(C) 2012 Omar Torres-Carvajal. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
We describe a new species of Imantodes from the Chocó region of northwestern Ecuador. The new species differs most significantly from all other congeners in lacking a loreal scale. We analyze the phylogenetic relationships among species of Imantodes based on two mitochondrial genes, and postulate that the new species and Imantodes lentiferus are sister taxa. A key to the species of Imantodes from Ecuador is presented.
Chocó, Dipsadinae, Ecuador, Imantodes, snakes, systematics
The New World colubrid snake clade Dipsadinae Bonaparte 1838 includes more than 400 extant species assigned to approximately 25 taxa traditionally ranked as genera (
Studies on phylogenetic relationships and species limits among dipsadines are scarce. However, recent work provides strong evidence from DNA sequence data for a clade containing Imantodes and Leptodeira, although monophyly of Imantodes remains controversial (
Three species of blunt-headed vine snakes are known from Ecuador; Imantodes inornatus and Imantodes lentiferus occur west and east of the Andes, respectively, whereas Imantodes cenchoa is known from both versants (
All type specimens of the new species described in this paper are listed in the type series below, and were deposited at the Museo de Zoología, Pontificia Universidad Católica del Ecuador, Quito (QCAZ), the Museo Ecuatoriano de Ciencias Naturales, Quito (DHMECN), and the Amphibian & Reptile Diversity Research Center at The University of Texas at Arlington, USA (UTA). Specimens of other species of Imantodes examined in this study are listed in the appendix. Snout-vent length (SVL) and tail length (tL) measurements were recorded to the nearest millimeter. All other measurements were made with digital calipers and recorded to the nearest 0.01 mm. Sex was determined by noting the presence of hemipenes, everted or by tail dissection. Partially everted hemipenes were prepared following standard techniques (
Total genomic DNA was digested and extracted from liver or muscle tissue using a guanidinium isothiocyanate extraction protocol. Tissue samples were first mixed with Proteinase K and lysis buffer and digested overnight prior to extraction. DNA samples were quantified using a Nanodrop® ND-1000 (NanoDrop Technologies, Inc), re-suspended and diluted to 25 ng/ul in ddH2O prior to amplification.
We amplified 1674 nucleotides (nt) encompassing two mitochondrial loci, NADH dehydrogenase subunit 4 (ND4, 651 nt) and cytochrome b (cyt-b, 1023 nt) from five individuals of Imantodes cenchoa, three of Imantodes lentiferus, three of the new species described herein and one of Leptodeira septentrionalis. Cyt-b was amplified using the primers Gludg, L14910, and H16064 (
Vouchers, locality data, and GenBank accession numbers of taxa and gene regions included in this study. Asterisks indicate new sequences obtained for this study.
| Taxon | Voucher | Locality | Genbank accession number | |
|---|---|---|---|---|
| Cyt-b | ND4 | |||
| Imantodes cenchoa | MPEGLJV 5763 | Brasil: Para | EF078556 | EF078508 |
| Imantodes cenchoa | JMD 1616 | Colombia: Chocó | GQ334486 | GQ334587 |
| Imantodes cenchoa | MHUA R-14290 | Colombia: Antioquia | GQ334484 | GQ334585 |
| Imantodes cenchoa | MHUA R-14500 | Colombia: Antioquia | GQ334485 | GQ334586 |
| Imantodes cenchoa | MVZ 149878 | CostaRica: Limón | EF078553 | EF078505 |
| Imantodes cenchoa | QCAZ 11115 | Ecuador: Santo Domingo de los Tsáchilas | *KC176244 | *KC176256 |
| Imantodes cenchoa | QCAZ 6300 | Ecuador: Esmeraldas | *KC176248 | *KC176260 |
| Imantodes cenchoa | QCAZ 4207 | Ecuador: Orellana | *KC176247 | *KC176259 |
| Imantodes cenchoa | UTA R-42360 | Guatemala: Izabal | EF078554 | EF078506 |
| Imantodes cenchoa | SIUCR 03724 | Panama: Cocle | EF078555 | EF078507 |
| Imantodes cenchoa | CORBIDI 3794 | Peru: Tumbes | *KC176245 | *KC176257 |
| Imantodes cenchoa | CORBIDI 8823 | Peru: San Martín | *KC176246 | *KC176258 |
| Imantodes chocoensis sp. n. | QCAZ 7978 | Ecuador: Esmeraldas | *KC176249 | *KC176261 |
| Imantodes chocoensis sp. n. | QCAZ 7984 | Ecuador: Esmeraldas | *KC176250 | *KC176262 |
| Imantodes chocoensis sp. n. | UTA R-60205 | Ecuador: Esmeraldas | *KC176254 | *KC176266 |
| Imantodes gemmistratus | UTA R-45922 | Guatemala: San Marcos | GQ334487 | GQ334588 |
| Imantodes gemmistratus | LSUMZ 39541 | Mexico: Sonora | EF078558 | EF078510 |
| Imantodes gemmistratus | UTA R-51979 | Mexico: Sinaloa | EF078557 | EF078509 |
| Imantodes inornatus | MHUA R-14540 | Colombia: Antioquia | GQ334488 | GQ334589 |
| Imantodes inornatus | ASL 307 | CostaRica | GQ334489 | GQ334590 |
| Imantodes inornatus | MVZ 204109 | CostaRica: Cartago | EF078559 | EF078511 |
| Imantodes inornatus | MVZ 204110 | CostaRica: Heredia | EF078560 | EF078512 |
| Imantodes lentiferus | MPEGLJV 5581 | Brazil: Para | EF078562 | EF078514 |
| Imantodes lentiferus | MPEGLJV 6880 | Brazil: Amazonas | EF078561 | EF078513 |
| Imantodes lentiferus | QCAZ 8377 | Ecuador: Pastaza | *KC176251 | *KC176263 |
| Imantodes lentiferus | QCAZ 8488 | Ecuador: Zamora Chinchipe | *KC176252 | *KC176264 |
| Imantodes lentiferus | QCAZ 9187 | Ecuador: Morona Santiago | *KC176253 | *KC176265 |
| Leptodeira septentrionalis | MHUA R-14403 | Colombia: Antioquia | GQ334528 | GQ334632 |
| Imantodes septentrionalis | QCAZ 10550 | Ecuador: Esmeraldas | *KC176243 | *KC176255 |
Editing, assembly, and alignment of sequences were performed with Geneious ProTM 5.3 (Drummond et al., 2010). Phylogenetic relationships were assessed under a Bayesian approach in MrBayes 3.2.0 (Ronquist and Huelsenbeck, 2003). The model of character evolution for each gene was obtained in JModeltest (Posada, 2008) under the Akaike information criterion. Genes were combined into a single dataset with two partitions, one per gene. Four independent analyses were performed to reduce the chance of converging on a local optimum. Each analysis consisted of five million generations and four Markov chains with default heating values. Trees were sampled every 1000 generations resulting in 5000 saved trees per analysis. Stationarity was confirmed by plotting the log-likelihood scores per generation in the program Tracer 1.2 (Rambaut and Drummond, 2003). Additionally, the standard deviation of the partition frequencies and the potential scale reduction factor (Gelman and Rubin, 1992) were used as convergence diagnostics for the posterior probabilities of bipartitions and branch lengths, respectively. Adequacy of mixing was assessed by examining the acceptance rates for the parameters in MrBayes and the effective sample sizes (ESS) in Tracer. After analyzing convergence and mixing, 500 trees were discarded as “burn-in” from each run. We then confirmed that the four analyses reached stationarity at a similar likelihood score and that the topologies were similar, and used the resultant 18, 000 trees to calculate posterior probabilities (PP) for each bipartition on a 50% majority rule consensus tree.
