(C) 2012 Masato Hirose. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Buchneria dofleini (Buchner, 1924), type species of Buchneria Harmer, 1957, was first described from material collected in 1904–1905 from Sagami Bay, Japan, but the type specimens had not been reexamined since the original description. In this study, I examined specimens of Buchneria from historical collections and material recently collected near Akkeshi, Hokkaido, Japan. Three Buchneria species were detected, two from Sagami Bay that Ortmann (1890) had placed in Escharoides, and one from Akkeshi that Androsova (1958) had described as Porella variabilis. I concluded that Buchneria dofleini is a junior synonym of Escharoides teres Ortmann, 1890; selected a lectotype for Escharoides teres among Ortmann’s syntypes; and established the new combination Buchneria teres (Ortmann, 1890), which becomes the type species of Buchneria. I also established the new combination Buchneria rhomboidalis (Ortmann, 1890) and selected a lectotype among Ortmann’s syntypes. Porella variabilis is transferred to Buchneria establishing the new combination Buchneria variabilis (Androsova, 1958). Here the three new combinations are redescribed and a key to the Japanese Buchneria species is provided. Finally, I transferred Buchneria to Bryocryptellidae on the basis of ovicell and orifice morphology. Therefore, Buchneria now includes a total of three species; Buchneria sinuata Harmer, 1957, a species from Indonesia that has hitherto been placed in this genus, is almost certainly not congeneric with other Buchneria. As far as is now known, Buchneria is endemic to northern Japan and the northern Sea of Japan.
Buchneria dofleini, Buchneria teres, Buchneria rhomboidalis, Buchneria variabilis, new combination, syno-nymy, distribution, Sagami Bay, Akkeshi
The status of Buchneria has not been evaluated subsequent to
I examined specimens from Sagami Bay and surrounding areas collected by Ludwig Döderlein (1880–1881), Franz Doflein and Karl Haberer (1904–1905), Emperor Showa (1918–1971), and most recently by the National Museum of Nature and Science Tokyo (2001–2005); see
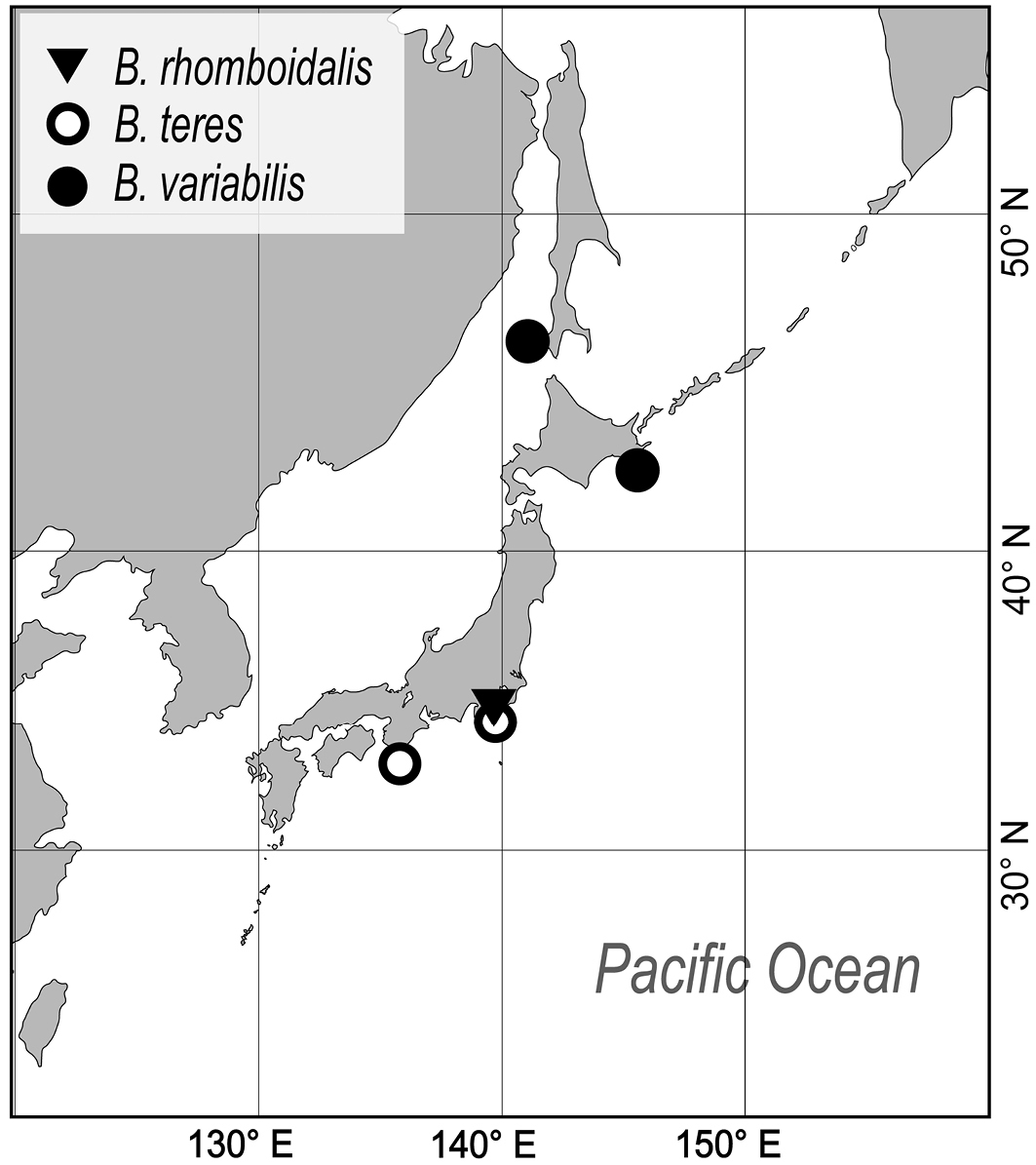
The author collected additional specimens from Sagami Bay by dredge from RV Tansei-maru (Japan Agency for Marine-Earth Science and Technology, JAMSTEC) and research boat Rinkai-maru (Misaki Marine Biological Station, The University of Tokyo) in November 2007 and February 2012, and outside Akkeshi Bay, Hokkaido, in July 2010 and 2011, by dredge from research boat Misago-maru (Akkeshi Marine Station, Hokkaido University) (Fig. 1).
Map showing the areas in Japan where species of Buchneria were collected.
