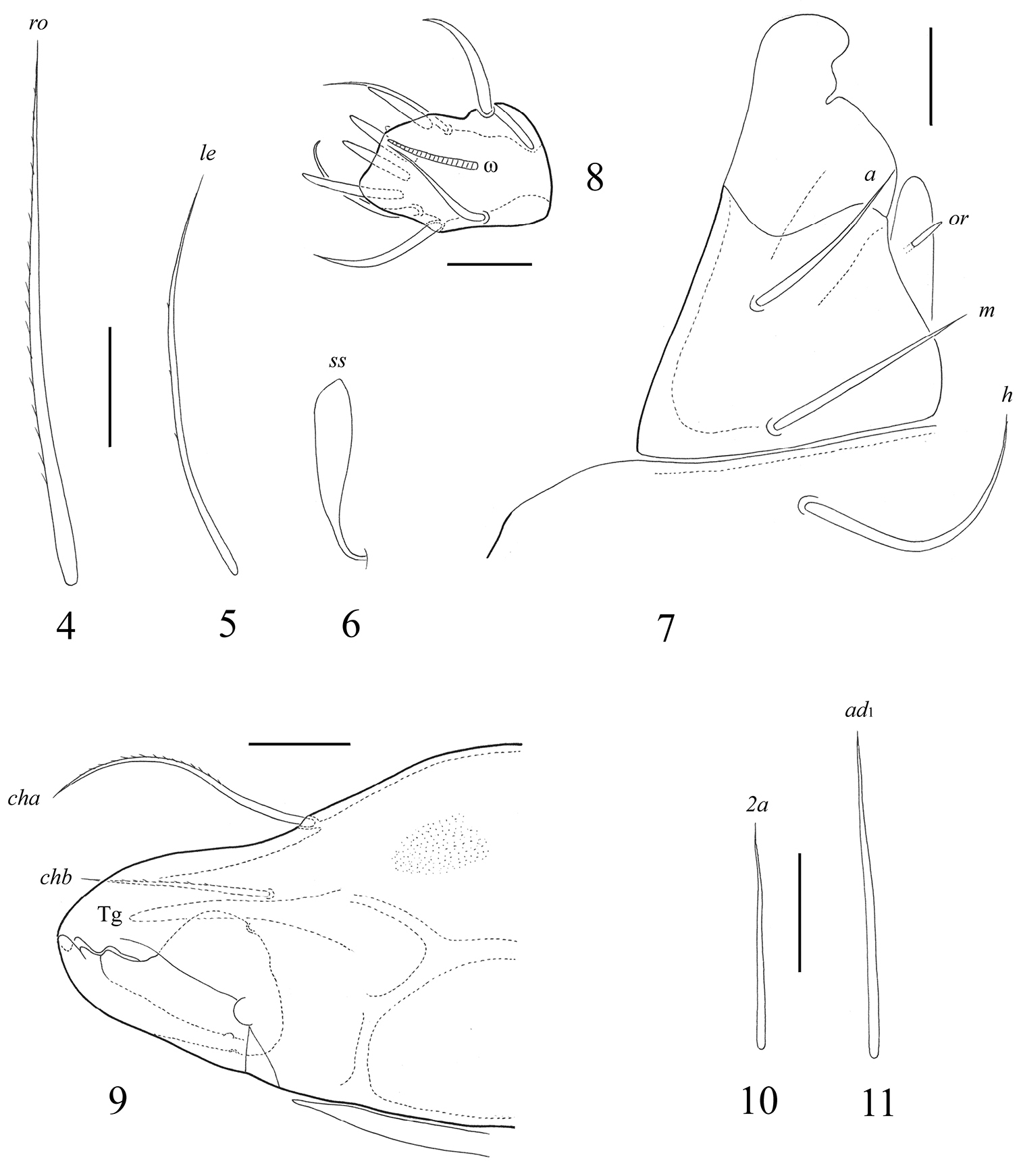
Prodorsum. Rostrum rounded. Lamellar lines (ce) strong, equal to half of prodorsum. Internal ridges (ci) present, very thin, reaching insertions of interlamellar setae. Anterior part of lamellar lines with short lateral ridges (cl), which are located perpendicularly to them. Rostral setae (ro, 69–82) setiform, weakly thickened, with short cilia, set on small tubercles. Lamellar setae (le, 41–45) setiform, thin, slightly barbed. Interlamellar (in) and exobothridial (ex) setae minute (1), poorly visible. Sensilli (ss, 32–36) curved backwards, with short stalk and longer clavate, smooth head.
