(C) 2013 Ricardo L. Pinto. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Pinto RL, Jocqué M (2013) A new species of Elpidium (Crustacea, Ostracoda) from bromeliads in Cusuco National Park, Honduras. ZooKeys 313: 45–59. doi: 10.3897/zookeys.313.4904
Passively dispersing aquatic invertebrates such as Ostracoda in restricted aquatic habitats such as bromeliads remain an intriguing observation considering the highly specialised dispersal vectors needed for efficient colonisation. Here we describe a new species of Elpidium, Elpidium merendonense sp. n., collected from bromeliads in the cloud forest from Cusuco National Park, Honduras. Elpidium merendonense sp. n. is a small to medium-sized species that can be easily distinguished from its congeners by its unique outgrowth at the posterior end of the left valve, visible especially in females. The species was common all through the park occurring at a wide range of altitudes and in different species of bromeliads. This finding is the first freshwater ostracod species described from Honduras and is in agreement with the prediction that the genus Elpidium contains a large number of species with small geographic distributions. We update the list of described species of Elpidium and present a key to species.
Timiriaseviinae, phytotelmata, dispersal, endemism, neotropics, cloud forest
Phytotelmata are plant structures that hold water, such as tree holes, flowers, husks or leaf brackets. These water bodies are completely rain dependent and often contain communities of highly specialised aquatic invertebrates (
The first discovery of an ostracod species in bromeliad tanks was published by
Studies on Elpidium remained predominantly taxonomic until relatively recently, when
In the present work, we report on a new species of Elpidium from Cusuco National Park, which consists of the first ostracod species collected from bromeliad phytotelmata in Honduras.
Material used for the species description was collected during the survey of aquatic invertebrate communities in bromeliads from 12th July to 22nd August 2006 in Cusuco National Park (CNP), Honduras. This survey is part of the yearly biodiversity survey by the ecovolunteer driven conservation organisation Operation Wallacea (UK) in CNP. CNP is situated in north-western Honduras, within the Merendón Mountain range. The core zone of the park consists of lower montane tropical rain forest (a mix of primary and secondary), with patches of primary cloud forest and upper montane rain forest characterised by high densities of bromeliads. Bromeliads sampled for Ostracoda were Tillandsia guatemalensis Smith and Catopsis sp. We sampled a total of 110 bromeliads in 12 different sites in the park, for a more detailed description of the study of aquatic invertebrates in bromeliads we refer to the protocol described in
For description, specimens were dissected under a stereomicroscope; valves were stored dry in micropaleontological slides and soft parts were mounted in permanent slides with CMC-9AF mounting medium (Masters Company Inc., Bensenville, Illinois, USA). Micrographs of valves and carapaces were obtained with a scanning electron microscope; line drawings of appendages and body parts were made under an optical microscope with the aid of a camera lucida.
All analysed material is deposited in the crustacean collection of the Museu de Zoologia da Universidade de São Paulo (MZUSP).
Higher taxonomy of the Ostracoda follows the synopsis by Horne et al. (2002).
(by original designation): Elpidium bromeliarum F. Müller, 1880.
Other species allocated: Elpidium inaequivalve Danielopol, 1980; Elpidium laesslei (Tressler, 1956) Danielopol, 1980; Elpidium maricaoensis (Tressler, 1941) Danielopol, 1980; Elpidium pintoi Danielopol, 1980; Elpidium purperae Danielopol, 1980.
Medium sized to relatively large ostracods; carapace broad, generally bigger in width than in height; ventral margin flat; pale to dark brown smooth surface; in dorsal and ventral view males with greatest width at midlength, females with posterior part expanded into a brood pouch carrying eggs and greatest width displaced posteriorly; strongly interlocking selvages along ventral margin leaving an anteroventral gap between left and right valve margins; hinge a crenulated cardinal bar on the smaller valve forming rudimentary anterior and well developed posterior teeth; A1 with 5 functional articles, the first one bearing a sub-apical expansion with a tuft of tiny setules on the dorsal margin; in males, A2 with a serrated apical claw on the terminal segment (no such serration in females); terminal segment of A2 with a small lobe (hyaline formation) in both males and females; second and third endites of the maxillule bearing two spatulate claws each; copulatory process of hemipenis a hook-like structure placed ventrally on the muscular body, near the base of distal lobe.