Specimens were observed by light microscope and scanning electron microscope (SEM). For SEM observation, part of each specimen was removed, soaked in a sodium hypochlorite solution to remove the soft tissue, rinsed in water, air dried, and mounted with double-sided adhesive tape or silver paste on an aluminum SEM stub. At Hokkaido University, mounted specimens were coated with Au in a Hitachi E-1030 sputter-coater and observed with a Hitachi S-3000N SEM at 15 kV accelerating voltage. At the SMF, specimens were coated with Pt-Pd and observed with a CamScan SEM. At ZSM, specimens were coated with Au in a POLARON SEM Coating System and observed with a LEO 1430VP SEM at 15 kV accelerating voltage. Fragments removed from specimens in the various collections, prepared and examined by SEM, and subsequently deposited in NMST are indicated in the text by the designation ‘NSMT Te’ (see Supplementary Table 1).
Measurements were taken from SEM images with ImageJ 1.37v software (Image Processing and Analysis in Java, Wayne Rasband, National Institutes of Health, USA; http://rsb.info.nih.gov/ij/ ). Measurements in the text are presented in millimeters, as ranges followed in parentheses by the mean and standard deviation. Sample sizes for measurements were n = 4–82, generally from more than one colony. Abbreviations used for measurements are as follows; ZL, zooid length; ZW, zooid width; OrL, orifice length; OrW, orifice width; AvL, suboral avicularium length; AvW, suboral avicularium width; OvL, ovicell length; OvW, ovicell width.
Taxonomy Order Cheilostomata Busk, 1852 Suborder Neocheilostomina d’Hondt, 1985 Infraorder Ascophorina Levinsen, 1909 Superfamily Lepralielloidea Vigneaux, 1949 Family Bryocryptellidae Vigneaux, 1949Palmicellaria dofleini Buchner, 1924 by original designation by Harmer (1957: 876) (= Escharoides teres Ortmann, 1890).
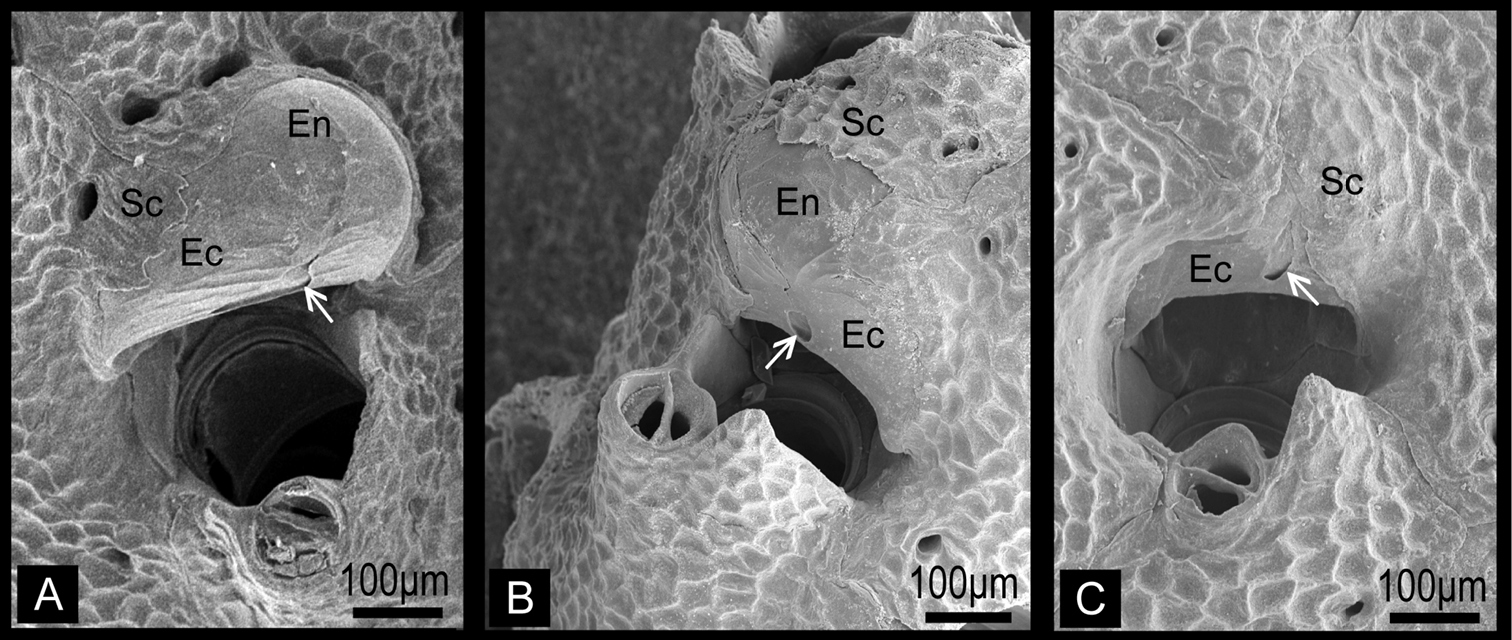
Colony erect, rigid, dichotomously or irregularly branching: branches cylindrical, flattened, or plate-like, fan shaped. Zooidal frontal shield uniformly tessellated, with a few areolar pores near margin or offset centrally in secondarily calcified wall. Orifice deeply immersed, without teeth on distal periphery, without lyrula or condyles, slightly concave or straight proximally; oral spines absent. Secondary orifice at colony surface cormidial, formed by contributions of secondary calcification from distal and lateral zooids. Suboral avicularium lies at proximal margin of secondary orifice, directed proximally or laterally, sometimes enlarged and occupying about half of frontal shield; small, conical tooth associated with avicularium projecting into secondary orifice (Fig. 2). Mandible of the suboral avicularium semicircular or spatulate, but never acute. Vicarious and other frontal avicularia absent. Ovicell globose, acleithral, and is produced by the distal zooid (Fig. 3). Both the endooecium and ectooecium are calcified. Endooecium is completely calcified, whereas ectooecium is not completely covering the endooecium (Fig. 3A, B). Immediately after formation, the ectooecium is then partially covered by the secondary calcification that is coming from the distal and neighbour zooids (Fig. 3B). Finally, the secondary calcification covers most of the ectooecium in the old parts of the colony, but a small area of proximal margin remains uncovered (Fig. 3C). Small basal pore chambers present.
