(C) 2013 Dan Cogălniceanu. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Cogălniceanu D, Rozylowicz L, Székely P, Samoilă C, Stănescu F, Tudor M, Székely D, Iosif R (2013) Diversity and distribution of reptiles in Romania. ZooKeys 341: 49–76. doi: 10.3897/zookeys.341.5502
The reptile fauna of Romania comprises 23 species, out of which 12 species reach here the limit of their geographic range. We compiled and updated a national database of the reptile species occurrences from a variety of sources including our own field surveys, personal communication from specialists, museum collections and the scientific literature. The occurrence records were georeferenced and stored in a geodatabase for additional analysis of their spatial patterns. The spatial analysis revealed a biased sampling effort concentrated in various protected areas, and deficient in the vast agricultural areas of the southern part of Romania. The patterns of species richness showed a higher number of species in the warmer and drier regions, and a relatively low number of species in the rest of the country. Our database provides a starting point for further analyses, and represents a reliable tool for drafting conservation plans.
Reptilia, species distribution, species range, biodiversity data, species richness, rarity
Reptiles are declining worldwide at an alarming rate (
Romania has five biogeographic regions (i.e., Alpine, Continental, Pannonian, Black Sea, and Steppic) out of the nine regions recognized by the European Union (
The diversity of reptiles is surprisingly high for a country with a mostly temperate and continental climate. There are 23 reptile species in Romania, out of which 12 species reach the limit of their geographic range (i.e., Testudo hermanni, Testudo graeca, Ablepharus kitaibelii, Lacerta trilineata, Podarcis tauricus, Podarcis muralis, Eremias arguta, Darevskia praticola, Eryx jaculus, Dolichophis caspius, Elaphe sauromates, Vipera ammodytes), while other two species are near their range edge in Romania (i.e., Zamenis longissimus, Vipera ursinii) (
