(C) 2013 Olavi Kurina. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Kurina O, Oliveira SS (2013) The first Cordyla Meigen species (Diptera, Mycetophilidae) from continental Australia and Tasmania. ZooKeys 342: 29–43. doi: 10.3897/zookeys.342.6045
A new species of Mycetophilidae, Cordyla australica sp. n., is described from continental Australia and Tasmania, representing the first Cordyla record in the region. A detailed description of its morphology with illustrations of male and female terminalia and a map of the collecting localities are provided. According to the structure of male terminalia, Cordyla australica sp. n. belongs to the Cordyla murina species-group that has 13 species worldwide. Within the group Cordyla australica sp. n. resembles Cordyla murina but has a unique outline of the hypoproct and medial branch of the gonostylus. The observed distributional pattern is restricted to the rainforest of eastern Australia and Tasmania.
Diptera, Mycetophilidae, Cordyla, new species, Australia, Tasmania
The genus Cordyla Meigen, 1803, a member of the tribe Exechiini of Mycetophilidae, is a well delimited monophyletic clade of fungus gnats (Diptera: Sciaroidea). Having been treated earlier also in Mycetophilini (since
Thirty-eight described Cordyla species are known worldwide at present, viz. twenty-four from the Palaearctic region (
The aim of this paper is to describe and illustrate the first Cordyla species from continental Australia and Tasmania and discuss its systematics.
The material was collected from seven localities in Tasmania using mostly Malaise traps, in few cases also pitfall traps or sweeping. A good amount of material comes from the Warra Long-Term Ecological Research Site (for details see
The habitus photo has been made in alcohol medium using the Canon 7D camera in combination with Canon MP-E65 (F2.8 1–5×) lens (see
The following acronyms are used for depositories:
AMSA Australian Museum, Sydney, Australia
ANIC Australian National Insect Collection, Canberra, Australia
IZBE Institute of Agricultural and Environmental Sciences, Estonian University of Life Sciences [former Institute of Zoology and Botany], Tartu, Estonia
MZUSP Museu de Zoologia da Universidade de São Paulo, Brazil
SMNH Swedish Museum of Natural History, Stockholm, Sweden
TFIC Tasmanian Forest Insect Collection, Hobart, Australia
Specimen information is available for download in Darwin Core 1.4 format at GBIF, the Global Biodiversity Information Facility, http://ipt.pensoft.net/ipt/resource.do?r=cordyla.
http://zoobank.org/22F4AE20-D2B3-42EE-9089-0A29BC3E9ECF
http://species-id.net/wiki/Cordyla_australica
Figs 1–15Holotype. 1♂, AUSTRALIA: Tasmania, Warra LTER: Manuka Road, Malaise trap, 43.07°S, 146.67°E, 20.iv.2004, R. Bashford leg., plot code: SSTSMA254, sample code FT30575 (mounted from alcohol, in AMSA).