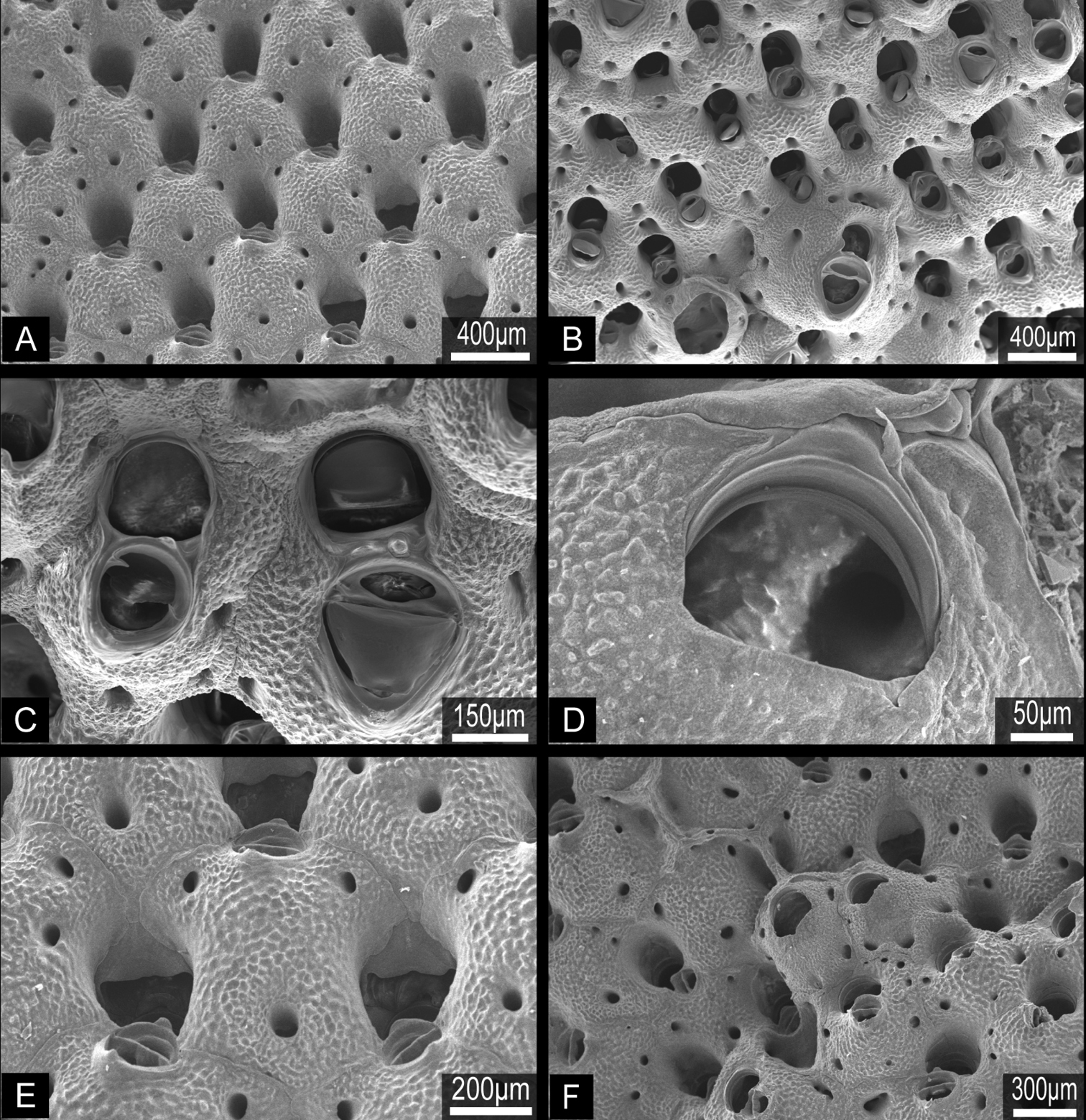
Orifices of three Buchneria species showing the small tooth distal to the suboral avicularium. A Buchneria teres B Buchneria rhomboidalis C Buchneria variabilis.
Ovicells of Buchneria teres showing various stages of development. A Younger stage ooecium showing smooth surface of endooecium and ectooecium with less secondary calcificationB Ooecium started covered by tessellated secondary calcification from neighboring zooidsC Ooecium almost covered by the secondary calcification with showing endooecium through the small proximal membranous window at ectooecium. Ec, ectooecium; En, endooecium; Sc, secondary calcification. Arrows indicate the proximal membranous window.
Excluding nominal Buchneria sinuata, Buchneria presently contains three species, which I redescribe here.
http://species-id.net/wiki/Buchneria_teres
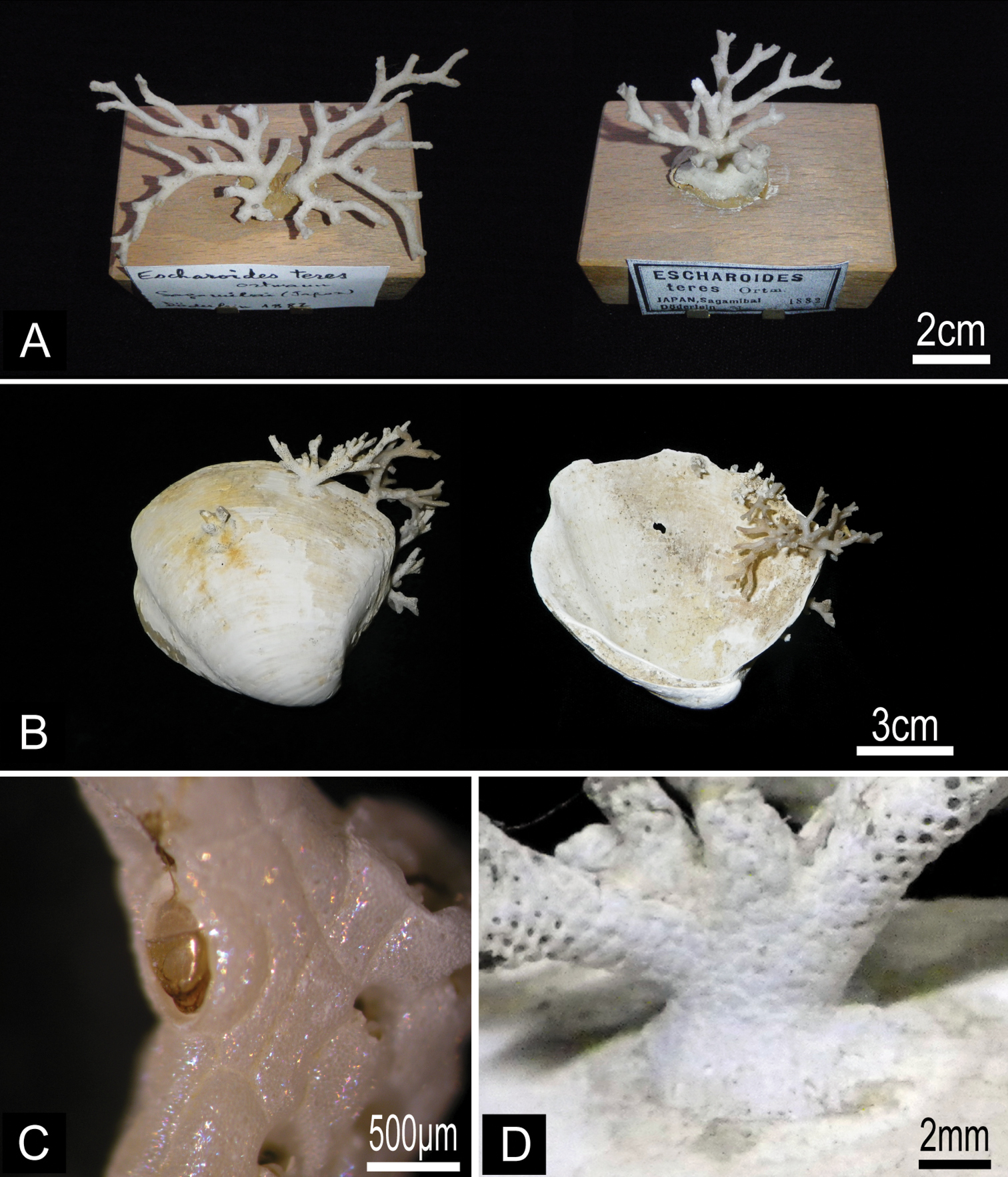
Figures 4, 5, 6Lectotype. Branched colony (MZS 36-2), collected by L. Döderlein, 1882, Sagami Bay. Paralectotype. Branched colony (MZS 36-1, 36-3; NSMT Te-738), collected by L. Döderlein, 1882, Sagami Bay. Other material examined. Fragment of colony ZSM 20043001, collected by F. Doflein, 17 October 1904, entrance of Tokyo Bay, 600 m depth; fragment of colony ZSM 20100261 collected by F. Doflein, 1904−1905, Sagami Bay; single small living colony on pebble and several fragments of living colonies (NSMT TeS-3, TeS-2), collected by NSMT from RV Shinyo-maru, 24 October 2003, Okinose, Sagami Bay (34°58.80'N, 139°31.50'E to 34°59.20'N, 139°31.20'E), 900−950 m depth, by dredge; fragments of colonies (NSMT TeS-4), collected by NSMT from research boat Rinkai-maru, 16 March 2001, SW of Hayama, Sagami Bay (35°11.46'N, 139°28.71'E to 35°11.64'N, 139°28.14'E), 432−580 m depth, by dredge; several living and dead colonies (NSMT TeS-5 to TeS-12) on dead Conchocele bisecta (Conrad, 1849) shells, collected by NSMT from RV Tansei-maru, 24 November 2007, ENE of Hatsushima, Sagami Bay (35°03.41'N, 139°12.55'E to 35°02.73'N, 139°13.73'E), 563−756 m depth, by beam trawl; single small dead colony on pebble (NSMT Te-876), collected by Nagai, 24 April 1997, SW of Shionomisaki, Wakayama Prefecture (33°24.91'N, 135°38.69'E to 33°24.95'N, 135°38.12'E), 500 m depth, by dredge.
ZL, 0.595−1.334 (0.978±0.160); ZW, 0.214−0.840 (0.450±0.133); n=65. OrL, 0.131−0.208 (0.171±0.021); OrW, 0.123−0.219 (0.187±0.018); n=32. AvL, 0.078−0.198 (0.110±0.017); AvW, 0.055−0.148 (0.086±0.016); n=82. OvL, 0.205−0.391 (0.311±0.049); OvW, 0.286−0.439 (0.365±0.043); n=29. Additional measurements: large suboral avicularium (LAv) length, 0.619−0.766 (0.682±0.076); LAv width, 0.398−0.453 (0.426±0.027), n=3.
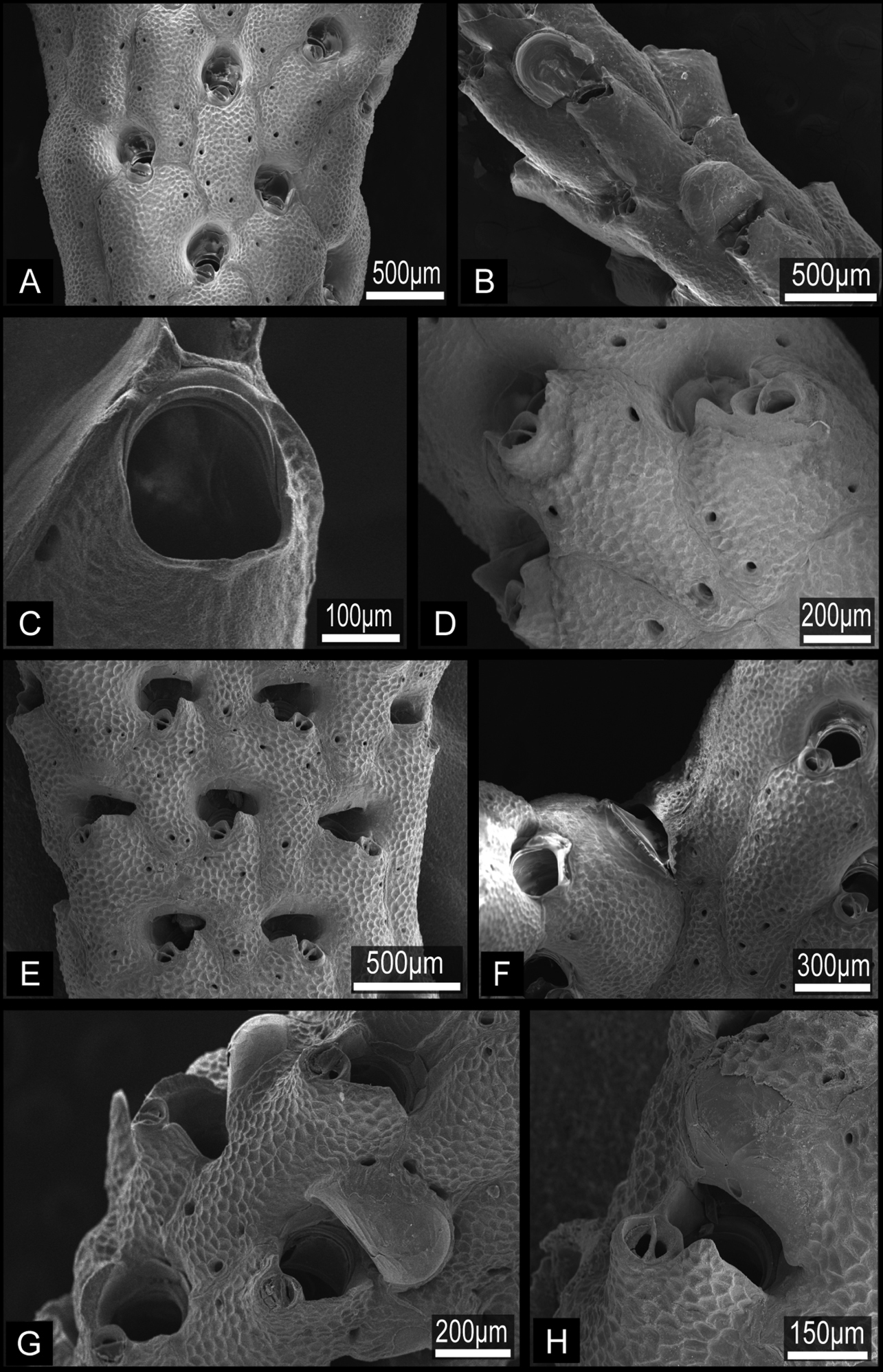
Colony erect, rigid, dichotomously branching, widely spreading, antler-like, terminal branches slender. Basal part of colony composed of both autozooids and kenozooids. Branches cylindrical, 1.39–4.76 mm wide (2.77±0.85 mm; n=25), with zooids opening all around, four or five zooids across in half-view (Fig. 4A, B). Autozooids subrectangular to oval, tapering proximally, cylindrical in younger ends of branches, arranged in quincunx; zooidal borders indistinct. Frontal shield convex, entirely tessellated with minute depressions, with two to six areolar pores offset from margin (Fig. 5A, B). Orifice (Fig. 5C) deeply immersed, elongated semicircular, about as wide as long, slightly concave proximally; lyrula and condyles absent. Oral spines lacking. Secondary orifice cormidial, bounded by contributions of secondary calcification from distal and lateral zooids, with suture lines often evident between the sectors; secondary orifice roughly oval in young zooids, complex in mature zooids, with suboral avicularium offset to