Citation: Zacharczenko B, Wagner DL, Hatfield MJ (2014) A new cryptic Sympistis from eastern North America revealed by novel larval phenotype and host plant association (Lepidoptera, Noctuidae, Oncocnemidinae). ZooKeys 379: 93–107. doi: 10.3897/zookeys.379.5765
A Triosteum-feeding species of Sympistis is described from eastern North America: Sympistis forbesi sp. n. Identity of the new species is most reliably determined from larval morphology and host plant association—both adult scaling and genitalic characters overlap with those of Sympisitis chionanthi, a Chionanthus and Fraxinus feeder.
Cryptic species, Triosteum, Caprifoliaceae, iridoid glycosides, unequal evolutionary rates
Sympistis Hübner is the second largest genus of North American macrolepidopterans, with 176 recognized species (
Our motivation for the description of the Triosteum-feeding Sympistis is that both Sympistis chionanthi and Sympistis forbesi are worthy, or are likely to become, conservation targets. Sympistis forbesi is believed to be extirpated from New Jersey (where Rummel first reported the species) and no extant colonies are known in New York (where Forbes and other Cornell lepidopterists knew it). We think it likely that it is declining or already extinct from much of its former eastern range due to decline in the abundance of Triosteum (feverwort), which fared better in the open agricultural landscapes of the two previous centuries. Increased grazing pressure by white-tailed deer is also thought to be a threat to the host plant and its herbivore fauna (
Below we describe the new Sympistis, illustrate the larval, pupal, and adult stages and provide a brief account of the biology of the new species.
The adult description of Sympistis forbesi is based on 45 pinned specimens from Iowa, Illinois and Minnesota. Seventy-one specimens of Sympistis chionanthi from Connecticut and New York were studied (n=71). The larval description of Sympistis forbesi is based on 15 preserved larvae and 65 larval images (GGC, ISIC, UCMS). Larvae were compared to 7 preserved larvae and 11 larval images of Sympistis chionanthi (CUIC, GGC, NYSM, UCMS). Genitalia of the male type and one female paratype were prepared and mounted according to
CNC Canadian National Collection, Ottawa, Ontario, Canada.
CUIC Cornell University Insect Collection, Ithaca, New York, USA
GGC George Godfrey Collection, Athens, IL, USA
CHC Chuck Harp Collection, Littleton, Colorado, USA
ISIC Iowa State Insect Collection, Iowa, USA
NDSU North Dakota State University, North Dakota, USA
NMNH National Museum of Natural History, Washington D.C., USA
NYSM New York State Museum, Albany, New York, USA
UCMS University of Connecticut, Storrs, Connecticut, USA
http://zoobank.org/A3005B5D-DCC6-42D1-B743-E1CD7A843763
http://species-id.net/wiki/Sympistis_forbesi
Figs 1, 2, 4, 5, 7, 8, 10, 11, 13, 14, 18–32, 34–36HOLOTYPE male (Fig. 1) IA: Boone Co., Little Bluestem Prairie [41°53'52N, 93°52'10"W], [larva] 29 May 2010, Mary Jane Hatfield, 051B-B10, [adult emerged] 9 September 2010, host: Triosteum perfoliatum; Genitalia CNC slide # ♂ 16516; Barcodes of Life Project # CNCLEP 81921, leg removed, DNA extracted. Deposited at UCMS, Storrs, Connecticut, USA. Paratypes (adults). (22 males, 23 females) Iowa: Polk Co., Snyder Farm [41°46'23.51"N, 93°29'21.38"W] [larva] 316 May 2009, Mary Jane Hatfield; DLW Lot: 2010E96, emerg: 29 August 2010, Host: Triosteum perfoliatum, (1 ♂) (UCMS); Polk Co., Snyder Farm [41°46'23.51"N, 93°29'21.38"W] [larva] 30 May 2009, Mary Jane Hatfield, emerg: fall 2009, Host: Triosteum perfoliatum, (1 ♀) (UCMS); Polk Co., Snyder Farm [41°46'23.51"N, 93°29'21.38"W], 9 May 2010 [larva], 004-P10, Mary Jane Hatfield, [adult] found dead 9 September 2010, Host: Triosteum perfoliatum (1 ♂) (UCMS); Boone Co., Little Bluestem Prairie [41°53'52N, 93°52'10"W], May 2010 [larva], Mary Jane Hatfield, 051-D-B10, emerged 19 September 2010, Host: Triosteum perfoliatum (1 ♀) (UCMS); Boone Co., Little Bluestem Prairie [41°53'52N, 93°52'10"W], 29 May 2010 [larva], Mary Jane Hatfield, 051C-B10, 13 September 2010 [emerged], Host: Triosteum perfoliatum (1 ♂) (UCMS); Boone Co., Little