Elpidium is closely related (
urn:lsid:zoobank.org:act:0300306E-1A75-4EB3-8C43-2221036F40A1
http://species-id.net/wiki/Elpidium_merendonense
Figs 1–4Cusuco National Park, Honduras at an altitude of 1840 masl. Geographical coordinates: 15.5133N, 88.2417W. Water accumulated in bromeliad (Tillandsia guatemalensis) leaf axils. Material collected on 15 July 2006 by M. Jocqué.
Holotype: a dissected male, with valves dried and coated for scanning electron microscopy stored in a micropaleontological slide and soft parts mounted in a permanent slide with CMC-9AF mounting medium (MZUSP 29072). Allotype: a dissected ovigerous female, with valves stored dry in a micropaleontological slide and soft parts mounted in a permanent slide with CMC-9AF mounting medium (MZUSP 29073). Paratypes: two males (MZUSP 29074 and MZUSP 29075), dissected and stored like the allotype; an ovigerous female (MZUSP 29076) dissected and stored like the holotype; three males (MZUSP 29077, MZUSP 29078 and MZUSP 29079) and three females (MZUSP 29080, MZUSP 29081 (carapace broken) and MZUSP 29082), dried and coated for scanning electron microscopy stored in micropaleontological slides; 25 males and 8 females kept whole in a vial with 70% ethanol (MZUSP 29083).
The species is named after the Merendón mountains in Honduras, where the specimens described in the present work were collected.
Small sized Elpidium (c. 700 μm). In dorsal and ventral views carapace relatively elongated for the genus (length/width c. 1, 4). In right lateral view carapace elongated (length/height c. 2), with left valve overlapping right valve on all margins, but very strongly at the posterior end of the carapace, especially in females, where this overlap produces a conspicuous outgrowth of the outer lamella, apparently without substantial change to the inner marginal structures. Ventral margin flat, with a subtle ventro-lateral ridge on each valve, at the edge of the flat area. Distal lobe on hemipenis triangular, with a pointed tip and with a finger-like projection at the base of the internal margin next to the dorsal seta; copulatory process a stiff hook-like structure, thick at the first half of its length and then quickly narrowing to the orifice at the tip.
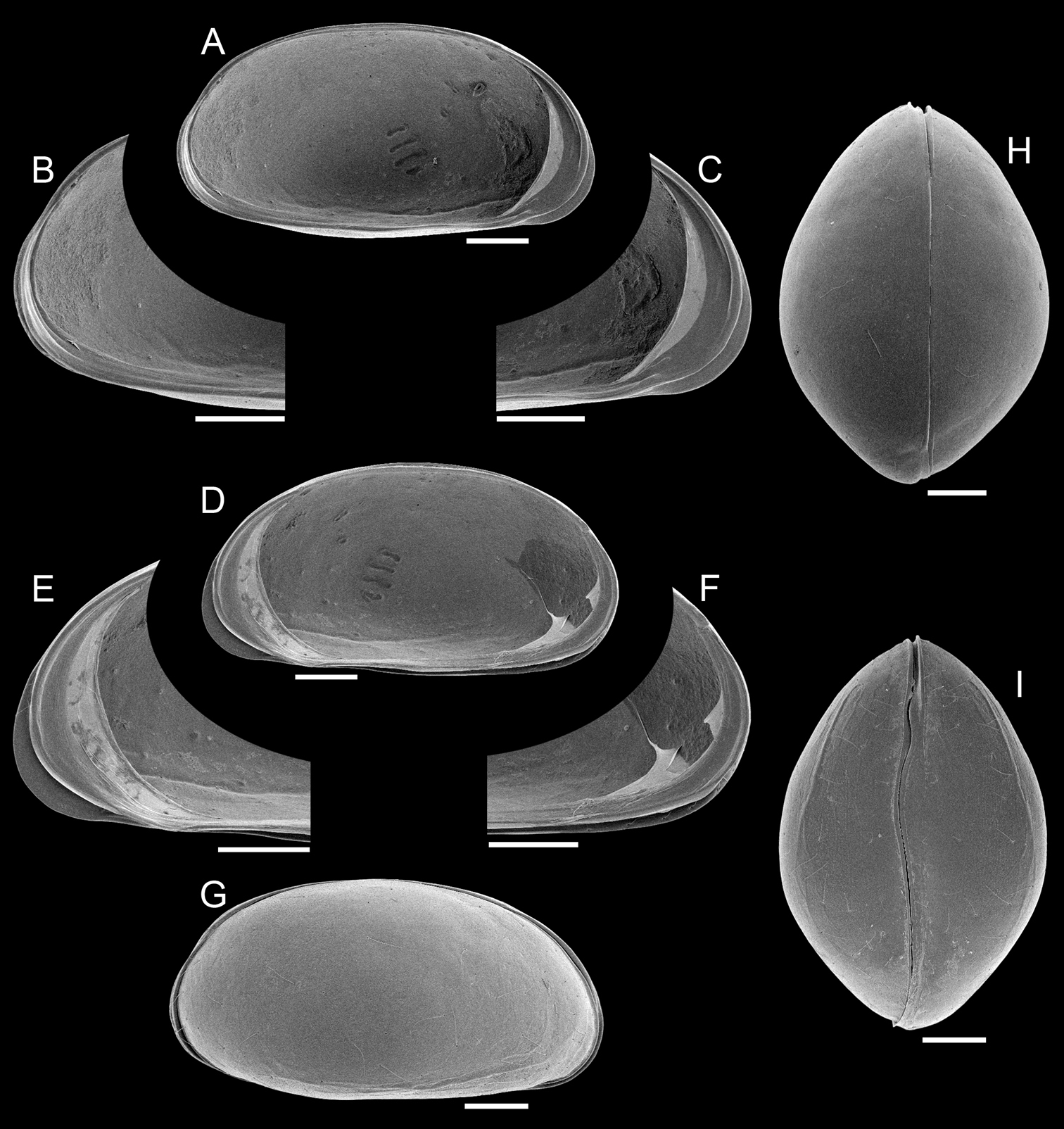
