(C) 2013 Sergei I. Golovatch. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Golovatch SI (2013) A reclassification of the millipede superfamily Trichopolydesmoidea, with descriptions of two new species from the Aegean region (Diplopoda, Polydesmida). ZooKeys 340: 63–78. doi: 10.3897/zookeys.340.6295
Two new species are described from caves in several Greek islands in the Aegean Sea: Galliocookia gracilis sp. n., a presumed troglobite from Rhodes, Dodecanese Islands, and Sphaeroparia simplex sp. n., likely a troglophile from Kithnos, Cyclades, and Chios, Eastern Sporades. These genera are assigned to the family Trichopolydesmidae Verhoeff, 1910. Because Sphaeroparia Attems, 1909, an Afrotropical genus, nicely bridges the gap, both morphologically and geographically, between the Euro-Mediterranean Trichopolydesmidae and the much more diverse, pantropical Fuhrmannodesmidae Brölemann, 1916, the latter family is considered as a new junior subjective synonym of the former, syn. n. Thus expanded, the family Trichopolydesmidae is rediagnosed and its position within the superfamily Trichopolydesmoidea refined. Because the families Mastigonodesmidae Attems, 1914, Macrosternodesmidae Brölemann, 1916 and Nearctodesmidae Chamberlin & Hoffman, 1950 are also formally synonymized with Trichopolydesmidae, syn. n., the Trichopolydesmoidea currently contains only two families, Trichopolydesmidae and Opisotretidae Hoffman, 1980.
Diplopoda, Trichopolydesmidae, Fuhrmannodesmidae, taxonomy, new species, cave, Greece
According to
Prompted by the discovery of two new species of trichopolydesmoids in caves on three Aegean islands of Greece, this superfamily is reclassified. One of the genera appears to so nicely bridge the gap between the Euro-Mediterranean Trichopolydesmidae and the much more diverse, pantropical Fuhrmannodesmidae, that the latter family is formally synonymized here with the former.
The material treated below had been taken in 1987 by Petar Beron, of the National Museum of Natural History, Sofia, Bulgaria (NMNHS), in caves on a few Aegean islands of Greece and was offered to me for treatment in August 2013, when I visited the NMNHS in the framework of the Russian-Bulgarian interacademician exchange programme. Most of the type material will be returned to the NMNHS, with only two paratypes retained for the collection of the Zoological Museum, State University of Moscow, Russia (ZMUM).
http://zoobank.org/97419B2E-E237-4A96-A507-2A9FBF4ACFC0
http://species-id.net/wiki/Galliocookia_gracilis
Figs 1–6Holotype ♂ (NMNHS), Greece, Rhodes Island, village Archangelos, Cave Coumellos, 02.05.1987, leg. P. Beron.
Paratypes: 1 ♂, 1 ♀, 1 ♂ subadult (NMNHS), same locality, together with holotype.
Differs from the other species of Galliocookia Ribaut, 1955, all three from caves in southern France (
To emphasize the highly gracile looks of this species.