Bluestem Prairie [41°53'52N, 93°52'10"W], 29 May 2009 [larva], Mary Jane Hatfield, 051B-B10, emerged 11 September 2010, Host: Triosteum perfoliatum; Barcodes of Life Project # CNCLEP 81922, leg removed, DNA extracted; Genitalia Slide CNC #16517 ♀ (UCMS); Boone Co., Little Bluestem Prairie, 41°53'53.83"N, 93°52'10.31"W, Sept. 2011, MJ Hatfield coll. (3 ♂) (2 UCMS, 1 ISIC); Boone Co., Little Bluestem Prairie, 41°53'53.83"N, 93°52'10.31"W, prairie remnant edge, larva May 5 2013, MJ Hatfield coll., 010E-B13 (3 ♀) (1 UCMS, 2 ISIC); Boone Co., Little Bluestem Prairie, 41°53'53.83”, N, 93°52'10.31"W, prairie remnant edge, larva May 5 2013, MJ Hatfield coll., 010C-1-13 (1 ♂) (ISIC); Kossuth Co., Algona, larva 20 May–14 June 2013, emerged 5 Sept. 2013, Matt Kenne coll. (1 ♀) (UCMS); Kossuth Co., Algona, larva 20 May–14 June 2013, emerged 10 Sept. 2013, Matt Kenne coll. (1 ♀) (UCMS); Kossuth Co., Algona, larva 20 May–14 June 2013, emerged 30 August 2013, Matt Kenne coll. (2 ♀) (UCMS); Kossuth Co., Algona, larva 20 May–14 June 2013, emerged 2 Sept. 2013, Matt Kenne coll. (1 ♂) (ISIC); Kossuth Co., Algona, larva 20 May–14 June 2013, emerged 3 Sept. 2013, Matt Kenne coll. (1 ♂) (NDSU); Kossuth Co., Algona, larva 20 May–14 June 2013, emerged 3 Sept. 2013, Matt Kenne coll. (1 ♀) (NDSU); Kossuth Co., Algona, larva 20 May–14 June 2013, emerged 6 Sept. 2013, Matt Kenne coll. (1 ♀) (ISIC); Illinois: Champaign Co., Mahomet, reared ex larva, 9–14 Oct. 1976, 27 Aug. 1976, 13 Aug. 1981, G. Godfrey coll. (7 ♂, 5 ♀) (CUIC); Cook Co., Elk Grove, [adults] bred 20–24 Aug. 1941 and 26–28 Aug. 1942, A.K. Wyatt (1 ♂, 3 ♀) (CUIC); Minnesota: Houston Co., Perkin’s Bluff Prairie, 43°47'8.85"N, 91°36'58.63"W, larva 11 May 2013, emerged 5 Sept. 2013, Mary Jane Hatfield coll. (3 ♀) (1 CNC, 1 CHC, 1 NMNH); Houston Co., Perkin’s Bluff Prairie, 43°47'8.85"N, 91°36'58.63"W, larva 11 May 2013, emerged 5 Sept. 2013, Mary Jane Hatfield coll. (1 ♂) (NMNH); Houston Co., Perkin’s Bluff Prairie, 43°47'8.85"N, 91°36'58.63"W, larva 11 May 2013, Mary Jane Hatfield coll. (4 ♂) (1 CNC, 1 CHC, 2 UCMS). Paratypes (larvae). Iowa: Polk Co., Snyder Farm [41°46'23.51"N, 93°29'21.38"W], Col: 29 March 2012 [larva], 9 May 2012 [preserved]; Mary Jane Hatfield, Host: Triosteum perfoliatum (UCMS); Story Co., Harker Savannah [41°54'6.73"N, 93°30'31.21"W], Col: 29 April 2012 [larva], 9 May 2012, [preserved] Mary Jane Hatfield, Host: Triosteum perfoliatum (UCMS); Story Co., Harker Savannah [41°54'6.73"N, 93°30'31.21"W], Col: 29 April 2012 [larva], 9 May 2012 [preserved]; Mary Jane Hatfield, Host: Triosteum perfoliatum (UCMS); Winneshiek Co., [43°27'51.96"N, 91°38'15.73"W], Col: 15 May 2012 [larva], 18 May 2012 [preserved], Mark Leoschke, Host: Triosteum perfoliatum (UCMS); Allamakee Co., [43°25'16.65"N, 91°16'54.53"W], Col: 23 May 2012 [larva], 24 May 2012 [preserved], Mark Leoschke, Host: Triosteum perfoliatum (UCMS); Boone Co., Danielle Wirth property (oak savannah) [41°52'11.29"N, 93°52'55.10"W], Col: 22 May 2010 [larva], 28 May 2010 [preserved], Mary Jane Hatfield, Host: Triosteum perfoliatum [dissected] (UCMS).
Adults of Sympistis forbesi and Sympistis chionanthi. 1 ♂ Sympistis forbesi HOLOTYPE, IA: Boone Co., Little Blue Stem Prairie, ex larva on Triosteum (UCMS) 2 ♂ Sympistis forbesi, IL: Champaign Co., Mahomet, ex larva on Triosteum (CUIC) 3 ♂ Sympistis chionanthi, NY: Tompkins Co., Ithaca, ex ova, reared on Fraxinus (CUIC) 4 ♂ Sympistis forbesi, IA: Boone Co., Little Blue Stem Prairie, ex larva on Triosteum (UCMS) 5 ♂ Sympistis forbesi, IL: Champaign Co., Mahomet, ex larva on Triosteum (CUIC) 6 ♂ Sympistis chionanthi, CT: Windham Co., Hampton, adult at light (UCMS) 7 ♀ Sympistis forbesi, IA: Boone Co., Little Blue Stem Prairie, ex larva on Triosteum (UCMS) 8 ♀ Sympistis forbesi, IL: Champaign Co., Mahomet, ex larva on Triosteum (CUIC) 9 ♀ Sympistis chionanthi, NY: Tompkins Co., Ithaca, ex ova, reared on Fraxinus (CUIC) 10 ♀ Sympistis forbesi, IA: Polk Co., ex larva on Triosteum (UCMS) 11 ♀ Sympistis forbesi, IL: Champaign Co., Mahomet, ex larva on Triosteum (CUIC) 12 ♀ Sympistis chionanthi, CT: Windham Co., Pomfret, adult at light (UCMS).
