(C) 2013 Giacinta Angela Stocchino. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Stocchino GA, Sluys R, Deri P, Manconi R (2013) Integrative taxonomy of a new species of planarian from the Lake Ohrid basin, including an analysis of biogeographical patterns in freshwater triclads from the Ohrid region (Platyhelminthes, Tricladida, Dugesiidae). ZooKeys 313: 25–43. doi: 10.3897/zookeys.313.5363
A new species of the genus Dugesia is described from the Lake Ohrid region in the western part of the Balkan Peninsula, forming the first fully documented species description for this genus in the Ohrid area. The morphological species delimitation is supported by complementary molecular, karyological, and cytogenetic data available from the literature. Therefore, species delineation is based on a truly integrative approach. Further, a short account on the degree of freshwater planarian endemicity in the Ohrid region is provided.
Platyhelminthes, Tricladida, Dugesia, integrative taxonomy, ancient lake, Ohrid, new species, endemicity
The oligotrophic karstic Lake Ohrid is located in the western part of the Balkan Peninsula on the Macedonian-Albanian frontier. With a limnological age of 2-5 million years it is considered to be one of the oldest lakes in Europe (
The first studies on the triclad fauna of the Ohrid area date back to the 1920’s with the first description of several new species of Phagocata Leidy, 1847 and Dendrocoelum Örsted, 1844 (cf.
In this paper we report on a new species of freshwater planarian of the genus Dugesia, forming the first fully documented species description for this genus in the Ohrid area. Our morphological species delimitation was supported by complementary molecular, karyological, and cytogenetic data available from the literature. Therefore, our species delineation is based on a truly integrative approach. Further, we provide a short account on the biogeographical patterns in freshwater planarians and their degree of endemicity in the Ohrid region.
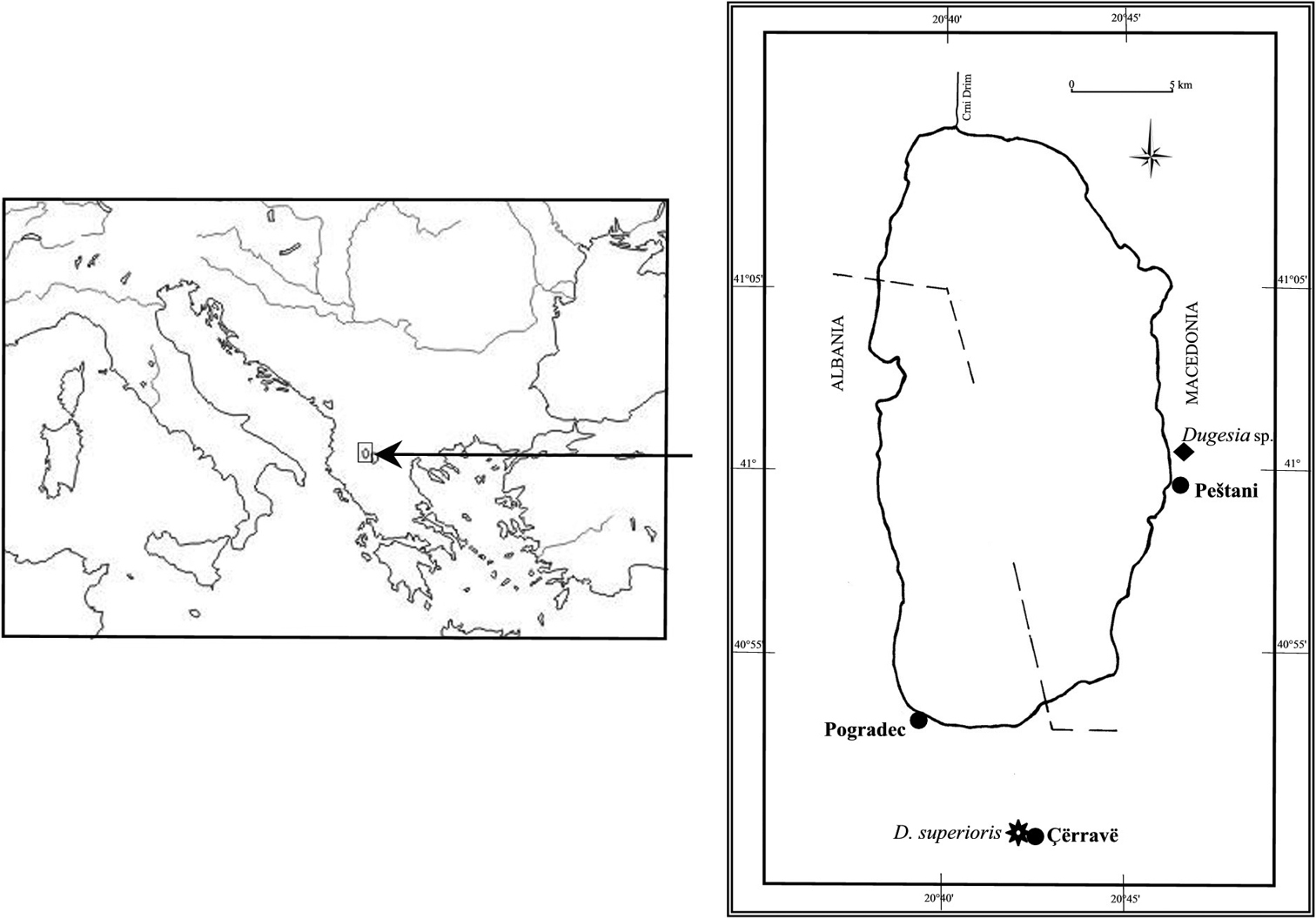
Planarians were collected in 1995 from the southern section of Lake Ohrid basin, near the town of Çërravë, along the Pogradec-Korçë road, ca. 10 km south-east of Pogradec, at an altitude of ca. 800 m asl (Fig. 1). The animals were found under pebbles and among vegetation in a rivulet, flowing along a steep meadow, joining a tributary stream of the lake. All individuals (n = 20) were asexual at collection. The collected specimens were transferred to the laboratory, reared in glass bowls under semi-dark conditions at 18 +/- 2 °C and fed with fresh beef liver.