Paratypes. 5♂♂, same as holotype (in alcohol, 2 in TFIC, 3 in IZBE); 8♂♂, same as holotype except 19.vii.2004, plot code: SSTSMA254, sample code: FT30660 (in alcohol, 3 in AMSA, 5 in IZBE); 15♂♂ 2♀♀, same as holotype except 1.vii.2005, plot code: SSTMID280 and sample code: FT36772 (in alcohol, in IZBE); 2♂♂, same as holotype except 1.vii.2005 and plot code: SSTEAS094, sample code FT36767 (in alcohol, in TFIC); 1♂, same as holotype except 2.v.2003, plot code: SSTTOP060, sample code FT29026 (in alcohol, in TFIC); 1♂, same as holotype except 1.iii.2005, plot code: SSTEAS318, sample code FT35684 (in alcohol, in IZBE); 2♂♂, same as holotype except 13.x.2002, plot code: SSTSMA254, sample code FT28944 (in alcohol, in IZBE); 3♂♂, same as holotype except 19.v.2004, plot code: SSTCON059, sample code FT30632 (in alcohol, in IZBE); 1♂, same as holotype except 19.v.2004, plot code: SSTTOP060, sample code: FT30634 (in alcohol, in IZBE); 1♂, same as holotype except 13.x.2002, plot code: SSTMID160, sample code FT7034 (in alcohol, in IZBE); 2♂♂, same as holotype except 1.iv.2005, plot code: SSTWES120, sample code FT35962 (in alcohol, in IZBE); 1♂, same as holotype except 2.iv.2007, plot code: SSTCON059, sample code FT40220 (in alcohol, in IZBE); 1♂, same as holotype except 3.ix.2007, plot code: SSTCON059, sample code FT40745 (in alcohol, in IZBE); 1♂, same as holotype except 5.iii.2007, plot code: SSTCON059, sample code FT40115 (in alcohol, in IZBE); 1♂ same as holotype except 24.iii.2000, plot code: SSTSMA663, sample code FT28616 (in alcohol, in IZBE); 2♂♂, Tasmania, Mount Warra - Mt. Weld alt. Transect 100, Malaise trap, 43.07S, 146.67E, 27.ii.2002, N. Doran & R. Bashford leg., plot code WR0100M, sample code FT5923 (in alcohol, in IZBE); 1♂, same as previous except 27.ii.2001, sample code: FT19 (in alcohol, in IZBE); 6♂♂, same as previous except 27.iv.2001, sample code: FT199 (in alcohol, in IZBE);1♂, same as previous except 27.ii.2001, plot code: WR0200M, sample code: FT26 (in alcohol, in IZBE); 3♂♂, AUSTRALIA, Tasmania, Ewart creek, 150m dstr bridge on A10, 221 m.a.s.l., Malaise trap, loc 12, 41°58.576'S, 145°27.708'E, 22.ii–03.iii.2006, Jönsson, N., Malm, T. & Williams, D. leg. (on slides, in SMNH); 23♂ 1♀ AUSTRALIA, Tasmania, Ewart creek, Malaise trap ethanol, 41°58'S, 145°28'E, 16.i–02.ii.1983, I.D. Naumann & J.C. Cardale leg. (1♂ 1♀ on slides, in MZUSP; 22♂♂ in alcohol, 5♂♂ in MZUSP other in ANIC); 23♂♂ 2♀♀, AUSTRALIA, Tasmania, Central Plateau, small creek flowing into Arthur’s Lake, 50m dst 1st bridge on gravel road from Rd B51 to Little Lake, 1006 m.a.s.l., Malaise trap, loc 17, 41°57.237'S, 146°51.928'E, 25.ii–04.iii.2006, Jönsson, N., Malm, T. & Williams, D. leg. (1♂ 1♀ on slides other in alcohol, 3♂♂ in IZBE other in SMNH); 5 ♂♂, AUSTRALIA, Tasmania, Cradle MTN NP. creek from Crater Lake to Ronny Greek 100m upstr broadwalk, 867 m.a.s.l., Malaise trap, loc 14, 41°38.667'S, 145°56.775'E, 23.ii–04.iii. 2006, Jönsson, N., Malm, T. & Williams, D. leg. (1♂ on slide other in alcohol, in SMNH); 1♂, AUSTRALIA, Tasmania, Southwest National Park, in forest 20m off Rd C607, 300m south off Creepy Crawly Walk, 573 m.a.s.l., Malaise trap, loc 9, 42°50.012'S, 146°22.866'E, 21.ii.–01.iii.2006, Jönsson, N., Malm, T. & Williams, D. leg. (in alcohol, in SMNH); 5♂♂, AUSTRALIA: Queensland, Brisbane Forest Park, Enoggera Creek at Scrub Road crossing, in tropical rain forest with Eucalypus spp., Malaise trap, 27°25'42"S, 152°50'33"E, 14–29.xi.1995, 1–7.xii.1995, 7–27.xii. 1995 and 28.xii.1995–4.i.1996, Irwin, M.E. leg. (in alcohol, 3 in ANIC, 2 in IZBE).