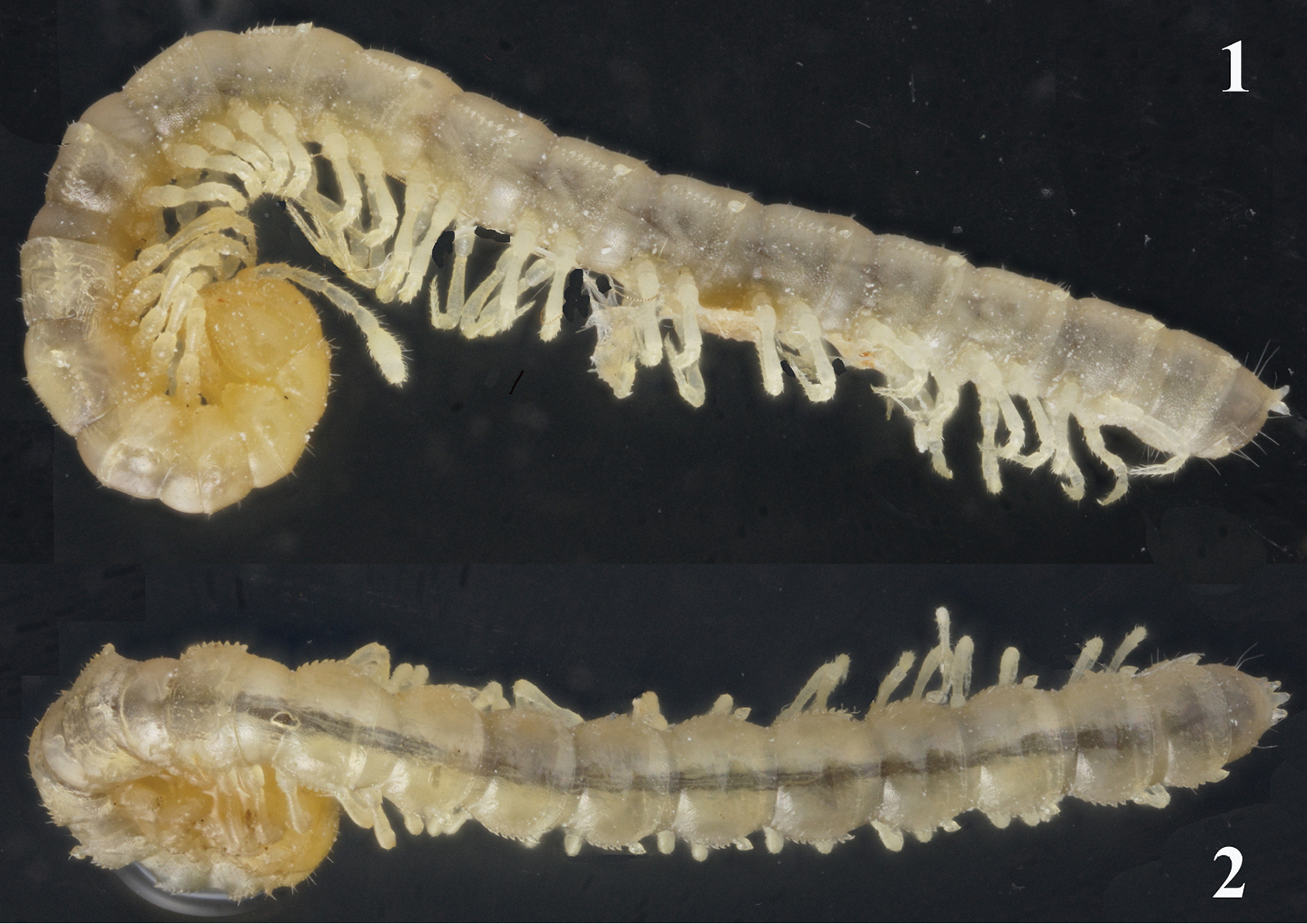
Length of adults ca 8.0 (♂) or 9.0 mm (♀), width of midbody pro- and metazona 0.8 and 0.9 mm (♂, ♀), respectively. Coloration in alcohol uniformly pallid to, in front body quarter, light yellowish (Figs 1, 2).
Body with 19 (♂) or 20 (♀) segments, strongly moniliform. Tegument smooth, mainly slightly shining, texture very delicately alveolate. Head densely pilose throughout; ♂ epicranial modifications absent, frons being regularly convex in both sexes. Antennae long and slender, reaching behind segment 2 when stretched dorsally (♂, ♀); antennomeres 2-4 and 6 subequal in length, but 6th clearly the highest (height being measured from ventral to dorsal margin) (Figs 1, 3); antennomeres 5 and 6 each with a distinct, round, distodorsal, compact field of sensilla.
Habitus of Galliocookia gracilis sp. n., holotype, lateral and dorsal views, respectively. Photos by K. Makarov, not taken to scale.
In width, head = segments 6-18(19) > 5 > 4 > 2 = 3 > collum; body suddenly tapering on telson. Collum ellipsoid, acutangular caudolaterally, like most of following metaterga with three transverse, rather regular rows of setae until segment 15 or 16, following metaterga with 3-4 less regular rows in front of a 4th or 5th regular row at caudal margin. Tergal setae very short, simple, sharp, only about 1/5-1/6 the length of a metatergum, a little longer only on collum and penultimate segment, mostly 3+3 in each row, but gradually growing to about 4+4 or 5+5 per row towards telson. A very faint transverse sulcus in caudal 1/3 of metaterga in front of caudal row of setae. Dorsum invariably convex. Paraterga poorly developed, especially so in ♀, visible starting from collum, invariably slightly declivous, set rather high (mainly at about upper quarter or third of midbody height in ♂ and ♀, respectively), slightly, but regularly rounded laterally; lateral margin of postcollum paraterga clearly serrate, with 6-7 subequal, often setigerous indentations in front of a somewhat (♀) or much (♂) larger, isolated, sometimes nearly pointed tubercle, this in pore-bearing segments turning into a conspicuous porostele (Figs 1, 2). This caudal tubercle or porostele of paraterga drawn caudolaterad, but never extending behind rear tergal margin. Pore formula normal: 5, 7, 9, 10, 12, 13, 15-18(19), ozopores round, dorsal, quite evident due to their porosteles. Stricture between pro- and metazona wide, shallow and smooth. Limbus very finely microspiculate. Epiproct conical, rather long, but barely extending behind 2+2 conspicuous, pre-apical teeth, caudalmost of which unusually strong and upturned (Figs 1, 2). Hypoproct trapeziform.
Sterna clearly separated, unmodified. Legs rather long and slender, without modified setae, ca 1.3–1.4 (♂) or 1.1–1.2 (♀) times as long as midbody height, femora and tarsi longest, claw short (Fig. 4). Epigynal ridge very low, inconspicuous.