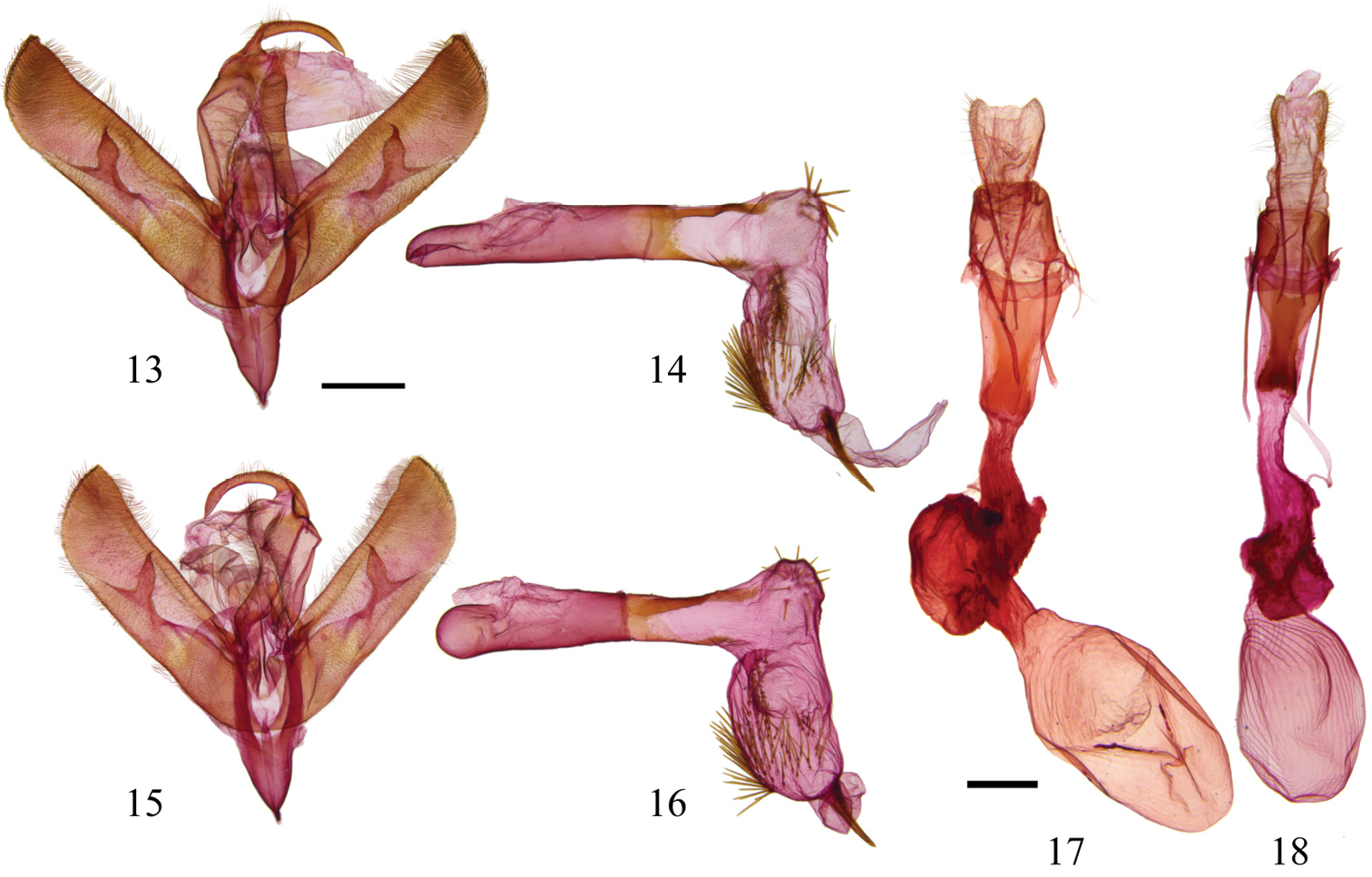
Sympistis forbesi and Sympistis chionanthi genitalia 13 Sympistis forbesi HOLOTYPE male, IOWA: Boone Co., Little Blue Stem Prairie, Genitalia CNC slide # 16516 ♂; scale = 1 mm 14 aedoeagus, same data 15 Sympistis chionanthi male, MANITOBA, Cartwright, Genitalia CNC slide # 16515 ♂ 16 aedoeagus, same data 17 Sympistis chionanthi female, SASKATCHEWAN, 8 mi NW Stewart, 1800’, Genitalia CNC slide # 13192 ♀; scale = 1 mm 18 Sympistis forbesi paratype female, same data as male, Genitalia CNC slide # 16517 ♀.
We name the species after William T. Forbes, North America’s premier lepidopterist over a 40-year period from 1920 to 1960. Forbes’ understanding of the species and higher-level taxonomy of eastern Macrolepidoptera was extraordinary, with the vast majority of his taxonomic decisions standing the test of time (and additional data). His four-volume treatise on the Lepidoptera of New York and Neighboring States remains the definitive work on eastern moths, especially for most Microlepidoptera.
