(C) 2013 Yongting Luo. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Luo Y, Segers H (2013) On Pulchritia new genus, with a reappraisal of the genera of Trichotriidae (Rotifera, Monogononta). ZooKeys 342: 1–12. doi: 10.3897/zookeys.342.5948
During the study of rotifers collected in Eastern DR Congo, we rediscovered specimens that correspond to Monostyla dorsicornuta Van Oye, 1926. This species, which we redescribe, had not been seen since it’s summary description, and lacked type material. Our analysis reveals that the animal belongs to Trichotriidae rather than to Lecane (presently considered to include Monostyla) or Lecanidae, but is nevertheless characterised by a foot structure that is remarkably convergent to that of Lecanidae, and different from all other genera of Trichotriidae. We conclude that the species and the closely related South American Macrochaetus kostei (José de Paggi, Branco & Kozlowsky-Suzuki, 2000) belong to a new genus of Trichotriidae; the two offer a rare example of African-South American vicariance in rotifers.We further provide emended diagnoses of the remaining genera of Trichotriidae, to conform these to the new information and to address some inconsistencies in these.
Africa-South America vicariance, biogeography, Macrochaetus, taxonomy
On the occasion of the 2010 International Year of Biodiversity and the 50th anniversary of the independence of the Republic of Congo, an international expedition explored swamps, rivers and other water bodies along a ~1750km stretch of Congo River Northwest of Kisangani (
The samples collected during the 2010 International Congo River expedition contained an abundance of rotifer material. It also contained numerous specimens of what we believe to be an enigmatic species of which only a brief description by