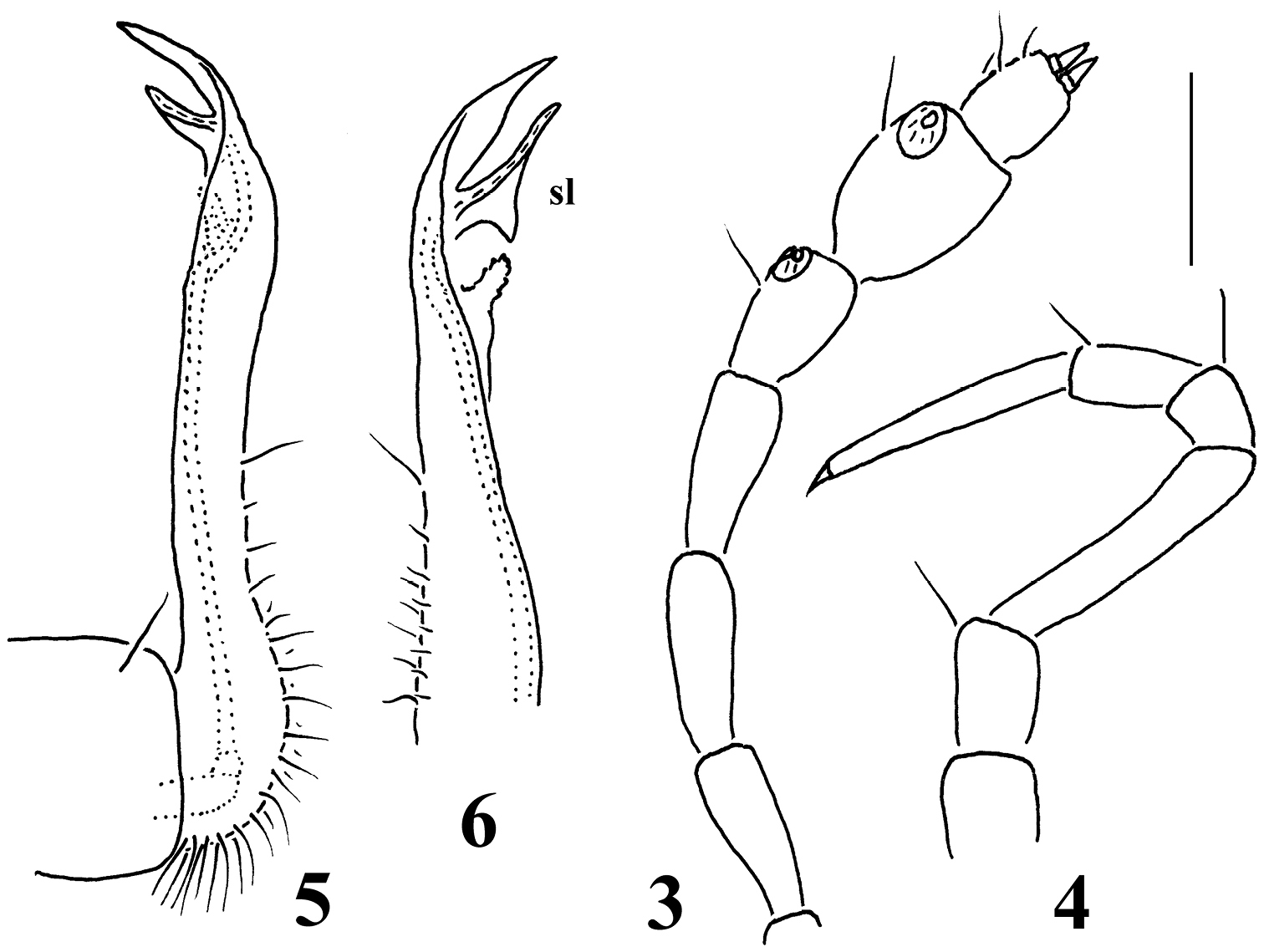
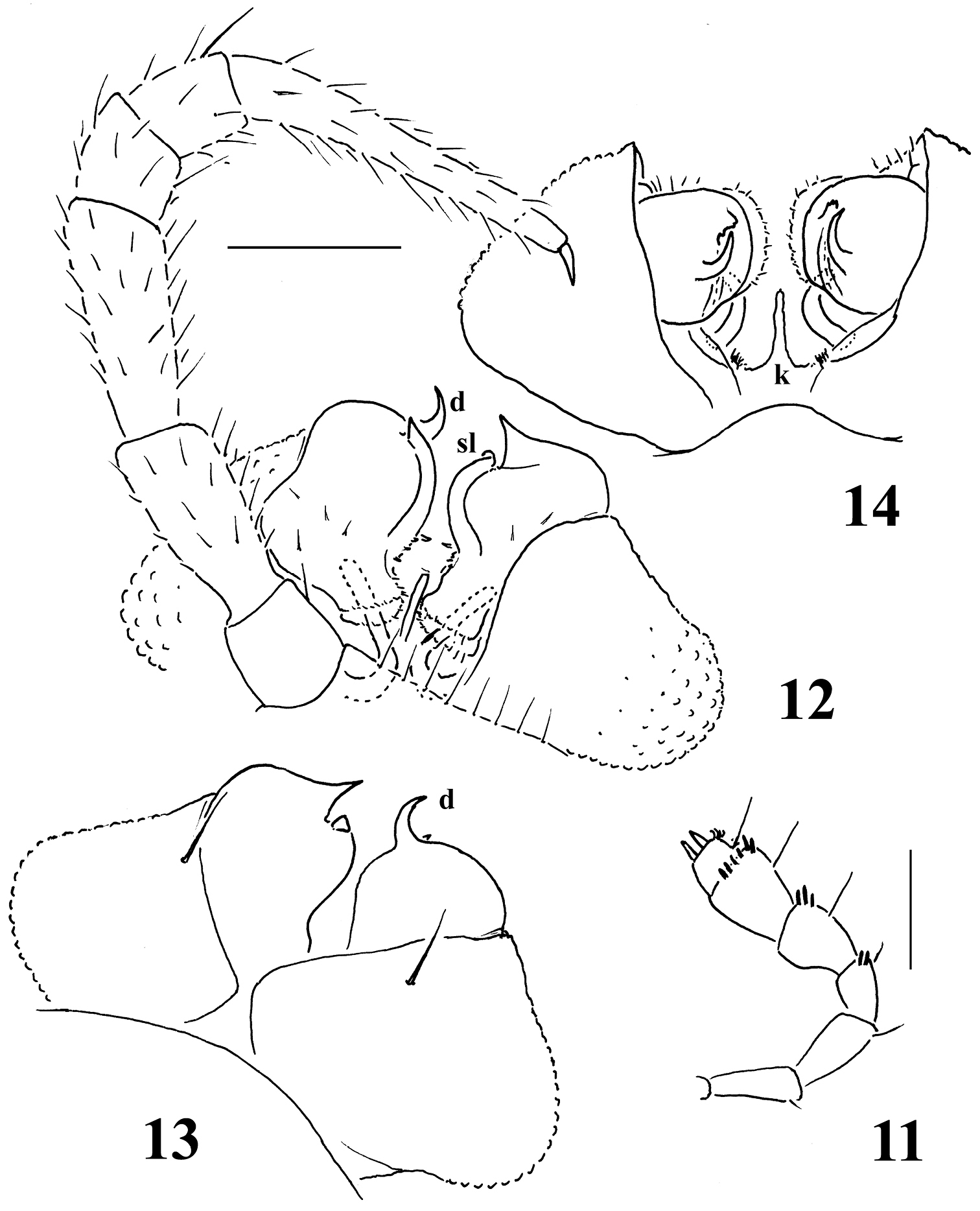
Galliocookia gracilis sp. n., holotype 3 antenna, lateral view 4 leg 9, 5, 6 right and left gonopods, lateral and sublateral views, respectively. Scale bars: 3, 4 0.2 mm; 5, 6 0.4 mm.
Gonopod aperture transversely oblong-oval, taking up most of ventral part of metazonite 7. Gonopods (Figs 5, 6) with small, subglobose, evidently exposed, medially fused coxae, each carrying only 1 long seta distolaterally and a long, curved cannula distomesally; a scaly texture on lateral face absent. Telopodite suberect, slender and long, up to about its half being taken up by an elongate, setose prefemoral part; acropodite slightly helicoid, its apical lobe subacuminate, with a pre-apical, lobe-shaped, subtriangular solenomere (sl) and a short, papillate, rounded stump a little proximally. Both prefemoral part and acropodite strictly coaxial, in situ directed forward and parallel to each other. No traces of accessory seminal chamber or hairy pulvillus.
Among the Euro-Mediterranean genera of Trichopolydesmoidea, only some show a deeply bipartite and strongly curved gonopod telopodite, the prefemoral part of which is quite elongate, but lies more or less transversely to strongly angular, largely (sub)parallel telopodites and extends across the nearly entire ventral width of segment 7. Such are Trichopolydesmus Verhoeff, 1898 (together with Banatodesmus Tabacaru, 1980), Bacillidesmus Attems, 1898, Napocodesmus Ceuca, 1974 and Caucasodesmus Golovatch, 1985. In