(C) 2013 Paulina D. Jenkins. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Jenkins PD, Abramov AV, Bannikova AA, Rozhnov VV (2013) Bones and genes: resolution problems in three Vietnamese species of Crocidura (Mammalia, Soricomorpha, Soricidae) and the description of an additional new species. ZooKeys 313: 61–79. doi: 10.3897/zookeys.313.4823
Recent investigations of Southeast Asian white toothed shrews belonging to the genus Crocidura have revealed discrepancies between the results of morphological and molecular studies. The following study concerns three species of Crocidura occurring in Vietnam, namely Crocidura attenuata, Crocidura tanakae and Crocidura wuchihensis, and an undescribed fourth species revealed by molecular analysis. For many years Crocidura attenuata has been known to occur in Vietnam but, until very recently, the morphologically similar and comparably sized Crocidura tanakae was believed to be restricted to Taiwan. Following several molecular studies over the last few years, this species is now believed to be considerably more widespread and recognised as occuring also in Vietnam. The results of one of these recent molecular studies also revealed the presence of an undescribed species of Crocidura, similar in size and morphology to Crocidura wuchihensis, which is herein described. Data are provided on geographical variation in Vietnam and the problems of defining morphologically similar yet molecularly disparate species are discussed.
Crocidura, new species, morphology, molecular analysis, geographical variation
From the late 1990s there have been several intensive surveys of the small mammal fauna in various localities in Vietnam, resulting in the discovery of a number of species new to science. Before that time only three species of Crocidura had been recorded from Vietnam: Crocidura attenuata Milne Edwards, 1872, Crocidura fuliginosa (Blyth, 1855) and Crocidura indochinensis Robinson & Kloss, 1922 (
Molecular studies were also being carried out during this period. Significant studies included those of
While the molecular studies of Vietnamese material confirmed some of the results of the contemporaneous morphological studies, a number of anomalies were equally revealed, indicating the presence of several morphologically similar but molecularly distinct taxa. Investigation of these incongruent results is the subject of this current study.
Crocidura attenuata Milne Edwards, 1872 described originally from Szechuan, China, was regarded as a widespread and common species known throughout much of Asia, including many localities from northern to southern Vietnam.
Crocidura tanakae Kuroda, 1938 from Taiwan was originally described as a full species but was subsequently considered to be either a synonym or subspecies of Crocidura attenuata (
Although known from few specimens at any one location Crocidura indochinensis Robinson & Kloss, 1922 was considered to have a wide, disjunct distribution, occuring in a few widely separated locations in Vietnam and extralimitally in Myanmar and China (
Crocidura wuchihensis Shaw, Wang, Lu and Chang, 1966 was originally described on the basis of two specimens from Hainan Island, China. Specimens collected from two localities in Vietnam (