Carapace (Fig. 1G–I). Small sized (length = 657–685 μm), with brown surface and sparse setae; surface smooth except for a subtle ventro-lateral ridge on each valve; elliptically elongated in lateral view (length/height=1.96), with greatest height just behind the mid-length; left valve overlapping right valve on all margins, strongly interlocking in antero-ventral, ventral and postero-ventral margins; ventral area flat; dorsal margin arched; posterior and anterior margins rounded, both produced towards the ventral side; oval shaped in ventral and dorsal views, with maximum width behind mid-length; dorsal margin straight in dorsal view; ventral margin sinuous in ventral view with well-marked ridges.
Elpidium merendonense sp. n., male. A Left valve, internal view B left valve, internal view, detail of postero-ventral margin C left valve, internal view, detail of antero-ventral margin D right valve, internal view E right valve, internal view, detail of antero-ventral margin F right valve, internal view, detail of postero-ventral margin G right lateral view H dorsal view I ventral view. A–F holotype, MZUSP 29072; G paratype, MZUSP 29077; H paratype, MZUSP 29078; I paratype, MZUSP 29079. Scale bars: 100 µm.
Left valve (Fig. 1A–C). In internal view, anterior and posterior margins rounded, produced towards the ventral side; ventral margin nearly straight; dorsal margin arched; calcified inner lamella well developed anteriorly, with a short line of concrescence near the valve margin leaving a vestibule; vestibule less developed posteriorly, but present; prominent selvage running on all margins except in the middle of dorsal margin; a short flange is formed at the antero-ventral margin; central muscle scars consisting of 4 spots arranged in a vertical row relatively separated from each other and a round small frontal scar.
Right valve (Fig. 1D–F). In internal view, anterior and posterior margins rounded, produced towards the ventral side; ventral margin nearly straight; dorsal margin arched; calcified inner lamella well developed anteriorly, with a short line of concrescence near the valve margin leaving a vestibule; vestibule less developed posteriorly but present; prominent selvage running all around, forming the hinge structures on the dorsal margin; a continuous flange is present, being wide on the antero-ventral margin, very narrow at the mouth region and narrow on the ventral and postero-ventral margins; central muscle scars consisting of 4 spots relatively separated from each other arranged in a vertical row and a round small frontal scar.
Hinge (Fig. 1A–F). A long (c. 3/4 of the valve length) cardinal ridge is present on the right valve, forming at each end, respectively, a small anterior tooth and a large posterior tooth; the ridge is slightly crenulated, especially at the posterior end; RV with complimentary groove and sockets.
Pigmented naupliar eye present; carapace less pigmented at the eye region.