(not included in paratypes due to quality of material from sticky traps). 1♀, AUSTRALIA: New South Wales, Werrikimbe National Park, 31°16'50"S, 152°03'19"E, 1045m, sticky trap on Eucalypus saligna, 1.xii–7.xii.1997, E. Tasker leg., WS-FC-127-6 (K377114, in alcohol, in AMSA); 1♀, AUSTRALIA: New South Wales, Werrikimbe National Park, 31°10'23"S, 152°09'45"E, 1060m, sticky trap on Eucalypus saligna, 1.xii–7.xii.1997, E. Tasker leg., WS-KF-127-6 (K377115, in alcohol, in AMSA); 1♀, AUSTRALIA: New South Wales, Carrai State Forest, 30°58'48"S, 152°17'06"E, 975m, sticky trap on Eucalypus obiqua, 11–16.i.1998, E. Tasker leg., CR-RO-018-4 (K377116, in alcohol, in AMSA); 1♀, AUSTRALIA: New South Wales, Werrikimbe National Park, 31°16'50"S, 152°03'19"E, 1045m, sticky trap on Eucalypus campanulata, 1.xii–7.xii.1997, E. Tasker leg., WS-FC-127-3 (K377117, in alcohol, in AMSA); 2♀♀, AUSTRALIA: New South Wales, Carrai State Forest, 30°59'45"S, 152°16'23"E, 930m, sticky trap on Eucalypus campanulata, 3.xii–8.xii.1997, E. Tasker leg., CS-FZ-127-4 (K377118, in alcohol, in AMSA); 1♂1♀, AUSTRALIA: New South Wales, Carrai State Forest, 30°59'45"S, 152°16'23"E, 930m, sticky trap on Eucalypus saligna, 3.xii–8.xii.1997, E. Tasker leg., CS-FZ-127-5 (K377119, in alcohol, in AMSA); 1♂, AUSTRALIA: New South Wales, Carrai State Forest, 30°54'19"S, 152°17'36"E, 1055m, sticky trap on Eucalypus campanulata, 3.xii–8.xii.1997, E. Tasker leg., CC-DP-127-4 (K377120, in alcohol, in AMSA); 1♀, AUSTRALIA: New South Wales, Carrai State Forest, 30°54'35"S, 152°16'26"E, 1090m, sticky trap on Eucalypus obiqua, 3.xii–8.xii.1997, E. Tasker leg., CC-FK-127-3 (K377121, in alcohol, in AMSA); 4♂♂, AUSTRALIA: New South Wales, Werrikimbe National Park, 31°16'50"S, 152°03'19"E, 1045m, sticky trap on Eucalypus obiqua, 3.vii–8.vii.1998, E. Tasker leg. WS-FC-078-1 (K377122, 1♂ in slide, 3♂♂ in alcohol, in AMSA); 1♀, AUSTRALIA: New South Wales, Carrai State Forest, Feltons Knob, 30.9097S, 152.2739E; 1090m, 24.iv–30.iv.1998, E. Tasker, P. German leg., CC-FK-048-3 (K377123, in alcohol, in AMSA); 2♂♂1♀, AUSTRALIA: New South Wales, Werrikimbe National Park, 31°16'50"S, 152°03'19"E, 1045m, sticky trap on Eucalypus obiqua, 3.vii–8.vii.1998, E. Tasker leg., WS-FC-078-3 (K377124, in alcohol, in AMSA); 1♂1♀, AUSTRALIA: New South Wales, Carrai State Forest, 30°54'33"S, 152°16'28"E, 1075m, sticky trap on Eucalypus campanulata, 3.xii–8.xii.1997, E. Tasker leg., CC-CR-127-2 (K377125, in alcohol, in AMSA); 2♂♂, AUSTRALIA: Tasmania, King William Creek Site, 43 08 84E, 5 22 76 00N [these label data are unclear, the approximate geographic coordinates are 42°12'S, 146°8'24"E], pitfall, 23.ii.2000, M. Driessen leg. (K377126 and K377128, in alcohol, in AMSA); 1♂, AUSTRALIA: Tasmania, Lake St Clair, Site: SCRW, sweep, 28.viii.1999, (K377127, in alcohol, in AMSA); 43♂♂, AUSTRALIA: Victoria, Coopracambra National Park, Beehive creek, 27 Km NNE Cann R., 347 m, Malaise traps, 37°20'01"S, 149°14'12"E, 5.xii.2004–12.i.2005, C. Lambkin & N. Starick leg., ANIC sample 2608 (material from ANIC in donation to SSO, housed at the Universidade de São Paulo, campus of Ribeirão Preto).
Male (Fig. 1). Total length 2.4–3.7, 2.9 [3.1] mm (n=10).
Cordyla australica sp. n. 1 male habitus 2 head with antennae and maxillary palpi, closer view 3 three apical segments of maxillary palpus. Scale bar = 1 mm (1), 0.2 mm (2) and 0.1 mm (3).
