(C) 2013 M. Antonio Todaro. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Todaro MA, Perissinotto R, Bownes SJ (2013) Neogosseidae (Gastrotricha, Chaetonotida) from the iSimangaliso Wetland Park, KwaZulu-Natal, South Africa. ZooKeys 315: 77–94. doi: 10.3897/zookeys.315.5593
Among the mostly benthic gastrotrichs, the Neogosseidae (Gastrotricha, Chaetonotida) are particularly interesting from an evolutionary point of view in virtue of their planktonic lifestyle; yet, they are poorly known and uncertainties concerning morphological traits hamper accurate in-group systematics. During a recent survey of meiofauna in the iSimangaliso Wetland Park, South Africa, two species of Neogosseidae were found in a freshwater pond near Charter’s Creek on the Western Shores of Lake St Lucia. Based on morphological traits, one species has been identified as Neogossea acanthocolla, originally described from Brazil, while the other, affiliated to the genus Kijanebalola, is proposed as new to science. Using a combination of differential interference contrast and scanning electron microscopy, fine anatomical details were observed and are here discussed in a larger taxonomic framework, especially regarding Kijanebalola devestiva sp. n. Results have also provided reasons for a revision of the diagnostic traits of Kijanebalola, Neogossea and the whole Family Neogosseidae. Besides expanding awareness about the biodiversity hosted by South Africa’s first UNESCO World Heritage Site, our study will be beneficial to future phylogenetic studies of the Gastrotricha based on morphology, by allowing the selection and/or a more precise character coding of traits of phylogenetic relevance.
Gastrotricha, meiofauna, new species, taxonomy, Kijanebalola, Neogossea, diagnoses
Most freshwater gastrotrichs (Chaetonotida, Paucitubulatina) have an epibenthic, periphytic or interstitial lifestyle, however, there are several species that conduct a pelagic and/or semi-planktonic existence (e.g.,
The origin and evolution of planktonic gastrotrichs remain largely unknown (
Kijanebalola includes the type species Kijanebalola dubia Beauchamp, 1932 from an Ugandan lake and Kijanebalola canina Kisielewski, 1991, from a pond in the State of Parà, Brazil. The status of a third species, described as a rotifer, Eretmia cubeutes Gosse in
We report here on two interesting Neogosseidae found during an ongoing survey of the meiofauna and macrobenthos of the iSimangaliso Wetland Park (
Samples containing gastrotrichs were collected on 11 February 2013 from a 310 m × 70 m freshwater pond located near Charter’s Creek on the Western Shores of Lake St Lucia, iSimangaliso Wetland Park, South Africa. The pond was unevenly divided in two interconnected-pools by a white road; its maximum depth was about 1-1.5 m and the water surface almost completely covered by aquatic vegetation, constituted mainly of blue waterlilies, Nymphaea nouchali. Collection was carried out in both pools by operating from the edge of the road with a hand-held plankton net (15 cm diameter, 25 μm mesh) used to scoop up water from the thick vegetation. The geographic coordinates of each site were recorded by means of a Garmin GPS-12 portable receiver, while the physico-chemical characteristics of the water were measured by means of a YSI 6600 multiprobe system. A list of the main characteristics of the sites surveyed is reported in Table 1 and a comprehensive map along with photos taken at the time of sampling are provided in the Appendix: Figure 1S.
Physico-chemical data and geographic coordinates of the four sampling sites investigated at the pond near Charter’s Creek on the Western Shores of Lake St Lucia, iSimangaliso Wetland Park, South Africa. Pool A represents the larger pool situated on the right side of the road, travelling from Charter’s Creek towards St Lucia town. Pool B is the smaller pool on the left side of the same road (see also supplementary file Figure 1S).
| Variable | Pool A | Pool B | ||
| 1 | 2 | 3 | 4 | |
| Temperature (°C) | 24.8 | 24.9 | 22.8 | 22.9 |
| Conductivity (mS/cm) | 0.24 | 0.25 | 0.25 | 0.40 |
| Salinity | 0.12 | 0.12 | 0.12 | 0.19 |
| Dissolved O2 (%) | 9.7 | 15.4 | 5.5 | 6.0 |
| Dissolved O2 (mg/L) | 0.78 | 1.23 | 0.46 | 0.5 |
| pH | 8.1 | 7.3 | 6.7 | 6.6 |
| Turbidity (NTU) | 21.5 | 17.6 | 16.8 | 14.3 |
| Surface light intensity (µmol.cm-2.s-1) | 184.9 | 222.4 | 242.6 | 179.1 |
| Bottom light intensity (µmol.cm-2.s-1) | 20.97 | 16.73 | 32.48 | 3.93 |
| Depth (m) | 0.5 | 0.7 | 0.60 | 0.80 |
| Geographic coordinates | Latitude 28°15'19"S, Longitude 32°23'37"E | Latitude 28°15'19"S, Longitude 32°23'38"E | ||
A total of four 1 L plastic jars filled with water, some debris and little vegetation from each pool (2+2 stations) were brought back to the laboratory at the University of KwaZulu-Natal (Durban) within 24 hr and analysed during a one-week period. To extract gastrotrichs, samples were stirred with a plastic pipette and aliquots of the sediment-water mixture were poured into 10 cm diameter plastic Petri dishes and scanned under a Wild M5 stereo-microscope. The animals of interest were picked-out with a micro-pipette, transferred to a slide in a drop of 1% MgCl2 solution and studied live (
Abbreviations used in the text are as follows: CT cephalic tentacles; PhIJ, pharyngeo-intestinal junction; PhL, pharynx length, TL, total length.
urn:lsid:zoobank.org:act:53BA8B47-3BC3-4AD1-9B5F-1CBD5F9F34DB
http://species-id.net/wiki/Kijanebalola_devestiva
Figs 1–6Roadside freshwater pond near Charter’s Creek, Lake St Lucia, Western Shores, iSimangaliso Wetland Park, South Africa (Lat. 28°15'19"S, Long. 32°23'37"E; Table 1, Figure 1S).
