(C) 2013 George E. Ball. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Ball GE, Shpeley D (2013) Western Hemisphere Zuphiini: descriptions of Coarazuphium whiteheadi, new species, and Zuphioides new genus, and classification of the genera (Coleoptera, Carabidae). ZooKeys 315: 17–54. doi: 10.3897/zookeys.315.5293
Based on small samples (exemplars) analyzed with morphological methods, including detailed descriptions and illustrations, this study treats primarily the Zuphium genus-group in the Western Hemisphere, which comprises two precinctive genera: Coarazuphium Gnaspini, Vanin & Godoy, 1998 (type species Parazuphium tessai Godoy & Vanin, 1990) and Zuphioides gen. n. (type species Zuphium mexicanum Chaudoir, 1863). The genus Coarazuphium includes six troglobitic species from Brazilian caves, and one probably hypogaeic (troglophilic) species from the mountains of Oaxaca, in Mexico (Coarazuphium whiteheadi, sp. n., type locality, ridge top, in western Oaxaca, Mexico, at 2164 m, 35 km north of San Pedro Juchatengo, 16.462N, 97.010W). The epigaeic genus Zuphioides includes 23 species, with its geographical range extended from Neotropical temperate Argentina in southern South America, northward through the tropics to north temperate southeastern Canada, in the Nearctic Region. Keys are provided to the species of Coarazuphium and to thegenera of Western Hemisphere Zuphiini.
Taxonomy, Zuphiitae, classification, key, anophthalmy, phylogenetic relationships, brachyptery, cave inhabitants, laboulbenealian fungi
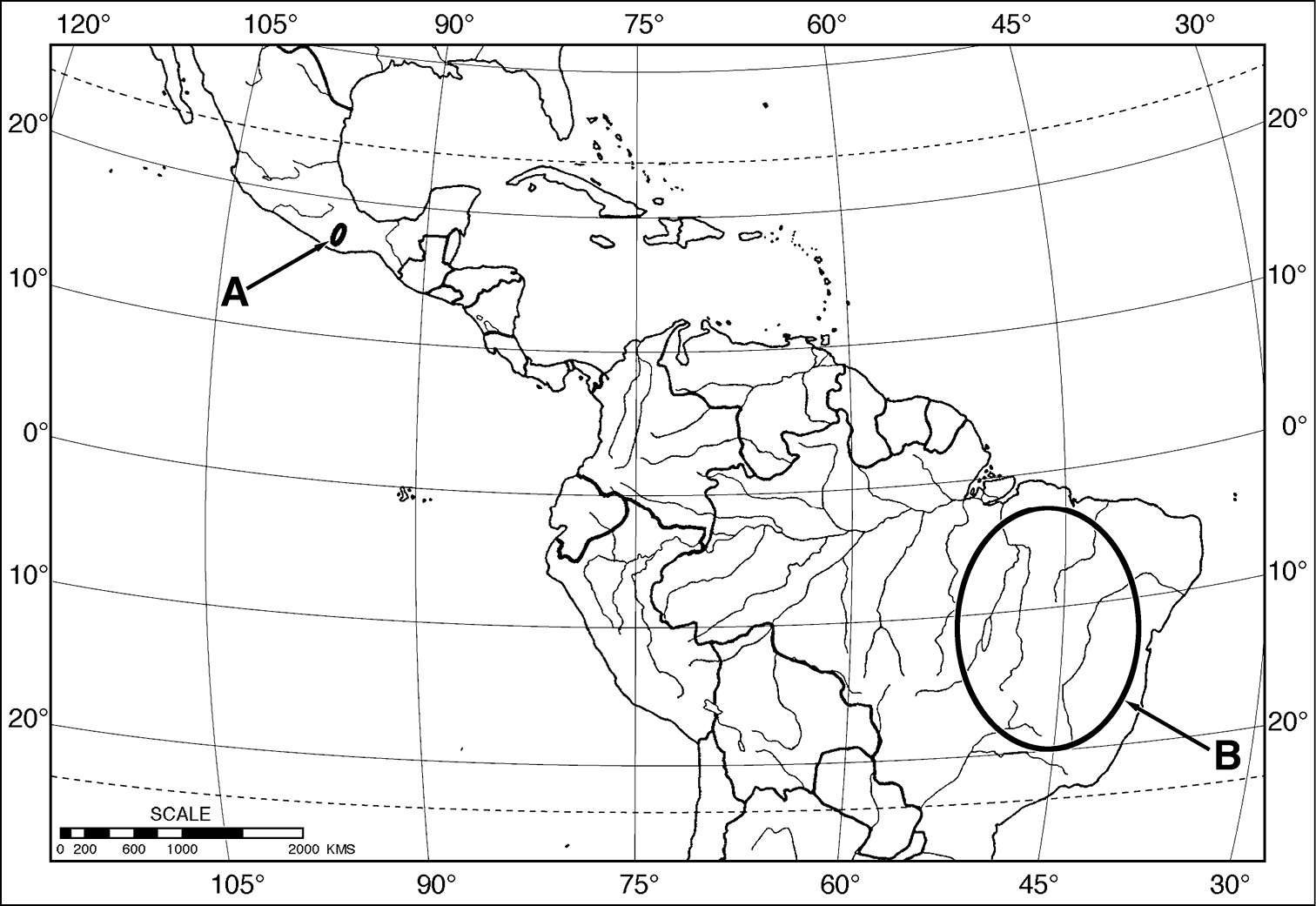
The taxonomic investigation reported herein, began with discovery of an undescribed Mexican species of Coarazuphium Gnaspini, Vanin & Godoy. Considering that Coarazuphium was known previously only from caves in eastern Brazil, its known range extension into the northern part of Middle America (Map 1) was reason to examine it closely, with the expectation of broadening our concept of the genus, and perhaps even of its tribe, the Zuphiini.
Although the six known Brazilian species had been clearly described and well illustrated, a more detailed comparison of Coarazuphium with other zuphiines seemed desirable. Closer comparisons with the geographically widespread, morphologically similar Zuphium of authors were undertaken, beginning with a sampling of the Western Hemisphere species, and extending into the Eastern Hemisphere taxa, principally those from Western Europe and eastern Africa, with emphasis on the wide-ranging olens species group.
We broadened our study by placing Coarazuphium and Zuphioides (see below) in the context of a short review, based on the literature, of the Western Hemisphere zuphiine genera. We also took into account the classification of the Zuphiini (see Appendix I).
This study is based on examination of 94 specimens of Zuphiina, from the Western Hemisphere Nearctic and Neotropical Regions, and Eastern Hemisphere Palaearctic, Afrotropical, and Oriental Regions. Much of the material was in the Strickland Museum, Department of Biological Sciences, University of Alberta (UASM). Through the generosity of Terry L. Erwin, a sampling of zuphiines was received from the Department of Entomology, United States National Museum of Natural History, Smithsonian Institution, Washington, D. C., U.S.A. 20560 (USNM).
Measurements. Measurements were made with an ocular micrometer in a Wild M5 stereoscopic microscope, at 50×. Measurements of external body parts and abbreviations used for them in the text are:
HL Length of head - linear distance from apex of extended left mandible to posterior margin of the postocciput;
HW Width of head - maximum distance across head, including eyes;
A1L Length of antennomere 1 - linear distance from base of antennomere 1 to apex of antennomere 1;
A2-4L Length of antennomeres 2–4 - linear distance from base of antennomere 2 to apex of antennomere 4;
PL Length of pronotum - linear distance from anterior to posterior margin, measured along the midline;
PWM Maximum width of pronotum - greatest linear transverse distance;
EL Length of elytra - linear distance from humerus to apex;
EW Width of elytra - maximum distance across the elytra.
OBL Overall Body Length is the sum of HL, PL, and EL. Values for ratios for species were computed, using the measurements above: A1L/A2-4L; HW/PW. These numerical data are illustrative rather than definitive.
To express quantitatively proportions of the phallus, three measurements were made, using left lateral and dorsal aspects as illustrated in Figs 10 and 13:
PL Length of phallus - measured in a straight line from basal to apical margin;
PAL Length of apical portion - measured in a straight line from apical margin of periostial area to apical margin;
PSW Width of phallus - maximum transverse distance across the shaft, in ventral aspect.
These measurements were combined as two ratios PAL/PL; and PSW/PL. These numerical data are illustrative rather than definitive.
Preparation of material. Dissections were made by using standard techniques. Genitalia and other small structures were preserved in glycerine in microvials and pinned beneath the specimens from which they were removed. Larger structures and those that were gold-coated for study with the SEM were glued to cards pinned beneath the specimens from which they were removed.
Micrographs of isolated structures were taken with a JEOL JSM 6301 FXV field emission SEM. Line drawings of selected body parts were prepared by using a camera lucida on a Wild W5 stereoscopic microscope. Plates were prepared by using Adobe Photoshop CS 4.
Citation of figures. Figures included in the present publication are cited in the text as “Fig.” Those previously published are cited “fig.”
Label data. For type material, the information on each label is reproduced as exactly as is possible using ordinary type. Information on each label is enclosed in quotation marks; as well, a semicolon marks the end of a label. A slash mark (/) indicates the end of each line of text.
Most of the terms used to designate details of structures are found in textbooks of general entomology, or are used by coleopterists, generally. Other words, used to designate particular structures or parts thereof, are not in general use, though they have been used by us in previous publications. We provide information about these words here, as well as names that have been changed for certain structural features.
Microsculpture. A “sculpticell” is the space on the surface of the cuticle enclosed by adjacent microlines of the integumental system of microsculpture (
Chaetotaxy. This term refers to the so-called “fixed setae”, which are the long, evidently tactile, commonly encountered setae on carabids: dorsal labral; clypeal; supraorbital; stipital; submental; mental; glossal; palpigeral; pronotal; elytral parascutellar, discal, and umbilicate (or lateral); coxal, trochanteral, femoral, and tarsomeral; abdominal sternal ambulatory (sterna IV, V, VI); and abdominal sternal terminal (sternum VII, near posterior margin). Standard leg setae were not included because of difficulty in distinguishing them from the general body setation; but see
Body parts. The term “segment” is restricted for use to those body parts that reflect embryonic somites; thus, somite-like portions of the abdomen are referred to as segments. Abdominal segments are designated by Roman numerals corresponding to their respective somites. The first complete sternum is III, and the last one normally exposed is VII. For numbering the genital somites, we follow
Head. The term “head capsule” in the Zuphium genus-group is restricted to that portion of the head that extends from the anterior margin of the clypeus to the broad part of the occiput that precedes the more or markedly narrowed posterior part of occiput + postocciput.
