(C) 2012 Rob van Soest. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Among the thousands of non-tetractinellid (monaxonid) Demospongiae species, less than twenty possess polyactine (usually three- or four-claded) megascleres. These are currently assigned to two closely related genera, viz. Cyamon Gray and Trikentrion Ehlers, both members of the raspailiid subfamily Cyamoninae. The two genera are considered valid on account of differences in the shape and the ornamentation of the polyaxone spicules. Cyamon predominantly has four-claded equiangular spicules with all cladi spined or rugose, whereas Trikentrion usually has a majority of three-claded spicules on which spines are found only on a single basal clade. Nevertheless, the differences between the two genera appear to overlap in several known and newly discovered species, necessitating a revision of the two groups. Two new species of Cyamon were found to occur on inshore sandstone platforms off the coast of Mauritania. One of the new species, Cyamon amphipolyactinum sp. n., possesses unique small ‘double’ polyactine spicules in addition to the usual calthrops-like polyactine megascleres characteristic for Cyamon. The second new species, Cyamon arguinense sp. n., possesses polyactine megascleres of which only one of the cladi is spined the remaining three or more cladi being smooth, a feature that is considered characteristic of sponges of the genus Trikentrion. The type species of Cyamon, Cyamon vickersii (Bowerbank) appears to have been misinterpreted as a Caribbean species, because circumstantial evidence strongly indicates an Indian Ocean origin. This has the consequence that specimens recorded subsequently under the name Cyamon vickersii from various Western Atlantic localities are reassigned to Cyamon agnani (Boury-Esnault), a species originally described from Brazil. A new species, reported as Cyamon vickersii sensu Burton & Rao from the east coast of India, and available to us only as a single thick section mounted on a glass slide, is named Cyamon hamatum sp. n. The Cyamon membership of the only deep-sea species, Cyamon spinispinosum (Topsent) is drawn in doubt due to considerable morphological deviation from mainstream Cyamon. The type species of Trikentrion, Trikentrion muricatum (Pallas), is extensively described and discussed, and a neotype is assigned. West African Trikentrion laeve (Carter) is for the first time since its original description properly redescribed from the type material. The specimen recorded by Burton as Trikentrion laeve from Congo turned out to be different from the original material of Carter and is assigned to a new species, Trikentrion africanum sp. n. All species of both genera considered valid are reviewed, mostly based on the examination of type or other original specimens. Our revision shows the existence of twelve species of Cyamon and six species of Trikentrion. A key to the species is provided and remarks on the geographic distribution of both genera are made. Based on our study, the differences between Cyamon and Trikentrion are re-evaluated. Only one character absolutely distinguishes the two genera, the presence (Trikentrion) or absence (Cyamon) of trichodragmata. A further discriminating character is the possession of short thick styles (most Cyamon species) versus thick oxeas (many Trikentrion), but this is complicated by absence of the oxeas in three Trikentrion species. Although spination of the polyactine spicules in itself cannot serve to distinguish the two genera with certainty, those of Trikentrion are usually recognizable by excessive hook-like spines against a finer spination in Cyamon. Possibly, the polyactine spicules of both groups are non-homologous, with Cyamon polyactines derived from styles and Trikentrion polyactines from oxeas, but this remains to be further investigated.
Sponges, new species, revision, Cyamon, Trikentrion, polyactines, Raspailiidae
The revision presented below was inspired by the recent discovery of two new species, evidently belonging to the sponge genus Cyamon Gray, 1867 (Demospongiae, Poecilosclerida, Microcionina, Raspailiidae, Cyamoninae), growing on shallow-water sandstone ridges off the coast of Mauritania. Cyamon species are unusual among raspailiid sponges in possessing polyactine megascleres (mostly four- or three-claded) with all cladi spined. Most species of Cyamon are rare encrusting sponges recorded from seemingly random localities across the warmer waters of the globe (
Below, we describe four new species and review previously described species of both genera, pointing out aspects that appear to have been overlooked. We propose the synonymy of several previously accepted species, indicate a serious misinterpretation of the origin of the type species of Cyamon and provide extensive data on the type species of Trikentrion, including designation of a neotype. We demonstrate that the distinguishing characters of the two genera are eroded by intermediate conditions in new, but also in already known taxa, and discuss the remaining characters available for unambiguous genus assignment. We provide keys to the species and review the geographic distribution. We will refrain from taking decisions affecting the genus- and subfamily classification until such time that sufficient independent molecular support may become available. Recently, molecular evidence was presented that Raspailiidae, currently assigned to the suborder Microciona of the order Poecilosclerida (Hooper, 2002) is probably not closely related to the chela-bearing Poecilosclerida (
Specimens of Cyamon and Trikentrion present in the collections of the Zoological Museum of Amsterdam and the Rijksmuseum van Natuurlijke Historie at Leiden (together now the Naturalis Biodiversity Center) were available from West Africa (two new species from a locality off Mauritania shown in Fig. 3, old collection specimens from Ghana), from the West Indian region (Curaçao and Colombia), the Seychelles, Indonesia and North Australia. We obtained loans of type material of most species from the collections of BMNH, USNM, MNHN, SMF, and LACM. One of us (JH) additionally examined fragments of Cyamon vickersii (Bowerbank, 1864) and Trikentrion flabelliforme Hentschel, 1912 obtained on loan from ZMB and SMF respectively. Non-type material of species of both genera was obtained on loan from BMNH and USNM (see below for abbreviations), and one of us (JLC) examined fresh material of Cyamon koltuni Sim & Bakus, 1986 and Cyamon (=Trikentrion) catalina Sim & Bakus, 1986. Details of collection numbers and localities are provided below with each species.
Abbreviations of institutions cited in the text:
AHF-NHMLA Allan Hancock Foundation, Natural History Museum Los Angeles County, USA
BMAG Bristol Museum and Art Galleries, Bristol, UK
BMNH British Museum of Natural History, London, UK
LEB-ICML-UNAM sponge collection Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autonoma de Mexico (Estación Mazatlán), México
MNHN Muséum National d’Histoire Naturelle, Paris, France
RMNH Rijksmuseum van Natuurlijke Historie Leiden (now part of Naturalis Biodiversity Center)
SMF Senckenberg Museum, Frankfurt, Germany
USNM United States National Museum, Washington, USA
ZMA Zoological Museum Amsterdam (now part of Naturalis Biodiversity Center)
ZMB Zoologisches Museum Berlin, Germany
Terminology: We employ the collective word ‘polyactine’ for the spicules previously named acanthotriaenes by
Microscopic preparation: dissoluted spicule preparations for measurements and SEM observations were made by dissolving a small fragment of the sponge in concentrated HNO3 or in undiluted household bleach, subsequent rinsing at least five times in distilled water, the last time in ethanol 96%, and finally pipetting a spicule suspension on stub or slide to be dried in a stove. Thick sections of the sponge made for the study of the skeletal structure were air-dried on a hotplate or in a stove and embedded in Canada balsam. Measurements of spicules (minimum-average-maximum) were made of 25 spicules of each category for each individual, unless otherwise stated (e.g. long thin spicules were often broken so the required number of spicules could not be measured).
ResultsWe present the results in the following seven sections: a refined description and illustration of the type material of the type species of Cyamon, Cyamon vickersii, in which we argue that its original locality has been misinterpreted, followed by a description of recent (1993) Seychelles material considered to belong to Cyamon vickersii; description of two new Cyamon species from West Africa; descriptions and illustrations of all species assigned to Cyamon previously, including a new species based on misidentified material; a refined description of the specimens of the type species Trikentrion muricatum (Pallas, 1766) including assignment of a neotype; descriptions of the remaining species, including proposed synonymies and the description of a new species of Trikentrion based on misidentified material; we provide a key to the recognized species of Cyamon and Trikentrion; we make summary remarks on the geographic distribution of the two genera.
Phylum Porifera Class Demospongiae Order Poecilosclerida Suborder Microcionina Family Raspailiidae Subfamily CyamoninaeDictyocylindrus vickersii Bowerbank, 1864 (original designation).
(emended): Cyamoninae with skeleton consisting of a basal mass of polyactine spicules of which one or more cladi are spined or rugose in mature condition, supporting a plumose choanosomal skeletal arrangement of single or columnar groups of styles or subtylostyles with pointed ends outwards. Additional longer and shorter thin styles may be present in peripheral regions.
The styles are usually smooth, but in Cyamon spinispinosum (Topsent, 1904) both shorter and longer styles are spined (see below). In the type species, and several other species, thin short styles take the form of angulated and/or centrotylote strongylostyles, some of which have one end faintly or more heavily spined (see below). Polyactine spicules are genuinely polyaxone, with axial canals visible in all cladi. They are predominantly calthrops-like and have four cladi, but this may vary between two and eight cladi in some species. Usually, one of the cladi differs from the others by having a pointed spined apex, whereas the other cladi frequently have rounded ends, with prominent spined bulbs in several species, or they are occasionally entirely smooth, differing frequently also in length (either longer or shorter) from the other cladi. The spined pointed cladus is termed ‘basal’, under the assumption that it is homologous to the shaft of an ancestral echinating acanthostyle. The remaining cladi are here termed ‘lateral’, based on the assumption they are lateral proliferations of the acanthostyle head. One of the new species described below, has the polyactine spicules in two distinct categories, the smaller one of which is ‘amphipolyactine’ (see below).
Trikentrion Ehlers, 1870 shares the polyactines with Cyamon. According to the latest treatment of both genera (Hooper, 2002) the polyactines of Cyamon would have all the cladi spined, whereas those of Trikentrion would have only the basal cladus spined. If this distinction between Cyamon and its close relative Trikentrion in the cladus spination would be maintained, then four species originally described as members of Cyamon would need to be transferred to Trikentrion, Cyamon quinqueradiatum, Cyamon neon de Laubenfels, 1930, Cyamon argon Dickinson, 1945 and Cyamon catalina, as well as one of the new species described below. We will demonstrate below and in the Discussion that cladus spination does not coincide with other more compelling differences with Trikentrion and consequently we will not transfer (all) the mentioned taxa.
The species considered valid members of Cyamon are listed in Table 1 and their properties in Table 2.
Summary of taxonomic decisions on Cyamon and Trikentrion species
| Cyamon agnani (Boury-Esnault, 1973 as Timea): valid species |
| Cyamon amphipolyactinum sp. n.: new species |
| Cyamon argon Dickinson, 1945: valid species |
| Cyamon arguinense sp. n.: new species |
| Cyamon aruense Hentschel, 1912: valid species |
| Cyamon catalina Sim & Bakus, 1986: transferred to Trikentrion |
| Cyamon dendyi De Laubenfels, 1936: j. syn. of Cyamon vickersii |
| Cyamon hamatum sp. n.: new species based on misidentified material of Cyamon vickersii sensu Burton and Rao 1931 |
| Cyamon incipiens (Topsent, 1928 as Acantheurypon): j. syn. of Cyamon spinispinosum |
| Cyamon koltuni Sim & Bakus, 1986: valid species |
| Cyamon neon De Laubenfels, 1930: valid species |
| Cyamon quadriradiatum (Carter, 1880 as Microciona): species inquirenda |
| Cyamon quinqueradiatum (Carter, 1880 as Microciona): species inquirenda |
| Cyamon spinispinosum (Topsent, 1904 as Hymeraphia): valid species, atypical, possibly not a Cyamon |
| Cyamon toxifera Arndt, 1927: mixture of Cyamon agnani and Clathria (Microciona) ferrea (De Laubenfels, 1936) |
| Cyamon vickersii (Bowerbank, 1864 as Dictyocylindrus): valid species, type species, type locality proposed to be Indian Ocean, Central West Atlantic specimens transferred to Cyamon agnani |
| Trikentrion africanum sp. n.: new species, formerly Trikentrion laeve sensu Burton, 1948 |
| Trikentrion catalina (Sim & Bakus, 1986 as Cyamon): valid species |
| Trikentrion flabelliforme Hentschel, 1912: valid species |
| Trikentrion helium Dickinson, 1945: valid species |
| Trikentrion laeve Carter, 1879: valid species |
| Trikentrion muricatum (Pallas, 1766 as Spongia): valid species, type species |
| Trikentrion papillosa (Sollas, 1879 as Plectronella): j. syn. of Trikentrion muricatum |
Summary of characters and spicule data of the species of Cyamon and Trikentrion considered valid in this study.
| Genus | Species | Shape | height | Long thin style | Short thin style | short thin style centrotylote | Short thick style | Oxea | Polyactine cladi | Basal cladus | Lateral cladus | Tricho-dragmas |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyamon | Cyamon vickersii | massive | 30 mm | 1700–2200 × 14–22 | 347–490 × 3.5–7 | yes, spined | 361–678 × 15–32 | not present | 3–5 | 54–102 × 9–18 | 39–78 × 7–16 | not present |
| Cyamon | Cyamon amphipolyactinum sp.n. | encrusting | 3 mm | 1058–1643 × 6–12 | 288-456 × 2-4 | no | 204–558 × 9–33 | not present | (1) 3–6 (2) 5–10 | (1)21–51 × 3–10 (2) 18–30 × 1–4 | (1)22–51 × 3–10 (2) 9–24 × 1–3 | not present |
| Cyamon | Cyamon arguinense sp.n. | encrusting | 2–3 mm | 1229–1668 × 12–18 | 244–719 × 2.5–9 | no | not present | not present | 4–5 | 51–69 × 5–8 | 31–78 × 4–8 | not present |
| Cyamon | Cyamon agnani | encrusting | 3–5 mm | 960–2065 × 7–9 | 210–658 × 1.5–4 | no | 174–489 × 7–21 | not present | 3–5 | 32–66 × 3–10 | 30–87 × 4–10 | not present |
| Cyamon | Cyamon aruense | massive | 30 mm | 162–1760 × 9–16 | 302–426 × 1.5–4 | yes | 297–456 × 8–17 | not present | 3–5 | 48–84 × 5–11 | 29–54 × 4–8 | not present |
| Cyamon | Cyamon koltuni | encrusting | 1 mm | 900–1400 × 5–7 | not present | no | 150–425 × 10–25 | not present | 3–6 | 35–66 × 5–10 | 35–66 × 5–10 | not present |
| Cyamon | Cyamon neon | massive | 20 mm | 860–1290 × 6–10 | 191–306 × 1.5–3 | yes, spined | 270–468 × 14–24 | not present | 2–4 | 33–69 × 6–14 | 30–132 × 7–14 | not present |
| Cyamon | Cyamonargon | arborescent | 35 mm | 960 × 15 | 210–348 × 3–4 | yes, spined | 350–593 × 15–42 | not present | 2–5 | 33–78 × 6–22 | 30-162 × 5–21 | not present |
| Cyamon | Cyamon quadriradiatum | encrusting | not known | 1042 × 41 | 347 | yes? | not present ? | not present | 4 | 76 | 76 | not present |
| Cyamon | Cyamon quinqueradiatum | encrusting | 3 mm | 129–1989 × 3-33 | 492–698 × 3–5 | no | 129–1989 × 3–33 | not present | 4–5 | 45–93 × 4–11 | 31–51 × 3–7 | not present |
| Cyamon | Cyamon hamatum sp. n. | encrusting | unknown | 1300 × 30 | 272–355 × 2.5–5 | yes, spined | 421–604 × 16–31 | not present | 3–4 | 104–126 × 11–21 | 42–65 × 10–20 | not present |
| Cyamon | Cyamon spinispinosum | encrusting | 1 mm | not present | 302–366 × 7–10 | yes | 657–822 × 32–38 | not present | 3–8 | 90–234 × 9–14 | 15–36 × 6–12 | not present |
| Trikentrion | Trikentrion muricatum | arborescent | 200 mm | not present | not present | no | not present | 287–528 × 13–31 | 2–3 | 78–156 × 12–27 | 42–84 × 12 –27 | 57–102 × 4–18 |
| Trikentrion | Trikentrion laeve | arborescent | 45 mm | 750–1062 × 4–9 | 234–433 × 0.5–2.5 | no | not present | 175–242 × 6–13 | 2–4 | 59–89 × 10–15 | 47–75 × 9–13 | 32–60 × 4–11 |
| Trikentrion | Trikentrion flabelliforme | flabelliform or arborescent | 60–260 mm | 405–1034 x3–9 | 182–392 × 0.5–4 | no | not present | 135–340 × 5–22 | 2–4 | 96–123 × 10–17 | 51–84 × 9–17 | 35–88 × 6–12 |
| Trikentrion | Trikentrion helium | bladed bush | 70 mm | 952–3393 x18–42 | 372–510 × 2.5–3.5 | no | not present | not present | 2–4 | 66–144 × 8–30 | 96–192 × 7–36 | 84–123 × 10–15 |
| Trikentrion | Trikentrion catalina | flabelliform | 150 mm | 1400–5400 × 8–40 | 130–730 × 3–8 | no | not present | not present | 3–4 | 79–126 × 16–31 | 156–236 × 18–29 | 63–88 × 7–13 |
| Trikentrion | Trikentrion africanum sp.n. | thin branch | 65 mm | 295–1394 × 9–24 | 192–358 × 2–3 | no | not present | not present | 2–3 | 27–96 × 11–21 | 33–121 × 9–19 | 49–61 × 5–11 |
http://species-id.net/wiki/Cyamon_vickersii
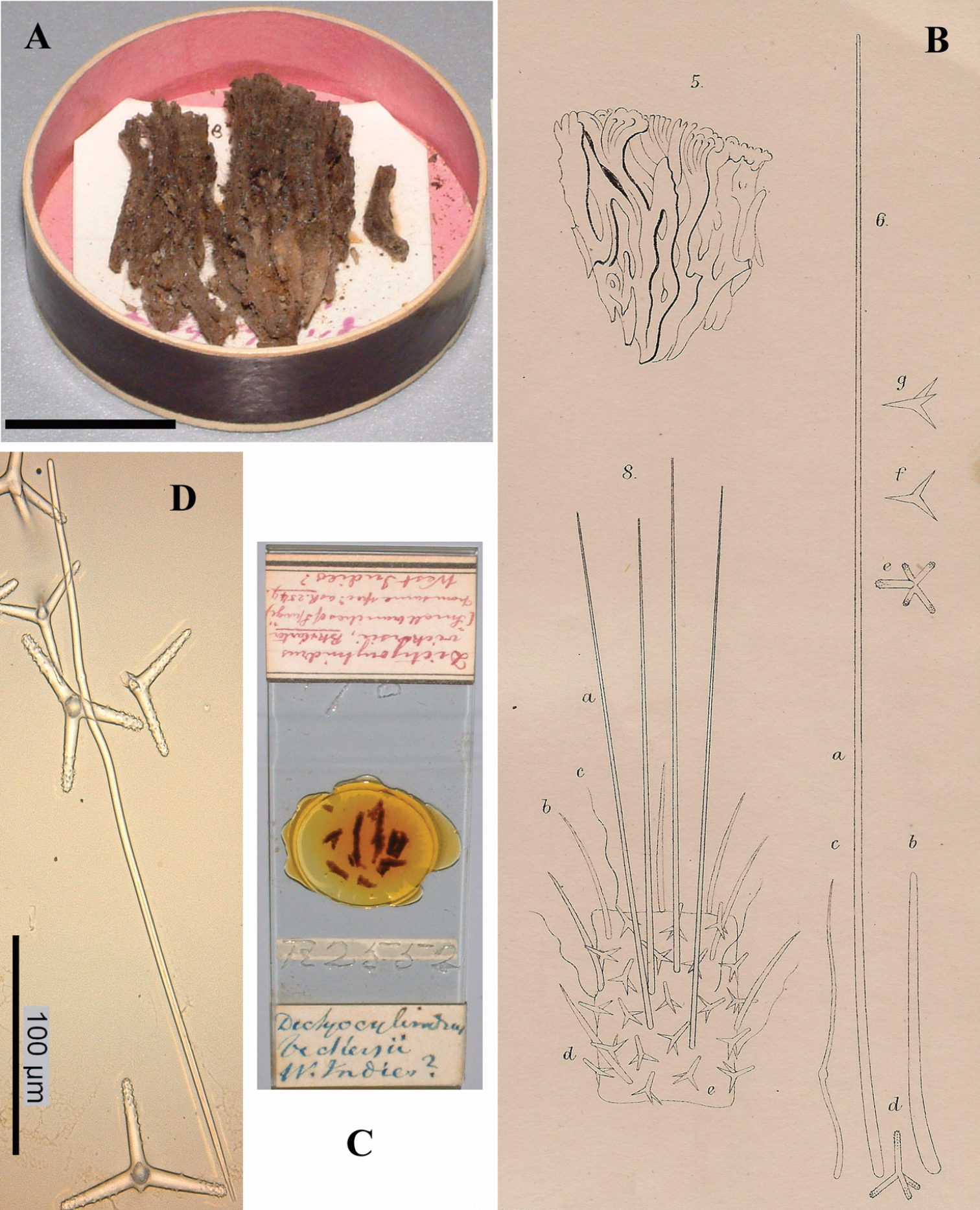
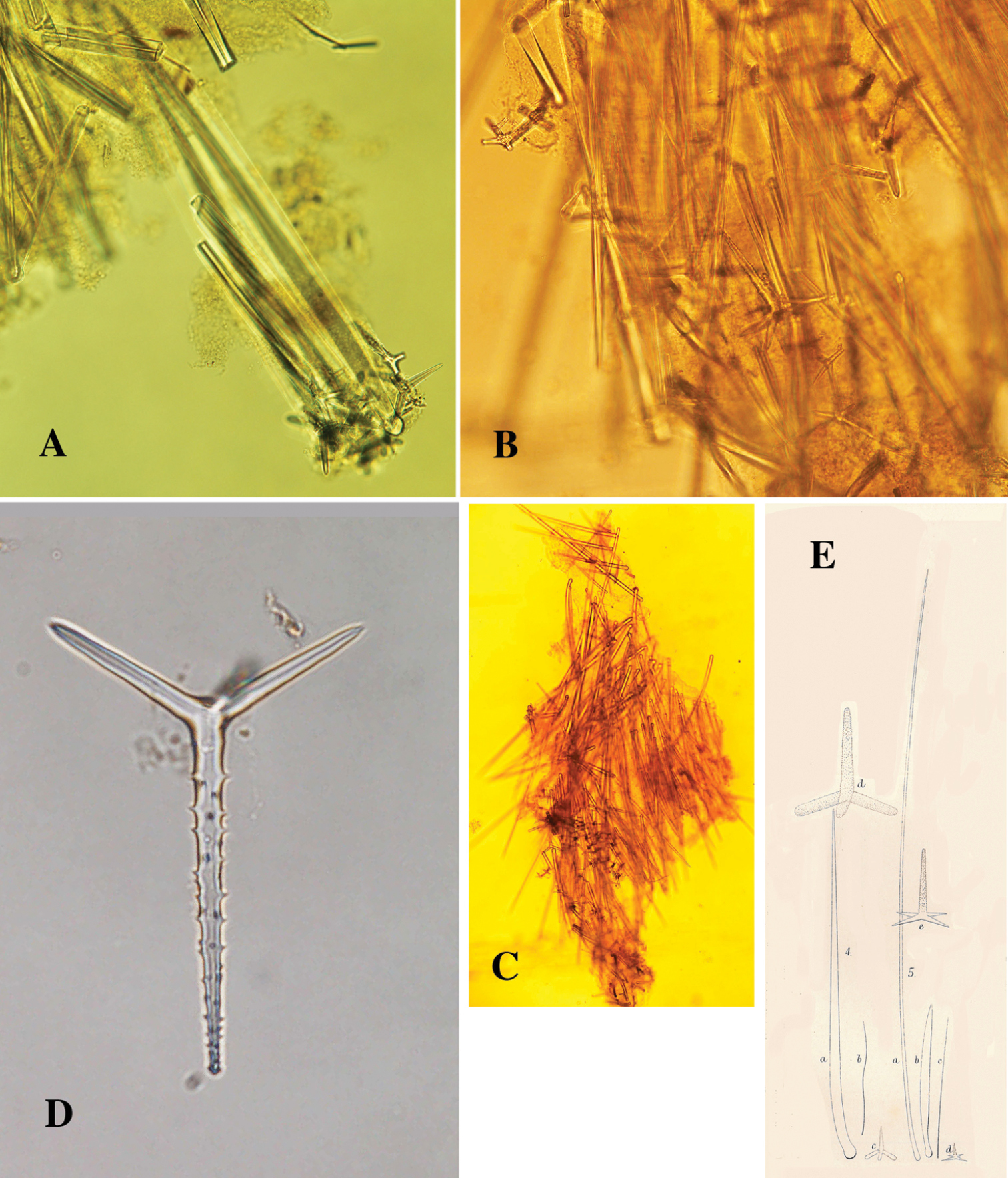
Figs 1A–D, 2A–DHolotypeBMNH 1877.5.21.1887, dry condition, labeled from Mr Vickers, Dublin, West Indies ?
