(C) 2013 Ricardo González-Muñoz. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: González-Muñoz R, Simões N, Tello-Musi JL, Rodríguez E (2013) Sea anemones (Cnidaria, Anthozoa, Actiniaria) from coral reefs in the southern Gulf of Mexico. ZooKeys 341: 77–106. doi: 10.3897/zookeys.341.5816
Seven sea anemone species from coral reefs in the southern Gulf of Mexico are taxonomically diagnosed and images from living specimens including external and internal features, and cnidae are provided. Furthermore, the known distribution ranges from another 10 species are extended. No species records of sea anemones have been previously published in the primary scientific literature for coral reefs in the southern Gulf of Mexico and thus, this study represents the first inventory for the local actiniarian fauna.
Anthozoa, Veracruz Reef System, Cayo Arenas, Alacranes Reef, Banco de Campeche, Yucatán
Sea anemones (order Actiniaria) are among the benthic and sessile invertebrates inhabiting the southern Gulf of Mexico (SGM) coral reefs. Nevertheless, sea anemones are typically overlooked in assessments of coral reefs biodiversity due to the poor taxonomic knowledge available on local species. Although some studies provide records of sea anemone species from some coral reefs in the SGM (
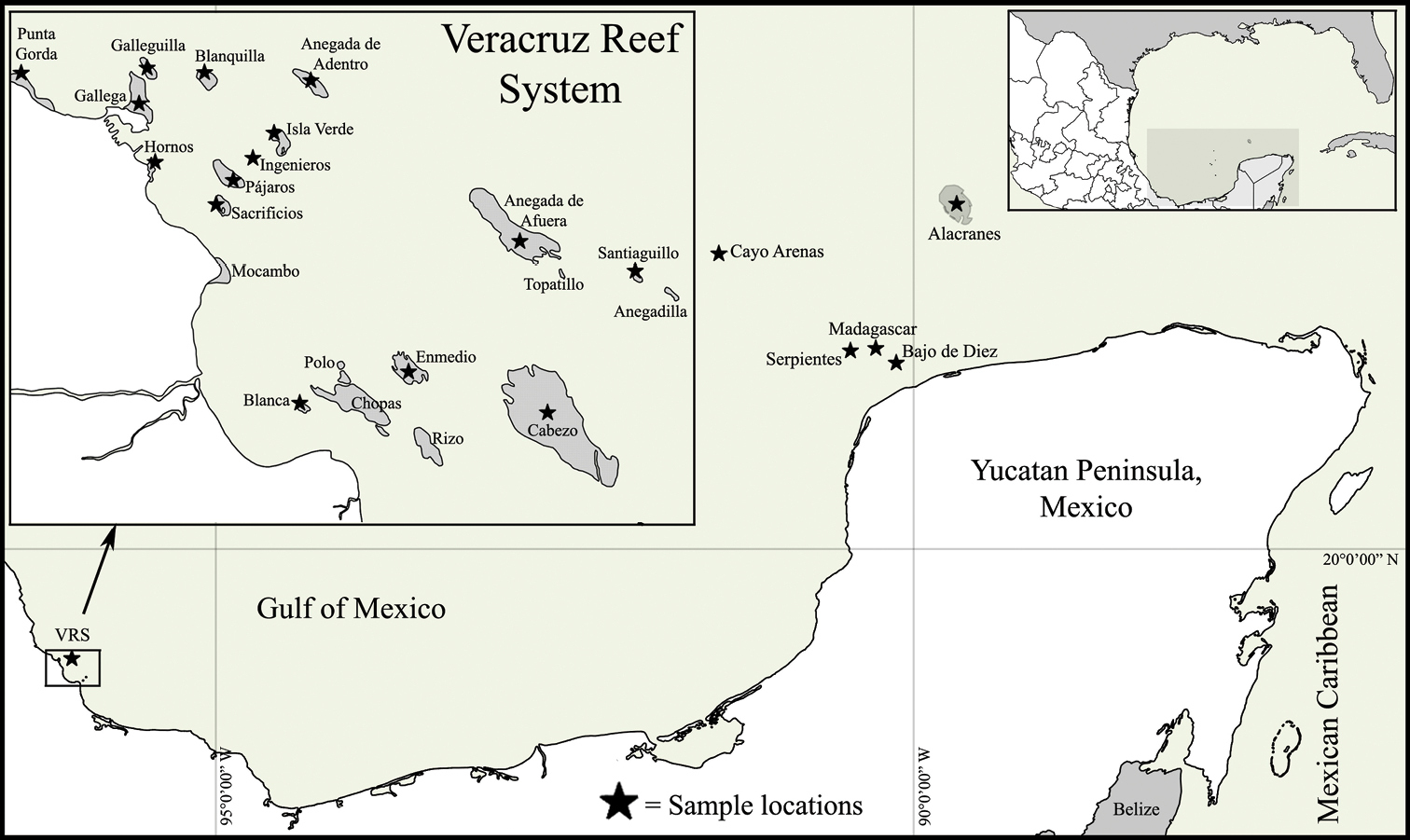
Map of the Southern Gulf of Mexico, indicating the localities sampled in this study.
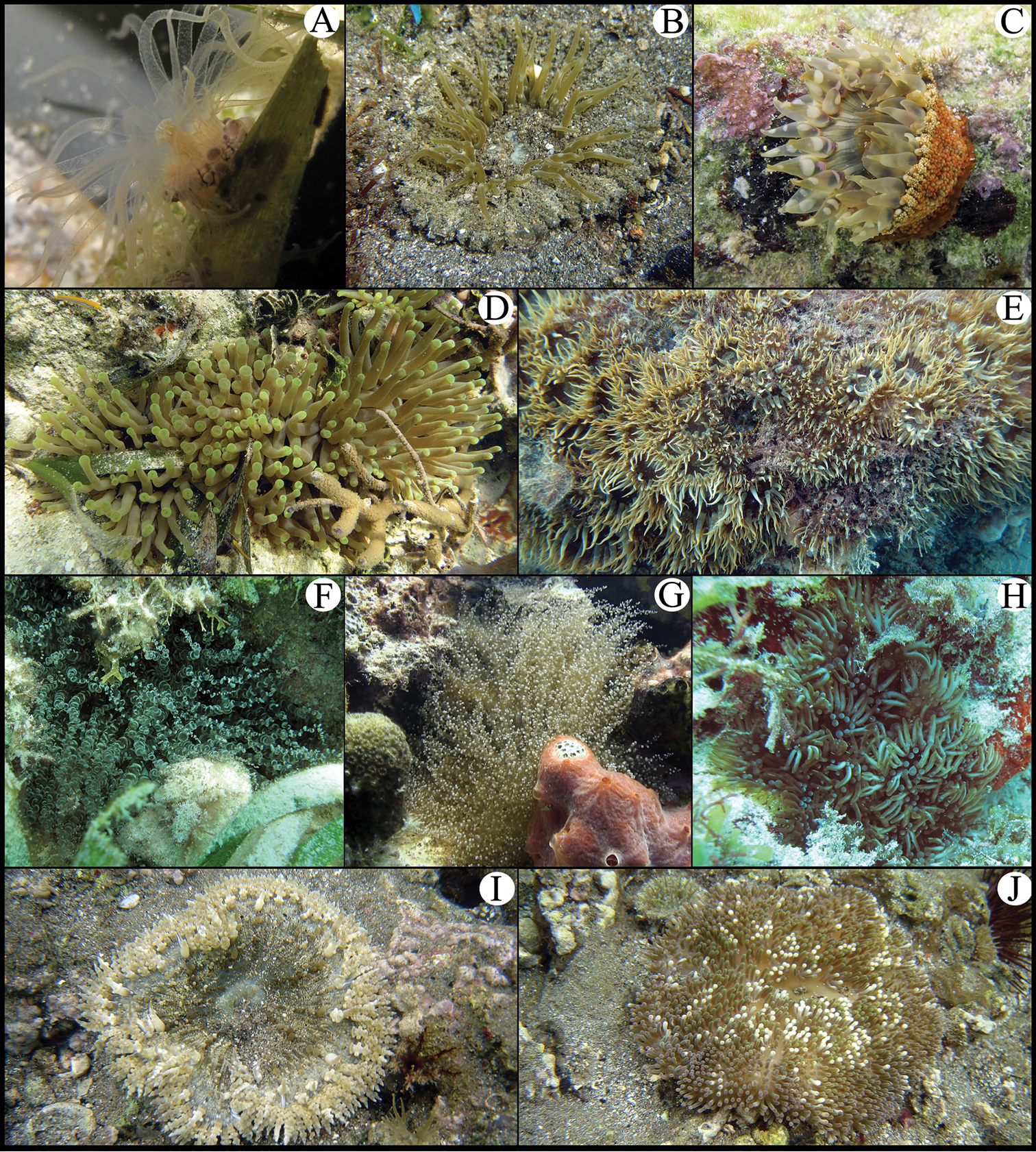
A Bunodeopsis antilliensis B Actinostella flosculifera C Bunodosoma granuliferum D Condylactis gigantea E Aiptasia pallida F Bartholomea annulata G Ragactis lucida H Lebrunia danae I Phymanthus crucifer J Stichodactyla helianthus.