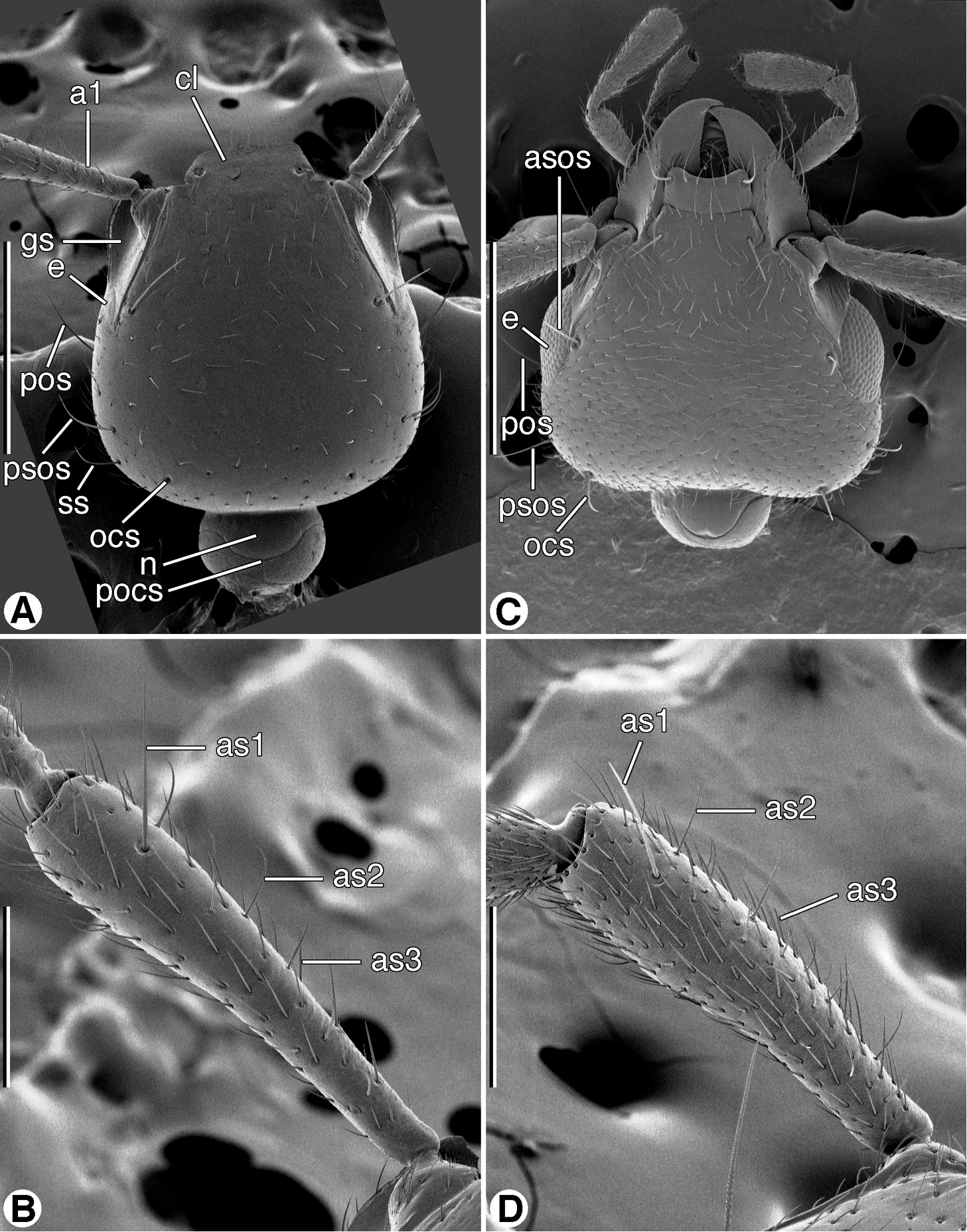
Eyes. Three conditions are recognized in the Zuphium genus-group: normal size (macrophthalmous), readily seen, convex, with distinct ommatidia (Fig. 3B); small (microphthalmous), virtually flat, without ommatidia, not readily seen (Fig. 3A); and absent (anophthalmous).
Antennae. Antennomere 1 (Figs 4B, 4D) exhibits two or three types of trichoid setae, based on size and declination: as1, single, erect, long; as2, row of several, erect, moderately long; and as3, more or less numerous, short, decumbent.
Mandibles.
Labium. The labium of the Zuphium genus-group is standard for Carabidae. For the combined glossae and paraglossae, we use the standard term ligula. The central sclerotized, apically setigerous, structure is the glossal sclerite. See Figs 8C and 8F.
Male tarsal vestiture. Two types of adhesive vestiture on the ventral surface of the fore tarsi are exhibited by males of the Zuphium genus-group: articulo-setae (Fig. 9A, as) and biseriate squamo-setae (See
Male genitalia. Based on economy of expression, in preference to the widely used terms “median lobe” and “internal sac”, we use “phallus” and “endophallus”, respectively. Such usage is also well established in the entomological literature treating the male reproductive system. The surface of the phallus treated by convention as dorsal is really the ventral surface, and vice versa (
Ovipositor (Figs 11A–11F) andfemale genital tract (Figs 12A, 12B and 13D). For naming the sclerites of the adephagan ovipositor and units of the female genital tract, we follow the system used by
Some of the morphological features that distinguish Coarazuphium from the Eastern Hemisphere widespread Zuphium olens species group (includes Zuphium olens (Rossi, 1790), type species of the genus) are shared with the Western Hemisphere species of Zuphium of authors (Table 1). From that distribution of character states, we conclude that the Western Hemisphere Zuphium of authorsforms an assemblage different from Coarazuphium andfromthe Zuphium olens species group, and this bridges the morphological gap between those two taxa. A reasonable taxonomic expression of this situation seems to us is to recognize three groups of generic rank: Zuphium (sensu stricto), Zuphioides new genus (= Zuphium of auothors), and Coarazuphium.
Comparison of diagnostic character state combinations exhibited by exemplars of Zuphium (sensu stricto), Zuphioides, new genus, and Coarazuphium Gnaspini, Vanin & Godoy.
| Taxa and character states | ||||
|---|---|---|---|---|
| No. | Characters | Zuphium (s. str.) ustum | Zuphioides mexicanum | Coarazuphium whiteheadi |
| 01 | Antennomere 1, row of erect setae | character state 01.1 absent |
character state 01.2 present Fig. 4D |
character state 01.2 present Fig. 4B |
| 02 | Posterior supraorbital seta | character state 02.1 absent |
character state 02.2 present Fig. 4C |
character state 02.2 present Fig. 4A |
| 03 | Prosternal setae | character state 03.1 absent |
character state 03.2 present |
character state 03.2 present Fig. 9B |
| 04 | Male adhesive setae | character state 04.1 biseriate | character state 04.2 pad-like, Fig. 9A |
character state 04.2 pad-like |
| 05 | Male genitalia, paraostial sclerites | character state 05.1 present Figs 13A, 13C |
character state 05.2 absent |
character state 05.2 absent |
| 06 | Female genital tract, bursal sac | character state 06.1 absent |
character state 06.2 present Fig. 12A |
character state 06.2 present Fig. 12B |
| 07 | Female genital tract, sec. spermathecal gland | character state 07.1 present Fig. 13D |
character state 07.2 absent |
character state 07.2 absent |
| 08 | Integument color | character state 08.1 rufous to piceous |
character state 08.1 rufous |
character state 08.2 testaceous |
| 09 | Head capsule form | character state 09.1 trapezoidal | character state 09.1 trapezoidal Fig. 4C |
character state 09.2 oviform Fig. 4A |
| 10 | Eyes | character state 10.1 macrophthalmous | character state 10.1 macrophthalmous Fig. 3B |
character state 10.2 microphthalmous Fig. 3A or anophthalmous |
| 11 | Metepisternum | character state 11.1 elongate |
character state 11.1 elongate |
character state 11.2 quadrate |
| 12 | Hind wings | character state 12.1 macropterous | character state 12.1 macropterous | character state 12.2 brachypterous-apterous |
| 13 | Ovipositor: gonocoxite 2 | character state 13.1 distal margin evenly rounded |
character state 13.1 distal margin evenly rounded Fig. 11F |
character state 13.2 distal margin deeply notched Fig. 11C |
Zuphioides mexicanum shares character states 01.2, 02.2, 03.2, 04.2, 05.2, 06.2, and 07.2 with Coarazuphium whiteheadi;
Zuphioides mexicanum shares character states 08.1, 09.1, 10.1, 11.1, 12.1, and 13.1 with Zuphium ustum;
Zuphium ustum exhibits uniquely character states 01.1, 02.1, 03.1, 04.1, 05.1, 06.1, and 07.1;
Coarazuphium whiteheadi exhibits uniquely character states 08.2, 09.2, 10.2, 11.2, 12.2, and 13.2.
Included are two precinctive genera of Zuphiini: Coarazuphium Gnaspini et al., and Zuphioides, new genus. They share the following features.
Recognition. With features of Western Hemisphere Zuphiini (see “Recognition”, Appendix I) and head markedly constricted posteriorly, a narrow neck formed by posterior part of occiput and postocciput; maxillary and labial palpi of about equal size and form, terminal palpomeres slightly widened apicad, apical margin obliquely truncate; antennomere 1 with short row of erect setae among the decumbent vestitural setae; prosternum with pair of long erect setae; male fore-tarsomeres 1-3 ventrally with adhesive vestiture of articulo-setae, in form of a broad pad; phallus dorsally without paraostial sclerites; endophallus with various microtrichial patches, without sclerites; ovipositor gonocoxite 2 very broad in dorso-ventral aspect without ensiform setae; female reproductive tract with terminal bursal sac, and without bulbous secondary spermathecal gland.
Description. Size and proportions. Small, OBL 4.13–4.40 mm; EW 1.36–1.48 mm; A1L/A2-4L 1.03-1.11; HW/PW 1.08.
Color. Body various, piceous to testaceous, or in life almost white; appendages slightly paler; vestiture flavous.
Habitus (Figs 1A, 1B). Flat, overall. Head (dorsal aspect) trapezoidal or oviform, subangulate or broadly rounded posteriolaterally anteriad markedly narrowed neck. Eyes (Figs 3A, 3B) convex, flat, or lacking. Pronotum narrow. Elytra parallel-sided or narrowed both anteriorly and posteriorly, lateral margins distinctly bowed.
Microsculpture. Dorsal surface of head (including clypeus) and pronotum smooth, microlines not evident; labrum and elytra with microlines fine (not easily seen at 50× or lower magnification), mesh pattern isodiametric. Ventral surface with microlines fine, generally transverse, sculpticells rather short.
Habitus, dorsal aspect, of: A Coarazuphium whiteheadi, new species B Zuphioides mexicanum (Chaudoir). Scale bars = 5 mm.
Luster. Dorsal surface of head and pronotum shiny, elytra somewhat duller.
Body vestiture and punctation. Dorsal surface of head and pronotum sparsely punctate, vestiture sparse. Elytra with punctation and vestiture dense, vestitural setae decumbent.
Fixed setae. Head (Figs 4A, 4C): clypeus one pair; head capsule with anterior pair of supraorbitals (asos) above eyes; posterior pair of supraorbitals (psos) posteriad eyes; one pair of postocular setae (pos) immediately behind eyes, laterally; one pair of occipital setae (ocs) posteriorly and mediad eyes, or lacking; also one pair posterior supernumerary setae (psus) laterad and close to posterior supraorbital setae, or lacking. Antennomere 1 (Figs 4B, 4D, as1) with single long seta distally, in addition to decumbent vestiture and row of small erect setae. Pronotum with two pairs of lateral marginal setae, anterior pair in anterior 1/8, posterior pair at posteriolateral angles. Prosternum with one pair setae anterioventrally (Fig. 9B). Elytra: each elytron anteriorly with parascutellar seta; lateral setae about 21 (one group anteriorly, one group posteriorly, and single seta medially. Abdominal sterna IV-VI with or without ambulatory setae, sternum VII with pair of long setae near posterior margin.
Head, dorsal aspect (Figs 2A, 2B). Occiput posteriorly and postocciput markedly constricted, in form of narrow neck, postoccipital suture evident. Frontoclypeal suture present (most species) or absent, with frons and clypeus fused (Coarazuphium pains,
Eyes. Small (microphthalmous), flat (Fig. 3A), ommatidia not evident at 50× or lower magnification; or, eyes normally convex (macrophthalmous, Fig. 3B), ommatidia evident at 50×.
Antennae (Fig. 1A). Filiform, extended about body length. Antennomere 1 (Figs 4B, 4D, a1) rather slender, slightly longer than antennomeres a2-a4; antennomere 2 short, about half length of a3; antennomeres 3-11 narrow, cylindrical, distinctly longer than wide.
Head, dorsal aspect, of: A Coarazuphium whiteheadi, new species B Zuphioides mexicanum (Chaudoir). Scale bars = 1 mm.
Head and prothorax, lateral aspect, of: A Coarazuphium whiteheadi, new species B Zuphioides mexicanum (Chaudoir). Legend: arrow indicates eye. Scale bars = 1 mm.