The holotype was extensively described by
The specimen consists of a barely coherent mass of columns, fragile, crumbly. Size approx. 3 × 2.5 × 0.6 cm. Colour now dark red-brown.
Skeleton: a branched columnar structure built by bundles of short thick styles supported at the base and along the column by masses of polyactines. The remaining spicules are not readily visible in the sections, so their positions are derived from Carter’s drawings (Fig. 1B): the columns are echinated by long and short styles and wavy strongylostyles.
Spicules (Fig. 2): long thin styles, short thin (strongylo-)styles, short thick styles, polyactines.
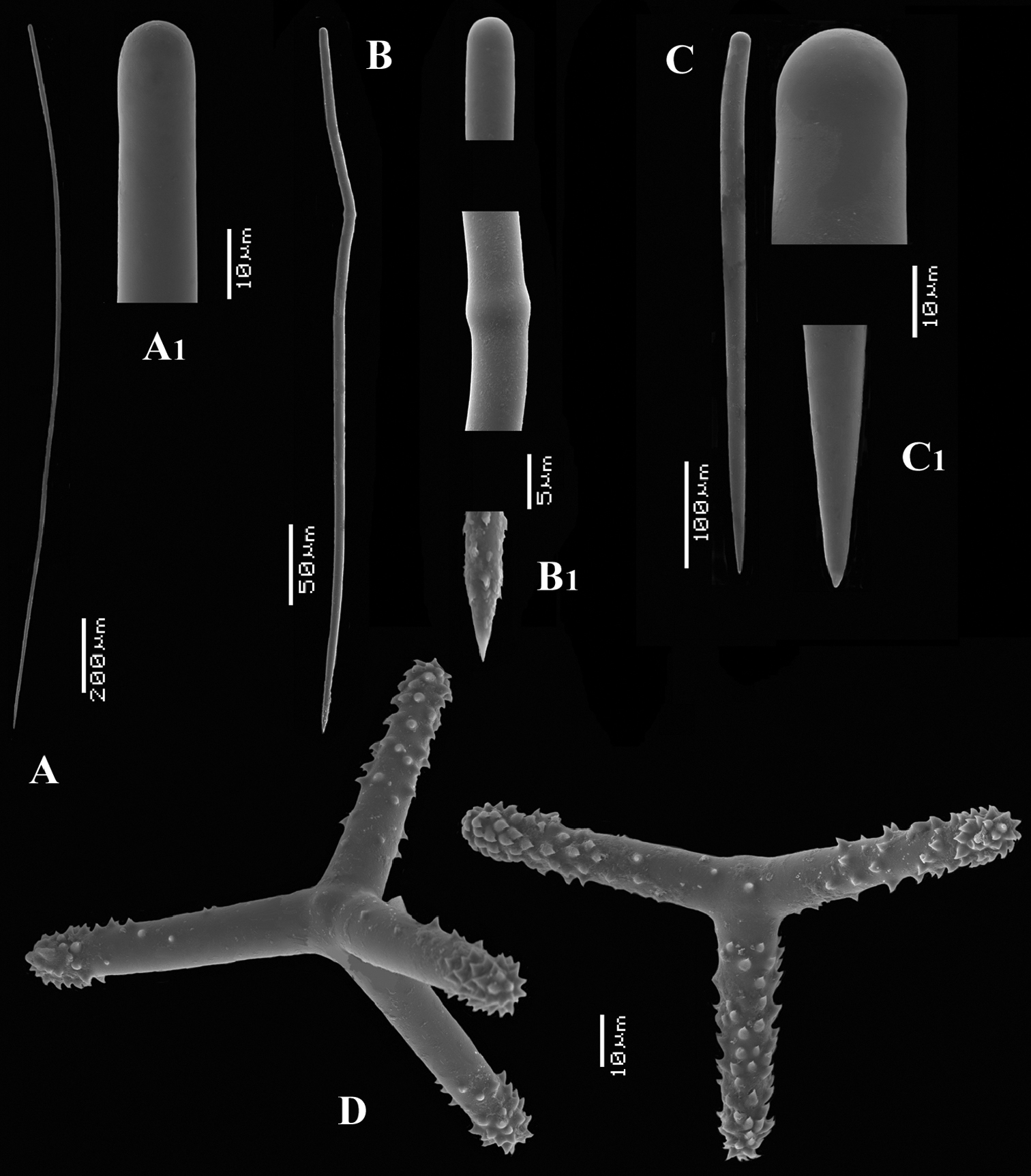
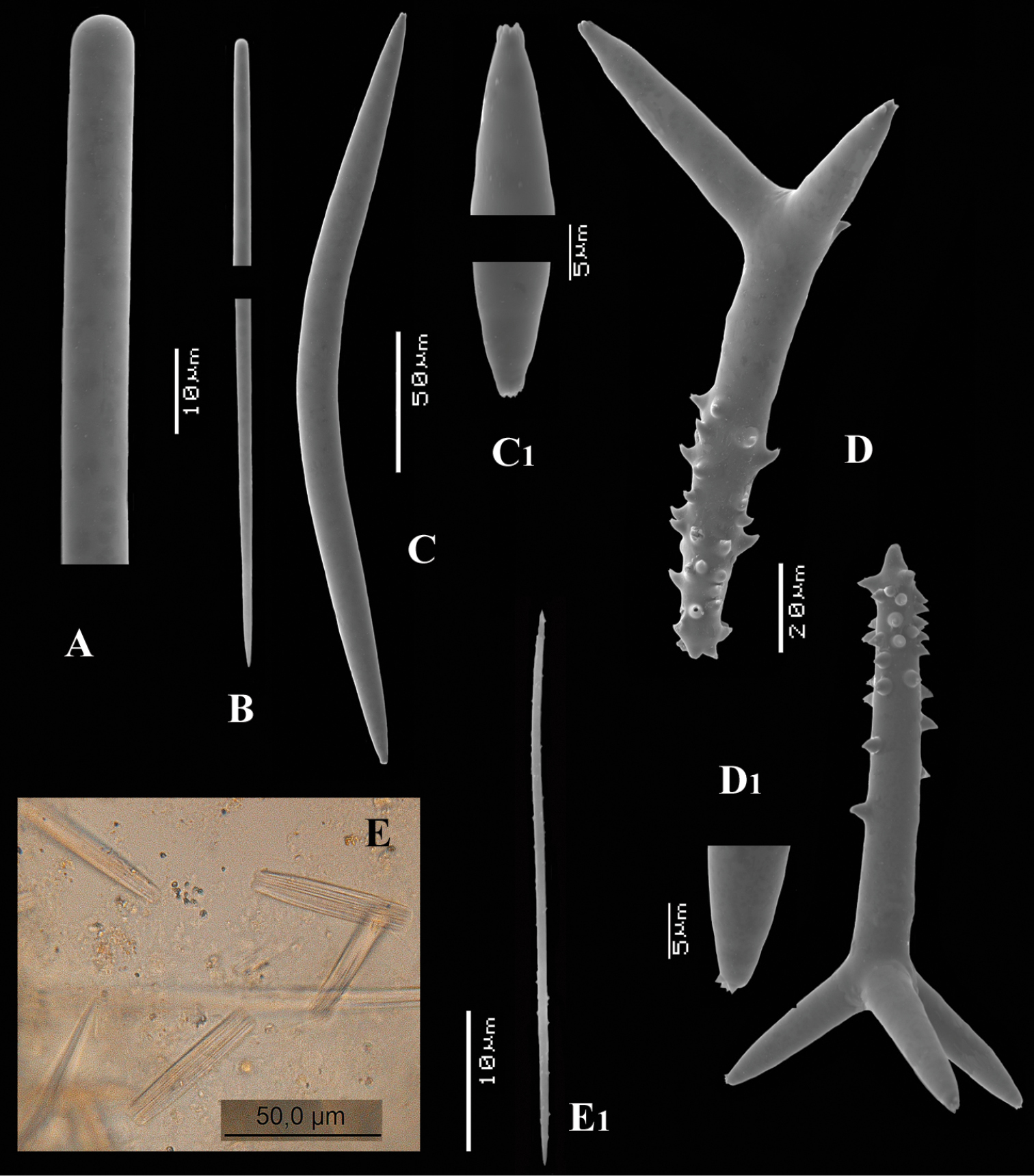
Long thin styles (Fig. 2A, A1) curved, usually broken, rounded end faintly constricted subterminally, 1785–2200 × 14–22 µm.
Short, thin, crooked or wavy, centrotylote styles (Fig. 2B, B1), sometimes strongylote, with the pointed end often swollen or mucronate, and faintly to markedly spined, 355–408.8–490 × 3.5–4.4–6 µm.
Short thick styles (Fig. 2C, C1), smooth, curved subterminally at the rounded end, 470–537.7–662 × 15–22.3–32 µm.
Polyactines (2D), robust, mostly equiangular, predominantly four-claded, three-claded forms also rather common, five-claded spicules rare and much smaller than the other; juvenile spicules almost entirely smooth, mature spicules with all cladi spined at the ends, which are also lightly swollen; only sparingly spined near the centre; all cladi approximately equal in length, basal cladi barely distinct from lateral cladi: basal cladi 55–62.5–69 × 10–12.6–16 µm, lateral cladi 50–65.6–78 × 9–12.4–15 µm.
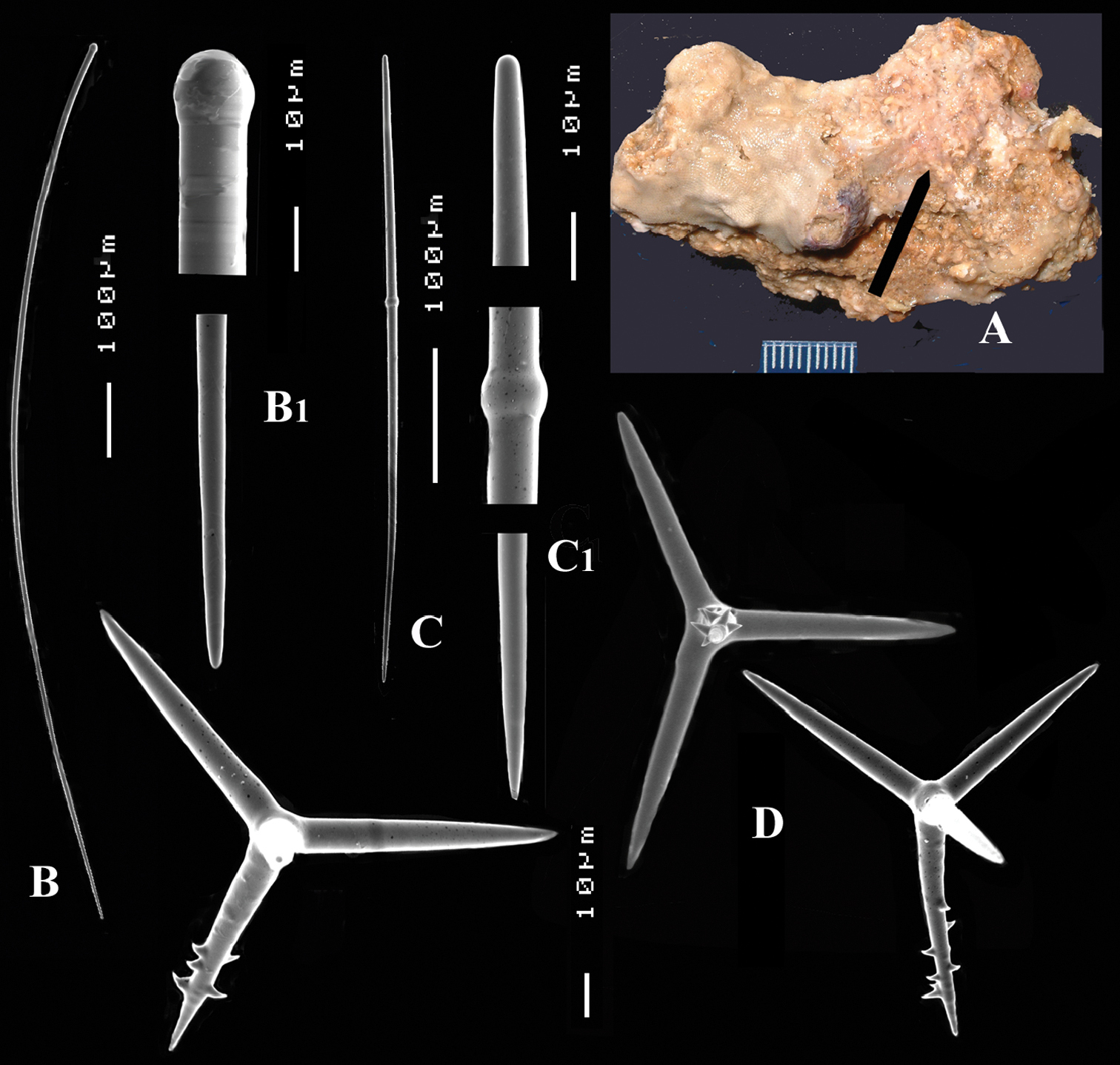
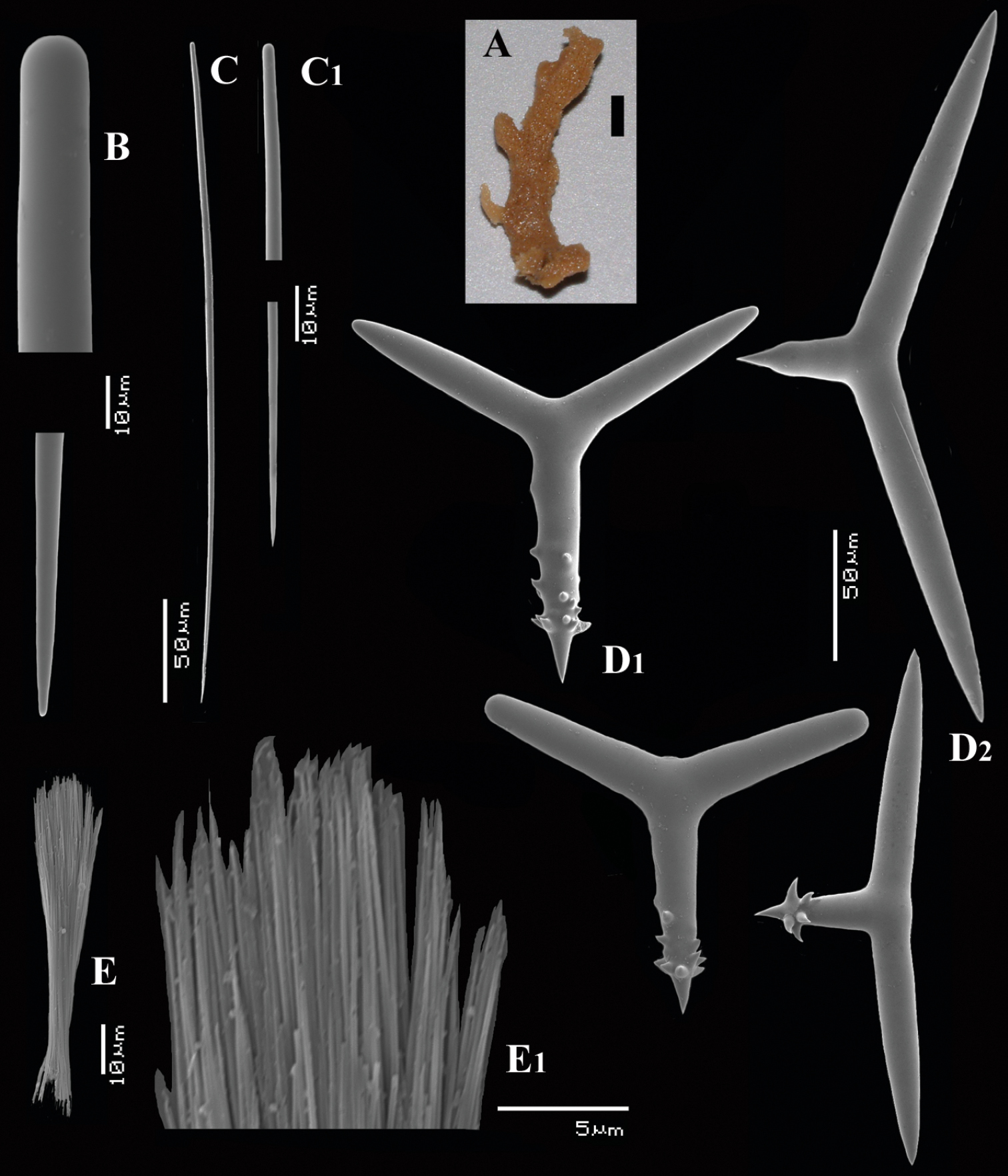
Cyamon vickersii (Bowerbank, 1864), holotype material, A holotype specimen BMNH 1877.5.21.188 (scale 1 cm) B illustrations from redescription of holotype by
Cyamon vickersii (Bowerbank, 1864), SEM images of spicules of the holotype BMNH 1877.5.21.188, A long thin style A1 details of apices of long thin style B short thin (strongylo-)style B1details of apices of short thin (strongylo-)style C short thick style C1 details of apices of short thick style D four-claded (left) and three-claded (right) polyactines.
Contrary to most other authors referring to Cyamon vickersii, we have become convinced that this species does not occur in the Western Atlantic. The evidence for this is two-fold.
(1) There is considerable uncertainty about the origin of the type specimen.
Spiculated inequi-angulated triradiate, with cylindrical entirely spined radii (Plate XXXVI. fig. 15). – From a fragment of a sponge presented to me by Mr. Vickers of Dublin, who thinks it probably came from the West Indies. This spiculum is an external defensive one. The triradiate rays are imbedded immediately beneath the dermal membrane, and the spicular ray is projected through it at right angles to its plane; they are very numerous.
The part of the sentence we placed in roman lettering contains the only factual information on the origin of the specimen, which was subsequently named Dictyocylindrus vickersii by
(2)
To conclude: specimens identical or similar to the type of Cyamon vickersii are reported from the Seychelles. Specimens recorded from the Western Atlantic are dissimilar to the type of Cyamon vickersii, a.o. by lacking the characteristic undulating spicules. For the Atlantic representatives, the name Cyamon agnani (Boury-Esnault, 1973) is available (see below).
