Citation: Hopkins WA, Moser WE, Garst DW, Richardson DJ, Hammond CI, Lazo-Wasem EA (2014) Morphological and molecular characterization of a new species of leech (Glossiphoniidae, Hirudinida): Implications for the health of its imperiled amphibian host (Cryptobranchus alleganiensis). ZooKeys 378: 83–101. doi: 10.3897/zookeys.378.6545
The hellbender (Cryptobranchus alleganiensis) is among the most intriguing and imperiled amphibians in North America. Since the 1970s and 80s, western populations of the Ozark and eastern subspecies in Missouri have declined by nearly 80%. As a result of population declines, the Ozark hellbender was recently federally protected as an endangered species, and the eastern subspecies was granted protection under CITES. Although habitat degradation is probably the biggest threat to hellbender populations, recent evidence suggests that pathogens including chytrid fungus and “flesh-eating” bacteria may also contribute to declines in Ozark hellbenders. Leeches, which are very common on Ozark hellbenders, have recently been implicated as possible vectors of disease among Ozark hellbenders but have not been described in eastern hellbenders or outside of Missouri and Arkansas. We discovered a population of leeches on eastern hellbenders in southwest Virginia and confirmed that the species of leech is within the genus Placobdella, but is morphologically and genetically distinct from all previously described leech species. We named the new species Placobdella appalachiensis sp. n. Moser and Hopkins, based on the mountainous region in which it was discovered. Our surveys over a three consecutive year period suggested that this leech species may be patchily distributed and/or have a narrow geographic range. We consistently detected leeches at one site (mean prevalence in 80 hellbenders = 27.5%; median intensity = 3.0 leeches per parasitized hellbender [range 1 – >250 leeches]) during three years of surveys, but we never found leeches in any of our other seven study sites in two streams (mean prevalence in 139 hellbenders = 0%). We found a significant positive relationship between hellbender body size and the intensity of parasitism, and we suggest the possibility that the behavioral ecology of adults leading up to reproduction may increase their encounter rates with parasites. We discuss the potential conservation implications of discovery of leeches in this stream, and make recommendations for future mitigation and monitoring efforts.
Hellbender, leech, disease, parasite, Hirudinida, Glossiphoniidae