Holotype: adult specimen 267 μm long shown in Figure 2, no longer extant (International Code of Zoological Nomenclature, Articles 73.1.1 and 73.1.4).
Thirteenspecimens (including the holotype) collected by the first author (10 from pond A and 3 from pond B, see Table 1). Seven specimens were observed alive and are not longer extant, while six were prepared for SEM analysis and are kept in the meiofauna collection of the first author (Ref. n. 2013-SA-01-02).
Above silty substratum, among vegetation.
A Kijanebalola with an adult length to 310 μm; body roughly barrel-shaped with head weakly separated from trunk and the rounded-off posterior end exhibiting medially a group of five spines; head with a pair of 26 μm long, club-like tentacles and a shallow cephalion; cuticular covering mostly smooth, except for a tiny patch of small triangular, keeled scales on the ventral side at the rear trunk; locomotor cilia arranged in tufts and interrupted bands on the head and 4 paired transverse bands along the trunk; three pairs of 14–16 μm long sensory bristles on the dorsal side, at U12, U22 and U92; mouth 13 μm in diameter, slightly protruding down forward and reinforced internally by 17-20 thick, longitudinal ridges; pharynx up to 67 μm, consisting of anterior spherical and posterior nosecone-shaped bulbs; PhIJ at U27; intestine straight, with anterior portion embracing the posterior portion of the pharynx; one pair of conspicuous protonephridia located adjacent to the intestine, from the PhIJ to about mid-body; parthenogenetic.
The specific name devestiva (from the Latin, devestivus, undressed), alludes to the general absence of cuticular ornamentations, such as scales and spines that cover the body of other congeneric species.
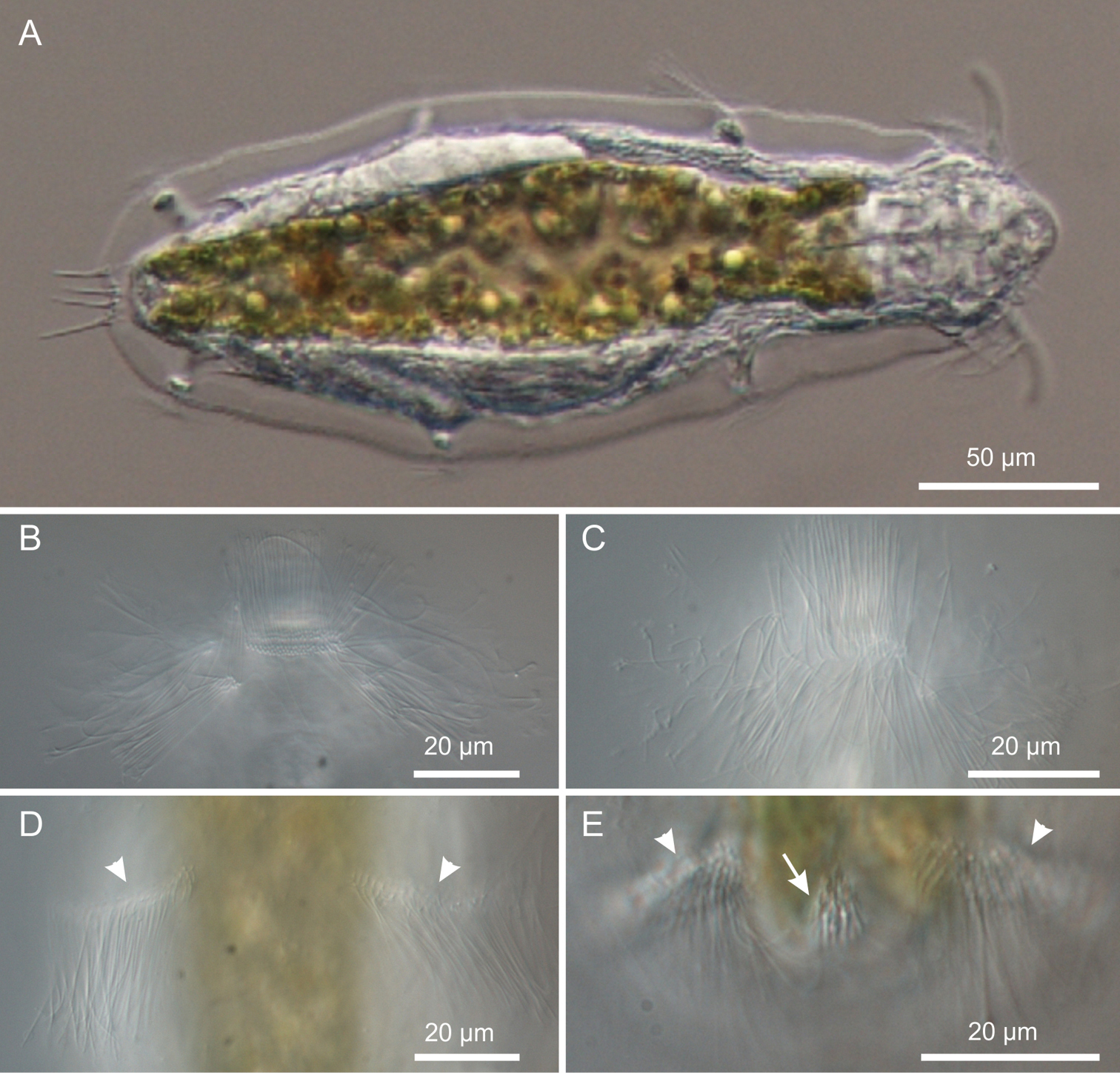
This description is mainly based on an adult specimen, 267 μm in total length (TL, posterior spines excluded). The body is roughly barrel-shaped with the head weakly separated from the trunk by a slight neck constriction and the posterior trunk region rounded-off, without paired lateral projections but exhibiting medially a group of five spines. Body widths at the head/neck/trunk/caudum are 57/55/90/32 μm, at U09/14/52/97, respectively. The head is provided with a pair of club-like tentacles projecting antero-laterally; they are 26 μm in length and insert ventro-laterally at U07; the hypostomion is absent; a shallow cephalion (10 × 4.5 μm) is appreciable only under SEM (Figure 5C). Under dissecting microscope, the animals appear swimming slowly in a rectilinear direction, with some following loose helicoidal trajectories. When purposely stimulated with a needle, specimens react by escaping aside, but never retracting the head inside the body; by contrast, most of the fixed specimens appear to have the head retracted to some extent (Figure 6A).
