(C) 2012 Samuel Gómez. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of the genus Nitocra Boeck, 1865, Nitocra taylori sp. n. is described from the St Lucia Estuary, Africa’s largest estuarine lake. It is also suggested that Nitocra sewelli husmanni Kunz, 1976 and Nitocra reducta fluviatilisGalhano, 1968 are granted full species rank as Nitocra husmanni stat. n. Kunz, 1976 and Nitocra fluviatilis stat. n. Galhano, 1968. Nitocra taylori sp. n. appears to be closely related to Nitocra husmanni. Unfortunately, the original description of the micro-characters of the species lacks the detail needed to make reliable comparisons between species of the genus Nitocra. The main differences observed are the number of spinules along the posterior margin of the anal operculum, length ratio of the exopod and endopod of the first swimming leg, shape of the outer spine on the male third endopodal segment of the third swimming leg, number of segments of the male antennule, relative length of the setae on the male baseoendopod of the fifth leg, shape of the male exopod of the fifth leg, relative length of the two setae of the male sixth leg, and shape of the female baseoendopod of the fifth leg. The current distribution of Nitocra taylori sp. n. is limited to the lake part of the estuary, an area which is most severely affected by the current freshwater deprivation crisis. During closed mouth conditions, these regions (South/North Lake and False Bay) are characterized by low water levels, high salinities and high turbidity levels. This suggests that Nitocra taylori sp. n. may favor these environmental conditions and the significant correlations found between the abundance of Nitocra taylori sp. n. and salinity and turbidity confirm this to a degree. Nitocra taylori sp. n. individuals are also able to withstand a wide range of fluctuations. They were recorded at turbidities ranging from 2 to 102 NTU, temperatures from 20.9 to 34.8 ºC and salinity levels ranging from 9.81 to 53.7 psu. However, in the current state of the system, salinity and temperature levels in the northern regions frequently exceed this value. Continued freshwater deprivation may, therefore, further limit the distribution range of this species.
Nitocra, taxonomy, ecology, St Lucia Estuary, South Africa
During the course of routine quarterly monitoring surveys undertaken during the last six years in the St Lucia Estuary, South Africa, several specimens of an unidentified harpacticoid copepod were retrieved from zooplankton samples.The St Lucia Estuary is the largest estuarine lake in Africa and has high priority for conservation as it forms part of South Africa’s first World Heritage Site - iSimangaliso (formerly Greater St Lucia) Wetland Park (
St Lucia characteristically experiences cyclical wet and dry phases, each lasting between four and ten years (
In light of the current crisis, research efforts have been directed at clarifying the biodiversity structure of the ecosystem and the effects of this stress on its functioning. A number of new and potentially endemic taxa have been identified recently from samples collected during routine monitoring surveys in the St Lucia Estuary (
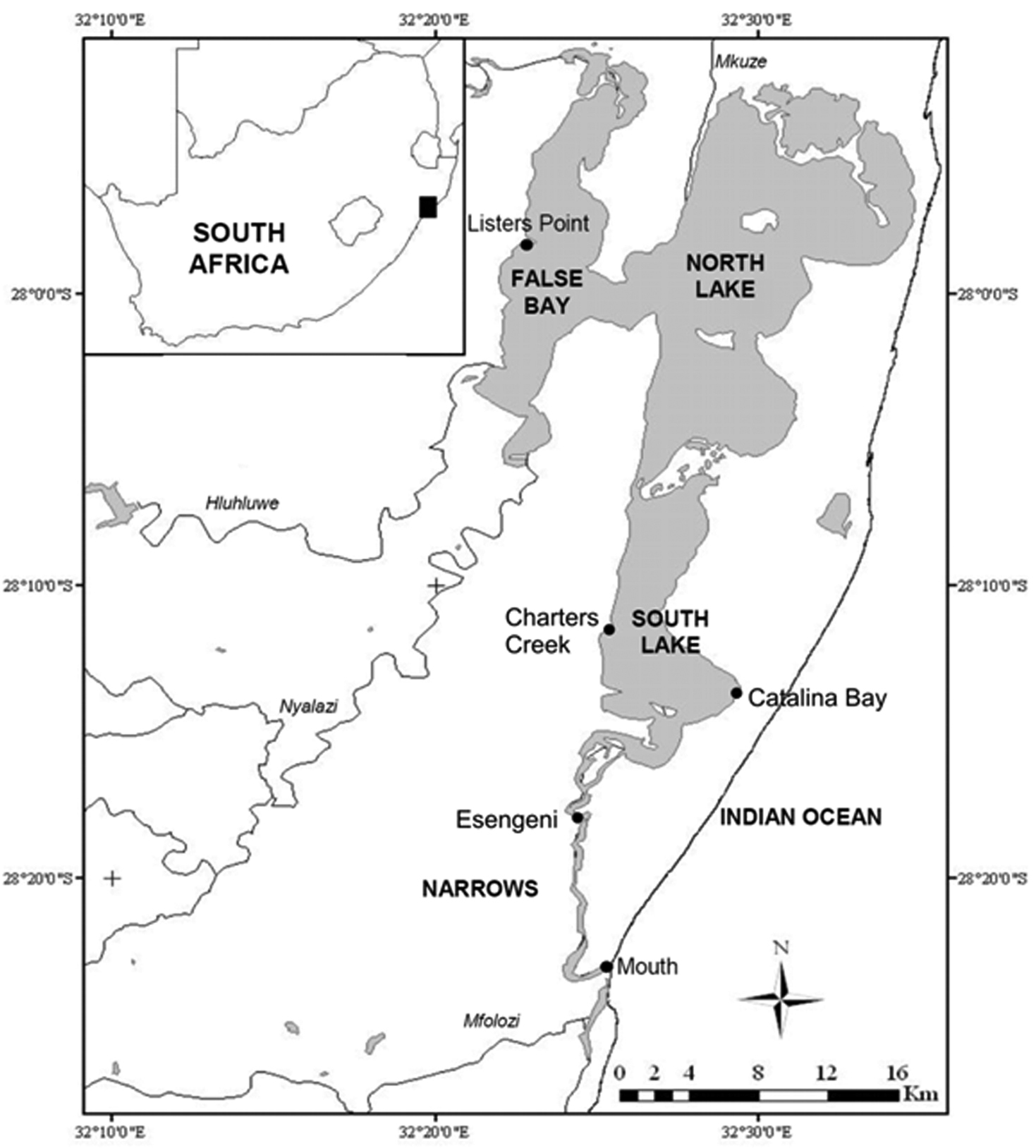
St Lucia Estuary showing the sampling stations and geographic location within South Africa.
Quarterly surveys were undertaken at five representative stations within the St Lucia Estuary, from February 2006 through to May 2011. These stations included the Mouth, Esengeni, Catalina Bay, Charters Creek and Listers Point (Figure 1). The study period covered three different hydrological phases, viz. a closed-mouth phase (February 2006 to February 2007), an open-mouth phase (March 2007 to August 2007), and a re-closed period (November 2007 to May 2011). Zooplankton together with physico-chemical data, were collected at each site on each sampling occasion.
Physico-chemical variablesPhysico-chemical measurements were taken with a YSI 6920 water quality logger, fitted with temperature (˚C), depth (m), conductivity (mS/cm), dissolved oxygen (mg.L-1), pH and turbidity (Nephelometric Turbidity Units or NTUs) probes.
Zooplankton samplingSingle daytime mesozooplankton samples were collected using an epibenthic sled (100 μm mesh). The sled was towed in the shallow waters near-shore at all stations, except at Esengeni, where a boat was used. The mouth of the net was semi-circular in shape (radius = 18.5 cm) and was mounted on a sled that was towed just above the sediment surface. Sampling with this sled, therefore, allowed the suitable collection of epibenthic harpacticoids. In regions where water depth was too shallow, or when zooplankton was too dense for the sled to be used, 30 L of water was passed through a 100 µm sieve. Samples were emptied into 500 mL bottles containing 4% phloxine-stained formaldehyde.
In the laboratory, samples were suspended in 0.5 to 5 L solutions, depending on the density of organisms. The main sample was then stirred vigorously so that all the organisms remained in a homogenous suspension. A 20 mL plastic vial attached to a metal rod was then used to withdraw three subsamples from mid-depth (
Univariate statistical analyses were conducted with SPSS version 19 for Windows, where a Pearson correlation was used to test for relationships between environmental variables and species abundance. This analysis was performed on log-transformed abundance data for all non-zero records.