contrast, the gonotelopodites in Verhoeffodesmus Strasser, 1959, Cottodesmus Verhoeff, 1936 and Occitanocookia Mauriès, 1980 have increasingly shortened prefemoral parts, being enlarged and laterally flattened distad, unipartite and mostly less strongly curved, in Cottodesmus and Occitanocookia also devoid of a solenomere, but sometimes supplied instead with what can be seen as a primordial accessory seminal chamber. In Trichopolydesmus, Heterocookia Silvestri, 1898, Ingurtidorgius Strasser, 1974 and, especially, Mastigonodesmus Silvestri, 1898, the solenomere is flagelliform, branching off near the base of the femorite. In all these genera, the gonotelopodites are strongly exposed, not sunken inside an obvious central coxal cavity (= gonocoel). A modest gonocoel seems to only be observed in Ingurtidorgius and Haplocookia Brölemann, 1915. This latter genus does resemble Galliocookia, but its gonopod telopodite is clearly curved, there is a small gonocoel and both sexes have 20 body segments (see also review by
The new species definitely belongs to Galliocookia, sharing with the other three congeners (see Diagnosis above) a small coxa and a slender, simple and suberect telopodite, the latter showing an extended prefemoral part strictly coaxial with the acropodite. Moreover, the solenomere is likewise modest, lobe-shaped and located distally. Even the presence of 19 or 20 body segments in the male and female, respectively, as well as of a normal ozopore formula, setose metaterga and laterally serrate/indentate paraterga coincide.