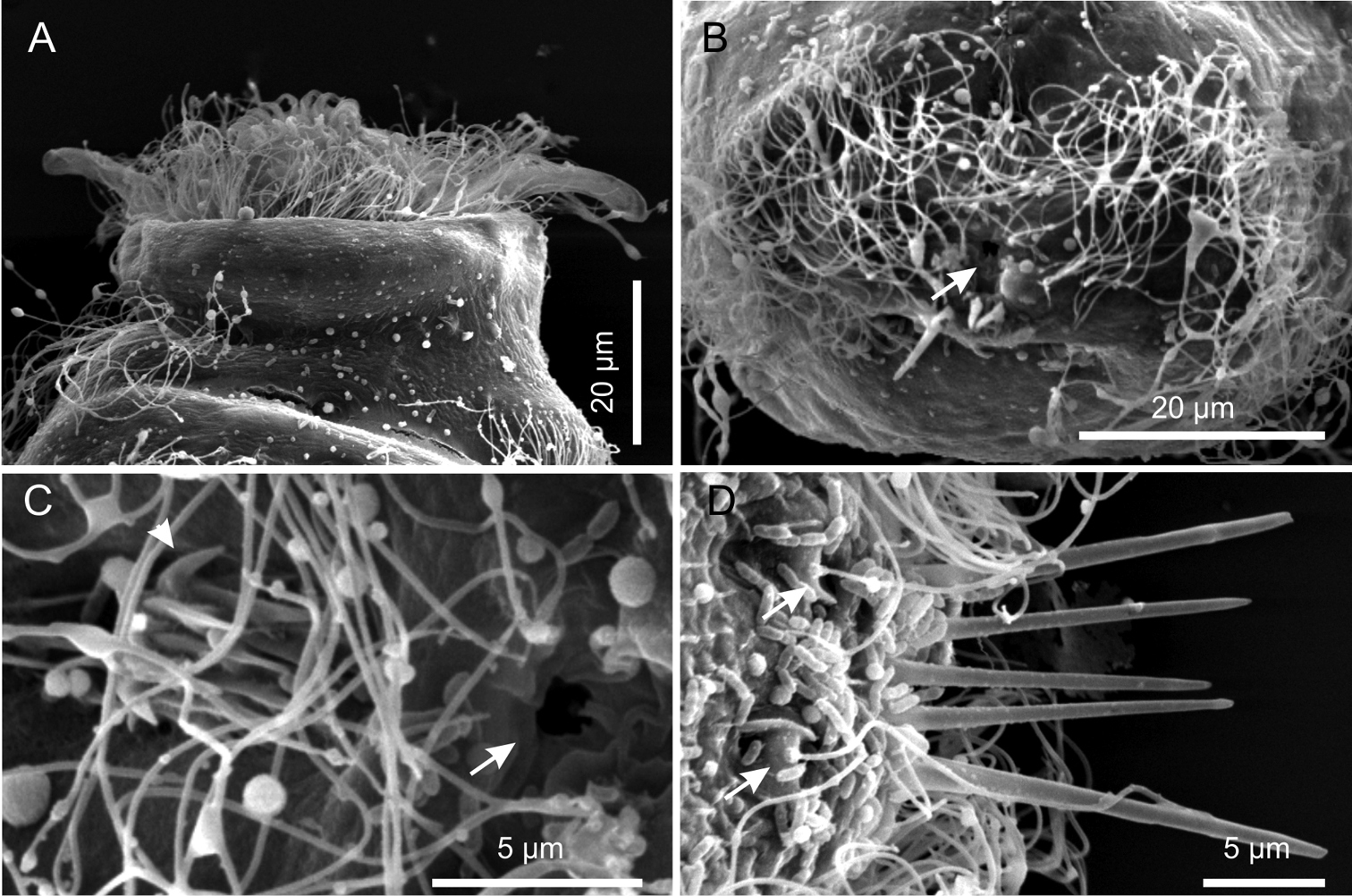
Cuticular armour. The body is covered by a smooth cuticle, except for a minute patch of keeled scales located on the ventral side of the posterior trunk region, at U93 (Figures 1B, 2E). The scales, arranged in 5–7 columns of 3–5 scales each, are very small (ca 1 μm) and may go undetected under light microscopy (DIC); when observed with a scanning electron microscope, they appear roughly triangular in shape and their keel continues in a proportionally long spiny process (Figure 6C). Five robust terminal spines, 15–24 μm in length, ornate the posterior end of the trunk; they are inserted dorsally to the anus (Figure 6B, D).