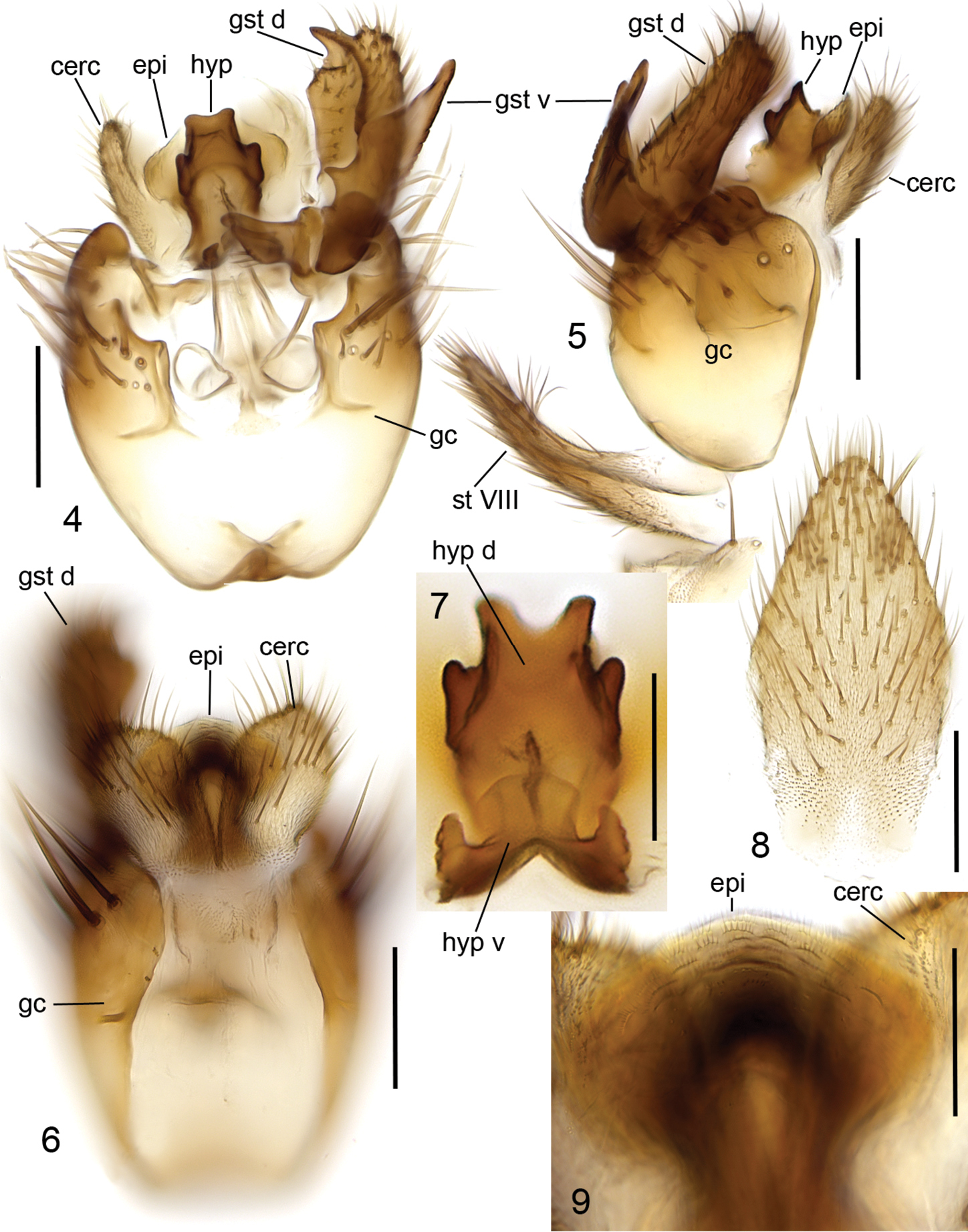
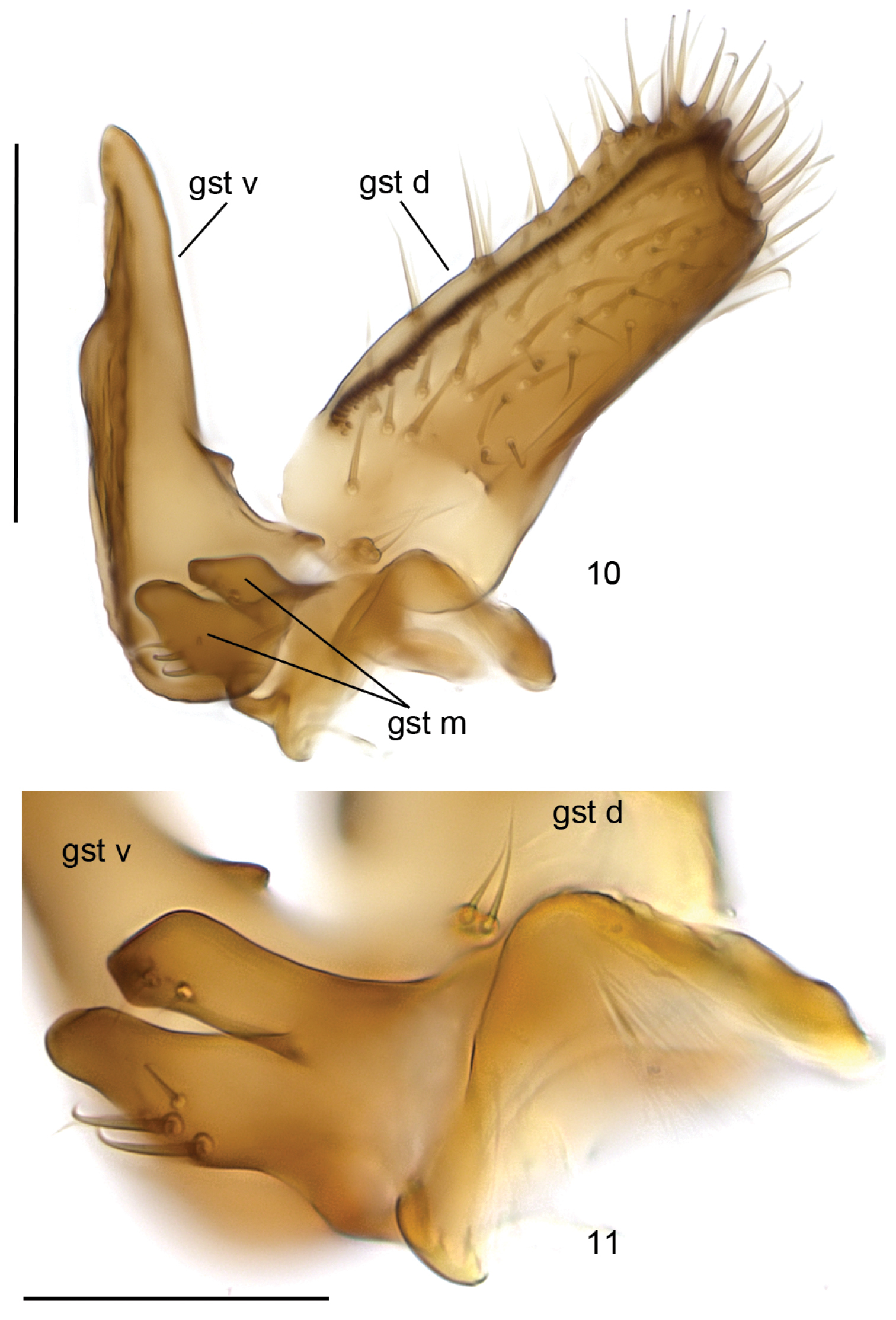
Head (Fig. 2) brown, mouthparts yellowish. Two ocelli encircled by dark brown areas, close to compound eyes. All three visible palpal segments (Fig. 3) setose, swollen antepenultimate segment blackish brown, succeeding segments light brown, basally pale. 4th segment slightly widening apically, 5th segment apically tapering. Swollen palpal segment 1.7–2.1, 1.9 [2.1] times as long as broad medially from lateral view, and 1.0–1.2, 1.1 [1.1] times as long as height of compound eye. Ratios of three apical palpomeres 1.0: 0.8–0.9, 0.8 [0.9]: 0.9–1.0, 1.0 [0.9]. Antenna light brown with 2+12 segments. Scape and pedicel with brown setae, flagellum with somewhat paler setosity. Scape elongate cup-shaped, 2.0–2.3, 2.1 [2.0] times as long as wide apically. Pedicel cup-shaped, 0.7–0.8, 0.8 [0.7] times as long as wide apically. Flagellomeres rectangular, about twice as wide as long. Apical flagellomere conical, about 1.6 times as long as wide basally. Thorax brown, mesonotum and hind margin of laterodergite somewhat darker. Anterior part of mesepimeron with a blackish patch leaving anteroapical corner light brown. Haltere with pale knob, stem basally pale and apically brown. All setosity on thorax brown. Scutum entirely covered with decumbent setae, scutellum with setae including two pairs of marginal bristles, laterals considerably shorter than internals. Antepronotum with setae including 4-5 [4] bristles, proepisternum with setae including 6–8 [8] bristles. Anepisternum with 4–6 [6] bristles at hind margin and with ca. 40 setae on its upper two third. Mesepimeron and katepisternum bare. Laterotergite with 4 bristles and ca. 10 setae. Mediotergite bare. Metepisternum with 3–5 [5] bristles and ca. 10 setae. Wing with yellowish tinge, otherwise