As mentioned before, the material of this study consists of samples collected during the 2010 International Congo River Expedition. Specimens of the target taxon were found in three qualitative, 4%-formaldehyde-preserved samples only, all from running water in rivers: sample KM-028 is from Lulu River near Basoko, KM-048 and KM-049 are from Lohulu River near Bomane, all DR Congo. The samples were collected by Papy Mongindo, Ernest Tambwe and Koen Martens using a either a 30- or a 50 µm mesh-width plankton net that was hauled through surface water (maximum 1 m depth) and the littoral.
Individual rotifer specimens were separated under a WILD M10 dissection microscope and examined and measured on an Olympus BX51compound microscope at high magnification using a micrometre eyepiece. Drawings were made using a camera lucida. Photographs were taken by a camera (Olympus C-5060) connected to the microscope. Stacks of photographs were combined used COMBINEZP (
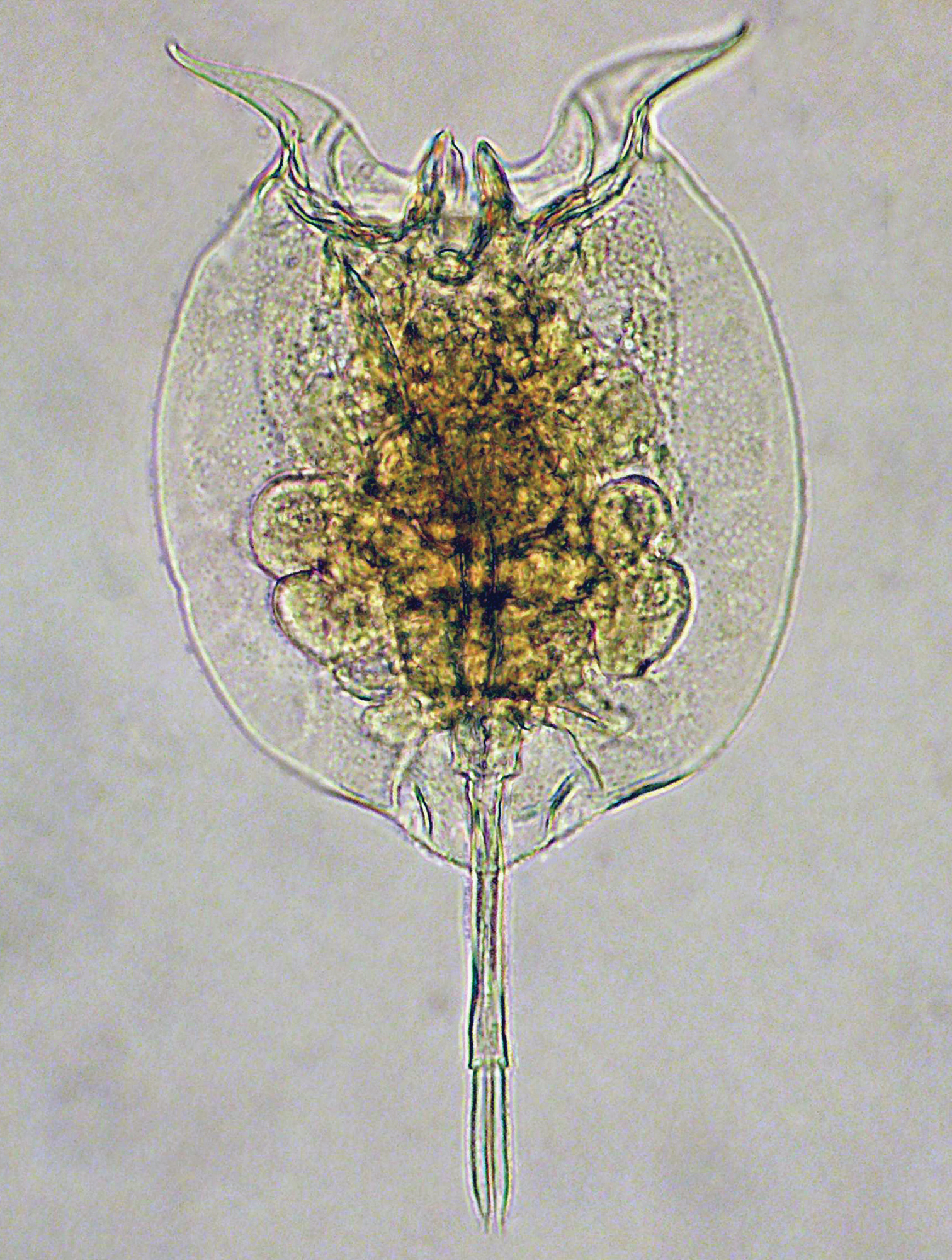
We found numerous specimens of our target species (Figs 1–2) which we identify, with some hesitation, as the species described as Monostyla dorsicornuta Van Oye, 1926, from the Ruki River near Eala, Congo. This description lacks detail and was considered to be based on some unrecognisable, poorly contracted rotifer by the authors of the candidate Rotifera part of the List of Available Names in Zoology (
Pulchritia dorsicornuta gen. n., comb. n., compound photomicrograph.
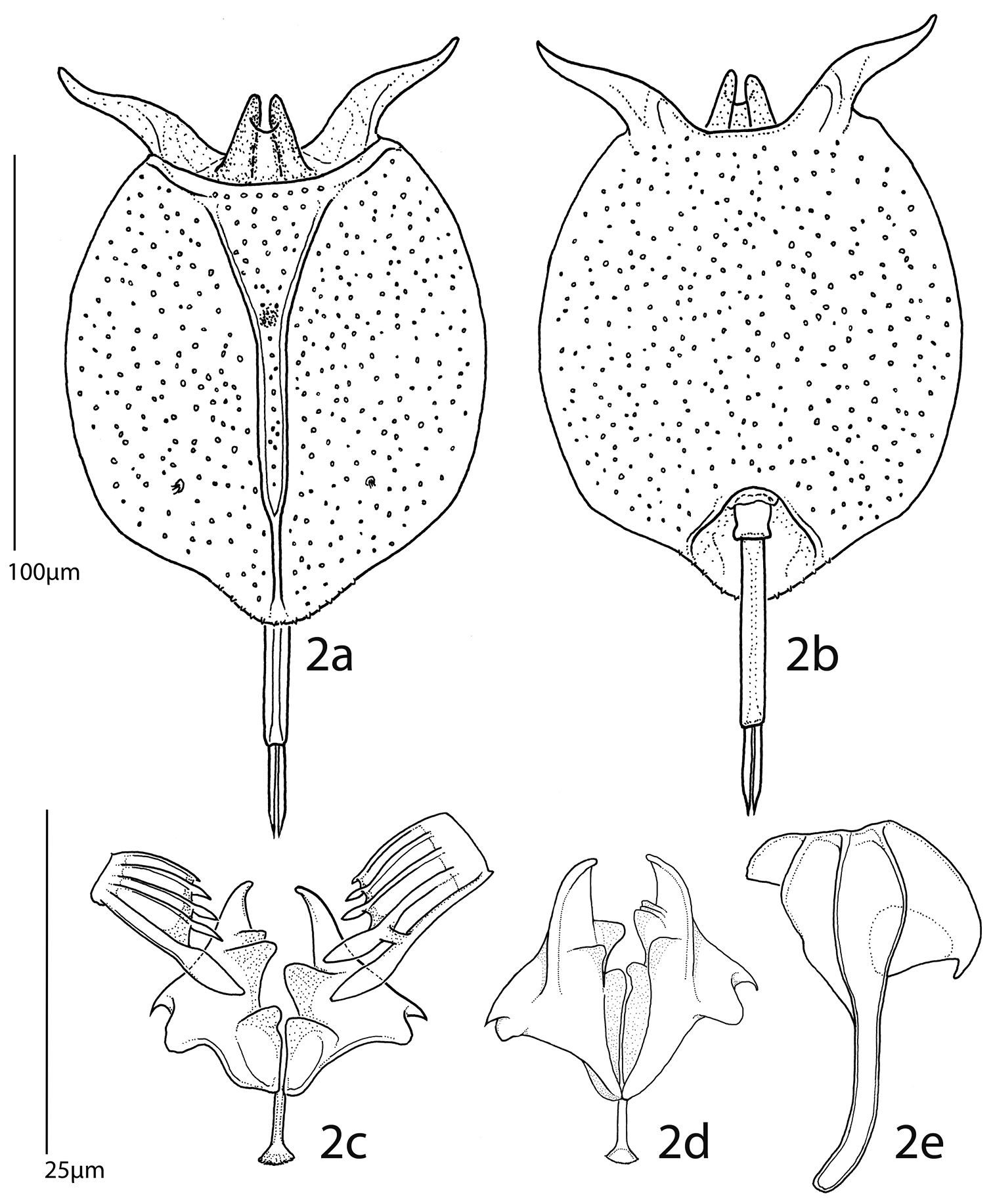
Pulchritia dorsicornuta gen. n., comb. n., a habitus, dorsal b habitus, ventral c–e trophi c unci and incus, frontal d incus, caudal e left manubrium, external. Scale bars: a–b= 100µm, c–e= 25µm.
By its trophi and lorica structure the species does not belong to Lecanidae as defined by
Trophi unspecialized, malleate; head, trunk and foot largely loricate, but head retractable. No discernible separate lorica plates or sulci on the trunk, but lorica stiffness not homogeneous. Lorica granulated and/or facetted. Distal part of trunk (anal segment) illoricate, separated from trunk proper. Foot with two pseudosegments and a pair of terminal toes.