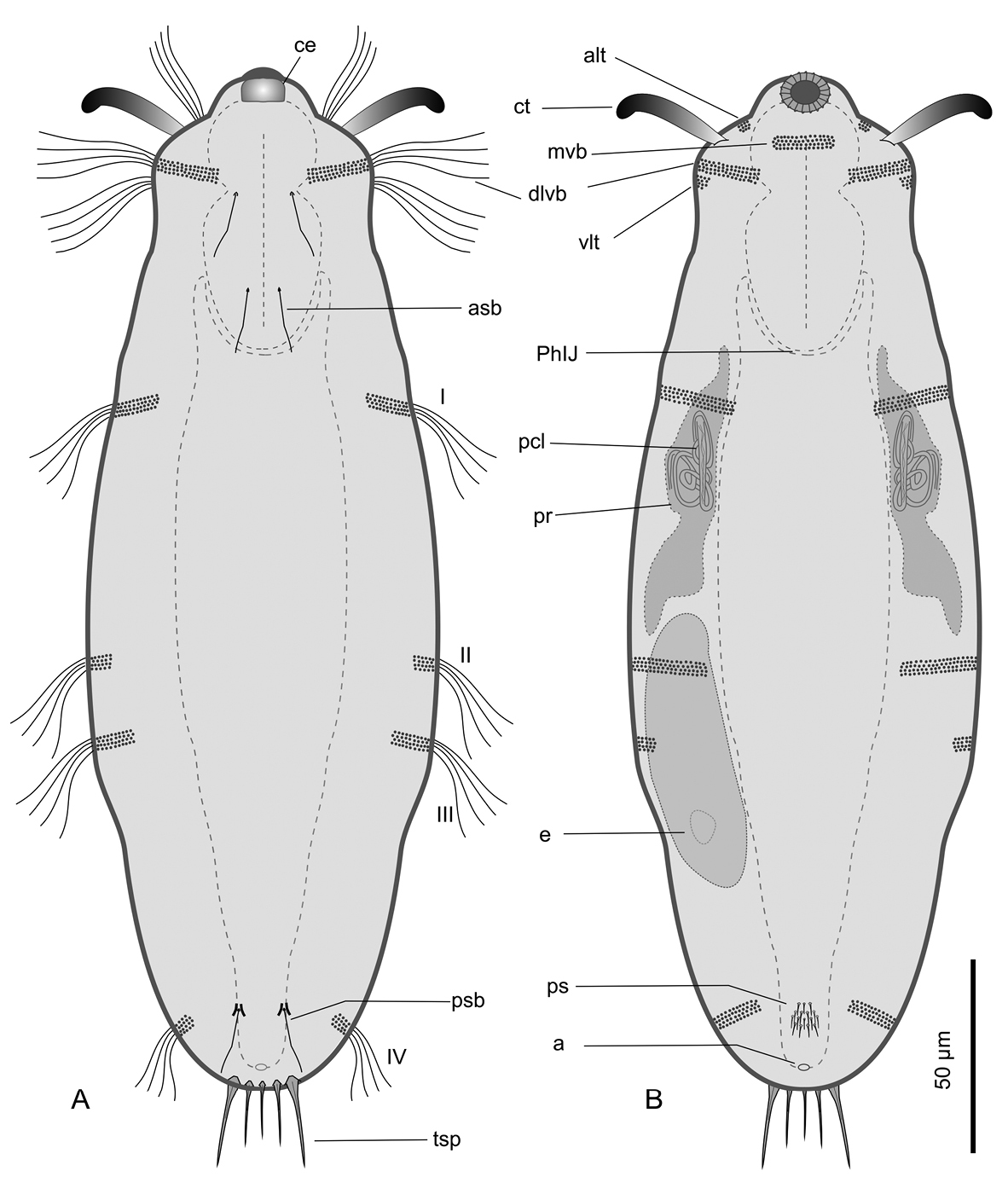
Kijanebalola devestiva sp. n. schematic drawings. A Dorsal habitus B Ventral habitus, showing some internal structures. a anus alt antero-lateral tuft of cephalic cilia asb anterior sensory bristle ce cephalion ct cephalic tentacle dlvb lateral band of cephalic cilia extending dorsally and ventrally e egg I-IV, first to fourth band of trunk ciliature mvb median ventral band of cephalic cilia pcl proximal canal cell lumen PhIJ pharyngeo-intestinal junction ps patch of keeled scales psb posterior sensory bristle tsp terminal spines vlt ventro-lateral band of cephalic cilia.
Kijanebalola devestiva sp. n. DIC photomicrographs. A habitus B, C close-up ventral view of theanterior region showing the ciliary bands D close-up ventral view of the mid-trunk region showing the second ciliary bands E close-up ventral view, of the posterior region, showing the fourth ciliary bands (arrowheads) and the residual patch of spined scales (arrow).
Ciliation. Locomotory cilia are arranged in tufts and interrupted bands around the head and paired transverse bands along the trunk (Figures 1, 2A–E, 5A, B). Most of the cephalic cilia have a ventral or ventro-lateral distribution; however, a precise organization is difficult to see due to their high density and relatively long span (16-18 μm in length). From anterior to posterior end, it is possible to discern the following groups: a pair of antero-lateral tufts, a median ventral band, a pair of lateral bands extending ventrally and dorsally, followed by a pair of ventro-lateral tufts (Figure 1). The trunk ciliature consists of four pairs of oblique short bands, with first (U32) and fourth (U94) inserted dorso-latero-ventrally, the second (U59) inserted latero-ventrally and the third (U66) latero-dorsally (Figures 1, 2D, E, 5A).
