(C) 2012 B. A. Venmathi Maran. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Redescriptions of two pennellid copepods, Peniculus minuticaudae Shiino, 1956 and Peniculus truncatus Shiino, 1956, are provided, based on postmetamorphic adult females collected from marine ranched fishes captured at Tongyeong marine living resources research & conservation center, Korea. Peniculus minuticaudae was collected from the soft fin rays of black scraper Thamnaconus modestus. It can be distinguished from the other two closely related congeners Peniculus ostraciontis Yamaguti, 1939 and Peniculus truncatus by having a well developed triangular-shaped abdomen; the abdomen is rudimentary in other two species. This is thefirst report of the occurrence of Peniculus minuticaudae in Korea. Peniculus truncatus was collected from the dorsal fin of Korean rockfish Sebastes schlegelii. It can be distinguished from Peniculus minuticaudae by the combination of a rudimentary abdomen, long neck and setae on leg 1 and from Peniculus ostraciontis by the long neck, slender trunk, and setae on leg 1. It is also shown that Peniculus truncatus captured from the same host in Korea was misidentified as Peniculus ostraciontis and hence, this is thesecond record of the occurrence of Peniculus truncatus in Korea. A key is provided for the 14 nominal species of Peniculus.
Copepod, pennellid, parasite, redescription, black scraper, rockfish, fins, identification, key
The genus Peniculus von Nordmann, 1832 belongs to the family Pennellidae Burmeister, 1835 and contains 14 nominal species (
Two species of Peniculus are redescribed from Korea in this study. They are Peniculus minuticaudae Shiino, 1956 and Peniculus truncatus Shiino, 1956. In Asia, nine species of Peniculus have so far been reported including six from India and three from Japan. The species reported from Japan are Peniculus minuticaudae, Peniculus truncatus and Peniculus ostraciontis Yamaguti, 1939 (
Peniculus truncatus was also identified and described by
The host Tetrosomus modestus have been cultured at a few localities along the southern coastal regions of Korea. At Tongyeong marine living resources research & conservation center (TMRC), several commercially important fishes were ranched under the marine ranching program in Korea by Korea Institute of Ocean Science & Technology (KIOST) from 1998 (
