(C) 2012 Pierfilippo Cerretti. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
New genus Neoethilla gen. n., is described to include two New World nominal species formerly recognized as valid species in Winthemia Robineau-Desvoidy: Exorista ignobilis van der Wulp and Winthemia antennalis Coquillett. Winthemia antennalis is proposed as a junior synonym of Exorista ignobilis syn. n. Neoethilla ignobilis comb. n. is removed from the Winthemiini and placed in the tribe Ethillini (Exoristinae) based on a study of the external features of adults, male terminalia, female reproductive system, and egg morphology. The small tribe Ethillini, not hitherto known from the New World, currently comprises fourteen genera worldwide. The phylogeny and systematics of the Ethillini and their relationships with related tribes are discussed and documented by descriptions and illustrations of relevant character states.
Ethillini, Exorista ignobilis van der Wulp , Nearctic Region, Neotropical Region, Winthemia antennalis Coquillett , new genus, new synonymy, phylogeny, systematics, Winthemiini
Van der
A second nominal species, Winthemia antennalis, was later described by
Winthemia antennalis was again treated as a Winthemia species, albeit provisionally, by
The enigmatic placement and identity of Winthemia ignobilis and Winthemia antennalis became a topic of discussion among us when several specimens provisionally identified as Winthemia antennalis were collected in the Gila National Forest of New Mexico, United States, during the field meeting of the North American Dipterists Society in 2007. Upon further study we have determined that Winthemia antennalis Coquillett is a junior synonym of Exorista ignobilis van der Wulp and that this taxon belongs not to the Winthemiini but to the Ethillini, a tribe hitherto unknown from the New World. We discuss below the characteristics of the Ethillini and propose a new genus for the single known New World species of this tribe.
Materials and methods SpecimensMale terminalia of Neoethilla ignobilis were dissected following the method described in detail by
BMHN Natural History Museum [formerly British Museum (Natural History)], London, United Kingdom (N. Wyatt).
CNC Canadian National Collection of Insects, Agriculture and Agri-Food Canada, Ottawa, Canada.
MZUR Museum of Zoology, Università degli Studi di Roma “La Sapienza”, Rome, Italy (A. Vigna Taglianti).
USNM National Museum of Natural History [formerly United States National Museum], Smithsonian Institution, Washington, United States (N.E. Woodley).
Label data of the holotypes of Exorista ignobilis and Winthemia antennalis are cited verbatim with the end of lines and labels indicated by the following symbols: /, end of a line and beginning of the next; //, end of a label and beginning of the next (from top to bottom on the same pin).