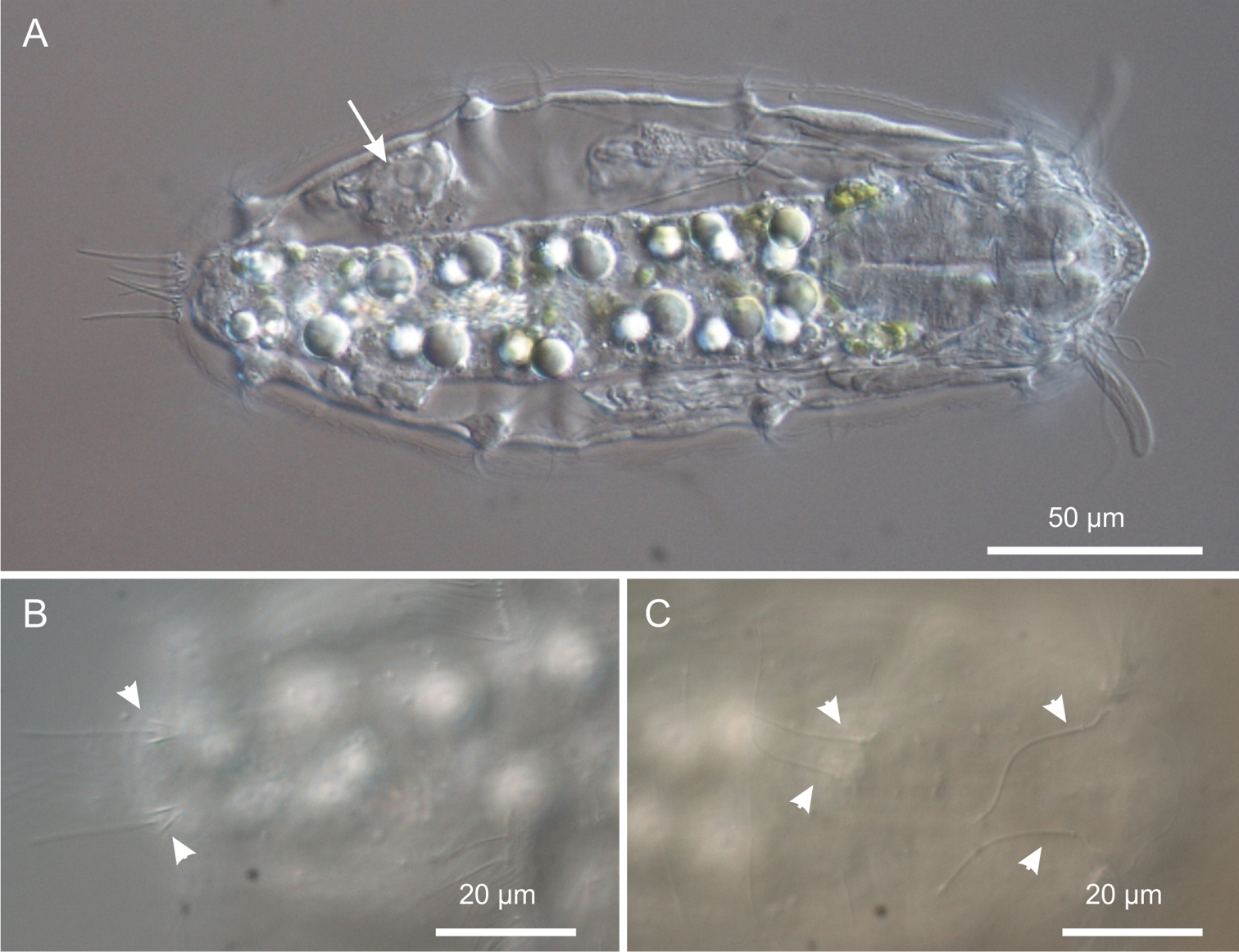
Three pairs of sensory bristles (14–16 μm in length) are present on the dorsal side at U12, U22 and U92, respectively (Figures 1A, 4B, C). The bristles of the first two pairs emerge from round pits, while posterior bristles originate directly from the cuticle and are flanked by two anteriorly-converging keels. Presence of additional sensory bristles hidden among the cephalic locomotor ciliation cannot be excluded.
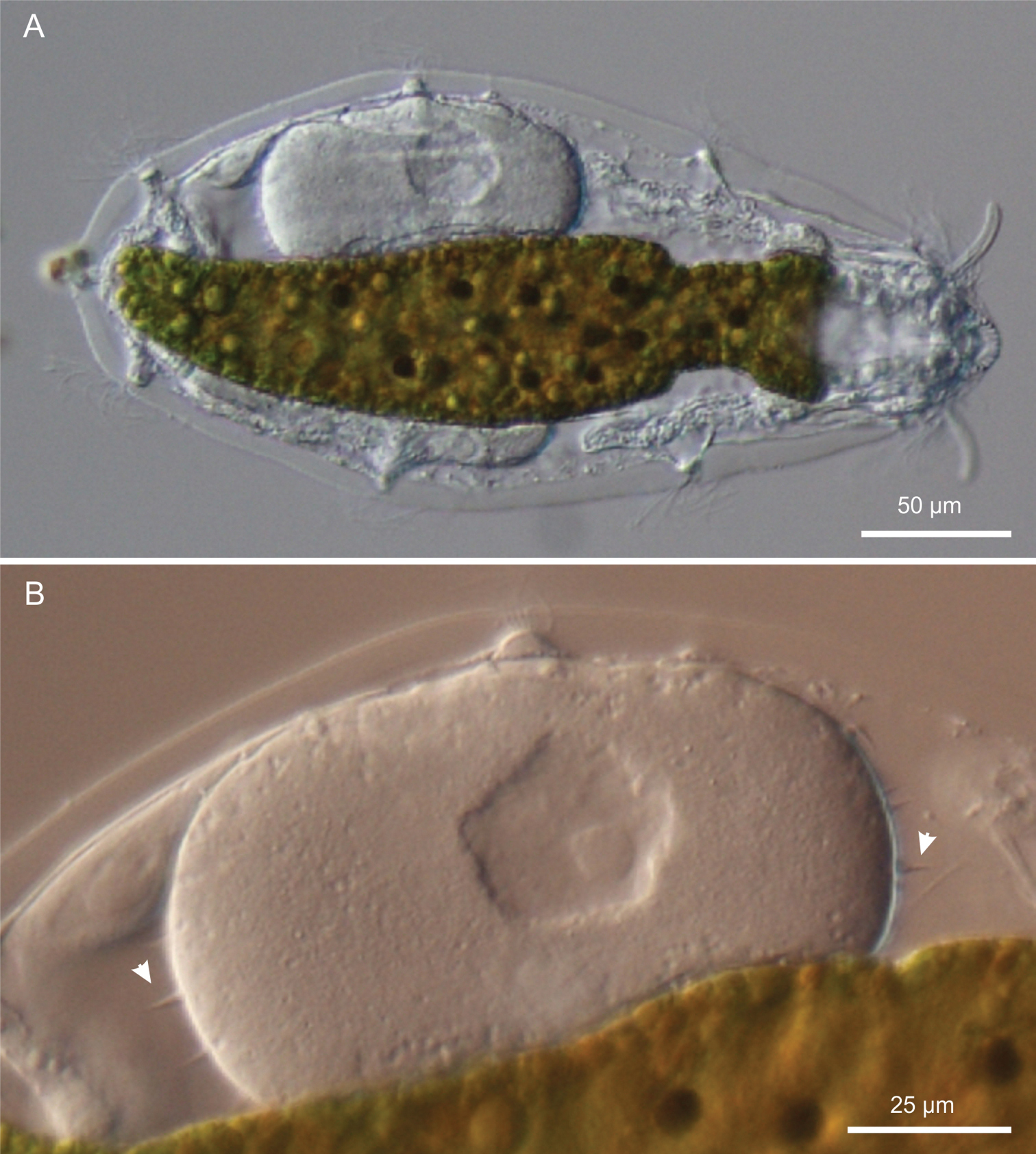
Kijanebalola devestiva sp. n. DIC photomicrographs. A habitus of a gravid specimen B close-up view of the inside egg with the shell bearing spine-like ornamentation (arrowheads).