The hellbender (Cryptobranchus alleganiensis) is among the most intriguing and threatened amphibians in North America. This giant salamander reaches total lengths of at least 74 cm (
It was recently postulated that leeches may transmit pathogens among amphibians, including Ozark hellbenders (
In this study we provide the first thorough documentation of leeches using eastern hellbenders as hosts, in a population far beyond the previously known range of leech infestations in Ozark hellbenders. Using a combination of morphological and molecular techniques, we identify this leech as a new distinct species. Based on three years of field surveys, we documented the prevalence and intensity of leech parasitism in two streams in the Upper TN River basin of Virginia. Given the importance of leeches as vectors of disease-causing organisms, we discuss the potential implications for the health and conservation of hellbenders.
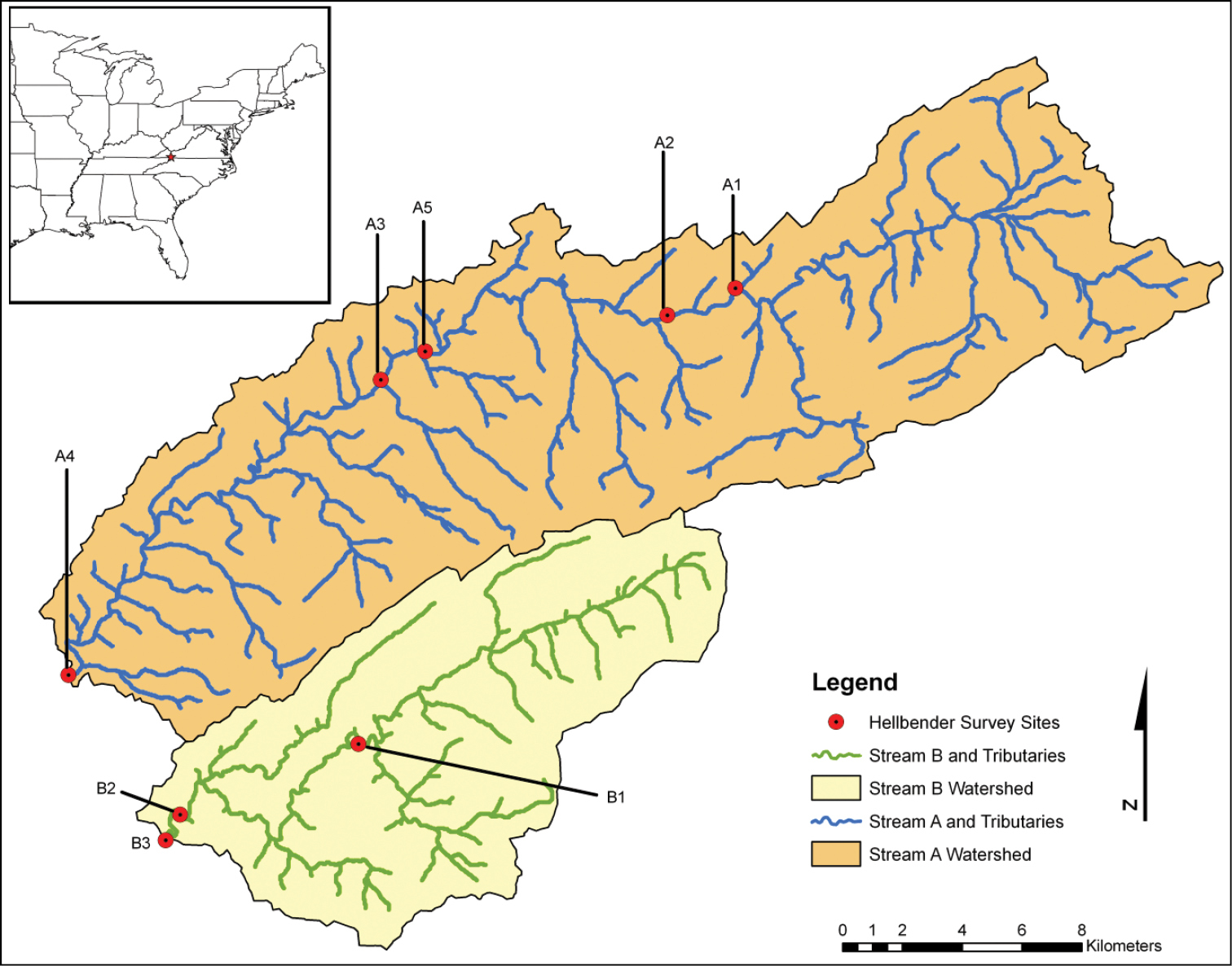
The two streams we surveyed in this study are located in the Tennessee River Basin and are part of survey efforts to understand the health and abundance of hellbenders in Virginia, USA (Figure 1). Because of the sensitive status of this species, we refer to the streams as Stream A and B. Both streams drain predominantly (> 70%) forested watersheds and still harbor relatively large populations of hellbenders. However, Stream A is increasingly subjected to a wide range of surrounding land use, including agriculture and development that threatens in-stream water and microhabitat quality.
Map of two streams in southwest Virginia, USA where hellbenders were collected from 8 stream reaches over 3 consecutive years. Leeches were only detected in stream reach A3.
Surveys for hellbenders were conducted for three consecutive summers in 2007–2009. In the first two years of our surveys, we collected hellbenders from 7 stream reaches (Figure 1), each 100 m long. Habitat characteristics and water quality for these reaches are reported elsewhere (
We collected hellbenders during diurnal surveys by turning rocks while skin-diving, which is the best method for obtaining all age classes of hellbenders (
Once we captured each hellbender, we transported it to the stream bank for processing. We measured total and snout-vent length (TL and SVL), weighed, and sexed (based on cloacal morphology of adults) each individual and subjected them to a physical exam that included enumeration and removal of leech specimens (see below). We then injected a passive integrative transponder tag (PIT tag) into the tail of each hellbender for future identification and released each individual under the rock where it was initially collected.
