(C) 2013 Lin Yang. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Yang L, Chen X-S (2013) Bambusananus cuihuashanensis, a new bamboo-feeding leafhopper species of Athysanini (Hemiptera, Cicadellidae, Deltocephalinae) from Shaanxi, China. ZooKeys 341: 107–113. doi: 10.3897/zookeys.341.5930
Bambusananus cuihuashanensis sp. n. (Hemiptera: Cicadellidae: Deltocephalinae: Athysanini), a new bamboo-feeding leafhopper species, is described and illustrated from Shaanxi Province of China. Checklist, host plants and distribution for each species of Bambusananus is given along with a key to all known species.
Bamboo leafhopper, Cicadomorpha, distribution, Homoptera, taxonomy
The leafhopper tribe, Athysanini, was established by Van Duzee in 1892 and is the largest tribe of Deltocephalinae, including 228 genera and 1123 species (
The leafhopper genus Bambusananus (Deltocephalinae: Athysanini) was established by
During on-going studies on species biodiversity of the bamboo-feeding leafhoppers in China, several specimens belonging to an undescribed species of Bambusananus were found. The purpose of this paper is to describe this new species, to summarize information on host plants and geographical distribution of the known species and to provide a key to species in the genus.
In the present paper, terminology follows
-
Bambusananus bipunctatus (Li, 1999) (in
Cai and Huang 1999 )Host plant. Bamboo (Indocalamus hirsutissimus Z. P. Wang & P. X. Zhang and Qiongzhuea communis Hsueh & Yi) (
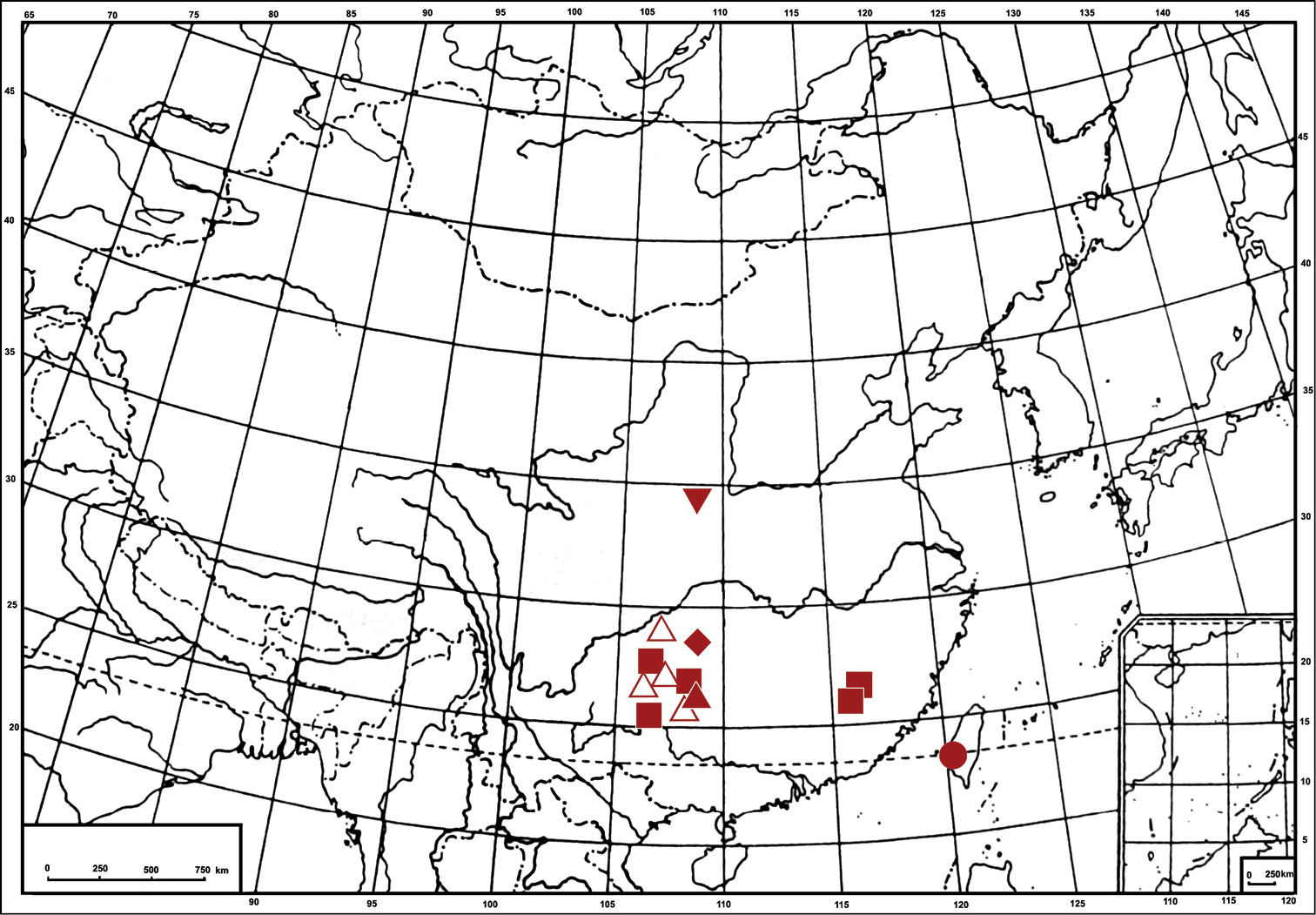
Yang and Chen 2012 ).Distribution. China (Guizhou, Fujian and Sichuan) (Fig. 12).
-
Bambusananus maculipennis (Li & Wang, 1993)
Host plant. Bamboo (Chimonobambusa pachystachys Hsuch & W. P. Zhang and Qiongzhuea communis Hsueh & Yi) (
Yang and Chen 2012 ).Distribution. China (Guizhou) (Fig. 12).
-
Bambusananus cuihuashanensis sp. n.
Host plant. Bamboo.