clear. Length 1.9–2.8, 2.3 [2.6] mm (n=10). Ratio of length to width 2.5–2.8, 2.6 [2.5]. All veins light brown. Radial veins seem darker because of setae on both surface; other veins bare. Crossvein r-m apically disjunct. M-stem about 4 times as long as r-m. R5 slightly sinusoid. M2 not reaching wing margin, broken 0.8–1.2, 1.0 [0.9] times of m-stem length before it. Cu-fork begins very slightly before medial fork. Legs yellow, with fore-femur infuscated ventrally and mid- and hind femurs infuscated at apical fifth. Tarsi seem darker because of dense brown setae. Hind coxa with 4–5 [5] posteroleteral bristles basally, with one lateral and one posterior bristle apically, and with ca. 25 weaker setae along posterolateral margin. Ratio of femur to tibia for fore-, mid- and hind legs: 1.3–1.4, 1.4 [1.4]; 1.0, 1.0 [1.0]; 0.9–1.0, 0.9 [0.9]. Ratio tibia to first tarsomere for fore-, mid- and hind legs: 1.1–1.2, 1.2 [1.1]; 1.2, 1.2 [1.2]; 1.4–1.5, 1.5 [1.5]. Fore-tibia with a spur about 0.5–0.6, 0.5 [0.6] of fore basitarsus; mid-tibia with anterior spur about 0.3–0.4, 0.4 [0.3] and with posterior spur about 0.6–0.7, 0.6 [0.6] of mid basitarsus; hind tibia with anterior spur about 0.5– 0.6, 0.5 [0.6] and with posterior spur about 0.6–0.7, 0.6 [0.6] of mid basitarsus. Abdomen with 3 or 4 segments dorsally brown, laterally and ventrally yellow; succeeding segments brown to dark brown. Terminalia (Figs 4-11) two-coloured: basal part of gonocoxite and cerci yellow; apical part of gonocoxite and gonostylus brown; sternite 8 seems brownish because of dense setosity. Sternite 8 ovate with bluntly rounded apex, basal quarter membranous and bare, setae on apical quarter somewhat stronger than rest of them. Gonocoxite slightly oblong, with broad ventral incision about half of gonocoxite height. Dorsal medial margin of gonocoxite