Notogaster. Lenticulus present, with amorphic borders. Notogastral region with 14 pairs of setiform, smooth notogastral setae and one pair of setal alveoli (c3). Setae c1, da (90–102) longer than c2, dm, la, lm (69–82), p1 (30–36), lp, h3 (28–32), dp, h1, h2 (20–24); p1, p2 shortest (4–6). Lyrifissures ia, im, ip, ih and ips and opisthonotal gland openings (gla) distinct, located typically for the genus.
Gnathosoma. Subcapitulum longer than wide (118–205 × 143–151). Subcapitular setae setiform, smooth; h (82–86) longer than a and m (both 53–57). Lips only with one spiniform seta (or, 10–12). Palps (123–131) with setation 0–2–1–3–9(+ω). All setae smooth. Solenidion weakly thickened, blunt-ended, pressed to surface of tarsus, not attached with eupathidium. Chelicerae (188–205) with two barbed setae; cha (69–73) longer than chb (45–49). Trägårdh’s organ (Tg) long, conical.
Epimeral and lateral podosomal regions. Apodemes 1, 2, 3 and sejugal well developed. Apodemes 2 and sejugal fused medially. Epimeral setal formula 3–1–3–2; setae setiform, smooth. Setae 1b (49–61) longer than 3b (41–49), 1a, 2a, 3a, 3c, 4a, 4c (24–36); 1c shortest (16–20). Pedotecta I (Pd I) of medium size, concave.
Anogenital region. Anogenital setae setiform, thin, smooth. Five pairs of genital (g1–g5), one pair of aggenital (ag), two pairs of anal (an1, an2) and the anterior two pairs of adanal (ad2, ad3) setae similar in length (28–32); only the first pair of adanal setae ad1 longer (41–53). Lyrifissures iad located in paraanal position.
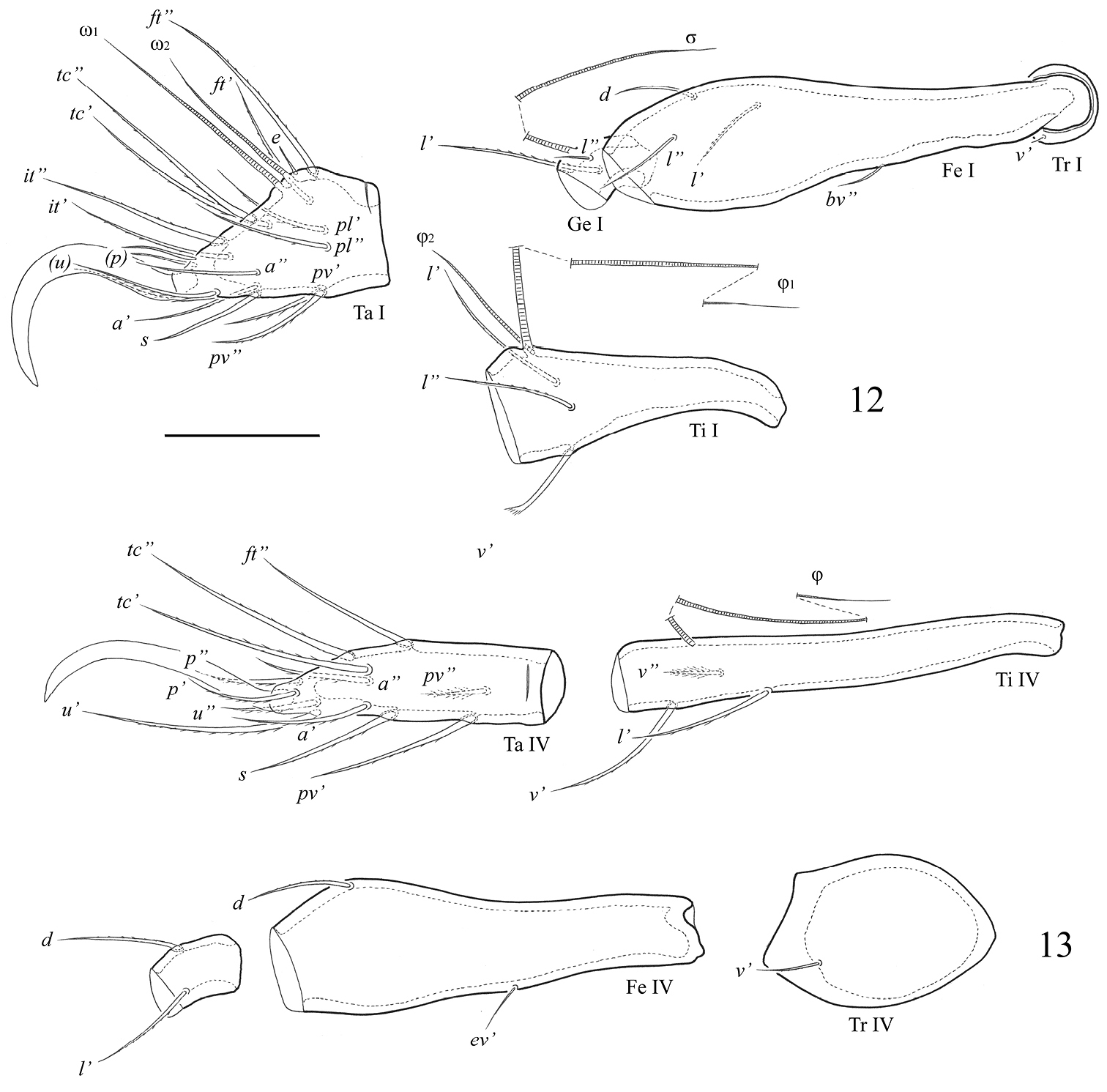
Legs. Claw of each leg large, smooth. Porose areas developed typically for the genus (
Leg setation and solenidia of adult Fortuynia smiti sp. n.
| Leg | Trochanter | Femur | Genu | Tibia | Tarsus |
| I | v’ | d, (l), bv’’ | (l), σ | (l), v’, φ1, φ2 | (ft), (tc), (it), (p), (u), (a), s, (pv), (pl), e, ω1, ω2 |
| II | v’ | d, (l), bv’’ | (l), σ | (l), v’, φ | (ft), (tc), (it), (p), (u), (a), s, (pv), ω1, ω2 |