The taxonomic conclusions of this study are based on the observation of morphological features and color patterns, as well as inferred phylogenetic relationships. We consider this information as species delimitation criteria following the general species concept of
Proposed standard English name: Chocoan blunt-headed vine snakes
Proposed standard Spanish name: Cordoncillos del Chocó
urn:lsid:zoobank.org:act:D47B3B06-B8B2–4FDC-A54E-3A1F99091044
– QCAZ 7984 (Figs. 1, 2), an adult male from 4 km N Durango, 1.0283°N, 78.5950°W (DD), 253 m, Provincia Esmeraldas, Ecuador, collected on 24 April 2007 by E. Carrillo-Ponce, I. G. Tapia, and E. E.Tapia.
– ECUADOR: Provincia Carchi: DHMECN 6753, Río San Juan, 1.1858°N, 78.5006°W (DD), 243 m, collected on 12 September 2009 by M. Yánez-Muñoz, L. Oyagata, and M. Altamirano; DHMECN 6757, Sendero Awa, 1.1643°N, 78.5071°W (DD), 257 m, collected on 16 September 2009 by M. Yánez-Muñoz, L. Oyagata, and M. Altamirano. Provincia Esmeraldas: UTA R-60205, San Lorenzo-Santa Rita, 1.0321°N, 78.7138°W (DD), 115 m, collected on 21 March 2008 by M. Alcoser, R. Betancourt, P. Loaiza L., L. Oyagata, S. Ramírez J., J. W. Streicher, C. Tobar, and E. N. Smith; QCAZ 7978, same collection data as holotype; QCAZ 10185, 4 km W Alto Tambo, 0.91241°N, 78.5809°W (DD), collected on 18 December 2009 by S. Poe, L. Gray, and I. Latella; QCAZ 10710, Playa de Oro, Estero Pote and Estero Angostura, lower part of Cotacachi Cayapas Ecological Reserve, 0.8285°N, 78.7220°W (DD), collected on 27 November 1994 by E. Toral-Contreras, V. Ortiz, and F. Nogales.
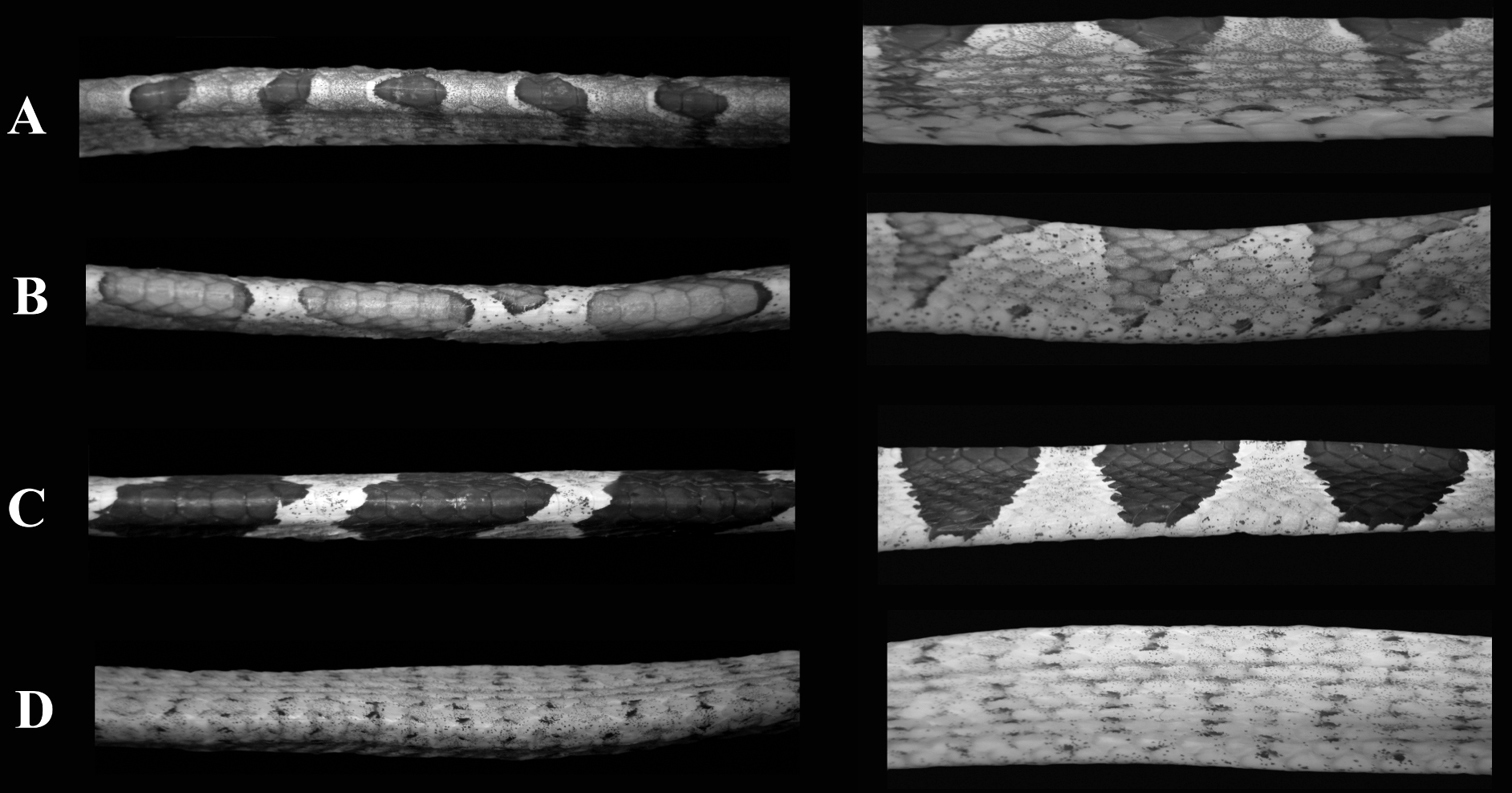
Imantodes chocoensis differs from all other known congeners in lacking a loreal scale. It can be further distinguished from its sister species (see Phylogenetic relationships) Imantodes lentiferus by having 17 longitudinal rows of dorsal scales at midbody and at nearly one head length anterior to the cloaca (15 in Imantodes lentiferus), more ventrals (t = 7.27, P < 0.001), more subcaudals (t = -4.31, P < 0.001), more postoculars (2–3, mean = 2.43 ± 0.51; 1–2, mean = 1.81 ± 0.39 in Imantodes lentiferus), more infralabials (12–15, mean = 13.21 ± 0.80; 9–12, mean = 10.68 ± 0.60 in Imantodes lentiferus), and smaller dark blotches on dorsum (Fig. 3). Among other species of Imantodes known from Ecuador, the new species differs further from Imantodes inornatus (N = 2–3) in having more ventrals (t = 6.74, P < 0.001), more subcaudals (t = -5.05, P = 0.002), more infralabials (9–11, mean 10.00 ± 0.89 in Imantodes inornatus), a longer head (head length/width 1.54–1.71, mean = 1.63 ± 0.07 in Imantodes chocoensis sp. n.; 1.29–1.61, mean = 1.45 ± 0.16 in Imantodes inornatus), and dark blotches on dorsum (dark spots and flecks in Imantodes inornatus; Fig. 3). The new species can also be distinguished from Imantodes cenchoa by having a single anal scale (vrs. two), fewer ventrals (t = 7.73, P < 0.001), fewer subcaudals (t = -4.04, P < 0.001), more infralabials (7–12, mean 9.92 ± 0.85 in Imantodes cenchoa), and dorsal dark blotches that include two or fewer vertebral scales and do not extend laterally onto ventrals (blotches are larger in Imantodes cenchoa and extend onto lateral tips of ventrals; Fig. 3). Scale counts and measurements of species of Imantodes from Ecuador are presented in Table 2.