Biogeographically, the discovery of a Galliocookia in the Aegean region, so far away from France, is remarkable, emphasizing a pan-Mediterranean distribution pattern of this currently purely cavernicolous, likely troglobitic genus.
http://zoobank.org/795A5831-ADEA-49EC-977F-AAE9878037B2
http://species-id.net/wiki/Sphaeroparia_simplex
Figs 7–17Holotype ♂ (NMNHS), Greece, Kithnos Island, village Dryopis near Phirès, Cave Katafyki, 08.05.1987, leg. P. Beron.
Paratypes: 1 ♂, 4 ♀♀, 1 ♂ subadult (19 body segments), 2 fragments (NMNHS) + 1 ♂, 1 ♀ (ZMUM), same locality, together with holotype; 1 ♂, 1 ♀, 1 ♀ fragment (head and first 12 body segments) (NMNHS), Greece, Chios Island, village Haghios Galos (Agiongalas, Haghia Gala), 65 km from town of Chios, Cave Hagiogalousaina, 12.05.1987, leg. P. Beron.
Differs from the other 30+ species of Sphaeroparia Attems, 1909, many of which have been reviewed by
To emphasize the highly simple gonopods in this species.
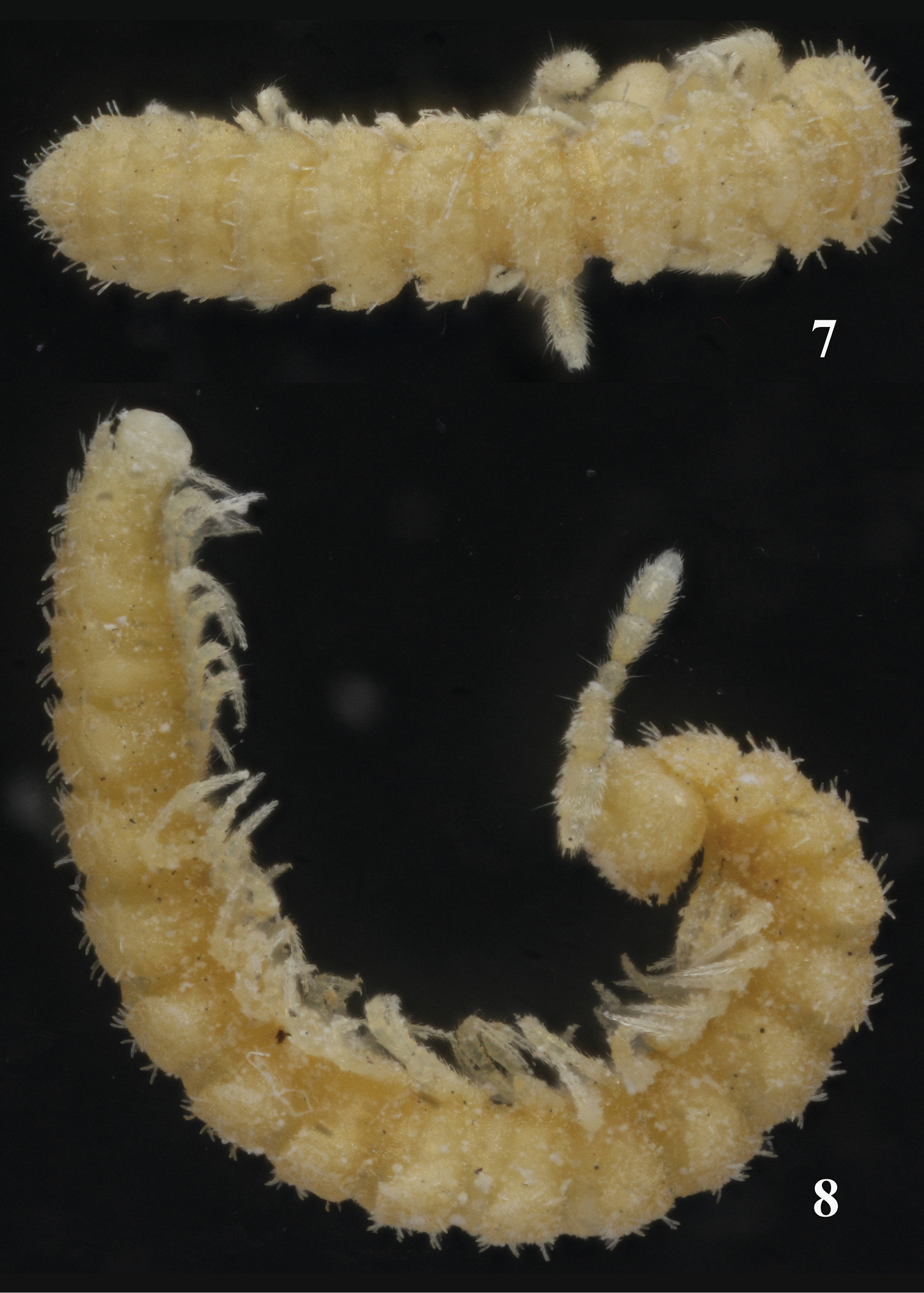
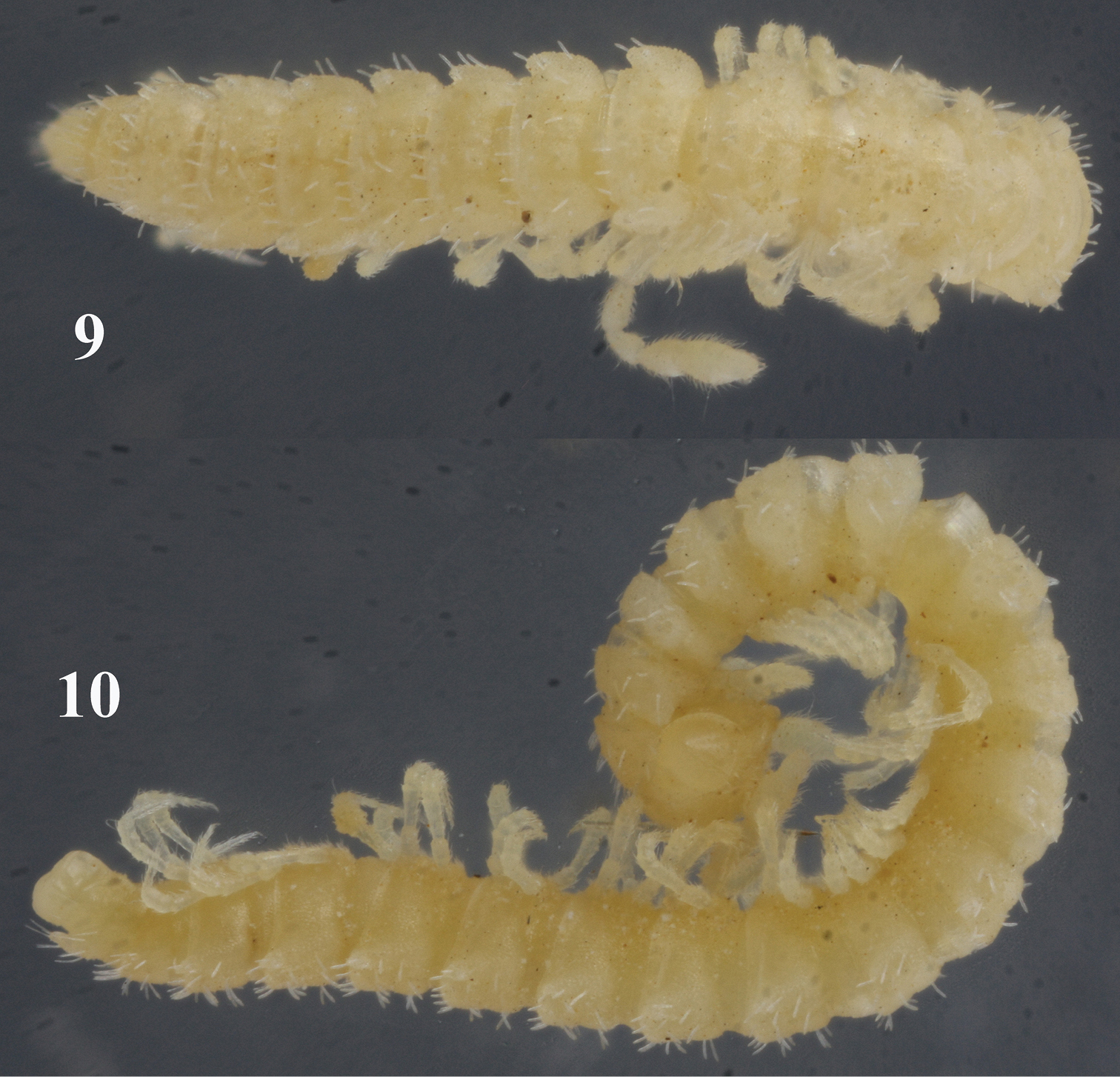
Length of adults ca 5.0–5.5 (♂) or 6.0–7.0 mm (♀), width of midbody pro- and metazona 0.55 and 0.7 mm (♂, ♀), respectively. Coloration in alcohol uniformly pallid to light yellowish (Figs 7–10).
Habitus of Sphaeroparia simplex sp. n., holotype, dorsal and lateral views, respectively. Photos by K. Makarov, not taken to scale.
Habitus of Sphaeroparia simplex sp. n., ♂ paratype from Kithnos, dorsal and lateral views, respectively. Photos by K. Makarov, not taken to scale.
