(C) 2012 Mathan Magesh. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Namalycastis jaya sp. n. (Polychaeta: Nereididae: Namanereidinae)is described from the southern coast of Kerala in southwest India. One important characteristic feature of the species is the lack of notochaetae in all parapodia, a characteristic that it shares with at least two other species, Namalycastis elobeyensis Glasby, 1999and Namalycastis hawaiiensis Johnson, 1903. It differs from Namalycastis elobeyensis by virtue of its smaller antennae, unequal eye size, bilobed acicular neuropodial ligule and multi-incised pygidium rim. Moreover, it differs from Namalycastis hawaiiensis by having fewer teeth on the serrated blades of the sub-neuroacicular falciger in chaetiger 10, and by possessing finely serrated falcigers in posterior segments. Beyond morphological analyses, molecular phylogenetics was used for the first time for Namalycastis to support population monophyly and recognition of the new species.The analysis, using both mitochondrial and nuclear data, corroborated the morphological analysis in suggesting that our specimens represent an as yet undescribed species, Namalycastis jaya sp. n., which forms a monophyletic group among the sampled nereidid taxa. Finally, a taxonomic key for Namalycastis species recorded from the Indian region is provided.
Annelida, Polychaeta, Nereididae, Namalycastis, 16s rRNA, 18s rRNA, COI, phylogeny, systematics, new species, India
Namanereidinae (Polychaeta: Nereididae) represents one of the most successful groups of colonizers of brackish waters (
Morphological variation between species of Namalycastis is often minute, presenting a possible problem for taxonomists. This is in part due to their simplified body form – lack of a notopodium and few types of chaetae – compared to other Nereididae. For example, Namalycastis abiuma was long considered a single, widespread species with a high level of intraspecific morphological variation, attributed to the differences in habitat choice. However, close investigations of the details of the serrations on the falciger blades of the species group revealed that American populations could be divided into at least two separate species, Namalycastis abiuma sensu Müller in Grube, 1871 and Namalycastis borealis Glasby, 1999. Nevertheless, Indo-Pacific populations of Namalycastis abiuma are still indistinguishable from their American counterparts. Because of the aforementioned, the inclusion of molecular data in association with phylogenetic analyses represents the first step in the molecular characterization of members of the genus and may assist with understanding the taxonomic boundaries within Namalycastis.
Here, we describe a new species of Namalycastis from the southwest coast of India on the basis of morphological investigations and corroborate the novelty of the species by phylogenetic analysis using both mitochondrial and nuclear loci. In addition, a morphological key to the different Namalycastis species recorded from India is provided.
Material and methodsIn March of 2009 and January of 2010, several polychaete samples were collected from the retting zone (upper intertidal zone characterized by accumulation of rotting coconut husks) of the Kadinamkulam estuary, near Thiruvananthapuram off the southwest coast of India. Specimens were sizeable enough to be collected by eye from rotting organic matter mixed with muddy sediments at the shoreline. The polychaetes associated with retting coconut husk were collected by breaking the coconut husk with hammer and chisel. Identifications were fascilitated by previous contributions and morphological keys (e.g.,
Total genomic DNA was extracted from the tissue samples following the extraction procedure of
Nucleotide sequences were deposited at NCBI (accession numbers HQ456363 and JN790065–67 for COI, HM138706 and JX483868–70 for 16S, and HQ157238 and JX483865- 67 for 18S) and type specimens were deposited in the collections of the WGRC- ZSI.
DNA analysesSequence reconciliation of forward and reverse sequences was carried out using BioEdit ver. 7.0.5.2 (
The following abbreviations are used in the text:
PSU Practical Salinity Unit
NCBI National Center for Biotechnology Information
WGRC, ZSI Western Ghats Regional Centre, Zoological Survey of India.