TerminologyMorphological terminology generally follows
urn:lsid:zoobank.org:act:2863EDC7-20B9-4102-AD19-4EF4CFFA6120
http://species-id.net/wiki/Neoethilla
Figs 1a–f, 2a–b, 4a–d, 5a–eExorista ignobilis van der Wulp, 1890, by present designation.
The compound name Neoethilla refers to the New (Latin, neo) World distribution and to the suspected close relationship of this genus with the Old World genus Ethilla Robineau-Desvoidy, 1863.
This generic description is based on a redescription of the single included species, Neoethilla ignobilis.
Length: 5.5–7.5 mm.
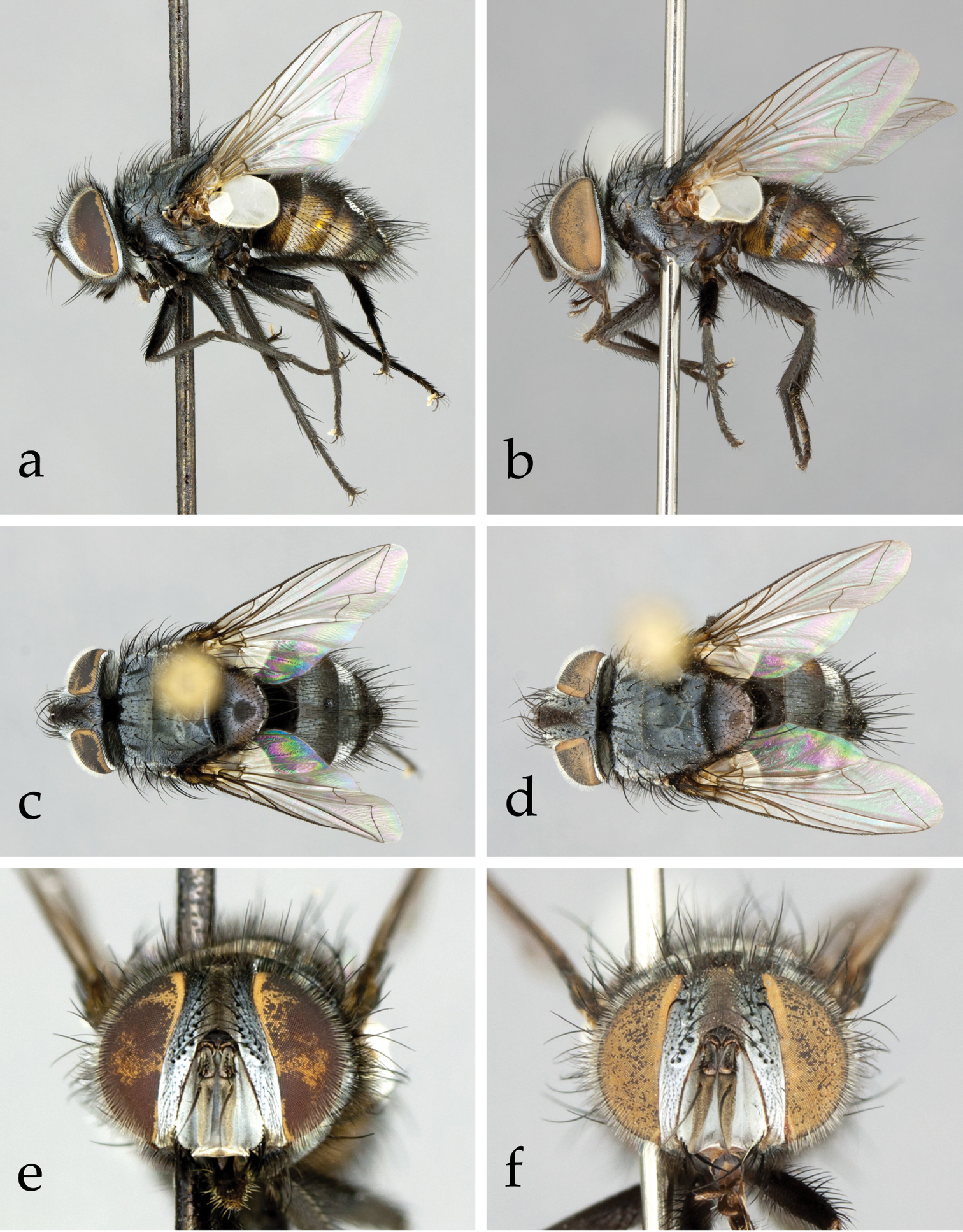
Colour: Head mainly black, covered with grey microtomentum. Palpus black to brown (usually paler in female). Thorax and legs entirely black. Abdomen mainly black but reddish yellow laterally (Fig. 1a–b). Tegula and basicosta black.
Head (Figs 1a–e, 2a): Large in dorsal view, about as wide as thorax; higher than long in lateral view. Compound eye densely covered with long ommatrichia (Fig. 1c–f). Frons at its narrowest point 2/3–6/7 (♂♀) as wide as eye in dorsal view (no significant sexual dimorphism in examined specimens). Frontal vitta (= interfrontal area) clearly widening anteriorly. Outer (= lateral) vertical seta not differentiated from postocular setae in male, well developed in female. Ocellar seta absent (Figs 1c–d, 2a) or very small; ocellar triangle with several short, proclinate setulae (Fig. 2a). Fronto-orbital plate of male with about three irregular rows of fine, medioclinate setae lateral to frontal setae. Seven to 10 frontal setae. Two or 3 upper (= dorsal) reclinate orbital setae, often not clearly differentiated from frontal setae. Proclinate orbital setae absent in male, 2 in female (Fig. 1a–b). Parafacial covered with proclinate, short, fine setae (Fig. 1a–b, 1e–f). Facial ridge straight in profile, with only a few setae above vibrissa on about 1/5–1/4 of its length. Vibrissa arising at about level of lower facial margin. Face concave; lower facial margin not visible in profile. Antenna slightly shorter than facial ridge. Postpedicel 3.0–3.5 times as long as pedicel. Arista bare, thickened on basal 2/5–1/2. First aristomere not longer than wide (usually shorter); second aristomere 1–2 times as long as wide. Genal dilation well developed. Gena in profile very narrow, about 0.10–0.15 times as high as compound eye (height measured in the same vertical plane as height of head). Postocular setae fine, relatively long and slightly bent anteriorly. Occiput flat, with 1–2 rows of black setulae behind postocular row. Prementum not more than 2 times as long as wide; palpus well developed, apically covered with setulae, often strongly clavate in female.