No updated distribution maps have been published since the publication of the milestone volume on Reptiles in the series Fauna of Romania fifty-two years ago (
We here present an updated overview of the distribution and diversity of reptiles in Romania. The aims of our study are to (1) map the distribution of reptile species in Romania and (2) analyze the spatial pattern of distribution data.
We extracted the occurrence records from four major sources: published data, museum collections, personal communications from specialists, and our own unpublished field data. The records were primarily stored and managed in a Microsoft Access database, and later imported it in an ESRI file geodatabase using ArcGIS Desktop 10.1 (ESRI, CA). We checked for data quality by (1) filtering the database for doubtful and erroneous records, (2) aggregating the known localities to a finer resolution, and (3) assessing the bias in sampling effort. Our own data were collected over a period of almost 25 years and it involved a large variety of methods. Since the majority of studies carried out were of ecology, the detailed distribution data was not made available in the resulting publications. Our own few publications presenting species distribution data were included in Appendix I. No voucher specimens were collected during our studies.
The distribution records that could not be georeferenced to an actual locality or toponym (e.g., occurrences assigned to mountain ranges, geographical provinces or hydrographic basins) or records with unspecified taxa within genera were not included in the geodatabase. Other doubtful or erroneous records such as species out of their known range or vagrant individuals sensu
The species taxonomy considered in the present paper is based on
We aggregated the occurrence records to the Universal Transverse Mercator (UTM) grid system at a spatial resolution of 25 km2 (UTM 5 × 5 km). The records with a spatial resolution of ≤ 25 km2 were assigned the corresponding UTM 5 × 5 km grid cell code using primarily the UTM index of localities (