. Scale counts and measurements of species of Imantodes from Ecuador. Range (first line) and mean ± SD (second line) are presented when appropriate. Sample size is presented in parentheses if different from that in heading.
| Character | Imantodes cenchoa N = 42 | Imantodes inornatus N = 6 | Imantodes lentiferus N = 30 | Imantodes chocoensis sp. n. N = 7 |
|---|---|---|---|---|
| Longitudinal scale rows on neck | 17 | 17 | 15–17 15.07 ± 0.37 | 17 |
| Longitudinal scale rows at midbody | 17 | 17 | 15 | 17 |
| Longitudinal scale rows anterior to cloaca | 17 | 13–15 13.67 ± 1.03 | 15 | 17 |
| Ventrals | 249–280 262.62 ± 6.22 | 203–219 210.67 ± 5.99 | 216–237 226.80 ± 5.03 | 232–251 243.14 ± 5.84 |
| Subcaudals | 155–189 (37) 165.95 ± 8.01 | 109–126 (5) 117.80 ± 6.18 | 130–151 (27) 139.85 ± 5.89 | 140–161 (6) 151.83 ± 7.41 |
| Anals | 2 | 1–2 1.17 ± 0.41 | 1 | 1 |
| Anterior temporals | 1–3 2.13 ± 0.51 | 1–2 1.08 ± 0.29 | 1 | 1–2 1.43 ± 0.51 |
| Posterior temporals | 2–5 2.80 ± 0.53 | 1–2 1.92 ± 0.29 | 1–3 2.07 ± 0.36 | 2 |
| Loreals | 1 | 1 | 1 | 0 |
| Preoculars | 1–3 1.36 ± 0.53 | 1–2 1.25 ± 0.45 | 1–2 1.03 ± 0.18 | 1 |
| Postoculars | 1–4 2.11 ± 0.38 | 2–3 2.08 ± 0.29 | 1–2 1.81 ± 0.39 | 2–3 2.43 ± 0.51 |
| Supralabials | 7–9 7.99 ± 0.33 | 8 | 7–9 8.05 ± 0.34 | 9 |
| Infralabials | 7–12 9.92 ± 0.85 | 9–11 10.00 ± 0.60 | 9–12 10.68 ± 0.60 | 12–15 13.21 ± 0.80 |
| Genials | 2 | 2 | 2 (28) | 2 |
| Head length/width | 1.35–1.80 1.56 ± 0.11 | 1.29–1.62 1.51 ± 0.13 | 1.37–1.91 1.62 ± 0.14 | 1.54–1.71 1.63 ± 0.07 |
| Tail length/Total length | 0.28–0.33 (37) 0.30 ± 0.01 | 0.27–0.30 (5) 0.28 ± 0.01 | 0.28–0.34 (27) 0.31 ± 0.01 | 0.29–0.32 (6) 0.31 ± 0.01 |
| Maximum SVL (cm) | 107.90 | 64.00 | 70.30 | 74.40 |
| Maximum Total length (cm) | 152.10 | 91.50 | 101.40 | 107.50 |
Male (Figs. 1, 2); SVL = 66.30 mm; tail length = 30.40 mm; head width = 7.98 mm; head length = 13.26 mm; head height = 5.37 mm.
Short, blunt head 1.7 times longer than broad and 2.5 times longer than deep; head abruptly distinct from neck, three times wider than thinnest part of neck and also slightly wider than greatest width of body; eye large and protuberant, occupying 27% of length of head, with elliptical pupil visible from anterior, lateral, dorsal, and ventral aspects; rostral 1.6 times wider than high, concave in anterior view, and narrowly visible from above; paired prefrontals extending anteroventrally to level of center of eye, each in contact with its mate and with frontal, supraocular, preocular, nasal, and internasal; frontal pentagonal, 1.6 times longer than wide (greatest width), and about 1.2 times longer than distance from its anterior edge to tip of snout; supraocular anteriorly narrow and posteriorly nearly as wide as greatest frontal width; broad parietals, about 1.3 times longer than wide; interparietal suture 1.2 times longer than length of frontal, and 1.4 times longer than distance from frontal to tip of snout; nasal plate single, centrally pierced by large naris (0.79 mm in diameter), in contact with rostral anteriorly, internasal dorsally, prefrontal posterodorsally, preocular posteriorly, and first and second supralabials ventrally; loreal absent; one large and high preocular; two postoculars (an extra tiny scale on left side ventrally), the lower somewhat less than half the size of the upper; temporals 2+2+3; supralabials 9, first and second in contact with nasal, fourth in contact with preocular, and fourth to seventh bordering the orbit; infralabials 13, with first six in contact with anterior genial, and sixth to eighth touching posterior genial; first pair of infralabials in contact medially behind mental; anterior and posterior genials nearly equal in length; gular scales with posterolateral apical pit.
Body higher than wide, rounded ventrolaterally; dorsal scales smooth, juxtaposed or subimbricate; dorsal body scales in 17 rows throughout; scales of vertebral row conspicuously enlarged, 2.5 times wider than adjacent dorsals, with concave posterior margins; ventrals 242; anal plate single; subcaudals 161.
Color in preservative of holotype (Figs 1, 2). Dorsal background light brown, with a longitudinal series of 63 dark brown middorsal blotches from head to cloaca; dark middorsal blotches longer anteriorly, 2–3 vertebral scales long, than posteriorly, 1–2 vertebral scales long, and extending laterally 1–3 (anteriorly) or more (posteriorly) dorsal scale rows, but never reaching ventral scales; each dark middorsal blotch irregularly bordered anteriorly and posteriorly by thin cream line; ventral aspect of body yellowish cream with dark brown spots and flecks; ventral aspect of tail yellowish cream with spots concentrating midventrally; dorsal surface of head light brown with several dark brown spots and two short dark stripes extending from posterior aspect of parietals to a point just posterior to head; ventral surface of head whitish cream.