Species descriptionA number of adult males and females were fixed in 4% formalin and preserved in 70% ethanol for taxonomic analyses and description. Observations and drawings were done at a magnification of 1000 x from whole and dissected specimens mounted in lactophenol with a Leica compound microscope equipped with phase contrast and a drawing tube. The type material was deposited in the collection of the Instituto de Ciencias del Mar y Limnología, Unidad Académica Mazatlán (Mexico) (EMUCOP) and in the collection of the Iziko South African Museum, Cape Town (South Africa) (SAM). The terminology proposed by
urn:lsid:zoobank.org:act:AF4F4062-2DF8-41B5-9089-B62EECAB56B1
http://species-id.net/wiki/Nitocra_taylori
Figures 2–9One female holotype (EMUCOP-080311-03) and one male allotype (EMUCOP-080311-06) preserved in alcohol, one female (EMUCOP-080311-04) and one male (EMUCOP-080311-05) dissected paratypes, and 15 adult females, 11 adult males, one CIII, three CIV and three CV paratypes preserved in alcohol (EMUCOP-080311-07) were deposited in the Copepoda collection of the Instituto de Ciencias del Mar y Limnología, Mazatlan Marine Biological Station; 26 additional paratypes (SAM-A45750) were deposited in the collection of the Iziko South African Museum; collected from Listers Point, St Lucia Estuary, South Africa; 8 March 2011; leg. N. K. Carrasco.
Listers Point, False Bay, St Lucia Estuary, South Africa (27°58'09.4"S, 32°22'48.11"E).
The species is named after Dr Ricky H. Taylor, former Regional Ecologist at Ezemvelo KZN Wildlife, St Lucia Estuary, for his invaluable help provided to research and his lifetime efforts towards the conservation of the St Lucia Estuary. The specific epithet is a noun in the genitive singular.
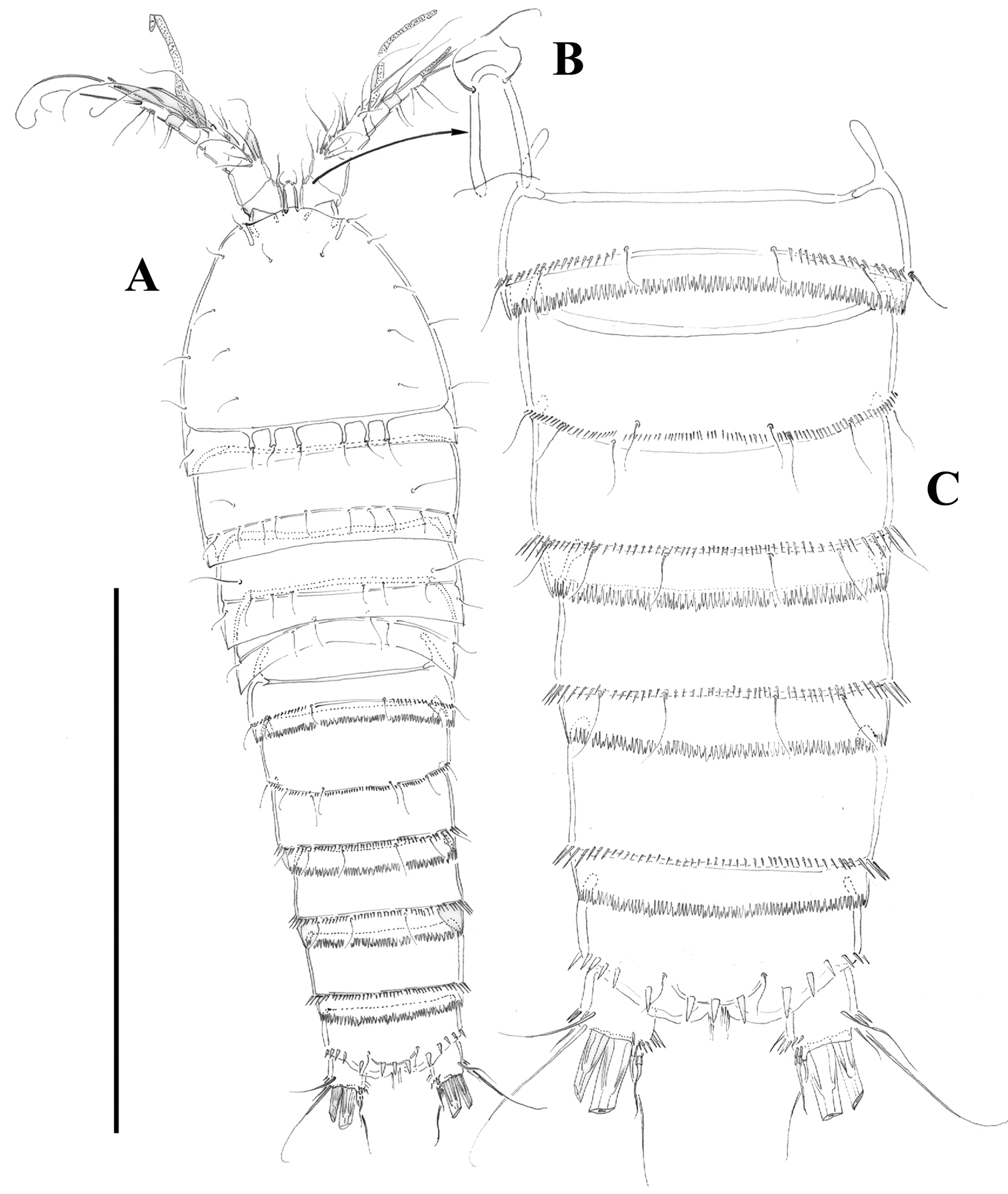
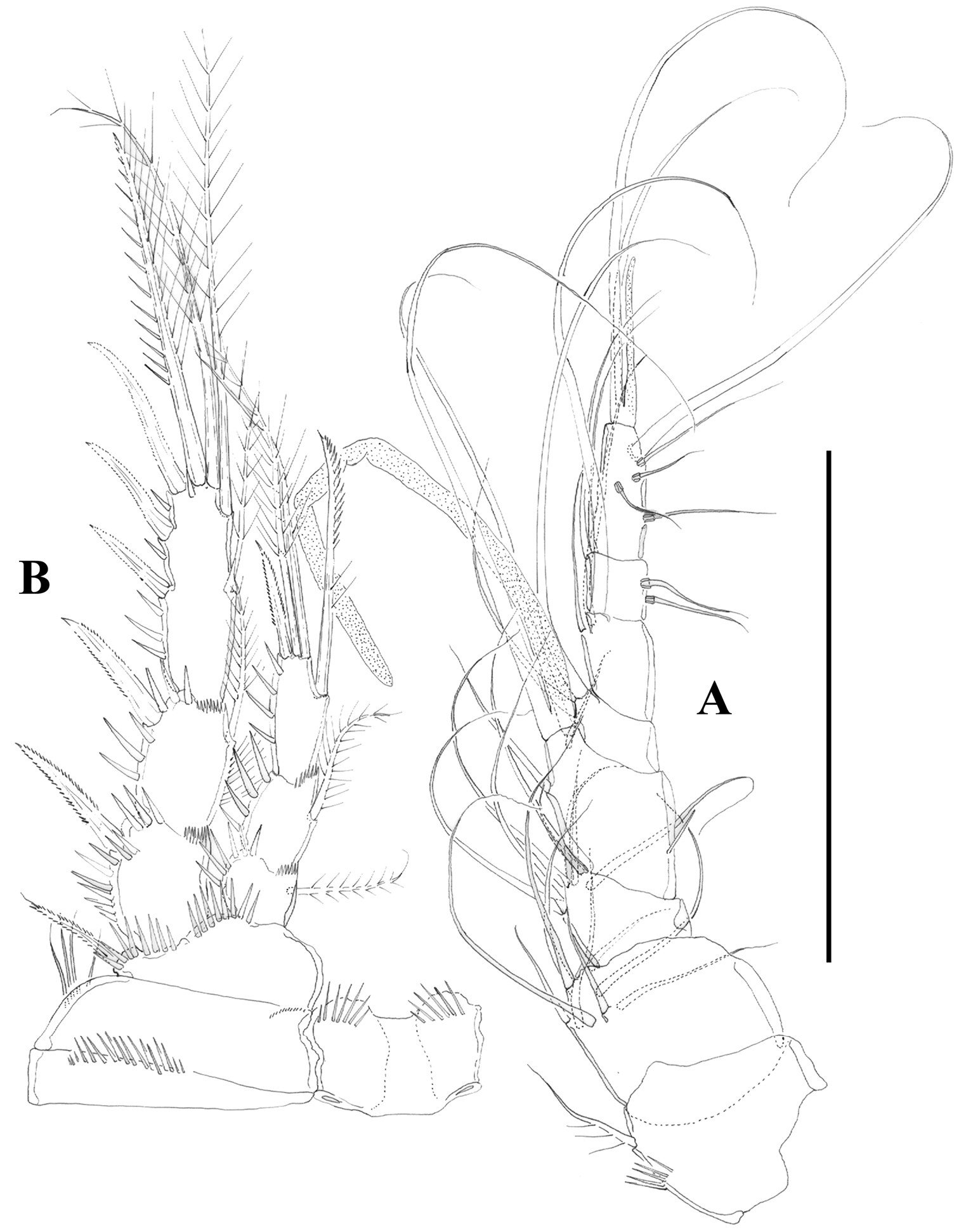
Female.Habitus (Figure 2A) tapering posteriorly; total body length measured from tip of rostrum to posterior margin of caudal rami ranging from 460 to 685 µm (mean, 537 µm; n= 11; holotype, 525 µm). Rostrum (Figure 2A, B) defined at base, elongate, small, barely reaching distal margin of first antennulary segment, with pair of sensilla subapically. Dorsal surface of cephalic shield and free prosomites without spinular ornamentation, with plain caudal frill (Figure 2A). P5-bearing somite with medially interrupted row of minute spinules close to posterior margin dorsolaterally, with deeply serrate caudal frill (Figure 2A, C). Subcuticular rib of genital double-somite with dorsolateral row of small spinules indicating former division between second and third urosomites (Figure 2A, C), but completely fused ventrally (Figs 3B, 10F); third urosomite with comparatively stronger spinules close to posterior margin dorsally and laterally, with median row of minute spinules ventrally, with deeply serrate caudal frill. Fourth and fifth urosomites as previous somite dorsally and ventrally, except for less and lack of sensilla in fourth and fifth urosomites, respectively. Anal somite somewhat shorter than previous somite, with strong spinules dorsally and laterally close to joint with caudal rami (Figs 2A, C, 3A), with comparatively smaller spinules ventrally (Figs 3B, C, 10F); rounded anal operculum with three strong spinules close to posterior margin, and flanked by pair of sensilla (Figs 2C, 3A). Caudal rami nearly as long as wide from dorsal view, but slightly longer than wide ventrally (Figs 2C, 3A, B, C, 10B), with seven setae as follows: seta I small, nearly as long as caudal ramus; seta II dorsal to seta I, about twice as long as the latter; seta III about twice as long as seta II, arising close to outer distal corner; setae IV and V well developed, the latter longest; seta VI arising from inner distal corner, slightly shorter than seta III; seta VII biarticulated, rather short, arising close to base of seta VI at inner distal corner.