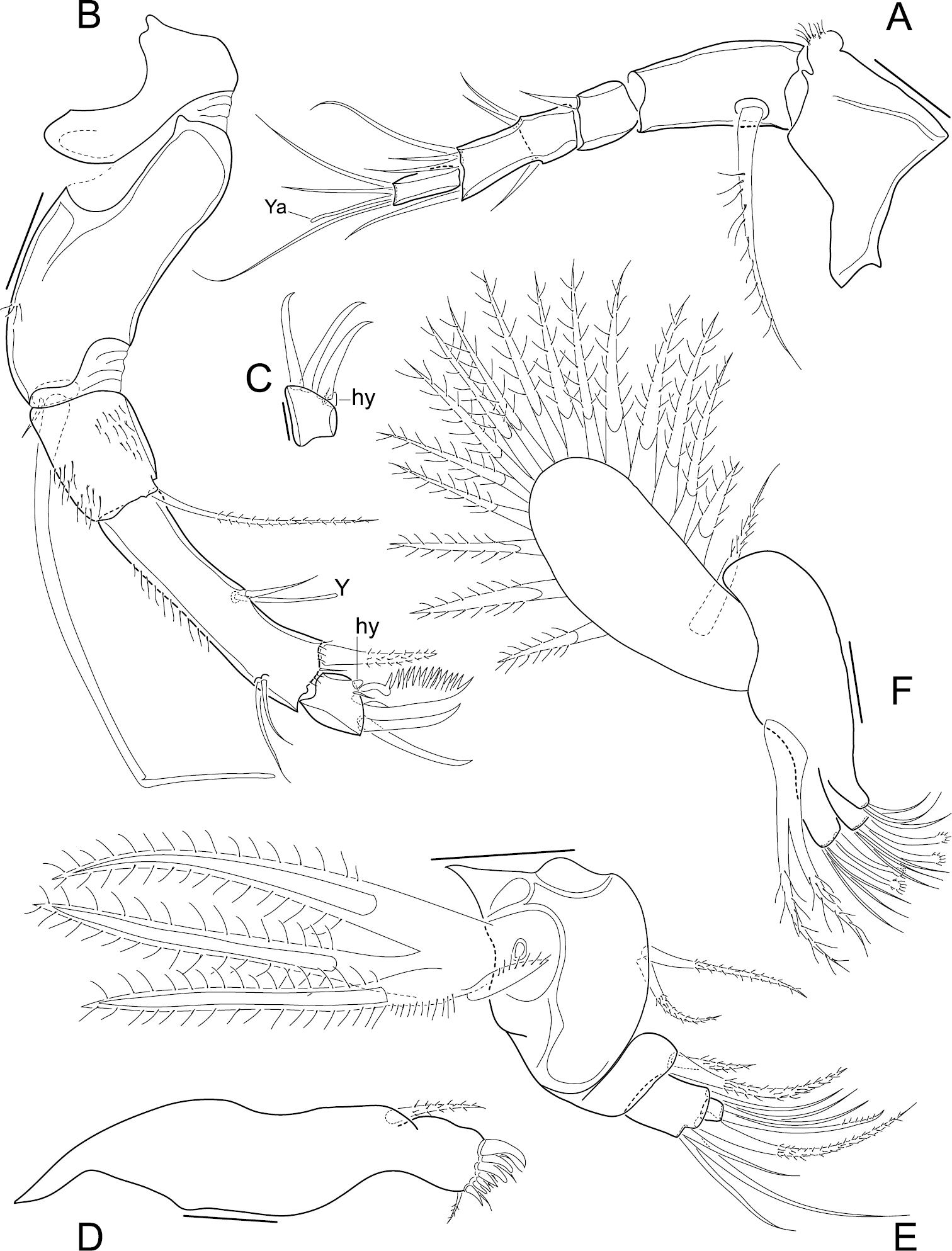
Antennula (Fig. 2A). 5 functional articles; first article relatively large, bearing on the dorsal margin a sub-apical expansion with a tuft of tiny setules; second article the longest with a ventro-proximal long and thick seta; third article small with a short dorso-apical seta; fourth article partially subdivided in two, medially (where the segment is subdivided) with two dorsal and one ventral setae, and apically with a long ventro-apical, two short and one long dorso-apical setae; fifth (terminal) article with two long setae, one short seta and a short aesthetasc (Ya).
Elpidium merendonense sp. n. A Antennula B Antenna C terminal segment of antenna D Mandibula E Mandibular palp F Maxillula A–B, F male specimen, holotype, MZUSP 29072 C female specimen, allotype, MZUSP 29073 D–E male specimen, paratype, MZUSP 29075. Scale bars: A–B, D–F: 50 µm; C: 20 µm. Ya = aesthetasc on terminal segment of A1. Y = aesthetasc on penultimate segment of antennae. hy = hyaline organ on second antennae.