ICZN International Code of Zoological Nomenclature
Systematics Order Phyllodocida Dales, 1962 Family Nereididae Blainville, 1818 Subfamily Namanereidinae Hartman, 1959 Genus Namalycastis Hartman, 1959urn:lsid:zoobank.org:act:2C0921DC-72BA-4CCD-8DC6-E2D134621931
http://species-id.net/wiki/Namalycastis_jaya
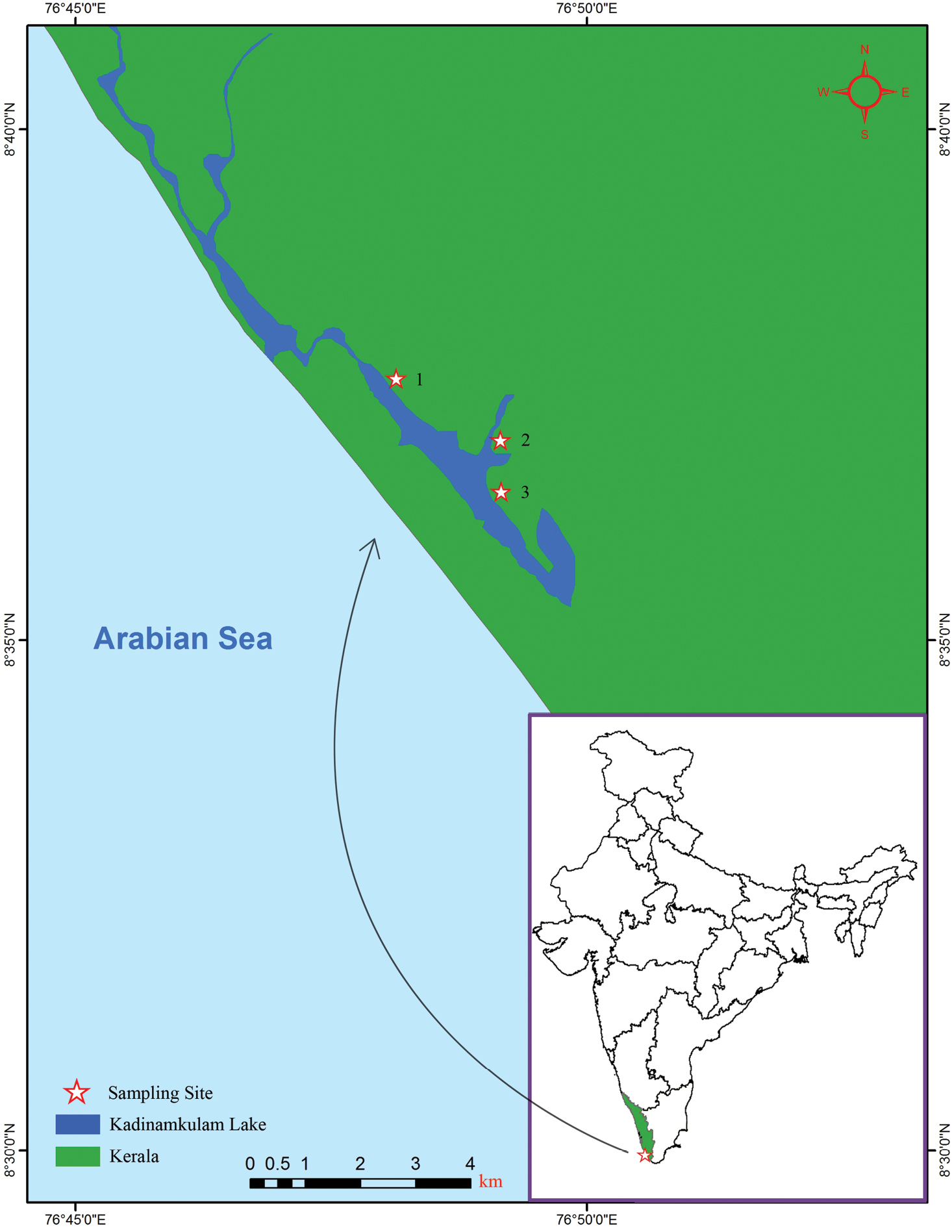
Figures 2a–k, 3a–lMurukkumpuzha retting zone, Thiruvananthapuram coast, Kerala, India, 8°36'57.47"N, 76°49'8.914"E (Fig. 1; site 2).
Map showing the positions of the collection localities of Namalycastis jaya sp. n. in Kerala , India. Site 1, Kadinamkulam estuary (8°37'33.34"N, 76°48'7.827"E); Site 2, Murukkumpuzha retting zone (8°36'57.473"N, 76°49'8.914"E); and Site 3, Kadinamkulam estuary (8°36'27.21"N, 76°49'9.474"E).