After having been kept in the laboratory for about one year, during which the strain notably increased in numbers due to asexual reproduction by fission, approximately 30% of the specimens shifted from the fissiparous reproductive mode towards a tendency to sexualize, i.e. to develop reproductive organs. These sexualized animals displayed the characteristic features of ex-fissiparous individuals: large body size, deve-lopment of the copulatory apparatus, hyperplasic ovaries.
Geographic distribution of Dugesia superioris (indicated by an asterisk) and Dugesia sp. NMNH 55294 (indicated by black diamond) in the Lake Ohrid region.
For morphological study sexualized specimens were fixed for 24 hours in Bouin’s fluid, dehydrated in a graded ethanol series, cleared in toluene, and embedded in synthetic paraffin. Serial sections were made at intervals of 5–7 μm and were stained with Harris’ haematoxylin-eosin, Mallory’s trichrome, or Pasini’s reagent.
The material is deposited in the Naturalis Biodiversity Center, Leiden, The Netherlands (collection code: ZMA), and in the Giacinta A. Stocchino collection (CGAS), University of Sassari.
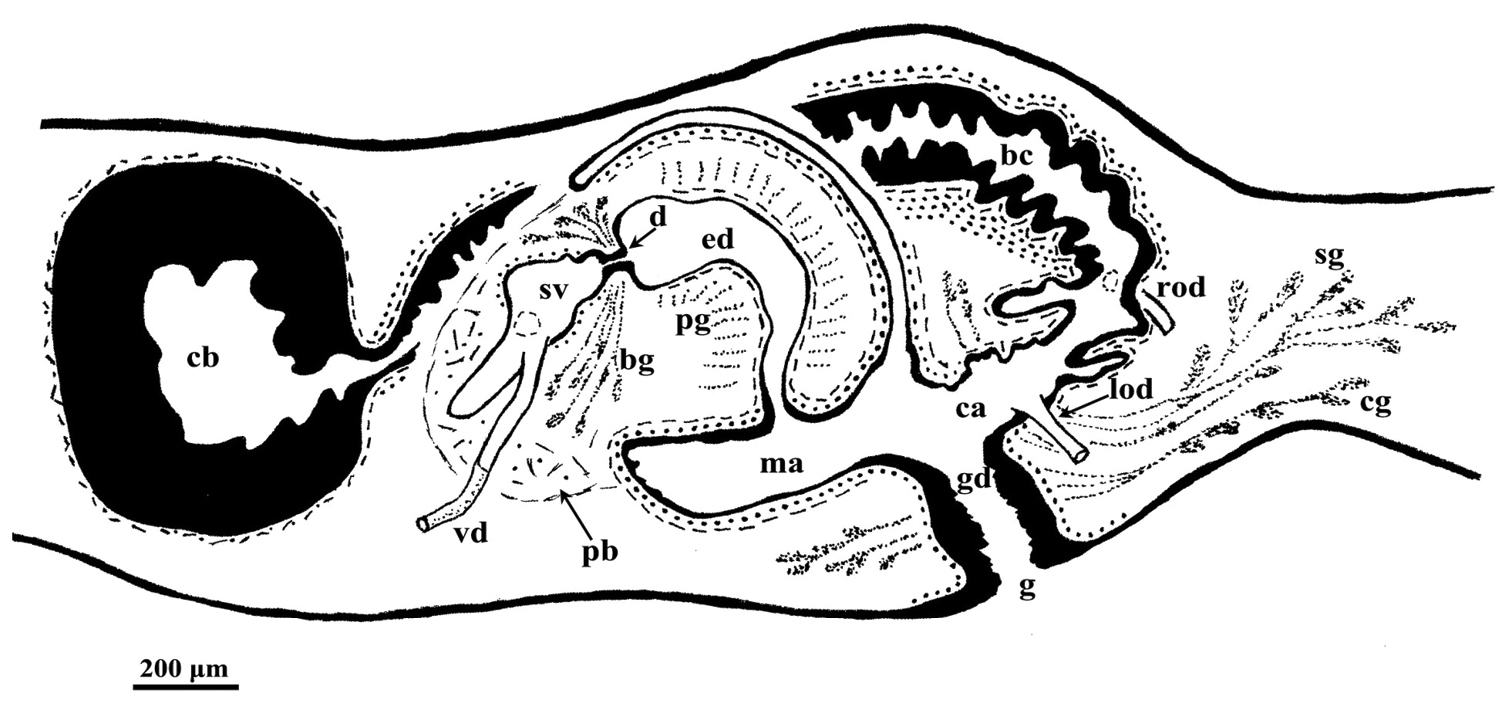
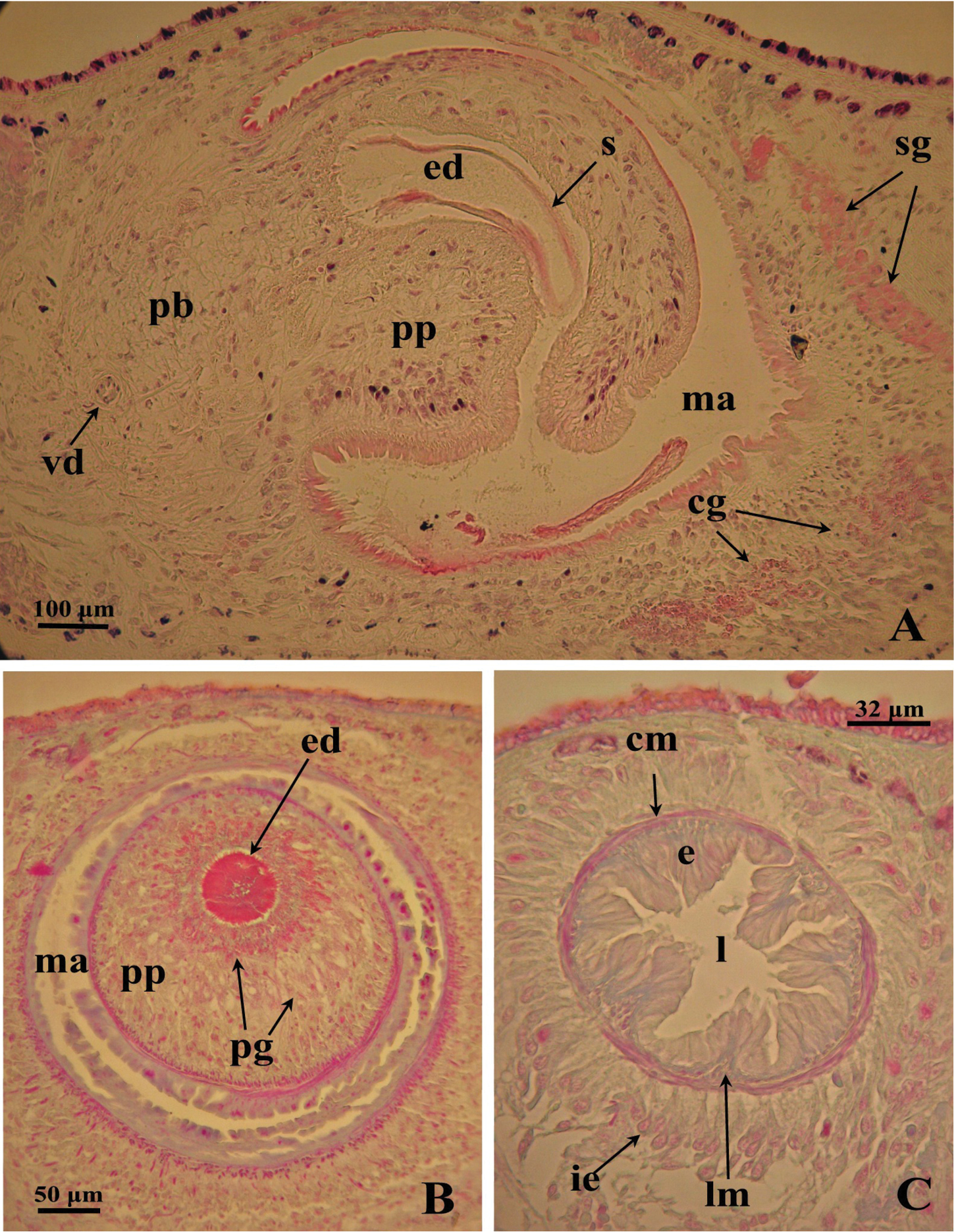
bc: bursal canal; bg: bulb glands; ca: common atrium; cb: copulatory bursa; cg: cement glands; cm: circular muscles; d: diaphragm; e: epithelium; ed: ejaculatory duct; g: gonopore; gd: gonoduct; ie: infranucleate epithelium; l: lumen; lm: longitudinal muscles; lod: left oviduct; ma: male atrium; pb: penis bulb; pf: penial fold; pg: penis papilla glands; ph: pharynx; pp: penis papilla; rod: right oviduct; s: spermatophore; sg: shell glands; sv: seminal vesicle; vd: vas deferens.
urn:lsid:zoobank.org:act:E1A595E2-6466-4F59-99CF-3E846A545332
http://species-id.net/wiki/Dugesia_superioris
Figs 1–4; Table 1Holotype: ZMA V.Pl. 7153.1, Çërravë, Pogradec District (40°50'56"N, 20°42'60"E), Lake Ohrid basin, Albania, August 1995, coll. P. Deri and N. Mazniku, one set of sagittal sections on 50 slides (stained in Harris’ haematoxylin-eosin).