Adult. Sympistis forbesi averages slightly smaller than Sympistis chionanthi. The scales over the thorax are smaller, more densely packed. In most individuals there are fewer white scales on the thorax and forewing: e.g., the costal margin, wing base, and orbicular and reniform spots have fewer white scales than most individuals of Sympistis chionanthi. Additionally, the anal dash is often more J-shaped and the fringe is only faintly checkered, lacking the pure white scales seen in many Sympistis chionanthi. In the hindwing, there is a more distinctive terminal line and the apex tends to have more black scales extending onto the fringe. The rami of male antennae through the basal half of the antenna average 0.50–0.65 mm in Sympistis forbesi, and 0.55–0.70 mm in Sympistis chionanthi. Larva. The larva provides unambiguous morphological characters that allow recognition of this new species. The last instar is mostly green with a reddish dorsum (red coloration is added through mid to late instars); there are no black or brown markings as in Sympistis chionanthi (in particular, the black subdorsal stripe characteristic of Sympistis chionanthi is absent from all instars of Sympistis forbesi). Body smaller, more elongate and modestly tapered at both ends, especially relative to the robust habitus of Sympistis chionanthi. Head width of ultimate instar of Sympistis forbesi 2.5–2.8 mm; head width of Sympistis chionanthi 3.0–3.2 mm. Spiracular height consistently smaller in Sympistis forbesi compared to Sympistis chionanthi – mean spiracular heights of A1–A6 are 0.30 and 0.36 mm, respectively. Mean crochet number of Sympistis forbesi on A3–A6 and A10 are 17, 19, 20, 21, and 20; mean crochet number of Sympistis chionanthi on A3–A6 and A10 are 27, 28, 32, 32, and 33.
Male. Forewing length: 14.5–16 mm (n=23, reared from wild larvae). Ground color warm gray. Head. Antenna biramous; rami approximately 0.50–0.65 mm through basal half of antenna. Forward-facing tuft of scales just above faint black line between eyes. Thorax. Gray, medial prothoracic tuft, edged with black, preceded by conspicuous transverse black line. Black edging of tuft continues laterad to wing base. Tegula steely gray, indistinct thick band of dark scales at back. Legs with mix of dark and light scales. Tarsi dark brown or black. Forewing. Thin, smoothly curved basal and antemedial lines. Thickened antemedial line tapering to inner margin. Orbicular spot gray centrally and pale gray peripherally, thinly edged with black. Medial line ill defined; field proximal to reniform spot with numerous dark scales, forming two dark fascia along costa above orbicular and reniform spots. Black line or open triangle in position of claviform spot. Postmedial line running parallel to medial line, connecting to base of reniform and looping around toward margin, finally connecting to dark fascia along costa. Anal dash usually crisp, occasionally absent, subtended by sharp or diffuse black spot basad, forming J-shape. Subterminal line forming black fascia at costa, but otherwise pale gray, weakly developed to nearly obsolescent. Fringe weakly checkered, without white scaling. Hindwing. Pearly white with thin, crisp terminal line except at apex where diffuse field of black scales extends through fringe. Postmedial line obsolescent in males. Abdomen. Mixture of light and dark scales and hairs. Whitish scales along posterior margin of pregenital abdominal terga. Male genitalia. Valves elongate, nearly parallel sided with flat-topped projection from apex; bulbous clasper with claw-like apex that curves mesad; corona of fine setae of variable lengths. Juxta poorly differentiated. Uncas curved, gradually tapering, apex drawn into fine, curved spine. Saccus V-shaped, drawn into point anteriorad. Aedeagus cylindrical, variously sclerotized with vesica bearing approximately one dozen spines on elbow-bend and numerous longer, narrower spines over bulbous subapical region; terminus armed with single stout spine nearly 1 mm in length (as large as uncus).
Female. Forewing length: 14–16.5 mm (n=10, reared from wild larvae). Similar to male, but with substantially more fuscous scaling in submarginal region of hindwing; often with faint postmedial band. Antenna simple, without rami. Female genitalia. Posterior and anterior apophyses slender, elongate, ca. 2.5 × length of sclerotized portion of A8; lamella antevaginalis sclerotized, winged anteriorally and posteriorally, with posterior part about ostium bursae cleft and thus appearing somewhat flipper-like; anterior end less flared, ca. ½ width and only shallowly cleft, and more strongly sclerotized. Appendix bursae well developed; ovate corpus bursae ca. 2 × size of appendix bursae with parallel thickenings most evident posteriorad.