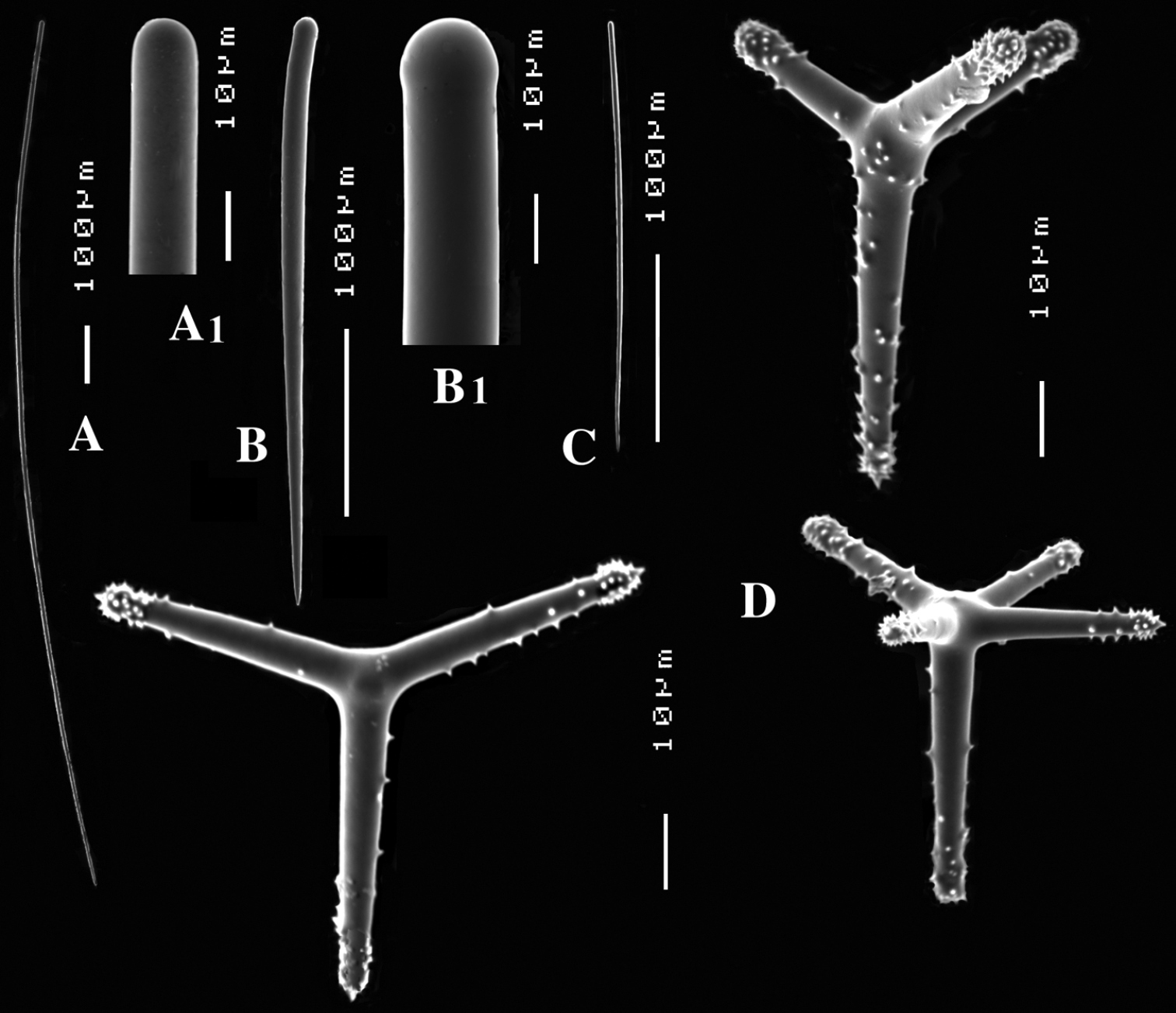
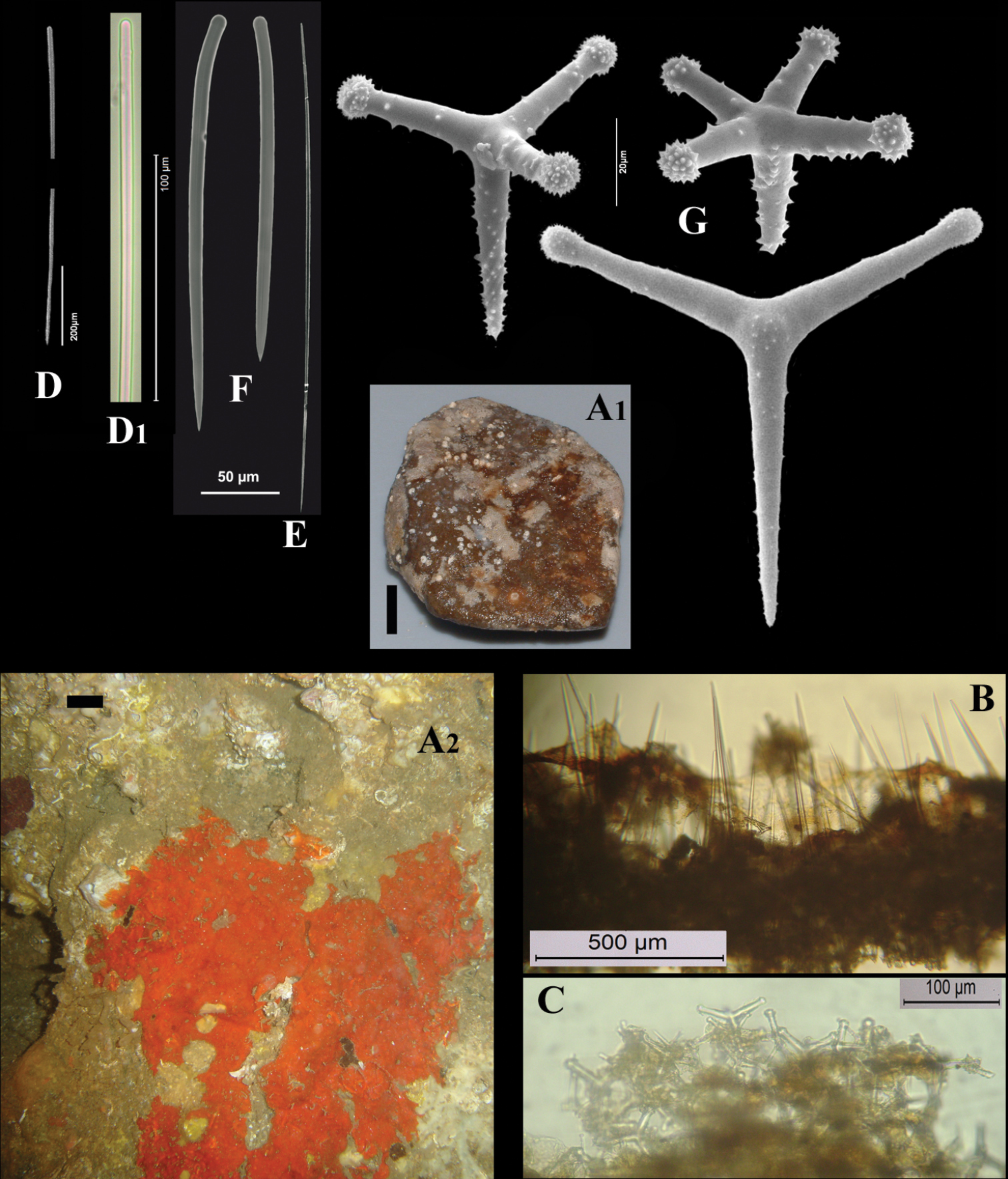
Cyamon vickersii (Bowerbank, 1864), ZMA material (Por. 10660) from the Seychelles A ‘strawberry’ shape (scale 1 cm) B long thin style C short thick styles D short thin (strongylo-)styles D1 details of apices of short thin (strongylo-)style E polyactines F overview of spicules.
Figs 3A–F
Three samples, ZMA Por. 11729, preserved in alcohol, Seychelles, Amirante Islands, N of Poivre Island, 5.7333°S, 53.3333°E, Netherlands Indian Ocean Programme, Leg E, stat. 776/05, rectangular dredge, depth 43–48 m, coll. R.W.M. van Soest, 29–12–1992.
ZMA Por. 10660, preserved in alcohol, Seychelles, Amirante Islands, NE of D’Arros Island, 5.4S, 53.3167E, Netherlands Indian Ocean Programme, Leg E, stat. 750/09, rectangular dredge, depth 48–53 m, coll. R.W.M. van Soest, 26–12–1992.
ZMA Por. 12558, preserved in alcohol, Seychelles, N of Aride Island, 4.1833S, 55.6667E, Netherlands Indian Ocean Programme, Leg E, stat. 716/09, rectangular dredge, depth 40 m, coll. R.W.M. van Soest, 19–12–1992.
N.B.: Dendy’s (1922) specimen labeled and described as Cyamon vickersii, BMNH 1931.1.1.19, Amirante, Sea Lark Expedition, 60 m, was examined and photographed by J.H. (Hooper, 2002: Fig. 17) but could not be found in the collection of the Natural History Museum in 2011 (Ms Emma Sherlock, in litteris).
Strawberry-shaped sponge (Fig. 3A), forming a single semiglobular mass with microlobate surface. Color red or orange-red (alive), dark brown-red in alcohol. Consistency firm, barely compressible. Specimens now looking clathrate due to loss of thin surface membrane, still present in places. Size of largest specimen 3 × 2 × 2 cm.
Skeleton: condition described as columnar, consisting of hillock-like masses of polyactines, variable in thickness up to 2 mm, supporting thick plumose bundles of thick styles, which in turn are peripherally surrounded by short thin strongylostyles. Rare long thin styles are not present in all slides.
Spicules (Figs 3B–F): long thin styles, short thick styles, strongylostyles, polyactines, overview presented in Fig. 3F.
Long thin styles (Fig. 3B), very rare, invariably broken in small pieces, largest piece found in our slides 300 × 12 µm; according to Dendy they can reach 1700 × 14 µm. We reconstructed a long style from several pieces found on the SEM stub (Fig. 3B).
Strongylostyles (Figs 3D, D1), angulated, often faintly centrotylote, with unequal endings, smoothly rounded at one end, spined-mucronate at the other, 294–347.1–402 × 4–5.6–7 µm.
Short thick styles (Fig. 3C), characteristically curved in the upper half and provided with a faint tyle, shape of spicule fusiform, smooth, occasionally strongylote, 361–538.9–678 × 16–24.1–31 µm.
Polyactines (Fig. 3E), three- or four-claded in approximately equal proportions, a single five-claded form was observed in the slides (Dendy shows a reduced two-claded form). Basal cladi bluntly pointed, heavily spined apically, lightly spined along the shaft, lateral cladi ending rounded, equally heavily spined apically, less so along the shaft. In the center of the spicule there are usually no spines. Young growth stages are frequently entirely smooth. Basal cladi usually longer, 54–77.5–102 × 9–14.4–18 µm, than the lateral cladi, 39–58.9–78 × 7–13.1–16 µm, regardless of the number of cladi.
So far known with certainty from several localities throughout the Seychelles (Mahé and the Amirante Islands).
Sandy bottoms at 30–50 m surrounding reefs and atolls.
The ectosomal strongylostyles in Cyamon vickersii are reminiscent of those found in the type species of the Axinellidae genus Reniochalina (Reniochalina stalagmitis Lendenfeld, 1888), which
urn:lsid:zoobank.org:act:3AD5636E-F603-4011-9967-F2DF5D32350A
http://species-id.net/wiki/Cyamon_amphipolyactinum
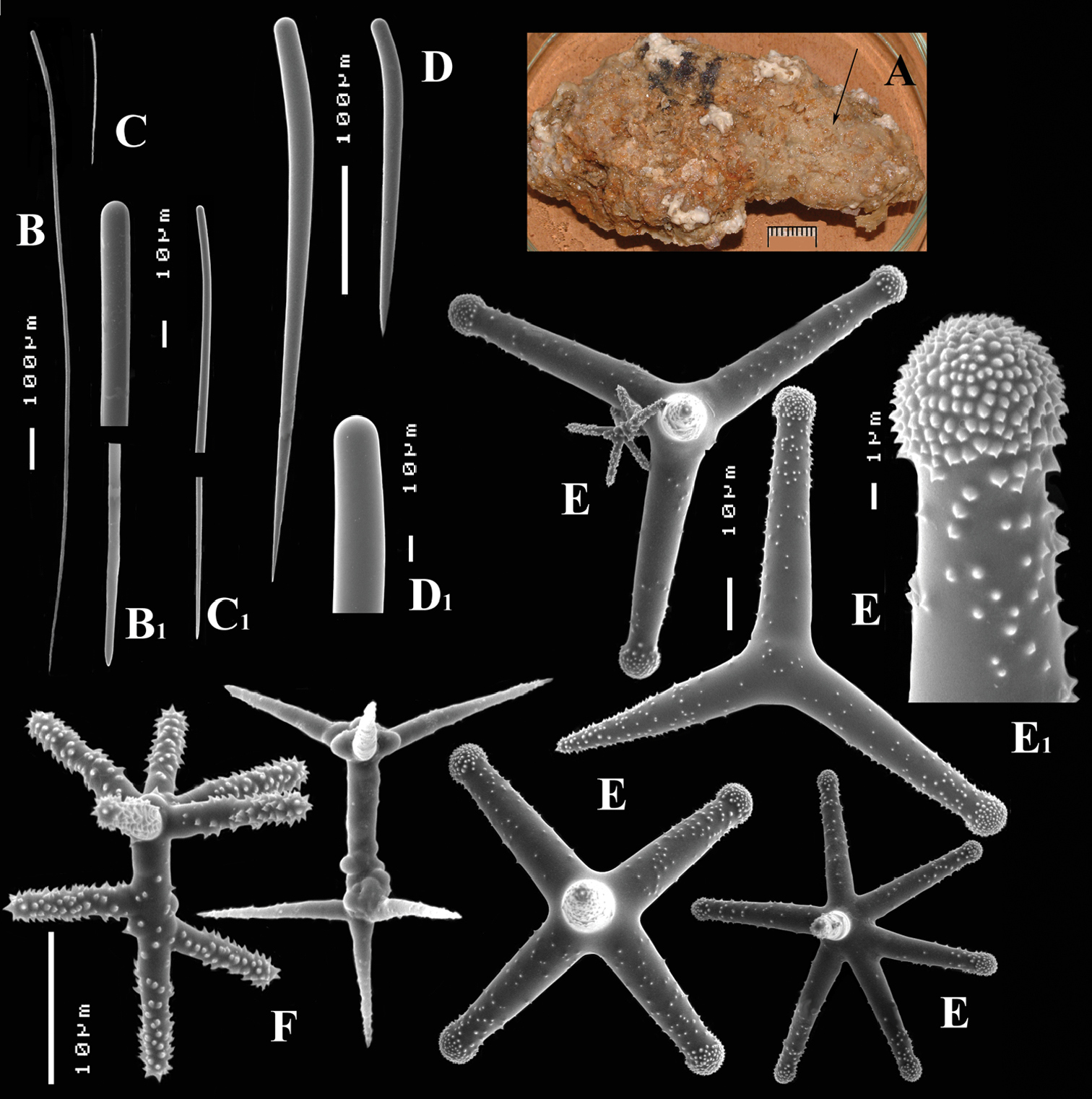
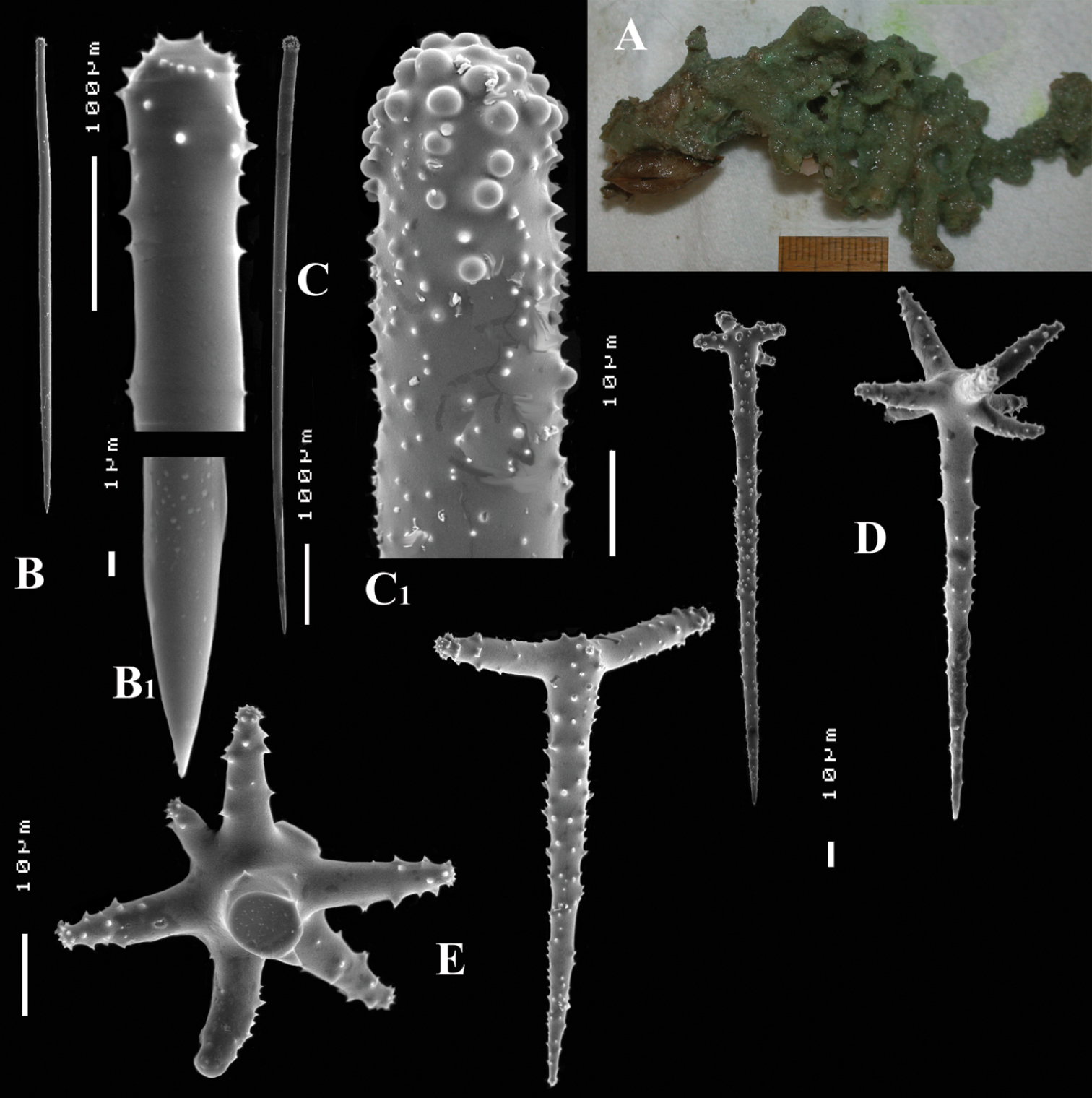
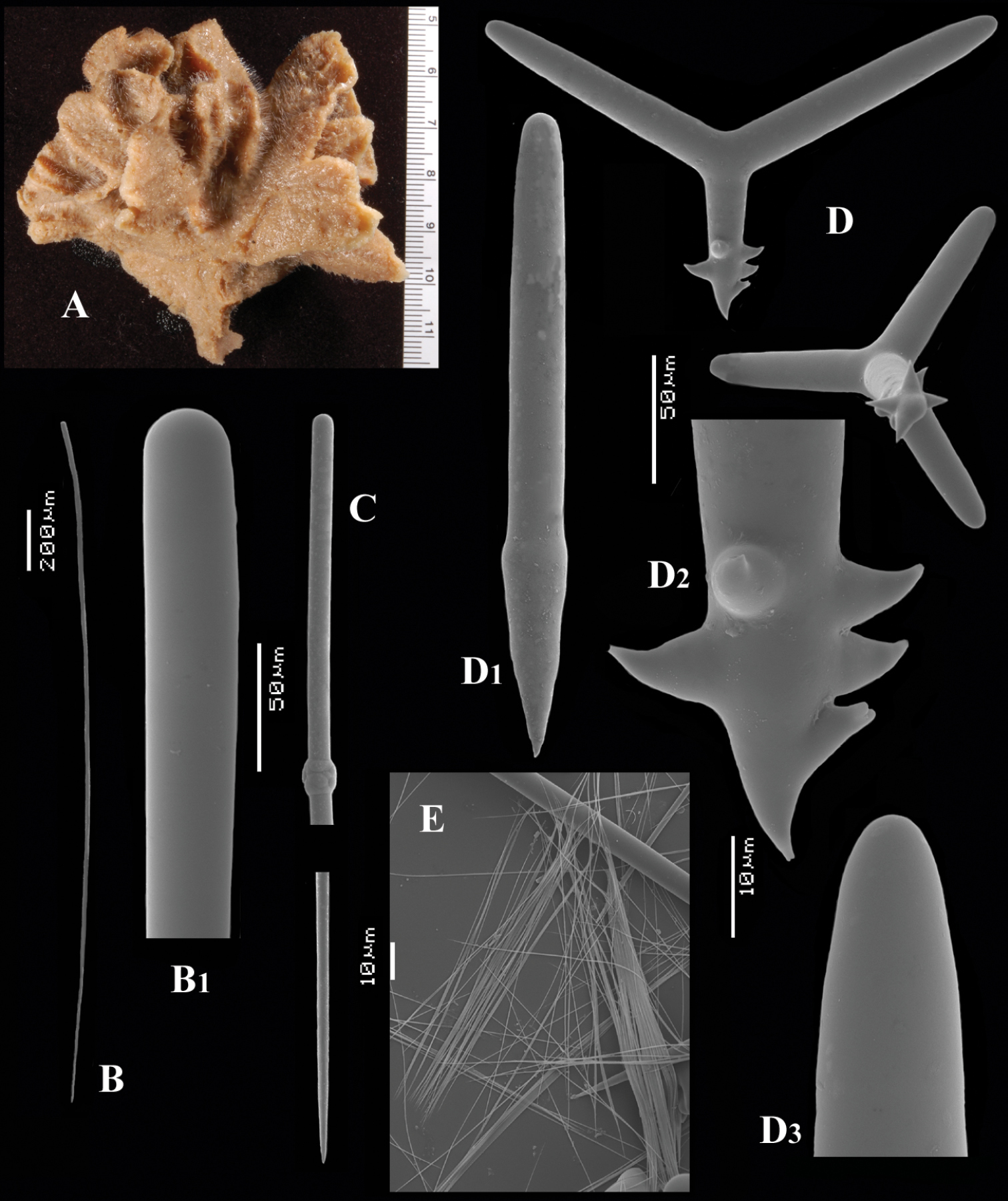
Figs 4A–E, 5Type specimen: HolotypeZMA Por. 22412, encrusting a stone, preserved in alcohol.