one side and a sharp, raised flattened peristomial flange on the other, often with a sinus between the two (Fig. 5D, E). Suboral avicularium lies on peristome periphery; small, circular, with complete pivot; semicircular mandible directed proximolaterally (Fig. 5D, H); orificial side of rostrum with a rounded-triangular tooth or flange (Fig. 5E). Zooids commonly have the small suboral avicularium replaced by a larger (Fig. 6A) or much larger, hypertrophied oval (Fig. 6B) avicularium (Fig. 6A, B), with the latter type sometimes displaced proximally toward the center of the frontal shield (Figs 4C, 6C). Another type of large avicularium occurs rarely at branch bifurcations (Fig. 5F), appearing almost as a crack in the bifurcation; twice as wide as long, 0.181 mm long by 0.373 mm wide (n=1). Interzooidal kenozooids lacking orifice are interspersed with autozooids on branches (Fig. 5A), but are often much more numerous on side of branch facing inward toward the colony axis than on outer side; kenozooids encircle the base of colony (Fig. 4D). Ovicell (Fig. 5B, G, H) globose, recumbent on distal zooid, roughly as wide as long when fully formed; ooecium smooth, proximal margin slightly curved, ectooecium is not completely covering the endooecium, leaving a large central membranous foramina and small proximal membranous window (Fig. 3A, B). Ectooecium is partially covered by tessellated secondary calcification from neighboring zooids with age (Fig. 5H). The proximal margin with the central pseudopore remains uncovered in the old parts of the colony (Fig. 3C).
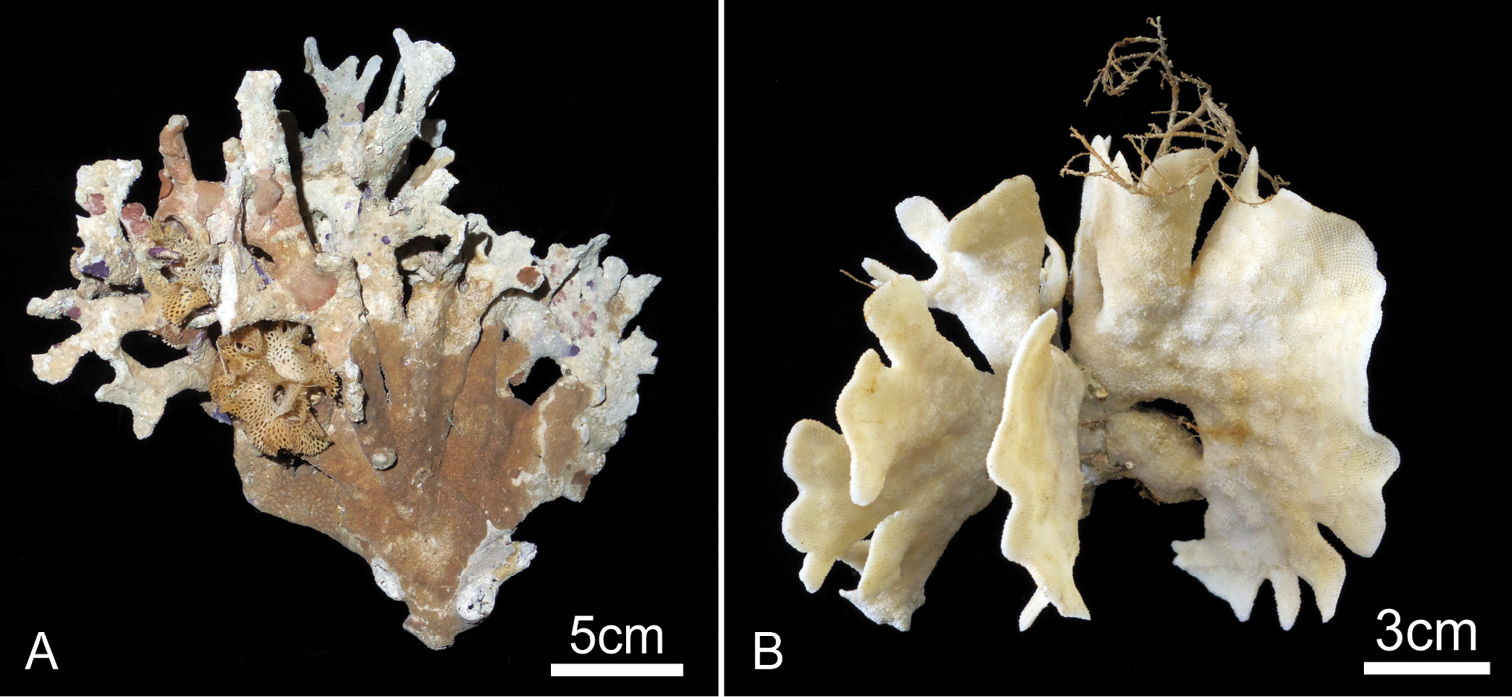
Buchneria teres comb. n. A Lectotype (MZS 36-2) and paralectotype (MZS 36-1) B Colonies on dead shells of Conchocele bisecta, NSMT TeS-8 and 10 C Large suboral avicularium with a semicircular mandible, NSMT TeS-2 D Kenozooidal base of the colony, NSMT TeS-8.