Antennule (Figure 5A) eight-segmented, surface of segments smooth except for spinular row on first segment. Armature formula as follows: 1-(1), 2-(9), 3-(8), 4-(3 + [1+ae]), 5-(2), 6-(3); 7-(4); 8-(5+acrothek). Fourth segment with one outer spinule. Acrothek consisting of two setae and one aesthetasc fused at their base.
Antenna (Figure 4A) with small coxa. Allobasis without abexopodal setae, and ornamented with one long spinule and a short row of minute spinules proximally. Free endopodal segment with inner spinules proximally and subdistally, with two lateral inner spines and one slender seta, and four single geniculate setae and one geniculate element fused basally to pinnate seta. Exopod one-segmented; with few spinules, and three setae (two pinnate spine like elements and one bipinnate seta).
Mandible (Figure 4B) robust; gnathobase with bi- and multicuspidate teeth, and one lateral seta. Mandibular palp two-segmented; first segment (basis) with some spinules and one seta; second segment (endopod) with one lateral and four apical setae.
Maxillule (Figure 4C). Arthrite of praecoxa with few spinules, with two surface setae, and three bare spines and two serrate/multicuspidate elements. Coxa with two elements. Basis seemingly with four setae; exopod vestigial represented by one seta; endopod two-segmented, first segment without any setae, second segment with two setae.
Maxilla (Figure 6A). Syncoxa with minute outer spinules; with one endite bearing three setae. Allobasis drawn into strong claw with one accompanying strong element. Endopod one-segmented, with two setae.
Maxilliped (Figure 6B) subchelate. Syncoxa with spinular rows and with one seta on inner distal corner. Basis unarmed, with longitudinal row of spinules, with some outer spinules distally. Endopod drawn into long and slender claw with one accompanying small seta.
P1 (Figure 4D, E). Intercoxal sclerite without spinular ornamentation; distal margin convex. Basis with inner and outer flagellate spine; with strong spinules at base of inner spine, between rami and at base of exopod. Exopod and endopod three-segmented; EXP1 without inner seta, EXP2 with plumose inner seta; EXP3 with one outer proximal bipinnate spine, two outer naked spines and two geniculate elements. Endopod three-segmented; slightly beyond EXP; first segment slightly shorter that second and third segments combined, reaching insertion level of inner seta of EXP2; first segment with inner seta ornamented medially with setules and with spinules along outer margin distally; second segment with plumose inner seta; third segment with one inner plumose seta apically, one median geniculate element, and one apical outer spine. Armature formula as below.
P2 (Figure 5B). Intercoxal sclerite with transverse spinular row distally on both lobes. Praecoxa with spinules close to joint with coxa. The latter with long outer setules and minute spinules close to outer and inner distal corner, respectively. Basis with outer spine; with strong spinules between rami and at base of EXP. Exopod three-segmented; first segment without setae, second segment with plumose inner seta; third segment with three outer bipinnate spines, one outer apical seta ornamented with strong spinules and setules along outer and inner margin, respectively, one inner apical plumose seta and one inner plumose seta. Endopod three-segmented, reaching proximal fourth of EXP3; first and second segments with inner plumose and short seta; third segment with one strong inner seta ornamented with few setules medially and with spinules along outer margin distally, two apical plumose setae and one outer bipinnate spine. Armature formula as below.
P3 (Figure 6C). Intercoxal sclerite, praecoxa and coxa as in P2. Basis as in P2 except for outer seta-like element in P3. Exopod as in P2. Endopod as in P2 except for additional inner element in P3ENP3 ornamented with setules proximally and with spinules along outer margin distally; reaching proximal third of EXP3.
P4 (Figure 7A). Intercoxal sclerite without spinules. Praecoxa (not shown), coxa and basis as in P3. Exopod as in P3 except for comparatively stronger inner distal seta of P4EXP3 ornamented with outer and inner spinules, and for outer apical seta ornamented with inner setules and outer spinules. Endopod as in P3, except for bipinnate inner proximal seta on P4ENP3; slightly beyond EXP2.
P5 (Figure 7B). Both legs separated. Exopod and baseoendopod not fused. Exopod ovate; with inner and outer spinules; with six elements; relative length of the setae from inner to outer element as follows: 0.73, 1, 0.38, 0.69, 0.38, 0.25. Endopodal lobe with five setae/spines; relative length of the setae from inner to outer element as follows: 0.30, 0.31; 0.30, 1, 0.52; with inner and outer spinules.
Armature formula of female P1-P5 as follows:
P6 (Figure 3D) represented by median plate in anterior half of second urosomite (first genital somite); each vestigial leg represented by one outer short and one inner long seta.
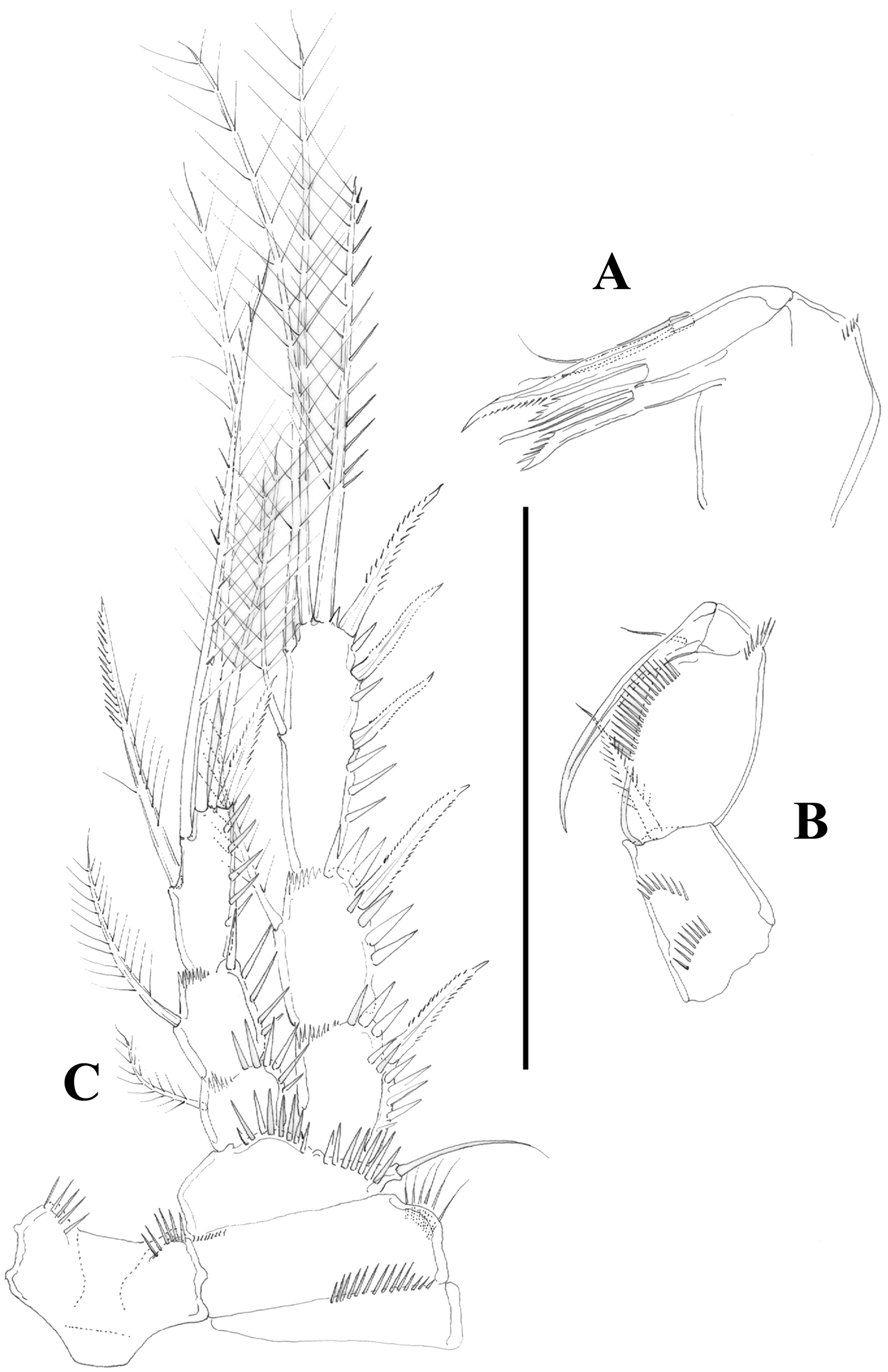
Male. Habitus (not shown) as in female, except for distinct second and third urosomites; total body length measured from tip of rostrum to posterior margin of caudal rami ranging from 385 to 520 µm (mean, 437 µm; n= 7; allotype, 490 µm). Ventral spinular ornamentation of third-sixth urosomites coarser and stronger than in female (Figs 8, 10D). Caudal rami (Figs 8, 10D) as in female.