Type locality: Mauritania, off Banc d’Arguin, 19.0833°N, 16.4167°W, on sandstone ridge, dredged, 12–18 m. coll. R.W.M. van Soest & J.J. Vermeulen, Mauritania II Exped. Stat. 49, 11–06–1988.
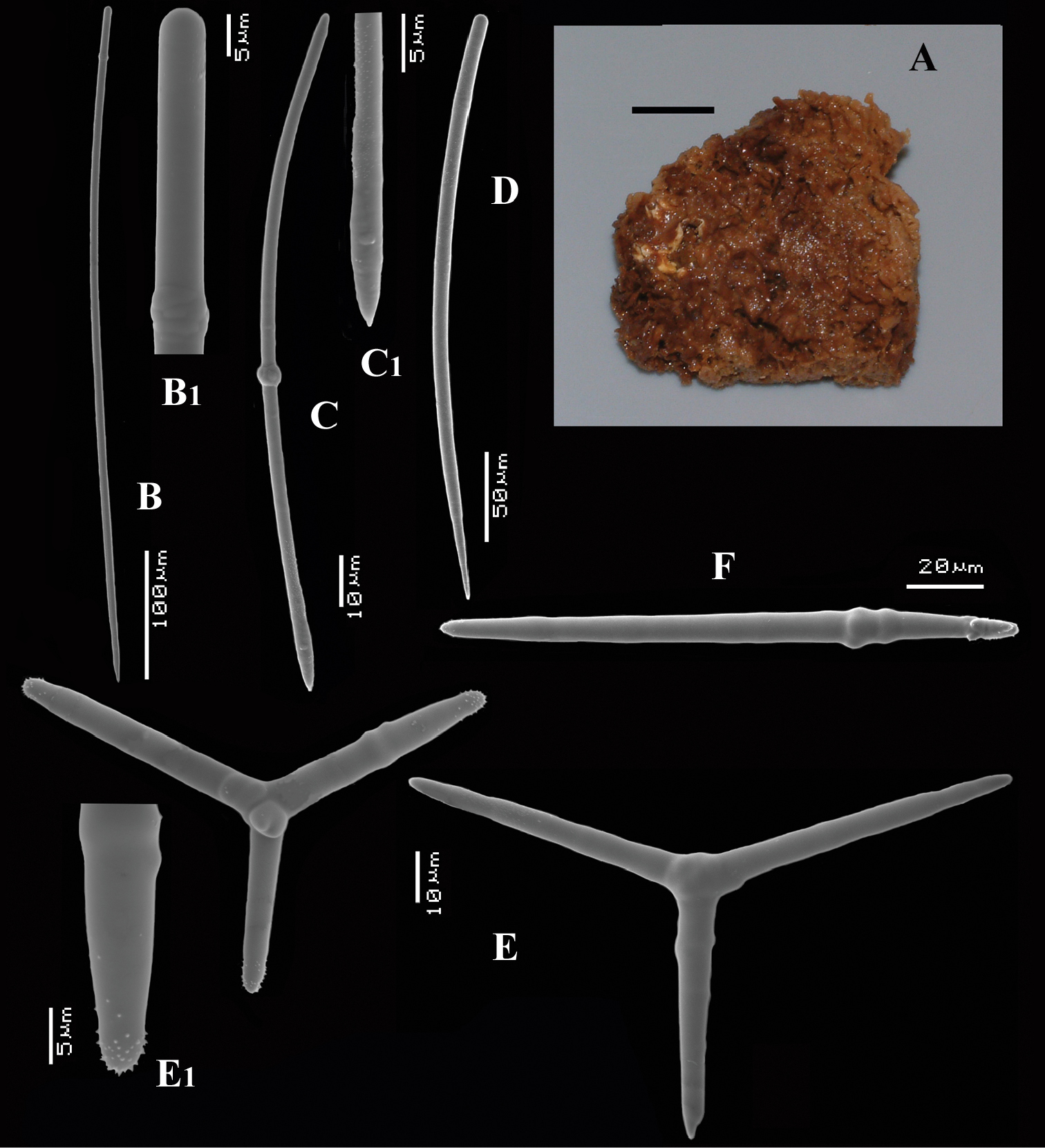
Encrusting a sandstone flake accompanied by several other encrustations (position of holotype indicated by arrow in Fig. 4A). Lateral size of holotype approximately 4x3 cm, thickness up to 3 mm. Color red in life, light orange brown in alcohol. Surface irregularly grooved and venous. Consistency soft, easily damaged.
Skeletal structure: A basal mass of polyactine spicules pierced by erect single or bundled thick styles, alternated by long thin styles protruding beyond the surface. At the periphery, the long styles are surrounded by bouquets of thin (tylo-)styles.
Spicules: of five types, long thin styles, short thin styles, short thick styles, large polyactines and small double polyactines.
Long thin styles (Figs 4B, B1), flexuous or curved snake-like, most were broken in the slides, size (based on 7 complete spicules): 1058–1294.0–1643 × 6–9.3–12 µm.
Short thin styles (Figs 4C, C1), curved, faintly tylote at the base, 288–374.9–456 × 2–3.2–4 µm.
Short thick styles (Figs 4D, D1), characteristically curved in the upper half, heads relatively thick with lower half narrowing strongly towards a sharp point, size varying strongly, 204–352.1–558 × 9–17.4–33 µm.
Large polyactines (Figs 4E, E1), in full-grown condition with all cladi ending in prominent, heavily spined knobs (Fig. 4E1) except one, the basal cladus, which is bluntly pointed. Cladi are less heavily spined towards the centre and at low magnification appear smooth. Growth stages may be partly or entirely without spines, but they are recognizable as unfinished by their irregularly undulating surface. The number of cladi varies between three and seven. In the largest spicules the cladi may be occasionally bifid. Basal cladi usually slightly shorter than the remaining cladi. Overall length of cladi regardless of condition is 18–51 × 3–10 µm.
Three-claded forms (rare), basal cladus 36–39 × 8–9 µm, lateral cladi 39–51 × 7–10 µm.
Four-claded forms (most common), basal cladus 18–51 × 3–9 µm, lateral cladi 22–51 × 3–9 µm.
Five-claded forms (also common), basal cladus 21–36 × 6–10 µm, lateral cladi 30–48 × 7–10 µm.
Six-claded forms (rare), basal cladus 21–36 × 4–5 µm, lateral cladi 24–38 × 4–6 µm.
Small double polyactines (Figs 4E and F), here termed amphipolyactines as they are obviously proliferated at both ends of the basal cladus. At first glance they resemble amphiasters or metasters (family Pachastrellidae Carter, 1875), but when studied with SEM they are similar in structure and ornamentation to the larger polyactines, but lack the swollen apices of the cladi of the larger ones. Cladi number from 5 to 10 (average 6.4) and they are spined in full-grown condition, smooth when still unfinished. Longest axis, presumably homologous to the basal cladus, is 18–30 × 1–4 µm, cladi 9–24 × 1–3 µm.
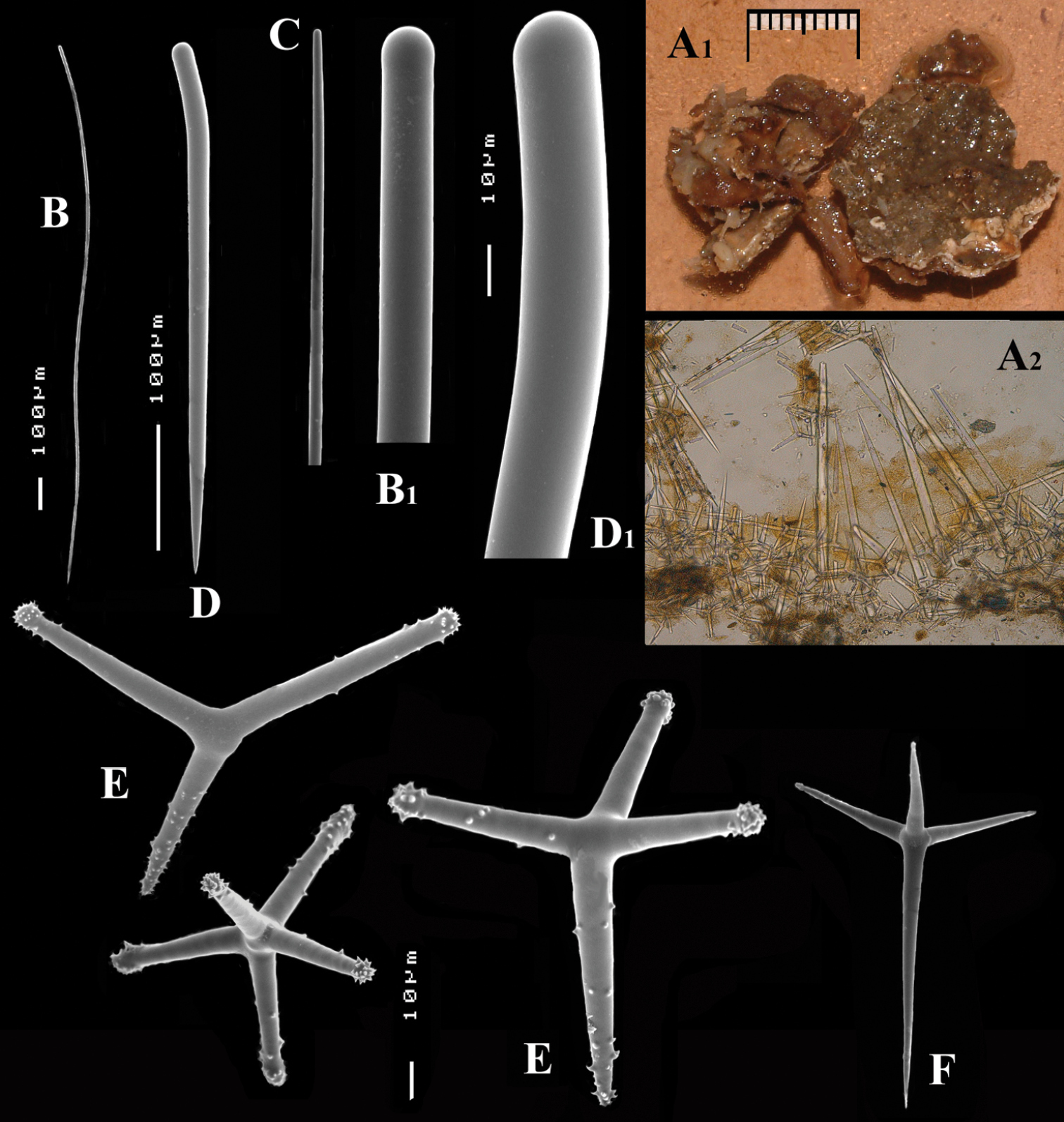
Cyamon amphipolyactinum sp. n., holotype ZMA Por. 22412, A shape (arrow) encrusting a fragment of sandstone (scale 1 cm) B long thin style B1 details of apices of long thin style C short thin style C1 details of apices of short thin style D short thick styles showing size variation D1 detail of head of short thick style E polyactines (three-, four-, five-, and seven-claded) and one amphipolyactine showing size differences E1 detail of bulbous end of lateral cladus F amphipolyactines full-grown and spined (left) next to incipient smooth spicule (right).
Map showing locality off the Mauritanian coast, where Cyamon amphipolyactinum sp. n. and Cyamon arguinense sp. n. were collected during the Netherlands Mauritania II Expedition, June 1988.
The name is an adjective that reflects the possession of unique small double polyactines, unprecedented in Cyamon and sponges in general.
(Fig. 5). So far known only from the sandstone ridges of coastal flats of the Banc d’Arguin, Mauritania, West Africa.
In shallow-water (12–18 m), highly sedimented environments, in the company of many other sand dwelling sponges such as Ciocalypta Bowerbank, 1862 and Polymastia Bowerbank, 1864 (cf.
The new species stands out among all described Cyamon and Trikentrion species by having unique double micro-polyactines. Further striking characters of the new species are the prominent heavily spined bulbous knobs of the large polyactines, which are only similarly developed in Californian Cyamon koltuni Sim & Bakus, 1986, and the high frequency of five-claded polyactines, which has been to that extent reported only for Cyamon quinqueradiatum (Carter, 1880) and Cyamon koltuni. The structure of the skeleton and the overall spiculation is shared with the type species of the genus, Cyamon vickersii and its close relative Cyamon agnani. Differences are the sizes of the spicules and the less prominent bulbous knobs on the cladi of the polyactines in the latter two species.
The remaining species appear more distinct with differences in the megascleres (apparent lack of thin styles in Cyamon spinispinosum and Cyamon koltuni), or the polyactine spicules (predominantly three cladi in Cyamon neon and Cyamon argon, smooth cladi except basal cladus in C. quinqueradiatum and Cyamon arguinense sp. n., irregular polyactines in Cyamon spinispinosum, lack of bulbous endings of the cladi and more densely overall spined in Cyamon quadriradiatum (Carter, 1880), and Cyamon aruense Hentschel, 1912).
urn:lsid:zoobank.org:act:0024C5CC-3BBE-4043-93C7-22F2766B7E13
http://species-id.net/wiki/Cyamon_arguinense
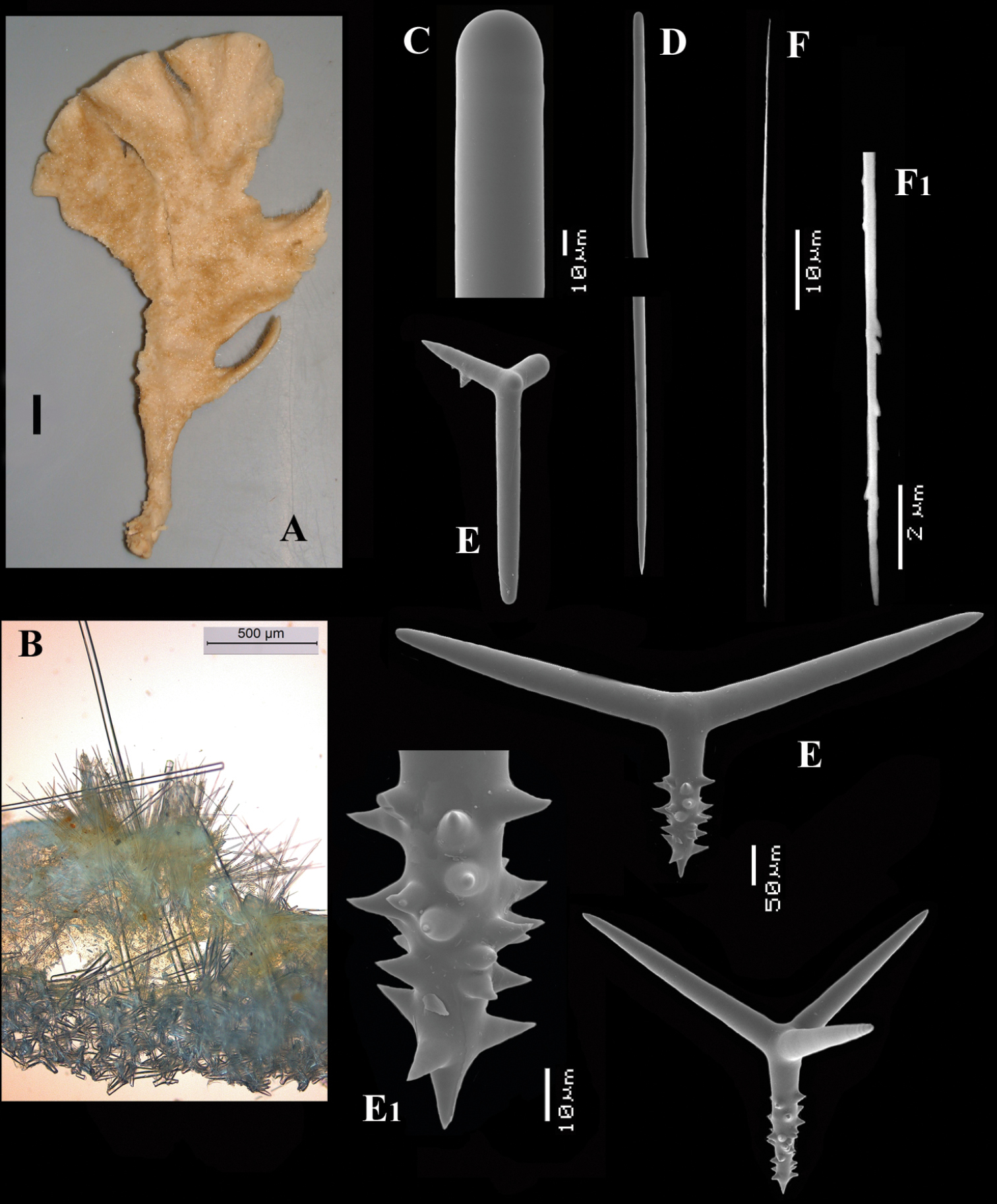
Figs 5, 6A–DType specimen: HolotypeZMA Por. 06723, encrusting a stone, preserved in alcohol.
Type locality: Mauritania, off Banc d’Arguin, 19.0833°N, 16.4167°W, on sandstone ridge, dredged, 12–18 m, coll. R.W.M. van Soest & J.J. Vermeulen, Mauritania II Exped. Stat. 49, 11–06–1988.
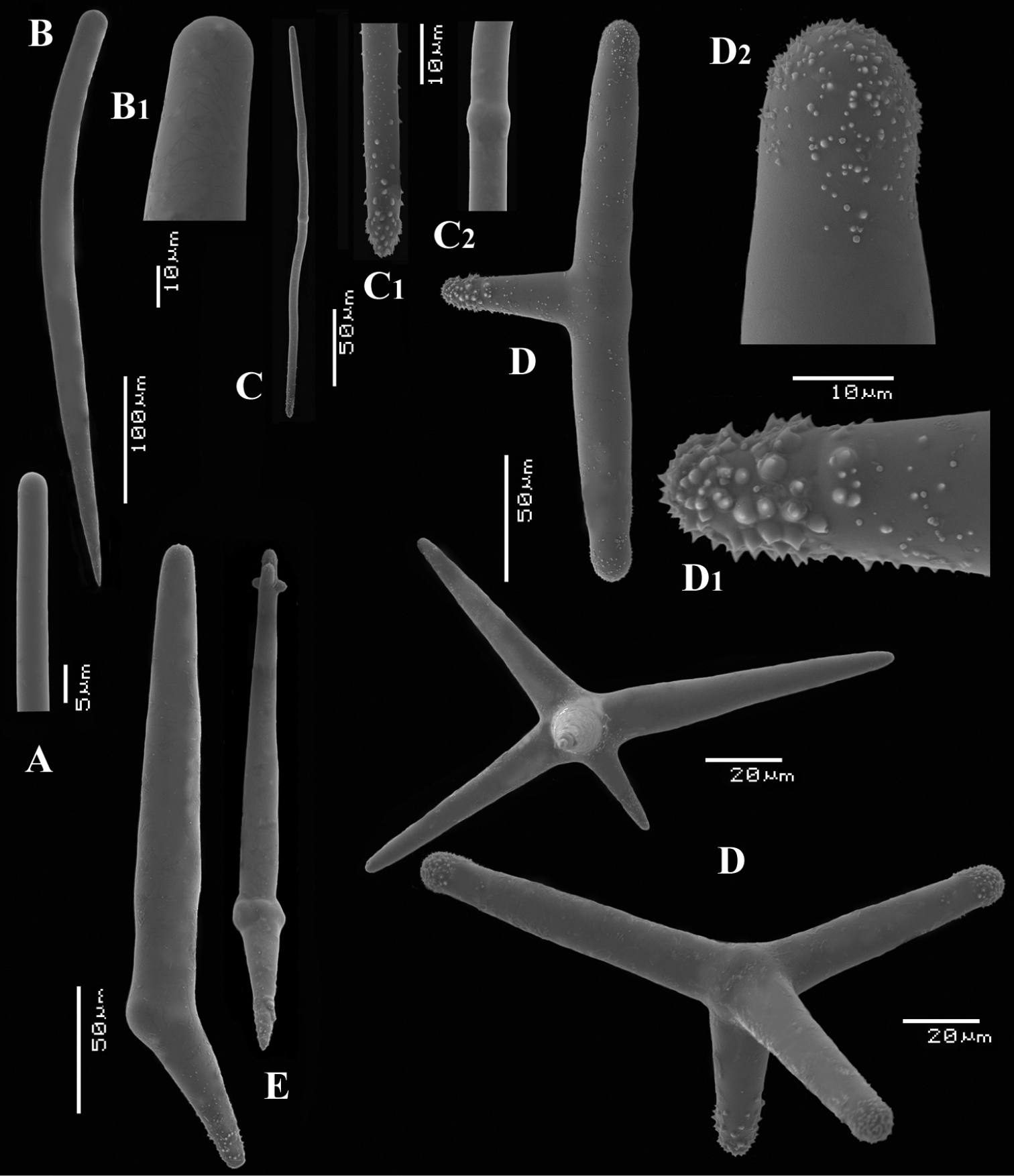
Thin crust, (Fig. 6A) hispid surface. Color red (alive), dirty white (alcohol). Consistency soft, easily damaged, size 2.5 × 1.5 cm × 2–3 mm.
Skeleton: columnar bundles of megascleres issuing from a basal layer of polyactines. Columns consist of a single long subtylostyle sheathed in a tight bundle of fusiform centrotylote styles; bundles separate, interconnected only near the substratum.
Spicules of three types: subtylostyles (assumed to be homologues of the long thin styles), centrotylote styles (assumed homologues of the short thin styles), polyactines (short thick styles apparently lacking).
Long thin (subtylo-)styles (Fig. 6B, B1) with prominent heads, and bluntly rounded pointed ends, 1229–1482.1–1668 × 12–13.9–18 µm.
Short thin styles, fusiform, centrotylote (Fig. 6C, C1), tyle slightly excentric, rounded end tapering, 244–521.5–719 × 2.5–6.4–9 µm.