Hosts and localities of collections of Pennellids (Copepoda: Siphonostomatoida) from Korea and Japan.
| Pennellid | Host | Infected site | Host order: family | Locality | Reference |
|---|---|---|---|---|---|
| Peniculus minuticaudae Shiino, 1956 | Stephanolepis cirrhifer (Temminck and Schlegel, 1850) [= Monacanthus cirrhifer] | Fins | Tetraodontiformes: Monocanthidae | Shirahama, Wakayama Prefecture, Japan |
|
| Stephanolepis cirrhifer | Fins | Monocanthidae | Oita Prefecture, Japan |
|
|
| Thamnaconus modestus (Günther, 1877) | Fins | Monocanthidae | Oita Prefecture, Japan |
|
|
| Thamnaconus modestus | Fins | Monocanthidae | Tongyeong, Gyeongsangnam-do, Korea | Present study | |
| Peniculus ostraciontis Yamaguti, 1939 | Tetrosomus gibbosus (Linnaeus, 1758) [= Ostracion gibbosum] | Head | Tetraodontiformes: Ostraciidae | Pacific Ocean, Aziro, Kanagawa Prefecture, Japan |
|
| Tetrosomus concatenatus (Bloch, 1785) [= Rhinesomus concatenatus] | Head | Ostraciidae | Sagami Bay, Japan |
|
|
| Peniculus truncatus Shiino, 1956 | Sebastes oblongus (Günther, 1877) [= Sebastichthys mitsukurii] | Fins | Scorpaeniformes: Sebastidae | Off Wagu, Mie Prefecture, Japan |
|
| Sebastes schlegelii Hilgendorf, 1880 | Fins | Sebastidae | Haklim fish farm, Kamak Bay, Jeollanam-do, Korea |
|
|
| Sebastes schlegelii | Dorsal Fin | Sebastidae | Tongyeong, Gyeongsangnam-do, Korea | Present study |
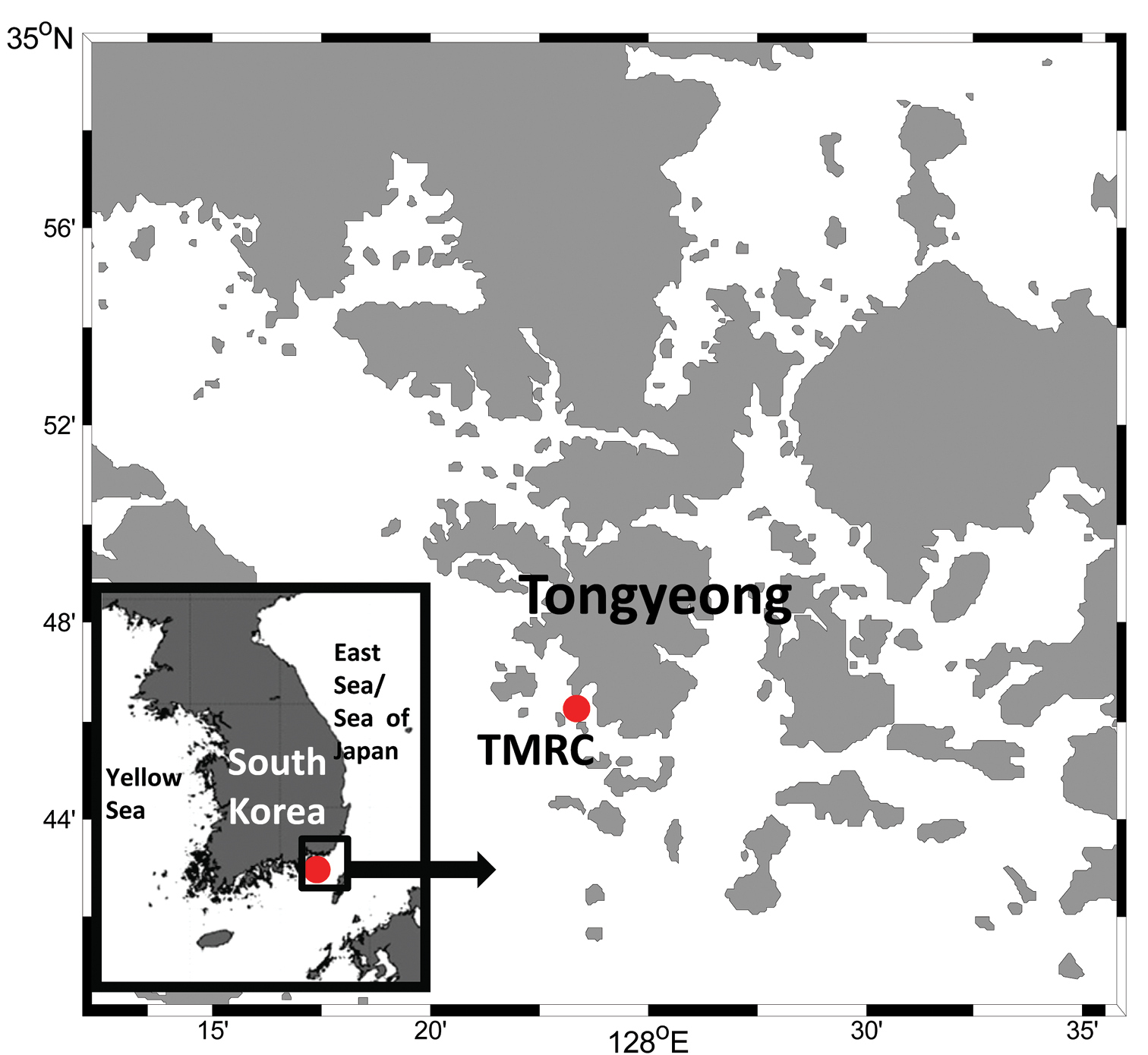
The pennellids were carefully removed from the fin rays of the marine ranched Tetrosomus modestus and Stephanolepis schlegelii at TMRC, Tongyeong, Gyeongsangnam-do, Korea (Figure 1) and they were preserved in 70% ethanol. Preserved copepods were cleared in a drop of 85% lactic acid or lactophenol prior to examination using an Olympus BX51 phase contrast microscope. Selected specimens were measured intact using an ocular micrometer and/or dissected and examined according to the wooden slide procedure of
Map showing the marine ranched fish farming facility, Tongyeong marine living resources research & conservation center (TMRC), Tongyeong, Gyeongsangnam-do, Korea
http://species-id.net/wiki/Peniculus_minuticaudae
Figures 2, 310 ♀♀ (NIBRIV0000245080) and 2 ♀♀ (MABIK CR00178439) from Thamnaconus modestus, Tongyeong, Gyeongsangnam-do, Korea, 20 September 2011.