Paratypes: CGAS Pla 6. 1, ibid., sagittal sections on 43 slides (stained in Harris’ haematoxylin-eosin); CGAS Pla 6. 2, ibid., sagittal sections on 12 slides (stained in Mallory’s trichrome); CGAS Pla 6. 3 ibid., transverse sections on 135 slides (stained in Pasini’s reagent); ZMA V.Pl. 7153.2, ibid., transverse sections on 131 slides (stained in Harris’ haematoxylin-eosin); CGAS Pla 6. 4, ibid., transverse sections on 60 slides (stained in Harris’ haematoxylin-eosin); ZMA V.Pl. 7153.3, ibid., horizontal sections on 21 slides (stained in Harris’ haematoxylin-eosin).
Dugesia superioris is characterized by the presence of the following features: dorsal course of the ejaculatory duct; subterminal opening of the ejaculatory duct; asymmetrical openings of the oviducts into the bursal canal; openings of vasa deferentia at halfway along the seminal vesicle; plump penis papilla; small diaphragm; triploid chromosome complement of 24 + 1B-chromosomes.
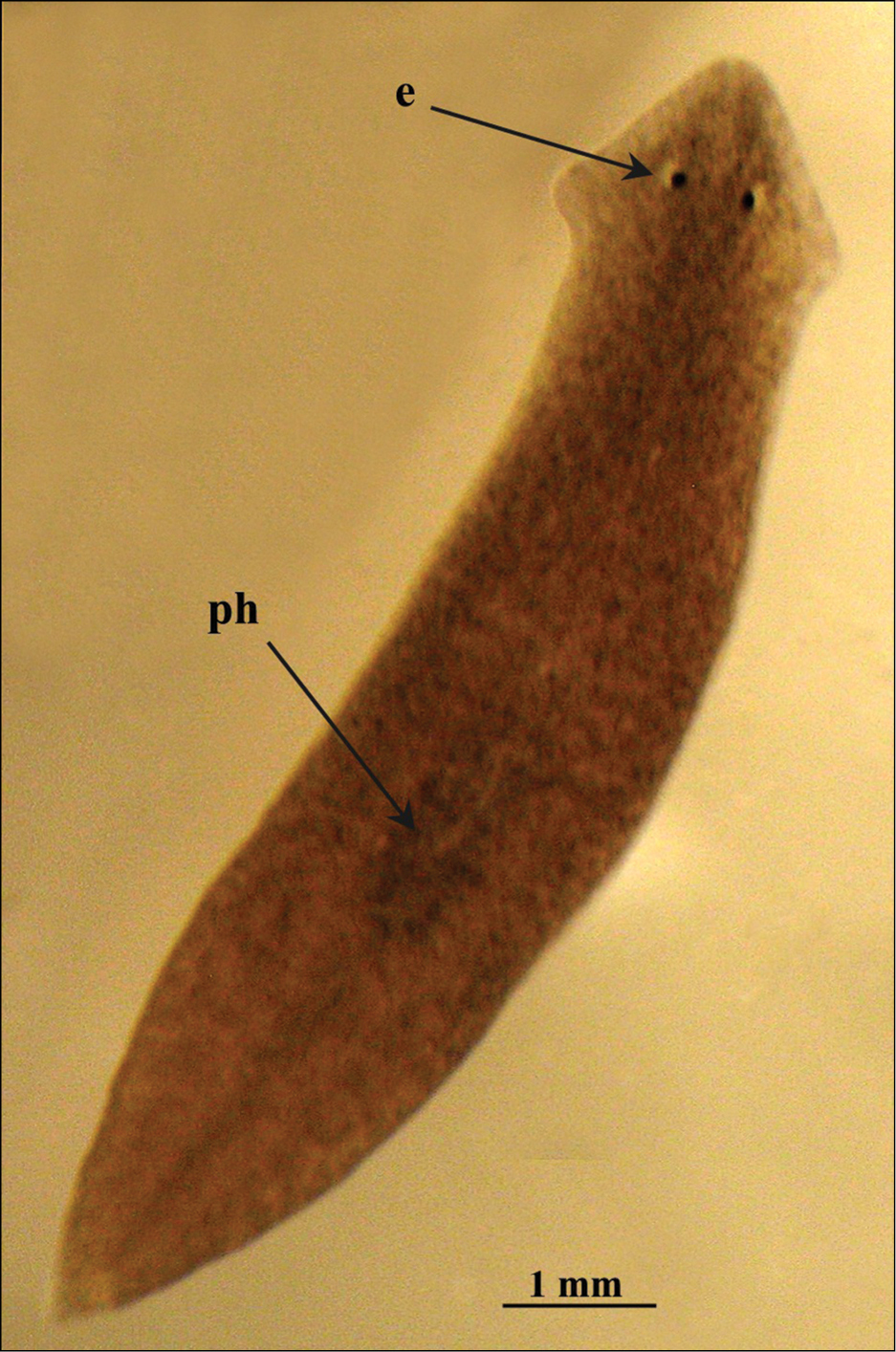
Body size of living fissiparous specimens ranged from 7–10 mm in length and 1.5–2 mm in width (Fig. 2). Sexualized specimens were about 13–16 mm in length and about 3 mm in width. Two eyes are present in the middle of the head, and unpigmented auricular grooves are marginally placed just posteriorly to the eyes. The colour is uniformly brown dorsally, and pale ventrally.