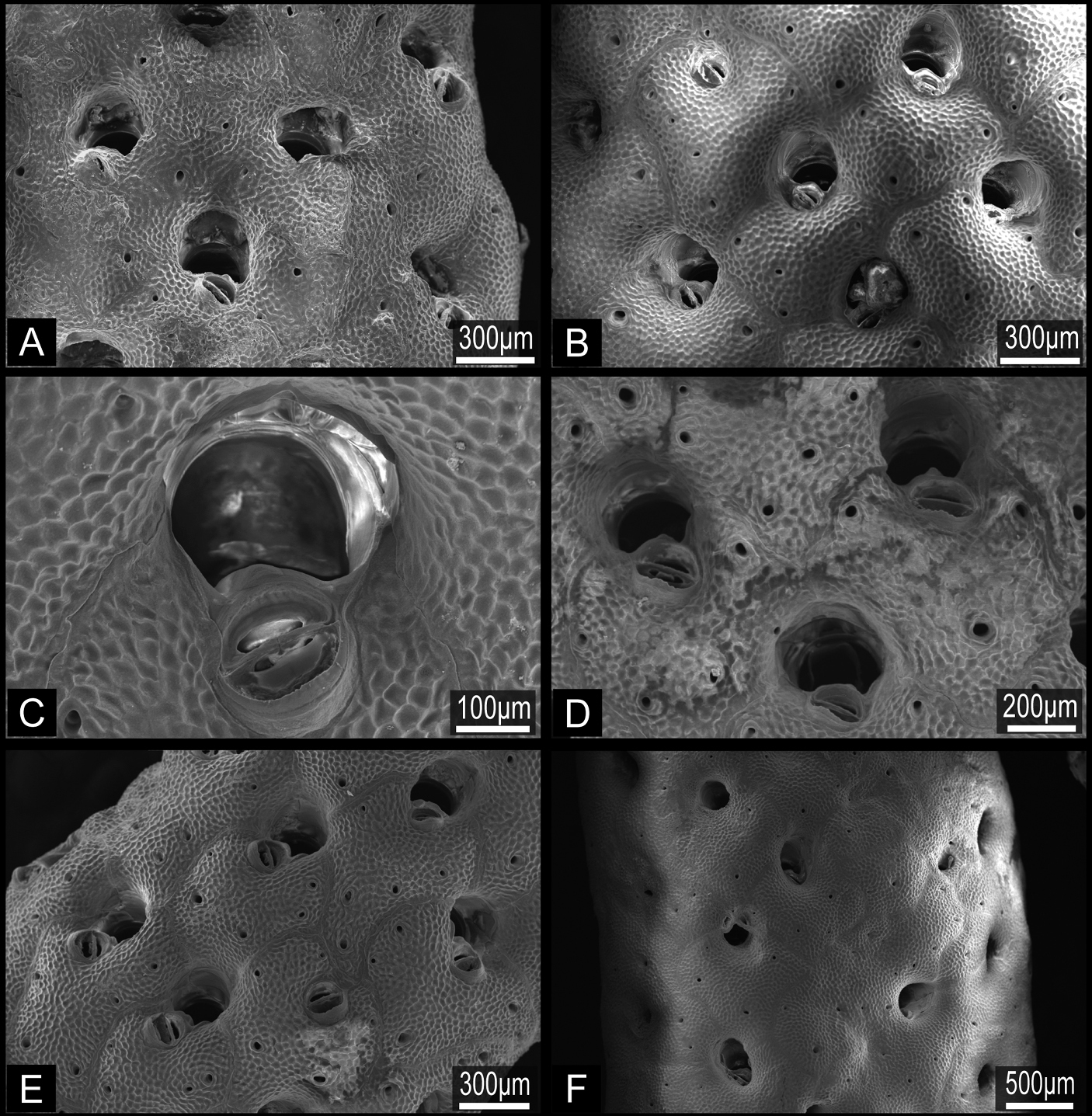
Buchneria teres comb. n., scanning electron micrographs. A Part of a branch showing autozooids with few frontal pores, NSMT TeS-2 B Younger part of a paralectotype branch showing rectangular zooids and semicircular ovicells, NSMT Te-738 (original MZS 36-3) C Orifice without lyrula or condyles, NSMT TeS-6 D Autozooid (right) with a developing peristomial labium of an intramural bud, NSMT TeS-3 E Ovicellate zooids with well-developed peristomial labia, ZSM 20100261 F Large avicularium at a branch bifurcation, NSMT TeS-2 G Zooids with young ovicells, NSMT TeS-6 H Ooecium partly covered by secondary calcification from surrounding zooids, ZSM 20100261.
Buchneria teres comb. n., suboral avicularia. A Two different sizes of suboral avicularia in adjacent zooids, ZSM 20043001 B A large, projecting suboral avicularium, NSMT TeS-2 C A large suboral avicularium appearing offset to the center of the frontal shield, NSMT TeS-2.