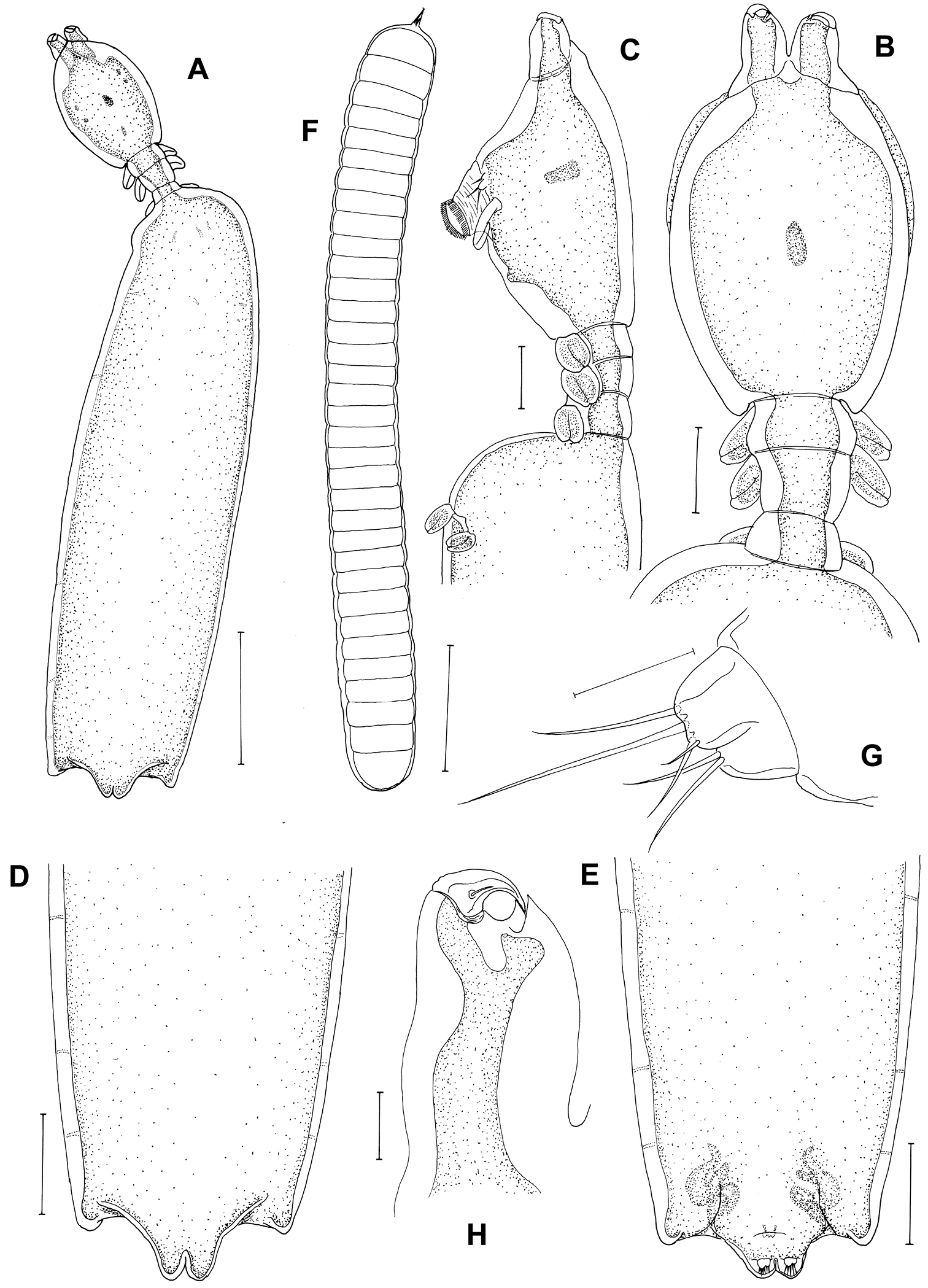
Postmetamorphic adult female. Body (Figure 2A), 2.42 (2.12–2.73) mm long (n=10) comprising oval head, slender neck, large trunk and reduced abdomen. Head (cephalothorax) ovoid, longer than wide, with blunt pointed apex (Figure 2B, C). Short slender neck (Figure 2C) consisting of three somites bearing legs 1, 2 and 3. Fourth pedigerous somite incorporated into trunk. Trunk large, cylindrical, longer than wide, bearing leg 4 proximally (Figure 2C). Abdomen slightly triangular-shaped (Figure 2D, E) long with subterminal caudal rami on ventral surface and projecting posterior tip with anal indentation. Egg sacs long and uniseriate with 33–40 eggs (Figure 2F). Caudal rami (Figure 2G) bearing 2 long, 3 medium sized subequal, 1 small setae. Antennule not observed. Antenna (Figure 2H) 2-segmented, chelate; proximal segment