Inner and outer pharyngeal musculature is bilayered, i.e. without an extra, third, outer longitudinal muscle layer. The ovaries are hyperplasic, with several scattered masses at a short distance behind the brain, filling up the entire dorso-ventral space. A degenerative condition is clearly evident in the ovaries, in that maturation of the oocytes is regular up to the beginning of the diplotene stage, whereas diplotenic oocytes show progressive cytoplasm vacuolation, followed by collapse of the entire cell content and by cell necrosis.
Dugesia superioris. Habitus of a living fissiparous specimen.
The anterior portion of the infranucleated oviducts is expanded to form a seminal receptacle that arises in the middle of the ovarian masses at a poorly defined position, dependent upon the hyperplasic condition of the ovaries. The oviducts run ventrally in a caudal direction up to the vaginal area and open asymmetrically into the distal section of the bursal canal. The right oviduct opens dorsally to the left one. The latter opens very close to the point where the canal communicates with the common atrium (Fig. 3). The very abundant shell glands open at the level of the left oviducal opening.
Dugesia superioris. Holotype ZMA V.Pl. 7153.1, sagittal reconstruction of the copulatory apparatus (anterior to the left).
The testes are situateddorsally and extend from just anterior to the ovaries to the posterior end of the body. The testes generally are under-developed in that the majority of germ cells are represented only by spermatogonia (ca. 90%). In only some specimens, and then in only a few follicles, mature sperms are present. However, in all cases anomalies were observed, such as irregularly shaped spermatids and spermatozoa. Vitellaria are located between the testes and the intestinal branches.
The large sac-shaped copulatory bursa is lined by a columnar, glandular epithelium bearing basal nuclei and it is surrounded by a thin layer of muscles. From the mid-posterior wall of the bursa the bursal canal runs in a caudal direction, to the left of the copulatory apparatus. Posteriorly to the gonopore the bursal canal recurves antero-ventrally and, subsequently, opens into the posterior section of the atrium. The bursal canal is lined by a pleated epithelium with cylindrical, infranucleated, and ciliated cells and is surrounded by a thin, subepithelial layer of longitudinal muscles, followed by a thicker layer of circular muscle. Ectal reinforcement is absent (Figs 3, 4C). At its distal section, near the atrium, the bursal canal shows several deep folds.
Dugesia superioris. Photomicrographs of the copulatory apparatus. A Holotype ZMA V.Pl. 7153.1, sagittal section showing the penis bulb and the penis papilla with the ejaculatory duct B Paratype CGAS Pla 6. 3, transverse section of the penis papilla and the ejaculatory duct surrounded by numerous glands C Paratype CGAS Pla 6. 3, transverse section of the bursal canal.
The moderately developed penis bulb, rich in glands, consists of intermingled longitudinal and circular muscle fibres. It houses an elongated seminal vesicle, which extends through the entire length of the penis bulb. The anterior half of the seminal vesicle is tubular in shape, while its distal, posterior section is considerably expanded.
The vasa deferentia penetrate the antero-lateral wall of the penis bulb and open separately and symmetrically into the seminal vesicle at a position about halfway along the vesicle. No spermiducal vesicles were observed in any of the specimens examined. The seminal vesicle, lined with a flat epithelium and surrounded in its distal, posterior section by layers of circular muscle fibres, opens into the ejaculatory duct via a small diaphragm. The latter, located at the base of the penis papilla, receives the openings of very abundant bulb glands. The blunt penis papilla is lined with an infranucleated epithelium that is underlain with a thin subepithelial layer of circular muscles fibres, followed by a layer of longitudinal muscle fibres.
The ejaculatory duct follows a dorsally displaced course through the penis papilla and has a sub-terminal opening. The spacious lumen of the ejaculatory duct is lined by a cuboidal, infranucleated epithelium that is surrounded by a layer of longitudinal musclesand receives the abundant secretion of penis papillaglands; in the majority of examined specimens the ejaculatory duct contained an empty spermatophore (Fig. 4A, B). Both the bulb glands and the penis papilla glands secrete globules that stain purple in Pasini’s reagent (Fig. 4B). The acentral, dorsally displaced ejaculatory duct makes the penis papilla asymmetrical, with the ventral part being thicker than the dorsal one (Figs 3, 4A, B).
The genital atrium is lined by an infranucleated epithelium that is underlain by a subepithelial layer of circular muscle, followed by a layer of longitudinal muscle fibres. The common atrium communicates with a gonoduct that is lined by a columnar epithelium, which receives the openings of very abundant cement glands; the gonoduct communicates with the ventral gonopore (Fig. 3).
The specific epithet is derived from the Latin superius, located at a higher position, and alludes to the dorsally displaced course of the ejaculatory duct in the penis papilla.
Known from the type locality and, most likely, also from a second Albanian locality, viz. Voskopojë (see below).