Polyactines, (Fig. 6D) predominantly four-claded, (a few five-claded forms were observed), basal cladus with coarse recurved spines, lateral cladi entirely smooth, basal cladus 51–58.6–69 × 5–6.5–8, lateral cladi 31–55.7–78 × 4–6.1–8 µm.
The name is an adjective referring to the type locality: the Mauritanian nature reserve Banc d’Arguin, one of the richest faunal areas of the west coasts of Africa (cf.
(Fig. 5). So far known only from the sandstone ridges of coastal flats of the Banc d’ Arguin, Mauritania, West Africa.
In shallow-water (12–18 m), highly sedimented environments, in the company of many other sand dwelling sponges such as Ciocalypta and Polymastia (cf.
The single spined cladus of the polyactine spicules is an alleged feature of the genus Trikentrion, but in all other characters (growth form, monaxone spicules and skeletal arrangement) this is a typical Cyamon. It reminds strongly of Indian Ocean Cyamon quinqueradiatum, with which it shares the shape and upper length of the subtylostyles, the lack of differentiated long and short thick styles, and the size and single cladus spination of the polyactines. Differences are the predominantly five-claded polyactines and the shape and size of the stylote spicules in Cyamon quinqueradiatum. Long subtylostyles with prominent heads are shared with Indian Ocean Cyamon quadriradiatum but that species has all the cladi of the polyactines densely spined.
The new species was collected in the same dredge sample as Cyamon amphipolyactinum sp. n. (see above), but on a different sandstone flake (these provide hard substratum for sponges that would otherwise be buried in the sand). The two species differ sharply in the shape, size and ornamentation of the polyactines as well as in the shape and size of the styles.
Cyamon arguinense sp. n., holotype ZMA Por. 06723, A shape (arrow) encrusting a fragment of sandstone (scale 1 cm) B subtylostyle B1 details of apices of subtylostyle C short thin centrotylote style C1 details of apices and middle part of short thin centrotylote style D polyactines.
http://species-id.net/wiki/Cyamon_agnani
Figs 7A–D, 8A–FIn view of the proposed major change in the status of Cyamon specimens reported from the Western Atlantic, description of the available material is presented in two sections, first the holotype of Cyamon agnani, subsequently other specimens known from the area and proposed to be assigned to Cyamon agnani.
Figs 7A–D
HolotypeMNHN NBE 947, preserved in alcohol, Brazil, NE coast, Calypso stat. 97, 21.1667°S, 40.7°W, 12 m depth.
Small hispid crust, color ochre. Detachable skin. The material borrowed from MNHN measured a few mm2 encrusting a small piece of coral.
Skeleton: basal layer of polyactines, upon which megascleres are erected individually.
Spicules: long thin styles, short thick styles, polyactines.
Long thin styles, curved, variable in length, possibly in two size categories, but difficult to establish due to broken condition of most spicules, longest complete spicule 960 × 7 µm (Fig 7A).
Short thin styles were not mentioned in
Short thick styles (Fig. 7B), curved in the upper half, ending in a slight tyle, smooth, slightly variable in length and thickness, 183–236.7–315 × 7–9.3–12 µm.
Polyactines (Fig. 7D), with three to five cladi (usually four), cladi lightly spined along the shaft but with heavily spined endings, with a blunt ending in the basal cladus, and slightly inflated rounded endings in the lateral cladi. Basal cladi 32–38.5–48 × 3–4.8–7 µm, similar sized lateral cladi, 30–40 × 5 µm.
Cyamon agnani (Boury-Esnault, 1973), holotype MNHN NBE 947, A long thin style A1 detail of head of long thin style B short thick style B1 detail of head of short thick style C short thin style D polyactines.
Cyamon agnani (Boury-Esnault, 1973), ZMA Por 10539 from NE Colombia A1 shape (scale 1 cm) A2 cross section of skeleton B long thin style B1 detail of head of long thin style C upper part of short thin style D short thick style D1 detail of head of short thick style E polyactines F incipient polyactine showing smooth cladi.
The Cyamon nature of this material was previously detected by
Figs 8A–F
ZMA Por. 00828, holotype of Cyamon vickersii var. toxifera, preserved in alcohol, from Curaçao, Spaanse Water, on dead Porites coral, 12.076N, 68.858W, coll. C.J. van der Horst, field number 65a, 19–05–1920.
ZMA Por. 10539, preserved in alcohol, Colombia, Santa Marta region, El Morro, 15 m, 11.25N, 74.2167W, coll. B. de Jongh, 26–10–1989 (Fig. 1A2).
USNM 22456, preserved in alcohol, Florida, SE of Loggerhead Key, on a block of limestone dredged from 70 m, coll. M.W. de Laubenfels, 26 June 1932.
USNM 221078 (23563), preserved in alcohol, Florida, Northern Gulf of Mexico, Apalachee Bay, rock and sand, 29.785 – 29.8°N, 84.325°W, 11 m, coll. F. Little, 1956-57;
USNM 33518, preserved in alcohol, off South Carolina, RV Oregon (S.C. Mar. Res. BLM), stat. 0SO6, 32.4883°N, 78.8217°W, 48 m, collected by grab, 4 May 1981.
(Based on ZMA Por. 10539). Irregular encrustation (Fig. 8A1), with hispid, bumpy surface (preserved condition). Size 3 × 2.5 cm in lateral expansion, 3-5 mm in thickness. Colour (alive) red, (alcohol) red-brown. Consistency soft.
Skeleton (Fig. 8A2): basal mass of polyactine spicules penetrated by single short thick styles erect with heads embedded in the substrate. Long thin styles also erect on the substrate with rare short thin styles arranged around the peripheral protruding apices. This ‘raspailid’ feature was only observed in a few places.
Spicules: long thin styles, short thin styles, short thick styles, polyactines.
Long thin styles (Figs 8B, B1), complete ones with a wavy outline (Fig. 8B), but mostly broken in the slides, largest complete style 2065 × 9 µm, with smaller pieces varying down to 1170 × 7 µm.
Short thin styles, straight (Fig. 8C), 423–486.6–658 × 2–2.2–2.5 µm. We were unable to find a complete spicule on the SEM stub, so we only show a broken spicule in Fig. 8C.
Short thick styles, (Figs. 8D, D1) curved in the upper half, with a faint tyle, smooth, in a large size range, 174–358.2–489 × 9–14.4–21 µm.
Polyactines (Figs 8E–F), with three to five cladi (usually four), typically with all cladi mostly smooth but ending in a spined apex, the basal cladus usually bluntly pointed, the lateral cladi with inflated endings (Fig. 8E), early growth stages smooth and with all cladi pointed (Fig. 8F), cladi often of unequal length but without clear pattern of variation, basal cladi 39–56.4–66 × 6.5–8.3–10 µm, either longer or shorter than the lateral cladi, 36–61.6–87 × 4.5–7.6–10 µm.
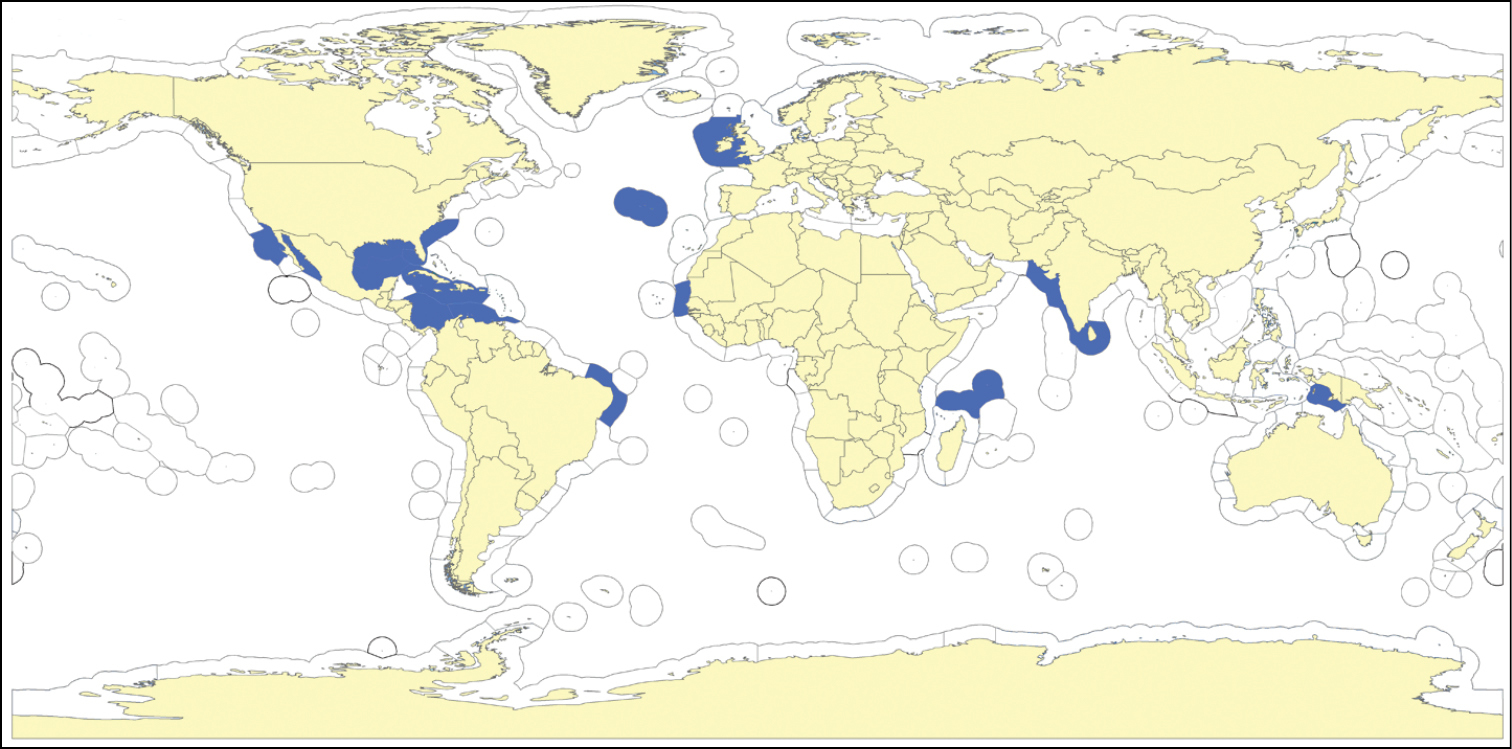
Greater Caribbean, Gulf of Mexico, South Carolina, N.E. Brazil.
Encrusting dead corals and other limestone substrates, 0–70 m.
The loan from the Smithsonian also included an undescribed specimen from South Carolina, USNM 33518. This had long thin styles of up to 2 mm, short thin styles 360–426 × 2–2.5 µm, short thick styles 410–500 × 22–23 µm, and polyactines (three- and four-claded) with basal cladi 48–93 × 12–15 µm and lateral cladi 45–49 × 12–14 µm.
We investigated the type material of Cyamon vickersii var. toxifera Arndt, 1927 (the name should be corrected to toxiferum to match the gender of the genus), ZMA Por. 00828, from Spaanse Water, Curaçao, and discovered that the toxas forming the basis of Arndt’s variety are clearly foreign. They form part of the spiculation of a microcionid sponge, readily identified as Clathria (Microciona) ferrea (De Laubenfels, 1936 as Fisherispongia) by its characteristic polytylote subtylostyles (see also description of Curaçao material of that species in
The spicule complement and the shape of the polyactines is broadly similar in the Brazilian type of Cyamon agnani and specimens recorded from Caribbean and Carolinean waters as Cyamon vickersii, but the latter may have long thin styles up to twice as long. The short thick styles and the polyactines also are on average clearly longer and more robust in Caribbean specimens. The geographic separation caused by the Amazonian outflow could be a barrier to gene flow between these shallow-water sponges, and the differences may thus have a genetic background. On the other hand, the Brazilian type material is only a single small specimen and variation in Brazilian waters may turn out to be as large as that in the Caribbean. Thus distribution and ecology for this species may be summarized as: tropical waters of Brazil, the Greater Caribbean and Gulf of Mexico, South Carolina, known from 0–70 m depth, usually encrusting dead corals and other limestone substrates.
http://species-id.net/wiki/Cyamon_aruense
Figs 9A–EFragment of holotypeSMF 1618, preserved in alcohol, Indonesia, Aru Islands, Straits of Dobo, 6°S, 134.8333°E, 40 m, coll. H. Merton, 20–03–1908.
The holotype is an encrusting sponge of 6 cm long and 3 cm wide growing over a haplosclerid sponge (Hentschel, 1912). The fragment of less than 0.5 × 0.5 cm and 1 mm in thickness (see Fig. 9A) examined by us was mixed with the haplosclerid in such a way that the microscopic slides were thoroughly contaminated with it. We have to rely on Hentschel’s remarks about shape and surface characters. The surface is hispid due to the long styles protruding from the sponge, which was grey coloured in alcohol, but shows a pale brownish colour in our fragment. Consistency not mentioned by Hentschel, but crumbly describes it best.
Skeleton: the usual basal mass of polyactinal spicules upon which relatively long styles are erected surrounded in the periphery by bundles of thin centrotylote styles. Thick short styles are singly erect on the substrate, buried in the basal mass of polyactines.
Spicules: long thin styles, centrotylote thin styles, short thick styles, polyactines.
Long thin styles (Figs 9B, B1), relatively rare, smooth, almost always broken in the slides so we cannot show a complete SEM image of them, heads smooth and not distinguished in width from the shaft, the other end gradually pointed. Longest style approximately 1620 × 16 µm, whereas Hentschel mentioned 1760 × 9–12 µm. Hentschel suggested a faint tyle, but we did not observe this.
Centrotylote thin styles (Fig. 9C, C1), smooth, curved, with a tyle near the middle of the spicule, but not exactly in the middle, the most common spicule of the monaxone spicule complement, 302–368.7–426 × 1.5–2.6–4 µm.
Short thick styles (Fig 9D, D1), relatively rare, smooth, often curved in the upper half, slightly fusiform, with a faint tyle, 297–389.8–456 × 8–13.9–17 µm.
Polyactines (Figs 9E) with 3-5 cladi, all of which are heavily spined with relatively coarse spines, without smooth areas, basal cladi rather blunt compared to those of other species, 48–68.9–84 × 5–8.1–11 µm, lateral cladi 29–40.6–54 × 4–6.7–8 µm.
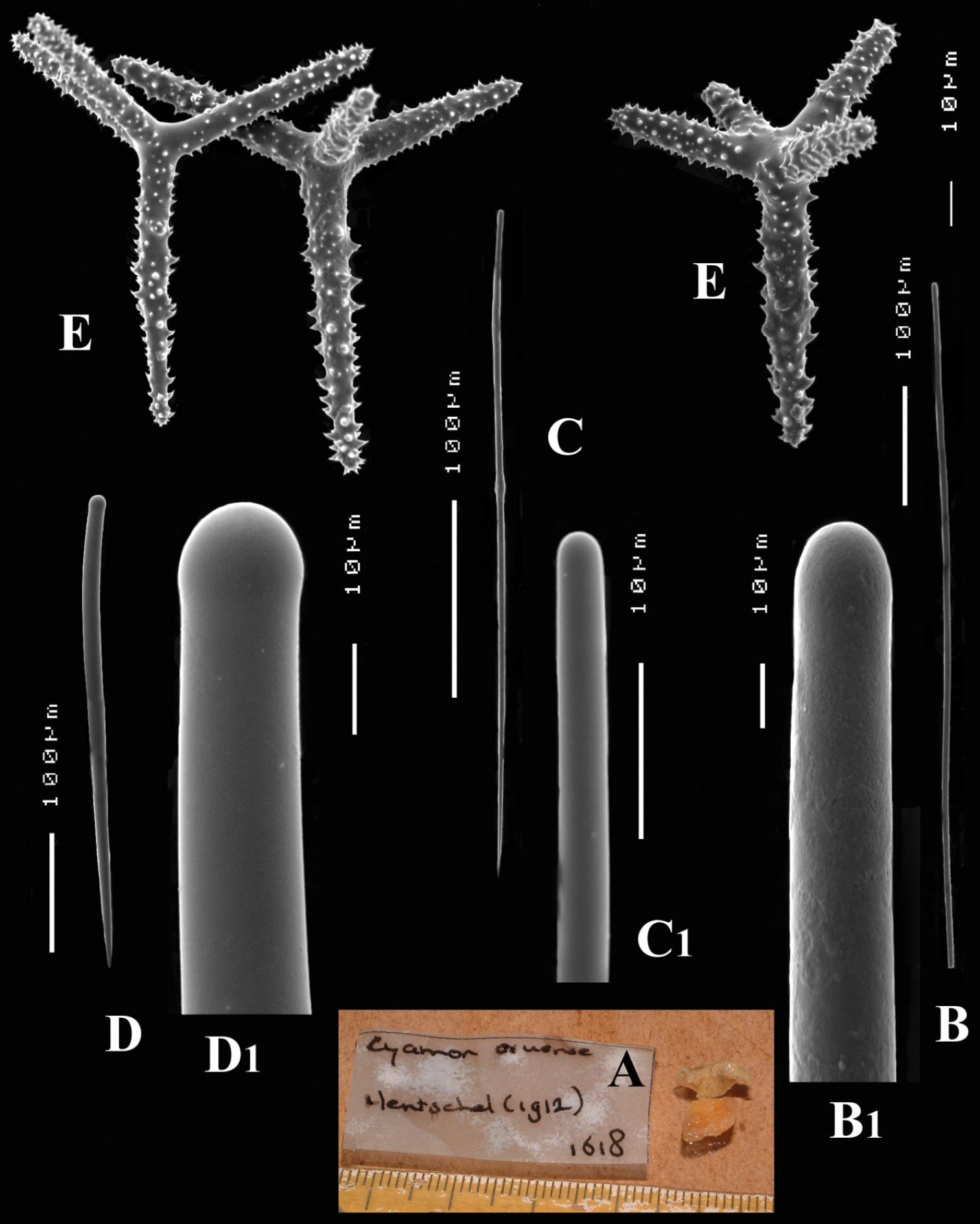
Cyamon aruense Hentschel, 1912, holotype SMF 1618, A fragments from holotype B long thin style (broken) B1 detail of head of long thin style C short thin style C1 detail of head of short thin style D short thick style D1 detail of head of short thick style E polyactines.
Only known from the Arafura Sea.
Deeper water on hard substrate.
The heavy spination of the polyactines appears to be a distinct feature of this species. Hooper’s (1991) redescription denies the occurrence in this species of centrotylote ectosomal thin styles, wheras these spicules appeared common in the fragment of the holotype examined by us. These spicules are comparable to those of Cyamon arguinense sp. n., rather than to those of Cyamon vickersii because they do not have the characteristic crooked shape and also are not rugose at the pointed end. The polyactines of this species appear somewhat similar to those of Cyamon quadriradiatum as described and drawn by
http://species-id.net/wiki/Cyamon_koltuni
Figs 10A–FHolotypeUSNM 33630, preserved in alcohol, California, Santa Catalina Island, Big Fisherman’s Cove, 33.45°N, 118.4833°W, 6 m.
LEB-ICML-UNAM 1497, preserved in alcohol, Mexican Pacific, Islas Marietas (Nayarit), Cueva Marietas, 20.7003°N, 105.5658°W, 11 m, coll. J.L. Carballo, 11–10–2006.
The holotype (Fig. 10A1) was received on loan from the Smithsonian Institution, but in view of the small crust and previous studies of it, including SEM examination (
Thinly encrusting (Fig. 10A1, A2) on rocks, color bright orange. Size of Mexican specimen 12 × 15 cm, thickness 1 mm. Surface very hispid.
Skeleton: a basal mass of polyactine spicules (Fig. 10B), upon which with styles are erected (Fig. 10C), no discernible skeletal organization due to thinness.
Spicules: long thin styles, short thin styles, short thick styles, polyactines.
Long thin styles (Fig. 10D, 10D1): rather straight, with faint subterminal tyle at the rounded end, 900–967–1400 × 5–5.9–7 µm.
Short thin styles (Fig. 10E), occasionally oxea-like with tapering thin endings, 265–370 × 2.5–5 µm.
Short thick styles (Fig. 10F): slightly curved and thickest subterminally near the faintly constricted rounded end, 150–316–425 × 10–14.7–25 µm.
Polyactines (Fig. 10G): three-six claded, cladi spined predominantly at the apices; basal cladi pointed, spined more heavily than the lateral cladi, which are provided with prominent bulbous apices, 35–46–66 × 5–8.9–10 µm.
Cyamon koltuni Sim & Bakus, 1986, A1 Californian holotype, USNM 33630, encrusting a rock (scale = 1 cm) A2 Mexican Pacific specimen LEB-ICML-UNAM 1497 B cross section of peripheral region C thick section of basal mass of polyactines D fragments of long thin style D1 microphoto of detail of rounded apex of long thin style E short thin style F short thick styles G three- to five-claded polyactines showing prominent bulbous ending of lateral cladi.
Southern California, Pacific coast of Mexico.
Under rocks and in caves in shallow water.
The enhanced bulbous endings of the polyactines is distinctive and is only matched by those of Cyamon amphipolyactinum sp. n., but that species differs clearly by possessing a smaller category of amphipolyactines. It is generally similar to Cyamon agnani, differing from that species in the sizes of the styles and the very prominent bulbous endings of the cladi of the polyactines.
http://species-id.net/wiki/Cyamon_neon
Figs 11A–HHolotype USNM 21412, preserved in alcohol, California, between Point Dunes and Newport, near San Pedro.
Paratype: BMNH 1929.9.30.5, two slides, Santa Catalina Island, California, 33.5°N.
Shape massively encrusting (Fig. 11A) with irregular conulose-villose surface. Size of specimen 4 × 3 cm in lateral expansion, 2 cm in thickness. Color (alcohol) red brown.
Skeleton: columnar, with thick short styles at the center of a mass of polyactines, with long thin styles protruding from this skeleton surrounded by shorter centrotylote styles.
Spicules: long thin styles, short thin centrotylote styles, short thick styles, polyactines.
Long thin styles (Figs 11B, B1), relative straight and robust, frequently with subterminal tyle 860–1041–1290 × 6–7.8–10 µm (De Laubenfels gives: up to 1560 × 12 µm).