The occurrences were characterized based on the year of observation into old (if recorded before 1990) and recent (if after 1990, i.e. post Cold-War period) records. The distinction is based on the type of studies published and the style of work. Most papers before 1990 did not indicate the year of observation/collection and some did not even differentiate between new and old records. After 1990 the trend was to publish new locations in pure faunistic studies done within administrative units. Moreover, after 1990 access was not restricted anymore in areas close to the border and it involved also major changes in landscape use and an overall reduction of threats, mostly caused by a reduction in the use of pesticides and fertilizers in agriculture, land abandonment, changes in local hydrology caused by the decline in irrigation intensity and the decline of mining and industrial activities. If the year of observation was not mentioned in the publications, mostly in our national and local journals, then we subtracted 3 to 5 years from the date of publication, based on our estimate of the delay between actual fieldwork and time of publication.
Based on the number of reptile records per UTM 5 × 5 km grid cell, we tested for spatial autocorrelation using Global Moran’s I statistic under the null hypothesis that the occurrence records were evenly distributed. If the null hypothesis is rejected, the occurrence records are more spatially clustered (Z > 0) or dispersed (Z < 0) than expected (
In order to assess the altitudinal distribution of each species, we extracted the mean altitude per grid cell from the SRTM digital topographic database (
We calculated the species richness at a spatial resolution of 50 × 50 km and 10 × 10 km without considering subspecies separately. Mapping the species richness at a coarser resolution reduced the potential bias in sampling effort and allowed us a better understanding and visualization of regional patterns (
We then calculated the Extent of Occurrence (EOO) as the minimum convex polygon, and the Area of Occupancy (AOO) as the total area of UTM 5 × 5 km grid cells where a species was reported (
Finally, we calculated a rarity index at a 50 × 50 km resolution (RI:
All spatial statistics analyses were performed using geoprocessing tools under ArcGIS for Desktop 10.1 (ESRI, CA). Non-spatial statistical analyses were performed in Minitab 16 (Minitab Inc., PA).
We extracted and georeferenced 18036 reptile records from published papers (66%), museum collections (2%), personal communication from specialists, and our own field surveys (32%). The published papers with the reptile distribution records used in this study are listed in Appendix I, while the dynamics in time of their publication is presented in Figure 1. Among the records, 87.1% were dated after 1990, and 12.9% before 1990 (Appendix II and Table 1). Compared to the national density of reptile records per 100 km2 of 0.029 in the GBIF dataset (http://data.gbif.org, accessed 30.03.2013), our database increased it to 7.5 reptile records per 100 km2.
The species’ Extent of Occurrence ranged from 6439 km2 (Testudo hermanni) to 233497 km2 (Natrix natrix), while species’ Area of Occupancy ranged from 125 km2 (Eryx jaculus) to 37346 km2 (Lacerta agilis, Table 1).
Number of publications with distribution data of reptiles in Romania (1823–2013). The reference line indicates the last country wide assessment published in 1961 (
The occurrence records for reptile species in Romania. EOO was estimated as 100% minimum convex polygon, and AOO was estimated as the total area of all UTM grid cell containing species records. Since not all UTM cells matched the 25 km2 area, the computed AOO is not a multiple of this value. Rarity index was calculated relative to a 50 × 50 km cell in order to check for regional patterns.
| Species | Total number of records | Old records (before 1990) | New records (after 1990) | Number of UTM5 squares | EOO (km2) | AOO (km2) | Rarity index |
|---|---|---|---|---|---|---|---|
| Emys orbicularis | 753 | 131 | 622 | 561 | 232748 | 13491 | 22.7 |
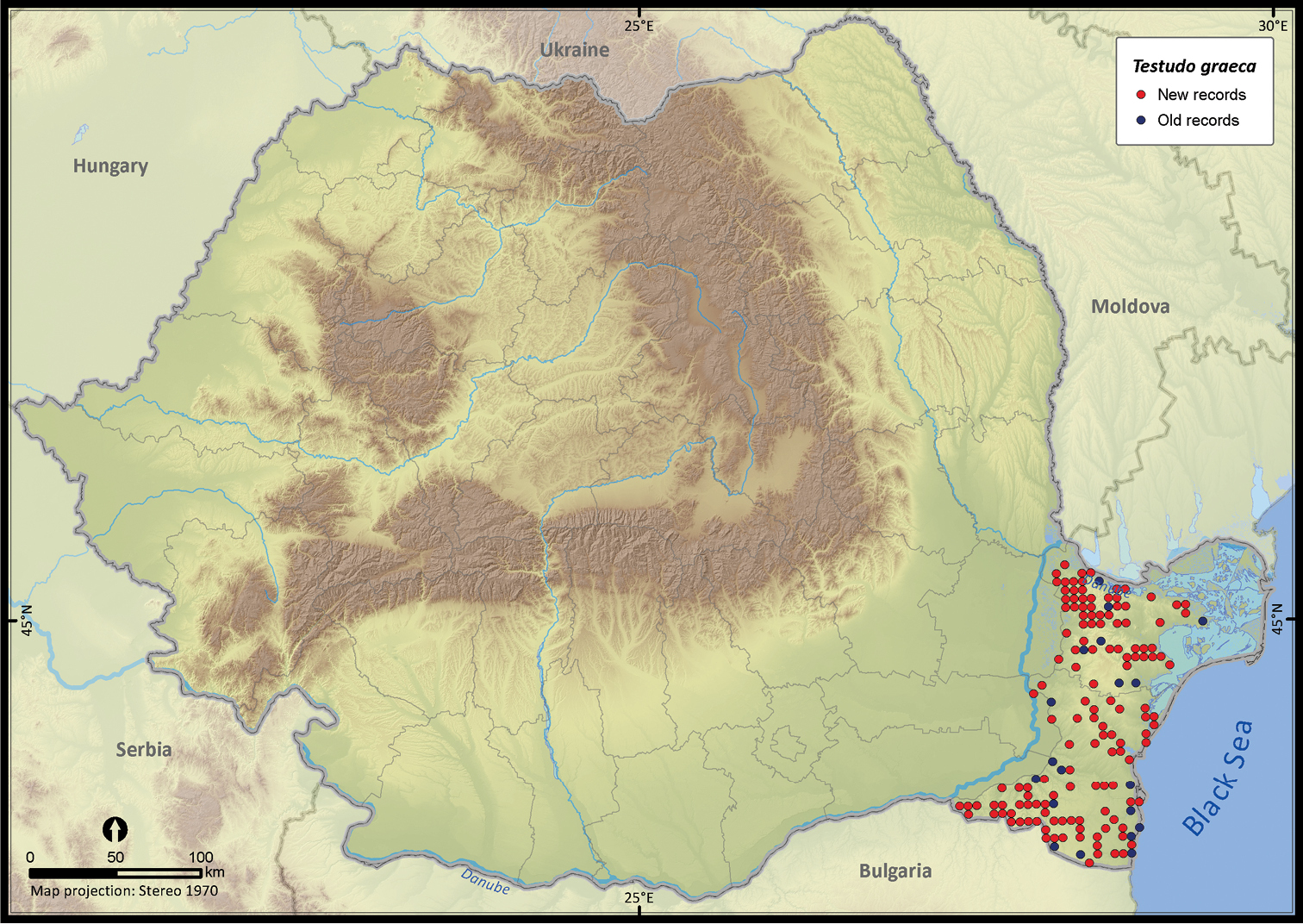
| Testudo graeca | 1159 | 117 | 1042 | 154 | 14441 | 3748 | 91.1 |
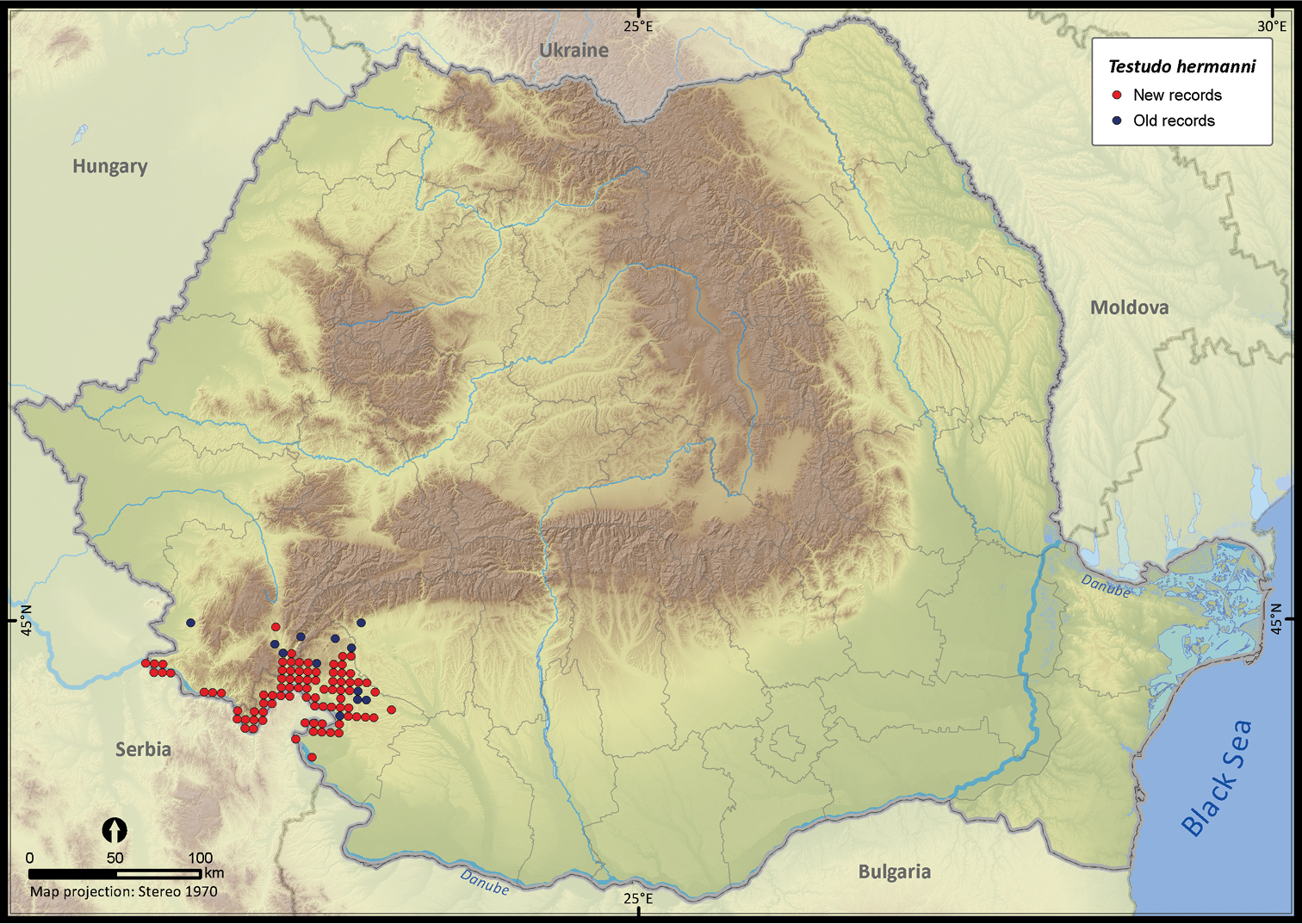
| Testudo hermanni | 891 | 83 | 808 | 96 | 6439 | 2120 | 93.4 |
| Anguis fragilis | 1006 | 111 | 895 | 728 | 211520 | 17994 | 38.2 |