SEM micrographs of head and antennomere 1, dorsal aspect. A and B Coarazuphium whiteheadi, new species C and D Zuphioides mexicanum (Chaudoir). Legend: a1, antennomere 1; as1, as2, as3, erect setae on antennomere 1; asos, anterior supraorbital seta; cl, clypeus; e, eye; gs, genal sulcus; n, neck; ocs, occipital seta; pocs, postoccipital suture; pos, postocular seta; psos, posterior supraorbital seta; ss, posterior supernumerary seta. Scale bars: A and C = 500 µm; B and D = 200 µm.
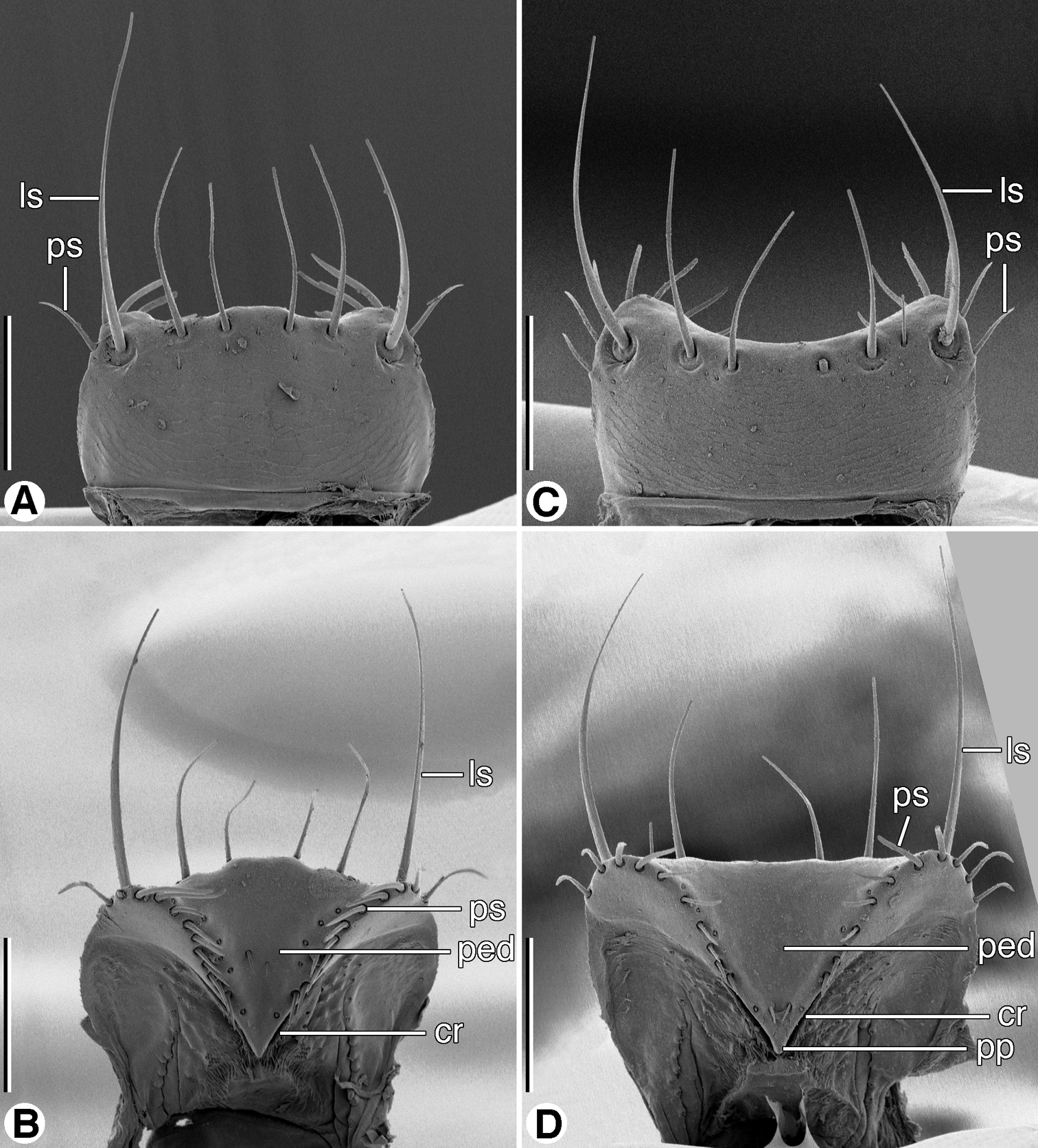
Labrum (Figs 5A, 5C). Rectangular, lateral margins rounded; anterior margin concave or irregularly convex; six dorsal setae, lateral setae longer than four medials. Epipharynx (Figs 5B, 5D) with pedium (ped) rather broad; parts standard (crepis cr, parapedial projection (pp), and parapedial setae (ps).
SEM micrographs of labra. A and B dorsal and ventral aspects, respectively, of Coarazuphium whiteheadi, new species C and D dorsal and ventral aspects, respectively, of Zuphioides mexicanum (Chaudoir). Legend: cr, crepis; ls, lateral seta; ped, pedium; pp, parapedial projection; ps, parapedial seta. Scale bars = 100 µm.
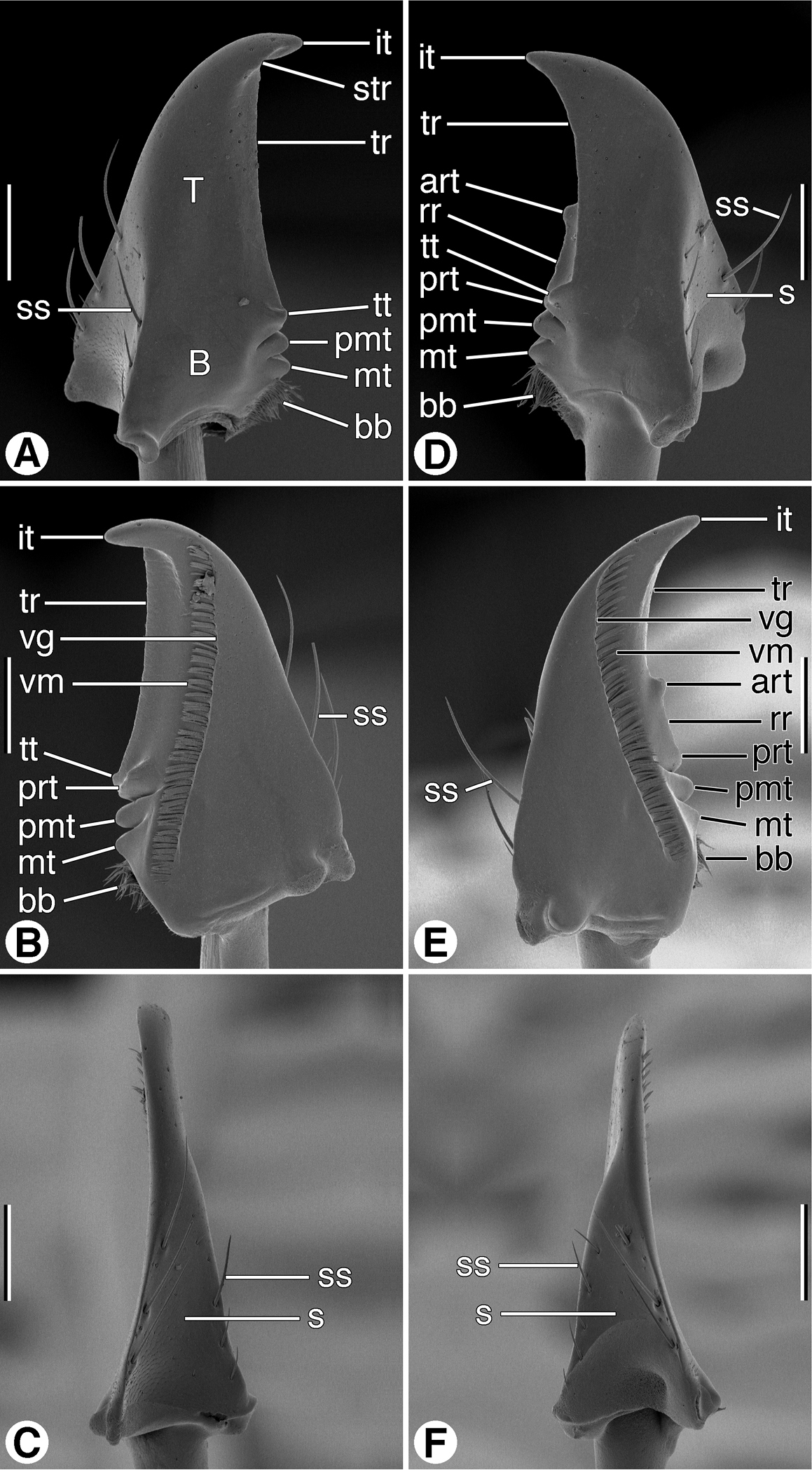
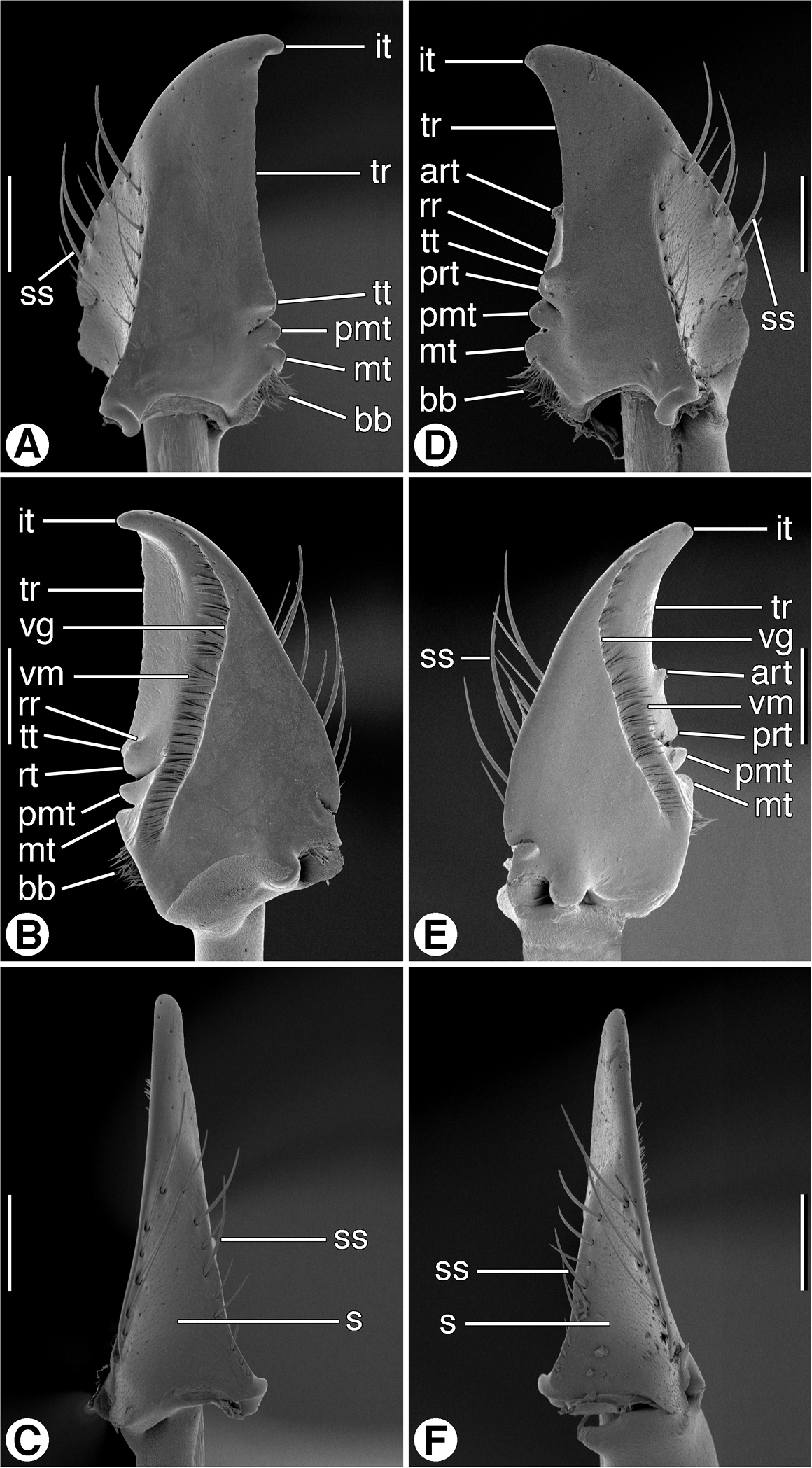
Mandibles (Figs 6A–6F and 7A–7F). Trigonal in form, with long rather slender terebra (T) and short basal area (B) with condyles; occlusal margin with long terebral ridge (tr), prominent incisor tooth (it), blunt terebral tooth (tt), small premolar (pmt) and molar (mt) teeth, and short basal brush (bb); ventrally with long ventral groove (vg) extended apically almost to mandibular margin, with moderately long and dense microtrichia (vm); lateral margin basally with distinct trianguloid scrobe (s), dorsal and ventral margins each with three moderately long setae (ss). Left mandible in dorsal aspect (Fig. 6A) apically with rather broad incisor tooth (it), short supraterebral ridge (str), shallowly concave terebral ridge (tr), moderately prominent terebral tooth (tt), and distinct premolar and molar teeth (pmt and mt); ventral surface (Figs 6B) with blunt retinacular tooth (rt), and short retinacular ridge (rr); other features as noted above. Right mandible, dorsal aspect (Figs 7A) with incisor tooth (it) shorter and less curved than in left mandible, terebral tooth (tt) not prominent; retinaculum prominent, with anterior and posterior teeth (art and prt, respectively), and intervening retinacular ridge (rr); terebral ridge (tr); premolar and molar teeth (pmt and mt, respectively) evident.