Holotype AQJ1 (ZSI/WGRC/IR/IV 2330), adult specimen collected from muddy sediment in Murukkumpuzha retting zone, 8°36'57.47"N, 76°49'8.914"E (Fig. 1; site 2) by M. Magesh on 31 March, 2009. Paratypes, four specimens: AQJ2–4 (ZSI/WGRC/IR/IV 2331, 2332 and 2337) collected in Kadinamkulam estuary, Thiruvananthapuram coast, Kerala, India, 8°37'33.34"N, 76°48'7.827"E (Fig. 1; Site 1); and AQPE1 (ZSI/WGRC/IR/IV 2191), collected in muddy sediment from Kadinamkulam estuary, Thiruvananthapuram coast, Kerala, India, 8°36'27.21"N, 76°49'9.474"E (Fig. 1; site 3). All paratypes collected by M. Magesh on 21 January, 2010.
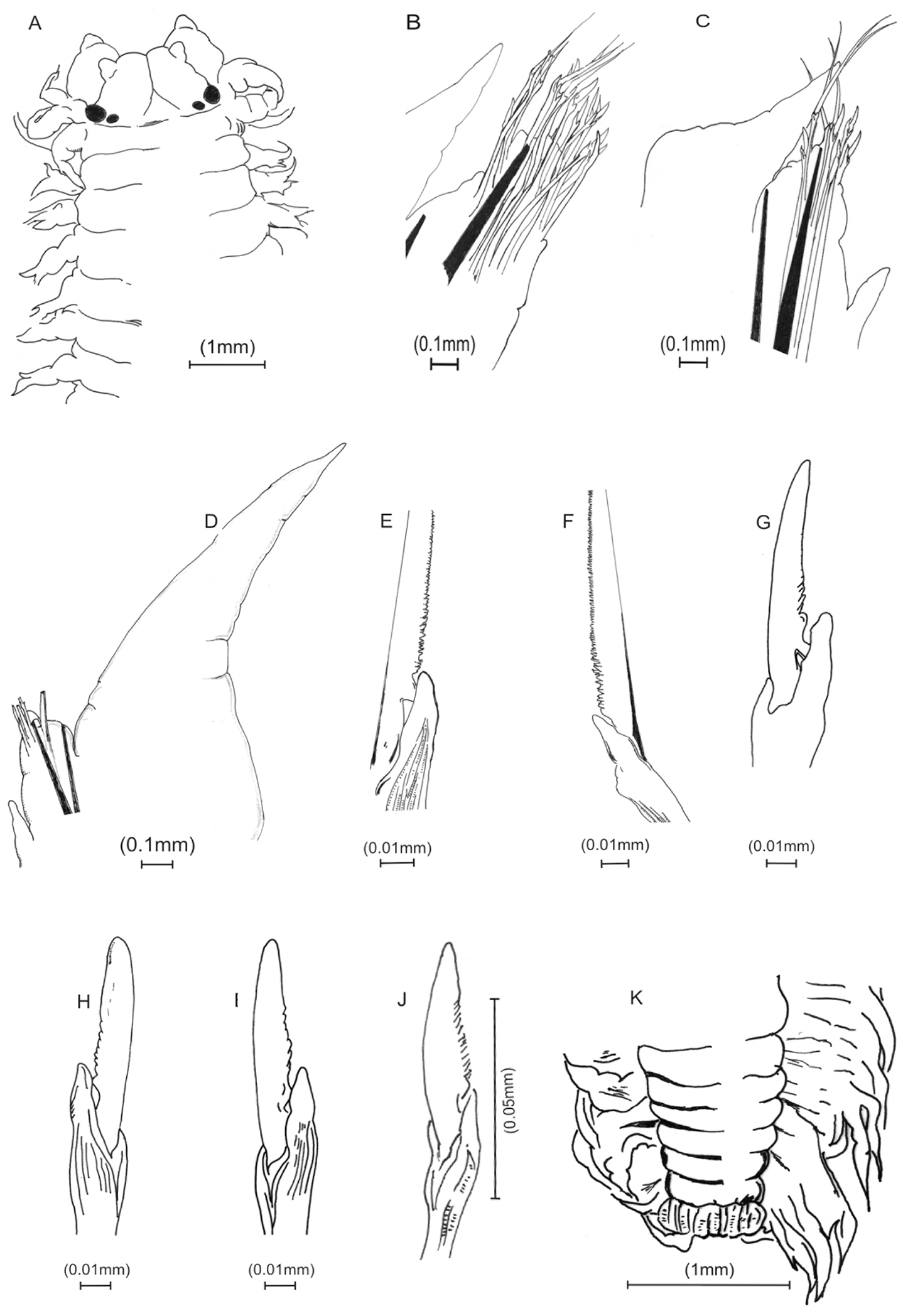
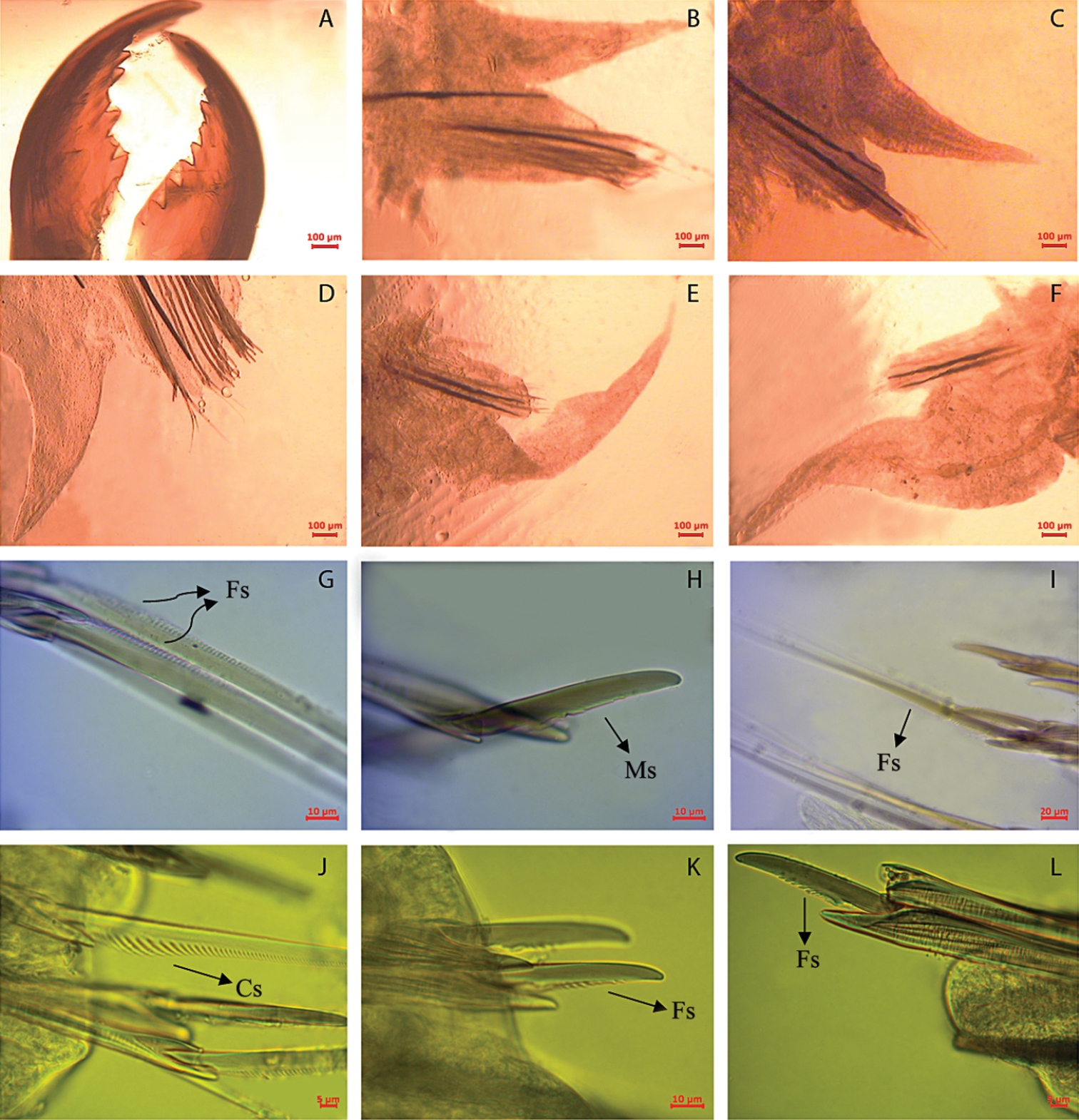
Holotype with body widest mid-anteriorly, tapering gradually anteriorly and posteriorly. Antennae small, distally subacute, aligned over mid-palps. Brown epidermal pigmentation present anterodorsally and posterodorsally.Prostomium triangular, deeply cleaved anteriorly. Longitudinal groove extending from tip to posterior part of prostomium, slightly indented laterally. Eyes 2 pairs, black, arranged obliquely, posterior pair considerably smaller. Posterodorsal tentacular cirri extending posteriorly