consisting of 2 pointed projections overlapping each other; terminal segment claw-like, acutely pointed with minute seta at base.
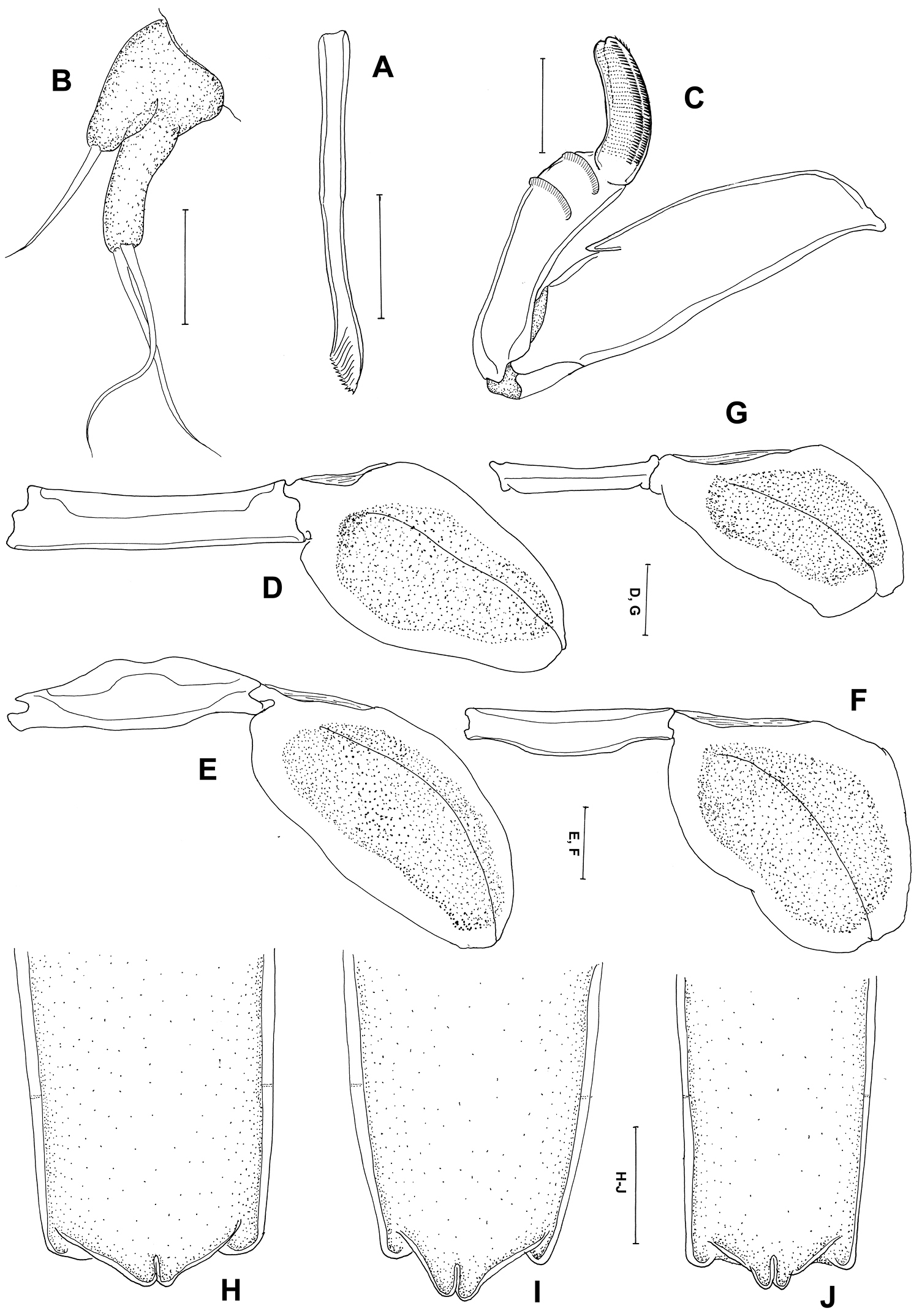
Mandible (Figure 3A) broad with 10 teeth terminally. Maxillule (Figure 3B) with 2 lobes having one and two long setae. Maxilla (Figure 3C) 2-segmented; proximal segment broad with spiniform small process, 2 rows of setules distally; distal segment blunt and curved with transverse striations and rows of spinules. Maxilliped absent. Legs 1 to 4 (Figure 3D–G) all represented by broad plate-like structures derived from the protopodal segments, without rami or seta. Leg 5 absent.
Some females showed variation on posterior end of trunk and abdomen (Figure 3H–J).
All fins of host fish.
Careful comparison between our material and the original description of Peniculus minuticaudae provided by
Peniculus minuticaudae Shiino, 1956. Postmetamorphic adult female. A Habitus, dorsal B Cephalothorax and free thoracic somites, dorsal C Cephalothorax and free thoracic somites, lateral D Posterior end of trunk with abdomen, dorsal E Posterior end of trunk with abdomen, ventral F Egg sac G Caudal ramus H Antenna, dorsal. Scale bars: A=500 μm; B–F=200 μm; G=25 μm; H=50 μm.
Peniculus minuticaudae Shiino, 1956. Postmetamorphic adult female. A Mandible, ventral B Maxillule, ventral C Maxilla, dorsal D Leg 1, ventral E Leg 2, ventral F Leg 3, ventral G Leg 4, ventral H–J variations of posterior end of trunk with abdomen, dorsal. Scale bars: A–C=25 μm; D–G=50 μm; H–J=200 μm.
http://species-id.net/wiki/Peniculus_truncatus
Figures 4, 54 ♀♀ (NIBRIV0000252624) and 1 ♀ (MABIK CR00178440) from Sebastes schlegelii, Tongyeong, Gyeongsangnam-do, Korea, 15 February 2012.