Sexual dimorphism expressed in the antennule, P1Basis, P3ENP, P5 and P6.
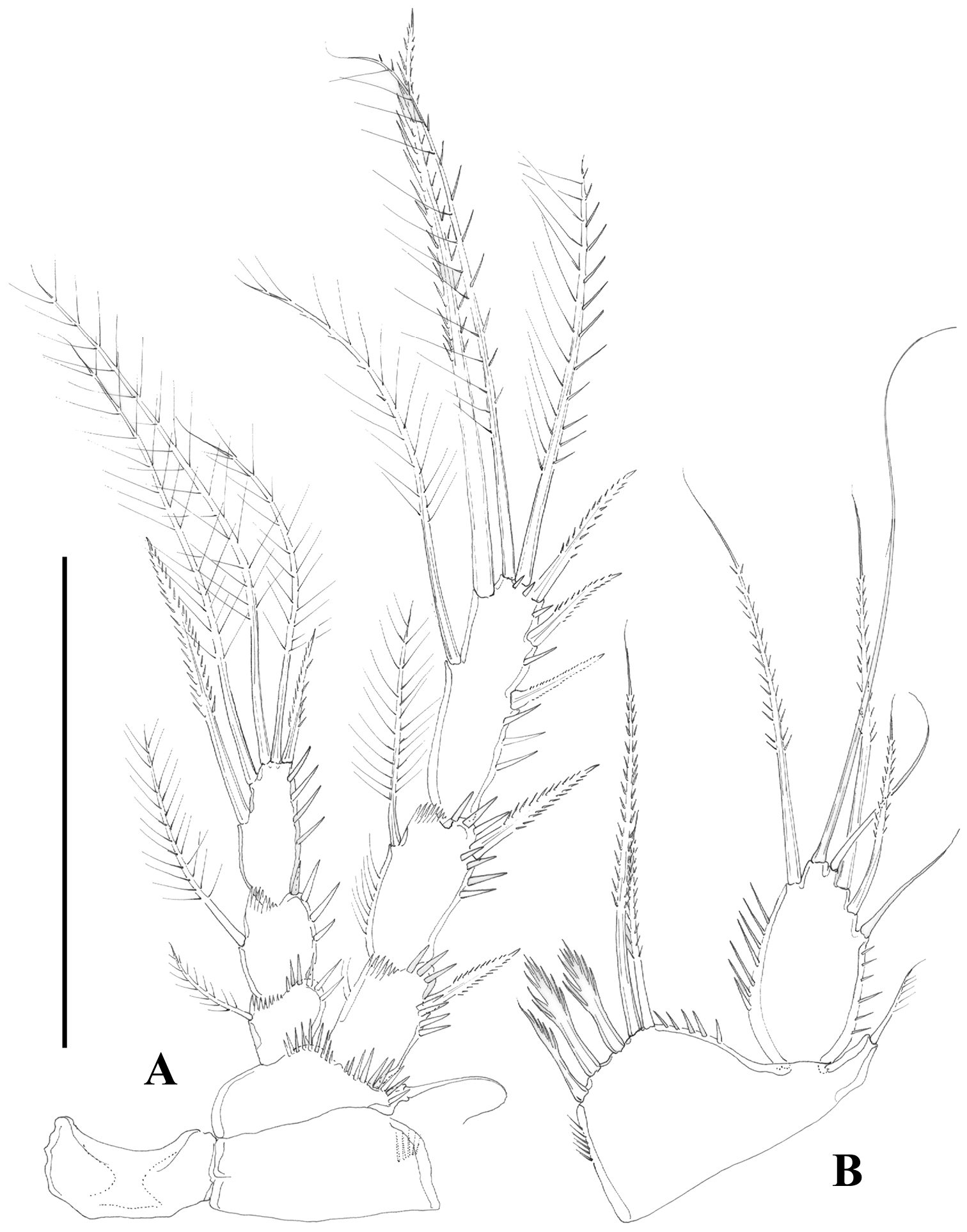
Antennule (Figure 9A) haplocer, nine-segmented; armature formula difficult to define, but most probably as follows: 1-(1), 2-(11), 3-(8), 4-(1), 5-(14+[1+ae]), 6-(1), 7-(2); 8-(1); 9-(9+acrothek); fifth, sixth and seventh (Figure 9B) and eight (Figure 9C) segments with modified setae and blunt spines/processes. Acrothek consisting of two setae and one aesthetasc basally fused.
Antenna (Figure 10E), mandible, maxillule, maxilla and maxilliped (not shown) as in female.
P1-P4 as in female, except for sexually dimorphic male P1 Basis (Figs 9D, 10A) and P3ENP (Figure 9E). The former with modified inner spine. The latter three-segmented; first and second segment as in female; third segment with outer longitudinal row of small spinules and armed with five setae/spines.
P5 (Figs 9F, 10C). Both legs fused medially. Exopod and baseoendopod separated. The former ovate, with six setae, relative length of elements from inner to outer margin as follows: 0.47, 0.35, 1.0, 0.44, 0.17, 0.39. Baseoendopodal lobe poorly developed, with three elements; relative length of elements from inner to outer margin as follows: 1.0, 0.79, 0.67.
P6 (Figs 9G, 10C) represented by two setae situated rather laterally, outer seta smaller than inner element.
Habitat characteristics. During closed-mouth conditions, the St Lucia Estuary was characterised by a reversed salinity gradient, with salinities ranging from near freshwater conditions at the Mouth and Narrows, to up to 200 psu at times in the northern regions of the lake (Table 1). Salinity levels were also more variable in the lakes than in the Mouth and Narrows region. Throughout the study period, salinity levels at the Mouth ranged from 3.2 to 37.6 psu, while at Listers Point they ranged from 18.3 to 216 psu. During closed-mouth conditions, water depth was also generally highest at the Mouth and Esengeni and shallower (~0.2 m) in the lakes (Table 1). High wind action, coupled with the fine sandy substratum in the lakes, also resulted in higher turbidity levels experienced here relative to the Mouth and Narrows. Listers Point and Charters Creek generally experienced the highest turbidities, while levels at the Mouth were usually at least one order of magnitude lower (Table 1). During open-mouth conditions, there was little disparity between sites in terms of the physico-chemical parameters measured. Salinity was generally within the range of sea water (~35 psu) across the estuarine lake and water levels in the lakes rose to approach the levels recorded at the Mouth and Narrows (Table 1).