Kijanebalola devestiva sp. n. DIC photomicrographs. A habitus of a subadult specimen showing developing egg (arrow) B close-up dorsal view of the posterior trunk region showing the two sensory bristles (arrow-heads) C close-up dorsal view of the anterior trunk and neck regions, showing two pairs of sensory bristles.
Digestive tract: The strong mouth ring is terminal and about 13 μm in diameter; it appears slightly protruding down forward and is reinforced inside by 17–20 thick longitudinal cuticular ridges, which protrude externally and bend on the outer contour (Figure 5). The pharynx is 64 μm in length and shows anterior and posterior bulbs separated by a noticeable constriction; the anterior bulb, deprived of cuticular reinforcement, is roughly spherical (28 × 24 μm), while the posterior one (30 × 40 μm) is more nosecone-shaped (Figures 1, 4A); PhIJ is at U27. The intestine is straight; in the adult it appears impressively filled with green material (Figures 2A, 3A) while in juveniles it is packed with translucent globules (Figure 4A). Peculiarly, the anterior portion of the intestine extends forward encircling a large part of the posterior portion of the pharynx (about half the length of the posterior bulb), resulting in this region much wider than the pharynx itself (36 μm); at the PhIJ and for a short tract the intestine is about as wide as the pharynx (28–30 μm), then it widens again reaching a maximum width of 45 μm at about mid-body (U52); after this point the gut progressively narrows until it joins the 5–6 μm sub-terminal anus at U97 (Figures 1, 6B, C).
Nephridial system. There is a pair of conspicuous protonephridia adjacent to the intestine; each protonephridium occupies an area extending from the PhIJ to about mid-body (U27-U53) and includes a clearly visible tubular canal containing two vibrating flagella, corresponding to the proximal canal cell lumen of
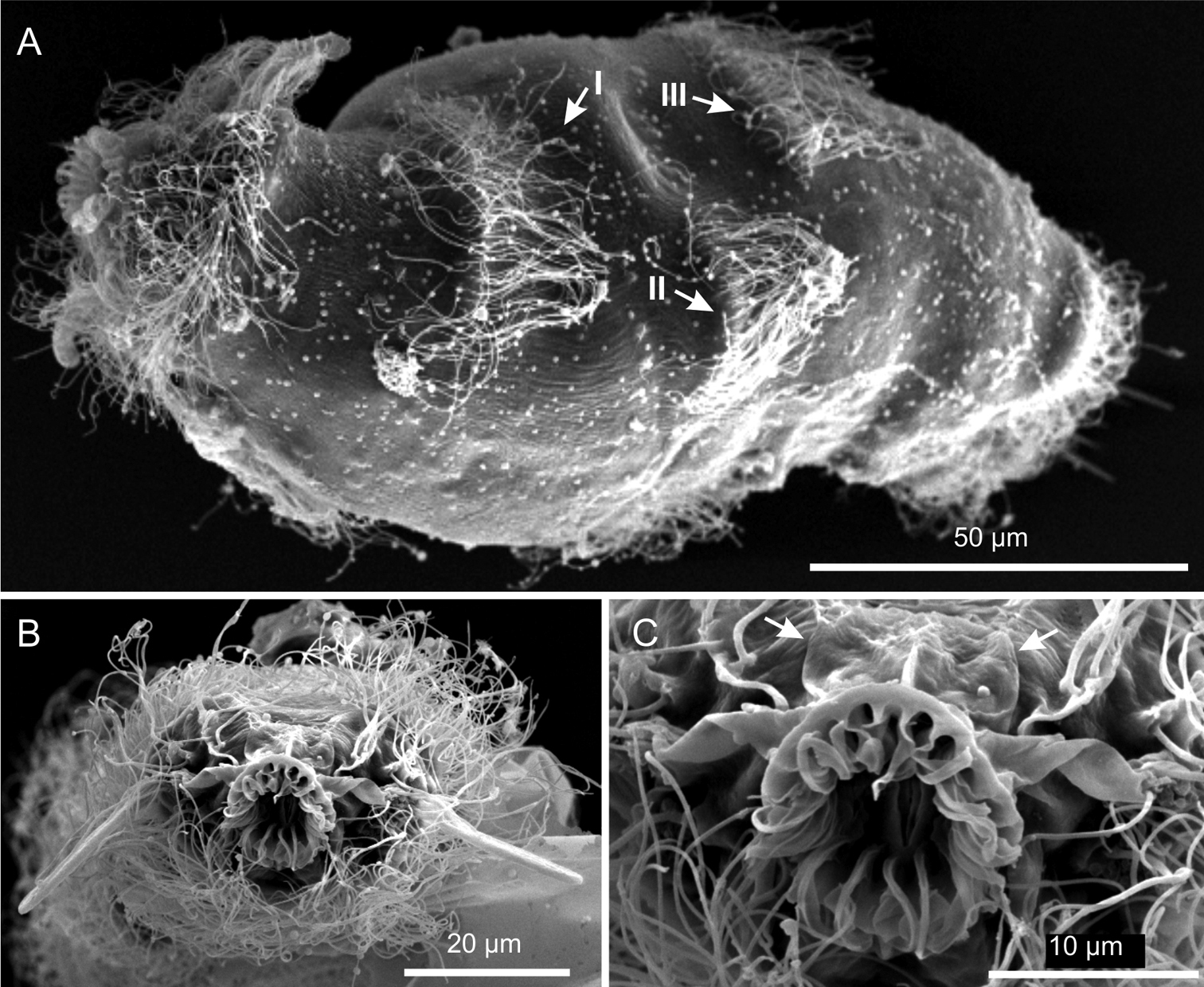
Kijanebalola devestiva sp. n. SEM photomicrographs. A habitus in a ventro-lateral view; note the arrangement of the first, second and third ciliary bands on the trunk region (number and arrows) B anterior region of a different specimen in a frontal view C close-up view of the mouth ring and cephalion (arrows).