The diagnostic autapomorphic feature for the family is the stiffening of the tegument of the head region, especially of the neck and lateral parts of the head, which in contracted specimens folds into a characteristic, more or less symmetrical shape protruding from the head aperture. The feature distinguishes family members from Brachionidae, Epiphanidae, Euchlanidae, and Mytilinidae who have an illoricate head; Lepadellidae has a characteristic sclerotized head shield overlaying the corona but the rest of the head is illoricate (Colurella, Lepadella), and not retractile (Squatinella). In contrast,
The classic diagnoses of Trichotriidae genera are problematic. They refer to features that are not present in all species of the genus (e.g., the purported synapomorphic dorsal spines in Macrochaetus), or features which appear to have been misinterpreted. This holds in particular for the structure of the foot which, in its basic form, consists of two foot pseudosegments bearing two toes, and is inserted on an illoricate terminal part of the trunk, termed the anal segment. This anal segment is a part of the trunk proper as it lies anterior to the (dorsal) anal opening. Its tegument is always relatively weakly sclerotized, which enables mobility of the rigid foot relative to the rigid trunk lorica, but which may also make it difficult to distinguish it from the trunk and/or from the two distal pseudosegments of the foot in contracted specimens. The structure is occasionally mistaken for a part of the foot and is then referred to as first of three foot pseudosegments. Note that in
In view of these inconsistencies, and awaiting a full review, preferably integrating both molecular and morphological data of genera in this and the related Euchlanidae and Mytilinidae, we tentatively propose emended diagnoses of the trichotriid genera, and propose a new genus to accommodate Monostyla dorsicornuta Van Oye, 1926 and Macrochaetus kostei José de Paggi, Branco & Kozlowsky-Suzuki, 2000.