Sagami Bay, Sagami Sea, Tokyo Bay, and off Kii Peninsula, at depths of 432–950 m. The collecting depth of the specimen (1921.11.7.9.) in NHMUK is 250–330 fathoms, which means 457–603 m; therefore, the depth given in
http://species-id.net/wiki/Buchneria_rhomboidalis
Figures 7, 8Lectotype. MZS 37-2 (NSMT Te-737), branched colony, collected by L. Döderlein, 1882, Sagami Bay. Paralectotype. MZS 37-1, branched colony, collected by L. Döderlein, 1882, Sagami Bay, 370 m depth. Other material examined. NSMT-Bry R256, Emperor Showa Collection, collected 8 February 1967, 5 km SW of Jogashima, Sagami Bay, 250–400 m depth; NSMT-Bry R267, Emperor Showa Collection, collected 18 March 1968, 4 km WSW of Jogashima, Sagami Bay, 200−220 m depth; NSMT TeS-1, coll. 14 May 2004, west of Ōshima, Sagami Sea (34°40.95'N, 139°17.92'E to 34°40.68'N, 139°18.22'E), 220−277 m depth, beam trawl, RV Tansei-maru; colony (NSMT Te-799), collected by H. Kohtsuka from research boat Rinkai-maru, 10 January 2012, SW of Jogashima, Sagami Bay (35°06.101'N, 139°34.284'E to 35°05.684'N, 139°34.061'E), 218–318 m depth; fragments of colonies (NSMT Te-796, Te-797), collected by M. Hirose from research boat Rinkai-maru, 24 February 2012, WSW of Jogashima, Sagami Bay (35°07.301'N, 139°33.365'E to 35°07.327'N, 139°32.978'E), 300–493 m depth.
ZL, 0.767−1.150 (0.948±0.100); ZW, 0.468−1.050 (0.735±0.116); n=32. OrL, 0.152−0.211 (0.177±0.019); OrW, 0.189−0.245 (0.222±0.023); n=14. AvL, 0.106−0.271 (0.171±0.034); AvW, 0.096−0.193 (0.144±0.024); n=28.
Colony erect, rigid, dichotomously branching, widely spreading (Fig. 7A). Branches flattened, multiserial, with zooids opening all around; 2.33−6.34 mm wide (3.34±0.88 mm, n=25), five to nine zooids across (Fig. 7B). Autozooids rhomboidal, arranged in quincunx (Fig. 7B), zooidal borders indistinct. Frontal shield convex, entirely tessellated with minute depressions, with two to four small areolar pores (Fig. 8A, B) offset from margin. Orifice subcircular, about as wide as long, smooth distally, proximal margin without sinus; lyrula and condyles absent (Fig. 8C). No oral spines. Orifice deeply immersed; aperture at colony surface roughly semicircular in outline, without sinus proximally; cormidial, bounded by contributions of frontal calcification from distal and one or two lateral zooids, with suture lines sometimes evident between the sectors (Fig. 8C, D). Suboral avicularium small, proximal to orifice on the peristome periphery; circular, with complete pivot, rostrum slightly elevated, slightly denticulate, semicircular mandible directed proximolaterally (Fig. 8C, D, E); distal tooth of suboral avicularium small, rounded-conical (Fig. 8D). No other avicularia were observed. On both the edges of branches and in older part of colony, interzooidal kenozooids lacking orifice (Fig. 8F) are interspersed with autozooids; kenozooids especially numerous in the basal part of colony. Ooecium imperforate, smooth, completely immersed, not evident from colony surface; proximal margin almost straight or slightly curved, rarely obscuring the distal edge of primary orifice in oviccelate zooids (Fig. 7C).
Buchneria rhomboidalis comb. n. A Lectotype in the Döderlein collection, MZS 37-2 B Enlargement of a branch of the lectotype C Enlargement of an orifice showing the proximal margin of the ooecium, NSMT Te-796.
Buchneria rhomboidalis comb. n., scanning electron micrographs. A Part of the lectotype, NSMT Te-737 (original MZS 37-2) B Specimen NSMT-Bry R256, showing rhomboidal autozooids with few frontal pores C Orifice with a round suboral avicularium, NSMT-Bry R267 D Suboral avicularia, each with a small, conical distal tooth, NSMT-Bry R267 E Autozooids, showing the slightly projecting rostrum of the suboral avicularia, NSMT-Bry R267 F Kenozooids interspersed with autozooids at the base of the colony, NSMT TeS-1.
Eastern part of Sagami Bay, and the Sagami Sea southwest of Jogashima and west of Ōshima, at depths of 200–493 m.
Examination of Ortmann’s (1890) type specimens revealed this species belongs not in Escharoides but in Buchneria, on the basis of the frontal shield with few pores, absence of oral spines, orifice without lyrula, immersed imperforate ooecium, and the suboral avicularia. Buchneria rhomboidalis is characterized by having rhomboidal zooids and flat branches. This species resembles Buchneria teres, but differs in having flat rather than cylindrical branches, in lacking a peristomial labium and sinus, and in lacking a large avicularium at branch bifurcations. The depth distribution of Buchneria rhomboidalis (200–493 m) is shallower than that of Buchneria teres (432–3660 m).
http://species-id.net/wiki/Buchneria_variabilis
Figures 9, 10Androsova’s type specimen (ZIN-1/3670) in Zoological Institute of the Russian Academy of Sciences (ZIN RAS), colony collected southwestern region of Sakhalin, Moneron Island (Kaibato), Sea of Japan, 36 m depth, (examined by micrographs); large erect colonies and fragments (NSMT Te-724 to Te-734; ZIHU 4130 and 4131), collected SE of Akkeshi Bay (42°48.37'N, 144°56.22'E) by M. Hirose from research boat Misago-maru, 6 July 2010, 116 m depth, by dredge; large erect colony and fragments (NSMT Te-790 to Te-794) collected SE of Akkeshi Bay (42°48.20'N, 144°55.43'E to 42°48.26'N, 144°54.91'E) by M. Hirose from research boat Misago-maru, 8 July 2011, 114−116 m depth, by dredge.
ZL, 0.558−0.921 (0.751±0.101); ZW, 0.408−0.882 (0.611±0.088); n=25. OrL, 0.135−0.223 (0.189±0.019); OrW, 0.130−0.226 (0.192±0.023); n=27. OvL, 0.124−0.444 (0.247±0.072); OvW, 0.104−0.395 (0.214±0.056); n=44.