bulging mesiad at apical third. Cerci setose, basally membranous and fused, apically rounded, protruding well over gonocoxite. Ventral margin of gonocoxite angular. Basal half of gonocoxite bare, apical half with strong bristles. Dorsal branch of gonostylus rectangular, apically drawn into a pointed lobe, with a sclerotized comb on its ventral surface, as long as branch height. Setosity homogeneous without any deviations. Dorsal branch of gonostylus with a basal tubercle on its ventral surface close to base of medial branch; tubercle with two apical setae. Ventral branch of gonostylus bare, subequal to dorsal branch, with serrated lateral margin and with a hump on basal third of medial margin. The apical third of ventral branch is well tapering in ventral view. Medial branch of gonostylus divided at apical two third into two subequal lobes: ventral lobe apically rounded, medially somewhat swollen, slightly curved dorsad, with three setae on its ventral margin medially; dorsal lobe apically angular with two setae on apical third. Epiproct campaniform with small setulae that arise in lines of 4 to 8 from small ridges. Hypoproct consists of basally connected dorsal and ventral parts: both parts are with well-outlined lateral shoulders, the dorsal part is apically notched while the ventral part is apically convex.
Cordyla australica sp. n., male terminalia. 4 ventral view 5 lateral view 6 dorsal view 7 hypoproct, ventral view 8 sternite VIII, ventral view 9 epiproct, dorsal view. Scale bar 0.1 mm (4, 5, 6, 8) and 0.05 mm (7, 9). Abbreviations: cerc = cercus; epi = epiproct; gc = gonocoxite; gst d = dorsal branch of gonostylus; gst m = medial branch of gonostylus; gst v = ventral branch of gonostylus; hyp = hypoproct; st VIII = sternite VIII.
Cordyla australica sp. n., gonostylus. 10 internal view 11 lobes of medial branch of gonostylus. Scale bar = 0.1 mm (10) and 0.05 mm (11).