| Eremias arguta | 85 | 23 | 62 | 30 | 10851 | 625 | 95.1 |
| Lacerta agilis | 2554 | 268 | 2286 | 1521 | 231768 | 37346 | 13.8 |
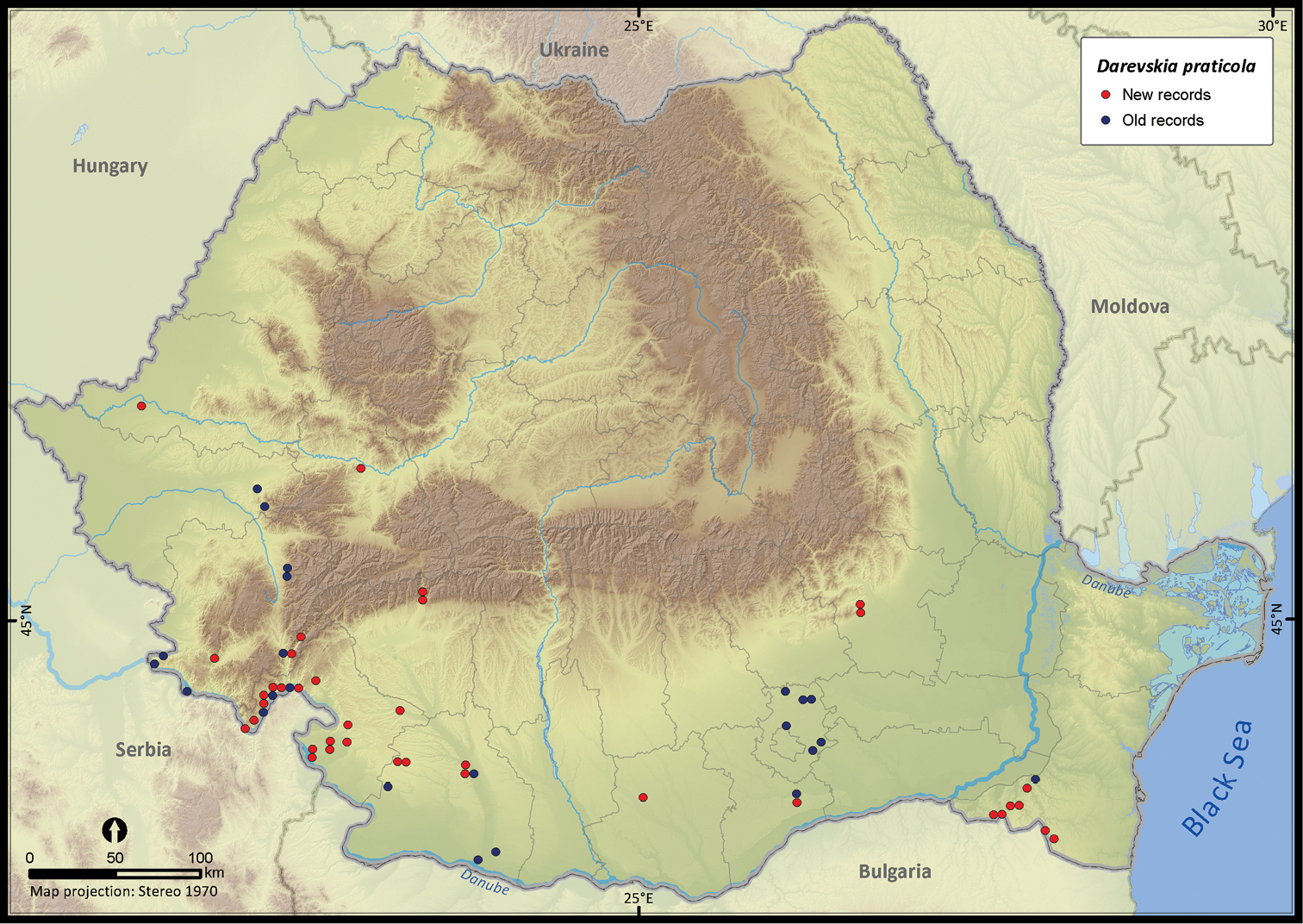
| Darevskia praticola | 93 | 34 | 59 | 60 | 80161 | 1449 | 84.5 |
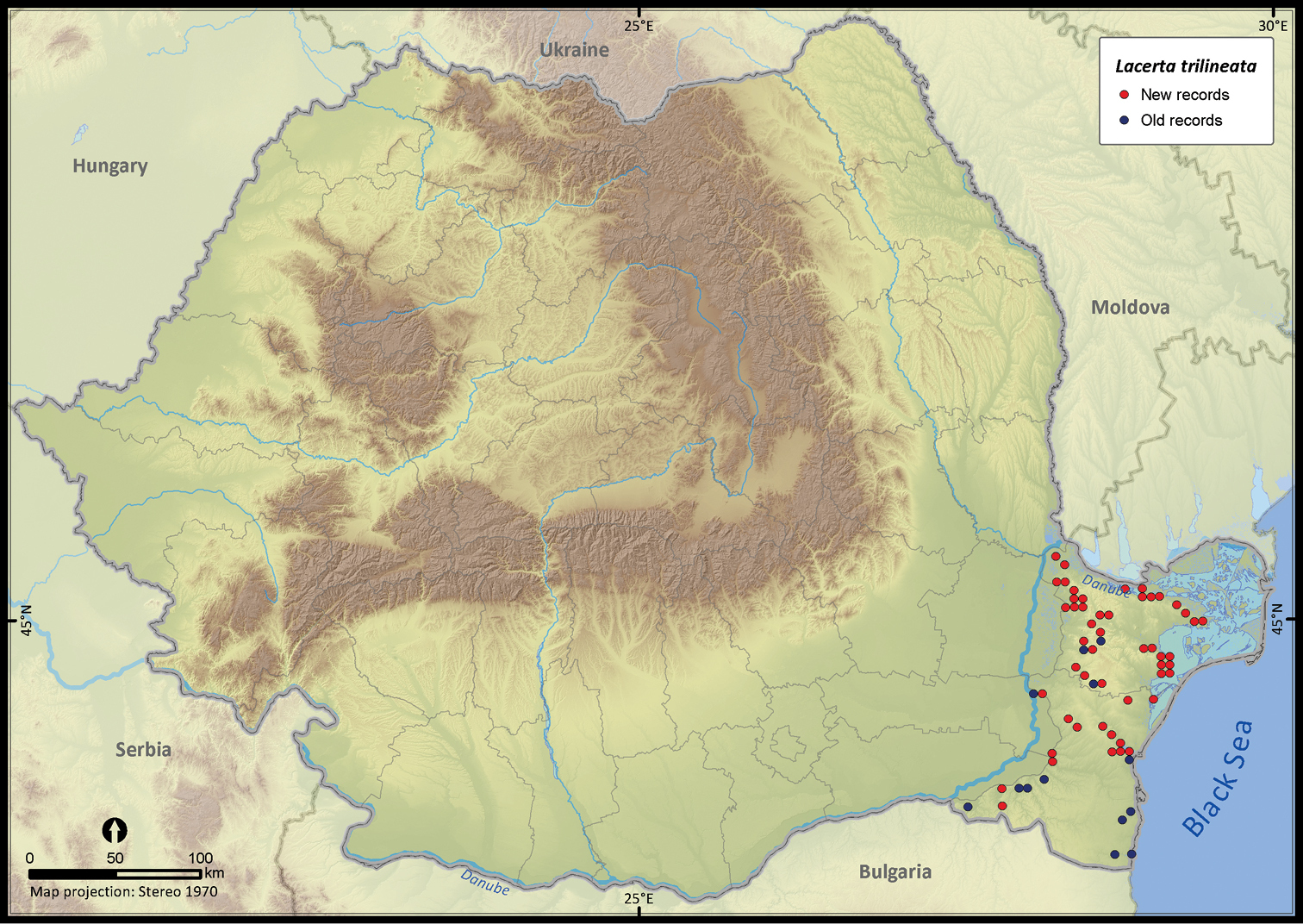
| Lacerta trilineata | 133 | 26 | 107 | 64 | 14028 | 1589 | 91.1 |
| Lacerta viridis | 2737 | 214 | 2523 | 1101 | 228111 | 26886 | 19.5 |
| Zootoca vivipara | 805 | 105 | 700 | 463 | 114513 | 11488 | 60.9 |
| Podarcis muralis | 672 | 111 | 561 | 374 | 167436 | 9093 | 56.9 |
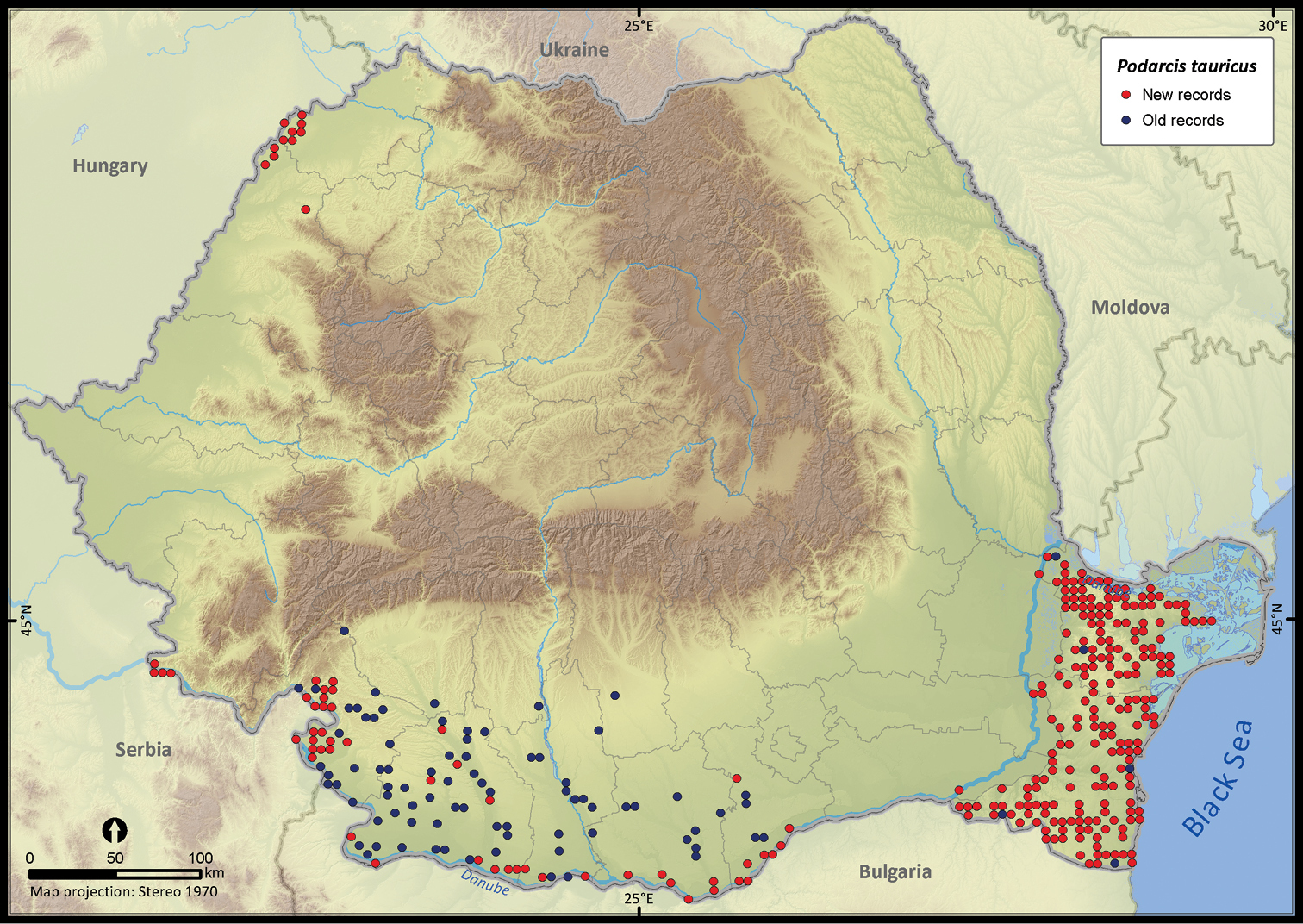
| Podarcis tauricus | 1399 | 188 | 1211 | 358 | 163668 | 8671 | 70.7 |
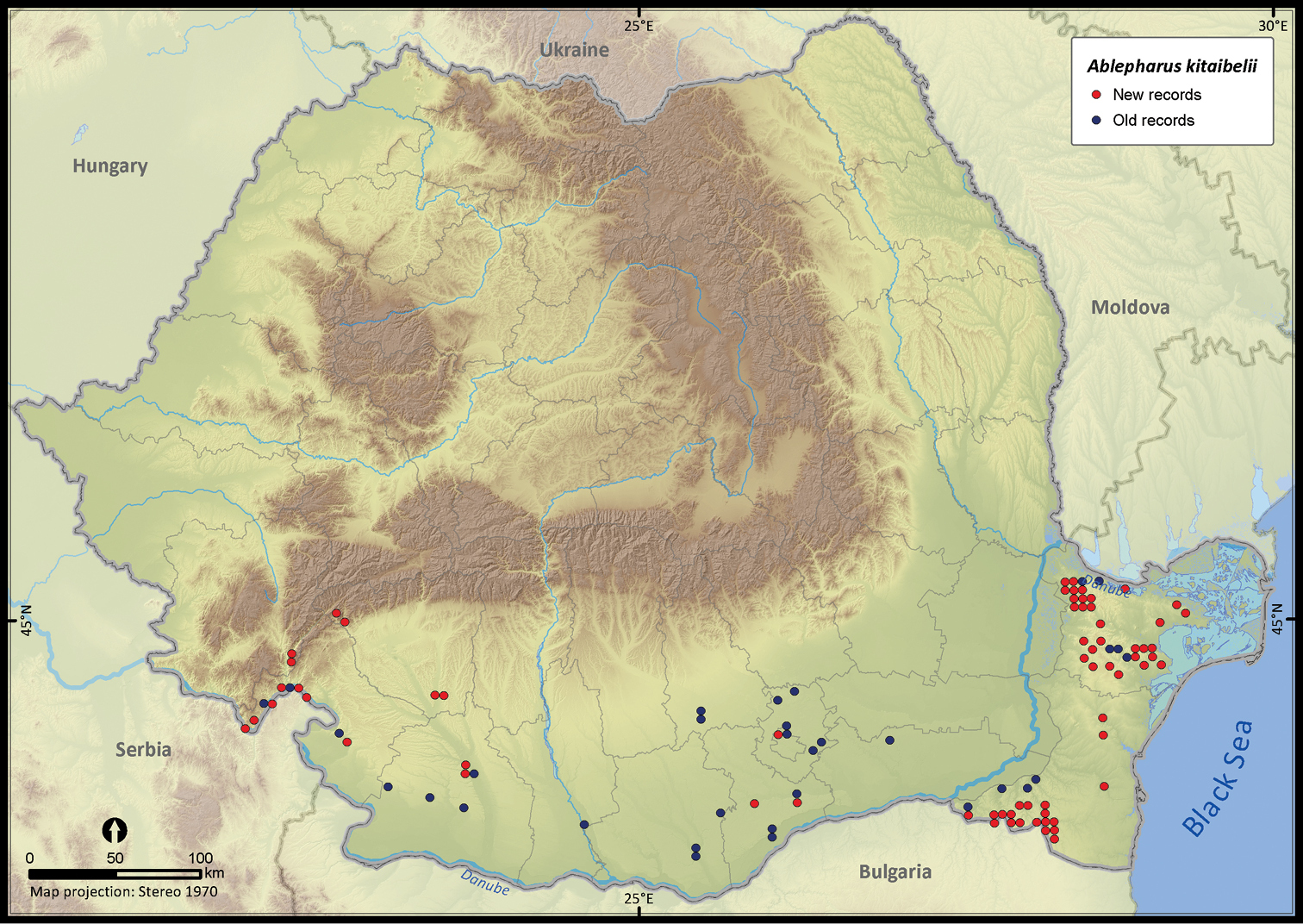
| Ablepharus kitaibelii | 250 | 56 | 194 | 100 | 63728 | 2413 | 80.4 |
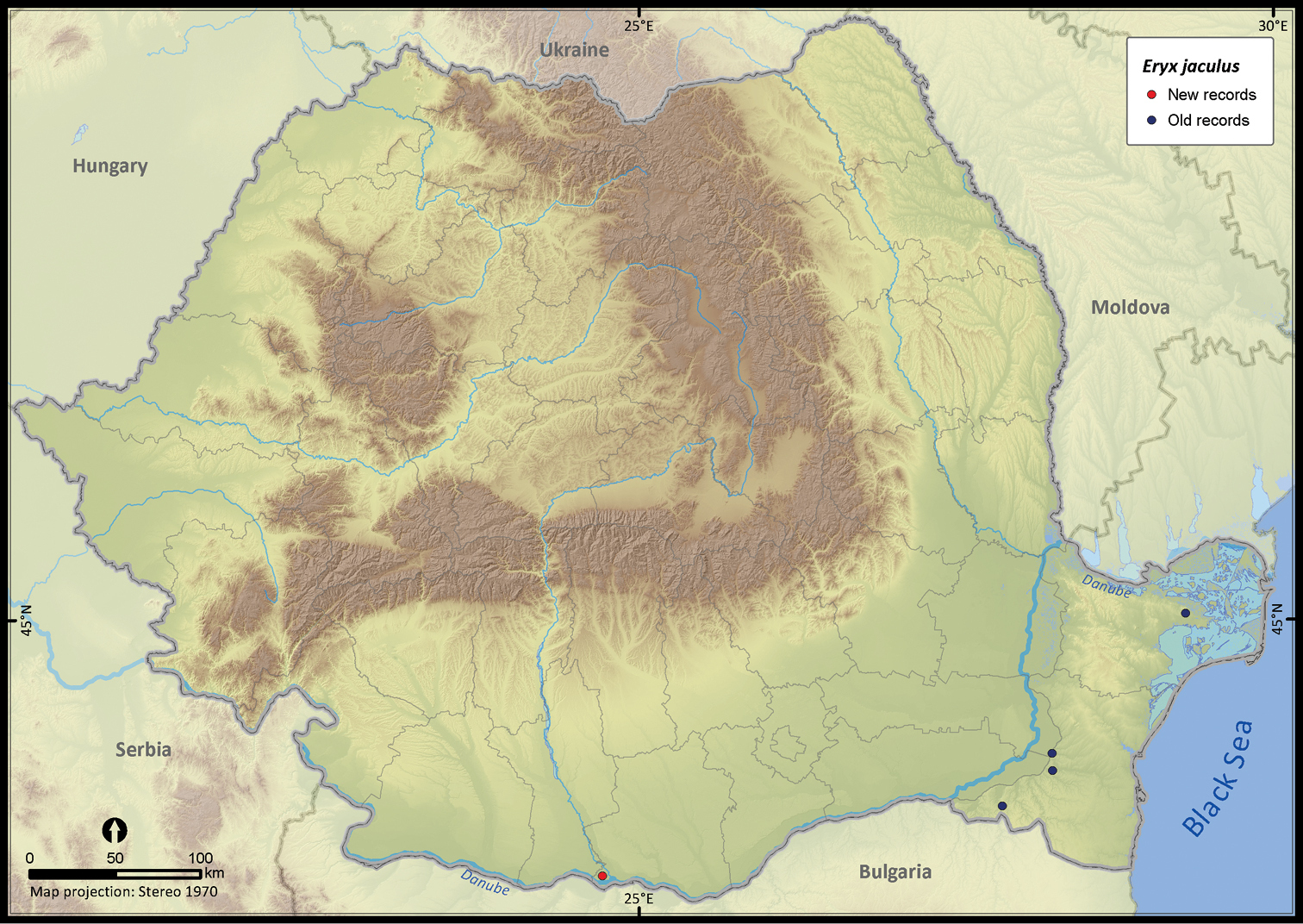
| Eryx jaculus | 5 | 4 | 1 | 5 | 10692 | 125 | 96.7 |
| Coronella austriaca | 527 | 98 | 429 | 389 | 213819 | 9581 | 39.8 |
| Zamenis longissimus | 500 | 73 | 427 | 363 | 201245 | 8868 | 50.4 |
| Elaphe sauromates | 47 | 15 | 32 | 27 | 17099 | 667 | 91.8 |
| Dolichophis caspius | 351 | 50 | 301 | 202 | 83585 | 4810 | 79.6 |
| Natrix natrix | 2180 | 163 | 2017 | 1361 | 233497 | 33202 | 15.4 |
| Natrix tessellata | 843 | 139 | 704 | 406 | 193909 | 9758 | 47.9 |
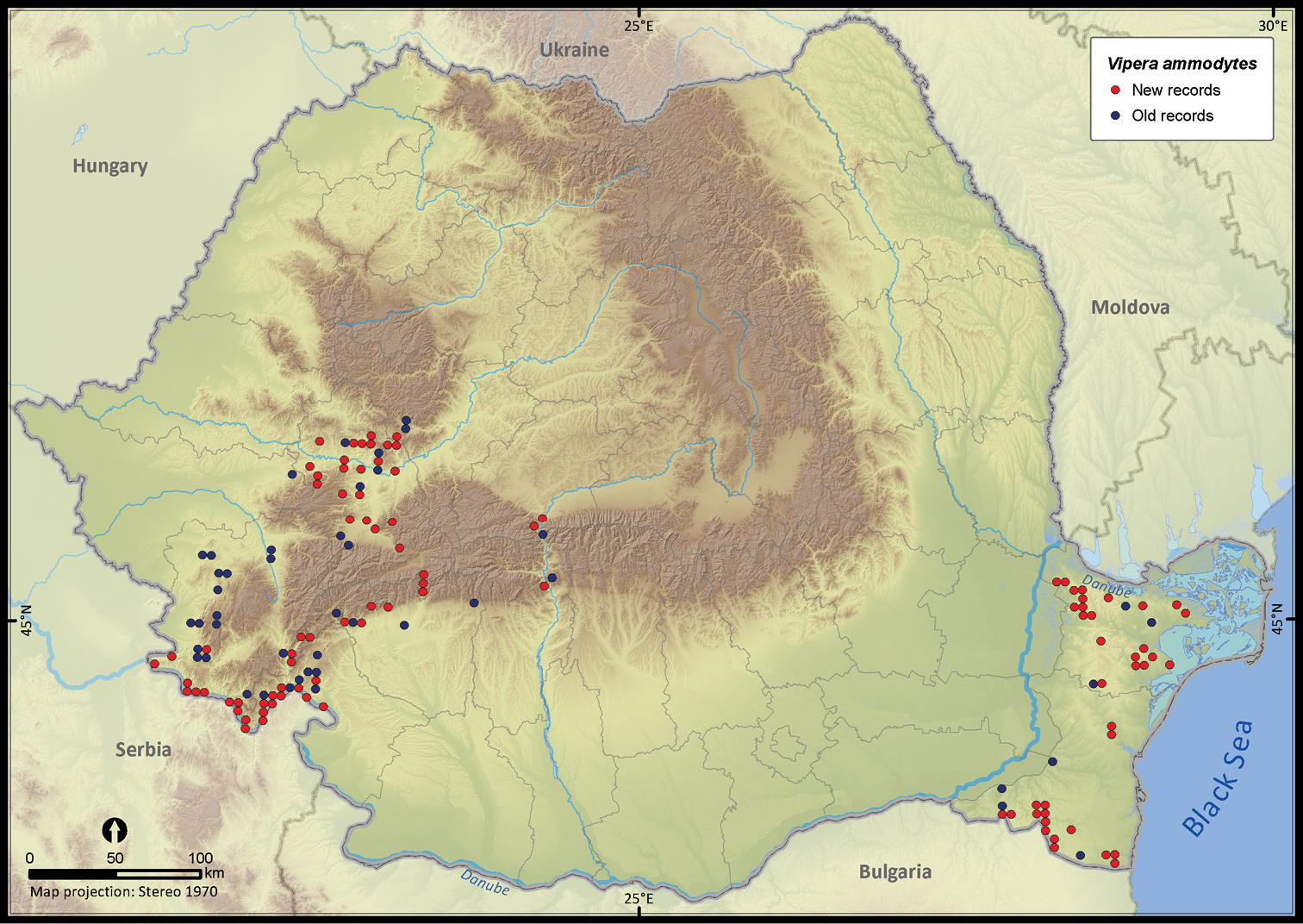
| Vipera ammodytes | 305 | 95 | 210 | 142 | 94177 | 3423 | 82.9 |
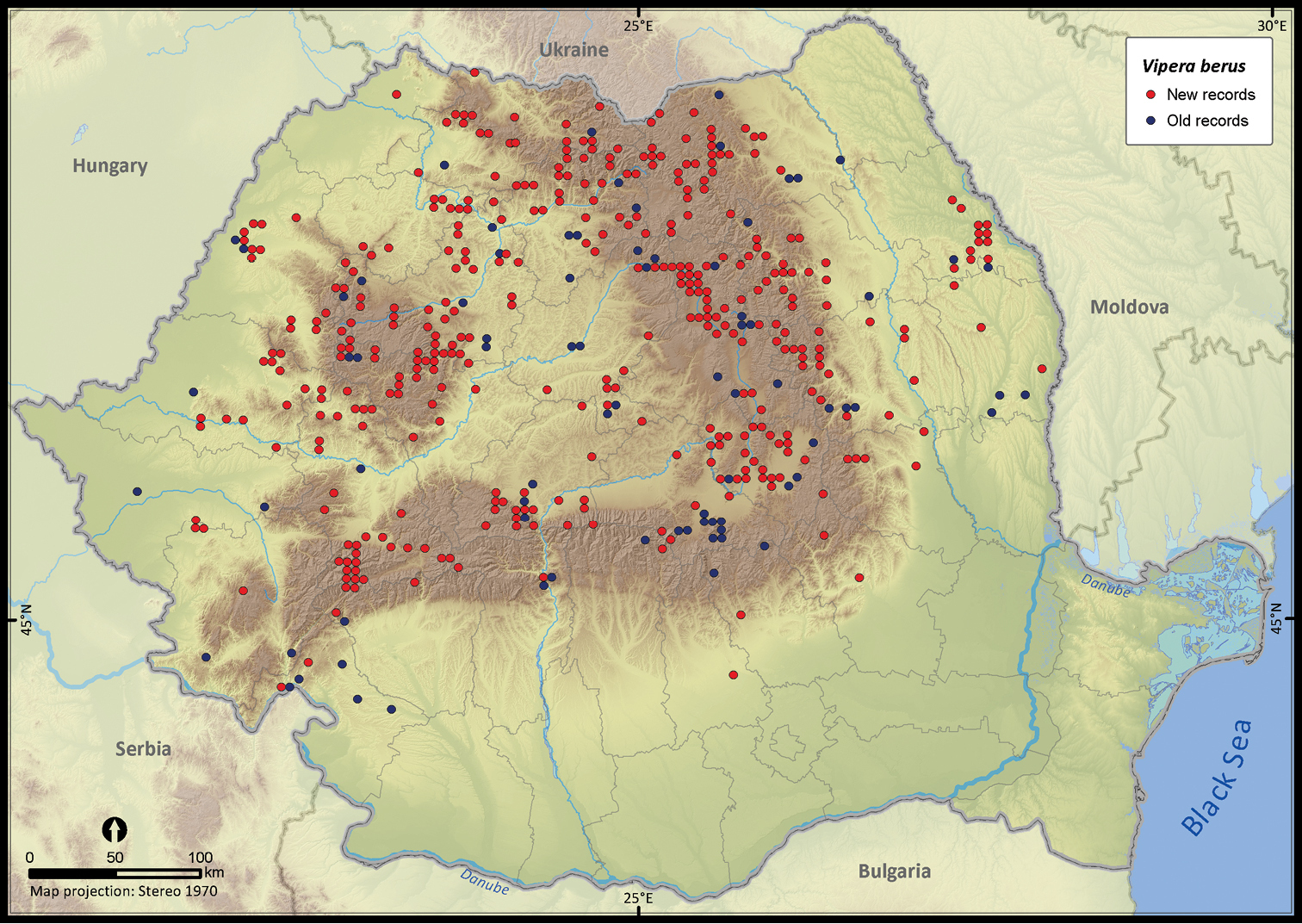
| Vipera berus | 663 | 138 | 525 | 464 | 144079 | 11515 | 47.1 |
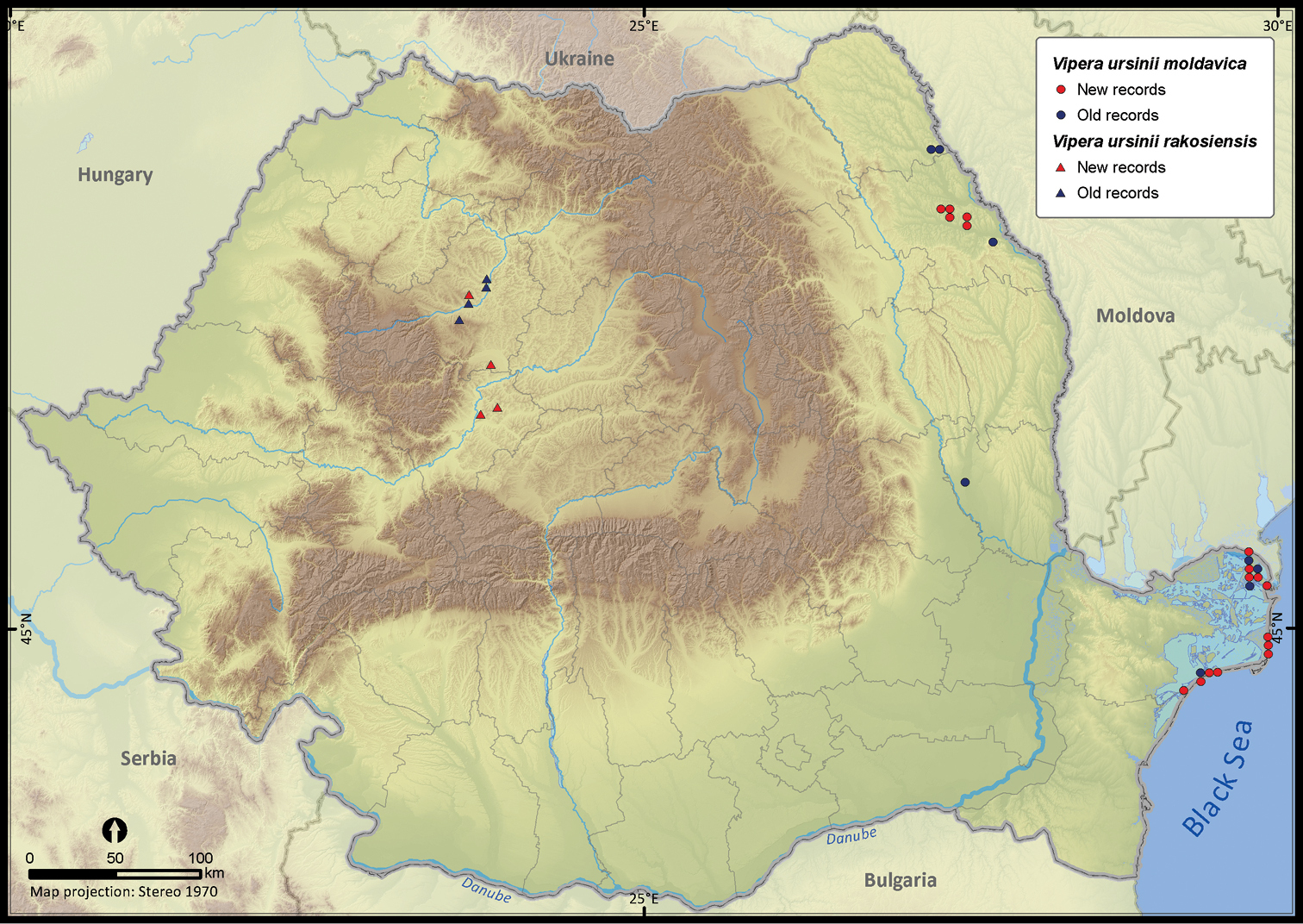
| Vipera ursinii | 78 | 35 | 43 | 33 | 67560 | 729 | 91.1 |
| Total | 18036 | 2277 | 15759 | - | - | - |
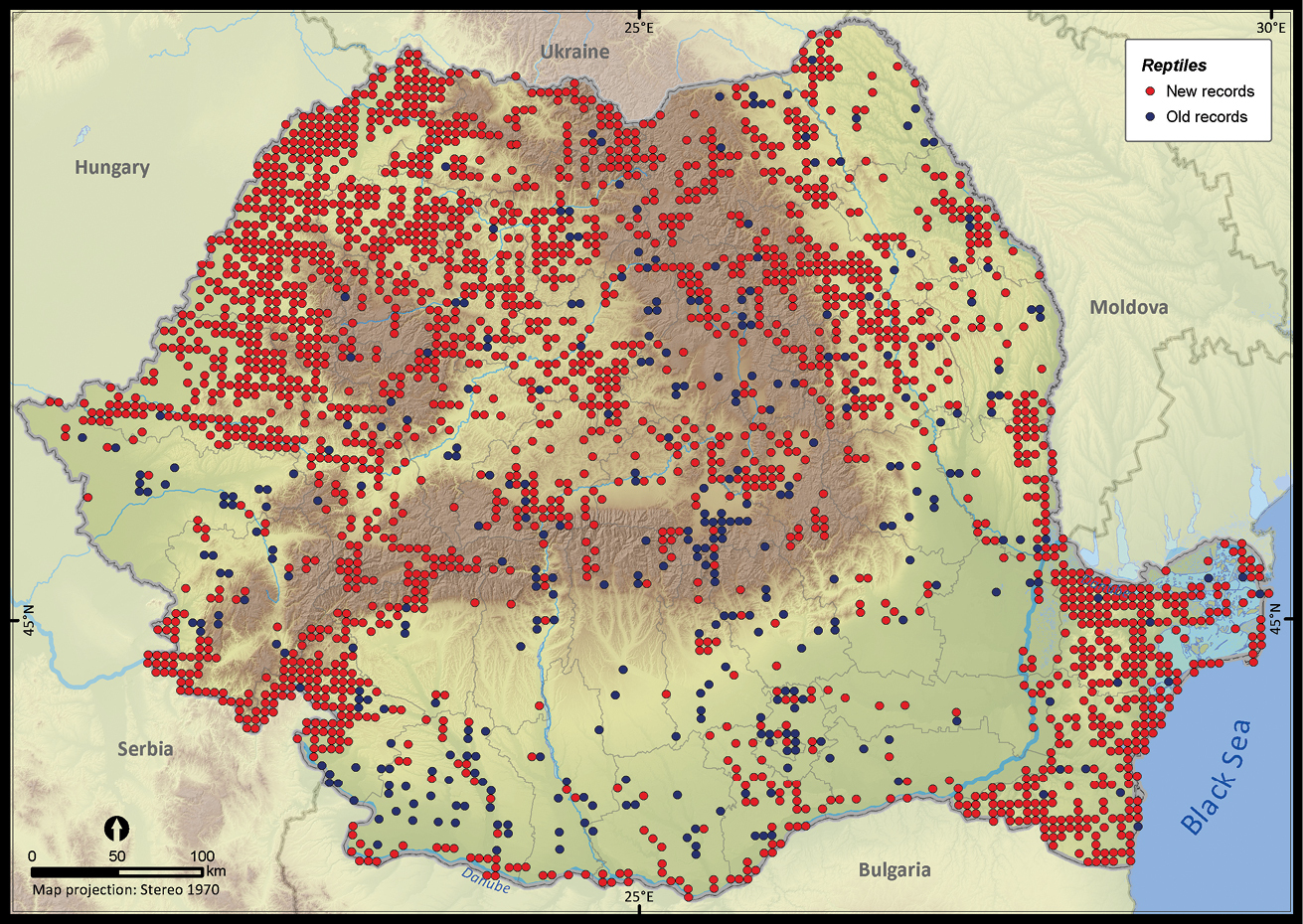
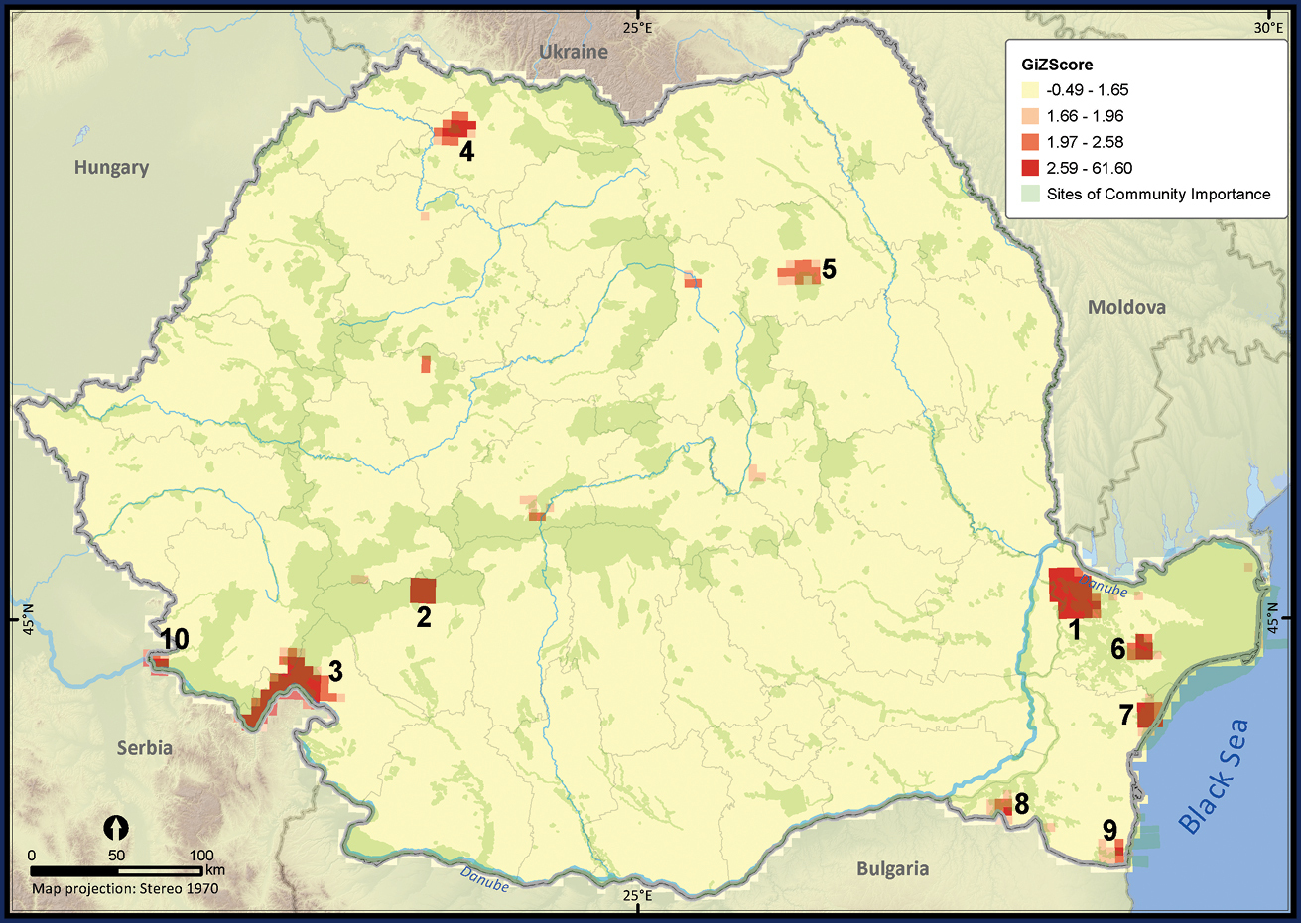
Only 27.7% of the total UTM 5 × 5 km grid cells in Romania contained the sighting of at least one reptile species (Figure 2). The number of cumulative reptile records per cell had a clustered pattern, supporting the hypothesis of an overall bias in sampling effort (Z = 19.98, p < 0.001). Getis Ord Gi* spatial statistic identified several isolated sites, mainly protected areas, as hotspots of sampling effort (Figure 3).