Distribution of sea anemones found on the coral reefs of SGM in the present study. The symbol “x” indicates localities of previous but not published records, “*” indicates new records for the locality found in the present study, and “†” indicates new records for Mexico.
| Veracruz Reef System | Campeche Bank Reefs | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Punta Gorda | Galleguilla | Gallega | Blanquilla | Anegada de Adentro | Hornos | Isla Verde | Pájaros | Isla Sacrificios | Ingenieros | Anegada de Afuera | Santiaguillo | Blanca | Isla de Enmedio | Cabezo | Bajo de Diez | Madagascar | Serpientes | Alacranes | Cayo Arenas |
| Bunodeopsis antilliensis Duerden, 1897 | * | * | ||||||||||||||||||
| Actinostella flosculifera (Le Sueur, 1817) | * | x | * | * | x | * | * | * | * | * | * | |||||||||
| Anemonia sargassensis Hargitt, 1908 | * | * | * | x | * | * | * | |||||||||||||
| Anthopleura pallida Duchassaing & Michelotti, 1864 † | * | |||||||||||||||||||
| Bunodosoma cavernatum (Bosc, 1802) † | * | * | * | * | * | * | * | |||||||||||||
| Bunodosoma granuliferum (Le Sueur, 1817) | * | * | ||||||||||||||||||
| Condylactis gigantea (Weinland, 1860) | * | * | x | * | ||||||||||||||||
| Isoaulactinia stelloides (McMurrich, 1889) † | * | * | * | * | ||||||||||||||||
| Aiptasia pallida (Agassiz in |
* | x | * | * | * | * | * | * | * | * | ||||||||||
| Bartholomea annulata (Le Sueur, 1817) | * | * | * | * | * | * | * | * | * | * | * | * | * | |||||||
| Ragactis lucida (Duchassaing & Michelotti, 1860) | * | * | * | * | ||||||||||||||||
| Lebrunia coralligens (Wilson, 1890) | x | * | * | x | * | * | * | * | * | * | * | |||||||||
| Lebrunia danae (Duchassaing & Michelotti, 1860) | * | |||||||||||||||||||
| Actinoporus elegans Duchassaing, 1850 † | * | * | * | * | * | |||||||||||||||
| Calliactis tricolor (Le Sueur, 1817) † | * | * | ||||||||||||||||||
| Phymanthus crucifer (Le Sueur, 1817) | * | x | * | * | * | x | * | * | * | * | * | * | * | * | ||||||
| Stichodactyla helianthus (Ellis, 1768) | * | x | * | * | * | x | * | * | * | * | x | |||||||||
Observations and collections of specimens were done at 20 coral reef localities of the SGM during 2009–2011 (Figure 1). Habitats sampled include sandy patches, seagrass meadows, rocky pavement, coral rubble, and coral patches in several zones of coral reefs, and depth and habitat characteristics were recorded. Specimens were collected by hand, either by snorkeling or SCUBA diving, using a small shovel, and hammer and chisel. Collected specimens were transferred to the laboratory and maintained in an aquarium to photograph their color in life. Specimens were relaxed using 5% MgSO4 seawater solution and subsequently fixed in 10% formalin in seawater. Measurements provided for pedal disc, column, oral disc and tentacles were obtained from living and relaxed specimens. Fragments of selected specimens were dehydrated and embedded in paraffin. Histological sections 6–10 µm thick were stained with hematoxylin-eosin (
Specimens were deposited in the Collection of the Gulf of Mexico and Mexican Caribbean Sea (Registration code: YUC–CC–254–11) of the Unidad Multidisciplinaria de Docencia e Investigación en Sisal (UMDI-Sisal) at the Universidad Nacional Autónoma de México (UNAM), and in the American Museum of Natural History (AMNH, accession number 65822). We followed the taxonomic classification and synonymies implemented in
http://species-id.net/wiki/Anemonia_sargassensis
Figure 3, Table 2Alacranes reef (22°31'35"N, 89°46'05"W; two specimens), Cayo Arenas reef (22°07'05"N, 91°24'17"W; three specimens), La Gallega reef (19°13'20"N, 96°07'39"W; two specimens), Ingenieros reef (19°08'41"N, 96°05'22"W; two specimens).
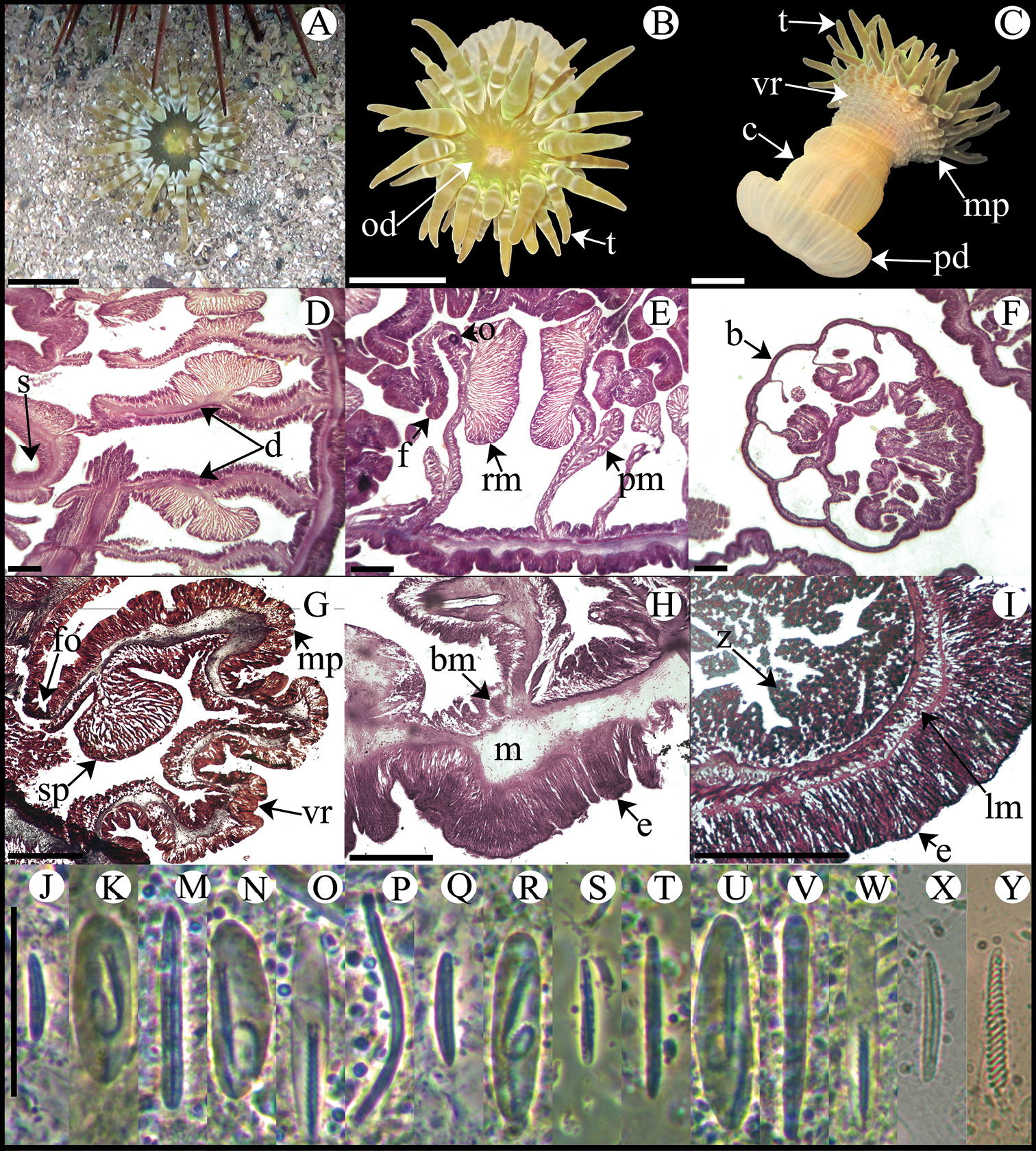
Fully expanded oral disc and tentacles 9–50 mm in diameter. Oral disc smooth, 4–22 mm in diameter, wider than column, dark-orange, brownish, greenish or dark-red, with white or yellowish endocoelic radial stripes tapering from tentacle bases (Figure 3A, B); mouth bright orange or pink (Figure 3B). Tentacles hexamerously arranged in 4–5 cycles (48–76 in number), moderately long (to 6–19 mm length), smooth, slender, tapering distally, inner ones longer than outer ones, contractile, dark-orange to reddish, sometimes with whitish or yellowish tips and pink or purple flashes (Figure 3A–C). Fossa well marked (Figure 3G). Poorly marked endocoelic marginal projections, 17–35, forming acrorhagi (Figure 3G), with holotrichs and basitrichs. Column cylindrical, short, smooth, 5–11 mm in diameter and 5–12 mm in height, dark-orange to dark-red. Pedal disc well-developed, 6–16 mm in diameter, wider than column (Figure 3C), bright-orange or pink. Mesenteries irregularly arranged in four cycles: first and second cycles perfect, others imperfect; more mesenteries proximally than distally (82–89 and 44–48 pairs respectively in specimens examined). Directives absent, 5–6 siphonoglyphs in specimens examined (Figure 3D, E). Gametogenic tissue not observed in specimens examined. Larvae observed in coelenteron of one specimen examined (Figure 3E). Retractor muscles diffuse to restricted; parietobasilar muscles weak with short mesogleal pennon (Figure 3F). Basilar muscles well-developed (Figure 3H). Marginal sphincter muscle endodermal, diffuse (Figure 3G). Longitudinal muscles of tentacles ectodermal (Figure 3I). Zooxanthellae present. Cnidom: basitrichs, holotrichs, microbasic b- and p-mastigophores and spirocysts (Figure 3J–U; see Table 2).