Kijanebalola devestiva sp. n. SEM photomicrographs. A anterior region of specimen with head partially retracted inside the body (ventral view) B posterior trunk region of different specimen, showing the fourth ciliary band and the anus (arrow) C close-up view of the posterior end showing the anus (arrow) and the residual patch of spined scales (arrows) D close-up view of the posterior end (dorsal view), showing theterminal spines and the sensorial bristles (arrows).
Reproductive tract. All the adult specimens were in parthenogenetic phase, with respective eggs at different stages of development.
Variability and remarks. The largest adult was about 310 μm in total length (terminal spines excluded) and was carrying a very big egg inside (73 × 107 μm, Figure 3A). Remarkably, the egg shell was ornated with spikes (Figure 3B); this is quite surprising because in freshwater Gastrotricha the shell ornamentation is believed to appear only after the egg has been laid, probably due to osmotic differences between the external vs internal milieu (
The general body appearance, the presence of club-shaped cephalic tentacles and the planktonic lifestyle that characterise Kijanebalola devestiva sp. n. suggest that the closest affiliation of the South African specimens lies with the Neogosseidae. Notwithstanding this, autoapomorphic traits of the new species make somewhat difficult its affiliation to any of the two currently recognized genera, Neogossea and Kijanebalola, based on their current diagnosis (see
Another potential difference between the two genera i.e., the presumed ability of Kijanebalola species to partially retract the head inside the body, can also be dismissed. Our observations with the new species demonstrate that retraction of the head is actually an artefact due to fixation (see above). Consequently, none of the above reported traits may be used to allocate our specimens to either of the two genera.
In summary, only a single character is apparently left to differentiate Neogossea and Kijanebalola; it pertains to the structure of the posterior part of the trunk, which appears truncate with a pair of postero-lateral projections, each provided with a tuft of relatively long spines in Neogossea, but rounded-off with a median group of spines in Kijanebalola. As the difference in this character between species of the two genera is consistent, it is reasonable to use it as diagnostic feature and as a solid synapomorphy for each genus.
Consequently, based on the round shape of the posterior trunk region of the iSimangaliso specimens, we propose that their closest affiliation is to the genus Kijanebalola. Therefore, the large size, the cuticular ornamentation reduced to an epaulet of scales on the ventral side of the trunk ending, and the number and size of the terminal spines are considered autoapomorphic characters that can easily distinguish Kijanebalola devestiva sp. n. from Kijanebalola dubia and Kijanebalola canina. Emended diagnoses are reported below.
2 adult specimens (1 measured and documented), South Africa, KwaZulu-Natal, roadside freshwater pond near Charter’s Creek, Lake St Lucia, Western Shores, 11 February 2013, MA Todaro legit.
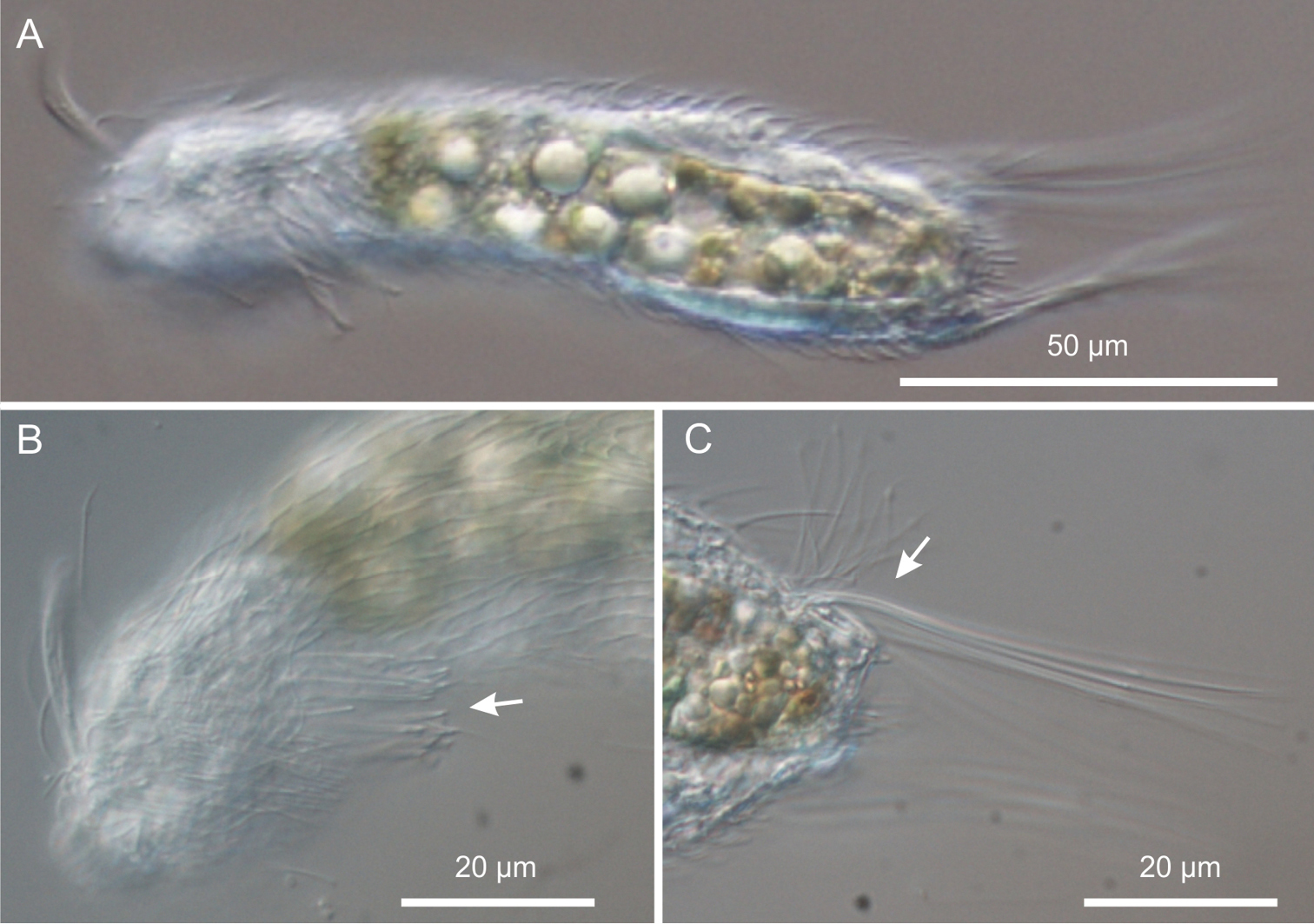
TL, 122 μm (posterior spines excluded); PhL, 34.5 μm; PhIJ at U36; CT, 24.5 μm; dorsal cuticular covering made up of about 16 columns of 17–24 trilobed scales bearing a short, simple spine; a group of 18 densely packed spines is present on the dorsal side of the neck; spines are 10–13 μm in length, rather thick and with a notched tip; posterior tufts of long spines made up of 7 spines each; spines are 45–50 μm in length and barbed, with the lateral denticle positioned at about 3/4 of the spine length.