| III | l’, v’ | d, l’, ev’ | l’, σ | l’, (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv) |
| IV | v’ | d, ev’ | d, l’ | l’, (v), φ | ft’’, (tc), (p), (u), (a), s, (pv) |
Roman letters refer to normal setae (e to famulus), Greek letters to solenidia. Single prime (’) marks setae on anterior and double prime (’’) setae on posterior side of the given leg segment. Parentheses refer to a pseudosymmetrical pair of setae.
Fortuynia smiti sp. n., adult: 1 dorsal view 2 ventral view (gnathosoma and legs not illustrated) 3 lateral view (gnathosoma and legs except femur II and trochanters II–IV not illustrated). Scale bar 100 μm.
. Fortuynia smiti sp. n., adult: 4 rostral seta 5 lamellar seta 6 sensillus 7 subcapitulum, right half of anterior part, ventro-lateral view 8 palptarsus 9 chelicera, anterior part 10 epimeral seta 2a 11 adanal setae ad1. Scale bar (4–7, 9–11) 20 μm, (8) 10 μm.
. Fortuynia smiti sp. n., adult: 12 segments of leg I, left, antiaxial view 13 segments of leg IV, right, antiaxial view. Scale bar 50 μm.
Holotype (male) and six paratypes (all males): Locality 02.
The holotype (in alcohol) is deposited in the collection of the Zoological Institute of the Russian Academy of Sciences, St. Petersburg, Russia; two paratypes (in alcohol) are deposited in the collection of the Siberian Zoological Museum, Novosibirsk, Russia; four paratypes (in alcohol) are deposited in the collection of the Tyumen State University Museum of Zoology, Tyumen, Russia.
Etymology. The species is named after our colleague, the renowned acarologist, Dr. Harry Smit (Netherlands Centre for Biodiversity Naturalis, Leiden, The Netherlands), who has collected the specimens of Fortuynia smiti sp. n.
Fortuynia smiti sp. n. is most similar to Fortuynia marina Hammen, 1960 from New Guinea (
We cordially thank Dr. Harry Smit (Netherlands Centre for Biodiversity Naturalis, Leiden, The Netherlands) for collecting of the oribatid mite material from New Caledonia. Open access to this paper was supported by the Encyclopedia of Life (EOL) Open Access Support Project (EOASP).