Antenna (Fig. 2B). Protopodite 2-segmented, the first one very short and the second one long, wide and curved; endopodite 3-articulated; first segment relatively short, bearing a long ventro-apical seta; second segment very long and narrow, dorsally with two sub-apical setae, one three thirds as long as the other, ventro-medially with a short seta and an aesthetasc (Y), and apically with two setae, one large and one minute; last segment small, with three claws, the ventral one strongly serrated and the other two slender, a minute seta and a tiny lobe (hyaline formation); exopodite with a very small seta and a spinneret seta.
Mandible (Fig. 2D–E). Coxa with 7 strong teeth and 6 setae on inner edge and a seta on outer edge (near the articulation with the palp); palp 4-segmented (basis + 3-segmented endopodite); basis externally with respiratory plate (exopodite) consisting of 3 rays and one reflexed seta, and internally with two setae, one two thirds as long as the other; first endopodal segment with two apical internal setae, one less than half as long as the other; second endopodal segment with an internal apical seta and 4 external apical setae, one short, two long and one intermediate; terminal endopodal segment with 2 setae and one slender claw, all equally long.
Maxillula (Fig. 2F). Internally with three endites, first one with 2 setae, second and third ones each with 3 setae and two claws, the latter with a conspicuous spoon-shaped apex; palp not segmented, tapering, with 2 apical setae; respiratory plate well developed, carrying a reflexed seta (i.e. reversed towards the front) and 16 long rays.
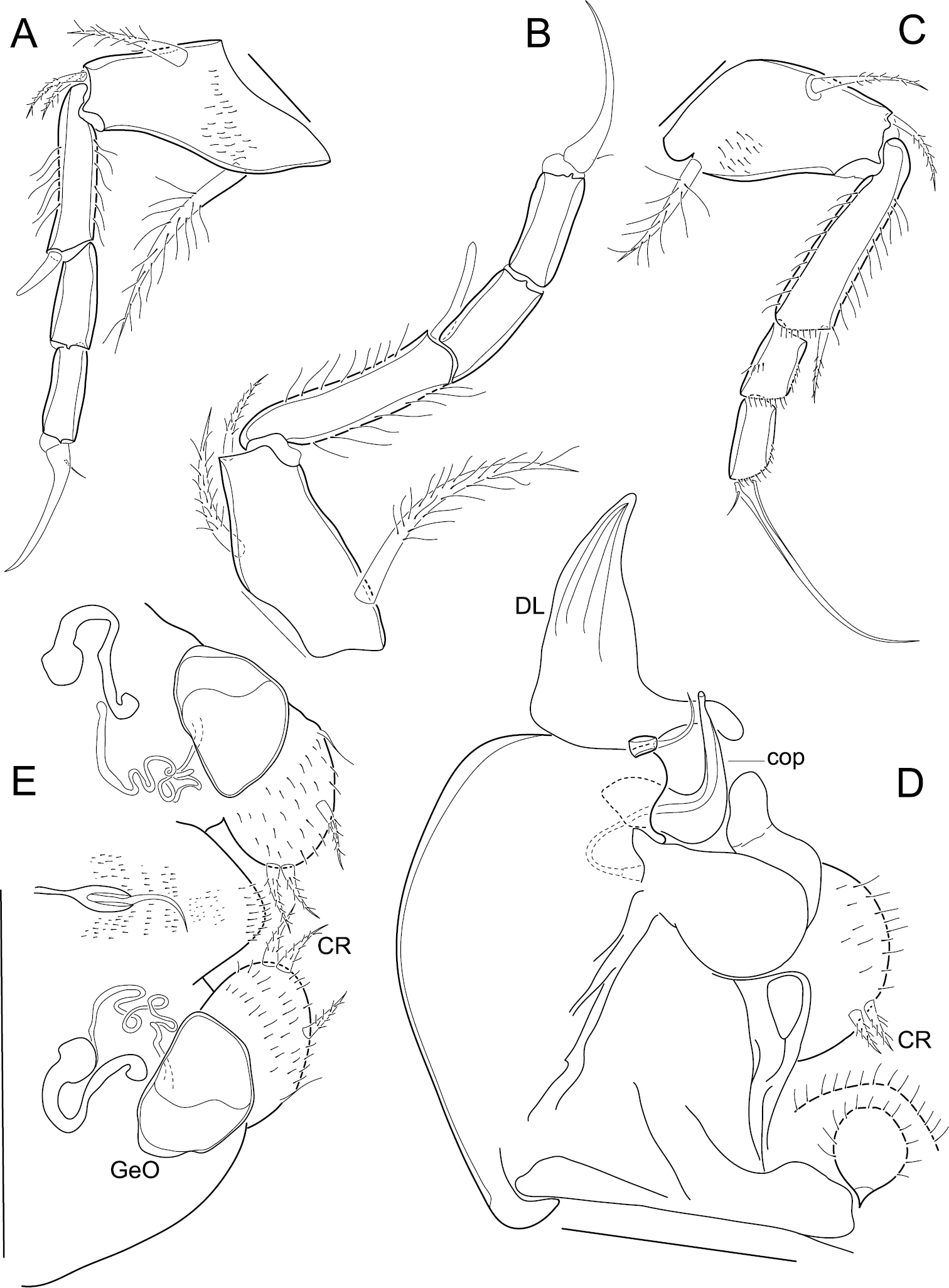
First thoracopod (Fig. 3A). 4-segmented; first segment with a long medio-proximal dorsal seta, a medium-sized medio-ventral seta and two stout short ventro-apical setae; second segment quite long, with a strong ventro-apical seta; third segment devoid of setae; terminal segment with an apical claw that bears a minute seta at its base.