Checklist of Tricladida from the Lake Ohrid hydrographic basin.
| Taxa | Lacustrine habitat | Adjacent waters of lake Ohrid | Endemic species | References |
|---|---|---|---|---|
| Dugesiidae Ball, 1974 | ||||
| Dugesia Girard, 1850 | ||||
| Dugesia superioris | _ | tributary rivulet | – | Present paper |
| Dugesia sp. | _ | Spring Elešec | ? | |
| Dugesia gonocephala (Dugès, 1830)(?) | _ | tributary streams and the effluent Crni Drim River | _ | |
| Schmidtea Ball, 1974 | ||||
| Schmidtea lugubris (Schmidt, 1861) | _ | stagnant waters of the Ohrid region; drainage ditch at Teferić | _ | |
| Dendrocoelidae Hallez, 1892 | ||||
| Dendrocoelum Örsted, 1844 | ||||
| Dendrocoelum adenodactylosum (Stanković & Komárek, 1927) | littoral, sublittoral, profundal zones | littoral cold springs; tributary streams; a tributary of the effluent Crni Drim River | _ | |
| Dendrocoelum albidum Kenk, 1978 | sublittoral zone | _ | + | |
| Dendrocoelum cruciferum (Stanković, 1969) | sublittoral zone | _ | + | |
| Dendrocoelum decoratum Kenk, 1978 | sublittoral and profundal zones | _ | + | |
| Dendrocoelum dorsivittatum Kenk, 1978 | profundal zone | _ | + | |
| Dendrocoelum jablanicense (Stanković & Komárek, 1927) | _ | Šum Spring; tributary streams | + | |
| Dendrocoelum komareki (Stanković, 1969) | sublittoral zone | _ | + | |
| Dendrocoelum lacteum (Müller, 1774) | sublittoral and profundal zones | stagnant waters | _ | |
| Dendrocoelum lacustre (Stanković, 1938) | sublittoral zone | _ | + | |
| Dendrocoelum lychnidicum (Stanković, 1969) | sublittoral zone | _ | + | |
| Dendrocoelum maculatum (Stanković & Komárek, 1927) | littoral zone | tributary streams; littoral springs | + | |
| Dendrocoelum magnum (Stanković, 1969) | sublittoral zone | _ | + | |
| Dendrocoelum minimum Kenk, 1978 | profundal zone | _ | + | |
| Dendrocoelum ochridense (Stanković & Komárek, 1927) | littoral, sublittoral, profundal zones | _ | + | |
| Dendrocoelum sanctinaumi (Stanković & Komárek, 1927) | littoral and sublittoral zones | tributary streams; littoral springs | + | |
| Dendrocoelum sinisai Kenk, 1978 | profundal zone | _ | + | |
| Dendrocoelum translucidum Kenk, 1978 | profundal zone | _ | + | |
| Planariidae Stimpson, 1857 | ||||
| Crenobia Kenk, 1930 | ||||
| Crenobia alpina montenegrina (Mrázek, 1904) | _ | springs; tributary streams and the effluent Crni Drim River | _ | |
| Phagocata Leidy, 1847 | ||||
| Phagocata maculata (Stanković, 1938) | sublittoral zone | _ | + | |
| Phagocata ochridana (Stanković & Komárek, 1927) | littoral, sublittoral, profundal zones | springs and pools | + | |
| Phagocata stankovici (Reisinger, 1960) | sublittoral and profundal zones | _ | + | |
| Phagocata undulata (Stanković, 1960) | sublittoral zone | _ | + | |
| Planaria Müller, 1776 | ||||
| Planaria torva (Müller, 1774) | _ | only one specimen at the mouth of the Studenčišta brook | _ | |
| Polycelis Ehrenberg, 1831 | ||||
| Polycelis tenuis Ijima, 1884 | _ | tributary streams | _ |
A karyological study by
A molecular cytogenetic comparison of several species and populations of the genus Dugesia revealed that these planarians from Pogradec besides two telomeric NOR loci, also have a ribosomal site located in an intercalated position on the long arm of one of the largest chromosomes (
More recently, a phylogeographic analysis of two Albanian populations, one from Pogradec and the other from Voskopojë (populations 30 and 31, respectively in
From Lake Ohrid only one species of Dugesia has been reported until now, viz. Dugesia gonocephala.