Distribution. China (Shaanxi) (Fig. 12).
-
Bambusananus furcatus Li & Xing, 2011
Host plant. Bamboo (Chimonobambusa angustifolia C. D. Chu & C. S. Chao) (
Chen et al. 2012 ).Distribution. China (Guizhou) (Fig. 12).
-
Bambusananus lii (McKamey & Hicks, 2007)
Host plant. Bamboo (
Li et al. 2011 ).Distribution. China (Taiwan) (Fig. 12).
-
Bambusananus yangae Xing & Chen, 2013
Host plant. Bamboo (Indocalamus sp.) (
Yang and Chen 2012 ).Distribution. China (Guizhou) (Fig. 12).
| 1 | Upper area of frontoclypeus with a large black transverse marking | 2 |
| – | Upper area of frontoclypeus without above marking (Fig. 2) | 3 |
| 2 | Aedeagal shaft with appendages long, reaching to middle of aedeagus (Fig. 11) | Bambusananus cuihuashanensis sp. n. |
| – | Aedeagal shaft with appendages short, only reaching to apical one-fifth of aedeagus | Bambusananus bipunctata (Li) |
| 3 | Ventral processes of male pygofer curved dorsad | Bambusananus maculipennis (Li & Wang) |
| – | Ventral processes of male pygofer with apical half curved ventrad (Fig. 6) | 4 |
| 4 | Appendages of aedeagal shaft branched | Bambusananus furcatus Li & Xing |
| – | Appendages of aedeagal shaft not branched (Figs 10, 11) | 5 |
| 5 | Aedeagal appendages arising from middle of aedeagus, straight, with apex directed apically; ventral margin of aedeagus without any teeth | Bambusananus yangae Xing & Chen |
| – | Aedeagal appendages arising from apical 1/3 of aedeagus, curved, with apex directed basolaterally; ventral margin of aedeagus with a row of teeth | Bambusananus lii (McKamey & Hicks) |
http://zoobank.org/4B853422-EEC6-41EB-AD6B-B1570C4E92E2
http://species-id.net/wiki/Bambusananus_cuihuashanensis
Figs 1–11Holotype: ♂, China: Shaanxi, Xi’an, Cuihuashan (108°57'E, 34°10'N), on bamboo, 37 Aug. 2008, J.-D. Li; paratypes: 2 ♂♂, 4 ♀♀, same data as holotype.
The new species is named after its locality, Cuihuashan, Shaanxi Province, China.