Neogossea acanthocolla. DIC photomicrographs. A habitus B anterior region showing the group of thick spines on the neck (arrow) C close-up of the posterior region of the trunk showing a tuft of long, barbed spines (arrow).
Morphometry and general appearance of the specimen from the iSimangaliso Wetland Park are in general accordance with data reported for the Brazilian Neogossea acanthocolla, the main peculiarity of which is the presence of the brush-like group of spines on the neck. A noticeable difference between the South American and African specimens lies in the type of scales covering the body: pedunculated and rhomboidal in the former, but ordinary (trilobed) and spined in the latter. However, as similar differences have been reported among members of the Brazilian populations (cf.
Paucitubulatina exhibiting weakly flattened body, 90–310 μm in length (posterior spines excluded). With a pair of club-shaped cephalic tentacles. Caudal furca and adhesive tubes absent. Posterior trunk with paired postero-lateral projection or rounded-off. Locomotor ciliature consisting of tufts and interrupted bands around the head and several pairs of tufts or short obliquely-running bands along the trunk. Cephalion and hypostomion present or absent. Body covered with scales, rarely naked with scales reduced to an epaulet on the ventral side; scales in general spined rarely pedunculated; spines simple, occasionally barbed. Trunk end with long spines distributed in paired lateral and/or unpaired median group. Mouth ring large and terminal, reinforced inside by thick longitudinal cuticular ridges, which may protrude externally. Pharynx strong with one to four bulbs. With a pair of protonephridia in the anterior trunk region. Parthenogenetic, with paired ovary. Male system unknown. Freshwater, semipelagic and/or planktonic.
Neogosseidae with body 90–200 μm in length (caudal spines excluded). Posterior end of body truncate, with a pair of postero-lateral projections, each provided with a tuft of long spines, simple or barbed, occasionally also with short claw-like structure. Cephalion and hypostomion present or absent. Body covered with fine and spined scales; scales trilobed or with edges often fused with basal cuticle; occasionally and only partially with pedunculated scales. Spines short and simple, occasionally partly long and barbed. Mouth ring large and terminal, reinforced inside by thick longitudinal cuticular ridges, protruding externally. Anterior half of pharynx consisting of terminal bulb followed by two smaller dilations; posterior half in the form of large bulb. Cuticular pharyngeal reinforcements lacking. Six species: Neogossea antennigera (Gosse, 1851) - type-species, Neogossea acanthocolla Kisielewski, 1991, Neogossea fasciculata (Daday, 1905), Neogossea pauciseta (Daday, 1905), Neogossea voigti (Daday, 1905) and Neogossea sexiseta Krivanek & Krivanek, 1959.
Neogosseidae exhibiting body 135–310 μm in length (caudal spines excluded). Posterior end of trunk rounded-off, with median group of spines and without lateral protrusions. Cephalion and hypostomion present. Body covered with convex scales with median keels and rudimentary spines, the latter tend to prolong in some trunk portions; occasionally body mostly naked with scales reduced to an epaulet on the ventral side. Pharynx consisting of at most two bulbs. Anterior pharynx portion at least as thick as posterior one and occasionally provided with strong and complex system of cuticular reinforcements. Mouth ring large and terminal, reinforced inside by thick longitudinal cuticular ridges, which may protrude externally.
Three species: Kijanebalola dubia Beauchamp, 1932 (type-species), Kijanebalola canina Kisielewksi, 1991 and Kijanebalola devestiva sp. n. The status of an additional species, originally described as a rotifer, i.e., Eretmia cubeutes Gosse in
The staff and management at iSimangaliso Wetlands Park Authority and Ezemvelo KZN Wildlife are thanked for their support and co-operation. We thank the Director, Nelisha Murugan, and the Principal Technician, Phillip Christopher, of the Microscopy and Micro-analysis Unit of the University of KwaZulu-Natal for kindly providing facilities and assistance towards the success of this study. We are also very grateful to Nelson Miranda, Nicola Carrasco and David Dyer for their invaluable assistance with field collections. Funding for this study was provided by the National Research Foundation (NRF, Pretoria), the University of KwaZulu-Natal (UKZN, Durban) and the University of Modena and Reggio Emilia, BIOTOME project.
Comprehensive map and DIC photomicrograph. (doi: 10.3897/zookeys.315.5593.app) File format: JPEG Image (jpg).
Explanation note: Figure 1S. Sampling location. Comprehensive map along with photos taken at the time of sampling. Figure 2S. Kijanebalola devestiva sp. n. DIC photomicrograph. Close-up of the protonephridial apparatus.