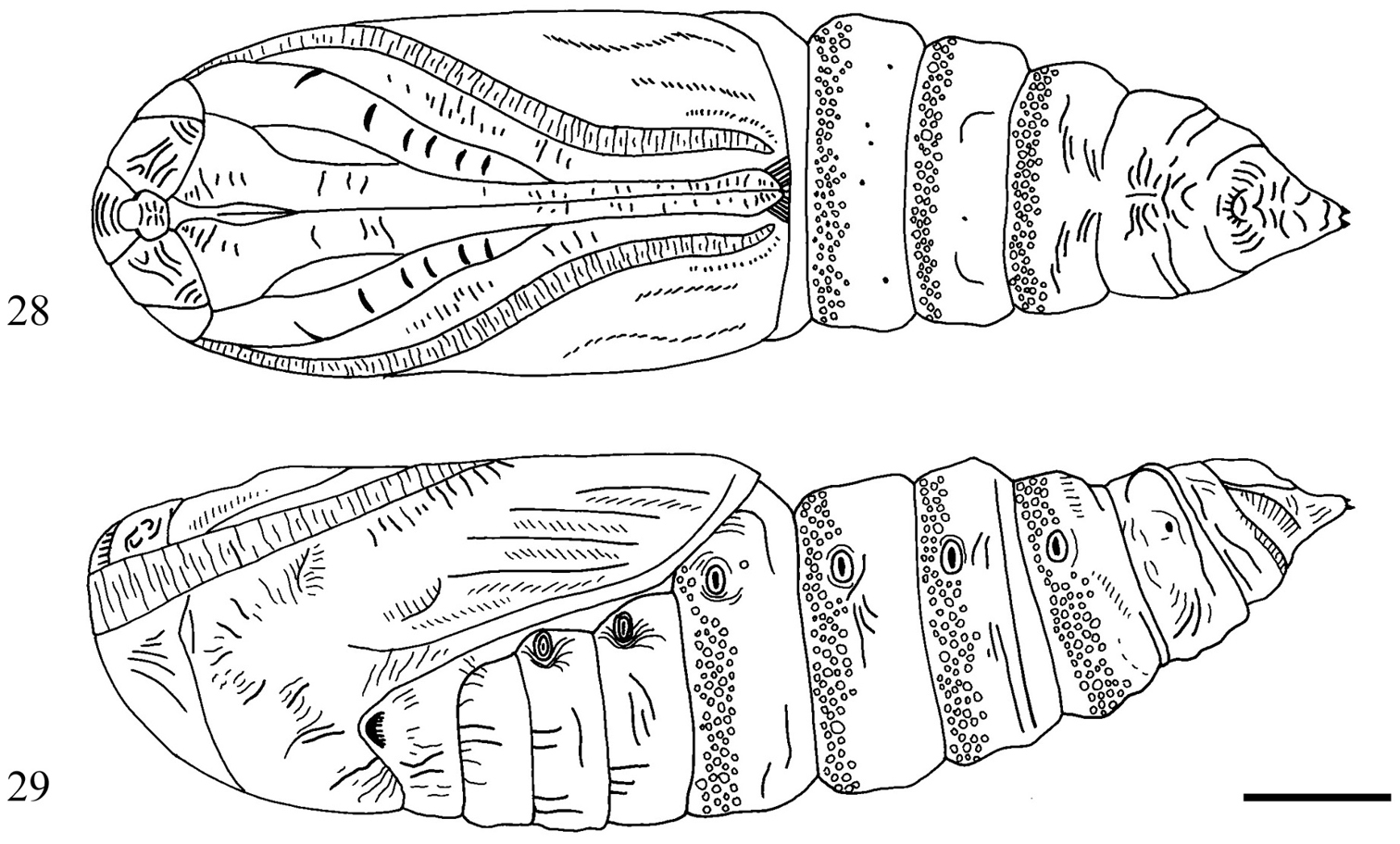
Description of pupa (Figs 28, 29). 16–19 mm long, 4.4–5.0 mm wide. Orange brown to deep chestnut brown, mostly smooth except for deeply pitted anterior portion of abdominal segments A4–A7. Primary setae extremely short, difficult to locate. Labial palpus visible, subequal to visible portion of profemur. Foreleg with cuminate apex, ending in abrupt spine. Proboscis extending just beyond antenna and midleg, nearly reaching end of wing. Labrum roughly shovel shaped with truncated apex. Eyepiece and frons ornamented with dense micro-ridging. Spiracular scars elongate, five times longer than wide. Cremaster ending in pair of minute thorn-like spines; cremaster deeply wrinkled and heavily sclerotized at base.
Description of living final instar (Figs 31, 32). Ground color sea to mint green with pink to red dorsum and pale longitudinal striping along sides of trunk; A8 modestly humped. Reddish dorsum composed of pink to pale red middorsal stripe flanked by darker red addorsal stripes; dorsal pinacula white. Mostly broken white pinstripe zigzags through D1 pinacula. Red dorsal area bounded by pale (green to white) subdorsal pinstripe. Two supraspiracular pinstripes edged below with darker green. Lateral stripe, greenish white, roughly equal to height of spiracles, extending along lower end of spiracles. Prolegs on A3 and A4 about half size of those on A5 and A6. Head pale to dark brown above, often with pink to reddish flush; labrum greenish white, shallowly rugose frons, gena with three whitish lines that anastamose about stemmata.
Description of living early instars (Fig. 30). All instars elongate, smooth, with numerous stripes; A8 modestly humped. First and second instars reddish brown and white, shiny; middorsal white stripe enlarged over anterior half of A8. Subspiracular white stripe thickened, enlarged to include each spiracle along trunk. All pinacula distinct, raised, brown black. First instar head width 0.15–0.16 mm. Second instar head width 0.40–0.56 mm. Third instar green and white, with brown-black pinacula; head width 0.90–1.00 mm. Fourth instar with small white pinacula as in final instar; head width 1.50–1.80 mm.