Body length (from apex of vertex to tip of forewings): male 4.75–4.85 mm (N = 2); female 5.75–5.90 mm (N = 4); forewing length: male 3.95–4.05 mm (N = 2); female 5.00–5.15 mm (N = 4).
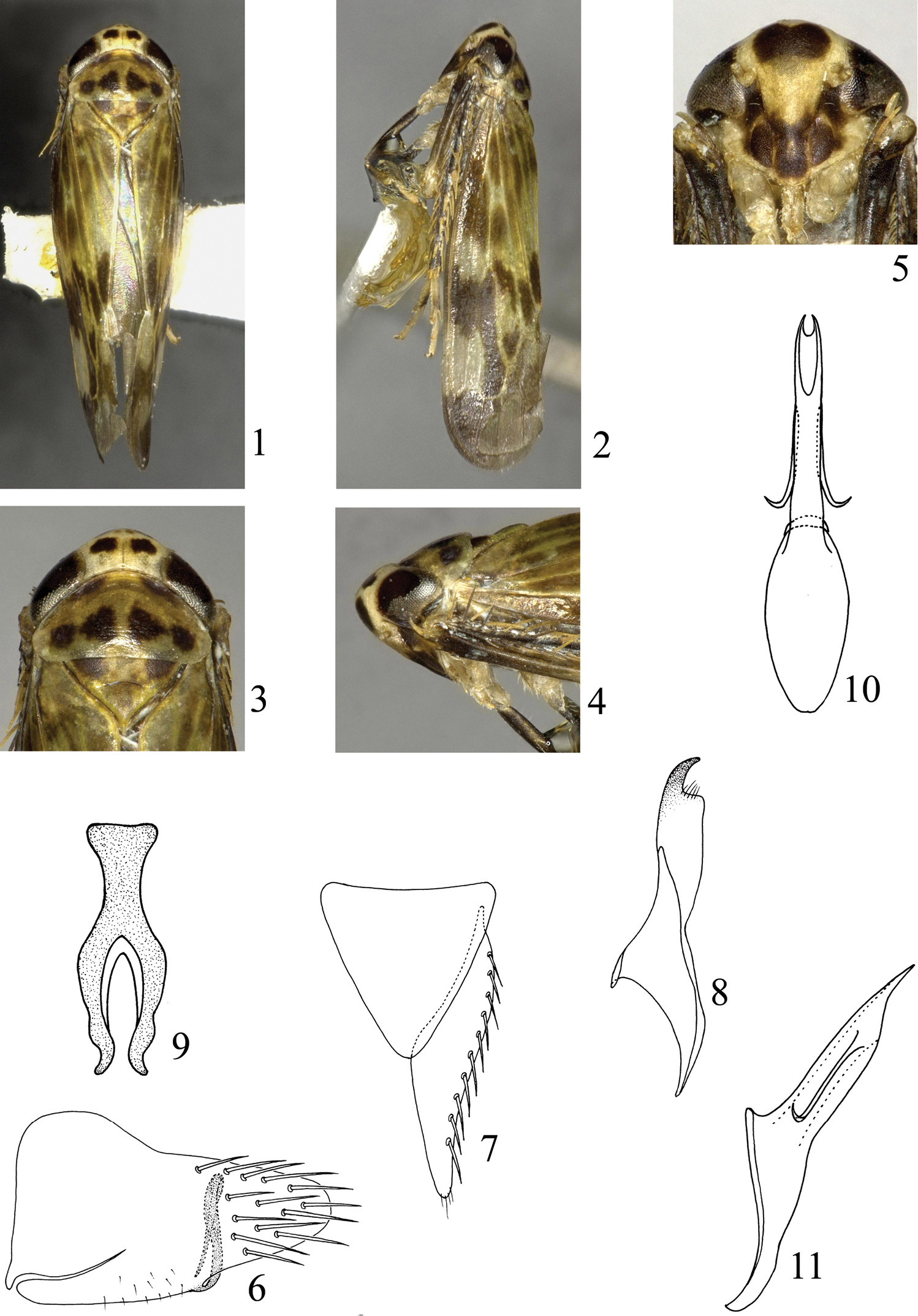
Crown (Fig. 1) pale yellowish white, two markings behind ocelli blackish brown. Eyes (Fig. 1) blackish brown, ocelli yellowish white. Frontoclypeus (Fig. 5) pale yellowish white, with lower area dark brown and a large kidney-shaped black marking at upper area; anteclypeus, lorums, genae with upper areas dark brown. Antennae (Fig. 5) pale yellowish brown. Pronotum (Figs 1, 3) pale yellowish brown, anterior areas with two dark brown markings, posterior areas with four blackish brown markings. Scutellum (Figs 1, 3) pale yellowish brown, with two brown markings basally. Forewing (Figs 1, 2) pale yellowish white to yellowish brown, veins yellowish white, with irregular blackish brown markings at median and posterior region. Thorax dark brown ventrally; legs brown to dark brown, except base of tarsus yellowish brown. Abdomen dark brown dorsally and ventrally, lateral margins of each segment pale yellowish white.