Neoethilla gen. n.ignobilis (New Mexico) a–d habitus a male in lateral view b female in lateral view c male in dorsal view d female in dorsal view d–e head in frontal view d male e female.
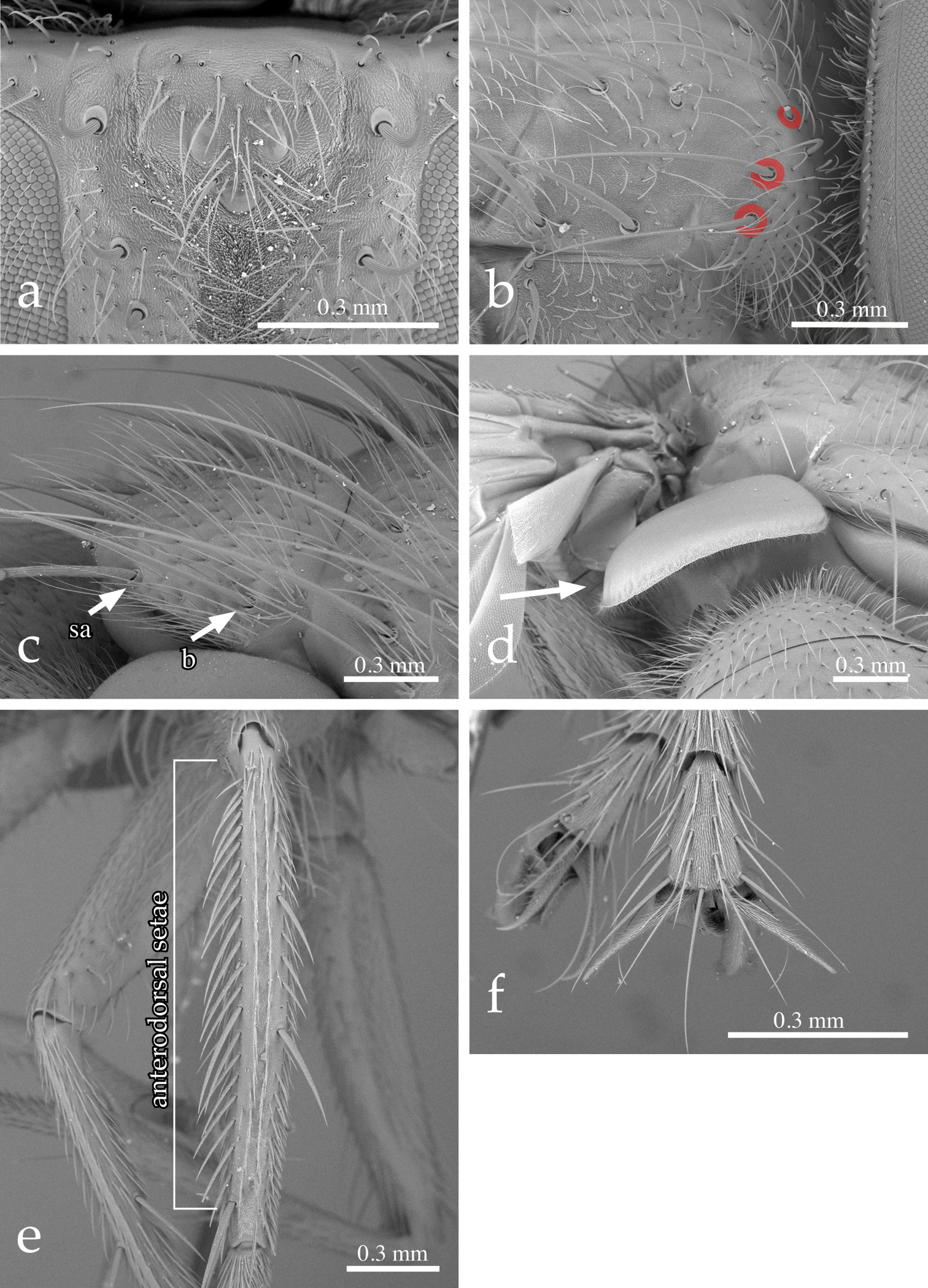
Thorax (Figs 1a–d, 2b–d): Postpronotum with 4 setae; 3 strongest, basal, arranged in a line (Fig. 2b). Scutum with 2–3 posthumeral setae, 1 + 3 supra-alar (first postsutural supra-alar seta at most as long as a notopleural seta), 0–1 + 3 intra-alar, 3 + 4 dorsocentral, 3 + 3 acrostichal setae. General hair-like setulae of scutum fine, relatively long and erect. Prosternum laterally setose. Proepisternal depression bare. Two katepisternal setae (the posterior one larger). Katepimeron setulose along its length. Anepimeral seta at most half as long as posterior katepisternal seta. Scutellum wider than long, covered with long, fine, erect setulae. Three pairs of marginal scutellar setae (basal, subapical, apical) (Figs 1c–d, 2c); basal and subapical setae about equal in size; apical pair shorter, crossed and sub-horizontal. Scutellum without discal setae. Anterior and posterior lappets of metathoracic spiracle unequal in size.