SEM micrographs of mandibles of Coarazuphium whiteheadi, new species. A and D dorsal aspect B and E ventral aspect C and F lateral aspect. Legend: art, anterior retinacular tooth; B, basal area; bb, basal brush; it, incisor tooth; mt, molar tooth; pmt, premolar tooth; prt, posterior retinacular ridge; rr, retinacular ridge; s, scrobe; ss, scrobal seta; str, supraterebral ridge; T, terebra; tr, terebral ridge; tt, terebral tooth; vg, ventral groove; vm, ventral microtrichia. Scale bars = 100 µm.
SEM micrographs of mandibles of Zuphioides mexicanum (Chaudoir). A and D dorsal aspect B and E ventral aspect C and F lateral aspect. Legend: art, anterior retinacular tooth; bb, basal brush; it, incisor tooth; mt, molar tooth; pmt, premolar tooth; prt, posterior retinacular ridge; rr, retinacular ridge; s, scrobe; ss, scrobal seta; tr, terebral ridge; tt, terebral tooth; vg, ventral groove; vm, ventral microtrichia. Scale bars = 100 µm.
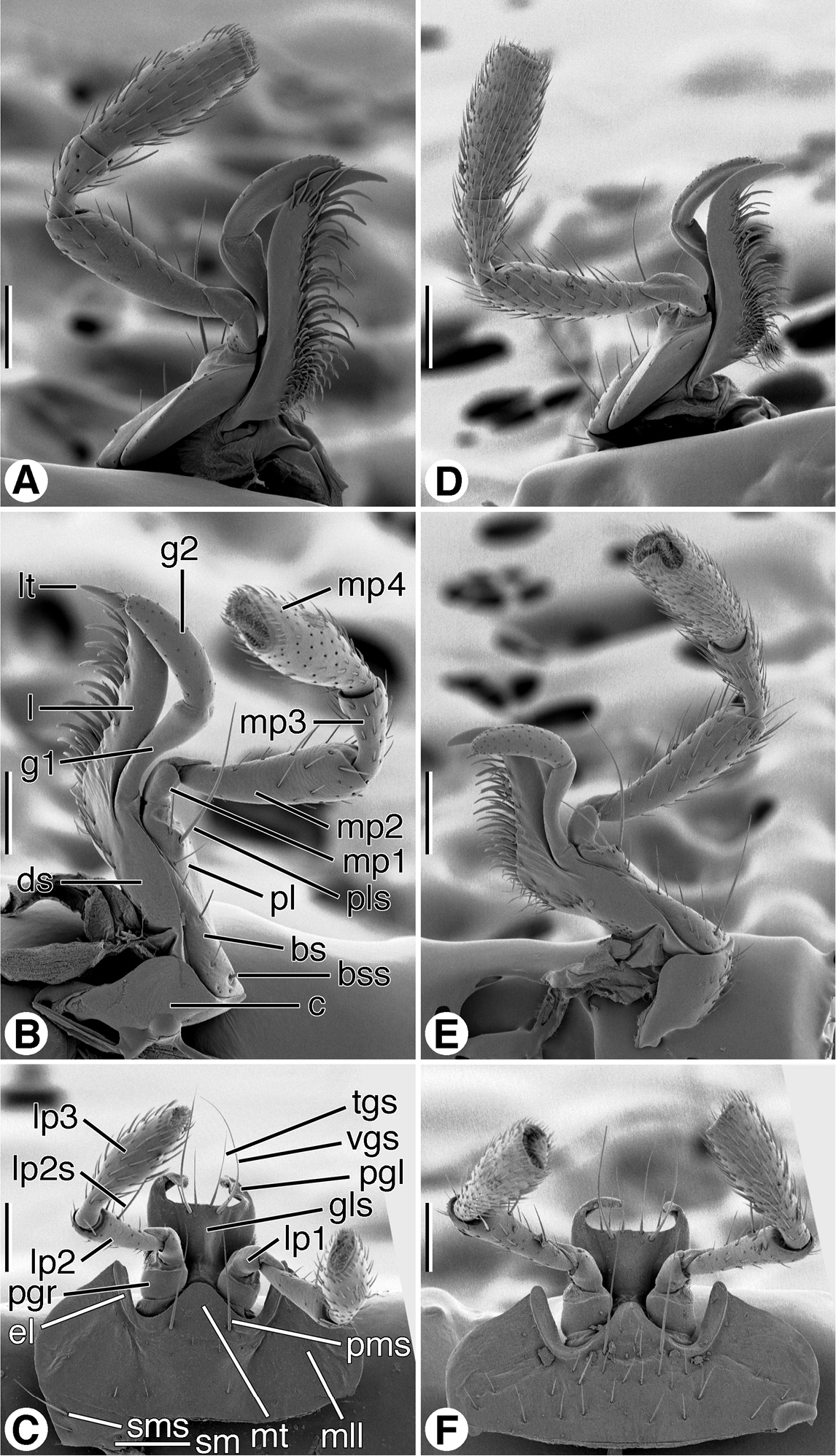
Maxillae (Figs 8A, 8B, 8D, 8E; left maxilla illustrated). Standard sclerites: cardo (c), stipes (basistipes (bs), dististipes (ds) and palpifer (pl)), galea (galeomere 1 (g1) and galeomere 2 (g2)); palpus with four palpomeres (mp1-mp4). Marginal setae: basistipital (bss) and palpiferal (pls). Lacinial occlusal margin densely setose, setae thick, moderately long. Galeomere 2 with numerous short erect setae; palpomeres 2 and 3 (mp2, mp3) sparsely setose; palpomere 4 (mp4) moderately densely setose. Palpomere 4 widened slightly distally, apical margin subtruncate.
SEM micrographs of left maxillae and labia. A–C Coarazuphium whiteheadi, new species D–F Zuphioides mexicanum (Chaudoir). Legend: bs, basistipes; bss, basistipital seta; c, cardo; ds, distitipes; el, epilobe; g1, galeomere 1; g2, galeomere 2; gls, glossal sclerite; l, lacinia; lp1, labial palpomere 1; lp2, labial palpomere 2; lp2s, labial palpomere 2 seta; lp3, labial palpomere 3; lt, lacinial tooth; mll, mental lateral lobe; mp1, maxillary palpomere 1; mp2, maxillary palpomere 2; mp3, maxillary palpomere 3; mp4, maxillary palpomere 4; mt, mental tooth; pgl, paraglossa; pgr, palpiger; pl, palpifer; pls, palpifer seta; pms, paramedial mental seta; sm, submentum; sms, submental seta; tgs, terminal glossal seta; vgs, ventral glossal seta. Scale bars = 100 µm.
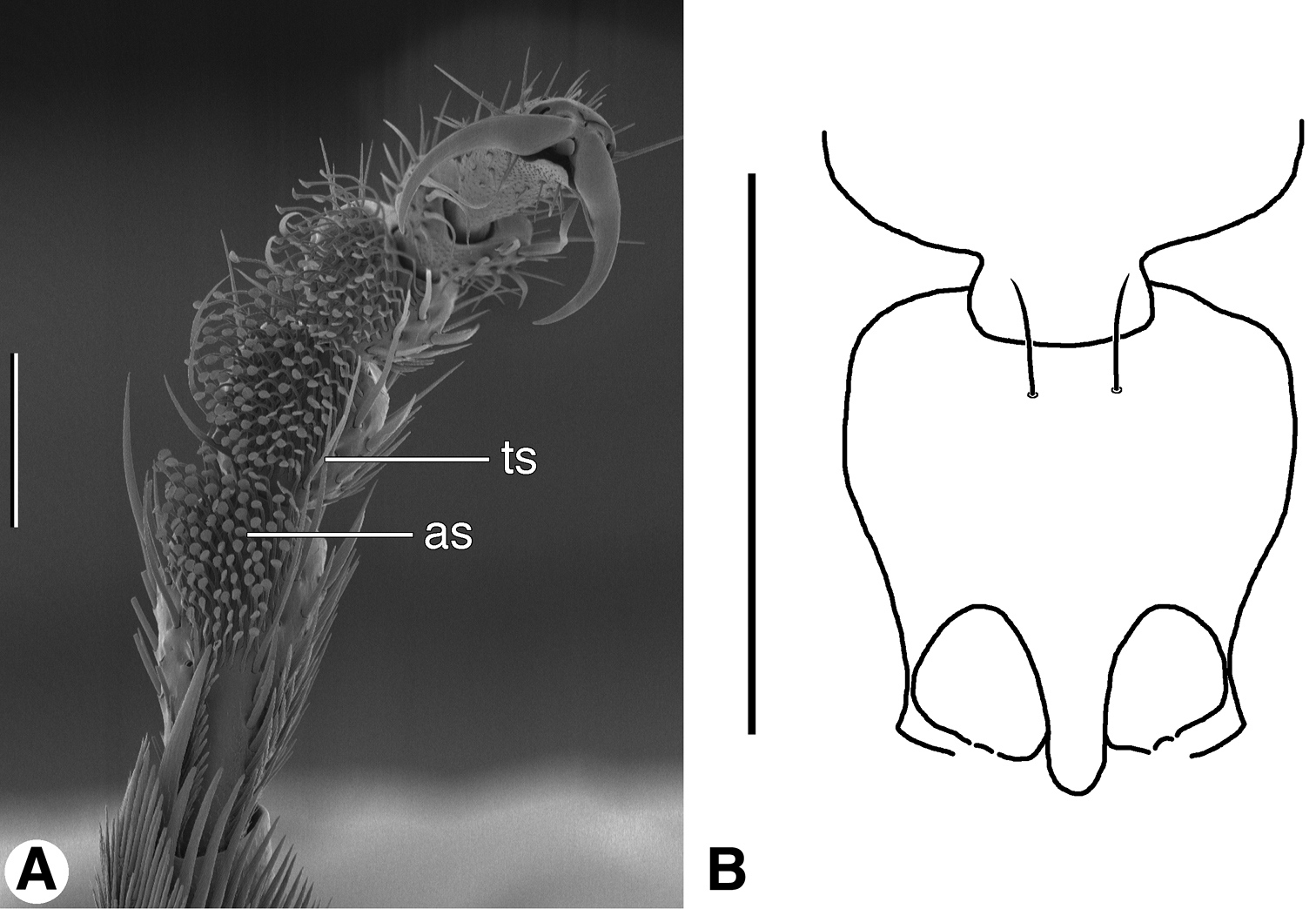
A SEM micrograph of fore tarsus of Zuphioides mexicanum (Chaudoir). Legend: as, articulo-seta; ts, trichoid seta. Scale bar = 100 µm. B Line drawing of prothorax and base of head, ventral aspect, of Coarazuphium whiteheadi, new species. Only medial pair of prominent prosternal setae illustrated, vestiture omitted. Scale bar = 1 mm.