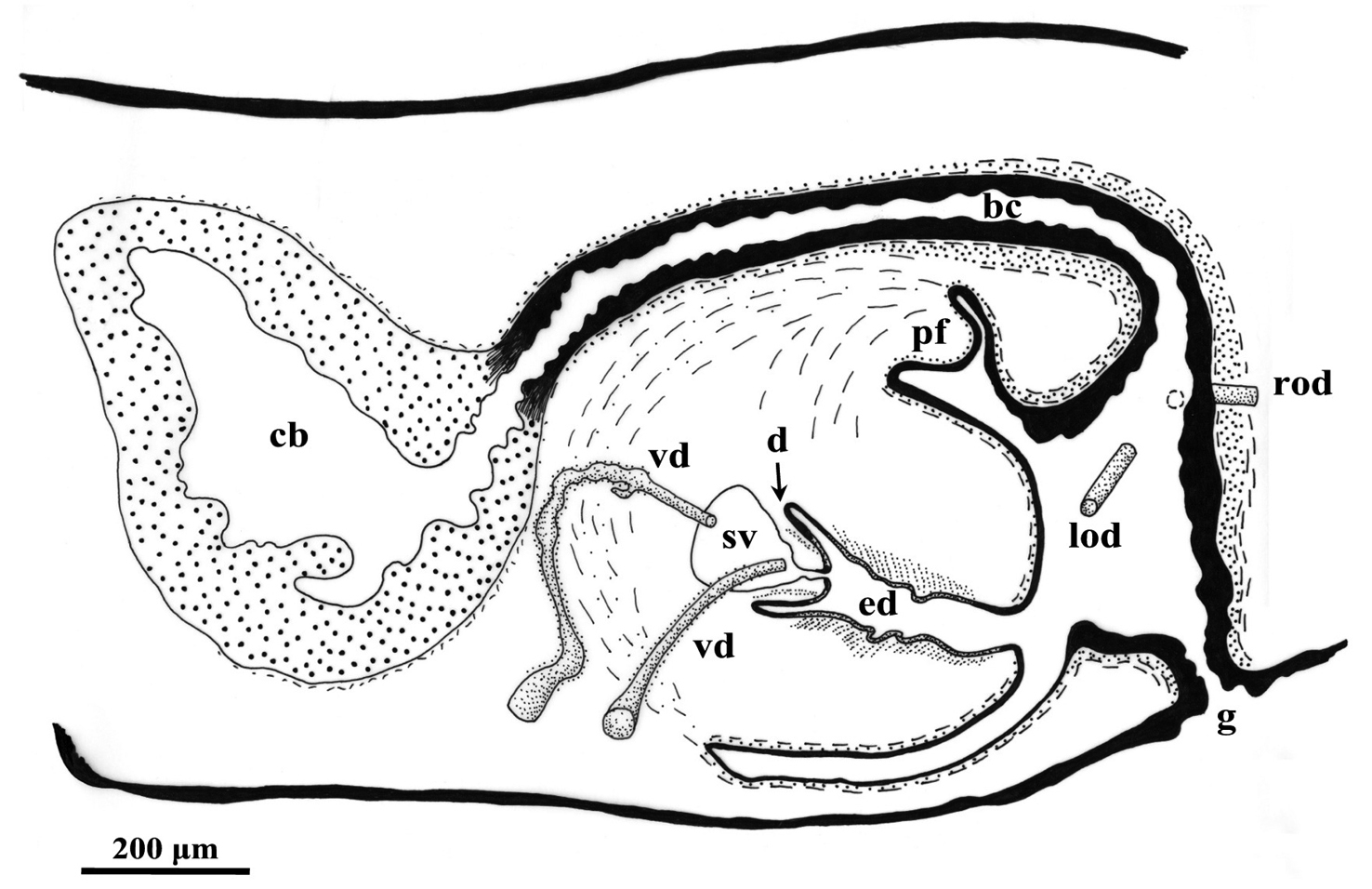
Sagittal reconstruction of the copulatory apparatus of Dugesia specimen NMNH 55294.
Out of a total of 27 nominal species of triclads reported from the Lake Ohrid region, 19 are endemic to this area (70% of endemicity) (Table 1). Most of these endemics (15) are restricted to the lake proper: Dendrocoelum albidum Kenk, 1978, Dendrocoelum cruciferum (Stanković, 1969), Dendrocoelum decoratum Kenk, 1978, Dendrocoelum dorsivittatum Kenk, 1978, Dendrocoelum komareki (Stanković, 1969), Dendrocoelum lacustre (Stanković, 1938), Dendrocoelum lychnidicum (Stanković, 1969), Dendrocoelum magnum (Stanković, 1969), Dendrocoelum minimum Kenk, 1978, Dendrocoelum ochridense (Stanković & Komárek, 1927), Dendrocoelum sinisai Kenk, 1978, Dendrocoelum translucidum Kenk, 1978, Phagocata maculata (Stanković, 1938), Phagocata stankovici (Reisinger, 1960), Phagocata undulata (Stanković, 1960). The species that live in the lake may inhabit only one of the three major bathymetrical zones of the lacustrine bottom (littoral, sublittoral and profundal) or can be found in two or more zones (Table 1). Onlythreespecies are endemic bothto the lake and adjacent water systems: Dendrocoelum maculatum (Stanković & Komárek, 1927), Dendrocoelum sanctinaumi (Stanković & Komárek, 1927), Phagocata ochridana (Stanković and Komárek, 1927).
Dendrocoelum lacteum (Müller, 1774) is a species with a very large distributional range across the Palaearctic Region that occursboth in the lake and in surrounding waters.
Dendrocoelum adenodactylosum is very common in the lake, in its tributary streams and springs and also in Lake Prespa, a nearby lake southeast of Lake Ohrid that is a major water supplier for the latter. Six species are found in surrounding streams and springs and do not occur in the lake proper, viz. Dugesia superioris, Dendrocoelum jablanicense (Stanković & Komárek, 1927), Schmidtea lugubris (Schmidt, 1861), Crenobia alpina montenigrina (Mrázek, 1904), Planaria torva (Müller, 1774), and Polycelis tenuis Ijima, 1884. Dendrocoelum jablanicense is endemic of the Lake Ohrid region, while the others concern widespread species.