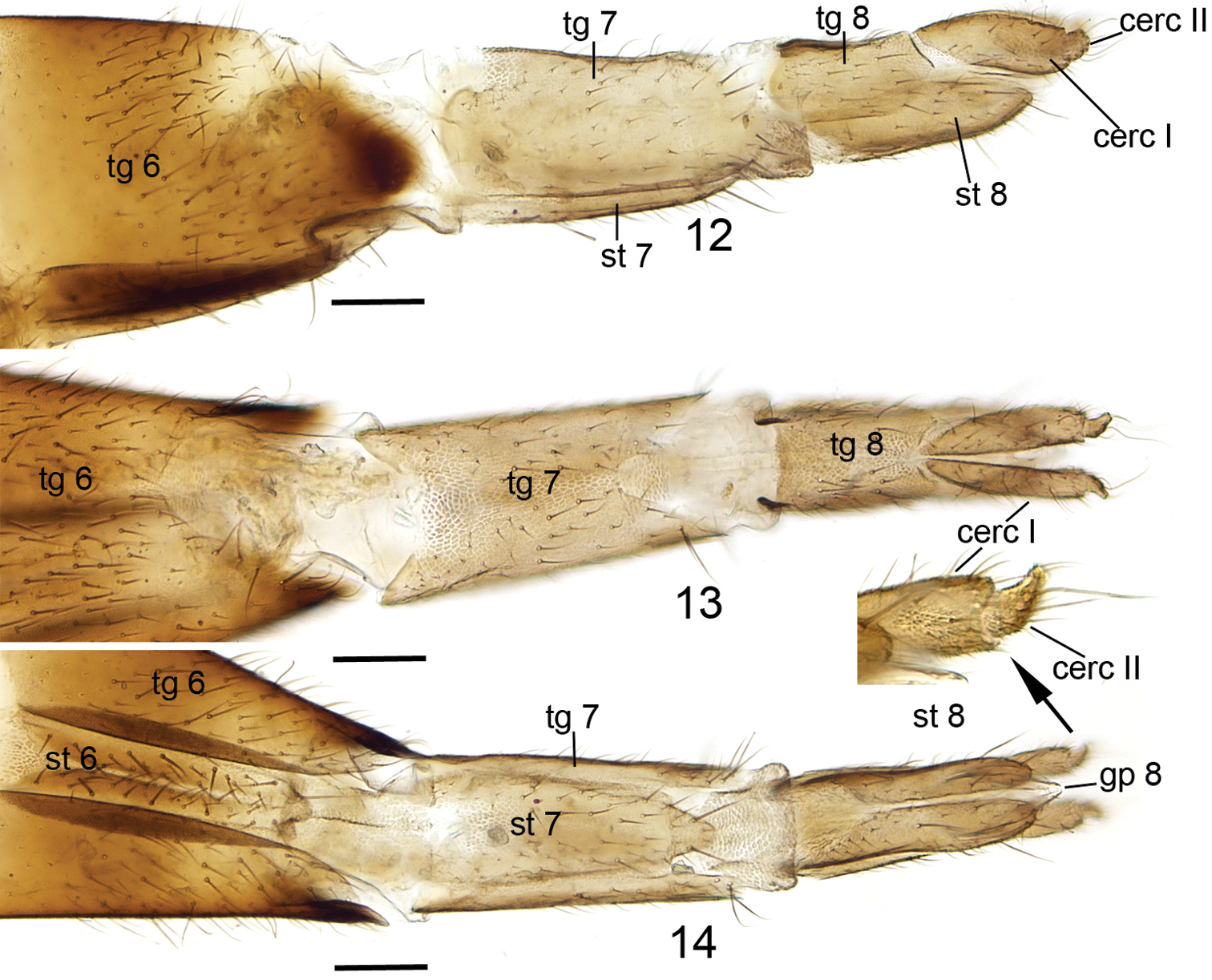
Female. Total length 2.2–3.4, 3.0 mm. Wing length 1.6–2.8, 2.2 mm. Ratio of length to width 2.5–2.9, 2.7. Antennae 2+9 segments. By setosity and coloration similar to male, except for entirely light brown abdomen in some specimen. Terminalia (Figs 12-14) light brown. Cercus two-segmented: apical segment small, apically tapering and bent laterad in ventral and dorsal views, with 2-3 long setae deviating from other setosity; basal segment long ovate, slightly sinusoidal and wider than apical segment. Gonapophysis VIII membranous, visible in ventral view, apically somewhat pointed. Tergite VIII rectangular, subequal to length of basal segment of cercus, apically angular, basally emarginated in dorsal view. Sternite VIII lateroapically conical, with deep ventral cleft. Tergite VII about twice as long as tergite VIII, with basal and apical broad incision dorsally and with a few apical stronger setae deviating from other setosity. Sternite VII apically conical, subequal to length of tergite VII. Tergite VI with conical and sclerotized apical edge laterally and with broad incision apicodorsally.
Cordyla australica sp. n. female terminalia. 12 lateral view 13 dorsal view 14 ventral view. Scale bar = 0.1 mm. Abbreviations: cerc= cercus, gp= gonapophysis, st= sternite, tg= tergite.
Unknown.
The species is named to indicate its discovery in Australia.
The species shows high variation (up to 35%) in body size that is, however, continuous and observed also in other Cordyla species (e.g. in European Cordyla crassicornis Meigen, 1818: OK pers. obs.) and colour variation including some specimens darker than others. Despite of that, we have not found any species level morphological differences within the studied material.