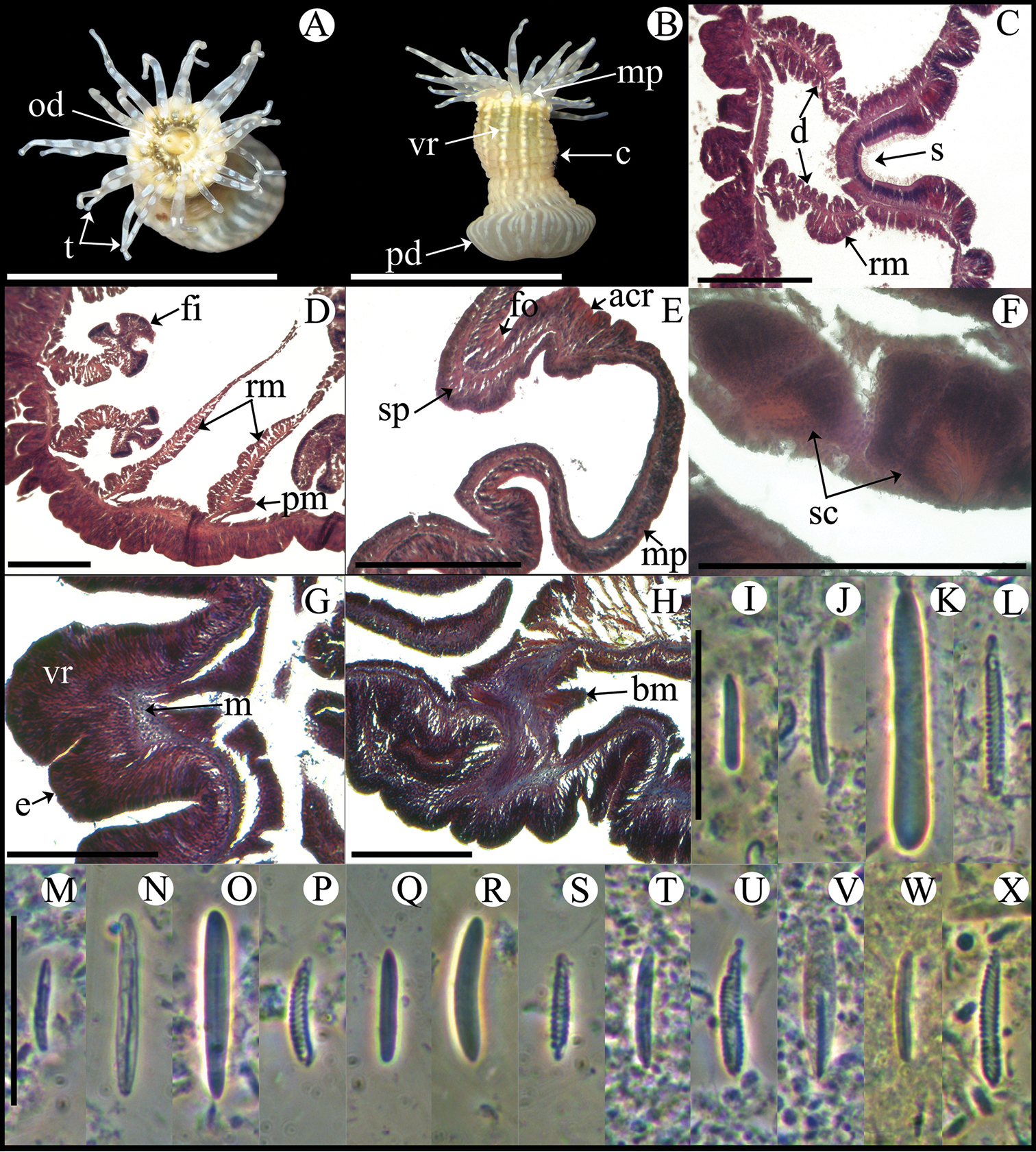
Anemonia sargassensis. A Live specimen in natural habitat B Oral view C Pedal disc view D Cross section through distal column showing mesenteries; arrows indicate siphonoglyphs E Detail of cross section through distal column showing a siphonoglyph F Detail of retractor and parietobasilar muscles G Longitudinal section through margin showing acrorhagi and marginal sphincter muscle H Longitudinal section through base showing basilar muscles I Cross section through tentacle J–U Cnidae.– acrorhagi: J small basitrich K basitrich L holotrich; actinopharynx: M small basitrich N microbasic p-mastigophore; column: O basitrich; filaments: P basitrich Q microbasic b-mastigophore R microbasic p-mastigophore; tentacle: S small basitrich T basitrich U spirocyst. Abbreviations.– acr: acrorhagi, bm: basilar muscle, e: epidermis, fo: fosse, g: gastrodermis, la: larvae, lm: longitudinal muscle, m: mesoglea, mp: marginal projection, od: oral disc, pd: pedal disc, pm: parietobasilar muscle, rm: retractor muscle, s: siphonoglyph, sp: sphincter, t: tentacle. Scale bars: A–C: 10 mm; D–I: 200 μm; J–U: 25 μm.
Size and distribution of preserved cnidae from specimens examined. “ml” and “mw” are the means (length and width respectively), “dl” and “dw” are the standard deviations (length and width respectively), all in µm. “#1” and “#2” is the number of capsules measured per each specimen examined, “p” is the proportion of animals examined with respective to the type of cnida present.
| Species | Tissue | Cnida | Capsule length (µm) | ml | dl | Capsule width (µm) | mw | dw | #1 | #2 | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anemonia sargassensis | Tentacle | Basitrich | 8.7–20.2 | 16.1 | 2.2 | 1.6–3.3 | 2.1 | 0.2 | 24 | 21 | 2/2 |
| Basitrich | 21.0–36.8 | 30.8 | 3.6 | 2.4–3.6 | 3.0 | 0.2 | 23 | 21 | 2/2 | ||
| Spirocyst | 15.1–40.0 | 25.3 | 7.0 | 2.2–3.5 | 2.9 | 0.3 | 21 | 20 | 2/2 | ||
| Actinopharynx | Basitrich | 13.9–33.5 | 24.9 | 3.9 | 2.1–4.0 | 3.2 | 0.4 | 20 | 26 | 2/2 | |
| Microbasic p-mastigophore | 16.8–24.9 | 19.7 | 2.2 | 3.3–5.6 | 4.5 | 0.6 | 6 | 9 | 2/2 | ||
| Column | Basitrich | 13.3–22.6 | 18.3 | 2.4 | 2.1–3.0 | 2.5 | 0.2 | 21 | 20 | 2/2 | |
| Acrorhagi | Basitrich | 14.6–26.4 | 20.1 | 3.0 | 2.1–3.2 | 2.4 | 0.1 | 20 | 22 | 2/2 | |
| Basitrich | 27.8–43.7 | 35.8 | 3.6 | 2.5–3.5 | 3.1 | 0.1 | 21 | 21 | 2/2 | ||
| Holotrich | 31.1–42.4 | 36.8 | 2.6 | 4.4–6.9 | 5.1 | 0.4 | 22 | 20 | 2/2 | ||
| Filament | Basitrich | 12.9–32.7 | 19.2 | 5.2 | 1.8–3.0 | 2.4 | 0.2 | 20 | 27 | 2/2 | |
| Microbasic b-mastigophore | 24.6–33.9 | 28.6 | 2.5 | 3.7–6.0 | 4.7 | 0.6 | 22 | 21 | 2/2 | ||
| Microbasic p-mastigophore | 15.0–24.5 | 20.4 | 2.3 | 3.7–5.9 | 4.8 | 0.5 | 21 | 22 | 2/2 | ||
| Anthopleura pallida | Tentacle | Basitrich | 12.6–20.6 | 16.8 | 2.0 | 1.7–2.6 | 2.1 | 0.2 | 23 | 21 | 2/2 |
| Spirocyst | 11.8–19.2 | 16.0 | 1.4 | 2.3–3.6 | 2.9 | 0.3 | 28 | 21 | 2/2 | ||
| Actinopharynx | Basitrich | 15.0–27.0 | 21.6 | 3.1 | 1.8–3.1 | 2.5 | 0.3 | 23 | 24 | 2/2 | |
| Basitrich | 10.1–18.0 | 14.0 | 1.7 | 1.5–2.4 | 1.9 | 0.1 | 15 | 22 | 2/2 | ||
| Spirocyst | 11.2–19.5 | 16.2 | 1.9 | 2.3–3.7 | 2.8 | 0.3 | 12 | 21 | 2/2 | ||
| Microbasic b-mastigophore | 20.9–28.3 | 24.6 | 2.7 | 2.8–4.4 | 3.3 | 0.5 | 5 | 1 | 2/2 | ||
| Microbasic p-mastigophore | 13.4–23.5 | 19.4 | 3.9 | 3.8–5.0 | 4.3 | 0.5 | 1 | 4 | 2/2 | ||
| Column | Basitrich | 14.7–19.3 | 17.2 | 1.2 | 2.8–4.2 | 3.2 | 0.3 | 22 | 22 | 2/2 | |
| Basitrich | 8.7–17.1 | 14.2 | 1.6 | 1.4–2.4 | 2.0 | 0.1 | 26 | 21 | 2/2 | ||
| Spirocyst | 10.3–14.7 | 12.6 | 1.5 | 2.3–2.6 | 2.5 | 0.1 | 5 | 1 | 2/2 | ||
| Acrorhagi | Basitrich | 12.2–25.3 | 16.6 | 2.7 | 1.7–2.6 | 2.1 | 0.2 | 25 | 23 | 2/2 | |
| Basitrich | 7.6–14.9 | 11.8 | 1.4 | 1.4–2.1 | 1.7 | 0.1 | 23 | 0 | 1/2 | ||
| Spirocyst | 11.3–23.9 | 17.8 | 2.6 | 1.9–3.5 | 2.6 | 0.4 | 22 | 20 | 2/2 | ||
| Holotrich | 17.9–39.3 | 31.8 | 3.8 | 2.4–4.7 | 3.6 | 0.5 | 29 | 25 | 2/2 | ||
| Holotrich | 21.1–36.5 | 27.9 | 4.2 | 2.3–3.3 | 2.8 | 0.2 | 24 | 0 | 1/2 | ||
| Microbasic p-mastigophore | 16.5–17.5 | 17.0 | 0.7 | 2.8–4.1 | 3.4 | 0.8 | 2 | 0 | 1/2 | ||
| Filament | Basitrich | 13.1–33.7 | 17.3 | 3.9 | 1.9–3.0 | 2.3 | 0.3 | 15 | 7 | 2/2 | |