Neoethilla gen. n.ignobilis (male, New Mexico) a vertex in anterodorsal view b right postpronotum and part of presutural portion of scutum in laterodorsal view [circles indicate basal postpronotal saetae] c scutellum in laterodorsal view [b = basal scutellar seta; sa = subapical scutellar seta] d lower calypter in posterior view e left hind tibia in dorsal view f fore claws.
Legs: Preapical anterodorsal seta of fore tibia about as long and stout as preapical dorsal seta. Mid tibia with 1 anterodorsal seta. Hind tibia with a row of moderately spaced, comb-like anterodorsal setae (Fig. 2e); 2 dorsal preapical setae. Preapical posteroventral seta of hind tibia not differentiated. Claws about as long as fifth tarsal segment in male (Fig. 2f), considerably shorter in female.
Wing (Figs 1a–d, 2d): Bend of vein M usually obtuse. Cell r4+5 open. Section of M between crossveins r-m and dm-cu longer than section between dm-cu and bend of M. Section of M between dm-cu and bend of M shorter than post-angular section of M. Vein R4+5 with a single setula at base dorsally and 0–1 ventrally. Lower calypter large and strongly convex, especially along its lateral and posterior margins (Fig. 2d).
Abdomen (Fig. 1a–d): Syntergite 1+2 with mid-dorsal depression extending to hind margin. Tergites 1+2 and 3 with a pair of fine median marginal setae, sometimes not differentiated from the general abdominal setae, and a pair of lateral setae; tergite 4 with a row of marginal setae; tergite 5 with scattered weak setae. Tergites 3 and 4 without median or lateral discal setae.
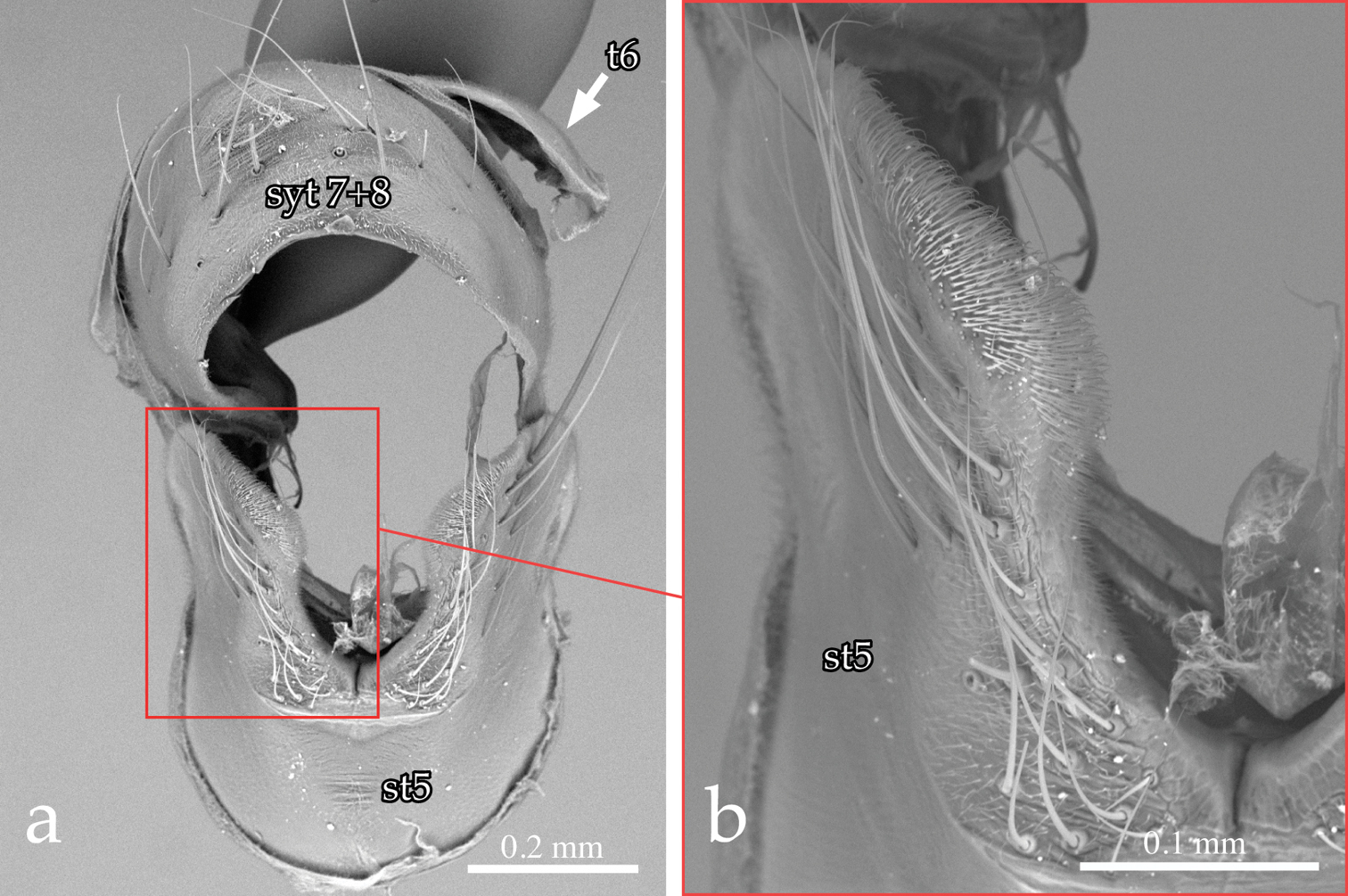
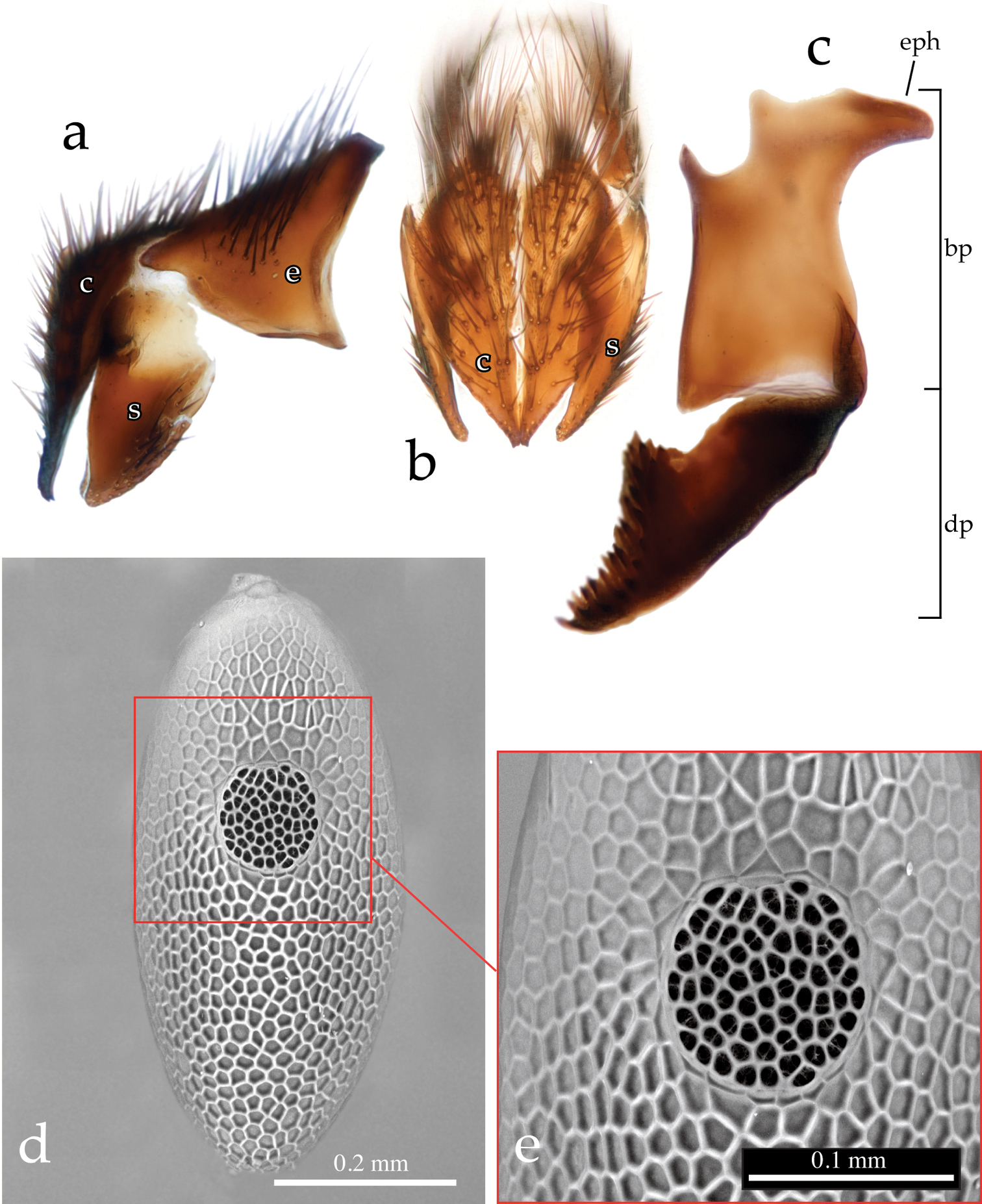