This morphological study draws on specimens from a wide range of geographical locations in Vietnam (see Fig. 1) and includes those specimens from which tissue samples were analysed in the papers by
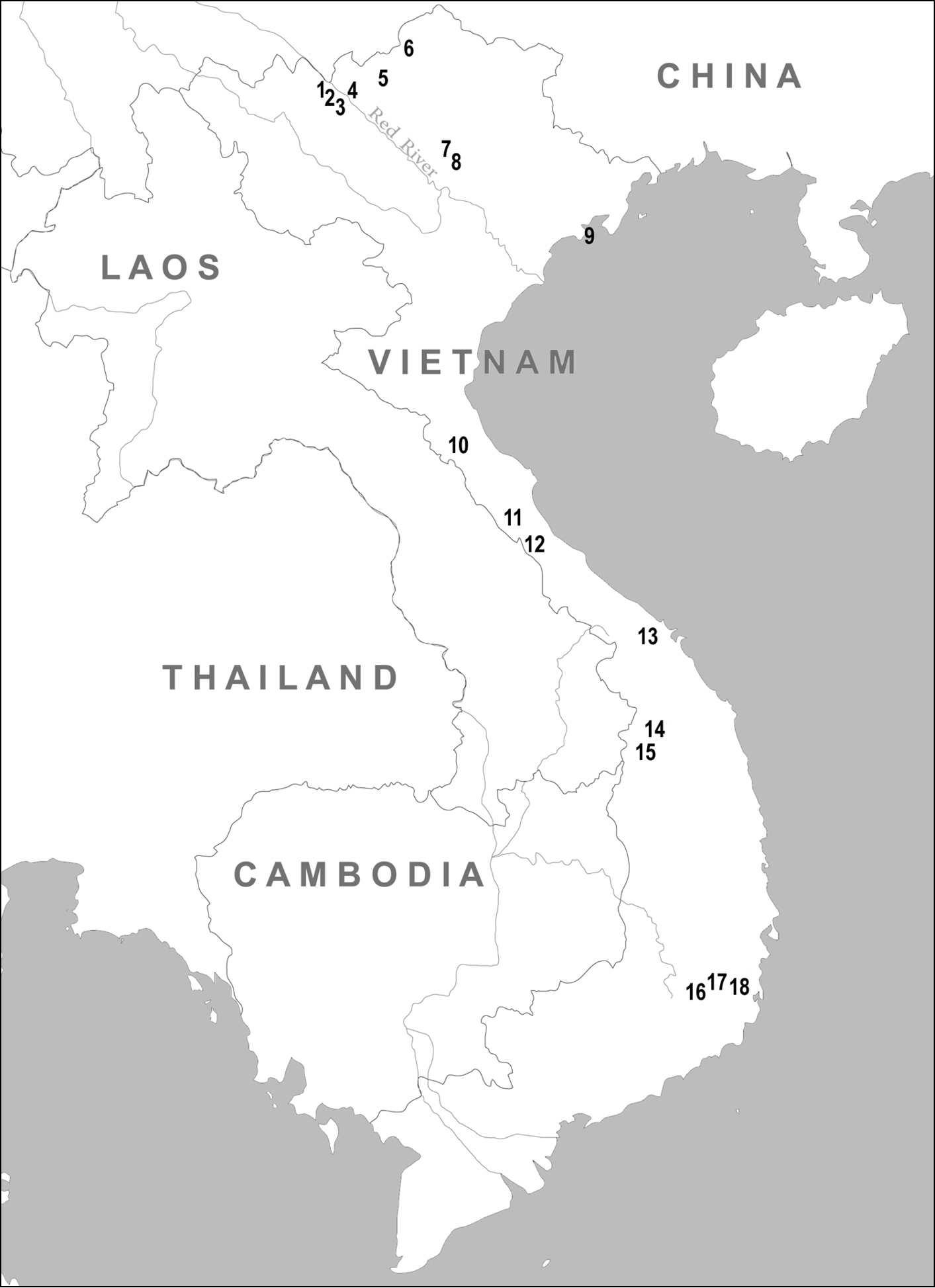
Geographical distribution of sampling localities in Vietnam: 1 Lao Cai Province, Ngai Tio 2 Lao Cai Province, Sa Pa District 3 Lao Cai Province, Van Ban District 4 Lao Cai Province, Thai Nien 5 Lao Cai Province, Pa Kha 6 Ha Giang Province, Mt. Tay Con Linh II 7 Tuyen Quang Province 8 Vinh Phu Province, Tam Dao 9 Hai Phong Province, Cat Ba Island 10 Ha Tinh Province, Huong Son District 11 Quang Binh Province, Phong Nha - Ke Bang National Park 12 Quang Tri Province, Huong Hoa Nature Reserve 13 Quang Nam – Da Nang Provinces, Ba Na Nature Reserve 14 Kon Tum Province, Ngoc Linh Mt. 15 Kon Tum Province, Dak To 16 Lam Dong Province, Da Lat 17 Lam Dong Province, Bi Doup - Nui Ba Nature Reserve 18 Khanh Hoa Province, Hon Ba Mt.
Measurements in millimetres were taken with digital callipers. Cranial and dental nomenclature follows that of
urn:lsid:zoobank.org:act:6016D7F3-D50E-4DBC-B571-EB54E5062D1E
ZIN 96433, genetic analysis code CVN108, BOLD Accession no. ABMIV114-08, field no. 132, male, body in ethanol, skull extracted, collected 25 May 2006 by A.V. Abramov.
Vicinity of Tram Ton Station of Hoang Lien National Park, north slope of Phansipan Mt. area, 6 km west of Sa Pa Town, Sa Pa District, Lao Cai Province, Vietnam, 22°21'N, 103°46'E, altitude 2200m above sea level.
ZIN 96262, genetic analysis code CVN93, GenBank no. HM587005, BOLD no. ABMIV100-08, field no. 13, male, collected 8 December 2005; ZIN 96264, genetic analysis code CVN94, GenBank no. HM587006, BOLD no. ABMIV101-08, field no. 15, female, collected 8 December 2005; ZIN 96269, genetic analysis code CVN99, BOLD no. ABMIV106-08, field no. 32, male, collected 15 December 2005; ZIN 96271, genetic analysis code CVN101, BOLD no. ABMIV108-08, female, collected 16 December 2005; ZIN 96274, genetic analysis code CVN102, BOLD no. ABMIV109-08, field no. 45, male, collected 17 December 2005; ZIN 96275, genetic analysis code CVN103, BOLD no. ABMIV110-08, field no. 46, male, collected 17 December 2005; ZIN 96276, genetic analysis code CVN104, BOLD no. ABMIV111-08, field no. 66, female, collected 22 December 2005; ZIN 96432, genetic analysis code CVN107, BOLD no. ABIOW074-08, field no. 131, male, collected 25 May 2006; ZIN 96434, genetic analysis code CVN109, BOLD no. ABIOW075-08, field no. 133, male, collected 25 May 2006; ZIN 96436, genetic analysis code CVN111, BOLD no. ABMIV116-08, field no. 136, male, collected 28 May 2006; ZIN 96438, genetic analysis code CVN113, BOLD no. ABMIV117-08, field no. 138, female, collected 28 May 2006; ZIN 96439, genetic analysis code CVN114, BOLD no. ABMIV118-08, field no. 139, female, collected 28 May 2006; ZIN 96442, genetic analysis code CVN117, BOLD no. ABIOW069-08, field no. 144, male, collected 31 May 2006; ZIN 99779, field no. 24, male, collected 10 May 2010. All bodies in ethanol, skulls extracted, collected by A.V. Abramov and A.V. Shchinov from the same locality as the holotype, altitude 1930–2200m above sea level.