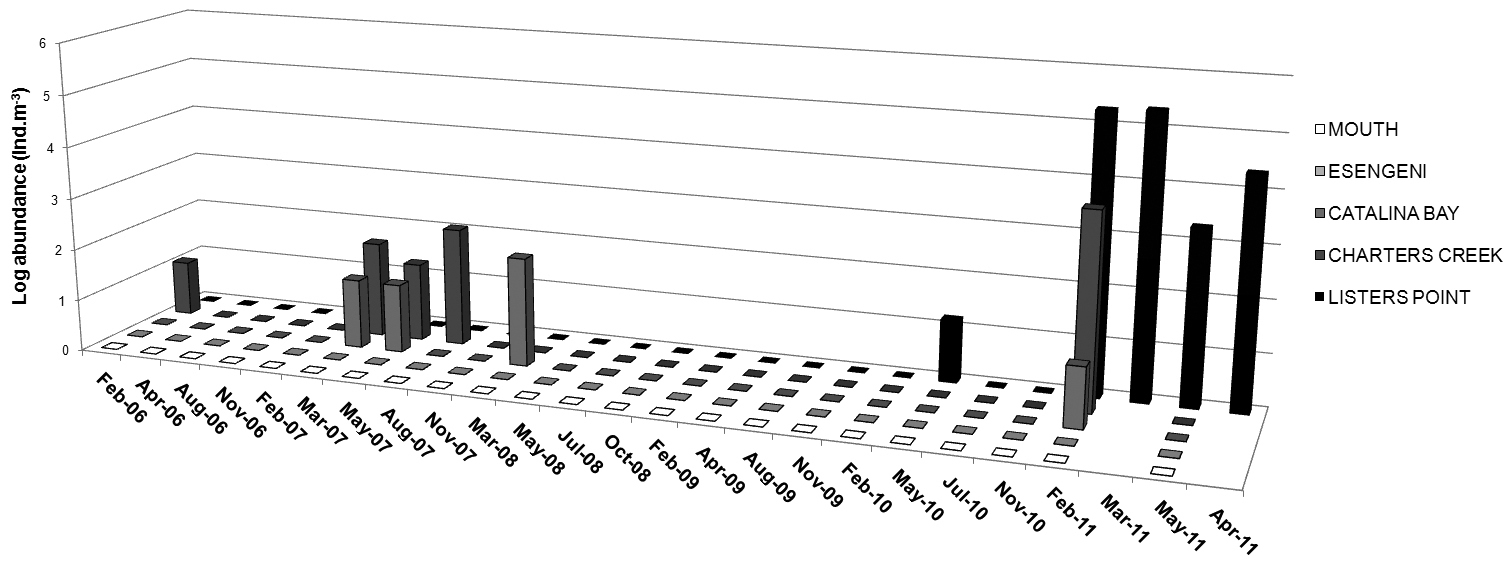
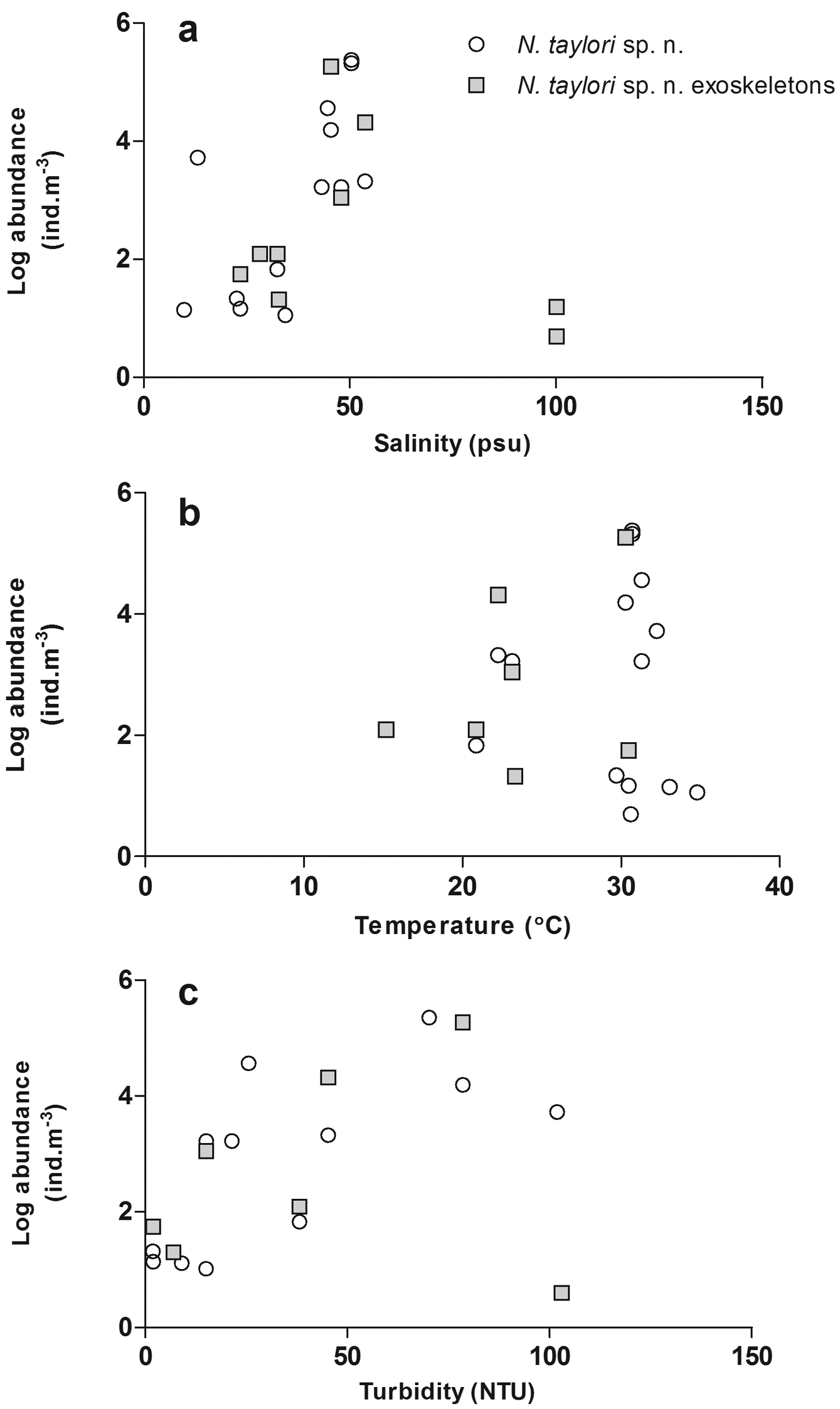
Occurrence of Nitocra taylori sp. n. through the study years has been irregular and the distribution has been limited to Catalina Bay and Charters Creek in South Lake and Listers Point in False Bay (Figs 1, 11). Nitocra taylori sp. n. was first recorded at Charters Creek in February 2006 in low densities (10.4 ind.m-3), while maximum densities (2.2 x 105 ind.m-3) were recorded at Listers Point in March 2011. These high densities followed heavy dilution of hypersaline waters after high rainfall in early 2011. Densities remained high in this region up until May 2011, after which salinities rose again above 53.7 psu and Nitocra taylori sp. n. virtually disappeared. Correlation analysis found significant positive correlations between the abundance of Nitocra taylori sp. n. and salinity (R = 0.621, p < 0.05, df = 12) and turbidity (R = 0.681, p < 0.05, df = 12). Nitocra taylori sp. n. individuals were able to withstand a wide range of fluctuations. They were found at salinity levels ranging from 9.81 to 53.7 psu, turbidities ranging from 2 to 102 NTU and temperatures from 20.9 to 34.8 ºC (Figure 12). In many instances specimens preserved in phloxine-stained formaldehyde did not take up the stain, but were rather completely transparent, resembling discarded exoskeletons. While these individuals were perfectly intact, it is unlikely that they were alive at the time of collection.
Nitocra taylori sp. n. Female. A habitus B rostrum, dorsal C urosome, dorsal. Scale bar: A=300 µm; B=75 µm; C=150 µm.
Nitocra taylori sp. n. Female. A anal somite and caudal rami, dorsal B urosome, ventral, showing P5 C left caudal ramus, ventral D P6 and genital complex. Scale bar: A=44 µm; B=100 µm; C=50 µm; D=71 µm.