Bambusananus cuihuashanensis sp. n. 1 Male habitus, dorsal view 2 Male habitus, lateral view 3 Head and thorax, dorsal view 4 Head and thorax, lateral view 5 Head and thorax, ventral view 6 Male pygofer, lateral view 7 Genital valve and left subgenital plate, ventral view 8 Left style, dorsal view 9 Connective, dorsal view 10 Aedeagus, ventral view 11 Aedeagus, lateral view.
External features as in generic description. Crown shorter medially than width between eyes (0.54:1). Pronotum longer medially than crown (2.10:1). Scutellum shorter medially than pronotum (0.89:1). Forewing longer medially than width at widest part (3.38:1).
Male pygofer (Fig. 6) with basal half nearly quadrate, then narrowing to apex, dorsal margin slightly sinuate, ventral margin broadly curved, smooth, apical margin acute and rounded, with several macrosetae at apical area; ventral process slender and long, slightly widening at middle, narrowing apically, arising from inner side of ventral margin, produced dorsad, then abruptly strongly curved ventrally. Genital valve (Fig. 7) triangular, basal width slightly longer than median length (1.06:1). Subgenital plate (Fig. 7) moderately narrow, triangular, inner margin nearly straight, outer margin slightly concave, narrowing apically, apex acute and rounded, with row of macrosetae laterally. Style (Fig. 8) broad at base, abruptly narrowing subapically and curved hook-like. Aedeagus (Figs 10, 11) with shaft broad at middle, narrowing basally and apically, gonopore at apex, paired appendages slender and long, apex acute, arising from apical one-fourth, directed basally, then laterally. Connective (Fig. 9) Y-shaped, stem robust and arms well developed, stem slightly shorter than arm (0.79:1).
Bamboo.
China (Shaanxi) (Fig. 12).
Geographic distribution of Bambusananus species: Bambusananus bipunctatus (Li) (■) Bambusananus cuihuashanensis sp. n.(▼) Bambusananus furcatus Li & Xing (▲) Bambusananus lii (McKamey & Hicks) (●) Bambusananus maculipennis (Li & Wang) (△) Bambusananus yangae Xing & Chen (◆).
This new species is similar to Bambusananus bipunctatus (Li, 1999) in general appearance, but can be distinguished by: pygofer in lateral view with ventral margin broadly smoothly rounded, without notch at base of ventral process (with notch in Bambusananus bipunctatus); connective with stem slightly shorter than arms (longer in Bambusananus bipunctatus); aedeagal shaft with appendages longer, in lateral view with apex reaching to middle of aedeagus (in Bambusananus bipunctatus, appendages shorter and apex only reaching to apical one-fifth of aedeagus). This new species is also similar to Bambusananus lii (McKamey & Hicks, 2007), but can be distinguished by: upper area of frontoclypeus with a large kidney-shaped black marking (lacking in Bambusananus lii); ventral margin of pygoger without any lobe (with a lobe near middle in Bambusananus lii); appendages of aedeagal shaft mostly straight basally, apex directed laterally (elbow-like and curved basally, apex directed caudad in Bambusananus lii); ventral margin of aedeagus without any teeth (with a row of teeth at middle in Bambusananus lii).
We are grateful to Dr. Ji-Chun Xing (Institute of Entomology, Guizhou University, China) for preparing figures of new species. This research was supported by the National Natural Science Foundation of China (31260178), China Postdoctoral Science Foundation founded project (2012M521719, 2013T60864) and the International Science and Technology Cooperation Program of Guizhou (20107005).