Male terminalia (Figs 3a–b, 4a–d, 5a–c): Sternite 5 with deep median cleft, outer lobe almost truncate along posterior margin (Fig. 3a–b). Tergite 6 large, plate-like (not divided into two sclerites nor indentate on posterior edge), bare; tergite 6 separated from tergite 5 and segment 7+8 by membrane. Cerci (Figs. 4a–b, 5a–b) almost flat, wide in posterior view (sub-ovoid), not fused medially (i.e., longitudinal medial suture complete), distally very slightly divided (Figs 4b, 4d, 5b). Surstylus long, wide and sub-triangular in lateral view, distal tip sometimes slightly bent posteriorly (Figs 4c, 5a). Posterior half of lateral surface of surstylus with several stout setae. Pregonite and postgonite not fused. Pregonite strongly recurved and pointed. Processi longi long, slender and well separated from each other. Epiphallus stout, well sclerotized, attached to basal portion of basiphallus (Fig. 5c). Connection between basiphallus and distiphallus strongly sclerotized (Fig. 5c). Lateroventral sclerites of distiphallus well developed, strongly sclerotized with robust spines lateroventrally (Figs 4a–b, 5c).
Neoethilla gen. n.ignobilis (male, New Mexico) [st5 = sternite 5; syt7+8 = syntergite 7+8; t6 = tergite 6] a sternite 5 and syntergite 7+8 b detail of left lobe of sternite 5.
Neoethilla gen. n.ignobilis (male, New Mexico) [c = cerci; hy = hypandrium; dp = distiphallus; pr = pregonite; s = surstylus] a cerci and right surstylus in lateral view b epandrial complex in posterior view c distiphallus and pregonite in left lateral view d distiphallus in dorsal view.
Female terminalia. Ovipositor short, not telescopic as in Winthemiini.