The presence records of reptiles per UTM 5 × 5 km grid cell in Romania. Records reported before 1990 were plotted as old records whereas those reported after 1990 were considered new records.
The hotspots of sampling efforts within Romania. The p value was < 0.05 when Z scores took values between 1.96 and 61.60, suggesting a highly clustered pattern in the number of reptile occurrences per UTM 5 × 5 km grid cell. The numbered hotspots were: 1 Măcin Mountains 2 Jiului Gorges 3 Iron Gates and Mehedinţi Plateau 4 Sweet chestnut Arboretum of Baia Mare and the surroundings of Baia Mare town 5 Goşman Mountains and the surroundings of Piatra Neamţ town 6 the Danube Delta and Babadag Forest 7 the Danube Delta and Histria Archaeological Complex 8 Canaraua Fetii – Iortmac 9 Hagieni – Cotu Văii Forest 10 Nera river mouth and Baziaş.
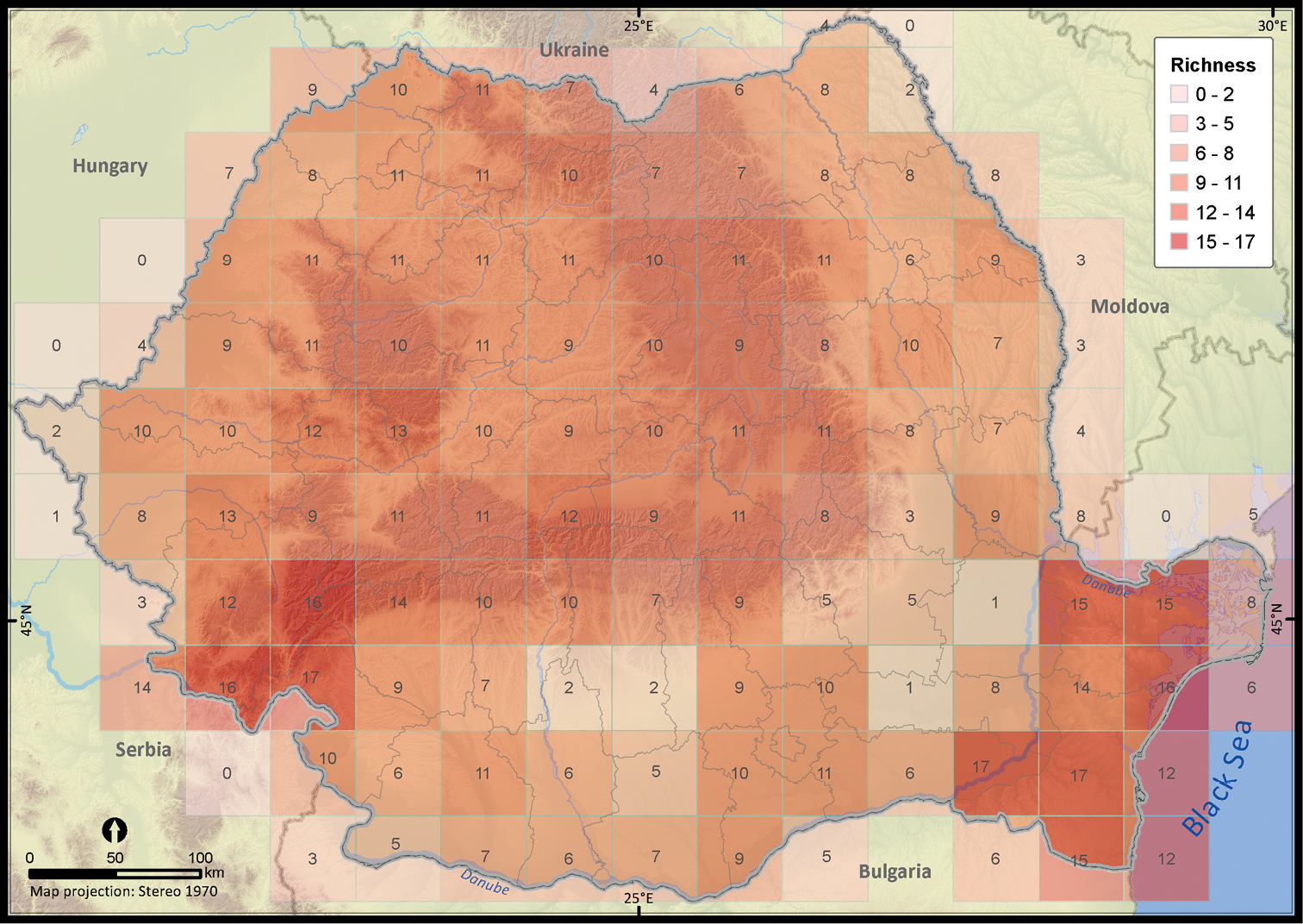
The species richness map highlighted a lower sampling effort in the vast agricultural areas in the southern part of Romania (Figure 4 and Appendix III). By contrast, the southeastern and southwestern part of Romania, with Mediterranean influences (
The reptile species richness at a 50 × 50 km grid resolution within Romania.
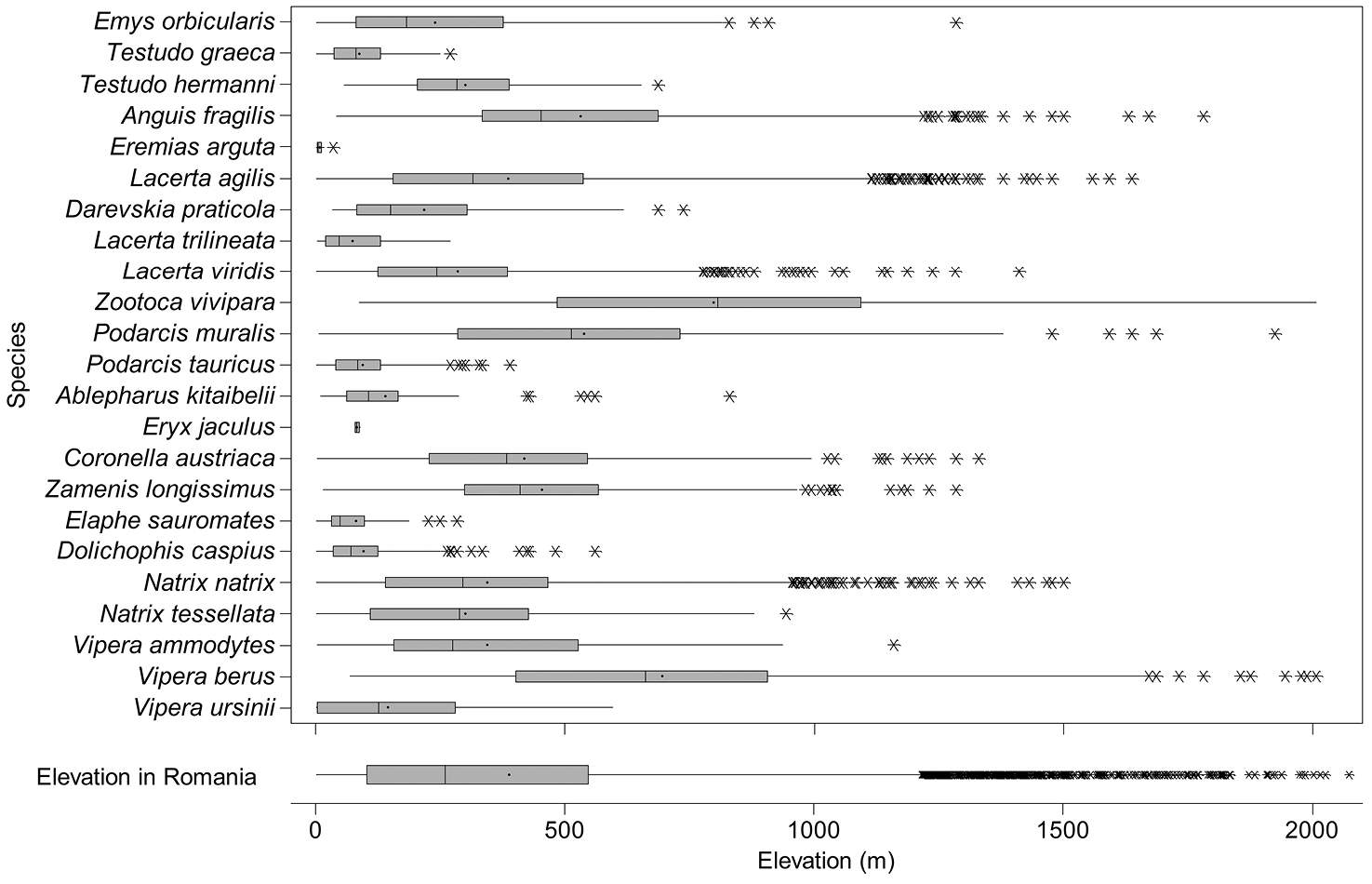
The altitudinal range of reptiles varied between 0 and 2075 m (Figure 5), with only Zootoca vivipara and Vipera berus occurring above 2000 m, while Eremias arguta (occurring at maximum 34 m) and Eryx jaculus (occurring at maximum 88 m) are clearly restricted to lowlands. The updated distribution maps of reptile species in Romania are presented in Figures 6–28.
The box and whisker plot of altitudinal distribution of reptile species in Romania. The boxes represent 25th–75th percentiles, upper and lower whiskers extends minimum and maximum data point within 1.5 box heights from the bottom and from the top of the boxes. Asterisks indicate outliers, lines and dots inside the boxes denote medians and means, respectively.
Emys orbicularis.
Testudo graeca.
Testudo hermanni.
Anguis fragilis.
Eremias arguta.
Lacerta agilis.
Darevskia praticola.
Lacerta trilineata.