Hemipenes (Fig. 4). The right hemipenis of the paratype DHMECN 6753 of Imantodes chocoensis was removed, fully everted and expanded (Fig. 4). The organ is bulbous and relatively long, 11.2 mm in length, and when adpressed to the outside of the tail it extends from the cloaca to the sixth subcaudal scale. The organ is longer than wide (width 46% of length), unilobed, symmetrical, calyculate, capitate, and arched towards the sulcal side. The sulcus spermaticus is simple, linear, semicentripetal, and thin, demarcated by thick bordering tissue at the base, particularly at the anterior border, and ending on the surface of the capitulum facing medially. The capitulum is ornamented with papillated calyces, spinulated proximally. The capitulum, approximately 45% the length of the hemipenis, is slightly demarcated by a groove, more prominent on the sulcal side and joining the sulcus spematicus. In the asulcate side the base of the capitulum has more prominent spines. Truncus covered by large spines, on the sulcate and asulcate and surfaces, 23 in total, 13 to the right of the sulcus spermaticus and 10 to the left, and has a few rows of small spines, two at the base on the asulcate side and two to three rows just right of the sulcus spermaticus.
Holotype of Imantodes chocoensis sp. n. in dorsal (left) and ventral (right) views. Photographs by OTC.
. Head of holotype of Imantodes chocoensis sp. n. in dorsal (top), lateral (middle) and ventral (bottom) views. Photographs by OTC.
Body segments of species of Imantodes from Ecuador in dorsal (left) and lateral (right) views. A Imantodes chocoensis sp. n. (DHMECN 6753, paratype) B Imantodes lentiferus (DHMECN 8345) C Imantodes cenchoa (DHMECN 7826) D Imantodes inornatus (DHMECN 5661). Photographs by MYM.
Right hemipenis of Imantodes chocoensis sp. n. (DHMECN 6753, paratype) in sulcal (A), asulcal (B), and lateral (C) views D close-up of distal end showing spines interrupted by sulci. Photographs by MYM.
Intraspecific variation in scale counts and measurements in Imantodes chocoensis sp. n. is presented in Table 2. Color in life of paratypes UTA R-60205 and DHMECN 6753 (Fig. 5) is similar to color in preservative of holotype; iris copper brown. Middorsal blotches from head to cloaca vary between 55–66; one specimen (UTA R-60205) had one middorsal blotch covering five vertebral scales.
Paratypes of Imantodes chocoensis sp. n. UTA R-60205 (top), DHMECN 6753 (bottom). Photographs by ENS and MYM.
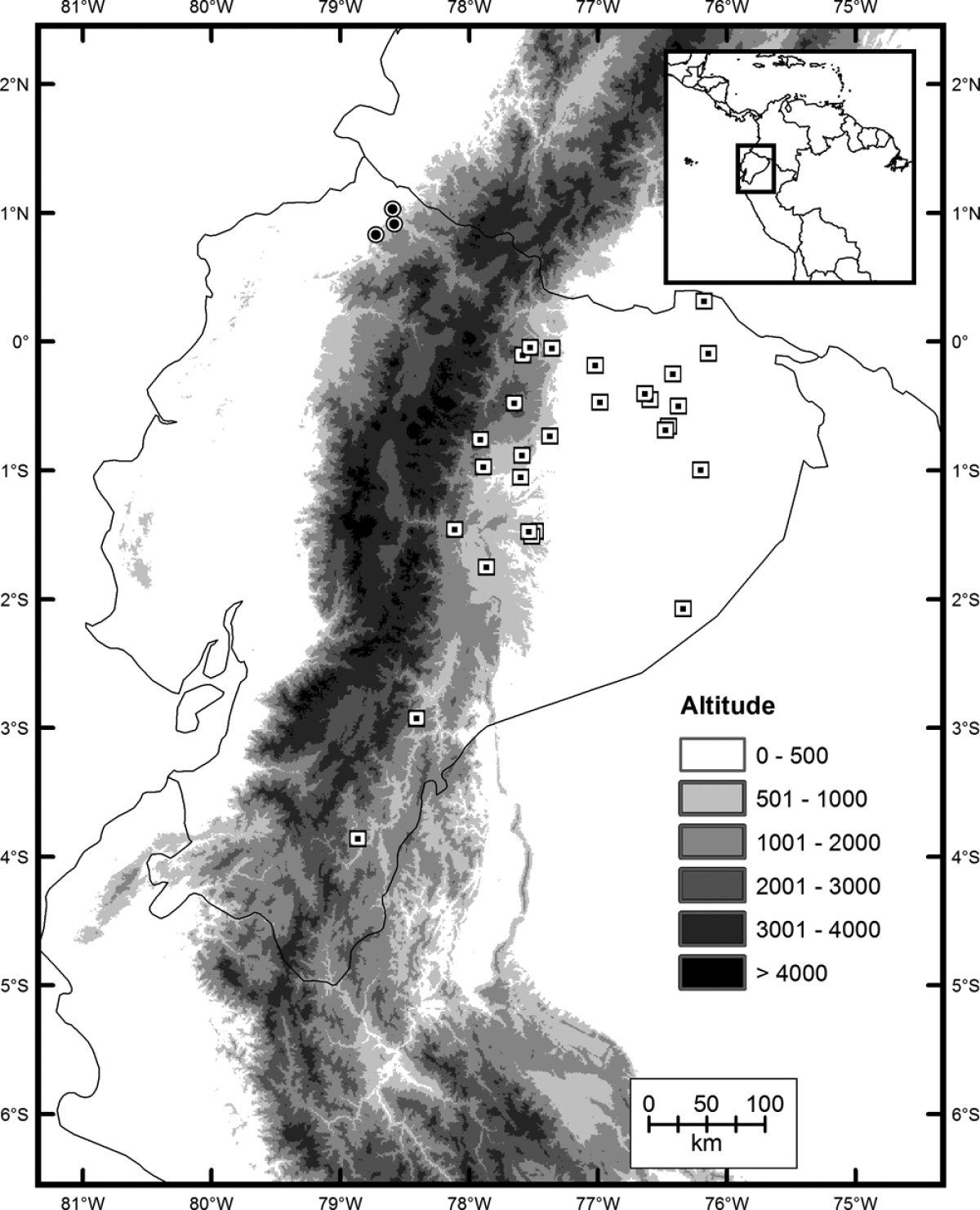
Imantodes chocoensis inhabits Chocoan rainforests on the Pacific coast in northern Ecuador (Fig. 6). It occurs in lowland evergreen forest (
Distribution of Imantodes chocoensis sp. n. (circles) and its sister species Imantodes lentiferus (squares) in Ecuador.