Egg. Plano-convex macrotype unembryonated; long-oval in dorsal view; anterodorsally operculate (Fig. 5d–e). Dorsal, convex surface of egg characterized by a strong polygonal micro-sculpturing.
Neoethilla gen. n. ignobilis a–c terminalia (male, Idaho) [c = cerci; bp = basiphallus; dp = distiphallus; e = epnadrium; eph = epiphallus] a epandrial complex in lateral view b epandrial complex in posterior view c phallus in lateral view d–e egg in dorsal view (Massachusetts) d habitus e detail of operculum.
Neoethilla is superficially similar to Winthemia because it has an enlarged compound eye covered with thick and long ommatrichia and parafacial covered with fine setulae. Moreover, Neoethilla and Winthemia both have a short first postsutural supra-alar seta, a comb-like row of anterodorsal setae on hind tibia and a fully setulose katepimeron. Neoethilla is distinguishable from Winthemia (i) in having the three strongest basal setae of postpronotum arranged in a line, (ii) in lacking the lateral scutellar setae and (iii) in having processi longi of male terminalia long, slender and well separated from each other. Females of Neoethilla have a short ovipositor and a dorsally operculate plano-convex egg. These characters, together with the strongly convex lower calypter, suggest that Neoethilla has an ethilline affiliation. Within this tribe the new genus is characterized by the following combination of character states: (i) parafacial fully setulose, (ii) gena very narrow (0.10–0.15 times as high as compound eye), (iii) ocellar setae absent or very reduced, (iv) three strongest basal postpronotal setae arranged in a line, and (v) lateral scutellar setae missing.