| Basitrich | 9.2–18.5 | 14.1 | 2.1 | 1.2–2.3 | 2.0 | 0.2 | 3 | 20 | 2/2 | ||
| Spirocyst | 10.9–19.2 | 15.5 | 2.1 | 1.9–3.5 | 2.6 | 0.3 | 20 | 4 | 2/2 | ||
| Microbasic b-mastigophore | 15.5–28.0 | 24.9 | 2.4 | 3.1–4.6 | 3.7 | 0.3 | 7 | 20 | 2/2 | ||
| Holotrich | 29.7–33.5 | 31.6 | 2.6 | 2.8–3.0 | 2.9 | 0.1 | 2 | 0 | 1/2 | ||
| Microbasic p-mastigophore | 17.2–23.4 | 20.5 | 2.2 | 4.2–4.8 | 4.5 | 0.2 | 1 | 4 | 2/2 | ||
| Bunodosoma cavernatum | Tentacle | Basitrich | 10.7–29.5 | 21.0 | 4.7 | 1.6–3.4 | 2.1 | 0.4 | 21 | 22 | 2/2 |
| Spirocyst | 13.2–22.6 | 16.8 | 2.3 | 1.7–3.7 | 2.5 | 0.6 | 20 | 23 | 2/2 | ||
| Actinopharynx | Basitrich | 21.0–27.2 | 24.7 | 1.2 | 2.8–3.5 | 3.2 | 0.1 | 22 | 20 | 2/2 | |
| Microbasic p-mastigophore | 16.3–21.1 | 18.5 | 1.5 | 3.7–6.0 | 4.9 | 0.5 | 4 | 22 | 2/2 | ||
| Column | Basitrich | 14.7–19.8 | 16.8 | 1.2 | 1.8–2.5 | 2.2 | 0.1 | 20 | 20 | 2/2 | |
| Basitrich | 20.8–28.4 | 24.8 | 1.6 | 2.5–3.9 | 3.0 | 0.2 | 31 | 21 | 2/2 | ||
| Acrorhagi | Basitrich | 17.2–28.8 | 22.8 | 3.5 | 2.1–3.5 | 2.7 | 0.4 | 21 | 20 | 2/2 | |
| Holotrich | 26.6–45.1 | 35.0 | 3.7 | 3.1–5.8 | 4.0 | 0.5 | 22 | 20 | 2/2 | ||
| Filament | Basitrich | 11.9–28.5 | 23.9 | 4.7 | 1.6–4.0 | 3.0 | 0.5 | 6 | 21 | 2/2 | |
| Microbasic b-mastigophore | 20.5–37.4 | 28.0 | 4.3 | 4.2–8.9 | 6.2 | 1.7 | 30 | 22 | 2/2 | ||
| Microbasic p-mastigophore | 14.4–23.1 | 18.7 | 2.9 | 3.2–6.7 | 4.6 | 0.9 | 20 | 21 | 2/2 | ||
| Isoaulactinia stelloides | Tentacle | Basitrich | 14.1–23.6 | 18.5 | 2.8 | 1.9–2.8 | 2.4 | 0.2 | 21 | 21 | 2/2 |
| Macrobasic p-mastigophore | 16.0–25.6 | 22.0 | 1.6 | 5.1–9.2 | 7.3 | 0.9 | 23 | 22 | 2/2 | ||
| Spirocyst | 12.2–22.2 | 17.4 | 2.6 | 1.9–3.0 | 2.4 | 0.2 | 21 | 21 | 2/2 | ||
| Actinopharynx | Basitrich | 11.7–18.5 | 13.7 | 1.7 | 1.6–2.7 | 2.1 | 0.2 | 21 | 21 | 2/2 | |
| Basitrich | 16.6–34.1 | 26.4 | 2.9 | 2.3–3.2 | 2.9 | 0.2 | 29 | 22 | 2/2 | ||
| Macrobasic p-mastigophore | 21.1–26.9 | 24.4 | 1.4 | 6.3–8.3 | 7.6 | 0.5 | 4 | 20 | 2/2 | ||
| Microbasic p-mastigophore | 18.0–28.6 | 25.2 | 2.7 | 4.1–5.7 | 4.9 | 0.4 | 10 | 5 | 2/2 | ||
| Microbasic b-mastigophore | 15.2–33.2 | 26.9 | 5.7 | 2.8–4.0 | 3.4 | 0.3 | 1 | 6 | 2/2 | ||
| Long, curved basitrich | 18.9–32.8 | 24.9 | 4.8 | 1.6–2.2 | 1.9 | 0.2 | 6 | 6 | 2/2 | ||
| Column | Basitrich | 11.8–15.7 | 13.5 | 0.9 | 1.9–2.8 | 2.3 | 0.1 | 23 | 21 | 2/2 | |
| Macrobasic p-mastigophore | 22.2–27.7 | 24.5 | 1.2 | 5.2–7.5 | 6.3 | 0.5 | 26 | 20 | 2/2 | ||
| Long, curved basitrich | 25.1–31.3 | 28.2 | 4.3 | 2.2–2.3 | 2.3 | 0.1 | 2 | 0 | 1/2 | ||
| Marginal projection | Basitrich | 11.1–13.8 | 12.3 | 0.7 | 1.8–2.8 | 2.3 | 0.2 | 26 | 20 | 2/2 | |
| Macrobasic p-mastigophore | 20.1–25.9 | 22.7 | 1.4 | 5.2–8.5 | 6.5 | 0.8 | 32 | 20 | 2/2 | ||
| Filament | Basitrich | 10.8–15.5 | 13.4 | 1.1 | 1.6–2.2 | 1.9 | 0.1 | 24 | 20 | 2/2 | |
| Basitrich | 17.6–31.8 | 22.2 | 3.4 | 1.7–3.1 | 2.3 | 0.3 | 20 | 21 | 2/2 | ||
| Macrobasic p-mastigophore | 23.3–29.3 | 26.0 | 1.4 | 5.9–8.1 | 7.1 | 0.4 | 20 | 20 | 2/2 | ||
| Microbasic p-mastigophore | 17.6–32.3 | 25.9 | 3.3 | 3.9–6.0 | 4.7 | 0.4 | 15 | 23 | 2/2 | ||
| Microbasic b-mastigophore | 29.3–39.7 | 34.1 | 2.4 | 3.2–4.9 | 3.9 | 0.4 | 20 | 22 | 2/2 | ||
| Long, curved basitrich | 17.0–29.7 | 24.1 | 5.5 | 1.5–2.2 | 1.9 | 0.3 | 4 | 0 | 1/2 | ||
| Lebrunia coralligens | Tentacle | Basitrich | 12.3–33.5 | 26.2 | 4.8 | 1.7–2.6 | 2.2 | 0.2 | 20 | 24 | 2/2 |
| Spirocyst | 17.1–29.9 | 23.8 | 3.5 | 2.8–5.5 | 4.1 | 0.6 | 2 | 21 | 2/2 | ||
| Microbasic p-amastigophore | 11.8–14.6 | 13.2 | 1.1 | 2.5–3.1 | 2.7 | 0.2 | 4 | 0 | 1/2 | ||
| Microbasic p-amastigophore | 29.0–68.7 | 48.6 | 9.0 | 4.4–7.1 | 5.6 | 0.6 | 20 | 21 | 2/2 | ||
| Pseudotentacle | Basitrich | 8.9–26.8 | 15.4 | 4.3 | 1.7–2.8 | 2.2 | 0.2 | 22 | 23 | 2/2 | |
| Microbasic p-amastigophore | 37.2–67.8 | 51.5 | 5.7 | 10.8–15.7 | 13.0 | 1.8 | 25 | 21 | 2/2 | ||
| Microbasic p-amastigophore | 11.7–25.9 | 17.2 | 2.8 | 2.3–4.6 | 3.3 | 0.4 | 20 | 20 | 2/2 | ||
| Actinopharynx | Microbasic p-amastigophore | 10.7–21.6 | 13.7 | 2.0 | 2.3–3.7 | 2.7 | 0.3 | 20 | 23 | 2/2 | |
| Microbasic p-amastigophore | 18.8–45.1 | 34.6 | 7.9 | 3.4–6.3 | 5.1 | 0.6 | 21 | 20 | 2/2 | ||