The specific epithet chocoensis is an adjective derived from Chocó, the very humid tropical region comprising the Pacific coast of northern Ecuador, Colombia and Panama (
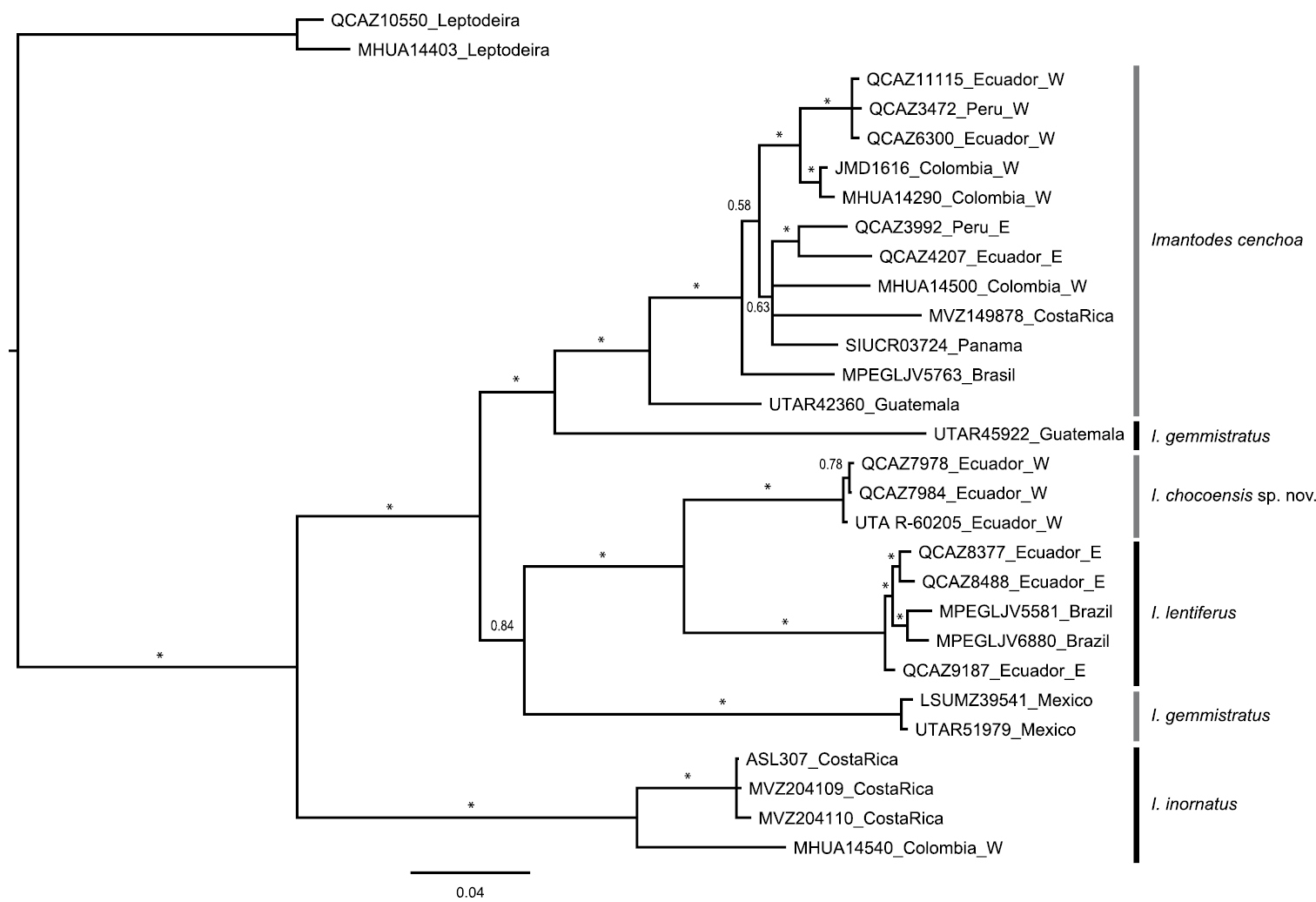
Selected models of evolution for sampled fragments of ND4 and cyt-b genes were HKY+I+G and TPM2uf+I+G, respectively. The resulting 50% majority rule consensus tree (Fig. 7) supports strongly (PP=1) a sister taxon relationship between Imantodes chocoensis sp. n. and Imantodes lentiferus, as well as the exclusivity (
Majority rule (50%) consensus tree of 18, 000 trees obtained from a Bayesian analysis of two mitochondrial genes (cyt-b, ND4) and 29 specimens. Asterisks correspond to posterior probability values > 0.99. Voucher numbers followed by country of collection are indicated for each terminal. E: east of the Andes, W: west of the Andes.
Monophyly of Imantodes remains controversial, but we refrained from testing it without better taxon and character sampling. Previous phylogenetic studies based on DNA sequence data have failed to support the monophyly of Imantodes as currently circumscribed (
| 1 | Longitudinal scale rows at midbody 17 | 2 |
| – | Longitudinal scale rows at midbody 15 | Imantodes lentiferus |
| 2 | Vertebral scales 2.5–4 times wider than adjacent dorsal scales; dorsal color pattern with conspicuous dark blotches(Fig. 3) | 3 |
| – | Vertebral scales similar in size or slightly wider than adjacent dorsal scales; dorsal color pattern with dark spots and speckles (Fig. 3) | Imantodes inornatus |
| 3 | Loreal present; dorsal blotches include more than two vertebral scales and extend onto edge of ventrals (Fig. 3) | Imantodes cenchoa |
| – | Loreal absent; dorsal blotches include two or less vertebral scales and do not extend laterally onto ventrals (Fig. 3) | Imantodes chocoensis |
We thank M. Altamirano and M. Morales for allowing access to DHMECN specimens. We also thank P. Venegas (CORBIDI, Lima, Peru) for the loan of Peruvian tissue samples, M.J. Pozo and J. Reyes for obtaining some of the DNA sequences used in this study, C.J. Franklin, L.A. Oyagata, and J.C. Streicher for field assistance, and P. Santiana for editing some pictures. Specimens of the new species described in this paper were collected under collection permits 008–09 IC-FAU-DNB/MA to PUCE, and 001–08 IC-FAU-DNBAPVS/MA to ENS, issued by Ministerio del Ambiente, República del Ecuador. Funds from Secretaría Nacional de Educación Superior, Ciencia y Tecnología del Ecuador were received by OTC (PIC-08–0000470) for DNA sequencing and MYM (PIC-08–0000478) for field work. Financial support for part of the field and laboratory expenses was provided by the Instituto Bioclon (Mexico) to ENS.