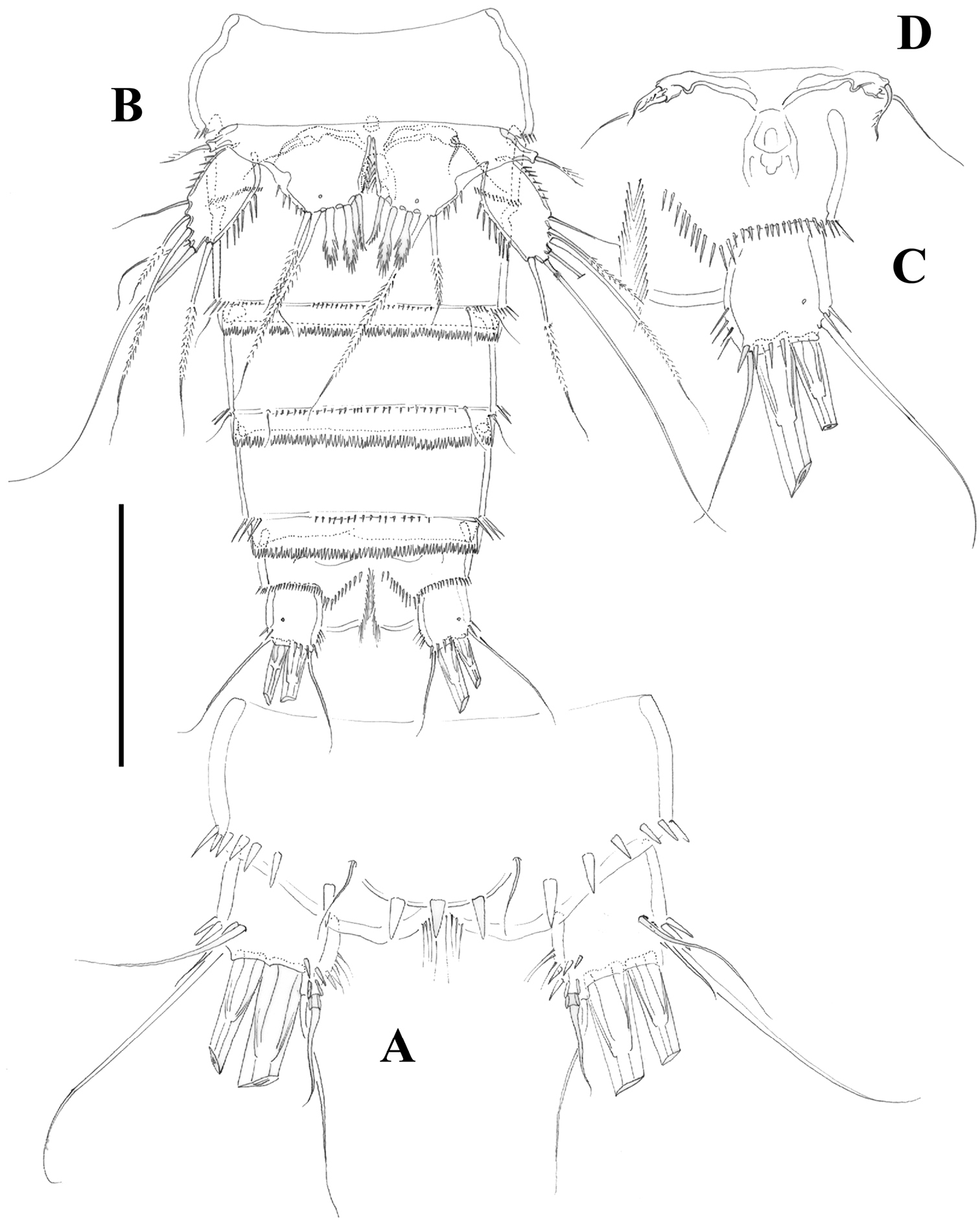
Nitocra taylori sp. n. Female. A antenna B mandible C maxillule D P1, anterior E intercoxal sclerite of P1, anterior. Scale bar: A–E=50 µm.
Nitocra taylori sp. n. Female. A antennule B P2, anterior. Scale bar: A=70 µm; B=100 µm.
Nitocra taylori sp. n. Female. A maxilla B maxilliped C P3, anterior. Scale bar: A, B=70 µm; C=100 µm.
Nitocra taylori sp. n. Female. A P4, anterior B P5, anterior. Scale bar: A, B=100 µm.
Nitocra taylori sp. n. Male. Urosome, ventral (P5- and P6-bearing somites omitted). Scale bar: 100 µm.
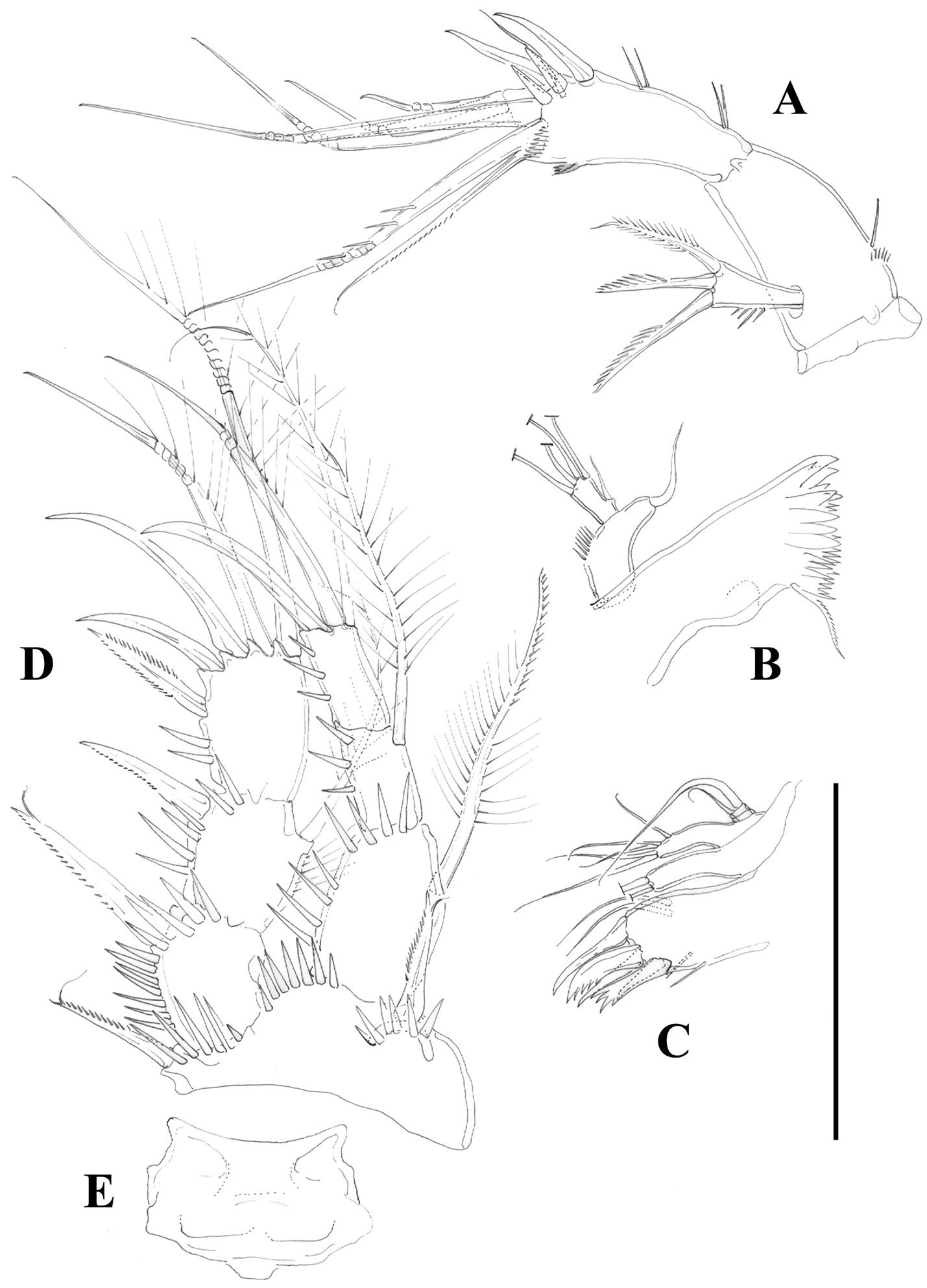
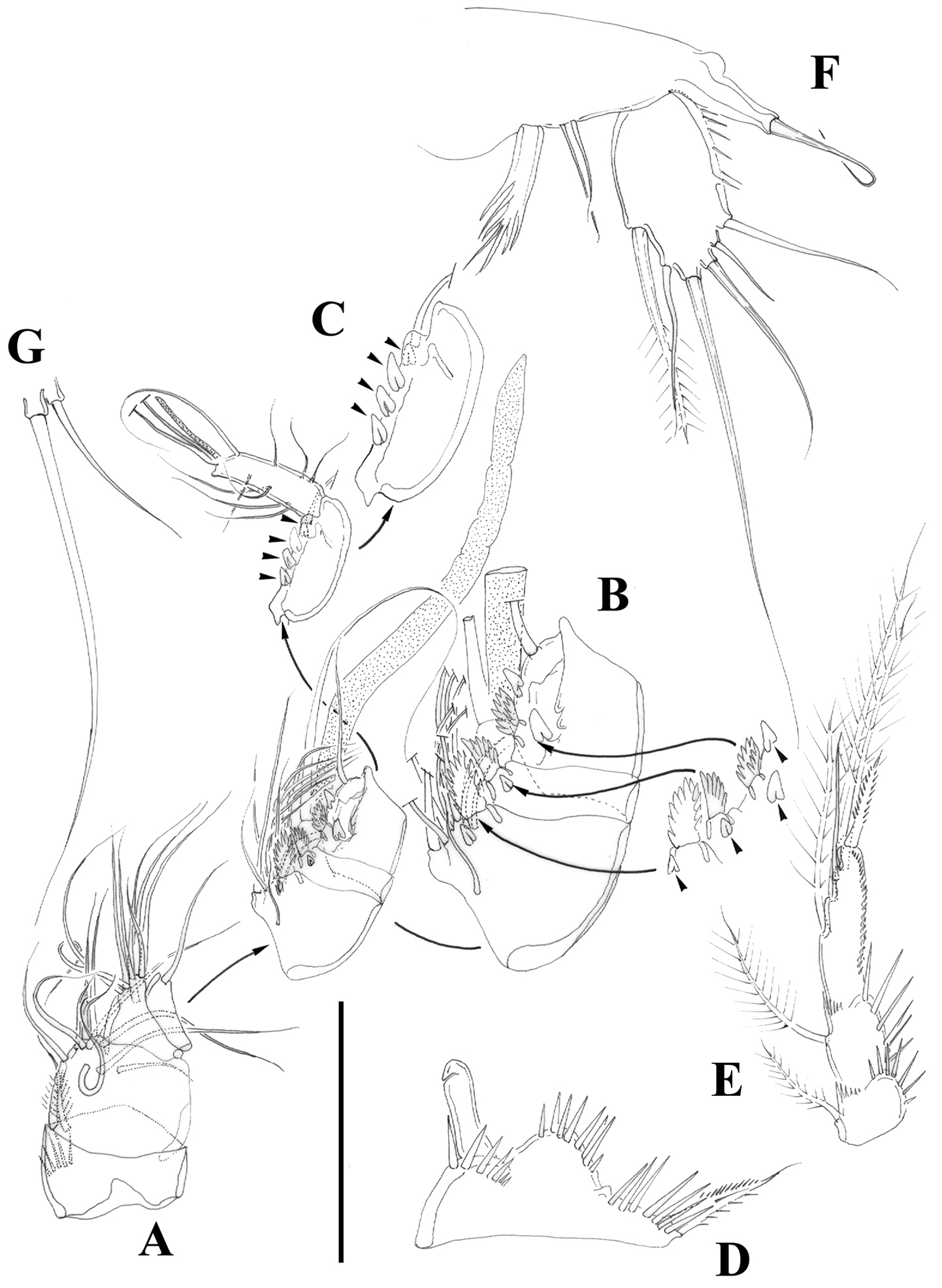
Nitocra taylori sp. n. Male. A antennule B fifth, sixth and seventh segments of the antennule, showing modified setae and blunt processes C eight segment of the antennule D P1 basis, anterior E P3ENP F P5, anterior G P6, anterior. Scale bar: A, E=50 µm; B, C=67 µm; D, F, G=35 µm.