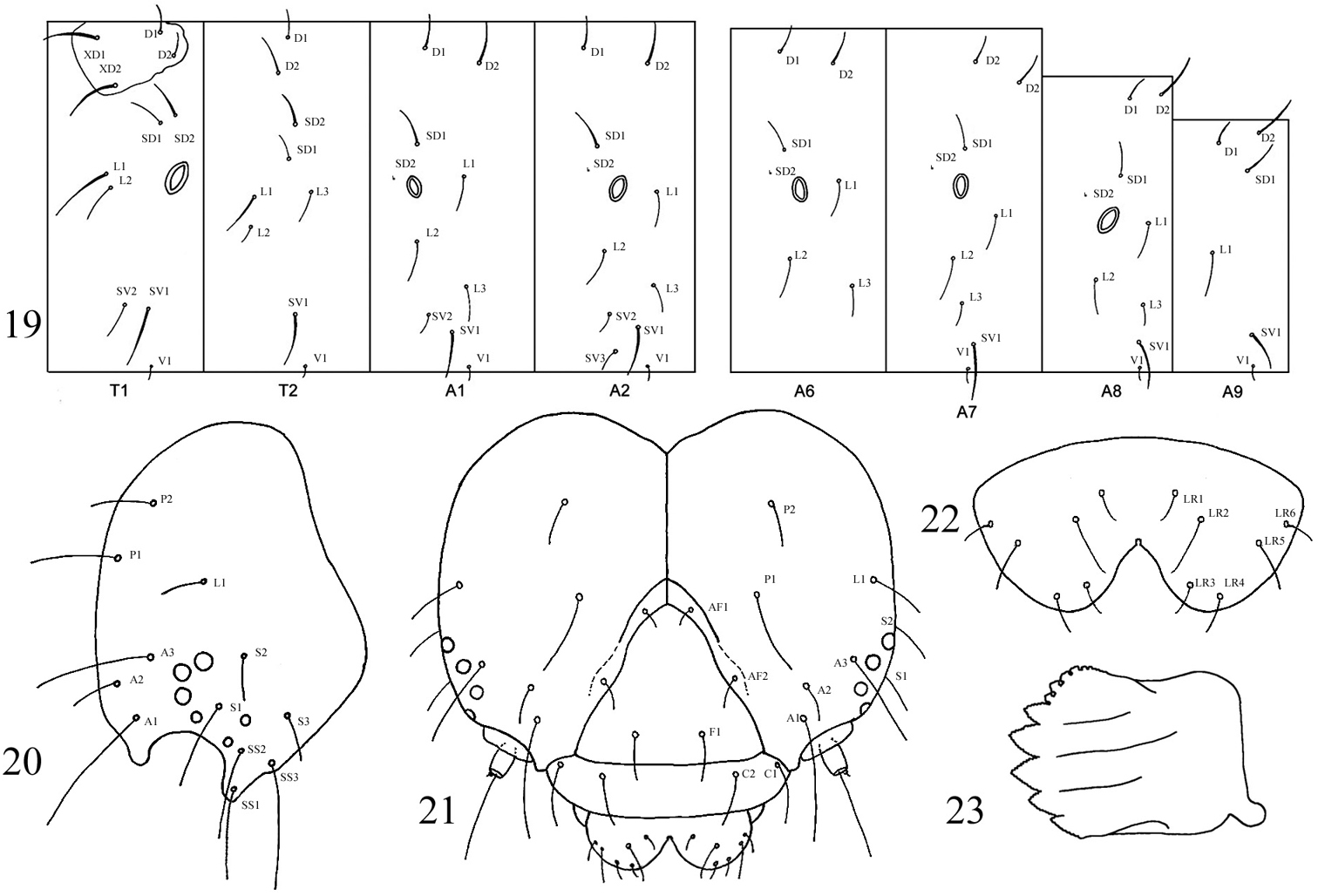
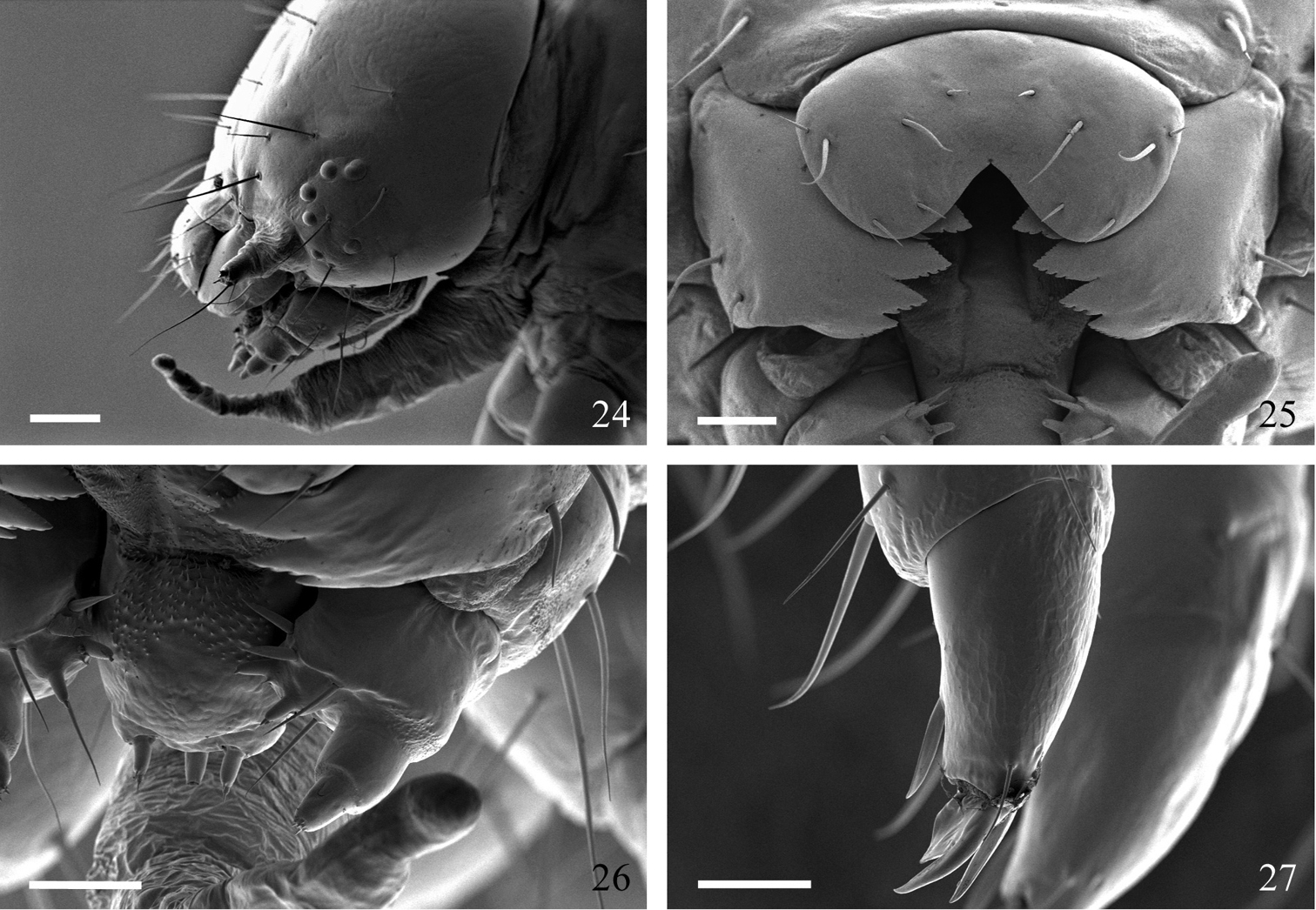
Description of preserved final instar (Figs 19–27). Head. Texture microtuberculate. Width 2.5–2.8 mm. Field of brown aggregated spots on vertex, especially between P setae and L1. Second group of spots caudad of S3. P1 2 × length of P2. A1 longest seta on head (Figs 20, 21, 24). V-shaped medial cleft about 2/5 labral depth (Figs 22, 25). Spinneret short, subequal to labial palpus (Fig. 26). Mandibles simple, inner surface mostly smooth (Fig. 23). Body. Length 34–44 mm. Integument smooth; lightly sclerotized prothoracic shield and anal plate. Primary setae short, most approximately 2 × height of spiracle on same segment (Fig. 19). Thorax. SV1 longest seta on thoracic segments, ca. 3 × height of prothoracic spiracle and 1½ × height of SV2; spiracular height: 0.30–0.32 mm. Prothorax with SD1 and SD2 free from shield, positioned above spiracle. L1 3 × longer than L2 on T2 and T3. Meso- and metathorax with D and SD setae more or less vertically aligned (Fig. 19). Thoracic legs with apical and subapical blade-like setae proximal to claws (Fig. 27). Abdomen. Two SV on A1, three SV on A2. L1 directly behind spiracle on A1–A6 and A8, displaced ventrad on A7. D2 becoming increasingly procumbent towards caudal end of body (Fig. 19). D2 seta on A8 and A9 arising from slightly elevated and pigmented, rearward-facing wart. A10 with D and SD setae on rearward facing warts. Spiracular height of A1 through A6 0.28–0.32 mm; height on A7 0.26–0.28 mm; height on A8 0.32–0.35 mm. Crochet numbers on A3–A6 and A10 as follows: 16–18, 17–22, 19–22, 20–23, and 20.
Sympistis forbesi larva. 19 chaetotaxy 20 head, lateral 21 head, frontal 22 labrum 23 mandible.
Sympistis forbesi middle instar. 24 head, lateral, with adenosma extruded; scale = 250 µm 25 labrum, mandibles, and oral cavity; scale = 100 µm 26 hypophryngeal complex (center left) and maxilla (center right); scale = 100 µm 27 prothoracic leg (note apical and subapical blade-like setae proximal to claws); scale = 100 µm.
We were unable to identify any consistent genitalic differences in either the male or female genitalia that distinguish Sympistis forbesi from Sympistis chionanthi. Our type series is based on reared material, as we know of no definitive structural or patterning characters that will assure certain identification of light-collected adults. Hence, we caution that features discussed in the diagnosis and description may be attributes more typical of reared (unflown) specimens. For example the slightly smaller size and darker coloration that we note above for Sympistis forbesi could be rearing artifacts.