Other material. FMNH 39029 Chapa [Sa Pa], Lao Cai Province; BMNH 1925.1.1.24; BMNH 1925.1.1.27 Ngai Tio, Lao Cai Province, 22°36'N, 103°40'E.
A small shrew distinguished by the mitochondrial genes cytochrome b (cyt b) and cytochrome oxidase c subunit I (COI) and by the shape of the talonid of the third lower molar (m3).
Size small (see Table 1) with a moderately long tail relative to head and body length (62–84%). Dorsal pelage dark greyish brown; tail dark grey dorsally, slightly paler below (see Fig. 2). Skull with a rounded, short rostrum: moderately broad interorbital region; rounded, relatively deep braincase with subangular superior articular facets and lambdoid crests just evident laterally near the junction with the mastoid (see Fig. 3). The first upper incisor is slender with a relatively small posterior cusp, less than half the height of the first upper unicuspid; posterolingual border of upper premolar (P4) deep and rounded, in close contact with the anterolingual margin of M1 in occlusal view; last upper molar (M3) relatively narrow. Lower incisor with two distinct cusps on the occlusal surface in unworn dentition; posterolingual cuspid present on lower premolar (p4); talonid basin of m3 broad and deep with an entoconid ridge and low entoconid (see Fig. 4).
Comparison of Crocidura indochinensis, Crocidura wuchihensis and Crocidura sapaensis. Crocidura guy and Crocidura zaitsevi B are included as representatives of the very small species in Vietnam. Measurements in millimetres are presented as the mean, standard deviation and range, followed by sample size in parentheses.
| Character | Crocidura zaitsevi B Bi Doup & Hon Ba | Crocidura guy Na Hang | Crocidura wuchihensis Mt Tay Con Linh II | Crocidura sapaensis Sa Pa | Crocidura indochinensis Bi Doup |
|---|---|---|---|---|---|
| Condyloincisive length | 15.4 ± 0.28 14.9-15.8 (15) |
15.4 ± 0.05 15.3-15.4 (4) |
16.4 ± 0.5 15.7-17.1 (6) |
16.6 ± 0.41 15.6-17.2 (20) |
18.1 ± 0.36 17.5-18.7 (10) |
| Condylobasal length | 14.8 ± 0.31 14.2-15.3 (15) |
14.9 ± 0.06 14.8-14.9 (4) |
15.7 ± 0.47 15.0-16.4 (6) |
15.9 ± 0.42 15.0-16.5 (20) |
17.4 ± 0.37 16.8-17.8 (10) |
| Upper toothrow length | 6.5 ± 0.14 6.3-6.8 (15) |
6.5 ± 0.14 6.4-6.7 (4) |
7.0 ± 0.25 6.6-7.2 (6) |
7.0 ± 0.17 6.5-7.2 (21) |
7.7 ± 0.23 7.3-8.0 (10) |
| Maxillary breadth at M2 | 4.5 ± 0.19 4.2-4.9 (15) |
4.5 ± 0.14 4.4-4.7 (4) |
4.9 ± 0.06 4.8-5 (6) |
4.8 ± 0.18 4.4-5.1 (21) |
5.2 ± 0.07 5.1-5.3 (10) |
| Braincase breadth | 7.2 ± 0.15 6.9-7.5 (15) |
7.2 ± 0.17 7.0-7.4 (4) |
7.5 ± 0.16 7.3-7.8 (6) |
7.7 ± 0.19 7.4-8.1 (19) |
8.2 ± 0.15 7.9-8.4 (10) |
| Braincase height | 3.7 ± 0.13 3.5-3.9 (14) |
3.6 ± 0.14 3.5-3.8 (4) |
4.0 ± 0.15 3.7-4.1 (6) |
4.1 ± 0.17 3.9-4.4 (19) |
4.4 ± 0.14 4.1-4.5 (10) |
| Head and body length | 52.7 ± 2.79 49-59 (15) |
49.5 ± 2.27 47-53 (4) |
60.6 ± 2.7 58-65 (5) |
57.4 ± 3.91 50-65 (20) |
63.5 ± 3.81 56-68 (10) |
| Tail length | 31.8 ± 1.2 30-34 (15) |
35.9 ± 1.4 34-37 (4) |
39.8 ± 2.28 37-42 (5) |
41.6 ± 2.48 37-47 (20) |
55 ± 2.79 50-58 (10) |
| Ratio of tail length to head and body length | 0.61 ± 0.02 0.55-0.65 (15) |
0.73 ± 0.03 0.69-0.77 (4) |
0.66 ± 0.04 0.63-0.72 (5) |
0.73 ± 0.06 0.62-0.84 (20) |
0.87 ± 0.05 0.81-0.98 (10) |
| Ratio of tail length to condyloincisive length | 2.1 ± 0.06 2.0-2.2 (15) |
2.3 ± 0.09 2.2-2.4 (4) |
2.4 ± 0.14 2.2-2.6 (5) |
2.5 ± 0.14 2.2-2.7 (20) |
3.1 ± 0.12 2.9-3.2 (10) |
Photograph of adult male Crocidura sapaensis (ZIN 99779).