Short thin styles (Figs 11C, C1), curved, centrotylote, often with mucronate slightly rugose pointed end, 191–242.8–306 × 1.5–2.4–3 µm.
Short thick styles (Fig. 11D), smooth, curved evenly, occasionally oxeote, 270–408.2–468 × 14–16.8–24 µm.
Polyactines (Figs 11E, 11E1, 11F) robust, largely smooth with cladi spined only at the apices (Fig. 11E1), or all cladi smooth. The three- or four claded forms vary widely in size and are sometimes reminiscent of Trikentrion spicules. Three-claded forms tend to have longer and thicker lateral cladi than the rare four-claded forms. Basal cladi in three-claded spicules are 33–48.8–63 × 8–11.7–14 µm, lateral cladi 72–95.7–132 × 7–12 µm, while four-claded forms have basal cladi 40–55.0–69 × 6–7.7–9 µm and lateral cladi 30–45.1–57 × 5–6.3–7 µm. There are very common diactinal polyactines (Fig. 11F), mimicking oxeas, but recognizable as reduced polyactines by centrotylote swellings and finely spined apices, size 123–158.3–202 × 7–10.2–14 µm.
Cyamon neon De Laubenfels, 1930, holotype USNM 21412, A massively encrusting shape with irregular surface (scale = 1 cm) B long thin style B1 detail of rounded end showing subterminal tyle C short thin centrotylote (strongylo-)style C1 detail of swollen roughened apex of short thin (strongylo-)style D short thick style E polyactines E1 detail of basal cladus of polyactine F diactinal polyactine.
Southern Californian Bight (San Pedro, Santa Catalina island, La Jolla).
On hard substrate, at depths 0–36 m.
Cyamon neon is unusual among Cyamon species by it possession of polyactines with smooth or barely spined cladi, the shape of many of the polyactines mimicking those of Trikentrion, and the occurrence of diactinal polyactines. The latter spicules are shared with Cyamon argon, which in most respects is similar to Cyamon neon. For a comparison between the two species see below in the remarks to Cyamon argon. The only other Cyamon species in the area is Cyamon koltuni, which differs substantially in the bulbous endings of the cladi of the polyactines and absence of the short thin styles.
http://species-id.net/wiki/Cyamon_argon
Figs 12A–C, Figs 13A–GHolotype of Cyamon argon, AHF-NHMLA L35535, D34, preserved in alcohol, Mexico, Cedros Island, South Bay, Hancock Pacific Expeditions, Velero Station 287–34, 28.09°N, 115.3°W, 18–27 m, among kelp, 10 March 1934.
Shape upright, bilobed thick branches (Fig. 12A), spreading out upwards, with longitudinal grooves and covered in rounded spiny projections and conules. Height and diameter 3.5 cm, stalk approximately 1.5 cm. Colour (preserved) red-brown. Consistency tough, barely incompressible.
Skeleton: axial-columnar, with surface projections formed by the outwardly directed columns (Fig. 12B) branching off from the axial region. Columns have a core of short thick styles and polyactines crowned at the surface by long thin styles accompanied by (rare) short thin centrotylote styles.
Spicules: long thin styles, short thin styles, short thick styles, polyactines.
Long thin styles (Fig. 13A), mostly broken in the slides, one complete one measured 960 × 15 µm.
Short thin centrotylote (Figs 13C, C1, C2), wavy to somewhat crooked, with one end rounded and the other mucronate-spined, 210–250.6–348 × 3–3.6–4 µm.
Short thick styles (Figs 13B, B1), smooth curved evenly, 350–480.5–593 × 15–32.3–42 µm.
Polyactines (Figs 12C, 13D-E) two-, three-, four- and five-claded, quite variable in shape and size. T-shaped spicules (Fig. 13D) similar to those found in Trikentrion are common. Basal cladi usually prominently spined (Fig. 13D1), lateral cladi finely spined (Fig. 13D2). No entirely smooth spicules were observed. Diactinal spicules (Fig. 13E) with swollen excentrical swellings and spined apices, often sharply angulated. Three-claded spicules with basal cladi 45–60.7–78 × 6–14.9–22 µm, lateral cladi 30–110.7–162 × 5–17.0–21 µm. Four-claded spicules have basal cladi 33–44.8–51 × 9–14.9–21 µm, lateral cladi 63–86.2–123 × 7–18 µm. Diactinal spicules: 204–245.1–312 × 18–22.8–31 µm.
Cyamon argon Dickinson, 1945, holotype AHF-NHMLA L35535 (D34), A shape (scale mm) (photo Phyllis Sun) B microphoto of cross section of skeleton showing columns of styles supported by polyactines C microphoto of a range of polyactine shapes.
Cyamon argon Dickinson, 1945, holotype AHF-NHMLA L35535 (D34), A detail of head of long thin style B short thick style B1 detail of head of short thick style C short thin centrotylote (strongylo-)style C1 detail of spined apex of short thin (strongylo-)style C2 detail of centrotylote part of short thin (strongylo-)style D polyactines D1 heavily spined basal cladus of polyactine D2 lightly spined lateral cladus of polyactine E diactine polyactines.
Pacific coast of North Mexico.
In kelp forest, 18–27 m.
As pointed out above, this species is close to Cyamon neon, and if more data on variation would become available, it is possible, in view of the nearness of both type localities that the two might be part of a single variable species. The following characteristics are similar between the two: long thin styles of 1000+ µm in length, the possession of short thin centrotylote styles with spined pointed apex (shared with Cyamon vickersii), smooth evenly curved short thick styles of 400-500 µm in length, polyactines consisting predominantly of three-claded polyactines with all cladi smooth except for the apices, short basal cladus compared to long lateral cladi, and the frequent occurrence of diactinal polyactines. However, there are also clear differences, which presently preclude synonymization of the two: shape bush-like in Cyamon argon, massively encrusting in Cyamon neon, thickness of short thick styles in Cyamon argon twice that of Cyamon neon, basal cladi of the polyactines distinctly spined in Cyamon argon whereas these are only rugose or even smooth in Cyamon neon, and finally the size (length but also thickness) of the lateral cladi in three-claded polyactines which are usually well over 200 µm long and 20 µm thick in Cyamon argon, whereas those of Cyamon neon are on average around 150 × 10 µm.
With Cyamon vickersii, this species shares a more elaborate, upright growth form, which is otherwise rare in the genus.
http://species-id.net/wiki/Cyamon_quinqueradiatum
Figs 14A–D, 14E (right)Seven slides from the collections of the Natural History Museum, BMNH 1954.2.23.8, made of Dendy’s (1905) topotypical material.
Carter’s specimen from the Gulf of Manaar is apparently lost from the collections of the National Museums Liverpool (Dr Ian Wallace, in litteris), no original slides have been found in the Natural History Museum (Ms Emma Sherlock, in litteris).
(Partly from Carter, 1880 and Dendy, 1905). Thinly encrusting, hispid, yellowish brown (alcohol) to cream color (dry). Dendy’s specimen was 1.1 cm in lateral expansion, 3 mm thick. Texture soft.
Skeleton (Figs 14A–C): bundles of subtylostyles and styles standing erect on the substratum, in the basal layer supported by polyactine spicules.
Spicules: predominant spicules are longer and shorter subtylostyles with a minority of thin styles and polyactines.
Subtylostyles, presumably a mixture of undifferentiated long thin styles and short thick styles, with prominent heads, usually lightly and gradually curved, in a large size range, which makes determining an average size meaningless: 129–1989 × 3–33 µm.
Thin styles, tapering gradually to thinly pointed curved ends, size range limited, 492–698 × 3–5 µm. Dendy believed these spicules to be growth stages of the subtylostyles, but we regard them, like Carter, as a separate spicule category.
Polyactines [Figs 14D, 14E(right)], predominantly five-claded (a few four-claded forms were observed), with the basal cladus relatively finely spined, the lateral cladi smooth, with mucronate, occasionally bifid ends, basal cladi 45–62.8–93 × 4–5.9–11 µm, lateral cladi 31–38.4–51 × 3–4.8–7 µm.
Cyamon quinqueradiatum Carter, 1880, images of Dendy’s (1905) non-type slides BMNH 1954.2.23.8 (A–E) and Cyamon quadriradiatum Carter, 1880 (E left), A–C various perpendicular sections showing long subtylostyles and basal polyactines of Cyamon quinqueradiatum D polyactine of Cyamon quinqueradiatum showing spined basal cladus and smooth lateral cladi E Cyamon quadriradiatum Carter, 1880 and Cyamon quinqueradiatum Carter, 1880, original drawings from Carter, 1880, E (right) Cyamon quinqueradiatum, right side of figure, showing long subtylostyle, short subtylostyle, thin style, and polyactine with spined basal cladus and smooth lateral cladi E(left) Cyamon quadriratiatum, left side of figure, showing long thick style, thin wavy spicule, and entirely spined polyactines.
Only known from the Gulf of Manaar.
Deep water (not specified).
As pointed out above, Mauritanian Cyamon arguinense sp. n. shares many features with Indian Ocean Cyamon quinqueradiatum, including the smooth lateral cladi and the lack of differentiation of the long thin and short thick styles. Although the Cyamon nature of this species has never been challenged, it is nevertheless obvious from the original description and drawing by
http://species-id.net/wiki/Cyamon_quadriradiatum
Fig. 14E (left)None. Type material apparently lost from the collections of the National Museums Liverpool (Dr Ian Wallace, in litteris), no slides have been found in the Natural History Museum (Ms Emma Sherlock, in litteris).
(From Carter, 1880). Thinly encrusting, hispid, color when dry dark brown. Spicules (Fig. 14E, left) of three kinds, long thick styles with a globular tyle, size given as 1042 × 41 µm, short thin ‘crooked’ styles, length 347 µm, and robust four-claded polyactines with all cladi entirely spined, length of cladus given as 76 µm.
Gulf of Manaar, Southeastern India.
No data.
This species needs redescription, but the long thick styles in combination with the densely spinous polyactines appear sufficiently distinct. Nevertheless there is a resemblance to the polyactines of Cyamon aruense, see above.
urn:lsid:zoobank.org:act:BA36E82A-F8CB-4FA4-B589-DDE8694C220D
http://species-id.net/wiki/Cyamon_hamatum
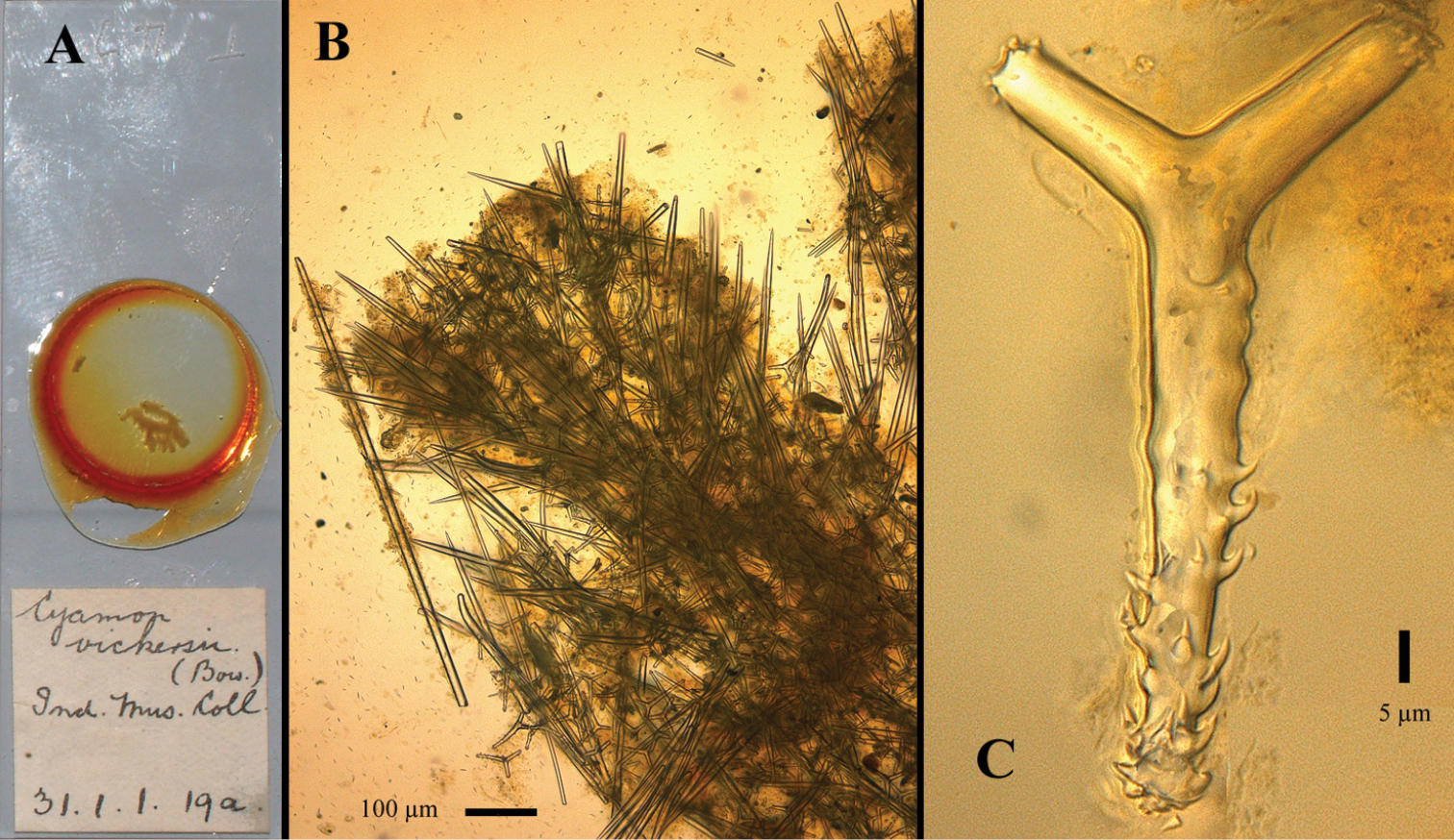
Figs 15A–CType specimen: Holotype (schizotype), 1 slide BMNH 1931.1.1.19a, labeled Cyamon vickersii (Bow.) Ind. Mus. Coll. in Burton’s handwriting. Presumably the type specimen was at one time lodged in the collections of the Indian Museum, Kolkata, India, but present whereabouts are unknown. It is likely housed in the Zoological Survey of India, Kolkata.
Type locality: India, 21 miles S.W.W. of Mangalore, 4 May 1888.
partly from
The single representative is a portion of a dull brown spherical mass. It agrees with the specimen described by Dendy (l.c.) except that the longest ray of the pseudactines bears a few recurved rays on the shaft and a crown of spines at the apex; the basal rays of these spicules have spines at the apex only; and the styli are very scarce. Locality. – 21 miles S.W.W. of Mangalore, S India (4th May 1888).
The slide (Fig. 15A) contains thick sections of the skeleton, showing the usual columnar structure of thick styles and polyactines (Fig. 15B). The slide allows the recognition and measurement of the spicule complement.
Spicules: long thin styles, short thin centrotylote styles, short thick styles, polyactines.
Long thin styles, not frequent, invariably broken, longest fragment measured 1300 × 30 µm.
Short thin styles, wavy outline, faintly centrotylote, under light microscopy mostly looking smooth but occasionally some spines are visible on the pointed end and also in at least one spicule two spines on the rounded end, 272–313.2–355 × 2.5–3.4–5 µm
Short thick styles, smooth, curved rather strongly near the rounded end: 421–495.6–604 × 16–19.9–31 µm.
Polyactines (Fig. 15C), predominantly three-claded, but occasionally four-claded, with long basal clades with prominent recurved hook-like spines and with short, stubby lateral cladi spined only at the bluntly rounded apices, basal cladi 104–114.2–126 × 11–14.8–21 µm, lateral cladi 42–47.8–65 × 10–11.7–20 µm.
Cyamon hamatum sp. n. (Cyamon vickersii sensu
The adjective hamatus (L.), means provided with hooks.
South India.
No data.
It is with some hesitation that we decided to name this scanty material as a valid new species. Although measurements of the megascleres conform to or are close to those of Cyamon vickersii, the shape and spination of the polyactines is distinctly different, as Burton & Rao already observed. With their strong hooks on the basal cladi and the peculiar short crowned lateral cladi the polyactines are different from any other known Cyamon.
Figs 16A–E
Twenty six samples in the ZMA Porifera collection, preserved in alcohol, all from Rockall Bank, approximately 55.4N, 15.8W, depth 500–900 m, collected during MOUNDFORCE 2004 and BIOSYS 2005 cruises with RV Pelagia. Type material:Monaco Oceanographic Museum, not examined.
Pale greenish encrustations (Fig. 16A) on deep-sea coral branches, surface irregularly conulose-hispid. Consistency soft. Dimensions up to 15 × 6 cm in widest expansions, thickness approximately 1 mm.
Skeleton: basal mass of polyactines, usually a single layer of spicules, with basal cladi pointing outwards and lateral cladi spread out on the substrate, taking up the position of echinating acanthostyles as in Hymedesmia or Clathria (Microciona). Single long styles with heads embedded in the layer of polyactines, surrounded by groups of short styles.
Spicules: long styles, short styles, polyactines.
Long styles (Figs 16C, C1) with upper parts heavily spined, becoming gradually smooth toward the pointed end, only a few were found to be complete, 657–737.2–822 × 32–35.5–38 µm.
Short styles (Figs 16B, B1), very abundant, heads slightly spined, shaft smooth, faintly polytylote, pointed end tends to be slightly mucronate, 302–324.1–366 × 7–8.4–10 µm.
Polyactines (Figs 16D-E), with 3–8 cladi, usually with a long and prominent basal cladus and short irregular lateral cladi (Fig. 16E), heavily spined without smooth areas, basal cladi 90–151.3–234 × 9–11.2–14 µm, lateral cladi 15–27.3–36 × 6–7.4–12 µm.
Cyamon spinispinosum (Topsent, 1904), ZMA Por. 19422, from SE Rockall Bank, North Atlantic A shape encrusting deep sea coral (scale = 1 cm) B short style, lightly spined at the head B1 details of apices of short style C long style, coarsely spined C1 detail of head of long style D various shapes of polyactines E detail of the cladome of a seven-claded polyactine.
Azores, Ireland, also Norway (P. Cárdenas, pers. comm.). A common North Atlantic bathyal species (
Encrusting deep-sea corals at depths from 500–900 m.
This is a deviating Cyamon with several unique features not shared by the majority of the species. Both monaxone megascleres are partially heavily spined, and the raspailiid feature of a long thin style surrounded by short thin styles is absent. The polyactines resemble echinating acanthostyles by their long basal cladus and crown of short irregular lateral cladi. These spicules may be assumed to bridge the gap between the polyactines with more or less equal length cladi and acanthostyles with heavily knobbed and spined heads such as found in some myxilline genera (Hymedesmia Bowerbank, 1864, Discorhabdella Dendy, 1924) and in the raspailiid genus Eurypon Gray, 1867. Additionally, it occurs in cold deep-sea habitats unlike all other Cyamon species. It is likely that this species does not belong in Cyamon, but we will await additional (molecular) evidence before removing it from the genus
Spongia muricata Pallas, 1766 (by monotypy).
(emended). Cyamoninae with reticulate skeleton containing polyactine spicules of which the basal cladi are provided with hook-like spines in mature condition, and if present choanosomal oxeas. Microscleres trichodragmas. Additional longer and shorter thin styles are usually present in peripheral regions.
Polyactine spicules are genuinely polyaxone, with axial canals visible in all of the predominantly three, occasionally four- or two cladi. As will be demonstrated below, none of the specimens of the type species we were able to examine, including the neotype, possess the raspailiid synapomorphy of peripheral long styles surrounded by short styles, despite Hooper’s (2002) description of the type species where such spicules were mentioned. Possibly, but unlikely, these spicules are present in living condition, because we only had dry old specimens available and the peripheral skeleton may have become abraded. It seems likely that Hooper’s (2002) description was based on a contaminated spicule slide. All other Trikentrion species do have the long and short styles as a peripheral skeletal feature, and in that sense the type species appears a deviating representative of the genus.
Trikentrion differs from Cyamon in its possession of choanosomal oxeas (whereas Cyamon has styles), but several species, Trikentrion catalina, Trikentrion helium Dickinson, 1945 and Trikentrion africanum sp. n., are lacking these spicules. The polyactines of Trikentrion differ from those of Cyamon in having only the basal clade provided with strong hook-like spines, with the lateral cladi smooth; also the shape is often Y- or T-shaped. As demonstrated above, these differences are not entirely exclusive, because Cyamon arguinense sp. n. and Trikentrion quinqueradiatum also have only the basal cladus spined, whereas Y- and T-shaped polyactines occur in Cyamon neon and Cyamon argon. Finally, all species of Trikentrion described below have abundant trichodragmas, which are entirely lacking in Cyamon species.
http://species-id.net/wiki/Trikentrion_muricatum
Figs 17A–D, 18A–E, 19A–D, 20A–DThe identity of the sponge named Spongia muricata by Pallas, which is assumed to be the type species of Trikentrion, is not straightforward. The first use of the name combination stems from
S. ramosissima, poris cylindricis subulatis prominentibus aequalibus multifidis hispidis, without further indication of where it had been collected or by whom. The Latin name muricata is generally considered to mean spined (after the name of a mollusk (Murex) yielding a purple dye, cf.
To conclude: the identity of Spongia muricata is not unequivocal, primarily due to the unrecognizable description of
A likely candidate is the assumed type of Trikentrion muricata housed in the Natural History Museum, London, BMNH 1872.10.19.1 (see Fig. 18A), with schizotype ZMB 7160, on the basis of which
The choice of a neotype again is complicated due to a recent discovery in the collections of the Naturalis Biodiversity Center at Leiden (NBC) of four old collection specimens, RMNH Por. 306 and 309, and ZMA Por. 02545 and 02546, which are sufficiently similar to Seba’s and Esper’s plates to raise the suspicion that they could belong to one of the original specimens of Spongia muricata.