We counted and noted the location of leeches on hellbenders before removing them from each hellbender. Leeches were gently secured flat between a cover slip and a glass slide before being relaxed using dropwise additions of 10% ethanol. Once relaxed, a subset of leeches was preserved in 95% ethanol and another subset was fixed in 10% buffered formalin before being preserved in 70% ethanol. The one exception to this protocol was a hellbender collected in 2007 that hosted so many leeches (> 250) that we were unable to count all of them due to constraints of our surveys (see results). In 2008 an additional subset of leeches were held alive in cold stream water to allow them to digest their blood meal before being shipped live or prepared as described above. Four specimens were pressed, stained with Semichon’s acetocarmine, and mounted in Canada balsam for examination by light microscopy according to techniques outlined by
We conducted molecular analyses on newly collected material according to
We prepared PCR Reactions using the Illustra PuRe Taq Ready-To-Go PCR beads from GE Health Care (Cat. No. 27-9559-01). Primers were purchased from Invitrogen and were comprised of 2 primers each for cytochrome c oxidase subunit I (CO-I) as specified by
We ran all statistical analyses in SAS 9.1 (SAS Institute Inc., Cary, NC, USA) or Microsoft Excel and recognized statistical significance at α < 0.05. Where appropriate, we tested for normality and homoscedasticity using Ryan-Joiners and Bartlett’s tests, respectively. Unless otherwise noted, we used raw data in statistical analyses.
Because leeches were only detected in one stream reach (see results), we did not statistically compare the incidence of leech parasitism across streams or reaches. Within the site where leeches were common, we compared prevalence (% of individuals harboring at least one leech) using a Fisher’s exact test. The intensity of leech infestation (number of leeches per parasitized individual) was compared among the three years using a Kruskal Wallis test because data were not normally distributed and transformation did not improve the distribution. Among individuals that were parasitized, we used linear regression to determine whether there was a relationship between body size (total length) and the number of leeches attached to individuals. We used a Chi-Square test and a Kruskal Wallis test to evaluate whether sex of adults differed in their probability of being parasitized or the number of leeches they harbored, respectively. We excluded the one individual with > 250 leeches from comparisons of size and sex because we did not have a precise count of leeches on this individual and it was a clear outlier compared to the rest of the population.
In total, we captured 219 hellbenders at our eight sites over the three year study. All age classes were detected in the two streams, from gilled larvae to large adults. Body mass of hellbenders ranged from 2.0 to 1, 040 g, total length ranged from 6.2–58.2 cm, and snout-vent length ranged from 4.1 to 37.1 cm.
We only detected leeches in stream reach A3, where they tended to be quite common (Table 1). No leeches were detected at any other sites, including nearby reach A5 which was added in 2009 to determine the upstream extent to which leeches could be detected. The species of leech was determined to be within the genus Placobdella, but was morphologically and genetically distinct from all species described to date (see Species Description below (Figures 2–4).
Prevalence and intensity of parasitism of eastern hellbenders by the leech Placobdella appalachiensis sp. n. in Stream Reach A3 in southwest Virginia, USA. No leeches were found in the other 7 stream reaches over 3 years of study. Prevalence represents the percentage of hellbenders parasitized by at least one leech. Intensity of parasitism is calculated as the number of leeches on parasitized individuals.
| Parameter | 2007 | 2008 | 2009 | All YRS COMBINED |
|---|---|---|---|---|
| Prevalence | ||||
| Sample size | 17 | 16 | 47 | 80 |
| # Parasitized | 6 | 6 | 10 | 22 |
| Prevalence (%) | 35.3 | 37.5 | 21.3 | 27.5 |
| Intensity | ||||
| Sample Size | 6 | 6 | 10 | 22 |
| Mean Intensity | 44.8 | 13 | 6.6 | 18.8 |
| Median Intensity | 2.5 | 3.5 | 3.0 | 3.0 |
| Range | 1 – >250 | 1 – 40 | 1 – 24 | 1 – >250 |
Dorsal surface of Placobdella appalachiensis sp. n., Holotype USNM 1232924 collected from an adult eastern hellbender (Cryptobranchus alleganiensis) from stream reach A3 in southwest Virginia, USA. Scale bar equals 1 mm.
Ventral surface of Placobdella appalachiensis sp. n., Holotype USNM 1232924 collected from an adult eastern hellbender (Cryptobranchus alleganiensis) from stream reach A3 in southwest Virginia, USA. Scale bar equals 1 mm.