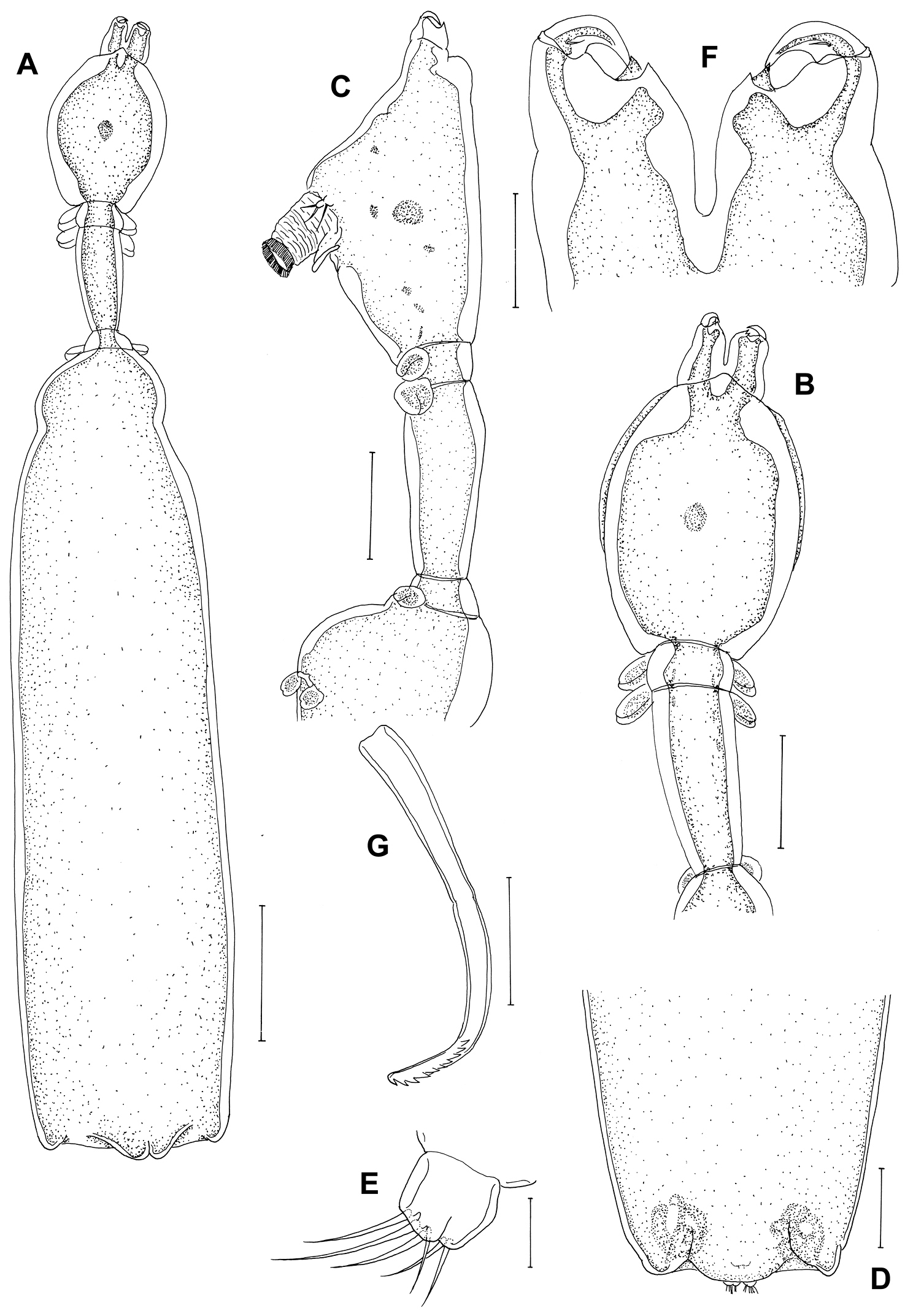
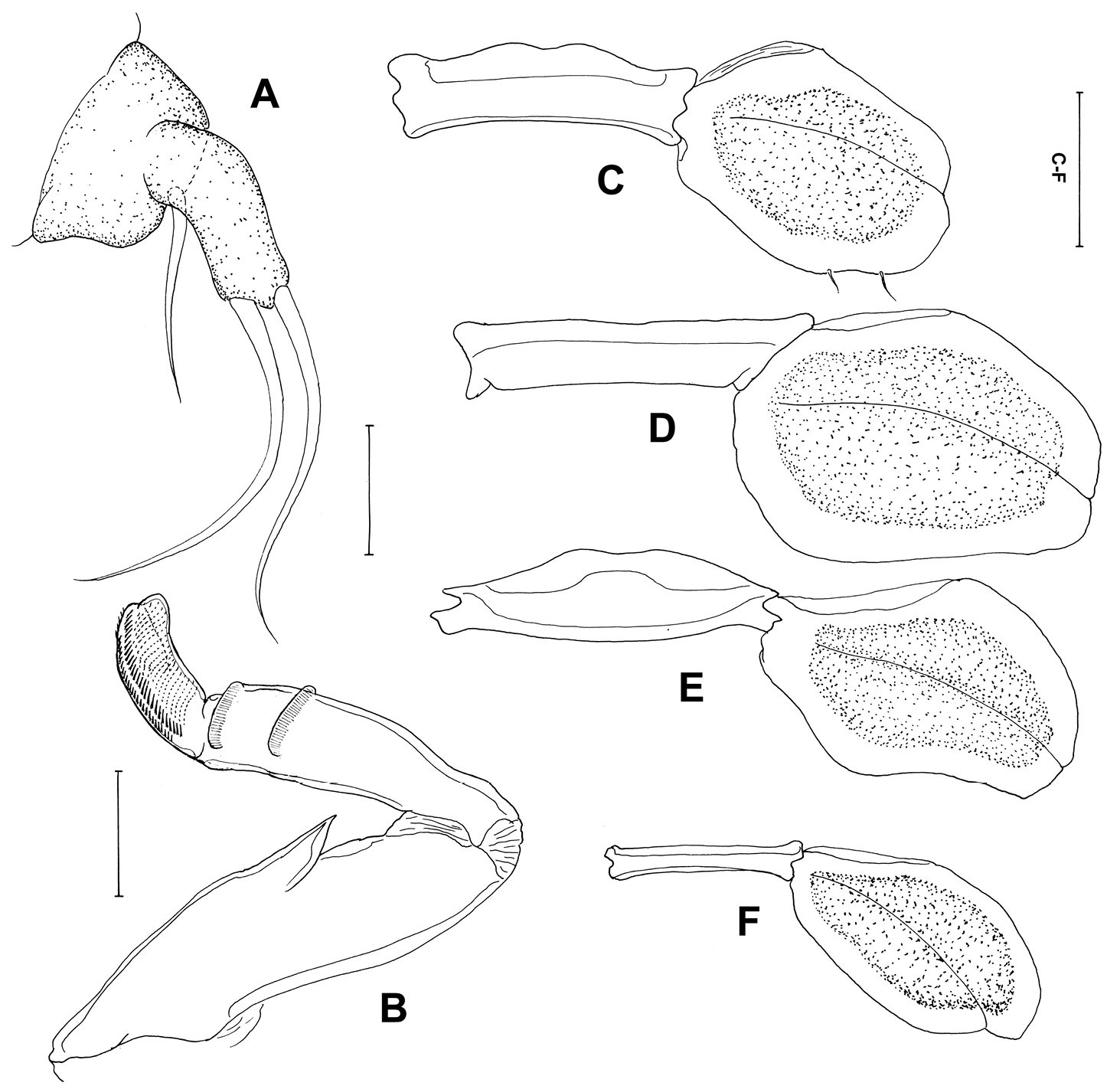
Postmetamorphic adult female. Body (Figure 4A), 4.59 (4.14–5.41) mm long (n=4) comprising oval head, long slender neck, large trunk and reduced abdomen. Head (cephalothorax) ovoid, flattened dorsally but convex ventrally with pair of rounded swellings anteriorly bearing antennae (Figure 4B, C). Mouth tube prominent, directed posteroventrally (Figure 4C). Neck long (0.47–0.55 mm) (Figure 4B, C), slender, comprising about one sixth of trunk length; consisting of three somites bearing legs 1, 2 and 3 (Figure 4B, C). Fourth pedigerous somite incorporated into trunk. Trunk slender, cylindrical, longer than wide, 6 times longer than neck, bearing leg 4 proximally. Abdomen (Figure 4D), reduced with subterminal caudal rami on ventral surface. Caudal rami (Figure 4E) bearing 6 setae. Egg sacs long and uniseriate with 30-37 eggs. Antennule not observed. Antenna (Figure 4F) 2-segmented, chelate; proximal segment bearing 2 pointed projections overlapping each other; terminal segment claw-like, acutely pointed with minute seta at base. Mandible (Figure 4G) moderate-sized, broad, provided with 10 teeth terminally.
Maxillule (Figure 5A) with 2 lobes having one short and two long setae. Maxilla (Figure 5B) 2-segmented; proximal segment broad with robust spiniform process, projecting laterally, 2 rows of setules distally; distal segment blunt and curved with transverse striations and rows of spinules. Maxilliped absent. Leg 1 (Figure 5C) forming blunt plate-like structure derived from protopodal segments, with 2 minute setae laterally. Legs 2–4 (Figure 5D–F) as for leg 1, but without seta. Leg 5 absent.