Locally common in Midwest, especially prairies. Most commonly found in Iowa, Illinois, and Minnesota. Believed to be extirpated from eastern portion of range in New York and New Jersey. Given that the genus Triosteum occurs from southern Canada to Texas and eastward, it is probable that the range of the new species is more extensive than circumscribed here.
So far as known larvae are specialists on members of the genus Triosteum, also known as horse-gentian or feverwort, of the family Caprifoliaceae. Nearly all our larval collections are from Triosteum perfoliatum (feverwort). We found a few larvae of what appeared to be the same species on Triosteum aurantiacum in Iowa. In the laboratory, larvae from Triosteum perfoliatum readily accepted and matured on Triosteum aurantiacum. In two separate instances, larvae were successfully reared to pupation on Fraxinus as well, a widely used host plant of Sympistis chionanthi. Sympistis forbesi larvae grew more slowly on Fraxinus, and maintained their typical green and pink coloration (MJH unpublished data, M. Keene personal communication). Sympistis forbesi is univoltine with a single generation that emerges, flies, and mates in late summer, mostly in early September. Females presumably lay eggs on or near the stems of Triosteum. Above-ground tissues of the host die and senesce over the winter. MJH has found first instars on unopened leaves that were just pushing forth from the ground in early March (in Iowa). Larvae complete their development by mid-June. Early instars feed exclusively on new leaves, principally of the apical meristem, before the leaves have had a chance to open and expand to full size. Where the moth is common and when collections are made through the first half the season, partially opened leaf fascicles often yield larvae that were not seen at the time of collection. Last instars also consume new leaves, but are content to feed on fully expanded leaves and flowers (Figs 34–36). All instars are cryptic in both color and habit. The late instars rest along a shoot head down, often near flowers, where their coloration is well matched to that of the stem and reddish-pink Triosteum petals and sepals. Densities can be high with more than a dozen larvae on a single shoot; on several occasions we noted cases where the larvae of Sympistis forbesi severely damaged the apical portions of their host plant. Prepupae form a slight cocoon below ground; the summer months are passed as a pupa.