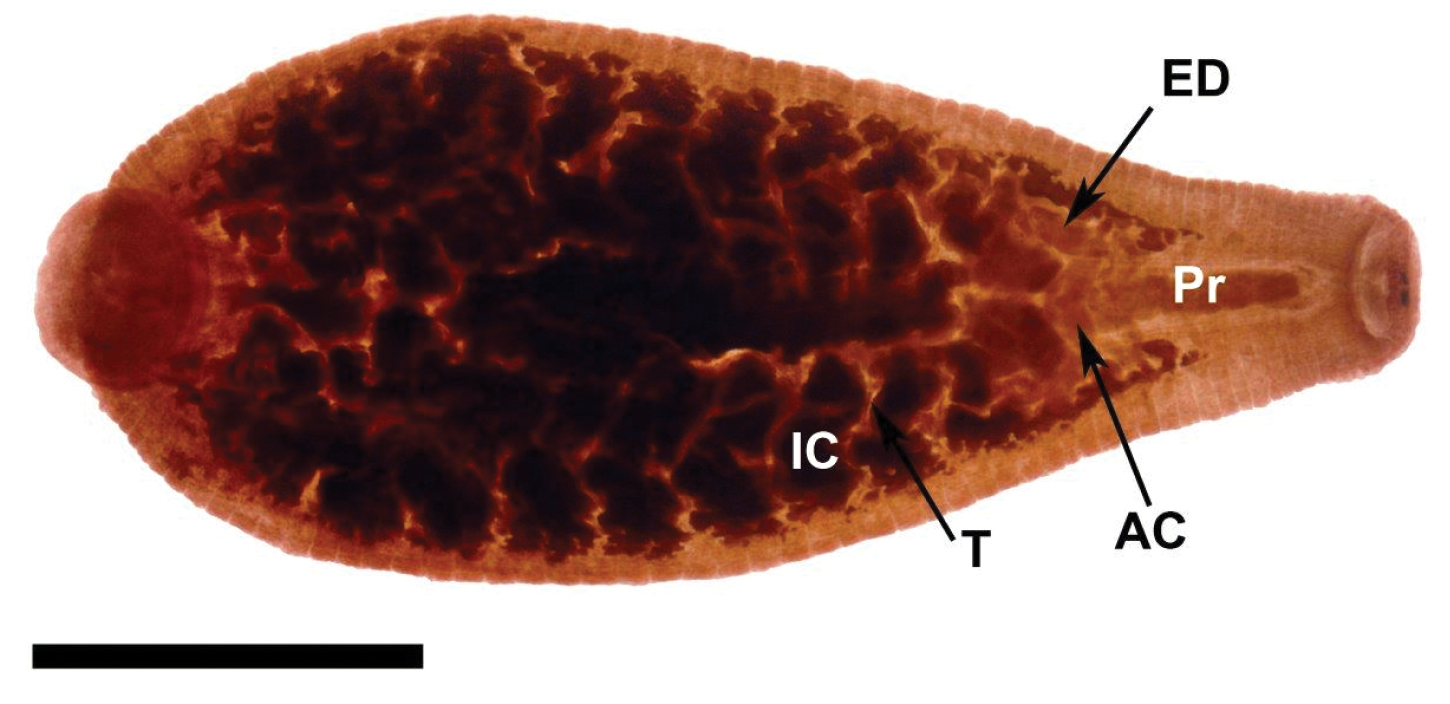
Internal anatomy of Placobdella appalachiensis sp. n., Paratype USNM 1232939 collected from an adult eastern hellbender (Cryptobranchus alleganiensis) from stream reach A3 in southwest Virginia, USA. Ventral view, atrial cornuae (AC), ejaculatory duct (ED), intestinal ceca (IC), proboscis (Pr), testisac (T). Scale bar equals 2 mm.