| Column | Basitrich | 9.0–14.0 | 10.9 | 1.0 | 1.6–2.6 | 2.1 | 0.2 | 24 | 20 | 2/2 | |
| Microbasic p-amastigophore | 12.1–23.5 | 14.8 | 1.8 | 2.7–4.0 | 3.3 | 0.3 | 23 | 21 | 2/2 | ||
| Filament | Microbasic p-amastigophore | 11.2–17.3 | 13.6 | 1.2 | 2.2–3.3 | 2.7 | 0.3 | 20 | 20 | 2/2 | |
| Microbasic p-amastigophore | 29.1–46.5 | 37.1 | 4.0 | 4.4–6.2 | 5.4 | 0.4 | 20 | 10 | 2/2 | ||
| Actinoporus elegans | Tentacle | Basitrich | 15.8–20.8 | 17.4 | 2.2 | 2.4–3.0 | 2.7 | 0.2 | 4 | 0 | 1/1 |
| Spirocyst | 26.6–37.6 | 32.8 | 3.2 | 2.2–2.9 | 2.6 | 0.1 | 23 | 0 | 1/1 | ||
| Actinopharynx | Basitrich | 25.6–32.5 | 27.9 | 1.7 | 3.5–4.8 | 4.2 | 0.3 | 21 | 0 | 1/1 | |
| Microbasic p-mastigophore | 29.8–34.9 | 31.9 | 1.4 | 6.6–9.0 | 7.8 | 0.5 | 22 | 0 | 1/1 | ||
| Column | Basitrich | 10.1–24.9 | 17.8 | 3.7 | 1.5–2.6 | 2.1 | 0.2 | 22 | 0 | 1/1 | |
| Filament | Basitrich | 16.1–24.5 | 21.3 | 2.2 | 2.1–3.1 | 2.6 | 0.2 | 20 | 0 | 1/1 | |
| Microbasic p-mastigophore | 25.7–30.2 | 27.8 | 1.1 | 5.3–6.9 | 6.0 | 0.4 | 20 | 0 | 1/1 | ||
| Calliactis tricolor | Tentacle | Basitrich | 12.9–16.3 | 15.0 | 0.8 | 1.4–2.5 | 1.7 | 0.2 | 21 | 6 | 2/2 |
| Spirocyst | 16.9–29.1 | 22.9 | 3.1 | 3.0–4.9 | 3.9 | 0.6 | 0 | 21 | 1/2 | ||
| Actinopharynx | Basitrich | 13.3–25.5 | 19.3 | 4.4 | 1.4–3.3 | 2.3 | 0.4 | 24 | 21 | 2/2 | |
| Microbasic p-mastigophore | 13.4–18.2 | 16.0 | 1.0 | 2.3–3.1 | 2.6 | 0.2 | 20 | 0 | 1/2 | ||
| Column | Basitrich | 8.0–16.5 | 11.4 | 2.4 | 1.3–2.4 | 1.8 | 0.3 | 20 | 20 | 2/2 | |
| Filament | Basitrich | 13.7–26.2 | 19.5 | 4.1 | 1.9–3.0 | 2.3 | 0.2 | 20 | 21 | 2/2 | |
| Basitrich | 9.2–12.7 | 10.7 | 0.8 | 1.4–1.8 | 1.7 | 0.1 | 0 | 22 | 1/2 | ||
| Microbasic p-mastigophore | 14.2–24.1 | 17.6 | 2.8 | 2.3–4.6 | 3.3 | 0.7 | 22 | 21 | 2/2 | ||
| Acontia | Basitrich | 13.6–25.3 | 19.5 | 3.9 | 2.0–3.4 | 2.7 | 0.4 | 20 | 21 | 2/2 |
Anemonia sargassensis inhabits shallow waters of the lagoon reef zone, often above Thalassia testudinum blades, but is also found under stones and coral gravel, between 0.5–2 m. It is often reported on floating Sargassum (
Western Atlantic, from the northern coast of USA and Caribbean Sea, to the northern coast of Brazil (
Of the 20 valid species of Anemonia, four species have been recorded in the Gulf of Mexico and Caribbean Sea (
http://species-id.net/wiki/Anthopleura_pallida
Figure 4, Table 2Alacranes reef (22°22'54"N, 89°40'59"W; four specimens).
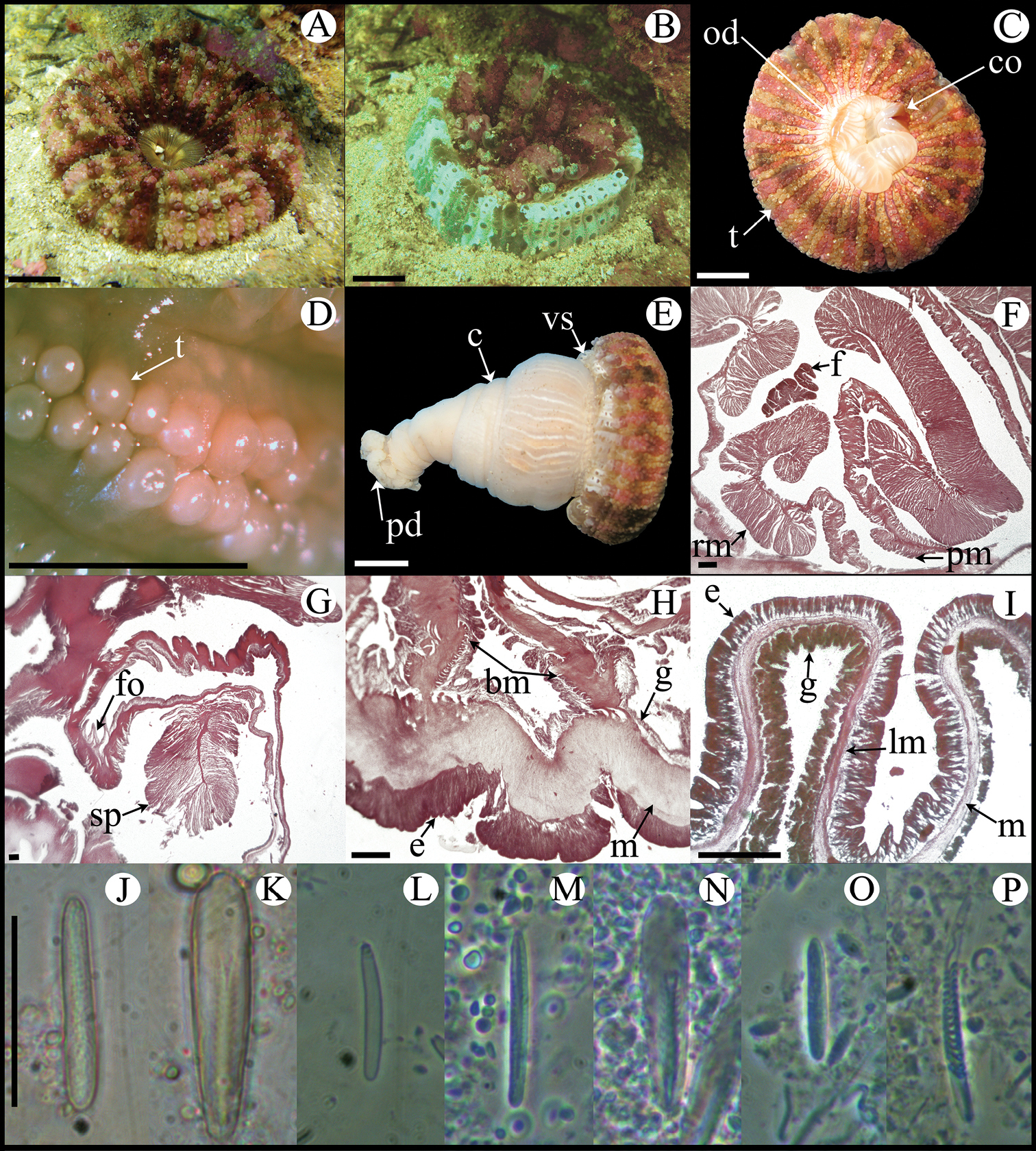
Fully expanded oral disc and tentacles 10–19 mm in diameter. Oral disc narrow, smooth, 3–8 mm in diameter, pale green or gray (Figure 4A). Tentacles hexamerously arranged in three cycles (24 in number), smooth, slender, relatively short (to 4–9 mm), tapering distally, inner ones longer than outer ones, contractile, whitish or gray, translucent, oral side with opaque white roundish spots (Figure 4A, B). Fosse well marked (Figure 4E). Column cylindrical, relatively elongate, 3–6 mm in diameter and 6–12 mm in height, with 12 longitudinal rows of verrucae from mid-column to distal margin (Figure 4B, G). Twelve endocoelic marginal projections forming acrorhagi (Figure 4B, E) with holotrichs, basitrichs, microbasic p-mastigophores, and spirocysts. Pedal disc well-developed, 4–8 mm in diameter, slightly wider than column (Figure 4B). Pedal disc and column white to pale green (Figure 4B). Mesenteries hexamerously arranged in 2–3 cycles: only first cycle perfect or first two cycles perfect and third imperfect; same number of mesenteries distally and proximally (12–32 pairs in specimens examined). Only first two cycles fertile (except directives); gonochoric (?), only spermatic cysts observed in specimens examined (Figure 4F). Two pairs of directives each attached to a well-developed siphonoglyph (Figure 4C). Retractor muscles diffuse; parietobasilar muscles well-developed with short mesogleal pennon (Figure 4D). Basilar muscles well-developed (Figure 4H). Marginal sphincter muscle endodermal, weak and diffuse (Figure 4E). Longitudinal muscles of tentacles ectodermal. Cnidom: basitrichs, microbasic b- and p-mastigophores, holotrichs, and spirocysts (Figure 4I–X; see Table 2).