Elpidium merendonense sp. n. A First thoracic leg B second thoracic leg C third thoracic leg D Hemipenis E Abdomen. A–D male specimen, holotype, MZUSP 29072 E female specimen, allotype, MZUSP 29073. Scale bars: A–C: 50 µm; D: 100 µm; E: 200 µm. DL = distal lobe on hemipenis. cop = copulatory process on hemipenis. CR = caudal ramus. GeO = genital operculum.
Second thoracopod (Fig. 3B). 4-segmented; first segment with a long medio-proximal dorsal seta, a medium-sized medio-ventral seta and a relatively short ventro-apical seta; second segment long, with a strong ventro-apical seta reaching tip of following segment; third segment devoid of setae; terminal segment with an apical claw bearing a minute seta at its base.
Third thoracopod (Fig. 3C). 4-segmented, quite slender; first segment with a medio-proximal dorsal seta, a medio-ventral seta and a ventro-apical seta; second segment quite long, with a slender ventro-apical seta; third segment devoid of setae; terminal segment with a very long and slim apical claw carrying a minute seta at its base.
Hemipenis (Fig. 3D). Consisting of a large rounded muscular body, an articulating distal lobe and a dorsal seta; distal lobe triangular, with a pointed tip and with a finger-like projection at the base of the internal margin, next to the dorsal seta; copulatory process a stiff hook-like structure, thick at the first half of its length and then quickly narrowing to the orifice at the tip; lower ramus (“crochet accessoire”) sinuous and with a rounded tip; caudal ramus a hirsute rounded lobe bearing a pair of setae.
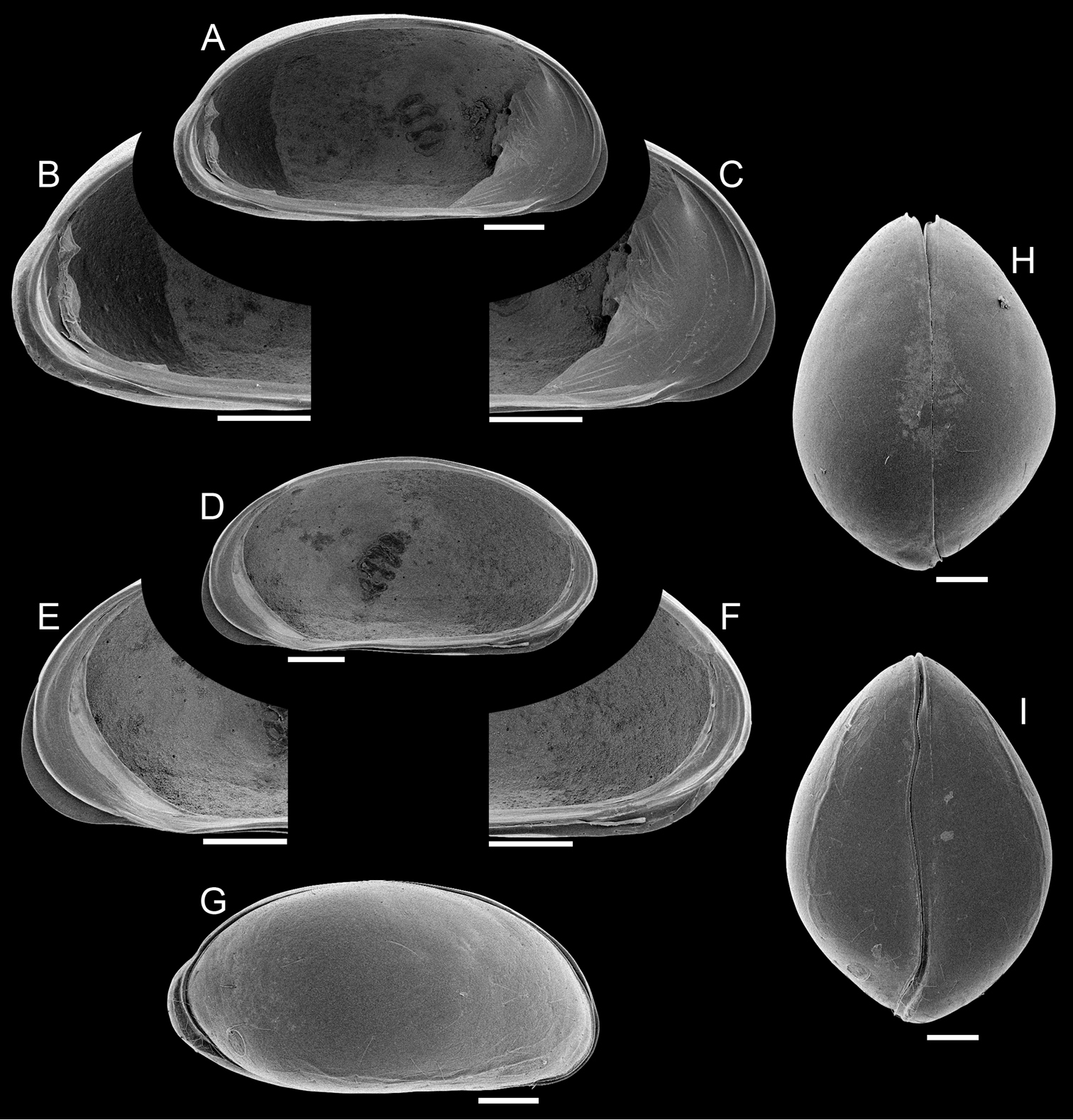
Carapace (Fig. 4G–I). Small sized (length = 697–722 μm), with brown surface and sparse setae; surface smooth except for a subtle ventro-lateral ridge on each valve; elliptically elongated in lateral view (length/height=1.96), with greatest height just behind mid-length; left valve overlapping right valve on all margins, strongly interlocking in antero-ventral, ventral and postero-ventral