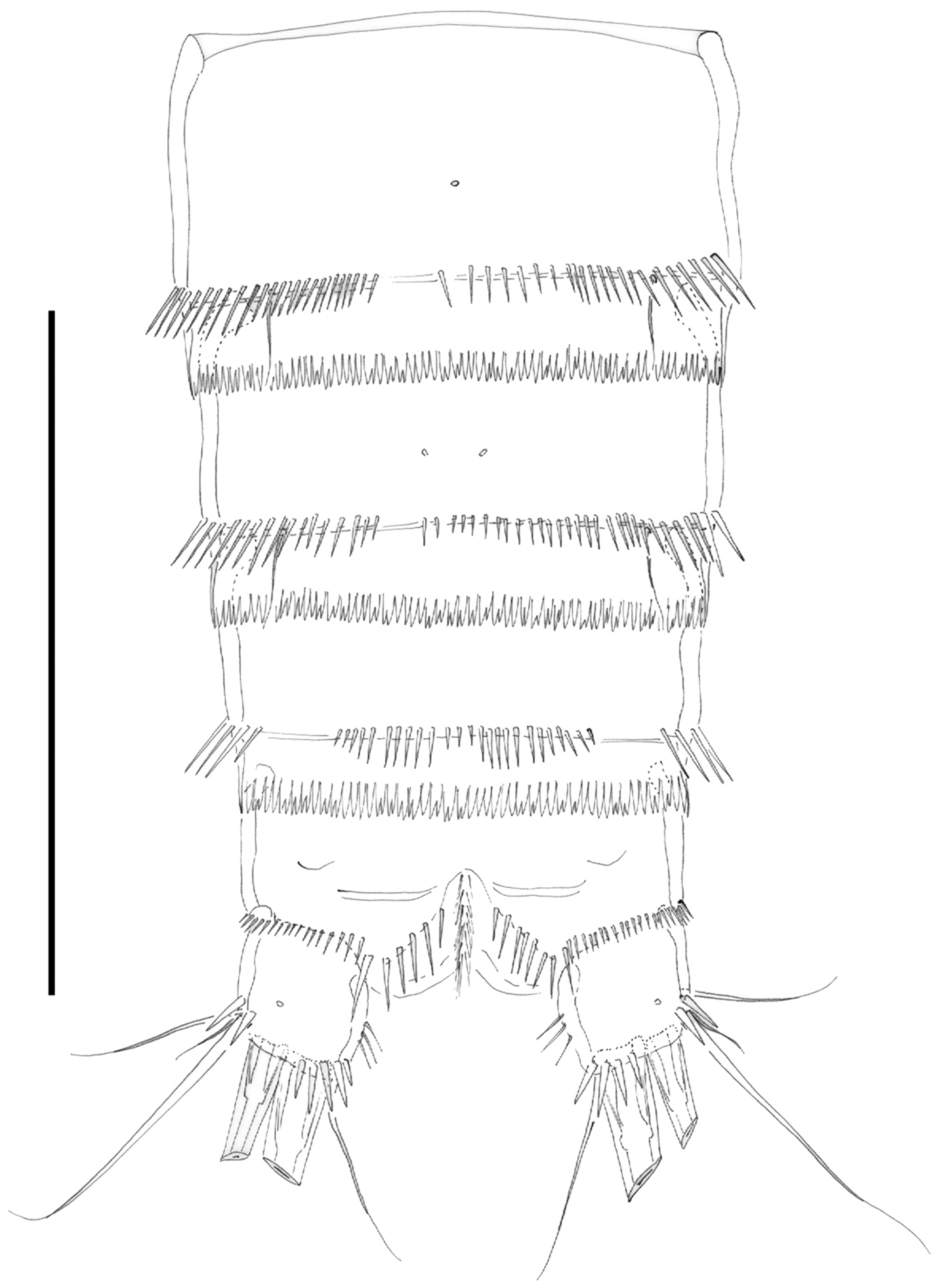
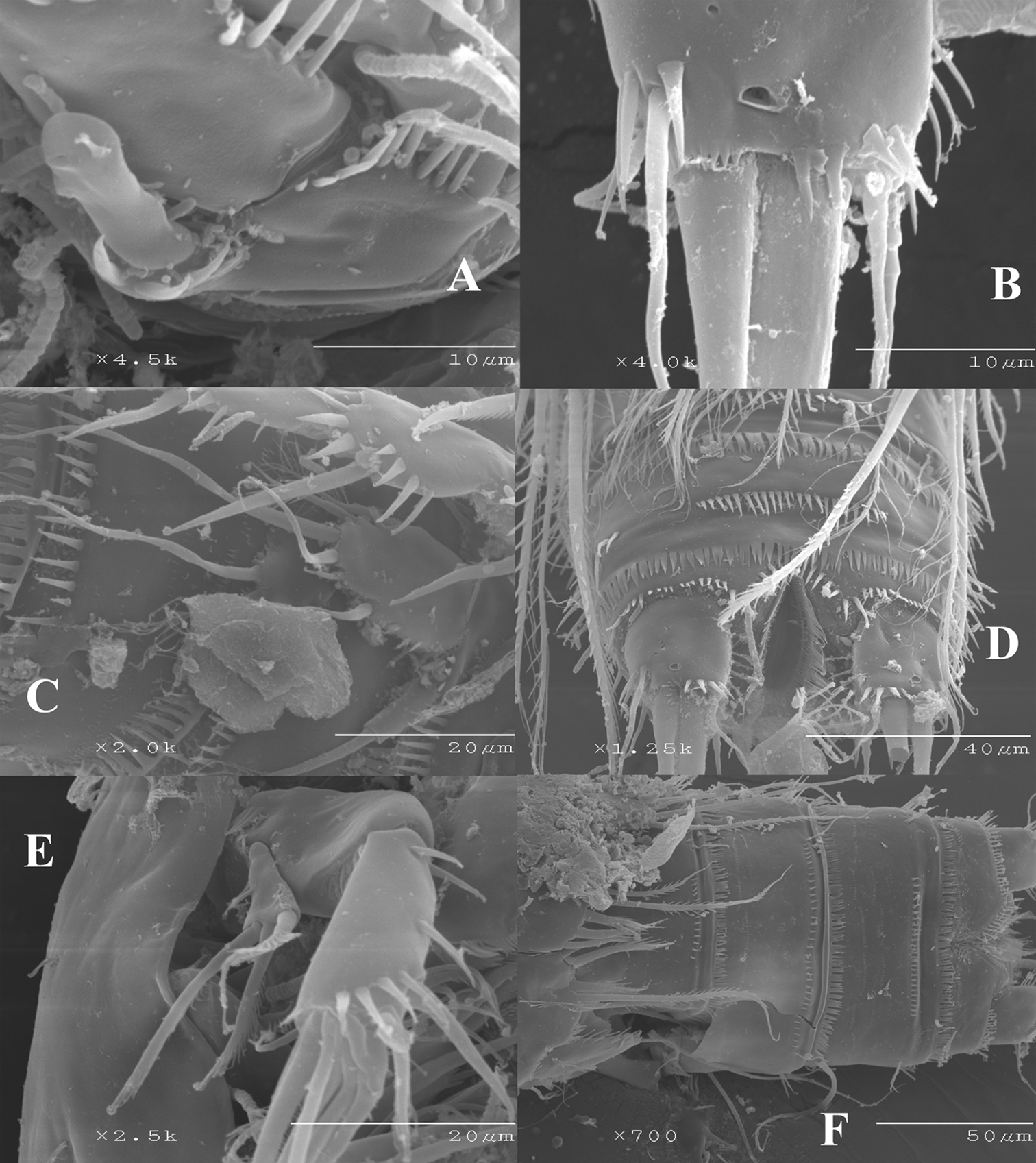
Nitocra taylori sp. n. SEM photographs A male P1 showing inner modified spine of basis B female caudal rami C male P5 exopod and P6 D posterior part of male urosome including caudal rami E male antenna F anterior part of female urosome.