to chaetiger 2. Jaws with 8 teeth, 4 subterminal and 4 ensheathed proximally.Acicular neuropodial ligule bilobed, superior lobe larger than inferior lobe. Dorsal cirri increasing in length posteriorly. Typically less than 4 sesquigomph spinigers in neuropodial supra-acicular fascicle in midbody. Notochaetae absent in all parapodia. Heterogomph chaetae with boss not lengthened. Supra-neuroacicular falcigers in chaetiger 10 with finely serrated blades, 9–12 teeth, approximately uniform in length. Sub-neuroacicular falcigers in chaetiger 10 with roughly serrated blades, about 20 teeth. Sub-neuroacicular spinigers in anterior region (up to segment 50) with blades finely serrated. Sub-neuroacicular spinigers in mid and posterior region (from about segment 70) with blades coarsely serrated proximally. Supra-neuroacicular spinigers in mid and posterior region with blades finely serrated. Pygidium with multi-incised rim, black with two lateral anal cirri.
Namalycastis jaya sp. n.Holotype: a anterior end, dorsal view b anterior parapodium from chaetiger 8 c mid-body parapodium from chaetiger 80 d posterior parapodium from chaetiger 230 e sub-neuroacicular spiniger, chaetiger 10 f sub-neuroacicular spiniger, chaetiger 30 g sub-neuroacicular falciger, chaetiger 10 h sub-neuroacicular falciger, chaetiger 80 i supra-neuroacicular falciger, chaetiger 80 j supra-neuroacicular falciger, chaetiger 120 k pygidium, dorsal view.
Namalycastis jaya sp. n.Holotype: a jaw pieces, ventromedial view b anterior parapodium from chaetiger 10 c parapodium from chaetiger 50 d parapodium from chaetiger 100 e posterior parapodium from chaetiger 210 f posterior parapodium from chaetiger 230 g sub-neuroacicular spiniger, chaetiger 10 h sub-neuroacicular falciger, chaetiger 109 i supra-neuroacicular spiniger, chaetiger 80 j sub-neuroacicular spiniger, chaetiger 210 k sub-neuroacicular falciger, chaetiger 210 l supra-neuroacicular falciger, chaetiger 20. Fs Finely serrated; Cs Coarsely serrated; Ms Medium serrated.
Named in honour of Dr. Jayalalithaa Jayaram (born 1948), the current Chief Minister of Tamil Nadu State of India, in recognition of her contributions to the field of education (especially for impoverished people) and scientific research. The specific epithet is considered to be a noun in apposition.
Known only from the Thiruvananthapuram coast of southwest India (but see note below).