Dugesia superioris differs from its congeners in particular in (a) the dorsal course of the ejaculatory duct, with its sub-terminal opening, (b) the asymmetrical openings of the oviducts into the bursal canal, and (c) the openings of vasa deferentia at about halfway along the seminal vesicle.
For the genus Dugesia a dorsal course of the ejaculatory duct was reported for the first time by
A subterminal opening of the ejaculatory duct, as found in Dugesia superioris, occurs in no less than 26 species of Dugesia: Dugesia bakurianica Porfirjeva, 1958, Dugesia biblica Benazzi & Banchetti, 1972, Dugesia leporii Pala et al., 2000, and Dugesia sicula Lepori, 1948, from the Western Palaearctic; Dugesia aethiopica Stocchino et al., 2002, Dugesia arabica Harrath & Sluys, 2013, Dugesia astrocheta Marcus, 1953, Dugesia lanzai Banchetti & Del Papa, 1971, Dugesia lamottei De Beauchamp, 1952, Dugesia neumanni (Neppi, 1904) and Dugesia myopa De Vries, 1988b from the Afrotropical Region; the other 15 species are distributed in the Oriental Region, Eastern Palaearctic and Australasian Region, viz. Dugesia andamanensis (Kaburaki, 1925), Dugesia austroasiatica Kawakatsu, 1985, Dugesia batuensis Ball, 1970, Dugesia bengalensis Kawakatsu, 1983, Dugesia burmanensis (Kaburaki, 1918), Dugesia deharvengi Kawakatsu & Mitchell, 1989, Dugesia indica Kawakatsu, 1969, Dugesia indonesiana Kawakatsu, 1973, Dugesia japonica Ichikawa & Kawakatsu, 1964, Dugesia leclerci Kawakatsu & Mitchell, 1995, Dugesia lindbergi De Beauchamp, 1959, Dugesia nannophallus Ball, 1970, Dugesia novaguineana Kawakatsu, 1976, Dugesia tamilensis Kawakatsu, 1980, and Dugesia uenorum Kawakatsu & Mitchell, 1995. However, in all of these species the ejaculatory duct is ventrally displaced, except for Dugesia bakurianica in which the ejaculatory duct is central. Therefore, a dorsal course of the ejaculatory duct and a subterminal opening of the duct represents a new diagnostic combination in the genus Dugesia.
The Pogradec population had already been subjected to karyological, cytogenetic, and phylogeographic studies before anything was known about the anatomy of the specimens (see above). All of these analyses pointed to a situation that this Dugesia population differs considerably from congeneric populations. Therefore, it was unsurprising that the anatomy of the Pogradec animals suggested also that they represent a new species. As a result of the cumulation of the evidences from these independent datasets, the present delineation of the new species is based on a truly integrative approach to taxonomy.
Studies on the phylogeny of Dugesia (
An asexual population of Dugesia sp. was collected in 2006 by R. Manconi from Voskopojë, an Albanian locality situated south-west of Lake Ohrid. Unfortunately, we have been unable to ascertain the taxonomic status of this population due to the lack of sexual specimens (Stocchino and Manconi, pers. obs.). However, according to the phylogeographic analysis of
That
Dugesia elegans from Rhodes differs from NMNH 55294 in the presence of a much larger seminal vesicle, a stubbier diaphragm, and the situation that its bursal canal epithelium is infranucleated (
The penial fold of Dugesia taurocaucasica is considerably larger than the one in NMNH 55294, while the fold is also traversed by the abundant secretion of cyanophilic glands, which discharge through the lining epithelium of the penial fold. Furthermore, in Dugesia taurocaucasica the ectal reinforcement layer on the bursal canal extends for a considerable distance towards the copulatory bursa (
The species Dugesia effusa differs from NMNH 55294 in the presence of a short, valve-like diaphragm, a large intrabulbar seminal vesicle, a highly glandular penis papilla, and symmetrical oviducal openings into the bursal canal.
Therefore, the Dugesia specimen NMNH 55294 may well represent a new species. However, on the basis of only the presently available material we refrain from describing it as new. Furthermore, the asymmetrical openings of the vasa deferentia into the seminal vesicle of this animal represents a highly unusual condition for a species of Dugesia and needs to be checked on additional material.
Present data support
This research was funded in part by the Regione Autonoma Sardegna (CRP-60215 “Conservazione e valorizzazione delle grotte sarde: biodiversità e ruolo socio-economico-culturale). G.A.S. acknowledges financial support by the “Fondazione Banco di Sardegna” and SYNTHESYS, a programme of the European Commission under the 7th Research and Technological Development Framework Programme (grant number: NL-TAF 2772). Completion of the manuscript was made possible by a grant from the Naturalis Biodiversity Center to R. Sluys. Dr. Jon Norenburg (National Museum of Natural History (NMNH), Washington, USA) kindly sent on loan the Dugesia specimen collected by R. Kenk. We thank Prof. G. Corso for her kind support. G.A.S. is grateful to Prof. M. Pala for her financial support in memory of her husband Prof. N.G. Lepori. R.S. thanks Prof. Dr. M. Kawakatsu for providing a copy of a cited publication.