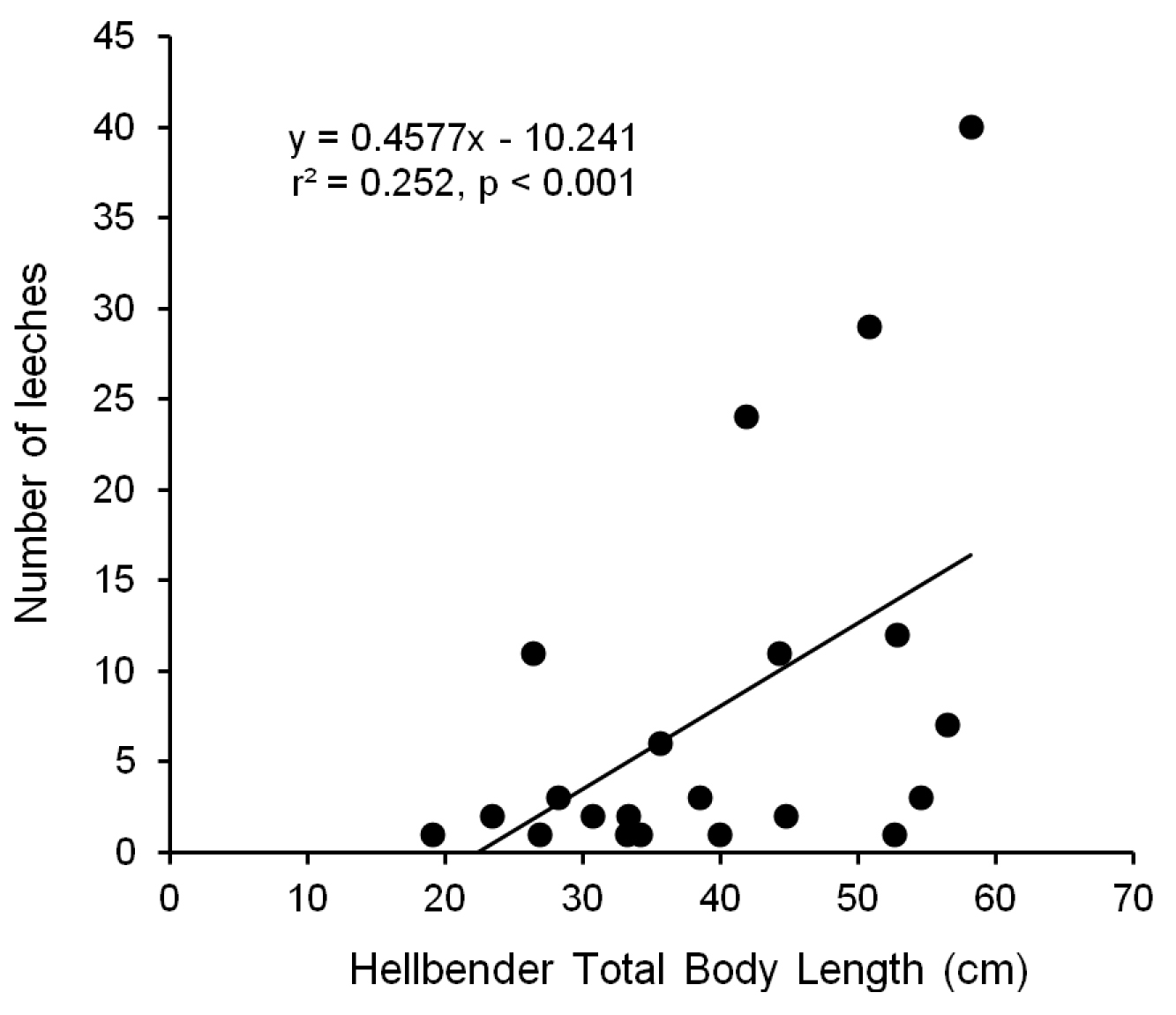
Within the site (A3) where leeches were prevalent, we found leeches on diverse size classes of hellbenders ranging from juveniles (19.1 cm TL) to large adults (58.2 cm TL). Leeches were primarily found on dorsal surfaces of the head, torso, and tail, but also on limbs and the gular region of the throat. Prevalence of leech parasitism was fairly consistent among years, ranging from 21.3–37.5% (p = 0.332). Likewise, median intensity of parasitism ranged from 2.5–3.5 leeches per parasitized hellbender among the three years (p = 0.942). There was a significant positive relationship between body size of hellbenders and the number of leeches they harbored, when all individuals (r2 = 0.12, p < 0.001) and when only parasitized individuals (r2 = 0.25, p < 0.001; Figure 5) were included in the model. Adult males and females (N = 22 and 26, respectively) were equally likely to harbor leeches (mean prevalence = 36.4% and 34.6%, respectively; p = 0.90). Of the individuals with leeches, adult males tended to harbor more leeches than females but there was no significant difference between the sexes (mean intensity = 38.1 and 10.0, respectively; median intensity = 6.5 and 2.0, respectively; p = 0.331).
Relationship between total body length (cm) of eastern hellbenders (Cryptobranchus alleganiensis) and the number of leeches (Placobdella appalachiensis sp. n.) they harbored. All hellbenders were collected from stream reach A3 in southwest Virginia, USA.