Comparison of crania of Crocidura wuchihensis (AMNH 274153), Crocidura sapaensis (ZIN 96433) and Crocidura indochinensis (ZIN 97668). Top row from left to right: dorsal views of the skulls of Crocidura wuchihensis, Crocidura sapaensis and Crocidura indochinensis, ventral views of the skulls in the same order. Lower row: left lateral view of skulls and mandibles from left to right of Crocidura wuchihensis, Crocidura sapaensis and Crocidura indochinensis.
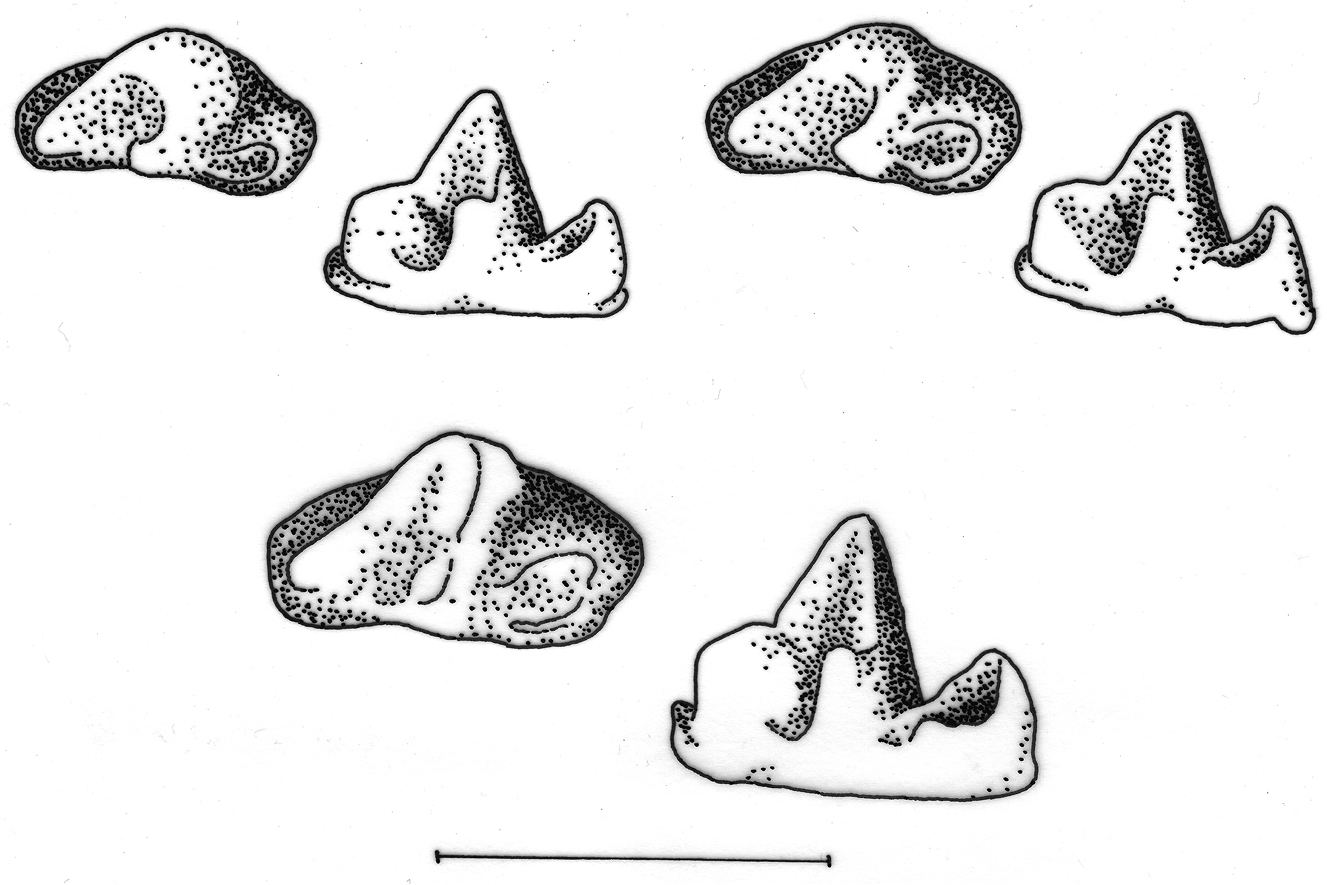
Occlusal (left) and lingual (right) views of right lower third molar to show differences in development of the talonid. Upper row left Crocidura wuchihensis AMNH 274168; upper row right Crocidura sapaensis ZIN 96439; lower row Crocidura indochinensis ZIN 97671. Scale equals 1 mm.