RMNH Por. 309 (see Fig. 18B) is labeled Raspailia xerampelina (Lmk) ? type (Spongia --- Lmk) without further information, and this specimen bears an overall strong likeness to Seba’s plate. RMNH Por. 306 (see Fig. 18C) is labeled Raspailia hispida (Mont.) type van Spongia muricata Lmk, Mus. Parijs, Kust van Guinée (translation: type of Spongia muricata Lamarck, from the Paris Museum, Coast of Guinea). If the specimen is compared to the plate of Spongia muricata of Esper one is compelled by the overall likeness of the two (though it is not an exact likeness). ZMA Por. 02545 (see Fig. 18D) is labeled Halichondria echidnaea Lmk no. 55 Kust van Guinea, ZMA Por. 02546 (see Fig. 18E) is labeled Halichondria echidnaea Lam / muricata Esper fide Lamouroux no. 62 Kust van Guinea. Both ZMA specimens bear some resemblance to Seba’s and Esper’s plates.
The skeleton and spicules of all five specimens conform with the descriptions of
The reason for the names on the labels of the specimens of the NBC and the referral to the Paris Museum is explained in
In view of the uncertain history of the NCB specimens and the more precise data available for the Natural History Museum, London specimen, we here designate BMNH 1872.10.19.1 as the neotype of Spongia muricata, the type species of the genus Trikentrion.
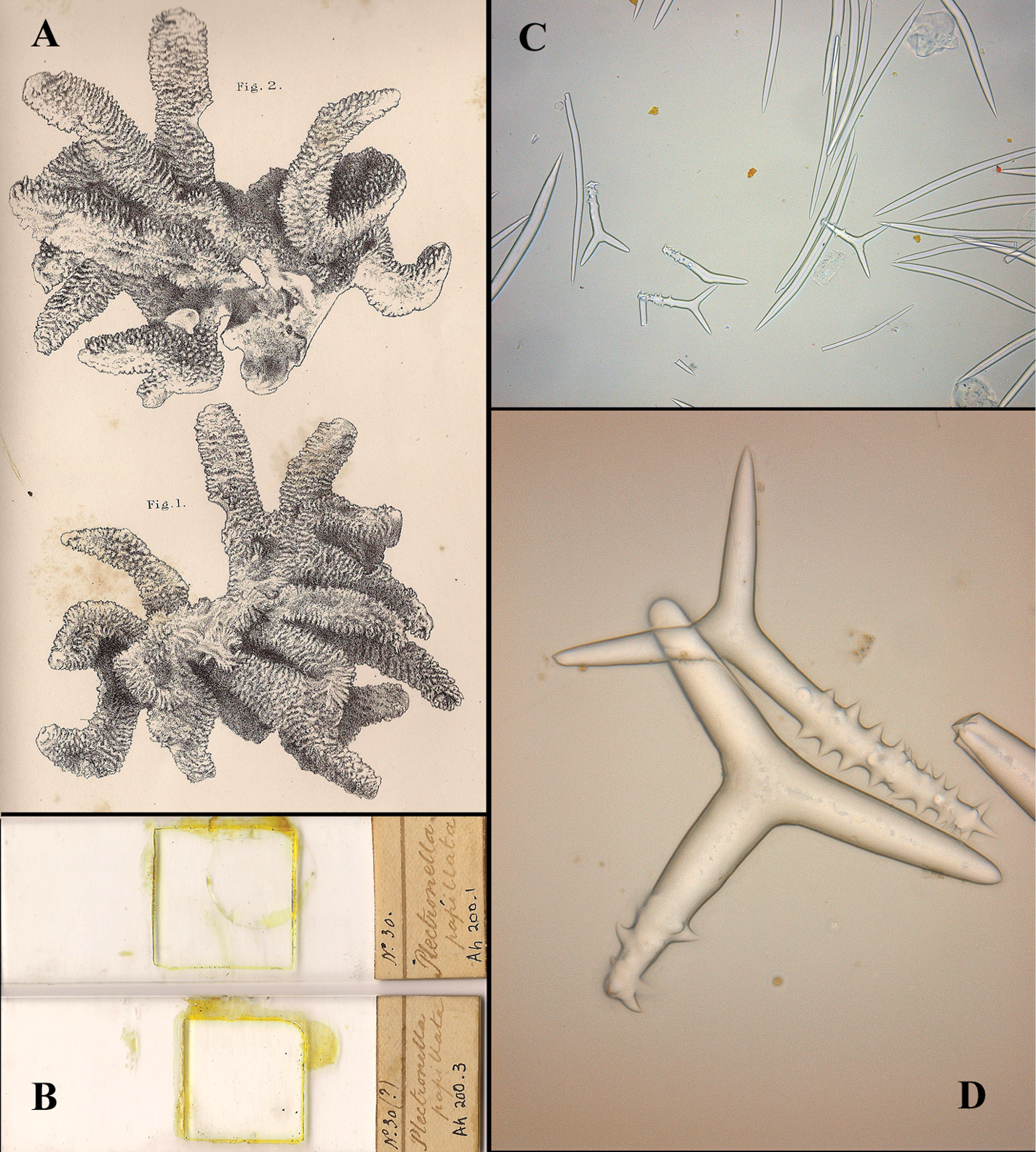
It is a pleasure to be able to announce that material of Plectronella papillosa Sollas, 1879, since long known to be a junior synonym of Trikentrion muricatum through its excellent description by Sollas, but otherwise never redescribed, has been discovered in the collection of the Bristol Museum and Art Gallery, in the form of 2 slides labeled No. 30 Ah.200.1, 200.3 (see Fig. 19B), containing cross sections of the skeleton and dissoluted spicules. We can confirm that Plectronella papillosa is a junior synonym and that details in the slides conform closely to those of Trikentrion muricatum (see Fig. 19C–D).
Neotype (designation herein): BMNH 1872.10.19.1 from Volta River, Fantee, Ghana, presented by Gov. Ussher. Schizotype ZMB 7160 of the same;
RMNH Por. 306, Spongia muricata Lamarck, Coast of Guinea;
RMNH Por. 309, Spongia xerampelina Lamarck, no further data;
ZMA Por. 02545, 02546, Halichondria echidnaea / muricata Lamarck, coast of Guinea;
BMAG Ah 200.1, 200.3, 2 slides labeled Plectronella papillosa no. 30, no further data.
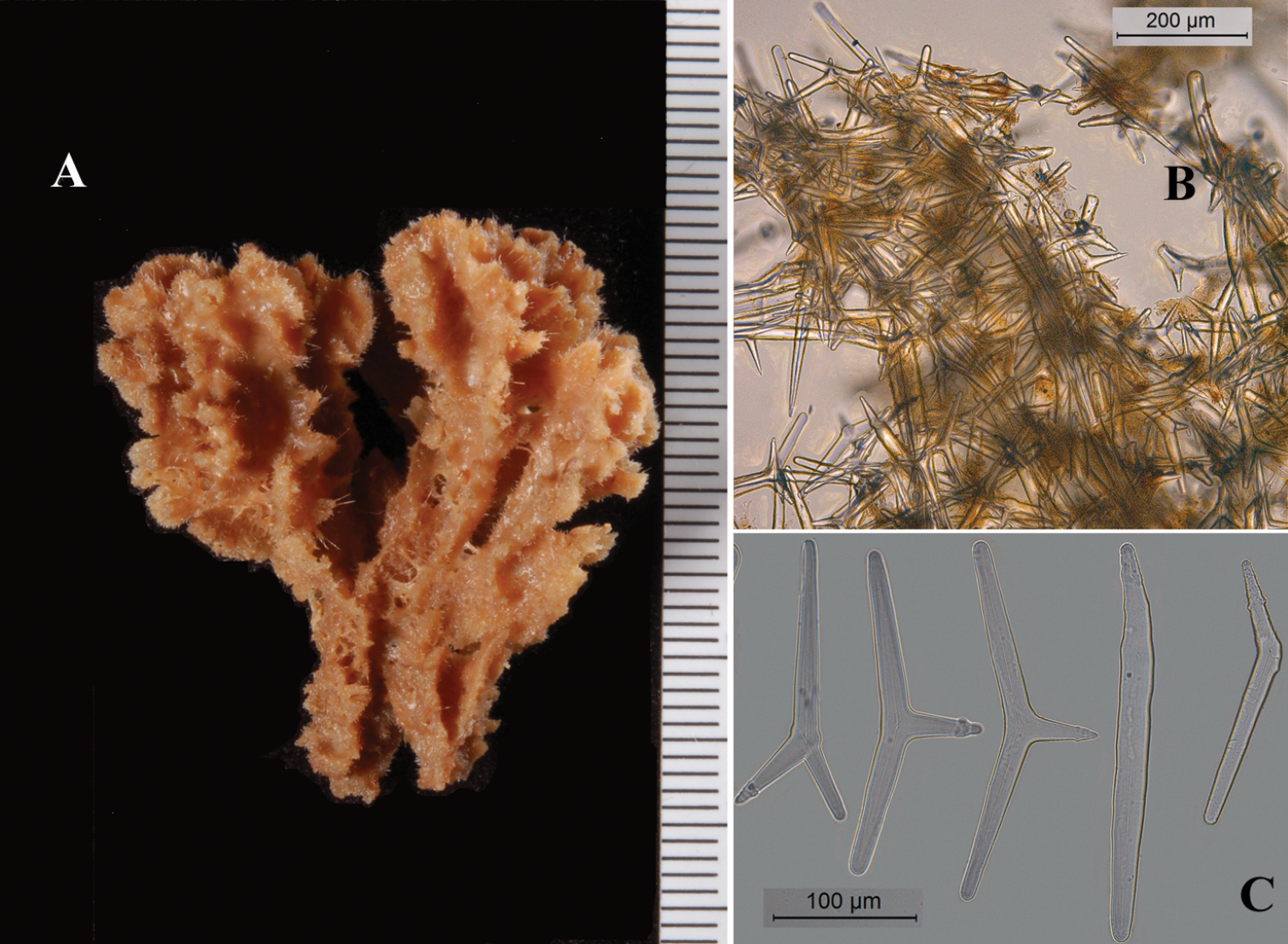
Wide basal holdfast upon which are erected groups of cylindrical branches, more or less in one plane, each branch usually with one or two dichotomous secondary branches, often also with anastomosing branches. Size of neotype (Fig. 18A) 13.5 × 12 × 5 cm of the whole group of branches, diameter of individual branches 1-1.5 cm. Sollas’ specimens (Fig. 19A as Plectronella papillosa) were described as being 20 × 20 cm, with branch diameter 2-3 cm. The other specimens are similar in size, but slightly smaller. Surface densely covered with broad, laterally flattened papillae, 1-4 mm in size (reminding of the surface projections of Ptilocaulis Carter, 1883). In some specimens the papillae are partially abraded (e.g. RMNH Por. 306, see Fig. 18C) giving the sponge a less striking aspect. Consistency (dry) hard, incompressible, crumbly. No live color has been reported in the literature, but color plates of Seba (Fig. 17A) and Esper (Fig. 17D) show a light orange brown color.
Skeleton (Fig. 20A): predominantly a wide-meshed reticulation of tracts of robust smooth oxeas, with little axial and extra-axial specialization. The polyactines are common in peripheral regions. No longer or smaller peripheral styles have been found in any of the examined specimens.
Spicules: Oxeas, polyactines, trichodragmas.
Choanosomal ‘true’ oxeas (Figs 19C, 20B, B1, not to be confused with diactinal polyactines), fat, fusiform, tapering gradually to sharp points, overall size (of all specimens examined) 222–376.2–528 × 13–19.9–31 µm, in the neotype: 287–351.5–432 × 13–17.2–26 µm.
Polyactines (Figs 19D, 20C), predominantly three-claded Y-shaped, rarely T-shaped, occasionally diactinal, with prominent hook-like spines on the basal clade (undeveloped spicules with smooth basal clade), and mucronate or nipple-like endings on many of the lateral cladi; overall size of basal clade (of all specimens examined) 78–111.7–156 × 12–19.2–27 µm, lateral cladi 42–67.2–84 × 12–16.7–27 µm, of neotype: basal clade 78–100.2–118 × 12–19.4–25 µm, lateral cladi 58–69.2–84 × 13–16.3–21 µm.
Trichodragmas (Fig. 20D), straight or sinuous, overall size (of all specimens examined) 57–82.0–102 × 4–9.7–18 µm, of neotype: 63–87.8–102 × 9–12.8–18.
Trikentrion muricatum (Pallas, 1766), early illustrations and original descriptions A
Trikentrion muricatum (Pallas, 1766), early museum specimens A BMNH 1872.10.19.1 redescribed by
Plectronella papillosum
Trikentrion muricatum (Pallas, 1766), neotype BMNH 1872.10.19.1 A microphoto of cross section of peripheral skeleton B oxea B1 detail of one of the apices of an oxea C polyactine C1 detail of apex of lateral clade D trichodragma.
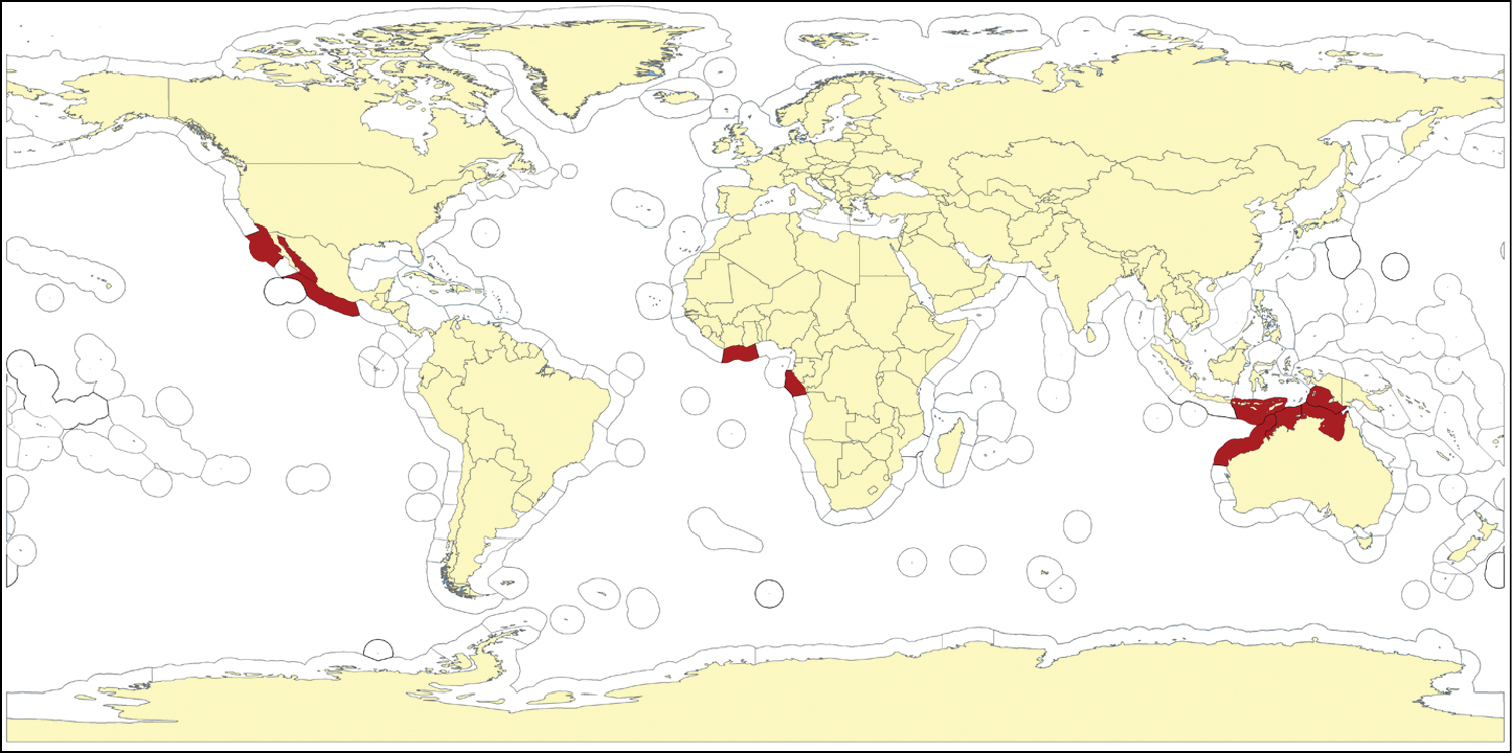
Tropical West Africa. Type from ‘Elmina, Guinea’ (Pallas, 1766; Esper, 1794), now situated in Ghana. Further specimens were reported mostly from Ghana (neotype (Carter, 1879): Volta; Burton, 1956: Gold Coast), or locality was unknown (Sollas, 1879), or more general (coast of Guinea).
Depth range: no definite data, but probably shallow water, growing on rocks.
The species must have been of common occurrence off the coast of Ghana in 18th century as there are a fair lot of specimens available from that age and region in several natural history museums. Curiously, no fresh material is known to exist, so the species remains ill-known. Trikentrion muricatum differs substantially from all other Trikentrion species described below in the lack of peripheral styles. Further differences are robust oxeas, up to twice as long and thick as those of the two other oxea-bearing species (Trikentrion laeve Carter, 1879 and Trikentrion flabelliforme), while the three remaining species (Trikentrion helium, Trikentrion catalina and Trikentrion africanum sp. n.) lack the oxeas entirely.
http://species-id.net/wiki/Trikentrion_laeve
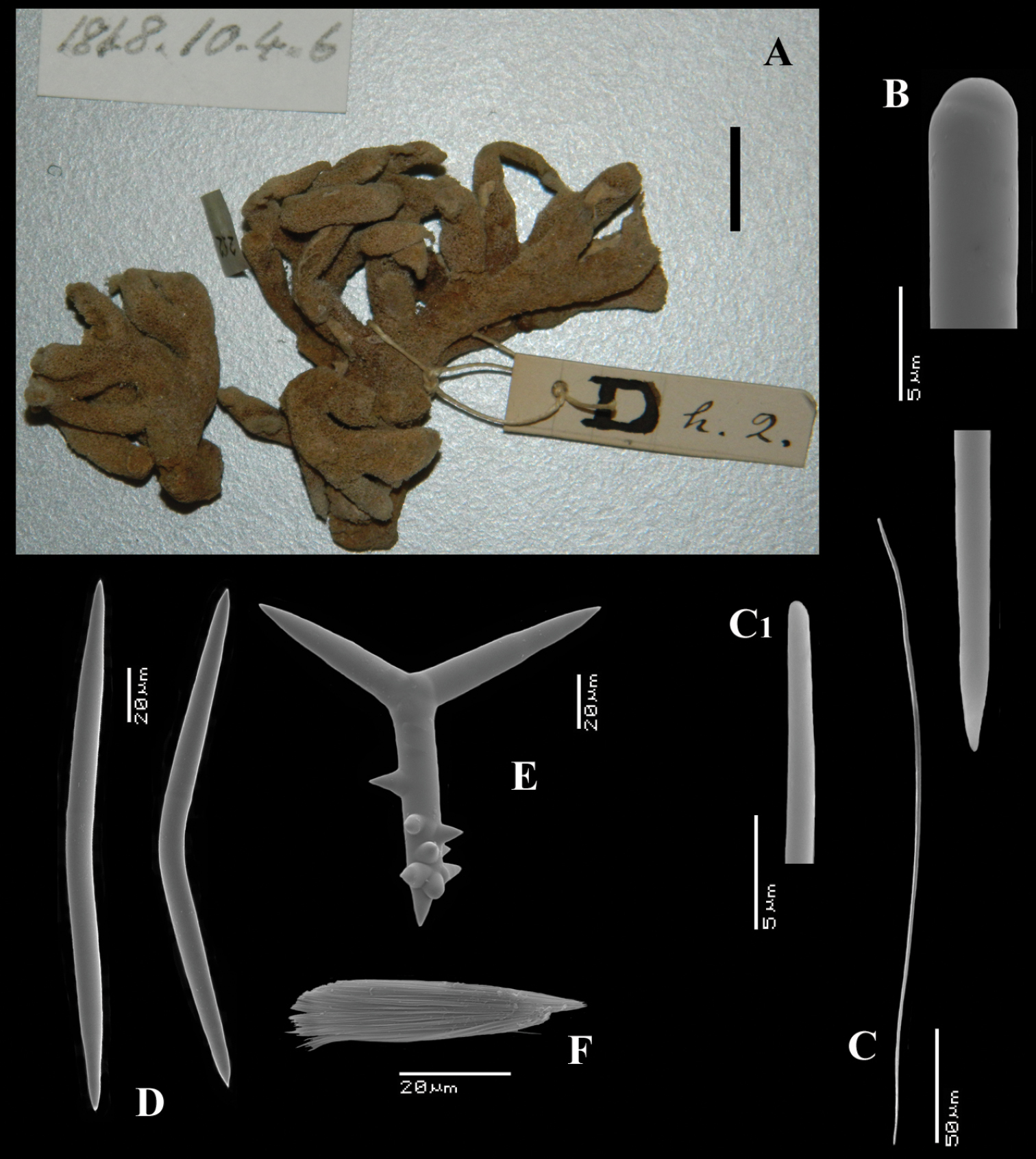
Figs 21A–FHolotype: BMNH 1848.10.4.6 (additional numbers Dh.2, 252), West Africa, coll. Rev. Allen; label text, presumably by Carter, reads Trikentrion Ehlers, very long acuates.
Multi-branched bush (Fig. 21A), with single stalk of 1.5 cm high, 0.8 cm diameter, from which cylindrical branches spread out dichotomously, ending in approximately 26 smaller terminally rounded branches. Size of entire specimen, which is broken in two unequal parts, 4.5 × 5.5 × 3 cm. Surface optically smooth, but microhispid, with punctate appearance. Consistency (dry) crumbly compressible, colour beige-purplish.
Skeleton: a comparatively loose reticulation of oxeas echinated sparingly with polyactines, forming rounded or squarish meshes of 150–200 µm diameter, with 5 or more oxeas to the sides, no axial specialization. Peripherally there are numerous long thin styles, accompanied by short thin styles.