Abundance of Nitocra taylori sp. n. (ind.m-3) in the St Lucia Estuary from February 2006– April 2011.
Abundance of Nitocra taylori sp. n. (alive and discarded exoskeletons) at different a salinity b temperature and c turbidity levels recorded in the St Lucia Estuary.
Physicochemical variables measured at each station during the different mouth phases (mean ± SE). NTU: Nephelometric Turbidity Units; DO: dissolved oxygen.
| Mouth state | Station | Depth(m) | DO(mg.L-1) | pH | Salinity(psu) | Temperature(ºC) | Turbidity(NTU) |
|---|---|---|---|---|---|---|---|
| Closed | Mouth | 2.05 ± 0.52 | 7.9 ± 0.52 | 8.02 ± 0.37 | 12.8 ± 1.67 | 26.5 ± 2.32 | 9.36 ± 5.82 |
| Esengeni | 1.38 ± 0.12 | 7.75 ± 1.13 | 8.37 ± 0.17 | 6.45 ± 1.52 | 24.5 ± 1.98 | 28.7 ± 3.76 | |
| Catalina Bay | 0.15 ± 0.04 | 9.06 ± 0.91 | 8.52 ± 0.14 | 14.5 ± 3.24 | 24.8 ± 2.58 | 42.8 ± 20.5 | |
| Charters Creek | 0.11 ± 0.02 | 8.38 ± 1.04 | 8.79 ± 0.22 | 24 ± 4.48 | 29.2 ± 2.5 | 33.2 ± 11.0 | |
| Listers Point | 0.07 ± 0.02 | 4.96 ± 1.52 | 8.17 ± 0.14 | 43.9 ± 21.58 | 29.8 ± 1.44 | 49.6 ± 12.7 | |
| Open | Mouth | 0.75 ± 0.35 | 7.57 ± 0.63 | 8.08 ± 0.14 | 34.6 ± 1.05 | 23.1 ± 1.6 | 24.4 ± 15.8 |
| Esengeni | 1.51 ± 0.21 | 5.23 ± 1.48 | 8.25 ± 0.07 | 37.4 ± 9.38 | 20.3 ± 2.98 | 45.6 ± 13.5 | |
| Catalina Bay | 0.33 ± 0.18 | 7.5 ± 1.26 | 7.99 ± 0.38 | 29.1 ± 3.26 | 23.7 ± 3.4 | 8.37 ± 4.18 | |
| Charters Creek | 0.53 ± 0.22 | 7.82 ± 1.52 | 8.11 ± 0.21 | 29.6 ± 3.1 | 24.5 ± 3.63 | 13.4 ± 5.96 | |
| Listers Point | 0.43 ± 0.28 | 6.91 ± 0.91 | 7.01 ± 1.01 | 35.1 ± 4.25 | 23.8 ± 2.73 | 32.8 ± 17.7 | |
| Re-closed | Mouth | 1.4 ± 0.23 | 6.85 ± 1.02 | 8.72 ± 0.26 | 15.3 ± 2.44 | 23.0 ± 0.87 | 20.0 ± 3.68 |
| Esengeni | 1.35 ± 0.13 | 7.51 ± 0.58 | 8.36 ± 0.17 | 9.39 ± 2.19 | 23.3 ± 0.81 | 60.3 ± 12.6 | |
| Catalina Bay | 0.21 ± 0.04 | 7.01 ± 1.18 | 8.61 ± 0.26 | 37.5 ± 5.25 | 24.8 ± 1.23 | 50.6 ± 10.5 | |
| Charters Creek | 0.2 ± 0.04 | 7.51 ± 0.39 | 8.65 ± 0.19 | 45.3 ± 5.3 | 26.3 ± 1.06 | 197 ± 61.2 | |
| Listers Point | 0.31 ± 0.05 | 7.53 ± 0.58 | 8.53 ± 0.19 | 87.4 ± 12.8 | 25.6 ± 1.73 | 155 ± 41.1 |
The phylogenetic relationships of the species within the genus Nitocra are still obscure. At first glance, three species groups can be recognized based on the combination of the armature formula of the P1EXP2-3. The less speciose group, composed only by Nitocra sewelli Gurney, 1927 and Nitocra platypus bakeri Chappuis, 1930, exhibits one inner seta and four elements on the P1EXP2 and EXP3 respectively. This two-species group is followed by that lacking inner armature on the P1EXP2, but with five setae on P1EXP3 (Nitocra reducta s. str. (
The creation of species and subspecies of Harpacticoida might appear, sometimes, based on questionable grounds. This holds true, as evidenced by
A similar case was observed for Nitocra sewelli.
At present and taking into account the rejection of all the described subspecies of Nitocra spinipes by
Within the most speciose group (see above), Nitocra australis Soyer, 1974, Nitocra fragilis, Nitocra spinipes, Nitocra intermedia Pesce, 1983, and Nitocra husmanni are unique in the combination of the number of setae/spines of the P2-P4Enp3 and P2-P4EXP3 (4, 5, 5 and 7, 7, 7, respectively) and number of inner setae on the P2-P4Enp1 (1, 1, 1). Note that
The South African material herein described agrees well with the description of Nitocra husmanni by
The nature of freshwater deprivation in the St Lucia Estuary has resulted in a northward gradient of drought effects. While regions in the south have recently been relatively protected from the drought, due to freshwater input from the Mpate and Mfolozi Rivers through the link canal (
The first record of Nitocra taylori sp. n. in the St Lucia Estuary dates back to 2006, when low densities were collected from Charters Creek, which is situated on the western shore of South Lake. It is possible that this species was present in earlier assessments conducted by
During this study, Nitocra taylori sp. n. individuals were recorded at salinity levels ranging from 9 to 53.7 psu. Many species of Nitocra exhibit wide salinity tolerance, since they occur in a variety of different habitats (rock pools, lagoons and sandy beaches), which naturally experience wide salinity fluctuations (
Species belonging to the genus Nitocra are also known to inhabit a wide range of sediment types (
Whether the main controlling factor is salinity, turbidity, water level, sediment composition, or a combination of all of them, the distribution of this new potentially endemic species is clearly limited to the lake part of the estuary, an area which is most severely affected by the current freshwater deprivation crisis. It appears that this species cannot tolerate salinity levels above 53.7 psu, however, in the current state of the estuary; salinity levels at Charters Creek and Listers Point often exceed this value. Continued freshwater deprivation would, therefore, further limit the distribution range of this species, but may also threaten its survival within the system. Up until now, research within the St Lucia Estuary has been focused at 5 representative stations, however, further investigations are needed in order to document the full extent of the distribution of Nitocra taylori sp. n. within the lakes. Additionally experimental studies on the salinity and temperature tolerance limits of this species would aid in the understanding of the physiological factors which affect its survival.
We are grateful to Holly Nel and Katrin Tirok (School of Life Sciences, University of KwaZulu-Natal) for their help during field work and to the iSimangaliso Wetlands Park Authority and the staff and management of EKZN Wildlife for their support during this project. We are indebted to Mrs Ma. Clara Ramírez Jáuregui (Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Unidad Académica Mazatlán, ) for her help with the search for bibliographic material and to Berenit Mendoza Garfias (Universidad Nacional Autónoma de México, Instituto de Biología) for the scanning microphotographs. Funding for the project was provided by the National Research Foundation (NRF, Pretoria), Marine and Coastal Management (DEA-MCM, Cape Town) and the World Wide Fund (WWF). R. Perissinotto is thanked for providing grant support to NKC and for critically reviewing earlier drafts of the manuscript.