Colony erect, rigid, robust, with thick, broad, strap-like branches at least 10 zooid widths across, or foliaceous, fan-shaped lobes; lobes or branches 0.86 to 8.04 cm wide (2.15±1.38 cm, n=25), multifurcate or irregularly lobed on distal margin; zooids open on both sides (Fig. 9). Broad lobes of some colonies are covered with conspicuous, closely spaced circular monticules (Fig. 9B). Autozooids oval, rounded hexagonal, or subrectangular in outline; strongly convex frontally, arranged in quincunx, zooecial borders indistinct; frontal shield tessellated, with four to eight areolar pores of irregular size along margin or offset more centrally (Fig. 10A, B). Orifice (Fig. 10C, D) semicircular, broader than long, slightly concave proximally, lyrula and condyles absent (Fig. 10D); deeply immersed with age. Oral spines lacking. Peristome deep, cormidial, formed by contributions of secondary calcification from distal and lateral zooids, with suture lines often evident between the sectors (Fig. 10E). Suboral avicularia approximately same size as orifice, located at margin of peristome; oval, with complete or incomplete pivot, rostrum slightly elevated distally, with a median tooth; mandible semicircular, directed proximally or proximolaterally (Fig. 10B, C). Rounded conical tooth on oral edge of avicularian rostrum conspicuous, projecting into secondary orifice (Fig. 10E). Hypertrophied suboral avicularia are frequent; often larger in area than orifice; distal end of rostrum elevated, pointed; rounded-triangular mandible directed proximally (Fig. 10B, C). I observed no other types of avicularia. Basal part of colony robust, composed of both interzooidal kenozooids and autozooids, borders indistinct. Ooecium imperforate, smooth, completely immersed by secondary calcification from the neighboring zooids, the proximal margin of the ooecium distinctly indented laterally and centrally, obscuring the distal edge of primary orifice in ovicellate zooids (Fig. 10E). Frontal budding frequent (Fig. 10F).
Buchneria variabilis comb. n. A Large, bushy dead colony with various encrusting epibionts, NSMT Te-726 B Large, fan-shaped living colony, NSMT Te-724; note the broad circular monticules at centre right.
Buchneria variabilis comb. n., NSMT Te-729, scanning electron micrographs. A Autozooids showing the strongly convex frontal wall with few pores B Autozooids with various sizes of suboral avicularia C Adjacent autozooids showing different sizes of suboral avicularia D Orifice without lyrula or condyles E Enlargement showing immersed orifices and the narrow, conical tooth distal to suboral avicularia F Overgrowth by frontal budding in the central part of the colony.
Moneron Island, SW Sakhalin, 36 m depth (
My material matches Androsova’s (1958) description of Porella variabilis. She mentioned that the tooth on the proximal margin of the peristome is associated with the suboral avicularium, and that the tooth is present when the avicularium abuts the proximal margin of the peristome, but absent when the avicularium is offset proximally from the peristome. This is the case in all known species of Buchneria.
Buchneria variabilis differs from Buchneria teres and Buchneria rhomboidalis in colony form and in having larger suboral avicularia.
To date, species of Buchneria have been only reported from the northwestern Pacific, where they appear to have a cold-temperate distribution (Fig. 11). Most records are from northern Japanese waters; I have not detected Buchneria species in field surveys in southern Japan (e.g., near Okinawa). The southernmost record of living Buchneria in Japan is Buchneria rhomboidalis and Buchneria teres from Sagami Bay, of which Buchneria teres has been considered as abyssal species (Buchner, 1924; Harmer, 1957) and was collected at depths of more than 400 m in Sagami Bay. Another, more northern Buchneria species in Japan showed shallower distribution; Buchneria variabilis occurred at 114–116 m depth near Akkeshi and at 36 m depth near Sakhalin (Androsova, 1958).
Map showing the known distribution of the three Buchneria species in the western Pacific.
While Buchneria might be endemic to this region, further sampling around the North Pacific rim, including deep-water sites, may expand the distributions of known species or detect additional species. Furthermore, as Buchneria resembles some other North Atlantic genera such as Porella, Palmiskenea, Marguetta and Porelloides, taxonomic studies of these other genera may also detect additional Buchneria species, and the close relationship may indicate a common history at times when there was a connection between the Pacific and Atlantic.
Taxonomic key to Japanese Buchneria species| 1a | Colony robust; branches thick and broad, fan-shaped distally; hypertrophied suboral avicularia often occur on the frontal shield | Buchneria variabilis comb. n. |
| 1b | Colony more delicate, branches cylindrical or flattened, slender distally; hypertrophied suboral avicularia rare or absent | 2 |
| 2a | Branches flattened; zooids rhomboidal; hypertrophied suboral avicularia absent | Buchneria rhomboidalis comb. n. |
| 2b | Branches cylindrical; zooids rectangular, hypertrophied suboral avicularia present | Buchneria teres comb. n. |
I thank Dr. Shunsuke F. Mawatari (Hokkaido University Museum) for valuable assistance in the study of the historical collections. I thank Dr. Bernhard Ruthensteiner and Mrs. Eva Lodde (Zoologische Staatssammlung München) for valuable advice and assistance in the study of the Doflein and Haberer Collections; Madame Marie-Dominique Wandhammer and Madame Marie Meister (Musée Zoologique Strasbourg) for kind help, assistance in observations, and the loan of the Döderlein Collection; Dr. Hiroshi Namikawa (Showa Memorial Institute of the National Museum of Nature and Science) for advice and assistance, and the loan of the bryozoan collection of Emperor Showa; and Mr. Hisanori Kohtsuka (Misaki Marine Biological Station, The University of Tokyo) for crucial support with collecting in Sagami Bay. Special thanks are due to Dr. Joachim Scholz and Mrs. Brigitte Lotz (Senckenberg Forschungsinstitut) for assistance during my stay in Germany. I thank Miss Mary Spencer Jones (Natural History Museum London) for valuable advice and assistance in the survey of the Harmer’s and Androsova’s material. I thank Mrs. Elly Beglinger (Naturalis, Leiden) for assistance in the survey of Harmer’s material. I thank Dr. Nina Denisenko (Zoological Institute of the Russian Academy of Sciences) for sending the photographs of Androsova’s type specimen. I thank Dr. Dennis Gordon (National Institute of Water & Atmospheric Research), Dr. Andrew Ostrovsky (University of Vienna), and two reviewers Dr. Björn Berning (Oberösterreichische Landesmuseen) and Dr. Kevin Tilbrook (Queensland Museum) for valuable advice and suggestions on the manuscript. I thank Professor Matthew Dick (Hokkaido University) for checking my English and providing crucial suggestions. This study was supported in part by the 21st Century COE Program “Neo-Science of Natural History” at Hokkaido University, funded by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and a Fellowship from the Japan Society for the Promotion of Science (No. 20-3856).
Specimens examined in this study. (doi: 10.3897/zookeys.241.3175.app) File format: Microsoft Office Excel file (xls).
Explanation note: The deposit column indicates the museum where specimens reside: MZS, Musée Zoologique Strasbourg, ZSM, Zoologische Staatssammlung München, NSMT, National Museum of Nature and Science Tokyo (now located in Tsukuba).