Included species and examined specimens
http://species-id.net/wiki/Neoethilla_ignobilis
UNITED STATES. Arizona: 1 ♂, Cochise County, Chiricahua Mountains, 1760 m, 31°52'26.1"N, 109°13'55.8"W, 18.VIII.2007, P. Cerretti leg. (MZUR). 1 ♂, [Cochise County], Chiricahua Mountains, Ash Spring, 1860m, 31°52.3'N, 109°14.7'W, 20–21.IX.2004, J.E. O’Hara leg. (CNC). 3 ♀♀, [Cochise County], Huachuca Mountains, Ramsey Canyon, 1680 m, 2.V.1967, D.M. Wood leg. (CNC). 1 ♂, Cochise County, [Huachuca Mountains], Ida Canyon, 1800 m, 31°23.5'N, 110°19'W, 22–24.X.2001, G. & M. Wood leg. (CNC). 1 ♂, Coconino County, 2 km w. Sunset Crater National Monument, 2100 m, 23–24.VII.1982, J.E. O’Hara leg. (CNC). California: 2 ♂♂, [Riverside County], Elsinore, 13.VII.1931 (CNC). Idaho: 1 ♂, Craters of the Moon National Monument, 8.VII.1965, D.S. Horning Jr. leg. (CNC). Maryland: 1 ♂, Calvert County, Port Republic, 21.VIII.2001, D.M. Wood leg. (CNC). Massachusetts: 1 ♀ (with egg), Plymouth County, Onset, 29.VI–6.VII.1983, W.J. Morse leg. (CNC). New Mexico: 1 ♂, Grant County, 21 km n. Silver City, Cherry Creek Campground, 2100 m [not 2250 m or 7400’ as printed on some labels from this locality], 32°54.8'N, 108°13.6'W, 16–19.VIII.1982, J.E. & W.M. O’Hara leg. (CNC). 1 ♂, same data except 14–16.VIII.1983, J.E. O’Hara leg. (CNC). 1 ♂, same data except 26.V.1991 (CNC). 2 ♂♂, same data except 16.IX.1994 (CNC). 2 ♂♂, same data except 15–16.VIII.1999 (CNC). 2 ♂♂, Grant County, n. Silver City, Gomez Peak (hilltop), 2200 m, 32°50'N, 108°17'W, 23.IX.2004, J.E. O’Hara leg. (CNC). 1 ♂, same data except 24.IX.2004 (CNC). 1 ♂, same data except 27.VIII.2006 (CNC). 1 ♂, same data except 9.VIII.2007 (CNC). 1 ♂, same data except 4.V.2010 (CNC). 1 ♂, same data except 11.X.2010 (CNC). 8 ♂♂, same data except 9.VIII.2011 (CNC). 6 ♂♂, same locality, 12.VIII.2007, P. Cerretti leg. (MZUR). 5 ♂♂, Grant County, n. Silver City, Eighty Mountain (hilltop), 2275 m, 32°50.9'N, 108°18.0'W, 26.VIII.2006, J.E. O’Hara leg. (CNC). 1 ♂, same data except 15.VIII.2007 (CNC). 3 ♂♂, same locality, 15.VIII.2007, D.M. Wood leg. (CNC). 1 ♀, Grant/Sierra County, 63 km e. Silver City, Emory Pass, 2500 m, 32°54'N, 107°45'W, 12.VIII.2007, G.& M. Wood leg. (CNC). 3 ♂♂, Torrance Co., 14 km s. Cedarvale, North Peak (hilltop), 2250 m, 34°16.4'N, 105°43.5'W, 7.VIII.2007, J.E. O’Hara leg. (CNC). South Carolina: 1 ♂, Oconee Co., Sumter [as “Sumpter”] National Forest, Hunt Camp, 1.V.1997, M. Wood leg. (CNC).
Neotropical: Mexico (Guerrero); Nearctic: widespread in continental United States (
The Ethillini are a small tribe of Exoristinae formerly thought to be exclusively Old World in distribution. Most Ethillini share a number of character states with several members of Winthemiini but the relationships between these tribes remain unclear as they have not been investigated with a rigorous cladistic approach. The tribe can be defined by the following character states:
(1) Lower calypter strongly convex (Fig. 2d) – this is a very rare condition in the Tachinidae (as well as in other Calyptratae) and could arguably be considered apomorphic. Excluding Mycteromyiella Mesnil, 1966 and Calliethilla Shima, 1979, whose systematic placements in Ethillini are questionable (see also
(2) katepimeron usually entirely covered with fine setulae – within the subfamily Exoristinae this character state is shared with practically all known Winthemiini, a few Exorista and the exoristine genus Crassicornia Kugler, 1980 (cf.
(3) female ovipositor short (i.e., non-telescopic) – this could be a plesiomorphic condition compared to the long and telescopic one present in all Winthemiini.