Imantodes cenchoa – COLOMBIA: Meta: UTA R-3364–65, Serranía de la Macarena, peak near Caño Sardinata, 30 Km WSW Vista Hermosa, 1300 ft; COSTA RICA: Limón: UTA R-12903, Approximately 17 km WSW Puerto Limón, between Río Blanco and Río Toro, 150 m; ECUADOR: Carchi: QCAZ 4419–20, Chical, road to San Pablo, 0.9211°N, 78.1809°W, 1248 m; Cotopaxi: QCAZ 1203, San Francisco de las Pampas, 0.4332°S, 78.9667°W, 1600 m; QCAZ 2771–72, 6 km west of Guasaganda, 0.8206°S, 79.1171°W, 350 m; QCAZ 8888, Naranjito, Bosque Integral Otonga (BIO), 0.4147°S, 79.0007°W; Esmeraldas: QCAZ 2248, La Mayronga, Lagarto, 1.0420°N, 79.2800°W; QCAZ 6669, Alto Tambo, El Placer, La Carolina, 0.7044°N, 78.2011°W, 500 m; QCAZ 7589–90, Bilsa, 0.6201°N, 79.9307°W; QCAZ 7930, Reserva Ecológica Bilsa, 0.3588°N, 79.7180°W, 590 m; UTA R-55946, road Alto Tambo - San Lorenzo, 1.33311°N, 78.61687°W, 336 m; UTA R-55947, road Lita - San Lorenzo, 1.07750°N, 78.65785°W, 91 m; UTA R-55948, Tunda Loma Lodge, 1.18333°N, 78.75349°W, 37 m; QCAZ 7979–81, 4 km N Durango 1.0283°N, 78.5950°W, 253 m; QCAZ 10203, near Lita, 0.8886°N, 78.5288°W; QCAZ 10706, lowlands of Reserva Ecológica Cotacachi Cayapas, Playa de Oro, Pote and Angostura estuaries, 0.8284°N, 78.7220°W; Guayas: QCAZ 9118, Bosque Protector Cerro Blanco, 2.1758°S, 80.0216°W, 213 m; Morona-Santiago: UTA R-37985–86, Limón; Napo: QCAZ 8422–23, río Hollín, 0.6950°S, 77.7307°W; Orellana: QCAZ 1742, 1752, Parque Nacional Yasuní, Block 16, Maxus road between Pompeya south and Iro, 0.6755°S, 76.3552°W, 110 m; QCAZ 3423, Parque Nacional Yasuní, km 80 road to Pompeya-Iro, Belle river bridge area, 0.8401°S, 76.3017°W; QCAZ 3650, Parque Nacional Yasuní, Estación Científica Yasuní, 0.6750°N, 76.3892°W, 220 m; QCAZ 8920–21, Florencia, 0.8966°S, 75.4370°W; QCAZ 9141, 9143, Parque Nacional Yasuní, road to Pompeya - Iro, km 10, 0.4598°S, 76.5931°W, 271 m; QCAZ 9528, southern bank of río Napo, Eden, 0.4983°S, 76.0711°W, 216 m; QCAZ 9551, northern bank of río Napo, San Vicente, 0.6790°S, 75.6511°W, 196 m; QCAZ 1738, Parque Nacional Yasuní, Block 16, Maxus road between Pompeya south and Iro, 0.6755°S, 76.3552°W, 100 m; El Oro: QCAZ 8992, Bella María, near Valle Hermoso, 3.5116°S, 79.8202°W, 282 m; Pastaza: QCAZ 4255, road to El Triunfo, Arajuno, 1.2325°S, 77.6876°W, 800 m; QCAZ 8161, around Villano, AGIP petroleum camp, K4, 1.4706°S, 77.4868°W; QCAZ 8378, around Villano, AGIP petroleum camp, K10 Unidad 3, 1.4727°S, 77.5359°W, 430 m; QCAZ 8914, Bataburo Lodge, 1.2500°S, 76.6667°W, 220 m; UTA R-15852, Río Cononaco, S side, within a few km of Perú border; Pichincha: EPN 1315354, Los Bancos, sector Milpe; Santo Domingo de los Tsáchilas: QCAZ 11048, La Concordia, Bosque Protector la Perla, 0.0570°S, 79.3590°W; QCAZ 11115, Hostería Tinalandia, near Alluriquín, 0.2965°S, 79.0523°W; Sucumbíos: QCAZ 1488–89, Reserva de Producción Faunística Cuyabeno, 0.2597°S, 75.8886°W; QCAZ 2564, Reserva de Producción Faunística Cuyabeno, research station (PUCE), 0.0018°S, 76.1755°W; GUATEMALA: Alta Verapaz: UTA R-46623, Finca Rubelpec, 650 m; UTA R-46624, Finca San Juan, 590 m; UTA R-46625, Finca San Juan, 550 m; UTA R-26194, N slope Sierra de lad Minas, Finca Pueblo Viejo, W slope Río Tinajas/Río Chiquito divide, 5.25 air km SSE, 1200–1500 m; UTA R-26193, N slope Sierra de las Minas, Finca Pueblo Viejo, 4.0 air km SE Pueblo Viejo, 0 m; UTA R-26195, Vicinity of Pueblo Viejo; Baja Verapaz: UTA R-42430, Purulhá, Finca Sabó, 15°14.87'N, 090°9.87'W, 1170 m; Escuintla: UTA R-28376, Finca Medio Monte, 720 m; UTA R-22804, 26198, S slope Volcán de Agua, Finca Rosario Vista Hermosa; Huehuetenango: UTA R-44705, 44716, Barillas, Finca Chiblac Buena Vista, 15°53.18'N, 091°14.73'W, ca. 930 m; UTA R-45491, Barillas, Finca Chiblac Buena Vista camino a Las Nubes, 15°52.12'N, 091°13.95'W, 1330 m; UTA R-42305, Sierra de Los Cuchumatanes, Finca Chiblac Buena Vista (now Aldéa Buenos Aires), 15°52.97'N, 091°14.80'W, 975 m; Izabal: UTA R-28373, E slope Montañas del Mico, 12.0 km WSW Puerto Santo Tomás, 786 m; UTA R-28375, E slope Montañas del Mico, along Río San Ramoncito, above Las Escobas, 226 m; UTA R-26205, El Estor, El Chupón, 2.0 m; UTA R-26206, El Estor, El Zapotillo, 2 m; UTA R-38217, Livingston, Aldéa La Libertad, Km 285 a Petén, 75 m; UTA R-39234, Livingston, Sierra de Santa Cruz, Cerro 1019, 940 m; UTA R-39536, Los Amates, Quirigua, Sitio Arqueológico; UTA R-29871, Los Amates, Sierra del Espiritu Santo, Aldéa San Antonio; UTA R-32996, Los Amates, Sierra del Espiritu Santo, Aldéa San Antonio, ca. 500 m; UTA R-28381, Los Amates, Sierra del Espiritu Santo, S side Cerro del Nylon, 725 m; UTA R-29870, Montañas del Mico, 11.6 km WSW Puerto Santo Tomás, 744 m; UTA R-16022–23, Montañas del Mico, 5.1 km WSW Puerto Santo Tomás, Las Escobas, 104 m; UTA R-20833–34, Montañas del Mico, 5.1 km WSW Puerto Santo Tomás, Las Escobas, 150–250 m; UTA R-28374, Montañas del Mico, 5.1 km WSW Puerto Santo Tomás, Las Escobas, 150 m; UTA R-29868–69, Montañas del Mico, 8 km WSW Puerto Santo Tomás, 457 m; UTA R-46686, Montañas del Mico, Crest of Cerro Las Escobas, near Guatel Tower, 860 m; UTA R-38216, 39535, Morales, Sierra de Caral, Aldéa Negro Norte, ca. 1150 m; UTA R-42358, Morales, Sierra de Caral, Aldéa Negro Norte, ca. 1100 m; UTA R-32998, Morales, Sierra de Caral, Aldéa Negro Norte, Cerro Negro Norte, 1180 m; UTA R-37240, Morales, Sierra de Caral, Carretera