Labium, ventral aspect (Figs 8C, 8F). Standard sclerites: submentum (sm), mentum, and prementum; prementum comprised of a central glossal sclerite (gls), pair of lateral paraglossae (pgl), and pair of palpi, each of three articles (lp1- lp3), borne on a short broad palpiger (pgr). Submentum (sm) narrow rectangular sclerite, with pair of lateral setae (sms). Mentum transverse, with broad lateral lobes (mll), broad, blunt tooth (mt), broad epilobes (el); one pair of long paramedial setae (pms). Glossal sclerite (gls), fused laterally each side with basal part of narrow paraglossae (pgl), latter membranous, densely setose, extended distally beyond broad distal margin of glossal sclerite. Glossal sclerite with pair of longer ventral setae (vgs), and four shorter terminal marginal setae (tgs). Palpigers (pgr) cup-like, glabrous. Palpi each of three palpomeres (lp1- lp3), palpomere 1 short, glabrous; palpomere 2 long, slender, subcylindrical, and moderately densely setose, most setae erect, one pair on anterior margin (lp2s) long; palpomere 3 widened slightly distally, apical margin subtruncate.
Pronotum (Figs 1A, 1B). Anterior margin truncate, lateral margins markedly sinuate posteriorly, posteriolateral angles prominent, dentiform, slightly anteriad posterior margin; surface impressions (anterior and posterior transverse and median longitudinal) shallow; lateral grooves and posteriolateral impressions moderately deep.
Pterothorax. Metasternum moderately long or short; metepisternum longer than wide or quadrate.
Elytra (Figs 1A, 1B). Separate, not fused along suture. Each elytron more or less rectangular, lateral margin straight or bowed, humerus projected anteriorly, basal ridge sinuate; apical margin truncate, with narrow band of membrane. surface with striae very shallow, intervals almost flat.
Hind wings. Macropterous, or brachypterous, with each wing represented by short stub.
Abdomen. Sternum VII with apical margin truncate.
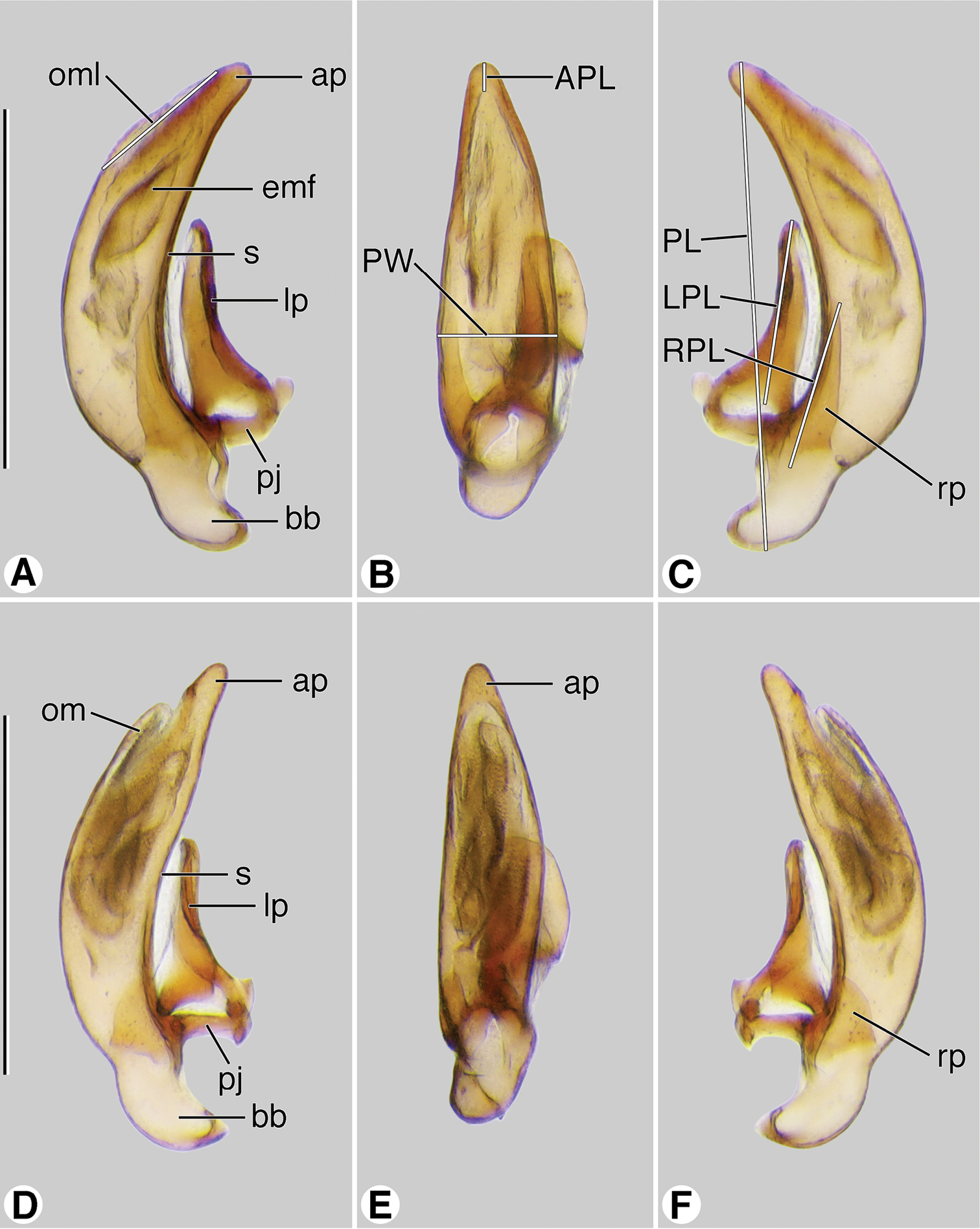
Male genitalia (Figs 10A–10F). Anopic. Phallus slightly curved ventrally, narrowed apically, apical margin rounded, apical portion very short; without paraostial sclerites. Left paramere (lp) conchoid or styliform. Right paramere (rp) short, broad, or styliform, relatively long.
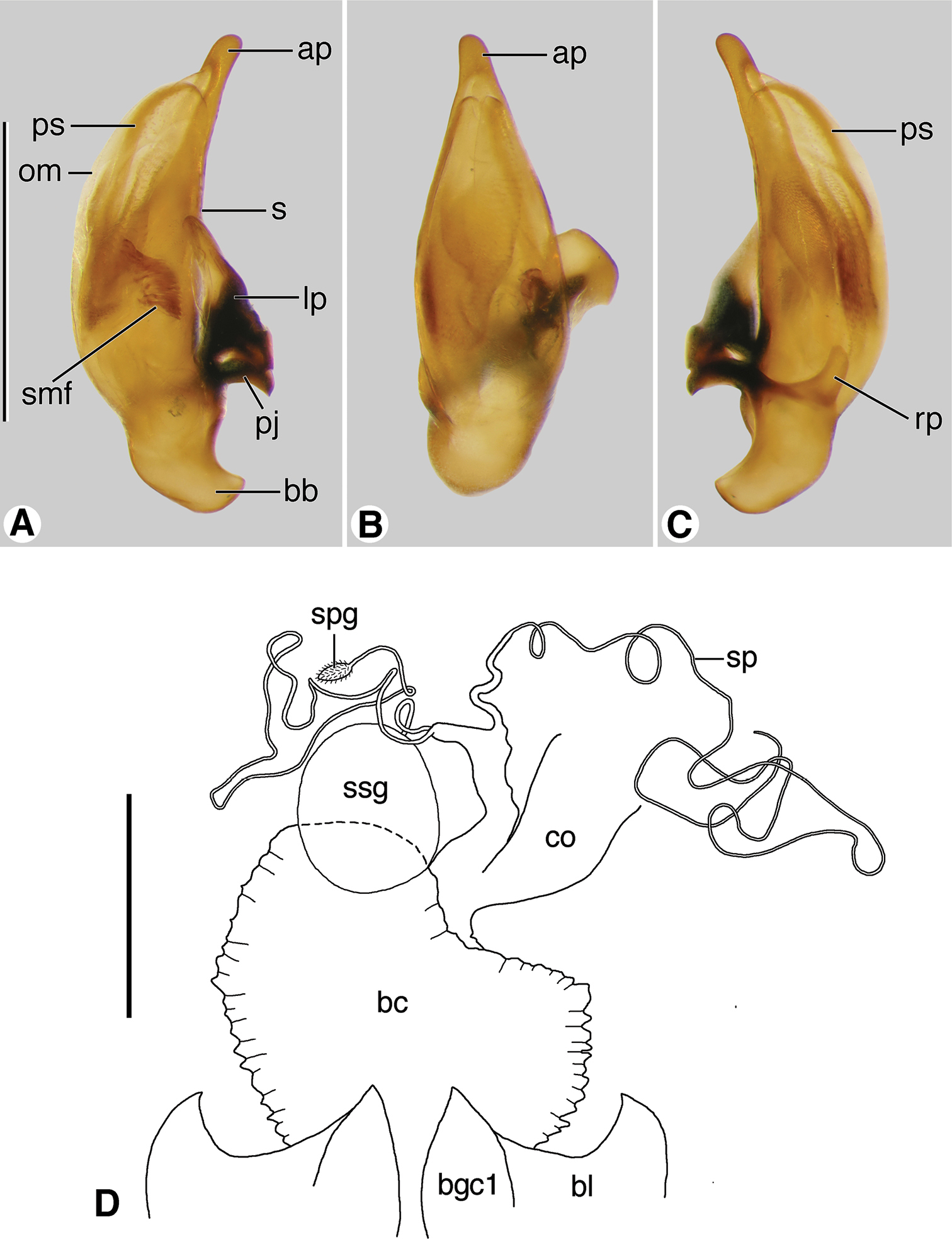
Digital images of male genitalia. A–C Coarazuphium whiteheadi, new species D–F Zuphioides mexicanum (Chaudoir). A, D left lateral aspect B, E dorsal aspect C, F right lateral aspect. Legend: ap, apical portion of phallus; APL length of apical portion ; bb, basal bulb of phallus; emf, endophallic terminal microtrichial field; lp, left paramere; LPL, left paramere length; om, ostial membrane; OML, periostial membrane length; pj, parameral juxta; PL, phallus length; PW, phallus width; rp, right paramere; RPL, right paramere length; s, shaft of phallus. Scale bars = 0.5 mm.