Monostyla dorsicornuta Van Oye, 1926.
Body, including head and foot, loricate; head retractile, foot non-retractile, consisting of a short basal, squarish and an elongate, cylindrical foot pseudosegment terminating in two equal toes. Anal segment strongly reduced. Trunk lorica ventrally relatively flat, dorsally with a Y-shaped keel, pustulated, rounded elliptical.
The name Pulchritia is derived from the Latin adjective pulcher, meaning “pretty, beautiful, handsome”. It refers to the beauty of its type species, Pulchritia dorsicornuta comb. n.
We recognize this genus as containing two species, Pulchritia dorsicornuta comb. n. and Pulchritia kostei (José de Paggi, Branco & Kozlowsky-Suzuki, 2000), comb. n.
The two share a number of features that clearly sets them apart from other Trichotriidae. Their rounded, dorso-ventrally flattened trunk shape reminds one only of Macrochaetus, while the anal segment being reduced is as in certain Trichotria (e.g., Trichotria buchneri Koste, Shiel & Tan, 1988, Trichotria brevidactyla Harring, 1913 (= Trichotria curta (Skorikov, 1914))). The peculiar keel formation of the dorsal lorica is somewhat similar to Trichotria buchneri only. The unique foot structure of the two species, however, can be considered synapomorphic and is superficially and probably functionally similar to the foot consisting of a single short foot pseudosegment and elongated, fused toes bearing terminal (pseudo)claws of some Lecane species.
Type material: Neotype (labelled: “Pulchritia dorsicornuta (Van Oye, 1926) Neotype. Lohulu River near Bomane, DR Congo, 24 May 2010 (KM-048)”) in Royal Belgian Institute of Natural Sciences, Brussels Belgium (IG32450, RIR 212).
Other material: Abundant specimens of the species were found in two localities: Lulu River near Basoko (sample KM-028: 1.2958°N, 23.6497°E (DD, GPS waypoint Mac 079), altitude. ca. 350 m asl., water temp. 25.8° C, conductivity 16.5 µS/cm), and Lohulu River near Bomane (samples KM-048, KM-049: 1.2486°N, 23.7280°E (DD, GPS waypoint Mac 089), altitude. ca. 410 m asl., water temp. 24.3° C, conductivity 30.4 µS/cm, oxygen 0.45 mg/l), both in Orientale province, DR Congo. All samples are from running water. One permanent trophi preparation, and nine permanent slides containing one, three slides containing two, and three slides containing three specimens. Deposited in RBINS and in the CSB-UK.
Pulchritia dorsicornuta comb. n. is unmistakable by the large, S-shaped antero-lateral projections of its ventral lorica. These are completely absent in its closest relative Pulchritia kostei comb. n.
Female (Figs 1, 2a–b; male unknown): Body: Head largely retracted in trunk lorica, with two lateral stiffened elements protruding from the head aperture. A pigmented spot (eye?) present. Trunk loricate, elliptic in outline, longer than wide, dorso-ventrally compressed. Ventral and dorsal plates fused laterally and caudally, leaving a broad head aperture and a smaller foot aperture. Dorsal plate medially with two semi-longitudinal ridges forming a Y-shaped double dorsal keel, fused to a single dorsal keel terminally. Posterior of dorsal lorica with a weakly protruding rounded margin bearing two pairs of short ridges over the foot aperture. Openings of the lateral antennae in posterior third of body, about halfway between dorsal keel and lateral margin of lorica. Dorsal head aperture margin concave. Ventral plate flat, with two protruding, weakly S-shaped and diverging spines antero-laterally, these separated by a shallow U-shaped sinus. Posterior of ventral plate with a well-defined foot aperture, with rounded anterior and diverging lateral margins. Anal segment indistinct, poorly developed (also in poorly contracted specimens). Foot subterminally, consisting of a short, bilaterally constricted first and an elongate, parallel-sided second foot pseudosegment. Two long, equal toes, these mostly parallel-sided, terminating in a sharp tip.
Trophi (Figs 2c–e) malleate, almost symmetrical. Fulcrum short, with a small basal plate; rami relatively flat, triangular, with rounded postero-lateral corners and short, curved alulae, inner margins with asymmetrical, protruding teeth-shaped structures. Left uncus with two large frontal and three minor dorsal webbed teeth, right with a single large frontal and four minor teeth, all minor teeth gradually reduced in size from frontal to dorsal. Manubria symmetrical, with elongate and weakly procurved shaft. Head broad, with clear ventral, median and dorsal chambers, anterior chamber with an additional rounded triangular apophysis, dorsal chamber with a recurved hook.