entre Quebradas y La Firmeza, 190 m; UTA R-42360, Morales, Sierra de Caral, Carretera Quebradas-La Firmeza, 990 m; UTA R-32997, Morales, Sierra de Caral, Cerro Bonillistas, 300 m; UTA R-38215, Morales, Sierra de Caral, La Firmeza, 825 m; UTA R-37241, Morales, Sierra de Caral, San Miguelito, 450 m; UTA R-37242, Morales, Sierra de Caral, San Miguelito, 470 m; UTA R-37243, Morales, Sierra de Caral, San Miguelito, 600 m; UTA R-37244, Morales, Sierra de Caral, San Miguelito, 550 m; UTA R-37245, Morales, Sierra de Caral, San Miguelito, 560 m; UTA R-46687, Morales: Sierra de Caral: Finca “San Silvestre”, 475m; UTA R-37239, Municipio de Morales, Sierra de Caral, along tributary of Río Bobos, E of San Miguelito, 625 m; UTA R-37238, Municipio de Morales, Sierra de Caral, road between Quebradas and San Miguelito, 100 m; UTA R-20832, Near Mariscos, 20 m; UTA R-46685, Polochic Valley, 4 mi w El Estor, 30 m; UTA R-42361, Quebrada El Branchi, 2.0–4.0 km NE Aldéa La Libertad, 50 m; UTA R-22805, Seshán, 900 m; UTA R-26207, Sierra de Santa Cruz, Cerro 1019, 980; UTA R-46681, Sierra de Santa Cruz, Exmibal Forest (first crest on road from El Estor to Finca Semuc) just W of El Estor, 560 m; UTA R-46682, Sierra de Santa Cruz, Exmibal Forest (first crest on road from El Estor to Finca Semuc) just W of El Estor, 650 m; UTA R-46683, Sierra de Santa Cruz, Exmibal Forest (first crest on road from El Estor to Finca Semuc) just W of El Estor, 650 m; UTA R-46684, Sierra de Santa Cruz, Exmibal Forest (first crest on road from El Estor to Finca Semuc) just W of El Estor, 805 m; UTA R-28377, Sierra de Santa Cruz, Finca Chacchilá, El Coco, ca. 300 m; UTA R-22168, 32999, Sierra de Santa Cruz, Finca Semuc; UTA R-28379, Sierra de Santa Cruz, Finca Semuc headquarters, ca. 500 m; UTA R-26199, Sierra de Santa Cruz, Finca Semuc, 1.0 km S headquarters, 445 m; UTA R-28378, Sierra de Santa Cruz, Finca Semuc, 1.0 km S headquarters; UTA R-29875, Sierra de Santa Cruz, Finca Semuc, 2 km S headquaters, Río Semuc, 400 m; UTA R-42359, Sierra de Santa Cruz, Finca Semuc, Casco, ca 450 m; UTA R-26196, Sierra de Santa Cruz, Finca Semuc, Chinamococh, 650 m; UTA R-26197, Sierra de Santa Cruz, Finca Semuc, Chinamococh, 600 m; UTA R-26200, Sierra de Santa Cruz, Finca Semuc, Chinamococh, 675 m; UTA R-29874, Sierra de Santa Cruz, Finca Semuc, S side Cerro Serujijá, 700 m; UTA R-29872, Sierra de Santa Cruz, Finca Semuc, S side Semococh, 580 m; UTA R-29873, Sierra de Santa Cruz, Finca Semuc, S side Semococh, 860 m; UTA R-26201, Sierra de Santa Cruz, S side Cerro Cana Tomasa, 475 m; UTA R-26202–04, Sierra de Santa Cruz, S side Cerro Cana Tomasa, 400 m; UTA R-26208–10, Sierra de Santa Cruz, Xiacam, 980 m; Petén: UTA R-39231, 7.9 km SW (by road) El Cruce, 740 m; UTA R-39233, ca. San José, N shore Lago Petén-Itzá; UTA R-50300, Gringo Perdido, NE side of Lago Petén-Itzá, near El Remate, 140 m; UTA R-46124, La Libertad, Parque Nacional, Sierra Lacandón, Distrito Yaxchilán; UTA R-39232, Near El Caoba, 400 m; UTA R-50299, on trail starting 1.6 km S of Tikal Visitor’ S Center; Quezaltenango: UTA R-20835, 22802–03, S slope Volcán Santa María, Finca El Faro, ca 4 km N El Palmar, 875 m; San Marcos: UTA R-42285, Area La Trinidad-Aldéa La Fraternidad, 1200–1940 m; UTA R-45685, Malacatán, Finca San Ignacio, 14°56.75'N, 092°2.50'W, 610–760 m; UTA R-39510, Municipio Esquipulas Palo Gordo, Aldéa Fraternidad, Finca La Esperanza, 1550–1890 m; UTA R-39329–35, San Rafael Pie de la Cuesta, Finca America El Vergel, ca. 1500 m; UTA R-45683–84, San Rafael Pie de la Cuesta, Finca America El Vergel, 14°56.30'N, 091°53.48'W, 1600 m; Sololá: UTA R-45687, San Lucas Tolimán, Finca Santo Tomás Pachuj, 14°36.53'N, 091°7.40'W, 1340 m; Zacapa: UTA R-32994, Sierra del Merendón, Finca San Enrique, Sur del Casco; UTA R-32995, Sierra del Merendón, Finca San Enrique, Sur del Casco, 800 m; UTA R-32993, Sierra del Merendón, Finca San Enrique, Sur del Casco, Cerro La Palmichera, 890 m; Unknown: UTA R-22167, No specific locality; HONDURAS: Olancho: UTA R-53268, Sierra de Agalta, 14.945N, 86.151W, 1195 m; Unknown: UTA R-24863, 25040–45, 24860–62, No specific locality; MEXICO: Nayarit: UTA R-53413, 12.4 road miles N of Las Varas, 21.21270°N, 104.99162°W, 749 m; Oaxaca: UTA R-2461, 13.5 from Veracruz-Oaxaca state line, 52 mi N La Ventosa, 17°10.80'N, 095°3.60'W; UTA R-52643, Municipio La Soledad, Piedra Ancha, 16.75417N, 95.45699W, 1135 m; UTA R-25819, Sierra Juárez, between Metates and Vista Hermosa, 17°40.20'N, 096°19.20'W, 900–1500 m (1200 m); UTA R-12336, Sierra Juárez, Metates, 17°42.00'N, 096°18.60'W, 902 m; UTA R-14148, Sierra Juárez, Metates, 17°42.00'N, 096°18.60'W; UTA R-12337, Sierra Juárez, Metates, 17.7 km S Valle Nacional, 17°42.00'N, 096°18.60'W, 914 m; Quintana Roo: UTA R-53411, Mex 180D, 21.05151°N, 87.06756°W, 13 m; San Luis Potosí: UTA R-4673, Hwy 85, 8.0 mi S Antiqua Morelos; Tamaulipas: UTA R-4672, Hwy 80, 1.0 mi E Nuevo Morelos; Veracruz: UTA R-3013, 0.2 mi N Los Mangos, 18°16.20'N, 095°7.20'W, 400 m; UTA R-3011, 0.2 mi N of Encinal, 18°15.00'N, 095°6.60'W, 370 m; UTA R-9410, 1.8 mi S Juan Diaz Covarrubias, 18°7.80'N, 095°9.60'W; UTA R-3017, 10 mi S Catemaco (bridge), 18°17.40'N, 095°7.20'W, 450 m; UTA R-3015, 2.4 mi S Los Mangos, 270 m; UTA R-3012, 3.2 mi S Catemaco (bridge), 18°22.20'N, 095°7.20'W, 400 m; UTA R-3016, 7.8 mi N Juan Diaz Covarrubias, 18°12.60'N, 095°7.20'W.