Anthopleura pallida. A Oral view B Lateral view C Detail of directives and siphonoglyph D Cross section through proximal column E Longitudinal section through margin showing acrorhagi and marginal sphincter muscle F Detail of spermatic cysts G Longitudinal section through distal column showing one verruca H Longitudinal section through base showing basilar muscles I–X Cnidae.– acrorhagi: I small basitrich J basitrich K holotrich L spirocyst; actinopharynx: M small basitrich N basitrich O microbasic b-mastigophore P spirocyst; column: Q small basitrich R basitrich S spirocyst; filament: T basitrich U spirocyst V microbasic p-mastigophore; tentacle: W basitrich X spirocyst. Abbreviations.– acr: acrorhagi, bm: basilar muscle, c: column, d: directives, fo: fosse, mp: marginal projection, od: oral disc, pd: pedal disc, pm: parietobasilar muscle, rm: retractor muscle, s: siphonoglyph, sc: spermatic cyst, sp: sphincter, t: tentacles, vr: verruca. Scale bars: A–B: 10 mm; C–H: 200 μm; I–X: 25 μm.
Anthopleura pallida inhabits the intertidal to shallow subtidal zone attached to coral on sandy shores, at 0.5 m. It is azooxanthellate and it broadcast spawns (
Western Atlantic, from Bermuda (
Currently there are three valid species of Anthopleura in the Gulf of Mexico and Caribbean Sea: Anthopleura krebsi (Duchassaing & Michelotti, 1860), Anthopleura texaensis (Carlgren and Hedgpeth, 1952), and Anthopleura pallida (
http://species-id.net/wiki/Bunodosoma_cavernatum
Figure 5, Table 2La Gallega reef (19°13'20"N, 96°07'39"W; thirteen specimens), Ingenieros reef (19°08'41"N, 96°05'22"W; two specimens).
Fully expanded oral disc and tentacles to 20–38 mm in diameter. Oral disc 10–22 mm in diameter, smooth, brown-yellowish, brown-reddish or pale olive-green, sometimes with white or yellowish radial stripes in endocoelic spaces of first two or three tentacular cycles (Figure 5A, B). Tentacles hexamerously arranged in five cycles (about 96 in number), smooth, simple, conical, moderately long (3–5 mm in length), tapering distally, inner ones longer than outer ones, contractile, olive-green, reddish or pale-orange (Figure 5A, B), often with white or yellowish spots on oral side and sometimes with purple flashes. Deep fosse (Figure 5I). Forty-eight endocoelic rounded marginal projections forming acrorhagi (Figure 5C, I) with holotrichs and basitrichs. Column cylindrical, 12–22 in diameter and 7–15 mm in height, densely covered with rounded vesicles, arranged in 96 longitudinal rows from margin to limbus (Figure 5C, G). Pedal disc well-developed, 12–19 mm in diameter (Figure 5C). Column and pedal disc light-brown, orange, reddish, yellowish or olive-green. Mesenteries hexamerously arranged in four cycles (48 pairs in specimens examined): first, second and some mesenteries of third cycle perfect, others imperfect; same number of mesenteries distally and proximally. All mesenteries fertile (except directives); gonochoric; oocytes and spermatic cysts well-developed in specimens collected in January and May (Figure 5E). Two pairs of directives each attached to a well-developed siphonoglyph (Figure 5D). Retractor muscles strong and restricted; parietobasilar muscles well-developed with a relatively long free mesogleal pennon (Figure 5E). Basilar muscles well-developed (Figure 5H). Marginal sphincter muscle endodermal, strong and circumscribed (Figure 5I). Longitudinal muscles of tentacles ectodermal (Figure 5F). Zooxanthellae present. Cnidom: basitrichs, microbasic b- and p-mastigophores, holotrichs and spirocysts (Figure 5J–T; see Table 2).
Bunodosoma cavernatum. A Live specimen in natural habitat B Oral view C Lateral view D Detail of directives; notice siphonoglyph E Cross section through proximal column showing oocytes F Cross section through tentacle G Longitudinal section through column showing vesicles H Longitudinal section though base showing basilar muscles I Longitudinal section through margin showing acrorhagi and marginal sphincter muscle J–T Cnidae.– acrorhagi: J basitrich K holotrich; actinopharynx: L basitrich M microbasic p-mastigophore; column: N small basitrich O basitrich; filament: P basitrich Q microbasic b-mastigophore R microbasic p-mastigophore; tentacle S basitrich T spirocyst. Abbreviations.– acr: acrorhagi, bm: basilar muscle, c: column, d: directives, e: epidermis, fo: fosse, g: gastrodermis, lm: longitudinal muscles, m: mesoglea, mp: marginal projection, o: oocyst, od: oral disc, pd: pedal disc, pm: parietobasilar muscle, rm: retractor muscle, s: siphonoglyph, sp: sphincter, t: tentacle, vs: vesicles. Scale bars: A–C: 10 mm; D–I: 200 μm; J–T: 25 μm.
Bunodosoma cavernatum inhabits shallow waters, attached to rocks and coral rubble, in the lagoon zone; between 2–6 m.
Western Atlantic, from North Caroline to Barbados; along the Caribbean Sea and Gulf of Mexico (
Currently four valid species of Bunodosoma have been reported in the Gulf of Mexico and Caribbean Sea (
http://species-id.net/wiki/Isoaulactinia_stelloides
Figure 6, Table 2La Gallega reef (19°13'20"N, 96°07'39"W; six specimens).
Fully expanded oral disc and tentacles to 24–38 mm in diameter. Oral disc smooth, slightly wider than column, 9–11 mm in diameter, light- or olive-green, sometimes with small white stripes near tentacles bases (Figure 6A, B). Tentacles hexamerously arranged in four cycles (about 48 in number), simple, smooth, moderately long (9–14 mm in length), conical, tapering distally, inner ones longer than outer ones, contractile, olive-green with white bands along entire length (Figure 6A, B). Deep fosse (Figure 6G). Twenty-four endocoelic marginal projections (Figure 6C, G) with basitrichs and macrobasic p-mastigophores. Column cylindrical, 8–12 in diameter and 13–22 mm in height, with approximately 48 longitudinal rows of verrucae along entire column, but more conspicuous distally (Figure 6C). Pedal disc well-developed, 9–16 mm in diameter (Figure 6C). Column, verrucae, and pedal disc light-brown or beige (Figure 6C). Mesenteries hexamerously arranged in three cycles (24 pairs in specimens examined): all cycles perfect; same number of mesenteries distally and proximally. First and second cycles fertile (except directives); hermaphroditic (?), only oocytes observed in specimens examined (Figure 6E). Developing polyps in coelenteron (Figure 6F). Two pairs of directives each attached to a well-developed siphonoglyph (Figure 6D). Retractor muscles strong and restricted; parietobasilar muscles well-developed with relatively long and thick free mesogleal pennon (Figure 6E). Basilar muscles well-developed (Figure 6H). Marginal sphincter muscle endodermal, strong and circumscribed, palmate (Figure 6G). Longitudinal muscles of tentacles ectodermal (Figure 6I). Zooxanthellae present. Cnidom: basitrichs, microbasic b-mastigophores, macrobasic and microbasic p-mastigophores, and spirocysts (Figure 6J–Y; see Table 2).