Body with 20 segments in both sexes. Tegument generally smooth, dull, texture very delicately alveolate. Head densely pilose throughout; ♂ epicranial modifications absent, frons being regularly convex in both sexes. Antennae rather short and clavate, nearly reaching end of segment 2 (♂) or collum (♀) when stretched dorsally; antennomeres 2, 3, 5 and 6 subequal in length, but both 5th and 6th clearly the highest (height being measured from ventral to dorsal side) (Figs 11, 15); antennomeres 4-6 each with a loose, indistinct, distodorsal group of increasingly long and numerous sensilla.
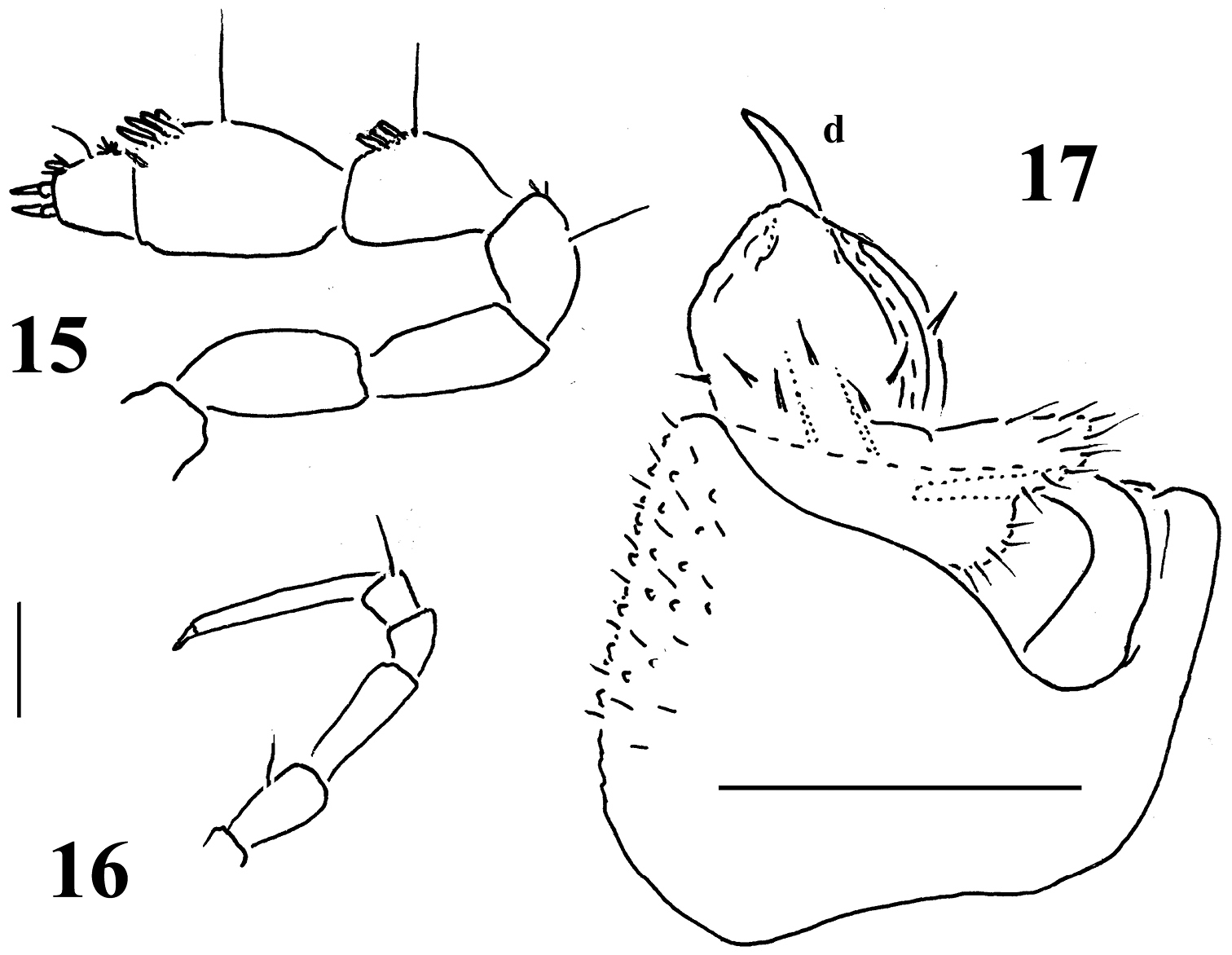
Sphaeroparia simplex sp. n., holotype (12, 13) and ♂ paratype (11, 14) from Kithnos 11 antenna, lateral view 12 leg 9 and both gonopods, caudal view 13, 14 both gonopods, oral and ventral views, respectively. Scale bars: 11 0.2 mm; 12–14 0.1 mm.
Sphaeroparia simplex sp. n., ♂ paratype from Chios 15 antenna, lateral view 16 midbody leg 17 left gonopod, mesal view. Scale bars: 0.1 mm.