In addition to the above states, all examined female ethillines have a macrotype plano-convex egg of white color. All the Exoristinae, most Phasiinae and the Eutheriini also have a plano-convex egg, or at least traces of a primitive planoconvex shape. This type of egg thus represents a plesiomorphic condition for Ethillini. It is worth noting that Ethillini show two reproductive strategies (
Worldwide the following genera fall well within the above limits: Amnonia, Atylomyia, Ethilla, Ethylloides Verbeke, 1970, Gynandromyia Bezzi, 1923, Nemorilloides Brauer & Bergenstamm, 1891, Neoethilla gen. n., Paratryphera Brauer & Bergenstamm, 1891, Phorocerosoma Townsend, 1927, Prosethilla Herting, 1984, and Zelindopsis Villeneuve, 1943. As mentioned above, Calliethilla and Mycteromyiella have a questionable ethilline affiliation because of the lack of the distinctive convex lower calypter.
The grasshopper parasitoids Gynandromyia and Phorocerosoma and the genus Zelindopsis (Phorocerosoma-group) share the following: postpronotum with five setae, with the three basal strongest arranged in a triangle; female sternites 4–6 very large and exposed from the ventrolateral margins of the corresponding tergites; and egg surface dorsally smooth with two aeropilar areas, irregular in shape. The number and disposition of postpronotal setae are exactly the same as in nearly all the Winthemiini, and the egg characteristics are shared with several other Exoristinae. However, the features of the female abdominal sternites appear to be unique within the Exoristinae and may represent a strong apomorphy in support of the monophyly of this group of ethilline genera. Interestingly, males of Mycteromyiella share with males of several species of the Phoroserosoma-group the presence of distinctive spots of setulae and microtrichia on the ventral side of abdominal tergites 4 and 5, and also its members are parasitoids of Phasmatodea (
All the remaining ethilline genera (Ethillini s. str.) share the following, probably derived, character states:
(1) postpronotum with the three strongest, basal, setae arranged in a line (Fig. 2b),
(2) male tergite 6 bare, wide and not indented antero-medially,
(3) male segment 7+8 very wide and platiform (cf.
(4) egg distinctly operculate (
The presence of operculate eggs is a very rare condition in tachinids. In most Exoristini an operculum is delineated by a line at the anterior end of the egg (cf.
Finally, two probably monophyletic groups may be identified within Ethillini s. str. (cf.
(1) long ovisac for storing fully embryonated eggs,
(2) pregonite and postgonite at least partly fused basally,
(3) connection between male sternite 6 and segment 7+8 on right side very narrow,
(4) intermedium (cf.
(5) epiphallus light-colored and attached to apical portion of basiphallus,
(6) ventrolateral sclerites of distiphallus greatly reduced, and
(7) dorsal connection between basiphallus and distiphallus almost membranous.
The second probably monophyletic group within Ethillini is the Ethilla-group, composed of Ethilla, Nemorilloides, Prosethilla and Neoethilla gen. n. These taxa share the following character states:
(1) short ovisac, females lay unembryonated eggs,
(2) pregonite and postgonite not fused,
(3) connection between male sternite 6 and segment 7+8 on right side very wide,
(4) intermedium very large,
(5) epiphallus massive and attached to basal portion of basiphallus (Fig. 5c),
(6) ventrolateral sclerites of distiphallus heavily sclerotized and covered with spinulae (Figs 4c–d, 5c), and
(7) dorsal connection between basiphallus and distiphallus heavily sclerotized (Fig. 5c).
We transfer the New World species Exorista ignobilis van der Wulp to the Ethillini and assign it to the new genus Neoethilla based on its distinctive characteristics. Neoethilla may share a sister-group relationship with Prosethilla, as evidenced by its lack of lateral marginal setae on the scutellum and similar egg morphology (operculum, microsculpture and shape very similar in these genera, judging from figures of Prosethilla by
We thank Norman Woodley (USNM) and Nigel Wyatt (BMNH) for assistance during examination of type material under their care; NW also kindly provided us with detailed images of the type specimen and labels of Exorista ignobilis. We are most grateful to Shannon Henderson and Alan Fleming (both at CNC) for the montaged images in figures 1a–f, to Massimo Lopresti and Giuseppe Lo Giudice (both at Centro Nazionale per lo Studio e la Conservazione della Biodiversità Forestale, Verona, Italy) for helping to compose figures 2–5, and to Hans-Peter Tschorsnig (Staatliches Museum für Naturkunde, Stuttgart, Germany) and an anonymous reviewer for their constructive comments on an earlier draft of this manuscript. This work was partially supported by U.S. NSF DEB-1146269.