margins; valve overlap very strong at the posterior end of the carapace, producing a conspicuous outgrowth of the outer lamella, apparently without substantial change to the inner marginal structures; ventral area flat; dorsal margin arched; posterior and anterior margins rounded, both margins produced towards the ventral side; valves oval shaped in ventral and dorsal views, with maximum width displaced towards the posterior end in comparison to the male, producing a brooding cavity; up to 10 eggs were observed in a brood (mean egg size = 4.87 ± 4.0 μm, N = 8); dorsal margin straight in dorsal view; ventral margin sinuous in ventral view with well-marked ridges.
Elpidium merendonense sp. n., female. A Left valve internal view, general B left valve internal view, detail of postero-ventral margin C left valve internal view, detail of antero-ventral margin D right valve internal view, general E right valve internal view, detail of antero-ventral margin F right valve internal view, detail of postero-ventral margin G right lateral view H dorsal view I ventral view. A–F paratype, MZUSP 29076; G paratype, MZUSP 29080; H paratype, MZUSP 29081; I paratype, MZUSP 29082.Scale bars: 100 µm.
Left valve (Fig. 4A–C). In internal view anterior margin rounded, produced towards the ventral side; posterior margin narrowly rounded, produced towards the ventral side, forming a bulge at the postero-ventral area; ventral margin nearly straight; dorsal margin arched; calcified inner lamella well developed anteriorly, with a short line of concrescence near the valve margin leaving a vestibule; vestibule less developed posteriorly, but present; prominent selvage running on all margins except in the middle of dorsal margin; a short flange is formed at the antero-ventral margin; at the postero-ventral region, with outer lamella expanded towards the posterior end; central muscle scars consisting of 4 spots arranged in a vertical row relatively separated from each other and a round small frontal scar.