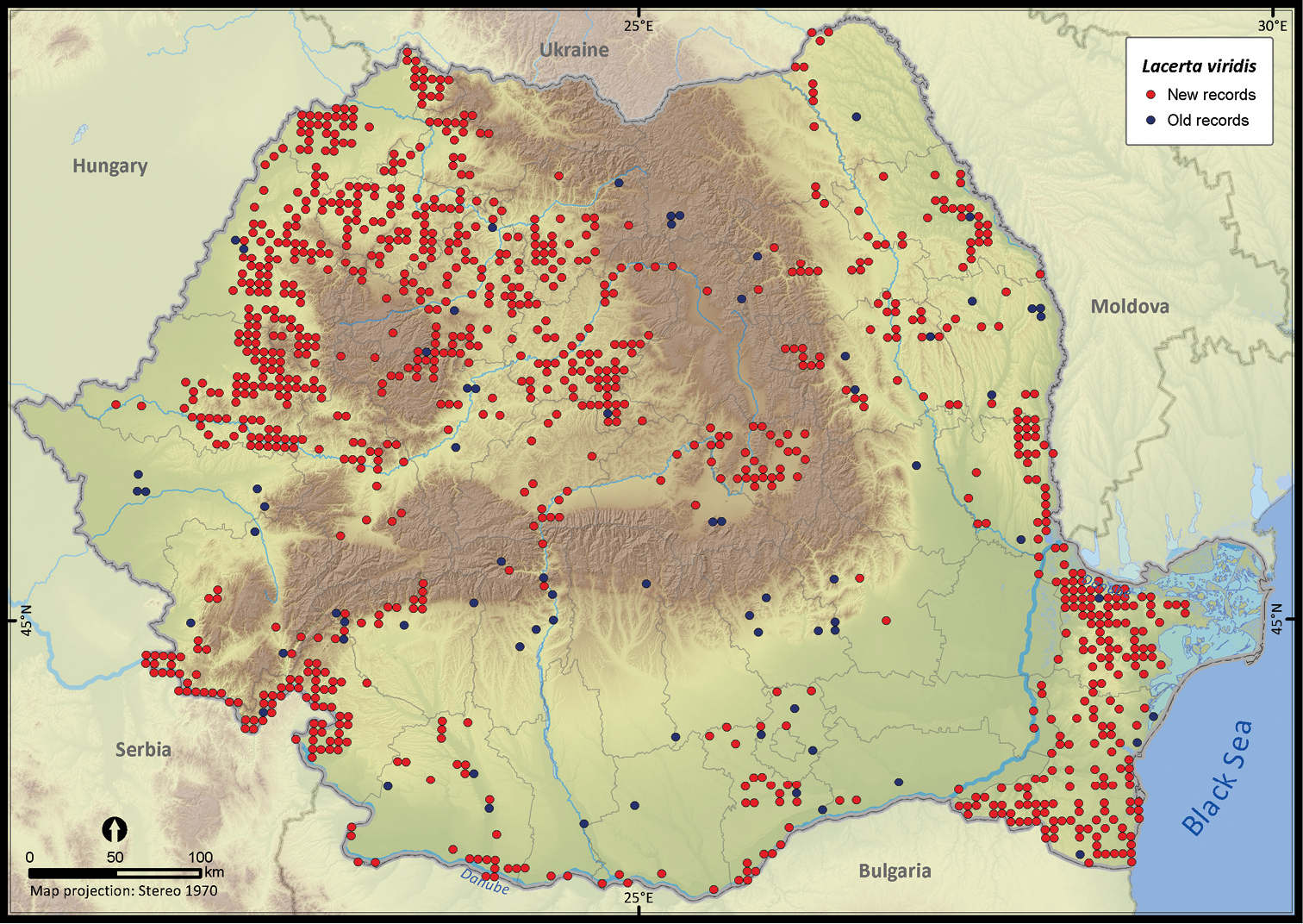
Lacerta viridis.
Zootoca vivipara.
Podarcis muralis.
Podarcis tauricus.
Ablepharus kitaibelii.
Eryx jaculus.
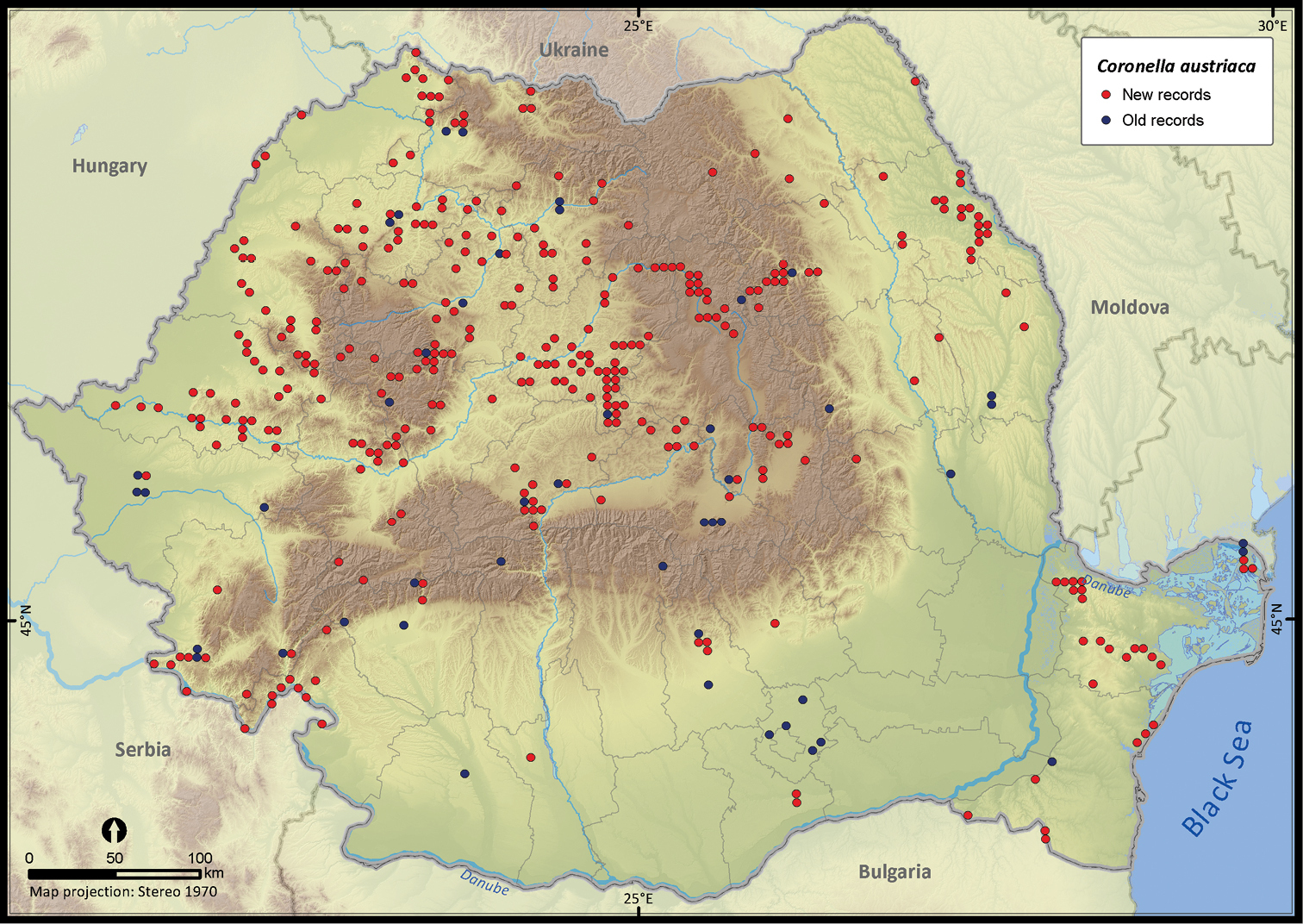
Coronella austriaca.
Zamenis longissimus.
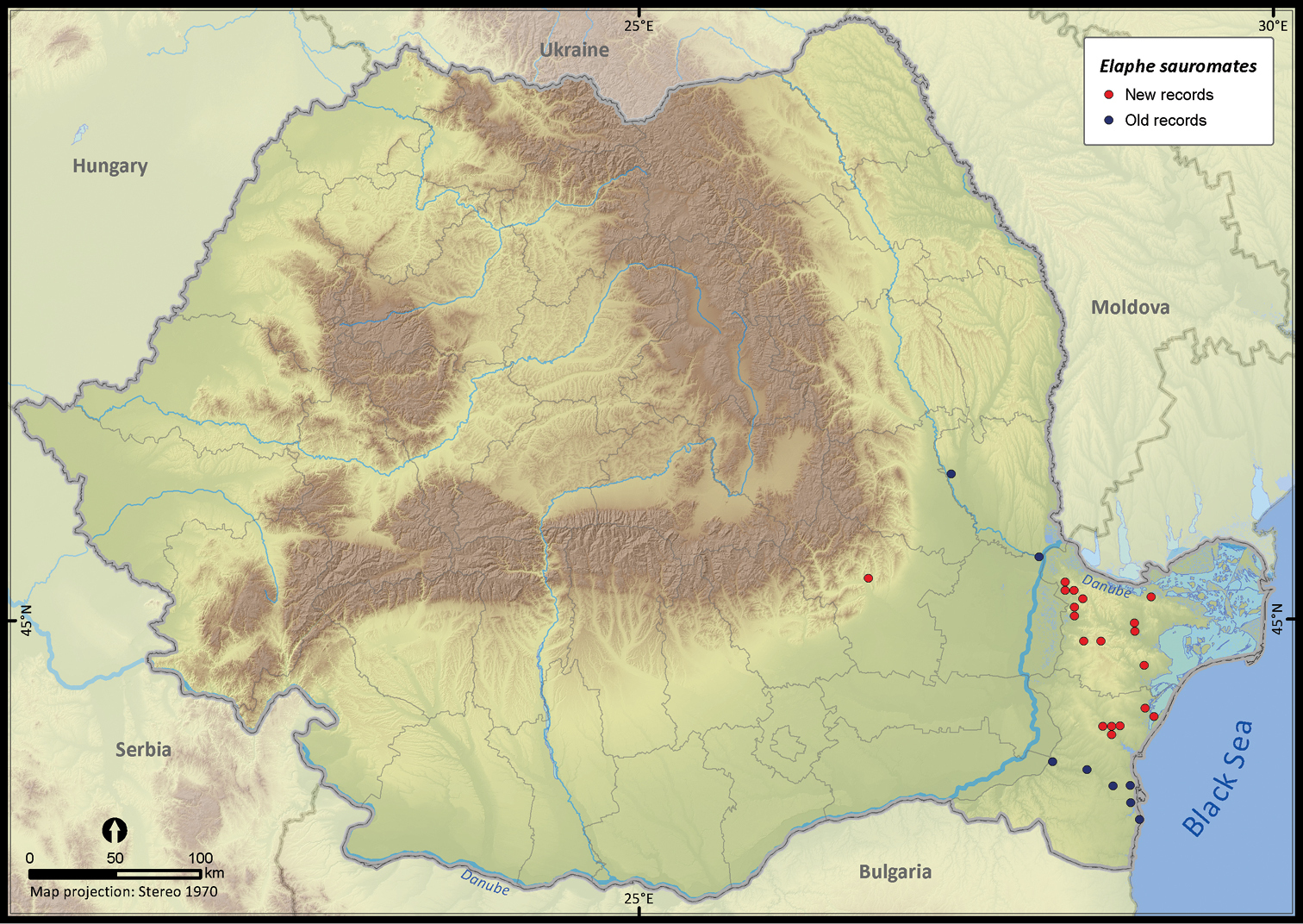
Elaphe sauromates.