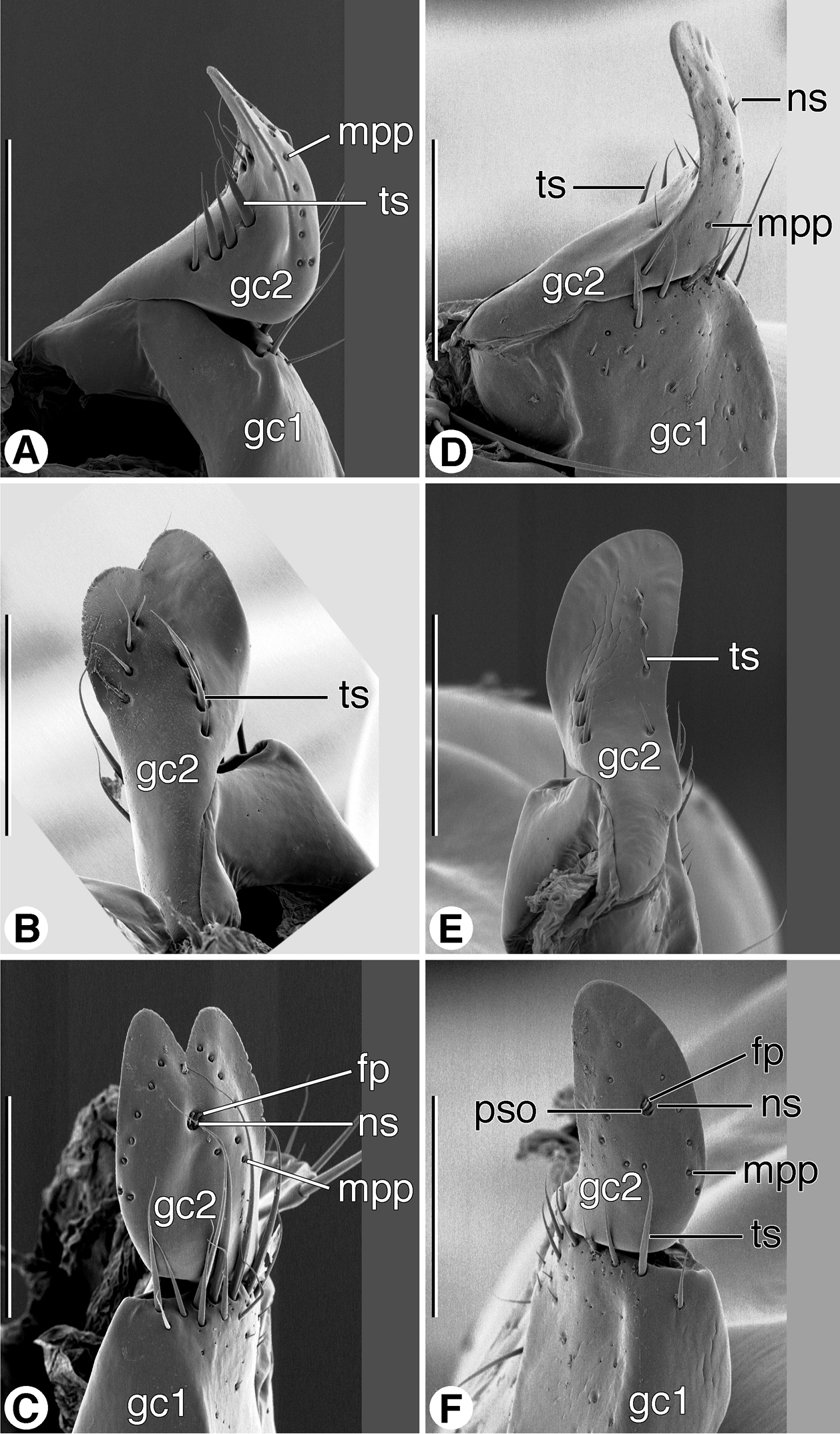
Female genitalia: ovipositor (Figs 11A–11F). Gonocoxite 1 (gc1) short, thick, with or without row of long trichoid setae distally on ventral and lateral surface. Gonocoxite 2 (gc2) short, thick; in lateral aspect falciform, apex pointed, variously provided with long trichoid setae dorso- and ventrolaterally; in dorsoventral aspect, broad, paddle-like, apex deeply notched or not; dorsal surface glabrous except for dorsolateral and ventrolateral trichoid setae (ts); ventral surface toward margins with row of pit pegs (mpp), preapical setose organ (pso) circuloid, with two furrow pegs (fp) and two very short nematiform setae (ns).
SEM micrographs of female ovipositor sclerites. A–C Coarazuphium whiteheadi, new species D–F Zuphioides mexicanum (Chaudoir). A, D left lateral aspect B, E dorsal aspect C, F ventral aspect. Legend: fp, furrow peg; gc1, gonocoxite 1; gc2, gonocoxite 2; mpp, marginal pit pegs; ns, nematiform seta; pso, preapical setose organ; ts, trichoid seta. Scale bars = 100 µm.
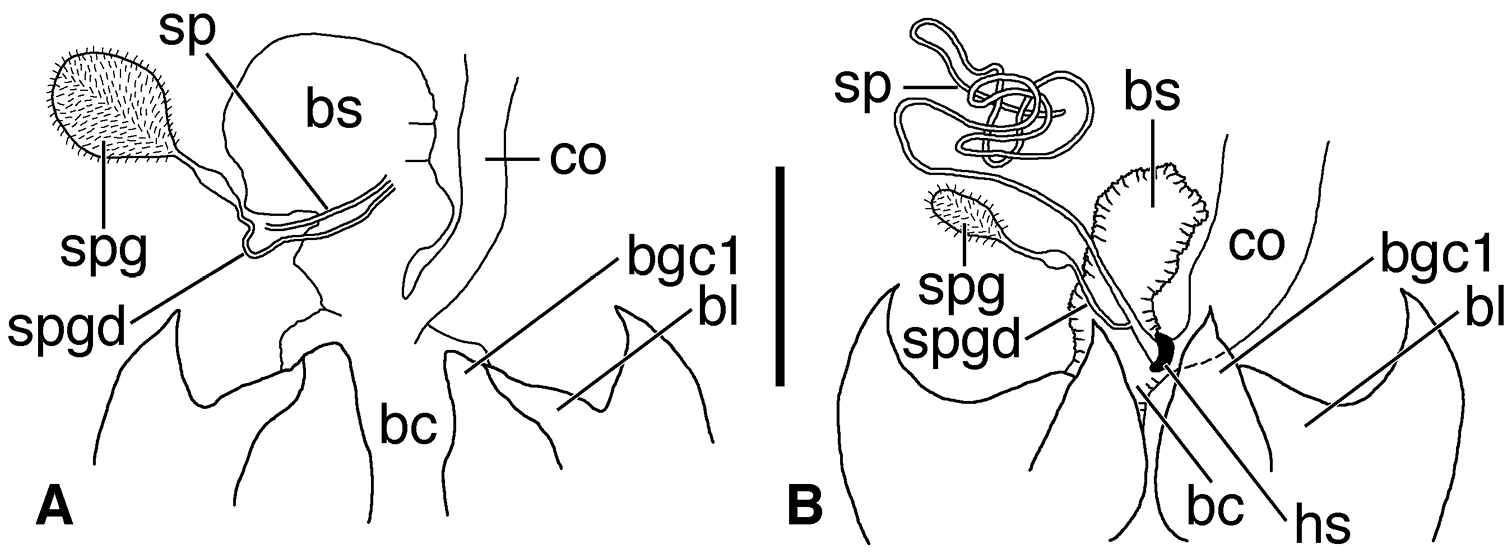
Female genital tract (Figs 12A, 12B). Bursa copulatrix (bc) ended in an expanded bulbous anterior extension (bs). Common oviduct (co) inserted in bursa copulatrix at base of its anterior extension. Spermatheca (sp) slender, long, inserted on or at base of bursal sac, beside insertion point of spermathecal gland duct (spgd); latter with swelling proximad spermathecal gland (spg). With or without helminthoid sclerite. Without secondary spermathecal gland.
Line drawings of female reproductive tract, ventral aspect. A Coarazuphium whiteheadi, new species B Zuphioides mexicanum (Chaudoir). Legend: bc, bursa copulatrix; bl, base of laterotergite; bs, bursal sacculus; bgc1, base of gonocoxite 1; co, common oviduct; hs, helminthoid sclerite; sp, spermatheca; spg, spermathecal gland; spgd, spermathecal gland duct. Scale bar = 0.25 mm.
Digital images of male genitalia and line drawing of female reproductive tract of Zuphium ustum Klug. A–C male genitalia, left lateral, dorsal and right lateral aspects, respectively D female reproductive tract, ventral aspect. Legend: ap, apical portion of phallus; bb, basal bulb of phallus; bc, bursa copulatrix; bl, base of laterotergite; bgc1, base of gonocoxite 1; co, common oviduct; lp, left paramere; rp, right paramere; om, ostial membrane; pj, parameral juxta; ps, paraostial sclerites; rp, right paramere; s, shaft of phallus; smf, spiny microtrichial field; sp, spermatheca; spg, spermathecal gland; ssg, secondary spermathecal gland. Scale bars: A – C = 1 mm; D = 0.5 mm.
Outline map of southern North America, Middle America and northern South America, showing generalized geographical range of species of Coarazuphium. A Coarazuphium whiteheadi, new species B Coarazuphium bezerra, cessaima, formoso, pains, tapiaguassu and tessai.
Geographical distribution. The range of this genus-group in the Western Hemisphere extends from Neotropical Argentina (
Included taxa. The Zuphium genus-group in the Western Hemisphere includes 30 species arranged in two genera: Coarazuphium Gnaspini et al. and Zuphioides new genus (Table 2).
List of names of species of Coarazuphium Gnaspini, Vanin & Godoy and Zuphioides, new genus.
| Coarazuphium bezerra Gnaspini, Vanin & Godoy 1998 |
| Coarazuphium cessaima Gnaspini, Vanin & Godoy 1998 |
| Coarazuphium formoso Pellegrini & Ferreira 2011 |
| Coarazuphium pains Alvarez & Ferreiera 2002 |
| Coarazuphium tapiaguassu Pellegrini & Ferreira 2011 |
| Coarazuphium tessai (Godoy & Vanin) 1990 |
| Coarazuphium whiteheadi Ball & Shpeley, sp. n. |
| Zuphioides aequinoctiale (Chaudoir, 1862) |
| Zuphioides americanum (Dejean, 1831) |
| Zuphioides argentinicum (Liebke, 1933) |
| Zuphioides batesi (Chaudoir, 1862) |
| Zuphioides bierigi (Liebke, 1933) |
| Zuphioides brasiliense (Chaudoir, 1872) |
| Zuphioides bruchi (Liebke, 1933) |
| Zuphioides capitum (Liebke, 1933) |
| Zuphioides columbianum (Chaudoir, 1872) |
| Zuphioides cubanum (Liebke, 1933) |
| Zuphioides delectum (Liebke, 1933) |
| Zuphioides exiguum (Putzeys, 1878) |
| Zuphioides exquisitum (Liebke, 1933) |
| Zuphioides flohri (Liebke, 1933) |
| Zuphioides haitianum (Darlington, 1935) |
| Zuphioides lizeri (Liebke, 1933) |
| Zuphioides longicolle (LeConte, 1879) |
| Zuphioides magnum (Schaeffer, 1910) |
| Zuphioides mexicanum (Chaudoir, 1863) |
| Zuphium vivinum (Liebke, 1933) |
| Zuphioides pseudamericanum (Mateu, 1981) |
| Zuphioides punctipenne (Bates, 1891) |
| Zuphioides pusillum (Chaudoir, 1862) |
| Zuphioides salivanum (Liebke, 1933) |
http://species-id.net/wiki/Coarazuphium
Parazuphium tessai Godoy & Vanin, 1990 (designated by
As explained by
With character states of Western Hemisphere Zuphium genus-group, restrictedas follows: size small; pronotum and dorsal surface of elytra moderately densely setose, setae decumbent; body markedly depressed; integument pale; head narrow, oviform, with posteriolateral margins broadly rounded; antennae elongate, antennomere 1 as long or longer than antennomeres 2-4; humeri narrowed, slightly or markedly so; metasternum short, metepisternum quadrate. Brachypterous or apterous. Male genitalia (Figs 10A–10C): phallus without dorsal paraostial sclerites. Female genital tract (Fig. 12A, 12B): without secondary spermathecal gland.
None required here. See
Confined to the Neotropical Region, the seven species of this genus are known only from southeastern Brazil (
The previously described species of genus Coarazuphium inhabit caves in one of two types of substrate (
Some specimens of Coarazuphium tapiaguassu were infested by laboulbenelian fungi. The fungi were not identified further. See this topic for Coarazuphium whiteheadi, below.