(in µm. N=12; range, mean).Total length (incl. foot): 180–205, 192; lorica width 92–122, 106; antero-lateral spine length 20–32, 27; head aperture width 37–58, 47; foot aperture width 29–40, 33; length 23–34, 28; first foot pseudosegment length 9–14, 11; second foot pseudosegment length 46–54, 48; toe length 26–32, 29.
Pulchritia dorsicornuta comb. n. is only known from the two localities cited above, and from Ruki River near Eala (
The main feature distinguishing Pulchritia dorsicornuta comb. n. and Pulchritia kostei comb. n. is the presence of well-developed antero-lateral spines in the former. As we observed only negligible variability of the antero-lateral spines of Pulchritia dorsicornuta comb. n., and as there are no indications at all of such spines in Pulchritia kostei comb. n., we can neither exclude nor confirm the possibility that this feature results from phenotypic plasticity and as such would not be taxonomically relevant. Examples of such environmentally induced spine development are common in rotifers, including Trichotriidae (
Macrochaetus subquadratus Perty, 1850.
Body, including head and foot, loricate; head retractable, foot not retractable, inserted on a large, relatively soft and broad anal segment covering an equally soft and relatively broad first foot pseudosegment, and a stiff, cylindrical terminal foot pseudosegment. Trunk lorica dorso-ventrally compressed, relatively wide, pustulated, circular or with angular corners in the anterior third, head and neck lorica plates with spinulets.
Most species of Macrochaetus are readily identified as belonging to this genus by the presence of long, conspicuous dorsal spines. However, three species of Macrochaetus (Macrochaetus aspinus Segers & Sarma, 1993, Macrochaetus danneelae Koste & Shiel, 1983, and Macrochaetus paggiensae Koste, 2000) lack these dorsal spines and their presence can therefore not be confirmed as generally diagnostic for the genus. On the other hand, small lorica spinulets are present dorsally, ventrally and marginally on the trunk lorica, and on the lorica of the head and neck regions. In particular the spinulets on the head and neck lorica appear to be synapomorphic for the genus. The foot consist of a large, relatively soft anal segment covering a relatively poorly sclerotized first foot pseudosegment and a terminal cylindrical foot pseudosegment bearing two separate toes. There are 14 species in this genus, several of which are endemic to South America (
Trichotria pocillum (Müller, 1776).
Body, including head and foot, loricate; head retractable, foot only partly retractable. Trunk lorica hexagonal in cross section, facetted, granulated, longer than wide, with parallel lateral margins in anterior part of the trunk. Head aperture nearly as wide as the trunk.
In comparison with Wolga, the anal segment is clearly discernible in almost all species but it is relatively weakly sclerotized; the two foot pseudosegments are cylindrical and strongly sclerotized. Retraction of the foot is not possible in those species in which the foot is situated terminally. Spines on the first foot pseudosegment and on the trunk lorica are present in most, but not all species (e.g., Trichotria pseudocurta Koste, Shiel & Tan, 1988). There are seven species in the genus (
Wolga spinifera (Western, 1894).
Tegument of head, and anterior part of trunk loricate, both head and foot entirely retractable. Anal segment relatively large, annulated; foot pseudosegments short, only the distal one sclerotized. Trunk lorica box-shaped, dorso-ventrally compressed, longer than wide, with relatively flat ventral and dorsal parts; facetted. No marginal spines or spinulets.
The published generic diagnosis refers to absence of an anal segment (
We thank Prof. Dr S. José de Paggi and Dr C. Castelo Branco for providing us with unpublished photographs of Macrochaetus kostei, and Dr C. Jersabek for photographs of a possible type specimen of Macrochaetus kostei deposited in Senckenberg Museum (SMF GP7650). We are grateful to Papy Mongindo, Ernest Tambwe (University of Kisangani) and Koen Martens (Royal Belgian Institute of Natural Sciences) who collected plankton samples during the 2010 Congo River expedition, and allowed us to examine these. We further wish to acknowledge Prof. Dr W.H. De Smet, Dr. C. Jersabek and Dr. R.J. Shiel for their valuable suggestions and corrections to the manuscript of this paper.