Dolichophis caspius.
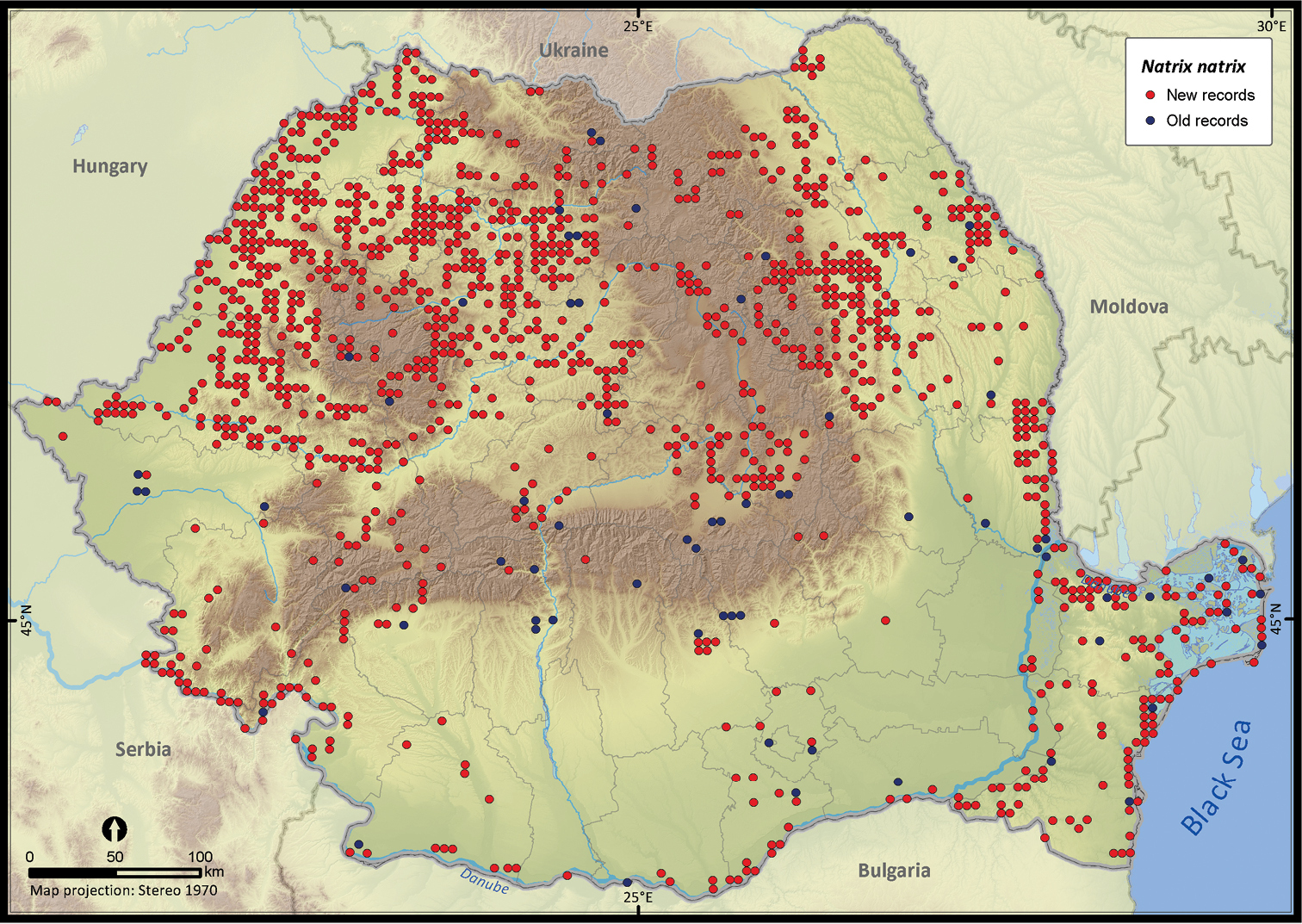
Natrix natrix.
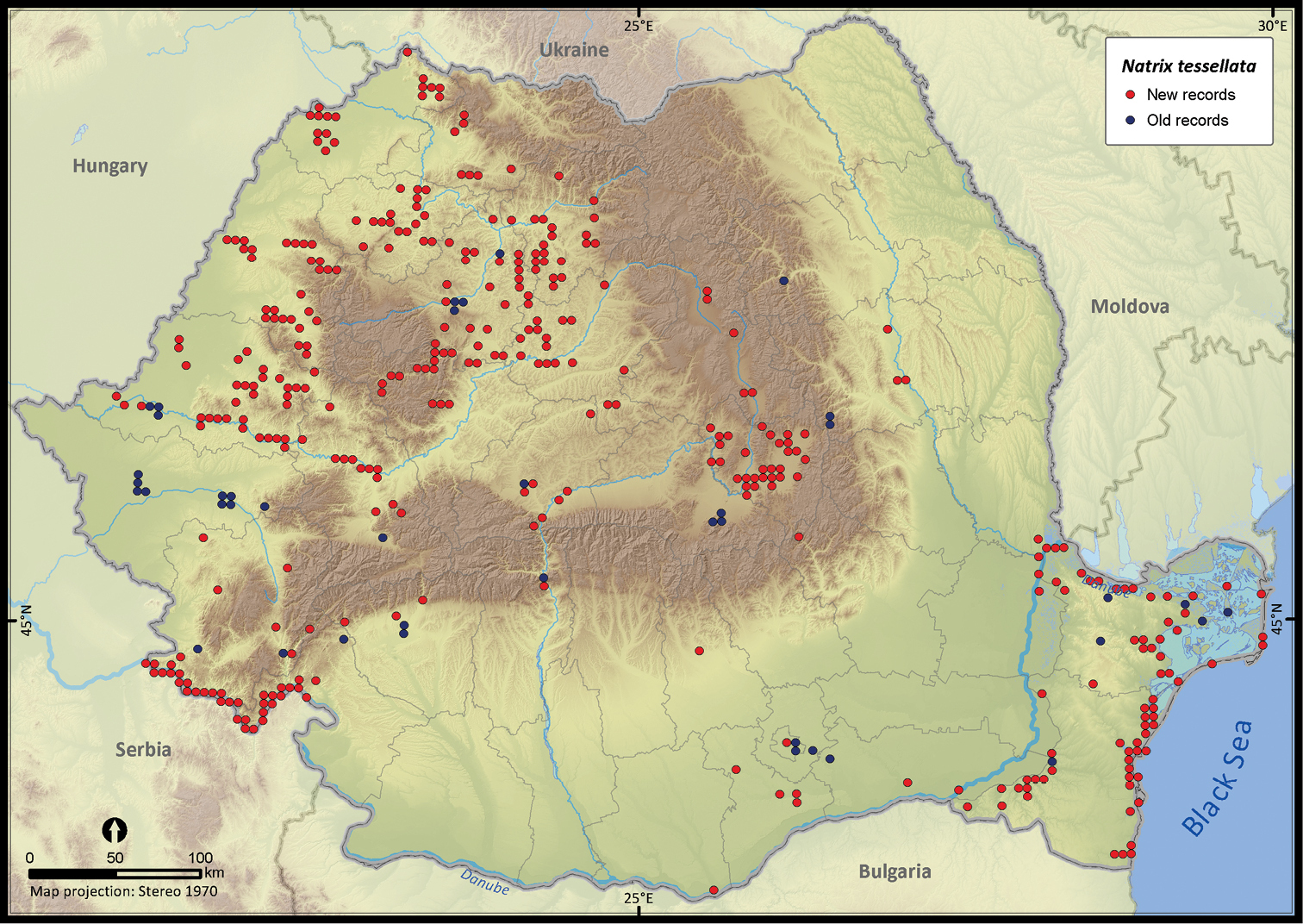
Natrix tessellata.
Vipera ammodytes.
Vipera berus.
Vipera ursinii moldavica and Vipera ursinii rakosiensis.
We here present the first comprehensive distribution database of the reptile species occurring in Romania. We fulfilled the major requirements of data quality by (1) filtering the large amount of data for doubtful and erroneous records, (2) aggregating the known localities to a fine resolution of 25 km2, and (3) assessing the bias in sampling effort and thus providing useful information for further analyses.
We addressed a series of issues related to the quality and relevance of occurrence records. The most conspicuous issue was the overall biased density of records, which might appear due to differences in species detectability. For instance, our database showed that the snake species with low detectability (
Another issue of concern was misidentification (e.g.,
Although individuals of Testudo hermanni were reported from south-east Romania (
The analysis of sampling effort revealed a significantly higher number of sightings per grid cell within certain areas, mostly protected areas, such as the Jiului Gorges National Park, the Iron Gates Natural Park or the Măcin Mountains National Park. That was a common pattern previously reported from other countries and for different taxa (e.g.,
The number of reptile species observed in Romania might increase in the near future with two species, namely Pseudopus apodus (
Several species were widespread across the country (e.g., Emys orbicularis, Lacerta viridis, Lacerta agilis, Natrix natrix, Natrix tessellata, Zamenis longissimus, Vipera berus), while others occurred only in the south of Romania (e.g., Testudo hermanni, Testudo graeca, Eremias arguta, Lacerta trilineata, Eryx jaculus, Elaphe sauromates, Dolichophis caspius). Lacerta viridis had the largest number of sightings mainly because it is a highly detectable species. Its AOO was surpassed only by Lacerta agilis and Natrix natrix, two other highly detectable species. Eryx jaculus is the rarest reptile in the country (see Table 1) with a single new report after 1990, based on a road-kill specimen (
The different biogeographic regions overlapping in Romania will face different types and levels of these changes (
We are grateful to the following persons for sharing their distribution data with us: Dr Arntzen Jan, Dr Bănărescu Petru (†), Dr Bereş Iosif, Buhaciuc Elena, Dr Gâldean Nicolae, Dr Hartel Tibor, Dr Kyek Martin, Dr Pârvulescu Lucian, Dr Oţel Vasile, Dr Skolka Marius, Sós Tibor, Talbot Neil, and Tallowin Oliver. We thank three anonymous reviewers for their constructive comments on this paper. This work was supported by two grants of the Romanian National Authority for Scientific Research, CNCS-UEFISCDI, project number PN-II-RU-TE-2011-3-0183 (principal investigator Laurenţiu Rozylowicz), and CNCS-UEFISCDI, project number PN-II-ID-PCE-2011-3-0173 (principal investigator Dan Cogălniceanu).
The publications used to compile the distribution of reptile species native to Romania. (doi: 10.3897/zookeys.341.5502.app1) File format: Microsoft Word file (doc).
The reptiles occurrence records within Romania per time intervals (1961 represented the publishing year of the last country wide assessment). (doi: 10.3897/zookeys.341.5502.app2) File format:Microsoft Word file (doc).
The reptile species richness at a 10 × 10 km grid resolution within Romania (SCI = Natura 2000 Sites of Community Importance). (doi: 10.3897/zookeys.341.5502.app3) File format: Microsoft Word file (doc).