The ultrastructural features (i.e., principally sensillar) observed with scanning electron microscopy by
In contrast, the standard structural troglobitic features of Coarazuphium (lengthening of antennae and legs, depigmentation, micro- or anophthalmy, and reduction of elytral length, elytral humeri, metathorax and metathoracic wings) plus details of male and female genitalia are evolutionarily informative. Based on eye loss and more elongate appendages,
The external features of Coarazuphium evidently evolved in parallel with, and independent of, four other zuphiine troglobite taxa: the remarkable Spanish Ildobates neboti Español, 1966; the Canary Islands Parazuphium feloi Machado (1997: 163); the Australian Nullarbor Speozuphium poulteri Moore (1995: 159) and Speothalpius grayi Moore (1995: 160). Another Canary Islands Parazuphium (Parazuphium damascenum canariense Machado, 1992: 580) exhibits these same reductive (though less developed) features, but it is evidently conspecific with the continental Palaearctic Parazuphium damascenum damascenum (Fairmaire, 1897). For a more detailed discussion of the matter see
Discovery of the Mexican species adds important details to the story of evolution of Coarazuphium. First, the marked geographical range extension (from southeastern South America to the southern part of the North American continent) shows that this genus was not confined to eastern Brazil. Further, the distribution pattern lends support to the hypothesis of Gnaspini et. al. (see above) that Coarazuphium originated in early Tertiary time (i.e., before the beginning of the drying trend that extended through much of the Cenozoic era). Second, the extra-speleal humid forest existence of Coarazuphium whiteheadi suggests that the basic troglobitic features of Coarazuphium (microphthalmy, brachyptery, depigmentation, etc.) evolved in surface habitats, though probably in forested deep leaf litter locations, and were in effect preadaptations for cave life.
An additional observation relating to evolutionary history of the Zuphium genus groupis that shared features of Zuphioides and Coarazuphium indicate that these two genera may be adelphotaxa, with Zuphioides retaining mostly ancestral features including life in lowland hygrophilous or mesophilous situations.
Key to species of genus Coarazuphium Gnaspini, Vanin & Godoy
| 1 | Anophthalmous ( |
Coarazuphium cessaima Gnaspini, Vanin & Godoy |
| 1’ | Microphthalmous. Maximum width of elytra near middle, or posteriad middle. Male genitalia: right paramere styliform or not, distinctly shorter than left paramere | 2 |
| 2(1’) | Elytron with apical margin truncate, not sinuate. Male right paramere styliform or broad | 3 |
| 2’ | Elytron with apical margin sinuate. Male right paramere broad, not styliform, distinctly shorter than left paramere | 5 |
| 3(2) | Head dorsally without setae posteriad the anterior supraorbital setae (Pellegrini and Ferreira 2011: 49, fig. 2A) | Coarazuphium tapiaguassu Pellegrini & Ferreira |
| 3’ | Head dorsally with one to three pairs of setae posteriad the anterior supraorbital setae | 4 |
| 4(3) | Labrum with anterior margin broadly concave. Prosternal setae two pair ( |
Coarazuphium pains Álvares & Ferreira |
| 4’ | Labrum (Fig. 5A) with anterior margin irregularly convex. Prosternal setae one pair (Fig. 9B). Male right paramere (Fig. 10C, rp) styliform, more than half length of left paramere. Mexico | Coarazuphium whiteheadi, sp. n. |
| 5(2’) | Head dorsally with three pairs of setae posteriad the anterior supraorbital setae ( |
Coarazuphium formoso Pellegrini & Ferreira |
| 5’ | Head dorsally with one or two pairs of setae posteriad the anterior supraorbital setae | 6 |
| 6(5’) | Head dorsally with two pairs of setae (posterior supraorbitals and occipitals, ) at posterior border of head capsule ( |
Coarazuphium bezerra Gnaspini, Vanin & Godoy |
| 6’ | Single pair of setae (posterior supraorbitals) at posterior border of head capsule ( |
Coarazuphium tessai (Godoy & Vanin) |
Only the following new species is treated here.
urn:lsid:zoobank.org:act:1CC227B6-FA52-40DC-8FC4-C25A060CE300
http://species-id.net/wiki/Coarazuphium_whiteheadi
Figs 1A, 2A, 3A, 4A, 4C, 5A, 5B, 6A–6F, 8A–8C, 9B, 10A–10C, 11A–11C, 12A, Map 1Three specimens, as follows. HOLOTYPE female, labeled: “MEXICO, Oaxaca/ 7100’ 21.8 mi./ n. Juchatengo/ VII.18-19.1966”; “George E. Ball/ D. R. Whitehead/ collectors” (USNM). PARATYPES two: female, labeled same as holotype (UASM); male, labeled: “MEXICO, Oaxaca/ Mt. Alban, near ruins/ Acacia scrub 6000 ft/ VI-3/4-82/ Rolf L. Aalbu, col.” (USNM).
Ridge top, Sierra de Miahuatlan, in western Oaxaca, Mexico, at 2164 m, 35 km north of San Pedro Juchatengo, 16.462N, 97.010W.
A Latinized eponym, masculine gender, genitive case, based on the surname of Donald R. Whitehead, now deceased, one of the collectors of the type series of this species.
See key, above.
Size and proportions. Small, OBL 4.13–4.40 mm; EW 1.36–1.48 mm A1L/A2-4L 1.03–1.11; HW/PW 1.08.
Color. Body testaceous, in life almost white; appendages slightly paler; vestiture golden.
Habitus (Fig. 1A). Flat, overall. Head capsule (dorsal aspect, Fig. 2A) oviform, broadly rounded posteriolaterally. Eyes (Fig. 3A) flat, hardly perceptible, ommatidia not evident at 50×. Pronotum narrow. Elytra narrowed anteriorly and posteriorly, lateral margins distinctly bowed.
Microsculpture. Dorsal surface of head capsule (including clypeus) and pronotum smooth, microlines not evident; labrum and elytra with microlines fine (not easily seen at 50× or lower magnification), mesh pattern isodiametric. Ventral surface with microlines fine, generally transverse, sculpticells rather short.
Luster. Dorsal surface of head and pronotum shiny, elytra somewhat duller.
Body vestiture and punctation. Dorsal surface of head and pronotum sparsely punctate, vestiture sparse. Elytra with punctation and vestiture dense, vestitural setae decumbent. Abdomen. Abdominal sterna IV-VI with punctation rather sparse, vestitural setae decumbent.
Fixed setae. Head (Fig. 4A, 4C): clypeus one pair; head capsule with anterior pair of supraorbitals (asos) above eyes; posterior pair of supraorbitals (psos) posteriad eyes; one pair of postocular setae (pos) immediately behind eyes, laterally; one pair of occipital setae (ocs) posteriorly and mediad eyes; also one pair posterior supernumerary setae (psus) laterad and close to posterior supraorbital setae. Antennomere 1 (Fig. 4B, as1) with single long seta distally, and row of several semi-erect setae more proximally (Fig. 4B, as2, as3). Pronotum with two pairs of lateral marginal setae, anterior pair in anterior 1/8, posterior pair at posteriolateral angles. Prosternum with one pair paramedial setae anterioventrally (cf. Fig. 9B). Elytra: each elytron anteriorly with parascutellar seta; lateral setae about 21 (one group anteriorly, one group posteriorly, and single seta medially). Abdominal sterna IV–VI each with one pair of ambulatory setae, sternum VII with one pair of long setae near posterior margin.
Head, dorsal aspect (Fig. 1A). Postoccipital suture evident. Frontoclypeal suture present, with frons and clypeus separate. Genal sulcus (Fig. 4A, gs) broad, ventral margin sinuous.
Eyes. (Fig. 3A) Small, flat, ommatidia not evident at 50×, or lower magnification.
Antennae. (Fig. 4A). Filiform, extended about body length. Antennomere 1 (a1) rather slender, slightly longer than antennomeres a2-a4; antennomere 2 short, about half length of a3; antennomeres 3–11 narrow, cylindrical, distinctly longer than wide.
Mouthparts. As described for Zuphium genus-group, above. Labrum (Fig. 5A).
Rectangular, lateral margins rounded; anterior margin irregularly convex; six dorsal setae, lateral setae longer than four medials. Epipharynx (Fig. 5B).
Mandibles (Figs 6A–6F) Maxillae (Figs 8A–8B). Labium, ventral aspect (Fig. 8C).
Pronotum (Fig. 1A). Anterior margin truncate, lateral margins markedly sinuate posteriorly, posteriolateral angles prominent, dentiform, slightly anteriad posterior margin; surface impressions (anterior and posterior transverse and median longitudinal) shallow; lateral grooves and posteriolateral impressions moderately deep.
Pterothorax. Metasternum short; metepisternum quadrate.
Elytra (Fig. 1A). Separate, not fused along suture. Each elytron more or less rectangular, but narrowed anteriorly and posteriorly, lateral margin thus distinctly bowed; humerus projected anteriorly, basal ridge sinuate; apical margin truncate, with narrow band of membrane; surface with striae very shallow, intervals almost flat.
Hind wings. Short stubs, brachypterous.
Abdomen. Sternum VII with apical margin truncate.
Male genitalia (Figs 10A–10C). Anopic. Phallus narrow (PW/PL 0.238), slightly curved ventrally, narrowed apically, apical margin rounded, apical portion very short (AL/PL 0.063), ostial membrane extensive (OM/PL 0.317). Left paramere (lp) conchoid. Right paramere (rp) styliform, relatively long (RP/LP 0.317).
Female genitalia: ovipositor (Figs 11A–11C). Gonocoxite 1 (gc1) with patch of long trichoid setae distally on ventral surface. Gonocoxite 2 (gc2) short, thick; in lateral aspect falciform, apex pointed, row of four long trichoid setae dorso- and ventro-laterally; in dorso-ventral aspect, broad, paddle-like, apex deeply notched; dorsal surface glabrous except for dorso-lateral and ventro-lateral trichoid setae; ventral surface toward margins with row of pit pegs (mpp), preapical setose organ (pso) circuloid, with two furrow pegs (fp) and two very short nematiform setae (ns).
Female genital tract (Fig. 12A). Bursa copulatrix (bc) ended in an expanded bulbous anterior extension (bs). Common oviduct (co) inserted in bursa copulatrix at base of the anterior extension. Spermatheca (sp, broken) slender, inserted on bursal sac beside insertion point of spermathecal gland duct (spgd); latter with swelling proximad spermathecal gland (spg). Without helminthoid sclerite. Without secondary spermathecal gland.
The specimens from the type locality were collected in the remains of a cloud forest. One was on the ground, in leaf litter; using a Bowie knife, the other was dug out of wet wood of a pine log, near the ground surface. The Monte Alban specimen was likely taken from a dark crevice, “where the temperature was much cooler than outside“ (Rolf Aalbu, personal communication). Judging from the structural features of the type material, this species may be troglophilic, but not troglobitic, as are its Brazilian congeners (
Attached to the holotype was a fungus of the species Rhachomyces zuphii Thaxter (Laboulbeniales: Laboulbeniaceae) (determiner of fungus not indicated on label), which is now attached to a pinned rectangular piece of clear plastic by a drop of balsam.
(Map 1). This species is known only from two montane localities in the Mexican state of Oaxaca.
Type specimens, only. For label details, see above.
urn:lsid:zoobank.org:act:C477A5DF-BA71-4B4D-B788-DC789857BC6D
http://species-id.net/wiki/Zuphioides
Zuphium mexicanum Chaudoir, 1863 (here designated).
A compound Latinized noun, treated as neuter, from the generic name Zuphium and oides, resembling; hence meaning “resembling Zuphium”.