Antenna (Fig. 2C). As in the male, except for the small terminal segment with three equally slender claws, a minute seta and a tiny lobe (hyaline formation).
Abdomen (Fig. 3E). Genital operculum rounded, internally connected by tubes to a trabecula; caudal rami two hirsute rounded lobes, each with two apical setae in juxtaposition, a medio-external seta and an inconspicuous external seta nearer the base of the caudal ramus; end of body rounded, with a dorsal seta inserted in a strongly chitinized structure.
Male. Holotype: length = 674 µm, height = 347 µm; Paratype MZUSP 29077: length = 685 µm, height = 349 µm; Paratype MZUSP 29078: length = 657 µm, width = 463 µm; Paratype MZUSP 29079: length = 677 µm, width = 461 µm. Female. Paratype MZUSP 29076, LV: length = 721 µm, height = 344 µm; Paratype MZUSP 29080: length = 715 µm, height = 355 µm; Paratype MZUSP 29081: length = 697 µm, width = 513 µm; Paratype MZUSP 29082: length = 722 µm, width = 518 µm.
The carapace of Elpidium merendonense sp. n. resembles that of Elpidium inaequivalve in being relatively elongated in dorsal view and having a wide valve overlap at the posterior end of the carapace. However, the valve asymmetry is more pronounced in Elpidium inaequivalve, while females of Elpidium merendonense sp. n. show a unique outgrowth at the posterior end of the left valve where it overlaps the right valve. Elpidium merendonense sp. n. and Elpidium laesslei both present a distal lobe on the hemipenis with similar shape, but the copulatory process and the lower ramus are smaller in the latter species. These two species can furthermore be distinguished by the shape of the carapace in dorsal view, which is broad and rounded in Elpidium laesslei and oval in Elpidium merendonense sp. n.
Elpidium merendonense was found only in water accumulated in leaf axils of bromeliads from 1, 400 to 2, 242m elevation in CNP. Only Tillandsia sp. were examined. In this altitudinal range, bromeliads occur all through the area in varying densities depending on microclimatic conditions. Specimens of this species were common in collections throughout the park, sometimes co-occupying bromeliads with a species of candonid ostracod and/or a species of Anomopoda (Ceriodaphnia laticaudata Müller, 1867). Ostracoda were more common in larger bromeliads.
Honduras has a complex topography with numerous cloud forested mountain tops characterised by a high endemicity, documented for several taxa such as plants (
With the present contribution, seven valid species are currently known in the genus Elpidium. Two of these, namely Elpidium maricaoensis and Elpidium laesslei, are known only from incomplete original descriptions and without records of males, whose reproductive structures bear the most important diagnostic features for identification. Nonetheless, it is still possible to confidently identify all seven species, and an identification key is presented here.
| 1 | Carapace surface smooth | 2 |
| – | Carapace surface pitted | Elpidium laesslei |
| 2 | Left valve overlapping right valve | 3 |
| – | Right valve overlapping left valve | Elpidium purperae |
| 3 | Female carapace small to medium size, not longer than 0.8 mm | 4 |
| – | Female carapace large, reaching more than 0.9 mm in length | Elpidium bromeliarum |
| 4 | in dorsal and ventral views, left valve with posterior end markedly longer than right valve, especially in females | 5 |
| – | Valves symmetric | 6 |
| 5 | Female carapace in dorsal view with length per width ratio smaller than 1.3 | Elpidium inaequivalve |
| – | Female carapace in dorsal view with length per width ratio bigger than 1.35 | Elpidium merendonense sp. n. |
| 6 | female carapace with greatest width at mid-length | Elpidium pintoi |
| – | female carapace with greatest width displaced towards the posterior end | Elpidium maricaoensis |
All seven known Elpidium species were recovered from water impounded inside bromeliads, and distribution of the genus seems to follow almost the whole geographic range of their host plants. Elpidium species have been recorded from a variety of altitudes from southernmost Brazil to Cuba: from near sea level (Elpidium bromeliarum; see
The presence of passively dispersing Ostracoda in tank bromeliads requires specialised dispersal vectors. Already in his earliest description of the genus,
We would like to thank the people from Buenos Aires, Cortecito and Tierra Santa around National Park Cusuco for collaboration and guidance on the numerous excursions in the forest. Also the staff and volunteers of Operation Wallacea for supporting the project, in particular Jonathan Kolby for constructive mental and physical support.