Isoaulactinia stelloides. A Live specimen in natural habitat B Oral view C Lateral view D Detail of directives showing a siphonoglyph E Cross section through proximal column F Detail of brooded juvenile G Longitudinal section through margin showing marginal sphincter muscle and marginal projection H Longitudinal section though base showing basilar muscles I Cross section through tentacle J–Y Cnidae.– marginal projection: J small basitrich K macrobasic p-mastigophore; actinopharynx: M basitrich N macrobasic p-mastigophore O microbasic p-mastigophore P long, curved basitrich; column: Q small basitrich R macrobasic p-mastigophore; filament: S small basitrich T basitrich U macrobasic p-mastigophore V microbasic b-mastigophore W microbasic p-mastigophore; tentacle: X basitrich Y spirocyst. Abbreviations.– b: brooded juvenile, bm: basilar muscle, c: column, d: directives, e: epidermis, fo: fosse, lm: longitudinal muscle, m: mesoglea, mp: marginal projection, o: oocyst, od: oral disc, pd: pedal disc, pm: parietobasilar muscle, rm: retractor muscle, s: siphonoglyph, sp: sphincter, t: tentacle, vr: verrucae, z: zooxanthellae. Scale bars: A–C: 10 mm; D–I: 200 μm; J–Y: 25 μm.
Isoaulactinia stelloides inhabits shallow waters in the lagoon reef zone, at 1–2 m, near Actinostella flosculifera, Stichodactyla helianthus, and the zoanthid Palythoa caribaeorum (Duchassaing & Michelotti, 1860). It lives with the column burrowed in the sand but the pedal disc attached to rocks and coral rubble. Although we only observed developing oocytes in the two specimens histologically examined, Isoaulactinia stelloides has been reported as a simultaneous hermaphroditic, internally brooding, often with developing polyps in the coelenteron (
Western Atlantic, from Bermuda to Barbados, and along the Caribbean Sea (
Currently Isoaulactinia has two valid species (
http://species-id.net/wiki/Lebrunia_coralligens
Figure 7; Table 2Isla Verde reef (19°13'26"N, 96°05'56"W; three specimens); Isla Sacrificios reef (19°10'36"N, 96°05'39"W; three specimens).
Fully expanded oral disc and tentacles to 18–22 mm in diameter. Oral disc smooth, 3–5 mm in diameter, beige and translucent (Figure 7B). Tentacles hexamerously arranged in 3–4 cycles (about 24–52 in number), moderately long (about 5–8 mm length), tapering distally, inner ones longer than outer ones, contractile, gray or beige, translucent, with tips whitish or yellowish and scattered bluish dots along the entire length (Figure 7B, C). Column short, smooth, 3–6 mm in diameter and 6–10 mm in height, bright-brown with faint stripes corresponding to mesenterial insertions. Column distally with 4–6 outgrowths (pseudotentacles). Pseudotentacles branched, ending in globular-shaped vesicles with batteries of macro- and micro-basic p-amastigophores and basitrichs; bluish with gray or brown circle in center (Figure 7A–C). Pedal disc well-developed, circular, 3–7 mm in diameter, light brown or beige, translucent (Figure 7C). Mesenteries hexamerously arranged in 2–3 cycles (12–24 pairs in specimens examined): first cycle perfect and sterile, others imperfect and fertile; more mesenteries proximally than distally (two and three cycles, respectively). Hermaphroditic (Figure 7G). Two pairs of directives each attached to a well-developed siphonoglyph (Figure 7D). Retractor muscles diffuse, strong; parietobasilar muscles with short and thick mesogleal pennon (Figure 7E, F). Basilar muscles relatively poorly developed (Figure 7H). Marginal sphincter muscle absent. Ectodermal longitudinal muscles in distal column. Longitudinal muscles of tentacles ectodermal (Figure 7I). Zooxanthellae present (Figure 7F). Cnidom: basitrichs, macrobasic and microbasic p-amastigophores, and spirocysts (Figure 7J–V; see Table 2).
Lebrunia coralligens. A Live specimen in natural habitat B Oral view C Pedal disc view D Cross section through distal column showing a siphonoglyph E Detail of retractor muscles F Detail of parietobasilar muscles G Detail of a mesentery showing oocytes and spermatic cysts H Longitudinal section through base showing basilar muscles I Cross section through tentacle J–V Cnidae.– actinopharynx: J small microbasic p-amastigopore K microbasic p-amastigophore; column: L small basitrich M small microbasic p-amastigophore; filament: N small microbasic p-amastigophore O microbasic p-amastigophore; tentacle: P basitrich Q microbasic p-amastigophore R small microbasic p-amastigophore S spirocyst; pseudotentacle: T basitrich U microbasic p-amastigophore V macrobasic p-amastigophore. Abbreviations.– bm: basilar muscle, d: directives, e: epidermis, lm: longitudinal muscle, o: oocyst, od: oral disc, pd: pedal disc, pm: parietobasilar muscle, ps: pseudotentacle, rm: retractor muscle, s: siphonoglyph, sc: spermatic cyst; t: tentacle, z: zooxanthellae. Scale bars: A–C: 10 mm; D–H: 200 μm; I: 100 μm; J–U: 25 μm; V: 20 μm.
Lebrunia coralligens inhabits narrow fissures of live coral with only the end of the pseudotentacles visible, between 3–6 m. During the day, the tentacles remain contracted and the pseudotentacles fully expanded allowing the zooxanthellae (particularly abundant in this area) to capture sunlight; at night the situation is the opposite, allowing tentacles to capture food (
Western Atlantic, from Bahamas to Brazil, and along the Caribbean Sea (
Currently there are two valid species of Lebrunia, both of them distributed in the Western Atlantic (
http://species-id.net/wiki/Actinoporus_elegans
Figure 8, Table 2La Gallega reef (19°13'20"N, 96°07'39"W; one specimen).
Fully expanded oral disc and tentacles up to 52 mm in diameter. Central part of oral disc smooth, narrow, to 16 mm diameter, beige; mouth oval with a well-developed conchula (Figure 8C). Tentacles small, vesicle-like, arranged in double radial rows covering almost entire oral disc, on endocoelic and exocoelic spaces, 24–26 tentacles per double row; reddish or pinkish rows of tentacles alternating with pale brown rows (Figure 8A–D). Deep fosse (Figure 8G). Column elongated, funnel-shaped, to 60 mm in height, wider distally than proximally; column diameter: distally 38 mm, mid-column 27 mm, proximally 13 mm (Figure 8E). Column with longitudinal rows of vesicles (6–8 vesicles per row) distally (Figure 8B, E). Pedal disc well-developed, narrow, 19 mm in diameter. Column and pedal disc white to pale-brown; mesenterial insertions visible distally (Figure 8E). Mesenteries irregularly arranged in three cycles (28 pairs in specimen examined): first cycle perfect, others imperfect. Gametogenic tissue not observed in specimen examined. Two pairs of directives, only one pair attached to a single well-developed siphonoglyph. Retractor muscles strong, circumscribed, with main muscle lamella divided in two parts; parietobasilar muscles strong with thick mesogleal pennon (Figure 8F). Basilar muscles well-developed (Figure 8H). Marginal sphincter muscle endodermal, strong and circumscribed, pinnate (Figure 8G). Longitudinal muscles of the tentacles ectodermal (Figure 8I). Zooxanthellae absent. Cnidom: basitrichs, microbasic p-mastigophores, and spirocysts (Figure 8J–P, Table 2).