Several hellbenders harbored very small (< 1–2 mm) leeches in all three years, providing evidence that leeches are successfully reproducing at the site and that hatchlings were likely using hellbenders for their first blood meal. In fact, one adult that we collected in 2007 harbored > 250 leeches, of which at least 200 were small hatchlings and juveniles. Of the 80 hellbenders collected at reach A3, 13 were recaptures in the second and/or third year of the study. There were no obvious patterns of parasite attachment across years for individuals that were recaptured. Five recaptured individuals did not harbor leeches in any of their capture years. Four individuals were not parasitized during their first year, but were parasitized when recaptured. In contrast, four individuals harbored fewer leeches in later years than in earlier captures, but these four individuals should be interpreted carefully since we removed voucher specimens from all parasitized hellbenders.
http://zoobank.org/33FF4843-BB57-4682-9D8F-8B2745BF57F0
http://species-id.net/wiki/Placobdella_appalachiensis
Figs 2–4Holotype (USNM 1232924) South Fork Holston River, Smyth County, Virginia.
Paratypes (USNM 1232925 – 1232942; YPM IZ 067799) South Fork Holston River, Smyth County, Virginia.
External morphology. Body very deeply to deeply ovoid and triannulate. Length of preserved specimens 2.5 – 10.4 mm long, mean + SE 5.2 + 0.4 mm (n = 23), width at widest point (in posterior half of body) 2.0 – 5.9 mm, mean + SE 3.4 + 0.2 mm (n = 23). Dorsum chocolate (#7B3F00) to russet (#80461B) brown with 6 rows of papillae (2 para-medial, 2 para-lateral, & 2 lateral) and many, thin, unpigmented, vertical lines (Figure 2; see hex color codes http://www.colorhexa.com). Para-medial, para-lateral, and lateral papillar rows begin at two points (just lateral to the anus) and the papillae are on the neural annulus. Apical cephalic region unpigmented, extending and tapering posteriorly through one thin nuchal band. Two pairs of eye spots (one pair much larger than the other) within cephalic unpigmented region, and occasionally separated by a little less than the diameter of the larger eyespot. Anal region and small genital bar unpigmented to diffusely pigmented with scattered unpigmented to diffusely pigmented small patches in between. Caudal sucker, 0.5 – 2.1 mm in diameter, mean + SE, 1.2 + 0.1 (n = 18), generally unpigmented with a few brown chromatophores and without papillae. Ventrum unpigmented with very sparse scattering of a few brown/green chromatophores (Fig. 3). Male and female gonopores in furrows and separated by 2 annuli.
Digestive system: Proboscis posteriad of the rim/lip of the oral sucker. Short, blunt-tipped proboscis, uniformly cylindrical, and in membranous sheath. Salivary cells scattered in the anterior third of the body (diffuse salivary glands) and slightly more abundant near the base of the proboscis. Salivary ductule bundles join with retractor muscles and attach at each side of the base of the proboscis. Flaccid esophagus extends from the base of the proboscis with one pair of saccular mycetomes. Seven pair of diverticulated crop ceca and the last pair extending posteriad into four sections and diverticulated. Four pair of simple, saccular intestinal ceca. Simple rectum opening to anus, located one annulus anteriad of the caudal sucker.
Reproductive system: (Male) Male gonophores slightly raised. Male atrium opening into ovoid or elloptoid atrial cornue extending laterally and anteriorly from male gonopore into robust, coiled, muscular ejaculatory ducts, recurving posteriorly to robust seminal vesicles and narrow vas deferentia connecting to testisacs. Six pairs of orbicular testisacs, each testisac located in the space between pair of crop ceca. (Female) Female gonopore simple, opening to pair of bifurcated ovisacs. Ovisac length depends on the reproductive condition of the leech.
Type host: Eastern Hellbender, Cryptobranchus alleganiensis alleganiensis (Daudin, 1803).
South Fork Holston River, Smyth County, Virginia.
USNM 1232924 (Holotype), USNM 1232925 – 1232942 (Paratypes), YPM IZ 067799 (Paratype).
Named for the Appalachian Region, where the leech is known to occur.
DNA Analysis.