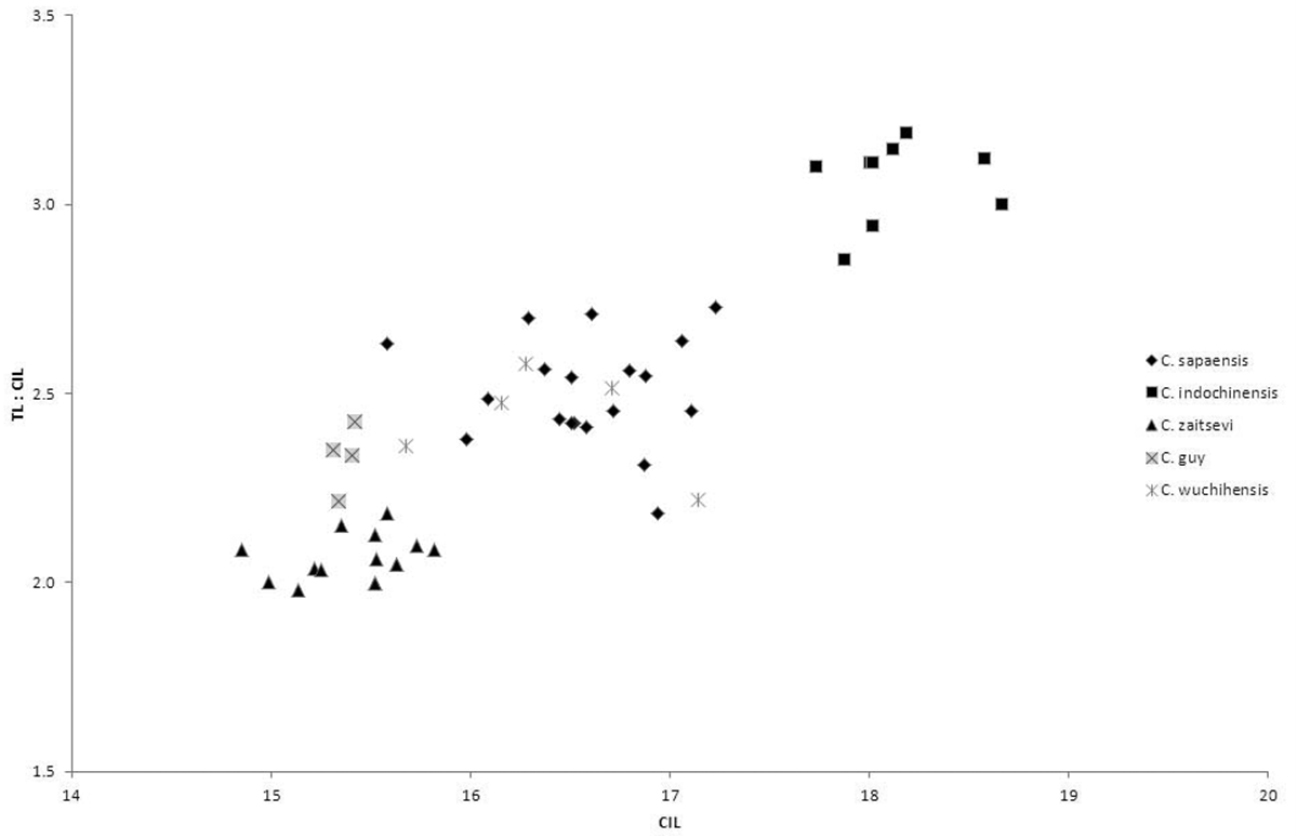
Crocidura sapaensis averages larger than the very small species of Crocidura recorded from Vietnam. The condyloincisive length is greater than that of Crocidura guy, Crocidura annamitensis and Crocidura kegoensis, within the upper part of the range of Crocidura zaitsevi and the braincase is deeper than that of all four small species. Crocidura wuchihensis and Crocidura sapaensis are in the same size range. Crocidura sapaensis is smaller than or at the lower end of the size range of Crocidura indochinensis with a relatively shorter tail (see Table 1 and Fig. 5).
Bivariate plot to show differences in skull size and relative tail length. Horizontal axis: condyloincisive length; vertical axis: ratio of tail length to condyloincisive length.