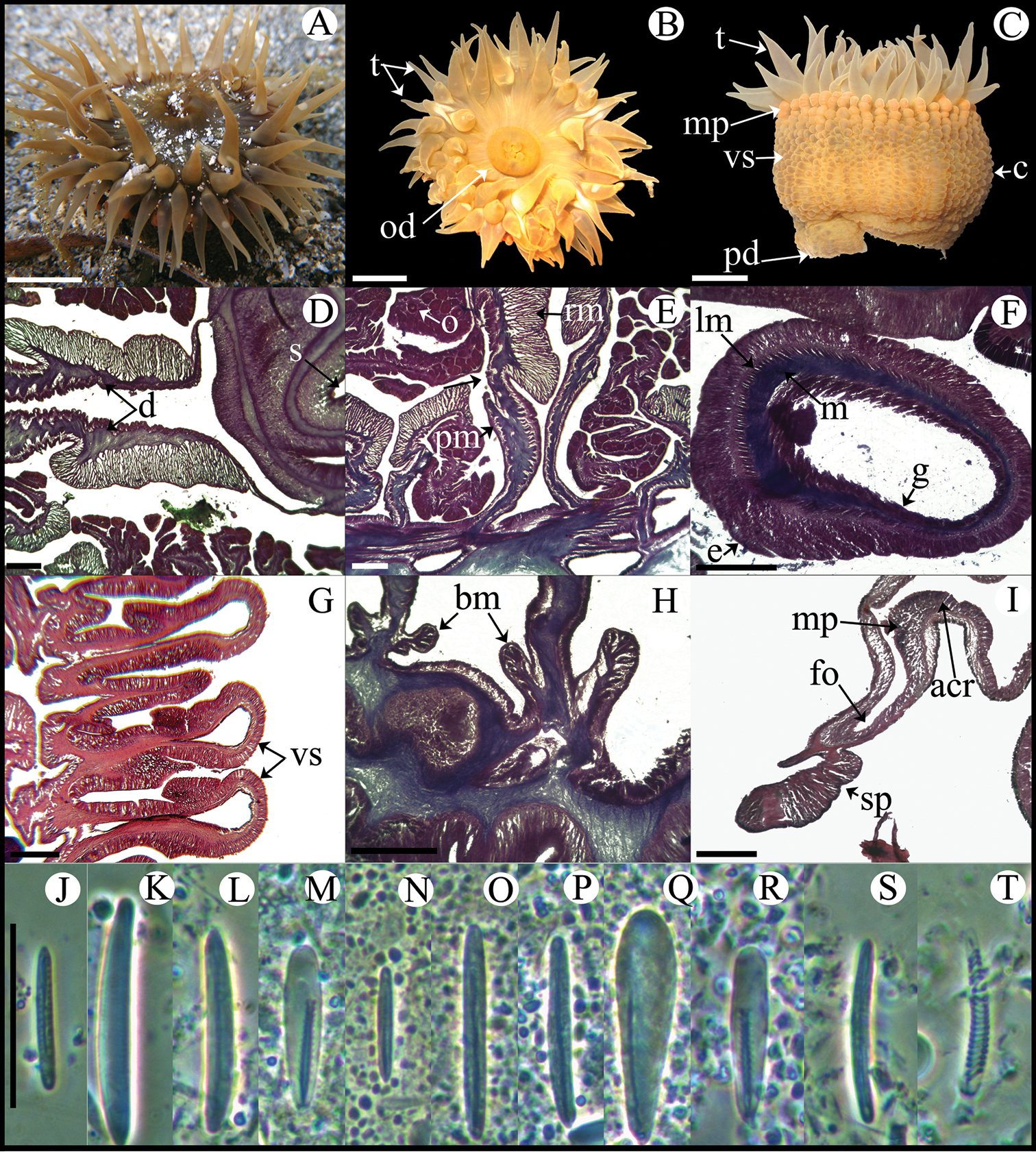
Actinoporus elegans. A Fully expanded specimen in natural habitat B Partially contracted specimen in natural habitat C Oral view D Detail of double rows of tentacles E Lateral view F Cross section through proximal column, showing retractor and parietobasilar muscles G Longitudinal section through column margin showing marginal sphincter muscle H Longitudinal section through base showing basilar muscles I Longitudinal section through tentacles J–P Cnidae.– actinopharynx: J basitrich K microbasic p-mastigophore; column: L basitrich; filament: M basitrich N microbasic p-mastigophore; tentacle: O basitrich P spirocyst. Abbreviations.– bm: basilar muscle, e: epidermis, f: filament, fo: fosse, g: gastrodermis, lm: longitudinal muscle, m: mesoglea, od: oral disc, pd: pedal disc, pm: parietobasilar muscle, rm: retractor muscle, sp: sphincter, t: tentacle, vs: vesicles. Scale bars: A–C, E: 10 mm; D: 2 mm; F–I: 200 μm; J–P: 25 μm.
Actinoporus elegans inhabits sandy bottoms, at 1–2 m; the column is burrowed in the sand but the pedal disc is strongly attached to rocks. When disturbed, it contracts the oral disc suddenly, completely burrowing the entire body.
Western Atlantic, from the northern coast of Brazil to Guadeloupe, Jamaica, and Curaçao (
Currently there are two valid species of Actinoporus: Actinoporus elegans and Actinoporus elongatus Carlgren, 1900 (
http://species-id.net/wiki/Calliactis_tricolor
Figure 9, Table 2Alacranes reef (22°31'35"N, 89°46'05"W; eight specimens), Serpientes reef (21°26'22"N, 90°28'25"W; five specimens).
Fully expanded oral disc and tentacles 9–48 mm in diameter. Oral disc smooth, wider than column, 3–20 mm in diameter, pale-brown translucent, with small white stripes in endocoelic spaces, sometimes forming a white ring; some specimens also with pink flashes (Figure 9A). Mouth bright yellow, orange, or white; often with purple ring around lips (Figure 9A). Tentacles hexamerously arranged in 5–6 cycles (96–192 in number), smooth, thin, short (2.5–15.5 mm), inner ones longer than outer ones, contractile (Figure 9A, B), tapering distally, pale-brown with a longitudinal row of white dots along entire length (Figure 9A, B); some specimens also with bright-pink flashes mainly at tips. Column trumpet-shaped in extended position, dome-shaped when contracted, 5–24.5 mm in diameter and 4–31 mm in height, divided into narrow, smooth capitulum and wrinkled-texture scapus (Figure 9B). Capitulum pale-brown to yellowish, scapus bright to dark orange often with small white stripes or white flashes slightly above limbus (Figure 9B). Pedal disc well-developed, circular to irregular, wider than column, 6–36 mm in diameter, with mesenterial insertions visible, pale-brown and translucent (Figure 9C). One or two rows of cinclides proximally, near limbus; dark-red or brown (Figure 9B). Mesenteries hexamerously arranged in four cycles; same number of mesenteries proximally and distally (to 48 pairs in specimens examined): first cycle perfect, others imperfect; third and fourth cycles poorly developed, without filaments or acontia. Gametogenic tissue not observed in specimens examined. Two pairs of directives each attached to a well-developed siphonoglyph (Figure 9E). Retractor muscles weak and diffuse; parietobasilar muscles poorly developed (Figure 9E, F). Basilar muscles poorly developed (Figure 9H). Marginal sphincter muscle mesogleal, strong, transversally stratified (Figure 9G). Longitudinal muscles of tentacles ectodermal. Acontia numerous, bright orange (Figure 9C), with basitrichs. Zooxanthellae present. Cnidom: basitrichs, microbasic p-mastigophores, and spirocysts (Figure 9J–Q; see Table 2).
Calliactis tricolor. A Oral view B Lateral view C Pedal disc view D Specimens on hermit crab shell E Detail of directives showing a siphonoglyph F Cross section through proximal column G Longitudinal section through margin showing the marginal sphincter muscle H Longitudinal section through base showing basilar muscles I Cross section through tentacle J–Q Cnidae.– acontio: J basitrich; actinopharynx: K basitrich L microbasic p-mastigophore; column: M small basitrich; filament: N basitrich O microbasic p-mastigophore; tentacle: P basitrich Q spirocysts. Abbreviations.– ac: acontia, bm: basilar muscle, c: column, ca: capitulum, ci: cinclides, e: epidermis, g: gastrodermis, lm: longitudinal muscle, m: mesoglea, od: oral disc, pd: pedal disc, rm: retractor muscle, sc: scapus, sp: sphincter, t: tentacle. Scale bars: A–D: 10 mm; E–I: 200 μm; J–Q: 25 μm.
Calliactis tricolor typically dwells on the shells of living hermit crabs often carrying more than one individual (Figure 9D), between 10–30 m. This peculiar symbiotic relationship has been widely studied (reviewed in
Western Atlantic, from the northern coast of USA to the northern coast of Brazil, along the Caribbean Sea and Gulf of Mexico (
Of the 18 valid species currently considered as valid of Calliactis, only two have been reported in the Gulf of Mexico and Caribbean Sea (
This work was partially supported by a grant from the Comisión Nacional de Ciencia y Tecnología (CONACyT) to R.G. for studies in the Postgraduate Program of Ciencias del Mar y Limnología (PCMyL, UNAM), and by CONACyT–SEMARNAT–108285 and DGAPA–PAPIME–PE207210 (UNAM) projects to N.S. All specimens were collected under consent of Mexican law, collecting permit approved by Comisión Nacional de Acuacultura y Pesca (Number 07332.250810.4060). We thank the Secretaría de Marina Armada de México (SEMAR), the Comisión Nacional de Áreas Naturales Protegidas (CONANP) and the staff at the Parque Nacional Arrecife Alacranes for their helpful assistance during field work. Dr. Horacio Pérez-España (Instituto de Ciencias Marinas y Pesquerías de la Universidad Veracruzana) provided support for field work; Dr. Leopoldina Aguirre-Macedo and M.S. Raúl Simá (Centro de Investigación y Estudios Avanzados Unidad Mérida, Instituto Politécnico Nacional), and M.S. Maribel Badillo-Alemán (UMDI-Sisal) provided access and support to histological facilities; M.S. Gemma Martínez-Moreno, Dr. Patricia Guadarrama-Chávez (UMDI-Sisal), B.S. José Antonio Martínez-Pérez, and B.S. Héctor Barrera-Escorcia (FESI-UNAM) helped with lab work and provided support in the microscopy lab; M.S. Alfredo Gallardo-Torres (UMDI-Sisal), B.S. Alejandro Córdova, B.S. Noé Salgado-Ortíz (FESI-UNAM), Professor Ariel Rolón, M.S. Geraldine García, M.S. Manuela Muhlia, M.S. Fernando Lazcano (PCMyL, UNAM) helped in the field, and Dr. Anastazia Banaszak helped with the English version of this paper. Comments of Dr. Lee van Ofwegen and one anonymous referee improved this manuscript.