Spicules: long thin styles, short thin styles, oxeas, polyactines, trichodragmas.
Long thin styles (Fig. 21B), rather curved, 750–921.8–1062 × 4–6.6–9 µm.
Short thin styles (Figs 21C, C1), often modified to thin oxeotes, wispy, curved, 234–312.9–433 × 0.5–1.4–2.5 µm.
Choanosomal ‘true’ oxeas (Figs 21D) (not to be confused with diactinal polyactines), straight, or more often centrotylote or abruptly curved, with pointed ends, very common, 175–204.1–242 × 6–9.6–13 µm.
Polyactines (Fig. 21E), usually three-claded, occasionally four-claded or diactinal, mostly Y-shaped, less often equiangular, with the basal ray provided with strong hook-like spines, basal cladi 59–69.6–89 × 10–11.9–15 µm, lateral cladi 47–63.4–75 × 9–10.7–13 µm.
Trichodragmas (Fig. 21F), straight or curved, 32–48.2–60 × 4–8.2–11 µm.
West Africa (Carter, 1879).
Probably from shallow water or washed up on the beach. No further data.
This is the first redescription after Carter’s report, which is accurate but deficient in omitting the trichodragmas and short thin styles. This is also the first depiction of habit of the specimen and with the details provided here the species is now at least properly described, but it remains ill known.
Trikentrion laeve Carter, 1879, holotype BMNH 1848.10.4.6, A shape of holotype specimen (size bar = 1 cm) B details of apices of long thin style C short thin style C1 detail of rounded head of short thin style D oxeas E polyactine F trichodragma.
http://species-id.net/wiki/Trikentrion_flabelliforme
Figs 22A–D, 23A–EHolotype missing from SMF, but a paralectotype fragment is present in the Natural History Museum, BMNH 1931.8.4.57, which was examined by JH in 2000, type locality: Indonesia, Aru Islands, 4–15 m depth.
ZMA Por. 02426, preserved in alcohol, Siboga Exped. Stat. 273, Aru Islands, Indonesia, pearl banks off Pulau Jedan, 5.4134°S, 134.6677°E, depth 13 m, 23–12–1899.
RMNH Por. 978, preserved in alcohol, Siboga Exped. Stat. 273, same data;
ZMA Por. 14022 and 14023, preserved in alcohol, East Point, Darwin, Northern Territories, Australia, 10 m, 29–11–1987, coll. J.N.A. Hooper nrs 8 and 9;
ZMA Por. 16049, dry old collection material without data.
Two distinct shapes, flabelliform (Fig. 22A), 6–26 × 4–19 cm high and wide, 0.2–1.4 cm thick) and digitate (Fig. 22B), up to 15 cm high, with flattened branches of up to 1.5 cm thickness (summary of many specimens described in Hooper, 1991). Flabelliform specimens may have blades at right angles (see Fig 22A). Frequently, the digitate specimens are infested with zoanthids (Fig. 22B). Surface optically smooth, microhispid, with characteristic pattern of fine meandering grooves. Texture firm. Colour orange-red, blood-red (shallow water) to beige (deep water).
Skeleton (Figs 22C–D): reticulated, square meshed or polyangular (Fig. 22D), with loose extra-axial and spongin-rich axial spicule tracts cored by oxeas, echinated by triactine polyactines; at the surface protruding long thin styles are surrounded by bouquets of short thin styles (Fig. 22C).
Spicules (Figs 23): Long thin styles, short thin styles, oxeas, polyactines, trichodragmas.
Long thin styles (Fig. 23A), curved, slim, 405–870.3–1034 × 3–7.3–9µm.
Short thin styles (Fig. 23B), thinly fusiform, 182–334.7–392 × 0.5–1.8–4 µm.
Choanosomal genuine oxeas (Figs 23C, C1), not to be confused with diactinal polyactines, evenly or more angularly curved, apices mucronate and many have minute spines visible under SEM (Fig. 23C1), sizes 135–287.7–340 × 5–16.8–22 µm.
Polyactines (Figs 23D, D1), rare in some specimens, predominantly three-claded, with prominent spines on the basal ray, and minute apical spines on the lateral rays (Fig. 23D1) visible only under SEM, occasionally strongly curved diactines or – often smaller – tetractines, basal cladi 96–109.5–123 × 10–13.1–17 µm, lateral cladi 51–70.0–84 × 9–12.6–17 µm.
Trichodragmas (Figs 23E, E1) with individual raphides showing rugose surface (Fig. 23E1), sizes 35–59.6–88 × 6–8.6–12 µm.
Trikentrion flabelliforme Hentschel, 1912, A flabellate specimen ZMA Por. 14023 from Darwin, North Australia (scale bar = 1 cm) B branching-digitate specimen RMNH Por. 978 infested with zoanthids from Aru Islands Indonesia (scale bar = 1 cm) C peripheral skeleton of ZMA Por. 14023 showing raspailiid character of long thin style sheathed in a bouquet of short thin styles D thick section of choanosomal skeleton of ZMA Por. 14023.
Figure 23. Trikentrion flabelliforme Hentschel, 1912, spicules of ZMA Por. 14023, A detail of rounded end of long thin style B details of short thin style C oxea C1 details of apices of oxeas showing minute spines D three- and four claded polyactines D1 detail of apex of lateral clade of polyactine showing minute spines E microphoto of trichodragmas E1 individual raphide dissociated trichodragma showing rugosities.
Arafura Sea, N and W Australia.
Shallow subtidal to offshore deeper water.
The species was erroneously attributed to
The two ‘growth forms’ are rather distinct, but distribution, skeleton, and spicules are similar and overlapping enitirely, making it impossible to separate the forms further. The digitate form is often overgrown with a zoanthid species, both in Australian (Hooper, 1991) and Indonesian (RMNH Por. 978) specimens. The shape of Trikentrion flabelliforme reminds of Californian Trikentrion catalina and Trikentrion helium, but spiculation in these species differs substantially by their lack of proper choanosomal oxeas. Comparative variation in shape is also recorded for Trikentrion helium (see below).
The apices of the oxeas and the polyactines show minute spines, which is here interpreted as a unique feature. It violates the rule that in Trikentrion only the basal, not the lateral cladi of the polyactines have spines, but there is little correspondence with the lateral cladus spination in Cyamon.
This is the only Trikentrion species that appears to be widespread and common. Chemistry of Trikentrion flabelliforme includes unique indoles (
We studied an Indonesian specimen from the ZMA collection labeled Trikentrion elegans Lendenfeld identified by Burton (ZMA Por. 02402, Siboga Exped. Stat. 303, Timor, Samau Island, Haingsisi, 10.2050S, 123.4591E, 23 m), which has the shape and skeletal structure of a small digitate Trikentrion flabelliforme, including ectosomal long thin styles (up to 1350 × 12 µm), short thin styles (300–400 × 1–3 µm), a choanosomal reticulation of robust oxeas (300–400 × 15–20 µm) and large amounts of trichodragmas (60–110 × 5–15 µm), but lacking polyactine spicules entirely. In view of the occasional rarity of these spicules observed in some specimens of Trikentrion flabelliforme, it is likely that it is a ‘deficient’ specimen of this species. Anecdotal records of Trikentrion flabelliforme from northern Australia have also occasionally encountered similarly deficient specimens (B. Alvarez, pers.comm.). The locality of the Siboga specimen is neatly inbetween the type locality and the North and West Australian localities.
http://species-id.net/wiki/Trikentrion_helium
Figs 24A–EHolotype AHF-NMHLA L-35535 (D33), preserved in alcohol, Hancock Pacific Expeditions, Mexican Pacific, Cedros island, South Bay, approximately 28.07°N, 115.3°W, 18–27 m depth, Velero Station 287–34, 10 March 1934.
Description. Undulating thin-bladed sheets together forming a bushy mass (Fig. 24A) of 7 × 5 × 5 cm. The surface bears a thick spicule brush of 3 mm thickness. Conistency firm, brittle. Colour reddish brown (alcohol).
Skeleton: built chiefly by polyactines (no oxeas), supporting the bases of long styles, which are surrounded by dense brushes of short thin styles.
Spicules: long thin styles, short thin styles, polyactines among which numerous diactinal forms, trichodragmas.
Long thin styles (Figs 24B, B1), variably thinner and thicker, but not divisible in two thickness categories, 952–1808.1–3393 × 18–25.8–42 µm.
Short thin styles (Fig. 24C), usually curved, and often with a subterminal tyle, 372–438.0–510 × 2.5–3.1–3.5 µm.
Polyactines (Fig. 24D), predominantly wide-angled triactines (Fig. 24D), with basal cladi provided with course conical spines (Fig. 24D2), lateral cladi usually much longer than basal cladi, with smooth, rounded endings (Fig. 24D3); basal cladi 66–105.4–144 × 8–22.1–30 µm, lateral cladi 96–146.5–192 × 7–23.6–36 µm; few, mostly smaller, tetractinal polyactines occur, with cladi 27–63 × 9 µm; more frequently diactinal reduced polyactines (Fig. 24D1) occur, asymmetrical, sometimes style-like, smooth, recognizable by an excentric swollen tyle, 192–235.2–306 × 13–19.8–27 µm.
Trichodragmas (Fig. 24E) abundant, occurring throughout the choanosomal and ectosomal regions, 84–100.7–123 × 10–12.1–15 µm. Individual raphides less than 0.5 µm in thickness.
Trikentrion helium Dickinson, 1945, holotype AHF-NMHLA L-35535 (D33), A shape of holotype (photo Phyllis Sun) B long thin style B1 detail of head of long thin style C details of short thin style D polyactines D1 reduced diactinal polyactine D2 detail of spination of basal clade of three-claded polyactine D3 detail of apex of lateral clade of three-claded polyactine E trichodragmas.
The holotype was collected in the Southern Californian Bight (Mexican Pacific).
Rocks and reefs at depths of 15–28 m.
The trichodragmas were not cited in the original description. Trikentrion helium shares the dominance of three-claded polyactines with relatively long lateral cladi with Trikentrion catalina (see below), to which it seems closely related. This species differs quite strongly from the other Trikentrion species by its possession of numerous diactinal or style-like reduced polyactines, which resemble, but clearly are not proper, oxeas like those of Trikentrion muricatum and Trikentrion flabelliforme. The spicules are recognizable as polyactines by the substantial difference between the smoothly rounded end, resembling the ends of the lateral cladi of the three-claded polyactines, and the dissimilar pointed end which shows an irregular surface and is connected to the other end by a swollen, often irregular middle part. Their lengths coincide with the added lengths of a lateral and a basal clade of the three-claded forms. Such reduced diactinal polyactines are also common in Cyamon neon.
The specimens described by
http://species-id.net/wiki/Trikentrion_catalina
Figs 25A–FHolotype USNM 33631, preserved in alcohol, California, Santa Catalina Island, Bird Rock, 33.45°N, 118.4833°W, on rocky cliff at 50 m depth, coll. K. McCleneghan.
Not examined: paratype BMNH 1985 (reg. nr. unknown), Santa Catalina Island, Ship Rock, on rock at 46 m depth, coll. R. Given.
Flabelliform sponge (Fig. 25A), measuring 15 × 8 by 0.4 cm, attached to rocks by a 3 × 0.6 cm stalk. Surface hispid. No oscules apparent. Consistency firm and leathery. Color reddish orange (alive), pale beige (alcohol).
Skeleton (Fig. 25B): choanosome densely packed with three-claded polyactines; ectosome with long, relatively thick styles surrounded by dense bouquets of short thin styles; trichodragmata commonly observed especially in the peripheral parts.
Spicules: long (thin) styles, short thin styles, polyactines, trichodragmas.
Long (thin) styles (Fig. 25C), usually broken and only a few could be measured: 1400–5400 × 8–40 µm, so not really thin.
Short thin styles (Fig. 25D), 130–611.3–730 × 3–5.6–8 µm,
Polyactines (Figs 25E, E1), predominantly three-claded, with spined shorter basal cladi (Fig. 25E1), occasionally with few or no spines on the basal cladi, and smooth, longer, relatively pointed lateral cladi; occasionally four-claded; size basal cladi 78–98.7–126 × 16–25.3–31 µm, lateral cladi 156–197.7–236 × 18–24.4–29 µm.
Trichodragmas: straight, with lightly spined raphides (Figs 25F, F1), 63–79.3–88 × 7–10.2–13 µm.
Santa Catalina Island, Southern California.
On rocks, from 46–50 m depth.
This species is assigned to Trikentrion, because of the flabellate shape resembling Trikentrion flabelliforme
Trikentrion catalina (Sim & Bakus, 1986), holotype USNM 33631, A shape of holotype specimen (scale bar = 1 cm) B cross section of skeleton C detail of head of long thin style D details of short thin style E three- and four-claded polyactines E1 detail of spined basal clade of polyactine F raphide F1 detail of raphide showing spination.
urn:lsid:zoobank.org:act:0807BE5A-BD22-4C6A-907D-3772E69CA479
http://species-id.net/wiki/Trikentrion_africanum
Figs 26A–EType specimen: Holotype BMNH 1939.2.20.9, preserved in alcohol.
Type locality: République du Congo, Pointe Noire, approximately at 4.7667°S, 11.8333°E, coll. E. Darteville, June 1938.
Upright flattened branch with two or three short side projections (Fig. 26A), with wider base and a cut-off upper ending, possibly the specimen is only a fragment as base and apex look damaged. Length of holotype 6.5 cm, diameter 1.5 cm at the base, 1 cm higher up. Side projections only on one side of the branch, less than 1 cm long and 0.5 cm thick, with rounded apex. Surface uneven, somewhat hispid. No apparent oscules. Consistency firm. Colour (alcohol) red-brown.
Skeleton: a dense mass of polyactines, towards the periphery surrounding long thin styles and short thin styles, which are embedded in the skeleton more so than in other Trikentrion species. No oxeas present, but T-shaped polyactines with very short basal clade appear to have taken the position of oxeas.
Spicules: long thin styles, short thin styles, polyactines, trichodragmas.
Long thin styles (Fig. 26B), smooth, straight, usually broken, so only a small number (five) were available for length measurements, 295–870.4–1394 × 9–14.6–24 µm.
Short thin styles (Fig. 26C, C1), straight or gradually curved, 192–241.1–358 × 2–2.3–3 µm.
Polyactines (Fig. 26D), basically three-claded, with the basal clade provided with strong conical spines near the apex. Two major morphological types appear dominant, those with almost equiangular outline (Fig. 26D1), and T-shaped forms with very short basal clade (Fig. 26D2), which is occasionally entirely smooth; basal cladi 27–51.3–96 × 11–13.7–21 µm, lateral cladi 33–96.3–121 × 9–13.9–19 µm.
Trichodragmas (Figs 26E, E1), straight or sometimes curved sinuously, up to 50 or more individual raphides with apical spines, 49–54.4–61 × 5–7.7–11 µm.
Trikentrion africanum sp. n., holotype BMNH 1939.2.20.9, A shape of holotype (scale bar = 1 cm) B details of long thin style C short thin style C1 details of short thin style D various shapes of polyactines E trichodragma E1 detail of trichodragma.
The name is anadjective referring to the type locality.
République du Congo.
Shallow water
The lack of choanosomal genuine oxeas is shared with Californian Trikentrion catalina and Trikentrion helium, but these species have flabelliform or bladed shape and much larger polyactine spicules.
Below the species of Cyamon and Trikentrion considered valid are keyed out. See Table 1 for a summary of recognized species and Table 2 for a summary of their characters.
| 1 | Trichodragmas absent, polyactines are predominantly four-claded or with more cladi, usually shaped equiangular, choanosomal megascleres if present thick styles | (Cyamon) 2 |
| – | Trichodragmas present, polyactines predominantly three-claded Y-shaped, choanosomal megascleres thick oxeas, sometimes absent, but no thick styles | (Trikentrion) 13 |
| 2 | Thicker and thinners styles both heavily spined on the head and more lightly spined along the shaft , polyactines irregular | Cyamon spinispinosum |
| – | All styles smooth, polyactines predominantly regular | 3 |
| 3 | Polyactines in two distinct size categories, the smaller of which is ‘double’ | Cyamon amphipolyactinum sp. n. |
| – | No double polyactines | 4 |
| 4 | Polyactines with only the basal cladi spined or rugose | 5 |
| – | Polyactines with all cladi spined or rugose | 6 |
| 5 | Thin styles fusiform and centrotylote | Cyamon arguinense sp. n. |
| Thin styles not centrotylote | Cyamon quinqueradiatum | |
| 6 | Ectosomal short thin styles with rugose or spined pointed end, often also with an angular bend | 7 |
| – | Ectosomal thin styles straight, lacking spines or rugose ending or they are entirely absent or not differentiated from long thin styles | 10 |
| 7 | Diactinal polyactines present (differentiated from true oxeas by a rugose or irregular condition of one of the apices) | 8 |
| – | No diactinal polyactines | 9 |
| 8 | T-shaped three-claded polyactines common, choanosomal styles averaging 30 µm in thickness, shape a little bush | Cyamon argon |
| – | Polyactines more regular, choanosomal styles averaging 16 µm in thickness, shape a massive encrustation | Cyamon neon |
| 9 | Polyactines predominantly three-claded, with a long basal cladus with hook-like spines and shorter only terminally spined lateral cladi | Cyamon hamatum sp. n. |
| – | Polyactines predominantly four-claded, with little distinction in length and spination of all cladi | Cyamon vickersii |
| 10 | Ectosomal thin styles have a faint centrotylote condition, polyactine spicules are heavily and entirely spined | Cyamon aruense |
| – | Ectosomal thin styles present but lacking a centrotylote condition | 11 |
| 11 | Short thick styles absen | Cyamon quadriradiatum |
| – | Short thick styles present | 12 |
| 12 | Polyactine spicules have swollen apices, but these are not developed into prominent knobs | Cyamon agnani |
| – | Polyactine spicules have prominent spined knobs on the lateral cladi | Cyamon koltuni |
| 13 | Shape rounded branches | 14 |
| – | Shape with flattened blades | 16 |
| 14 | Styles absent | Trikentrion muricatum |
| – | Styles present | 15 |
| 15 | Choanosomal genuine oxeas present | Trikentrion laeve |
| – | Oxeas absent | Trikentrion africanum sp. n. |
| 16 | Choanosomal genuine oxeas present | Trikentrion flabelliformis |
| – | Choanosomal genuine oxeas absent, but diactinal polyactines may be present | 17 |
| 17 | Shape a single large blade, with dense spicule pelt, styles up to 5.5 mm | Trikentrion catalina |
| – | Shape a bladed bush, hispid, but not with a dense pelt, styles up to 3.5 mm | Trikentrion helium |
With the new records from Mauritania, South Carolina and the reassigned Brazil record, the genus Cyamon appears to have a circumglobal warmer water distribution (Fig. 27), commonly observed in many shallow-water sponges (
Idealized global distribution of the genus Cyamon, showing presence of the genus in Marine Ecoregions of the World (
Species assignable to the genus Trikentrion are also found in the warmer waters of all three oceans (Fig. 28), but so far the genus is not recorded from the Central West Atlantic. In contrast, West African waters appear to have a concentrated occurrence of Trikentrion species.
Idealized global distribution of the genus Trikentrion, showing presence of the genus in Marine Ecoregions of the World (
It is likely that more species of both genera will be discovered in the near future.
DiscussionThe two genera were independently erected contemporarily (1867 vs 1870), but at first Cyamon was ignored (Higgin, 1877; Carter, 1879).
Putative similarities and differences of Cyamon and Trikentrion
| Character | State | Cyamon | Trikentrion |
|---|---|---|---|
| Shape | |||
| thinly encrusting | √ | – | |
| lobate | √ | √ | |
| branching | √ | √ | |
| flabellate | – | √ | |
| hispid surface | √ | √ | |
| Architecture | |||
| plumose | √ | – | |
| reticulate | – | √ | |
| raspailiid ectosome | √ | √ | |
| Ectosomal styles | |||
| long thin styles | √ | √ | |
| short thin styles | √ | √ | |
| Choanosomal megascleres | |||
| thick short styles | √ | – | |
| oxeas | – | √ | |
| Polyactines | |||
| equiangular | √ | √ | |
| sagittal | √ | √ | |
| all cladi spined | √ | – | |
| only basal cladus spined | √ | √ | |
| swollen apices | √ | – | |
| Trichodragmata | |||
| present | – | √ | |
Possibly, the position of the polyactines in the skeleton is different in the two genera: usually a basal or central concentration of these spicules in Cyamon and more peripheral or scattered throughout in Trikentrion, but more observations are necessary to confirm this feature.
The isolated occurrence of such unusual polyactine spicules in two genera that are otherwise likely to belong to monactine raspailiids could be interpreted as support for
On the basis of the current state of our knowledge, with, for example, a compelling similarity of polyactines of Cyamon arguinense sp. n. and Trikentrion catalina (compare Figs 6D and 25D, lower right), such a hypothesis lacks sufficient support, and likewise cannot yet be interpreted as support for
Elly J. Beglinger (Naturalis Biodiversity Center, Leiden) made most of the SEM photos. J.A. Cruz (Universidad Nacional Autonoma de Mexico, Estación Mazatlán) assisted with the research on Mexican Pacific species. Kathy Omura (Natural History Museum of Los Angeles County) kindly allowed reproduction of photos made by Phyllis Sun of the types of Cyamon argon and Trikentrium helium. The late Dr Jaap van der Land (Naturalis Biodiversity Center, Leiden) invited the first author to participate in the Netherlands Mauritania II Expedition June 1988. Jan J. Vermeulen (formerly Zoological Museum Amsterdam) assisted in the collection and preservation of Mauritanian sponge samples. For the loan of type and other specimen we are grateful to the curators and collection managers of the Natural History Museum, London (Ms Emma Sherlock and colleagues), Bristol Museum and Art Galleries (Ms Rhian Rowson), the National Museum of Natural History (Smithsonian Institution) (William Moser, Klaus Rützler), the Natural History Museum of Los Angeles County (Ms Kathy Omura), the Senckenberg Museum Frankfurt (Dorte Janussen), the Museum für Naturkunde, Berlin (Carsten Eckert).