Crocidura sapaensis and Crocidura wuchihensis are distinguished by differences in cyt b sequences. Crocidura sapaensis differs from Crocidura zaitsevi and Crocidura indochinensis in the cyt b and COI gene sequences.
Differences in the shape of the talonid of m3 in northern Vietnamese populations serve to distinguish Crocidura sapaensis and Crocidura wuchihensis (see Fig. 4). In specimens of Crocidura sapaensis from northern Vietnam the talonid basin is broad and deep with an entoconid ridge and low entoconid, whereas in Crocidura wuchihensis the talonid basin is narrow. In Crocidura indochinensis the talonid basin is broad and deep with a hypoconid, entoconid and marked entoconid ridge (see Fig. 4).
The new species is named after Sa Pa, the capital of Sa Pa District in Lao Cai Province of northern Vietnam, with the Latin suffix - ensis (belonging to).
The series of type specimens was collected from a variety of habitats in the vicinity of Tram Ton Station of Hoang Lien National Park: mixed evergreen forest; forested banks of small streams; open grassy glades (Fig. 6); primary forest with large trees at an elevation 1930–2200m (
Habitat typical of the area where Crocidura sapaensis was found.
Confirmed specimens of Crocidura sapaensis are recorded from Lao Cai Province, Sa Pa District on the basis of cyt b analysis and morphology of m3. On the basis of morphology, specimens from the northern part of Lao Cai Province, Ngai Tio (elevation 1450m) and from the vicinity of Cat Cat Village near Sa Pa Town (elevation 1400–1450m) in relatively close geographical proximity also probably belong to the same species.