According to structure of male terminalia, Cordyla australica sp. n. belongs to the Cordyla murina species-group as outlined by
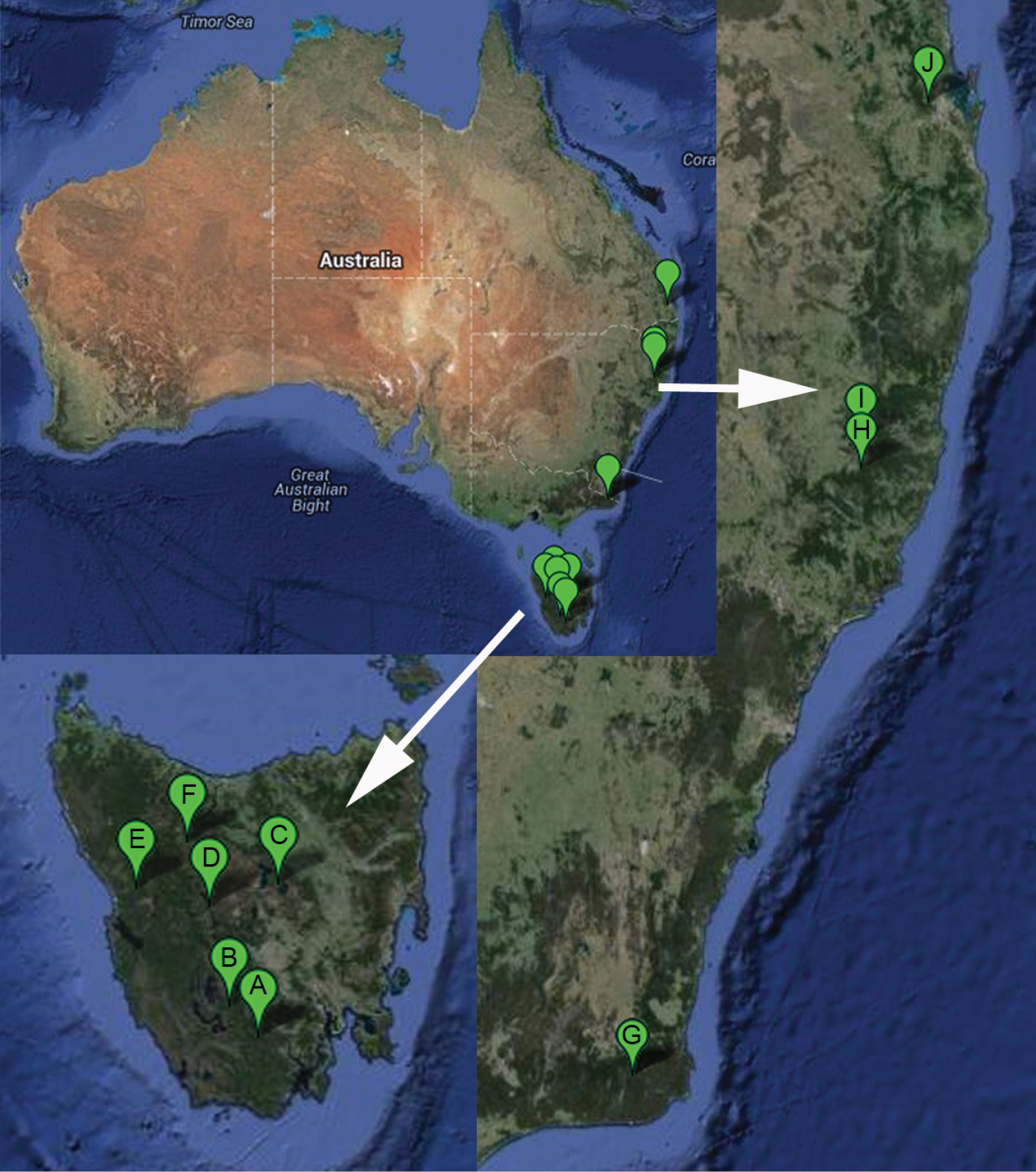
In spite of wide range of the studied Australian samples (SSO pers. obs.), Cordyla australica sp. n. is apparently found only in wet forest of eastern Australia and Tasmania (Fig. 15).
Collecting localities of Cordyla australica sp. n. in the continental Australia and Tasmania. A Tasmania, Warra long-term ecological research site B Tasmania, Southwest National Park C Tasmania, Central Plateau D Tasmania, King William Creek E Tasmania, Ewart creek F Tasmania, Cradle Mountain G Victoria, Coopracambra National Park H New South Wales, Werrikimbe National Park I New South Wales, Carrai State Forest J Queensland, Brisbane Forest Park.
OK was supported by the grants 9174 and 8583 of the Estonian Science Foundation and by target financing project SF0170160s08. SSO was supported by the postdoctoral research fellowship of the FAPESP (grants 2008/52324-6 and 2012/51577-3) and the Geddes Postgraduate Award 2010-2011 from the Australian Museum. We are grateful to Dr. M. Jaschhof (Müncheberg, Germany), Dr. Y. Brodin (Stockholm, Sweden) and Dr. D.R. Britton (Sydney, Australia) for loaning material for study. Dr. M. Jaschhof and Dr. D. Bickel (Sydney, Australia) are acknowledged for their help including comments on the material. Mr. P. Chandler (Melkshamn, United Kingdom), Dr. P. Kerr (Sacramento, USA) and an anonymous reviewer are thanked for their comments and suggestions on the manuscript.
Occurence data of Australian Cordyla. (doi: 10.3897/zookeys.342.6045.app) File format: Mircosoft Excel file (xls).
Explanation note: Occurence data of a new species – Cordyla australica Kurina & Oliveira, 2013 – based on material from Austarlian mainland (Victoria, New South Wales, Queensland) and Tasmania are provided.