Namalycastis jaya sp. n. resembles Namalycastis elobeyensis Glasby, 1999 and Namalycastis hawaiiensis Johnson, 1903 by virtue of lacking notochaetae. However, our new species differs from Namalycastis elobeyensis as the latterhas long antenna, equal size eyes, comparatively longer posterodorsal tentacular cirri, subconical acicular neuropodial ligule, tripartite pygidium and no epidermal pigmentation. Namalycastis jaya sp. n. also differs from Namalycastis hawaiiensis, by the latter possessing 35 to 70 teeth on the blades of the sub-neuroacicular falcigers in parapodia of chaetiger 10, mid-posterior falcigers with proximally coarsely serrated blades from chaetiger 120 (chaetiger 30 in smaller specimens) and by the absence of epidermal pigmentation. The lack of notochaetae sets Namalycastis jaya sp. n. apart from other Indian species, including Namalycastis indica Southern, 1921, the Namalycastis abiuma species group, Namalycastis fauveli Nageswara Rao, 1981 and the recently described species Namalycastis glasbyi Fernando and Rajasekaran, 2007. These other species typically have 1–3 notochaetae in at least some parapodia, except those in the anterior-most and posterior-most body. Namalycastis jaya sp. n. most closely resembles Namalycastis abiuma, but differs from the holotype of that species in having very short tentacular cirri (posterodorsal one only extending to chaetiger 2 as compared to chaetiger 5 in Namalycastis abiuma), in the very short, distally sub-acute antennae (antennae pointed and extending to end of palps in Namalycastis abiuma) and in lacking notochaetae (present from chaetiger 12 in Namalycastis abiuma). A key for taxonomic differentiation between species recorded from the Indian region is provided below.
Based on the above comparative account of the features used for identifying the species of the family Nereididae, the present species can be distinguished as a new species by the following combination of characters: (1) smooth and small antennae, (2) absence of notochaetae in all chaetigerous segments, (3) sub and supra-neuroacicular falcigers of parapodia 10 with finely serrated blades, (4) coarsely serrated teeth in sub-neuroacicular spinigers in mid-posterior region, (5) brown pygidium with multi incised rim and two lateral anal cirri, (6) jaws with 8 teeth, and (7) eye capsule protruded above the dorsal alignment of the head. In all of these features, the new species resembles Namalycastis meraukensis (var. zeylancia), described from Dondra, southern Sri Lanka by
This species is able to sustain in polluted (sulphide rich and odorous) sediments and is especially associated with decaying materials such as bark and retting coir; the salinity at the collection localities ranged from 5–22 psu.
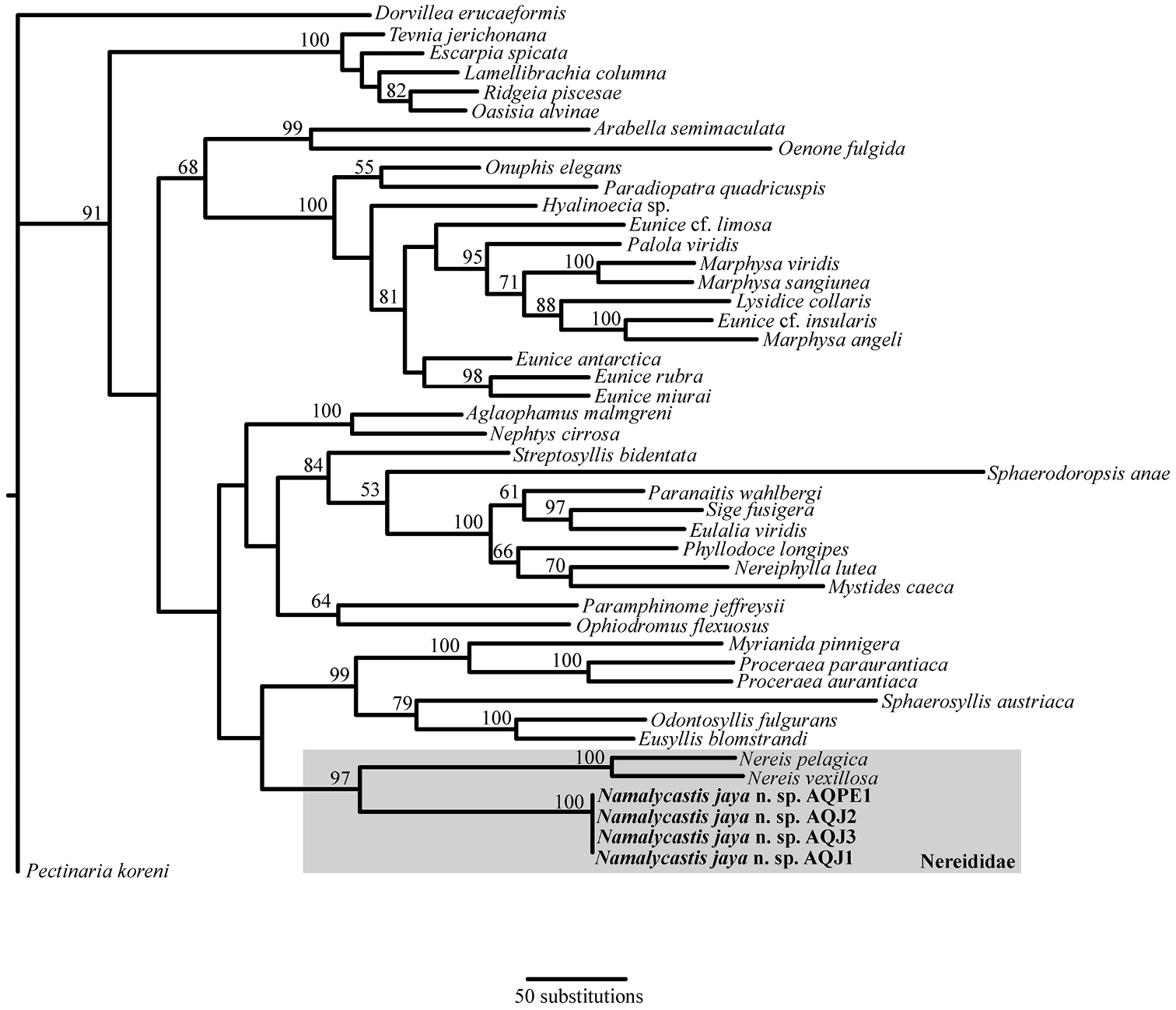