Populations of Crocidura wuchihensis identified on the basis of cyt b and those probably representing this species on the basis of morphology (from Pa Kha and Thai Nien, both in Lao Cai Province) all occur in northeastern Vietnam in localities to the east of the Song Hong (Red River). The observation that this river marks the border between the two species, with Crocidura wuchihensis to the east and Crocidura sapaensis to the west, was made by
The population of Crocidura wuchihensis recorded from Huong Son, Ha Tinh Province in the southern Annamites by
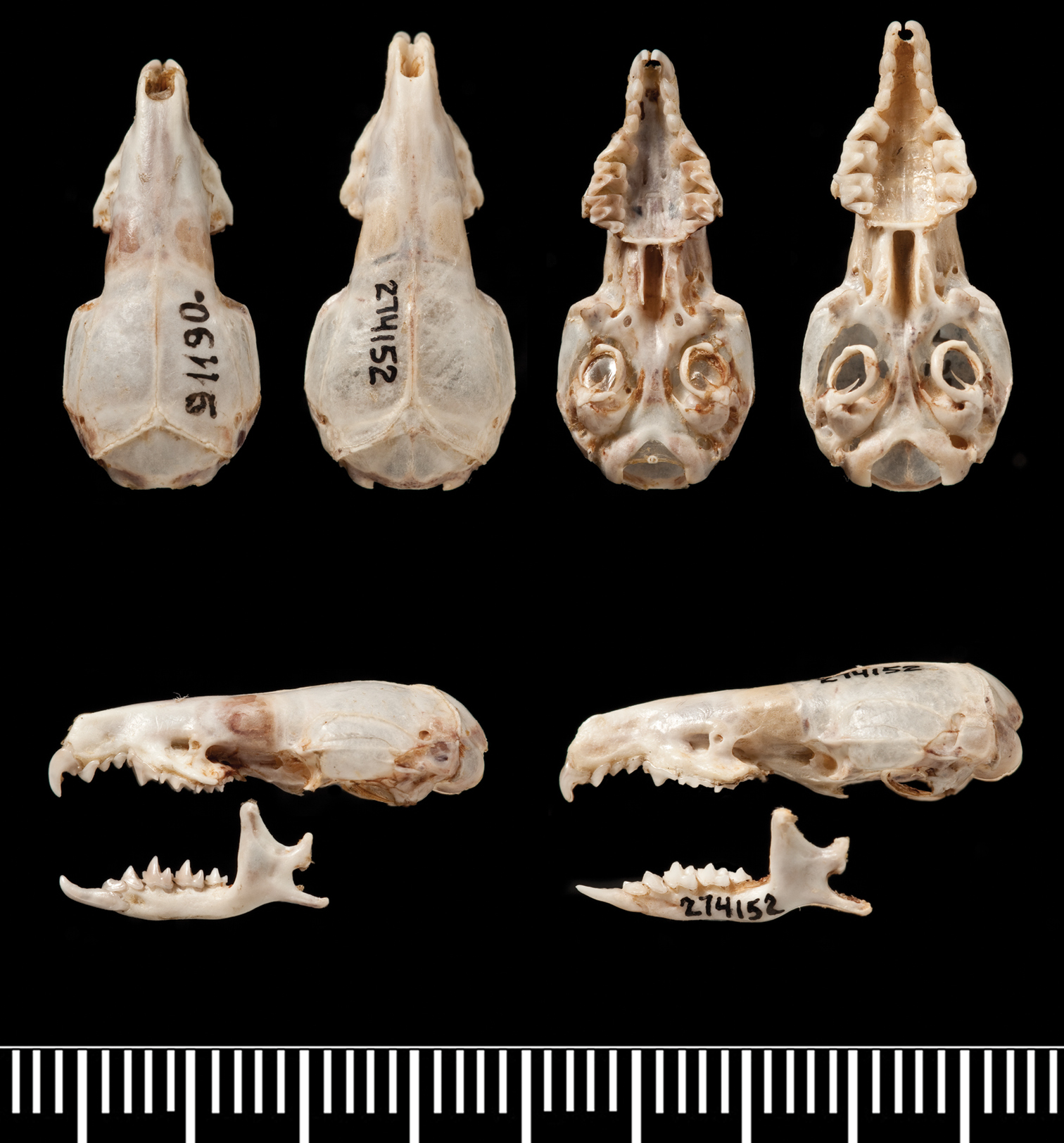
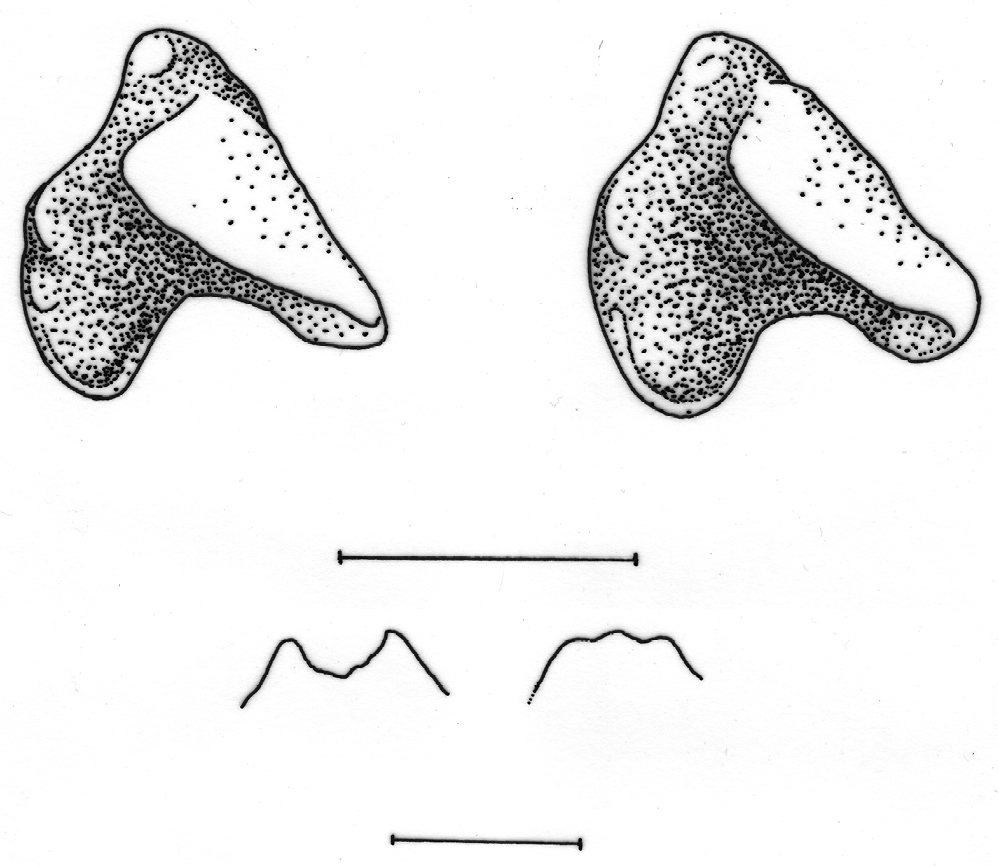
The population from Mt Tay Con Linh II, Ha Giang Province in northern Vietnam recognised by molecular analysis of cyt b as Crocidura attenuata, falls within the size range of Crocidura tanakae and both species are morphologically very similar in appearance. The two species may be separated by the following characters. The basioccipital region in Crocidura attenuata is narrow and ridged particularly anterior to the position of the basioccipital suture, whereas in Crocidura tanakae the basioccipital region is broad and flat to concave (see Fig. 7). The palatal suture in Crocidura attenuata is a rounded to flat-topped ‘n’ shape, whereas in Crocidura tanakae the suture is a shallow to more marked ‘m’ shape (see Fig. 8). The two species also differ in the shape of the talon of the upper premolar (P4). In occlusal view, the talon of Crocidura attenuata is broader and more angular than that of Crocidura tanakae, the lingual border is straight to concave, with the posterior border shallowly indented so that the whole tooth looks larger in occlusal view. In Crocidura tanakae the lingual border of P4 is rounded and the posterior border of the tooth is deeply indented (see Fig. 8).