Only on dorsal fin-rays.
Comparison between our material and the original description of Peniculus truncatus provided by
Peniculus truncatus Shiino, 1956. Postmetamorphic adult female. A. Habitus, dorsal B Cephalothorax and free thoracic somites, lateral C Cephalothorax and free thoracic somites, dorsal D Posterior end of trunk with abdomen, ventral E Caudal ramus, ventral F Antenna, dorsal G Mandible, ventral. Scale bars: A=500, μm; B–D=200 μm; E, G=25 μm; F=50 μm.
Peniculus truncatus Shiino, 1956. Postmetamorphic adult female. A Maxillule, dorsal B Maxilla, dorsal C Leg 1, ventral D Leg 2, ventral E Leg 3, ventral F Leg 4, ventral. Scale bars: A, B=25 μm; C–F=50 μm.
According to
The mean body length of Peniculus minuticaudae was 2.42 mm. It corresponds well to the body length (2.48 mm) of Peniculus minuticaudae reported from Oita Prefecture, Japan (
Peniculus truncatus was originally reported from Stephanolepis oblongus in Japan (
Peniculus truncatus has so far been reported from two species of the genus Sebastes, Stephanolepis schlegelii and S. oblongus and this pennellid appears to be host specific to rockfish (Table 1). Peniculus minuticaudae and Peniculus ostraciontis might be specific to file fish and puffer hosts, respectively (
(Modified from
| 1 | Cephalothorax with 4 large holdfast processes | Peniculus asinus Kabata |
| – | Cephalothorax without such processes | 2 |
| 2 | Cephalothorax with rounded swelling on ventral surface posterior to mouth tube | 3 |
| – | Cephalothorax without posterior swelling on ventral surface | 5 |
| 3 | Swimming legs apparently absent | Peniculus scomberi Gnanamuthu |
| – | Swimming legs with 4 pairs | 4 |
| 4 | Trunk about 11 times longer than wide | Peniculus trichuri Gnanamuthu |
| – | Trunk about 8 times longer than wide | Peniculus stromatei Gnanamuthu |
| 5 | Legs 3 and 4 closer together than legs 1 and 2 | Peniculus communis Leigh-Sharpe |
| – | Legs 3 and 4 further apart than legs 1 and 2 | 6 |
| 6 | Trunk conical-shaped | Peniculus furcatus Krøyer |
| – | Trunk between 3 and 4.5 times longer than wide | 7 |
| 7 | Mouth tube forming a massive posteriorly-directed proboscis | Peniculus clavatus Krøyer |
| – | Mouth tube not forming a massive posteriorly-directed proboscis | 8 |
| 8 | Cephalothorax ovoid | 9 |
| – | Cephalothorax cylindrical | Peniculus theraponi Gnanamuthu |
| – | Cephalothorax widest near posterior margin and tapering anteriorly | Peniculus elegans Leigh-Sharpe |
| 9 | Abdomen well developed; trunk longer than wide | Peniculus minuticaudae Shiino |
| – | Abdomen well developed; trunk longer than wide; with swelling on the head | 10 |
| – | Abdomen reduced; posterior margin of trunk more or less straight | 11 |
| 10 | High degree of ventral swelling on the head; neck constricted | Peniculus fistula Nordmann |
| – | Low degree of ventral swelling on the head; neck constricted | Peniculus elongatus Boxshall |
| 11 | Trunk 4.3 times longer than wide; neck less than half as long as cephalothorax | Peniculus ostraciontis Yamaguti |
| – | Trunk 3.3 times longer than wide; neck more than half as long as cephalothorax | Peniculus truncatus Shiino |
All authors declare that they do not have any conflict of interest.
We thank two anonymous reviewers for their constructive comments. Senior author is thankful to Korea Research Council of Fundamental Science and Technology (KRCF) and this work was formed part of Korea Institute of Ocean Science & Technology projects (PK08080, PE98746, PE98785). This work was partially supported by the National Institute of Biological Resources (NIBR), Korean project on the survey of Korean indigenous species and National Marine Life Collection program (project) sponsored by the Ministry of Land, Transport and Maritime Affairs, Korea (MABIK 2012-001-03) to SYM, HYS.