In order to rigorously test the familial placement of our species, the phylogenetic analysis used numerous specimens from various polychaete families as well as the only two members of Nereididae for which data were available for all of COI, 16S and 18S (Nereis pelagica Linnaeus, 1758and Nereis vexillosa Grube, 1849). The final molecular matrix contained 3789 aligned sites. The TNT analysis recovered 5 equally parsimonious trees, 8426 steps long and the strict consensus of these (Fig. 4) corroborates the morphological analysis in confirming the novelty of the species. The specimens of Namalycastis jaya sp. n.form a monophyletic group among the sampled nereidid taxa. This position is supported by a bootstrap value of 97% and the monophyly of the specimens received maximum support. The details of the remainder of the tree are presented in Fig. 4.
Strict consensus of five equally parsimonious trees from the TNT analysis (Length: 8426; CI: 0.357; RI: 0.543).Bootstrap support values are shown above the nodes and representatives of the new species are shown in bold font. See text for further discussion.
The monophyletic status of the mostly tropical or subtropical subfamily Namanereidinae has been confirmed by phylogenetic analyses (
Namalycastis jaya sp. n. represents the fifth species of Namalycastis recorded from India, the remaining species being the Namalycastis abiuma species group, Namalycastis indica, Namalycastis fauveli and Namalycastis glasbyi. We note that Namalycastis glasbyi, which is known only from the type locality, Mumbai, bears a close resemblance to Namalycastis indica (see
| 1 | Articulation of heterogomph chaetae with boss extraordinarily expanded; antennae minute | Namalycastis fauveli |
| – | Articulation of heterogomph chaetae with boss not extraordinarily expanded (equal or little longer); antennae extending beyond tip of prostomium | 2 |
| 2 | Notochaetae present in all or several parapodia; antennae distally pointed | 3 |
| – | Notochaetae absent in all parapodia; antennae distally sub-acute | Namalycastis jaya sp. n. |
| 3 | Anterior and posterior eyes more or less same size | 4 |
| – | Anterior eyes substantially smaller than posterior ones | Namalycastis glasbyi |
| 4 | Lengthy posterodorsal tentacular cirri reaching back to chaetiger 5–6 and tripartite pygidium | Namalycastis indica |
| – | Postero-dorsal tentacular cirri only reaching back to chaetiger 2–5; multi-incised pygidium | Namalycastis abiuma species group |
MM is grateful to Dr. A. Bijukumar, Department Head, Department of Aquatic biology and fisheries, University of Kerala, for his constant support. SK thanks the Wenner-Gren Foundation for generous support. Open access to this paper was supported by the Encyclopedia of Life (EOL) Open Access Support Project (EOASP).