Molecular comparison of 637 nucleotides of CO-I revealed differences of 0.2% to 1.3% (1–8 nucleotides) among four specimens of Placobdella appalachiensis sp. n. (GenBank KF990590–KF990593) collected from South Fork Holston River, Smyth County, Virginia. Differences of 17.7% to 19.1% (113 to 122 nucleotides) were found between four specimens of Placobdella appalachiensis sp. n. and seven specimens of Placobdella cryptobranchii (GenBank KF601755–KF601761) collected from Missouri. CO-I sequence data among four specimens of Placobdella appalachiensis sp. n. revealed differences of 18.7% to 19.6% (119 to 125 nucleotides) compared to five specimens of Placobdella ornata (GenBank JQ8128–JQ8132) collected from the type locality (West River, New Haven County, Connecticut), differences of 18.8% to 20.0% (120–127 nucleotides) compared to four specimens of Placobdella ornata collected from the type locality (Shivericks Pond, Falmouth, Barnstable County, Massachusetts) of Placobdella phalera (junior synonym of Placobdella ornata) (GenBank JQ812133–JQ812136), differences of 17.7% to 18.7% (113–119 nucleotides) compared to two specimens of Placobdella translucens (GenBank AY047328, JX122778), differences of 15.8% to 16.8% (101–107 nucleotides) from 1 specimen of Placobdella picta (GenBank AF116020), differences of 17.2% to 18.1% (110–115 nucleotides) from 1 specimen of Placobdella biannulata (GenBank AF116021), and differences of 17.9% to 19.0% (114–121 nucleotides) from 2 specimens of Placobdella sophieae (GenBank KF990594–KF990595) collected from Oregon.
Our study provides the first comprehensive description of a population of eastern hellbenders (Cryptobranchus alleganiensis alleganiensis) parasitized by leeches. We identified the leech as a new species, Placobdella appalachiensis, based on its distinct morphological and genetic characteristics. Importantly, leeches were only detected in one reach of the rivers we studied, suggesting that the population of leeches is either isolated or that they are patchily distributed within the river(s). Given the fact that leeches can transmit pathogens amongst individuals, our discovery may have important implications for hellbender conservation (
Ozark hellbenders (Cryptobranchus alleganiensis bishopi) are commonly parasitized by a congener, Placobdella cryptobranchii, in several rivers in Missouri and Arkansas (
Placobdella appalachiensis sp. n. shares morphological similarities with several other leeches. For example, it is also morphologically similar to Placobdella sophieae (
Leeches were common on hellbenders within the stream reach where they were detected. Overall, 27.5% of individuals collected in reach A3 harbored leeches, with a median intensity of infestation of 3 leeches per individual. However, the prevalence of parasitism we observed was substantially lower than that observed by Placobdella cryptobranchii on Ozark hellbenders in Missouri and Arkansas. In a survey of the North Fork White, Spring, and Eleven Points Rivers, 71% of sampled hellbenders hosted 1-140 leeches, with a mean infestation of 8.7 leeches per individual (
The fact that we only found leeches at one study reach within these two streams raises questions about the detectability and geographic distribution of this new species. The widespread prevalence of the Ozark hellbender leech makes it readily detectable within its previously known geographic range in Missouri and Arkansas (
Compared to previous studies examining leeches in Ozark hellbenders, ours is the first to detect a significant influence of hellbender body size on the intensity of parasitism by leeches. However,
It remains unclear as to whether Placobdella appalachiensis sp. n. is an eastern hellbender specialist or whether it uses multiple vertebrate host species. For comparison, the Ozark hellbender leech (Placobdella cryptobranchii) is not known to parasitize eastern hellbenders in Missouri and Arkansas where they occur in close geographic proximity to Ozark hellbenders, which is interesting given the similar habitat use and behavior of these two hellbender subspecies. However,
Our discovery of a new species of leech using eastern hellbenders as hosts has important implications for the health and conservation of these imperiled salamanders. Leeches influence the growth and survival of amphibians, are important vectors of disease, and have even been implicated as contributors to amphibian population declines.
This project would not have been possible without the dedicated assistance of M. Hepner, J. McPherson, B. Todd, S. Orlofske, J. Burke, and S. DuRant. C. Bodinof Jachowski, J.D. Willson, and S. DuRant provided additional technical assistance and/or comments that improved the paper. This work was possible because of supportive landowners (anonymity retained) who generously allowed us access to their property and the logistical assistance and support of Mike Pinder, Joe Ferraro, and Amanda Duncan. This project was funded by the VA Dept of Game and Inland Fisheries, The Fralin Life Science Institute, and the National Science Foundation (IOB-0615361).