In a neighbor-joining tree based on J. D. Lafontaine’s unpublished barcodes for 137 North American oncocnemidine noctuids (representing 687 individuals), Sympistis chionanthi (n=7; CT, Quebec, Ontario, Alberta) and the new species (n=2; both Iowa) grouped together in a “cluster” separate from other North American Sympistis, and each taxon was reciprocally “monophyletic, ” although the two groups differed by less than 1% from one another. In a second analysis, focused on “Adita-group” Sympistis that included 13 individuals from across North America, again the two Triosteum feeders grouped in their own cluster.
Sympistis chionanthi was described by J. E. Smith in
Sympistis chionanthi was described as being very rare in Georgia (
Although the adults of Sympistis forbesi and Sympistis chionanthi are difficult (and sometimes impossible) to distinguish even upon dissection, their larvae are distinct in size, coloration, habitus, and life history. Presumably these coloration and morphological differences reflect, at least in part, the structural differences in their preferred hosts. Triosteum is an herbaceous perennial that dies back to the ground each winter; Fraxinus and Chionanthus are trees. The brown, bark-like coloration of late instar Sympistis chionanthi is suggestive that larvae rest off of foliage by day and perhaps even near the ground along the trunk or off the host in leaf litter. We know of no brown noctuoid larvae that rest on foliage by day, and many, like Catocala Schrank, Melipotis Hübner, and Zale Hübner, may wander far from the foliage when not feeding. The coloration of last instar Sympistis forbesi (Figs 32, 33) is reflective of its preferred resting site: the green stems of feverwort. Likewise, it is our guess that the less robust body, smaller prolegs, and reduced crochet hook number of Sympistis forbesi reflect the fact that larvae rest adjacent to suitable foliage. By contrast, the caterpillars of Sympistis chionanthi on a mature ash or fringetree may well have to traverse meters in search of suitable food each night. Surprisingly, no differences in mandible morphology of the two sister taxa were noted.
Despite the differences between the hosts of Sympistis forbesi and Sympistis chionanthi, the plants share secondary metabolites which may elucidate how the ancestral host plant switch was able to occur. Triosteum are members of the Caprifoliaceae, whereas the hosts of Sympistis chionanthi (Chionanthus and Fraxinus) (
Prior to
Part of our interest in the new species derives from our desire to document instances where rates of phenotypic evolution in Lepidoptera differ markedly among life stages. For example, in Acronicta Oschenehimer and some notodontid genera (e.g., Datana Walker and Schizura Doubleday) larval phenotypes differ substantially among related species that are otherwise difficult to determine using external and genitalic features of the adults. Adults of Acronicta hastulifera (J. E. Smith) and Acronicta dactylina Grote are sometimes impossible to separate by eye or dissection, but each has a distinctive larva that readily distinguishes the second to final instars of both species (
Sympistis forbesi pupa 28 ventral 29 lateral.
Sympistis forbesi and Sympistis chionanthi larvae. 30 Sympistis forbesi second (upper) and third (lower) instars. IA: Boone Co., Little Blue Stem Prairie, May 2011, ex Triosteum perfoliatum 31 Sympistis forbesi middle instar, same collection data 32 Sympistis forbesi mature last instar, same collection data 33 Sympistis chionanthi mature last instar, NY: Albany Co., Albany, female fall 1995, ex ova reared on Fraxinus americana, DLW Lot: 1996F32.
Sympistis forbesi IA: Boone Co., Little Blue Stem Prairie, May 2011 on Triosteum perfoliatum 34 three larvae secreted in a leaf axil; note frass accumulation 35 larvae on new spring leaves; note two larvae on new leaf bundle and one on foreground leaf 36 last instar on a flower of Triosteum perfoliatum, matching the color of the flower and petioles.
We would like to thank J. Donald Lafontaine for his gracious contribution of DNA barcode data, dissections, specimens, and manuscript advice. Reviewers provided valuable suggestions that improved an earlier draft of the manuscript. George Godfrey sent us extensive notes and photographic images of Sympistis forbesi from Illinois. Jason Dombrowski and Jim Liebherr arranged for the loan of specimens from CUIC. John Franclemont’s interest in Adita generated much of the critical material for our studies. Jocelyn Gill prepared the genitalic figures. Molly McGovern, Dr. Danielle Wirth, Cindy Hildebrand, and Mark Leoschke helped locate caterpillars in Iowa. Marie Cantino and Bruce Goodwin of the Electron Microscopy Laboratory (University of Connecticut) assisted with the scanning electron microscopy images. Support for this work has been partially funded by Connecticut State Wildlife Grant from the Connecticut Department of Energy and the Environment to DLW.