With character states of Western Hemisphere Zuphium genus-group, restricted as follows: size small, pronotum and dorsal surface of elytra densely setose, setae decumbent, body depressed, integument piceous to rufotestaceous, head capsule posteriorly relatively broad, laterally broadly rounded, antennae elongate, antennomere 1 as long or longer than antennomeres 2–4. Humeri broadly rounded. Metasternum long, metepisternum longer than wide at base. Macropterous. Male genitalia: phallus without dorsal paraostial sclerites (Fig. 10D–10F; cf. Fig. 13A–C, ps). Ovipositor: Gonocoxite 2 (Figs 11D–F; cf. Figs 11A–C, gc2) short, thick; in lateral aspect falciform, apex pointed; in dorso-ventral aspect, broad, paddle-like, apex broadly rounded, not notched. Female genital tract: without secondary spermathecal gland (Fig. 12B; cf. Fig. 13D, ssg).
None required here. See description of Zuphioides mexicanum, below.
Members of this genus are mesophilous to hygrophilous, occupying wet meadows and flood plains principally in open sites, but also in shaded areas along streams, in tropical gallery forest. For more details, see
http://species-id.net/wiki/Zuphioides_mexicanum
Figs 1B, 2B, 3B, 4C, 4D, 5C, 5D, 7A–7F, 8D–8F, 9A, 10D–10F, 11D–11F, 12BFor Zuphioides mexicanum, two females, Oberthür-Chaudoir Collection, in front of the following box label: “mexicanum/ Chaud/ Mexique/ 57. Sallé”. LECTOTYPE female, labeled as follows: “33”/ “Ex Musaeo/Chaudoir” [red print]; “Lecto/type” [circular, ringed with red]; in Museum National d’Histoire Naturelle, Paris; PARALECTOTYPE labeled “ Ex Musaeo/Chaudoir” [red print]. Designated by
Indicated by
A Latinized eponym, nominative case, based on the name of the country in which the type locality is located.
Size and proportions. Small, OBL 4.88–4.96 mm; EW 1.60–1.62 mm A1L/A2–4L 0.90–1.00; HW/PW 0.92–0.97. (For seven species of Zuphioides: OBL 4.64–6.586 mm; EW 1.60–1.84 mm A1L/A2–4L 0.88–1.12; HW/PW 0.91–0.97).
Color. Body rufotestaceous; appendages slightly paler; vestiture testaceous.
Habitus (Fig. 1B). Flat, overall. Head capsule (dorsal aspect, Fig. 2B) trapezoidal, obtusely angulate posteriolaterally.
Eyes (Figs 3B). Convex, easily seen, ommatidia clearly evident at 50× (about 16 ommatidia are crossed on a horizontal diameter).
Pronotum. Narrow.
Elytra (Fig. 1B). Lateral margins straight, not bowed, parallel to one another.
Microsculpture. Dorsal surface of head capsule (including clypeus) and pronotum smooth, microlines not evident; labrum and elytra with microlines fine (not easily seen at 50× or lower magnification), mesh pattern isodiametric. Ventral surface with microlines fine, generally transverse.
Luster. Dorsal surface of head and pronotum shiny, elytra somewhat duller.
Body vestiture and punctation. Dorsal surface of head and pronotum sparsely punctate, vestiture sparse. Elytra and abdominal sterna III-VII with punctation and vestiture dense, vestitural setae decumbent.
Fixed setae. Head (Fig. 4C): clypeus one pair; head capsule with anterior pair of supraorbitals (asos) above eyes; posterior pair of supraorbitals (psos) posteriad eyes; one pair of postocular setae (pos) immediately behind eyes, laterally; one pair of occipital setae (ocs) posteriorly and mediad eyes; posterior supernumerary setae lacking. Antennomere 1 (Figs 4B, 4D, as1) with single long seta distally, in addition to decumbent vestiture and row of small erect setae (as2, as3). Pronotum with two pairs of lateral marginal setae, anterior pair in anterior 1/8, posterior pair at posteriolateral angles. Prosternum with one pair setae anterioventrally (Fig. 9B). Elytra: each elytron anteriorly with parascutellar seta; lateral setae about 21 (one group anteriorly, one group posteriorly, and single seta medially. Abdominal sterna IV-VI without evident ambulatory setae, sternum VII with pair of long setae near posterior margin.
Head, dorsal aspect (Fig. 4C). Occiput posteriorly and postocciput markedly constricted, in form of narrow neck, postoccipital suture evident. Frontoclypeal suture present. Genal sulcus (gs) prominent, but less so than in Coarazuphium (cf. Fig. 4A).
Eyes (Fig. 4B). Macrophthalmous, markedly convex, readily seen, ommatidia evident at 50×, or lower magnification.
Antennae (Fig. 4D). Antennae filiform, extended about body length. Antennomere 1 rather slender, slightly longer than antennomeres a2-a4; antennomere 2 short, about half length of a3; antennomeres 3-11 narrow, cylindrical, distinctly longer than wide.
Mouthparts. As described for Zuphium genus-group, above. Labrum (Fig. 5C). Anterior margin concave. Epipharynx (Fig. 5D). Mandibles (Figs 7A–7F). Maxillae (Figs 8D, 8E). Labium, ventral aspect (Fig. 8F).
Pronotum (Fig. 1B). Anterior margin truncate, lateral margins markedly sinuate posteriorly, posteriolateral angles prominent, dentiform, slightly anteriad posterior margin; surface impressions (anterior and posterior transverse and median longitudinal) shallow; lateral grooves and posteriolateral impressions moderately deep.
Pterothorax. Metasternum of average length; metepisternum elongate, lateral margin longer than anterior margin.
Elytra (Fig. 1B). Separate from one another, not fused along suture. Each elytron more or less rectangular, lateral margin straight; humerus projected anteriorly, basal ridge straight; apical margin truncate, with narrow band of membrane. Surface with striae very shallow, intervals almost flat.
Hind wings. Macropterous; wings folded beneath elytra at rest.
Legs. Male fore-tarsus (Fig. 9A).
Abdomen. As described for Western Hemisphere Zuphium genus-group.
Male genitalia (Figs 10D–10F). Phallus narrow (PW/PL 0.254), slightly curved ventrally, narrowed apically, apical margin rounded, apical portion very short (AL/PL 0.036), ostial membrane extensive (OM/PL 0.317). Left paramere (lp) conchoid. Right paramere (rp) short, ovoid in form (RP/LP 0.317).
Female genitalia: ovipositor (Figs 11D–11F). Gonocoxite 1 (gc1) with patch of long trichoid setae distally on ventral surface. Gonocoxite 2 (gc2) short, thick; in lateral aspect falciform, apex pointed, row of four long trichoid setae dorso- and ventro-laterally; in dorso-ventral aspect, broad, paddle-like, apex broadly rounded, not notched; otherwise, as described for Western Hemisphere Zuphium genus-group.
Female genital tract (Fig. 12B). Bursa copulatrix (bc) bulbous (collapsed in Fig. 12B), inserted at base of common oviduct (co). Spermatheca (sp) inserted near junction of common oviduct and bursa copulatrix, markedly elongate and slender, with helminthoid sclerite (hs) at base. Spermathecal gland duct inserted near base of spermatheca, with distinct swelling proximad spermathecal gland (spg). Without secondary spermathecal gland.
This species ranges from northern Mexico (states of Nuevo Leon, Nayarit, and Tamaulipas) northward to southwestern U.S.A (states of Arizona, New Mexico, and Texas;
Treatment of the Western Hemisphere zuphiines remains incomplete. The large geographical gap between the Brazilian and Mexican species of Coarazuphium causes one to wonder just how large the gap is. Surely, considering that this genus is likely to be an old (early Tertiary) resident of the Middle American highlands, we can expect therein additional species.
At hemisphere level, Zuphioides must be reviewed in detail, using as the principal basis the work of
The authors are pleased to thank: Rolf L. Aalbu (Entomology, California Academy of Sciences, San Francisco California) for information about the locality where he collected a paratype of Coarazuphium whiteheadi; Terry L. Erwin (Entomology, Smithsonian Institution, Washington, D. C.) for loan of specimens, and general advice about organization and structure of the manuscript; and David R. Maddison (Zoology, Oregon State University, Corvallis, Oregon), for advice about interpretation of molecular data in relation to classification of the Zuphiitae. Careful reviews of a previous draft of the submitted manuscript were made by our colleagues Douglas A. Craig and Bruce S. Heming. We are grateful for their considerable efforts, which led to a substantially improved manuscript. We are also grateful for the advice received from the referees, who reviewed the penultimate draft of our manuscript.
Based on morphological features, most authors have treated the Zuphiini as a “natural” group, meaning in phylogenetic parlance that it is essentially monophyletic. But a molecular analysis, based on two nuclear genes (28S rDNA and wingless) conducted by
Specimens of this tribe are small in size (overall length less than 7.00 mm), integument various (nearly white, testaceous to piceous), and generally pilose, head more or less constricted posteriorly, antennomere 1 (scape) elongate more than length of antennomeres 2+3 and wider than antennomeres 2-11; elytra with apical margin truncate, or subtruncate-sinuate, with a narrow membranous fringe; elytral interval 3 without fixed setae; tibiae without prominent spines. For a more detailed characterization of Zuphiini, see
The range of this tribe in the Western Hemisphere is co-extensive with the range of the Zuphium genus-group, as noted above.
Based on previous studies,
The following key distinguishes among the Western Hemisphere genus-groups and genera of Zuphiini. Note that among other recent authors,
| 1 | Maxillary and labial palpi similar to one another in size and proportions; maxillary palpomere 4 (Figs 8A–8F) not markedly enlarged. Head posteriorly markedly constricted as a narrow neck (Figs 1A, 1B). Male fore-tarsomeres 1-3 with adhesive vestiture pad-like, not biseriate (Fig. 9A) or with a single row of squamo-setae | Zuphium genus-group, 2 |
| 1’ | Maxillary palpi much larger than labial palpi; maxillary palpomere 4 markedly enlarged. Head posteriorly less constricted. Male fore-tarsomeres 1-3 with adhesive vestiture biseriate, or a single row of squamo-setae | 3 |
| 2(1’) | Eyes absent or flat, ommatidia not evident. Metepisternum about quadrate. Elytra with humeri markedly constricted, lateral margins broadly rounded (Fig. 1A) | Coarazuphium Gnaspini, Vanin & Godoy |
| 2’ | Eyes convex, normal size, ommatidia evident. Metepisternum elongate, longer than wide at base. Elytra with humeri broadly rounded, lateral margins straight, parallel to one another (Fig. 1B) | Zuphioides, gen. n. |
| 3(2’) | Head posteriorly constricted as very narrow neck (more or less as wide as diameter of one eye (cf. Fig. 1A)). Gonocoxite 2 of ovipositor without ensiform setae, with slender setae on dorsal margin, near base ( |
Mischocephalus genus-group, 4 |
| 3’ | Head posteriorly with neck thick (wider than diameter of one eye). Gonocoxite 2 with broad ensiform setae ( |
Patrizia genus-group, 5 |
| 4(3) | Elytron with lateral margin straight for most of length. Pronotal posteriolateral angles long, very prominent spines. Labium with submentum and mentum fused ( |
Mischocephalus Chaudoir |
| 4’ | Elytron with lateral margin broadly rounded. Pronotal posteriolateral angles short spines. Labium with submentum and mentum separated by a suture | Chaudoirella Mateu |
| 5(3’) | Pronotal posteriolateral angles not spined | Pseudaptinus Laporte de Castelnau |
| 5’ | Pronotal posteriolateral angles each a small, sharp spine | 6 |
| 6(5’) | Antennal socket limited above and below by a sharp carina, inferior carina better developed than superior one (clearly visible from above). Antennomere 1 relatively shorter (slightly shorter than antennomeres 2-4). Pronotum as long as wide; anterior angles more or less sharp. Integument generally glabrous | Metaxidius Chaudoir |
| 6’ | Antennal socket with superior and inferior carinae equally developed. Antennomere 1 relatively longer (as long or longer than antennomeres 2-4). Pronotum longer than wide. Pronotum and elytra setose | Thalpius LeConte |