Imantodes gemmistratus – COSTA RICA: Guanacaste: UTA R-44972, Liberia, Santa Rosa National Park; GUATEMALA: Escuintla: UTA R-22807, Finca Medio Monte, ca. 500 m; UTA R-22169–70, 22806, S slope Volcán de Agua, Finca Rosario Vista Hermosa; San Marcos: UTA R-45922, Malacatán, Finca San Ignacio, 14°56.75'N, 092°2.50'W, 610–760 m; UTA R-28380, San Pablo, Finca Palmira; Santa Rosa: UTA R-45651, Taxisco, Aldéa Madre Vieja (entre Monterrico and Ixtapa), 0 m; Sololá: UTA R-4496, San Juan Obispo; Unknown: UTA R-4706, No specific locality; MEXICO: Guerrero: UTA R-53968, Carretera Marquelia - San Luis Acatlán, 16.68388°N, 98.78722°W; Jalisco: UTA R-53967, Carretera Puerto Vallarta - La Cumbre Mex 200, 19.4 road miles S of Puerto Vallarta Plaza; Nayarit: UTA R-53539, Carretera Puerto Vallarta - Tepic, Mex 200, 21.20688°N, 104.95229°W, 86m; UTA R-53540, MX Hwy 200 S of Tepic, just S of Las Varas, 21.08391°N, 105.20233°W, 24 m; UTA R-53966, Carretera Tepic Las Vacas, Mex 200. 17.3 road miles N of Las Vacas, 21.21319°N, 104.94702°W, 879 m; Oaxaca: UTA R-52646, Camino Niltepec-El Palmar, 16.6808N, 94.57074W, 230 m; UTA R-6638, Cerro Baúl, 16°33.60'N, 094°10.20'W, 1700 m; UTA R-2460, 23.3 mi E La Ventosa, 16°33.60'N, 094°36.60'W; UTA R-8828, 5.9 mi S El Tejocotes, 17°13.20'N, 097°3.00'W; UTA R-6072, 8.0 km NE Tapanatepec, 16°24.60'N, 094°9.60'W, 274 m; Sinaloa: UTA R-4823, 3.6 mi E Concordia; UTA R-4376, 5.0 mi SE Villa Unión, 100 ft; Veracruz: UTA R-3014, 5.5 mi S Catemaco (bridge), 18°21.00'N, 095°6.60'W, 540 m; UTA R-3074, Los Tuxtlas, 3.6 mi S Catemaco, 18°22.80'N, 095°7.20'W, 390 m; UTA R-3081, On way back from Encinal; UTA R-6869, 2.1 mi N Juan Diaz Covarrubias, 18°9.60'N, 095°9.60'W; UTA R-6865, 4.4 mi N Juan Diaz Covarrubias, 18°10.20'N, 095°8.40'W; UTA R-9411, 5.4 mi S Juan Diaz Covarrubias, 18°10.80'N, 095°7.80'W; Yucatan: UTA R-53969, Mex 180D, 20.74248°N, 88.21232°W.
Imantodes inornatus – COSTA RICA: Cartago: UTA R-12904, 2.0 km W Pavones de Turrialba, 500 m; ECUADOR: Esmeraldas: QCAZ 3843, La Chiquita protected forest, 30 km San Lorenzo-Ibarra, 1.2333°N, 78.7600°W; DHMECN 5661, road San Lorenzo-Lita, 1.1979°N, 78.7305°W, 60 m; UTA R-55930–31, road San Lorenzo-Lita, 1.26219°N, 78.79528°W, 40 m; Manabí: EPN 8978, Maicito on road to Chone.
Imantodes lentiferus – COLOMBIA: Vaupés: UTA R-5175, Timbó; ECUADOR: Morona Santiago: QCAZ 9187, General Leonidas Plaza Gutiérrez (Limón), Napinaza, Quebrada río Napinaza, 2.9230°S, 78.4080°W; Napo: QCAZ 284–85, El Reventador, 0.0412°S, 77.5268°W; DHMECN 4591, Archidona, Cotundo, Narupa Biological Reserve, Fundación Jocotoco, 0.7583°S, 77.9103°W, 1800 m; Orellana: QCAZ 8476, Pata 3 and Chontayacu, Huataracu community, 0.1811°S, 77.0201°W, 346 m; QCAZ 10110, Parque Nacional Yasuní, 1 km E road to Maxus, km 38, 0.6539°S, 76.4518°W; Pastaza: QCAZ 8170, around Villano, AGIP petroleum camp, K4 Unidad 1, 1.4722°S, 77.4864°W; QCAZ 8197, around Villano, AGIP petroleum camp, K4 Unidad 3, 1.4697°S, 77.4874°W; QCAZ 8276, around Villano, Kurintza community, Kurintza Unidad 3, 1.5100°S, 77.5141°W, 387 m; QCAZ 8377, around Villano, AGIP petroleum camp, K10 Unidad 3, 1.4727°S, 77.5359°W, 430 m; DHMECN 4368, Montalvo, Nuevo Corrientes, Kurintza, 2.0721°S, 76.3397°W, 300 m; EPN 1276, Mera, 1.4567°S, 78.1114°W; EPN 6256, Arajuno, Parroquia Curaray, caserío Chuyayacu, 0.4744°S, 77.6505°W; EPN 6482, Arutam, 1.7500°S, 77.8666°W; EPN 8974, Shell, Taigsha; EPN 897677, no specific locality; Sucumbíos: EPN 11531, 11617, Gonzalo Pizarro, Lumbaqui, Aguarico protected Forest, 0.0492°S, 77.3567°W; DHMECN 160, Lago Agrio, Tarapoa, San Pablo de Kantesiya, 0.2499°S, 76.4166°W, 300 m; DHMECN 1572, Shushufindi, Limoncocha, 0.3999°S, 76.6333°W, 300 m; DHMECN 8345, Putumayo, Santa Elena, Block 27, 0.3667°N, 76.1869°W, 264 m; Zamora Chinchipe: QCAZ 8488, Guadalupe, Afluente del río Piuntza, Finca de Mesías San Martín, 3.8564°S, 78.8646°W, 1154 m; [Pichincha]: EPN 8975, Pachijal (in error), 0.1300°S, 78.7264°W; NO SPECIFIC POLITICAL UNIT: Oriente: EPN 897273, No locality data: EPN 9585.
Imantodes tenuissimus – MEXICO: Quintana Roo: UTA R-53970, Mex Hwy180D, 21.07407°N, 87.02190°W, 17 m; Yucatan: UTA R-53412, Carretera Yaxacalba - Tahdzibichen, 20.52230°N, 88.82845°W, 33 m.