Comparison of crania of Crocidura tanakae (ZIN 91190) and Crocidura attenuata (AMNH 274152). Top row from left to right: dorsal views of the skulls of Crocidura tanakae and Crocidura attenuata, ventral views of the skulls in the same order. Lower row: left lateral view of skulls and mandibles from left to right of Crocidura tanakae and Crocidura attenuata.
Above: occlusal view of left upper premolar of Crocidura tanakae (ZIN 91205) left and Crocidura attenuata (AMNH 274232) right. Below: palatal sutures of the same specimens in the same order. Scales equal 1 mm.
Populations of Crocidura tanakae in Vietnam show a distinct clinal variation in skull size, populations at higher latitudes averaging smaller in size than those at lower latitudes (see Table 2). Although sample sizes are small, this observation is a possible example of the converse Bergmann’s rule where body size decreases with latitude.
Latitudinal size variation in Crocidura tanakae. Measurements in millimetres are presented as the mean, standard deviation and range, followed by sample size in parentheses.
| Character | Lao Cai Prov., Van Ban Dist. 21°58'N | Ha Tinh Prov., Huong Son 18°21'N | Quang Tri Prov., Huong Hoa 16°56'N | Kon Tum Prov., Ngoc Linh 15°05'N | Lam Dong Prov, . Bi Doup 12°11'N |
|---|---|---|---|---|---|
| Condyloincisive length | 19.3 ± 0.47 18.5–20.0 (14) |
19.7 ± 0.47 18.4–20.4 (24) |
20.1 ± 0.38 19.6–20.6 (5) |
20.6 ± 0.36 20.1–20.9 (4) |
20.4 ± 0.52 19.6–21.3 (11) |
| Condylobasal length | 18.8 ± 0.48 17.9–19.4 (14) |
18.9 ± 0.48 17.7–19.6 (24) |
19.2 ± 0.36 18.8–19.6 (5) |
19.8 ± 0.29 19.4–20.0 (4) |
19.5 ± 0.52 18.8–20.6 (11) |
| Upper toothrow length | 8.3 ± 0.18 7.9–8.6 (14) |
8.5 ± 0.22 8.2–9.0 (24) |
9.0 ± 0.24 8.6–9.3 (5) |
9.1 ± 0.14 8.9–9.2 (4) |
9.0 ± 0.23 8.5–9.4 (11) |
| Maxillary breadth at M2 | 5.9 ± 0.17 5.6–6.3 (14) |
6.0 ± 0.19 5.6–6.4 (24) |
6.2 ± 0.17 6.0–6.4 (5) |
6.3 ± 0.18 6.1–6.5 (4) |
6.2 ± 0.21 5.9–6.6 (11) |
| Braincase breadth | 8.7 ± 0.31 8.3–9.2 (14) |
8.9 ± 0.19 8.3–9.1 (24) |
9.0 ± 0.14 8.7–9.1 (5) |
9.2 ± 0.36 8.9–9.6 (4) |
9.3 ± 0.26 8.9–9.6 (11) |
While Crocidura attenuata in Vietnam appears to occur only to the east of the Song Hong (Red River), in northeastern Vietnam (
For sister species that are recognised on the basis of morphology, cyt b distance values typically exceed 5% (
Crocidura tanakae is currently recognised as being widely distributed in Southeast Asia including southern China, Vietnam, Taiwan and the Philippines (
The field studies in Vietnam were possible due to the support of the Joint Vietnam-Russian Tropical Research and Technological Centre. We are grateful to the administration of the Hoang Lien National Park for providing us with an opportunity to carry out field surveys. AA thanks A.V. Shchinov, A.E. Anichkin, Vu Van Lien, A.L. Monastyrskii, S.V. Kruskop for their help and scientific expertise during the field work. This study was supported in part by the Russian Foundation for Basic Research (projects 11-04-00020, 12-04-93005).
PJ particularly thanks Darrin Lunde, National Museum of Natural History, Smithsonian Institution, Washington D.C. for generously allowing access to specimens collected at pivotal localities in Vietnam and for many discussions over the problems of identifying morphologically conservative shrews. For providing access to the collections in their care, we are especially grateful to Eileen Westwig and Neil Duncan, American Museum of Natural History, New York; to James Patton and Chris Conroy, Museum of Vertebrate Zoology, University of California, Berkeley and to William Stanley and John Phelps, Field Museum of Natural History, Chicago. As always, grateful thanks to Phil Hurst, Photographic Unit, Natural History Museum, London, for specimen photography and to Roberto Portela Miguez, Mammal Group, Natural History Museum, London for scanning documents. We thank two anonymous reviewers for their helpful and constructive comments and criticisms.
Specimens included in the morphological study. (doi: 10.3897/zookeys.313.4823.app). File format: Microsoft Word document (doc).
Explanation note: The supplementary file contains a list of all specimens included in this study.