In width, head = segments 6-16(17) > 5 > 4 > 2 = 3 > collum; starting from segment 17, body gradually tapering towards telson. Collum ellipsoid, lobuliform caudolaterally, like most of following metaterga with 3 transverse, rather regular rows of 3+3 setae on minute knobs until segment 16, of 4+4 setae in segments 17(18)-19. Tergal setae medium-sized, slender, bacilliform, mostly about 1/4 the length of a metatergum, a little longer only on collum and penultimate segment. Usually a very faint transverse sulcus between first 2 rows of setae. Dorsum invariably convex. Paraterga poorly developed, especially so in ♀, visible starting from collum, invariably slightly declivous, set rather high (mainly at about upper third of midbody height in both sexes), slightly, but regularly rounded laterally; lateral margin of postcollum paraterga very poorly indentate, on each side usually with 3 subequal setigerous indentations in front of a rounded caudolateral lobule, the latter increasingly well drawn caudad, but never extending behind rear tergal margin (Figs 7–10). Pore formula normal: 5, 7, 9, 10, 12, 13, 15-18(19), ozopores round, dorsal, rather indistinct, lying at base of caudolateral lobule near lateral margin. Stricture between pro- and metazona wide, shallow and smooth. Limbus very finely microspiculate. Epiproct conical, rather long. Hypoproct trapeziform.
Sterna clearly separated, unmodified. Legs rather long and slender (Figs 8, 10, 12, 16), without modified setae, ca 1.2–1.3 (♂) or 1.0–1.1 (♀) times as long as midbody height, tarsi longest, claw short. Epigynal ridge very low, inconspicuous.
Gonopod aperture evident, transversely oblong-oval, taking up most of ventral part of metazonite 7. Gonopods (Figs 12–14, 17) with large, transversely subglobose, evidently exposed, medially fused, medially clearly excavate coxites, each microgranular/scaly and micropilose laterally, carrying only 1 long seta distofrontally and a long, curved cannula distomesally; sternum plate-like, with a slender, central, not too strongly chitinized process (k) between coxites. Telopodites short, stout, subglobose, sac-shaped, very simple, only moderately exposed below coxites, deeply sunken inside a prominent gonocoel; prefemoral part as usual, setose, quite elongate, about as long as, but clearly set at an angle to, acropodite, the latter with a distinct seminal groove running entirely on mesal side and terminating on a vestigial lobuliform solenomere (sl) at base of a rather strong, only faintly curved, apical spine (d). Neither an accessory seminal chamber nor a hairy pulvillus.
This species seems to be only a troglophile, which occurs in caves on two remote islands in the Aegean Sea. On the other hand, Cave Katafyki is known to support at least two presumed troglobites, i.e. the woodlouse Cordioniscus kithnosi Andreev, 1986 (Isopoda, Oniscidea, Styloniscidae) (
The discovery of a Sphaeroparia in the Aegean region is even more remarkable than that of a Galliocookia. Indeed, Sphaeroparia is a rather large genus hitherto believed to be strictly Afrotropical, ranging from Liberia and Benin in western Africa, through Gabon and Zaire, to Kenya and Tanzania in eastern Africa (see review by
Naturally, similar general trends can be surmised to have occurred in the fuhrmannodesmids of Central America and the Afrotropical and Oriental realms, which also support fairly diverse faunas of this family. Remarkably, none of the genera of Trichopolydesmidae (see above), all confined to Europe and the Mediterranean, though demonstrating a certain degree of variation in the length and orientation of the gonopod prefemoral part, has a deep gonocoel. Furthermore, Galliocookia as one of the few genera where the gonocoxae are particularly small while the prefemoral part is still elongate, but already strictly coaxial with a very strongly exposed acropodite represents an evolutionary extreme, apparently the basalmost situation. The discovery of a Sphaeroparia in the eastern Mediterranean, of an Afrotropical genus demonstrating a very large and deep gonocoel for the small and only poorly exposed telopodites to be hinged into, clearly represents the opposite, evolutionarily obviously the most advanced extreme. Therefore, the Euro-Mediterranean Trichopolydesmoidea appear to show basically the same full range of evolutionary trends in the development of gonopod structures as do at least the properly assessed South American representatives now assigned to Fuhrmannodesmidae! Among the Afrotropical Trichopolydesmoidea, Sphaeroparia is certainly the most speciose genus and
Considering the above new evidence, I no longer hesitate to formally synonymize the family Fuhrmannodesmidae Brölemann, 1916 under Trichopolydesmidae Verhoeff, 1910, syn. n.
The following refined classification and diagnosis of the superfamily Trichopolydesmoidea, as well as of its two constituent families can be proposed (see also
Body largely polydesmoid, only 2–16 mm long, exceptionally up to 30 mm, usually ≤ 10 mm long, with 18–20 segments. Antennomeres 2-6 usually subequal in length, but 6th normally highest; 5th often, 6th only exceptionally, devoid of an apicodorsal field of sensilla. ♂ head sometimes with vertigial modifications (humps, fossae etc.). Paraterga at most with only thin calluses, often devoid of these, lateral margin entire, only exceptionally lobulate (Trilobodesmus Golovatch & Mauriès, 2007), typically at least faintly incised and setigerous; metaterga devoid of a cerategument, more often with 3-4, more or less regular, transverse rows of low, polygonal or rounded, setigerous bosses, but rather usually these latter either obliterated or pronounced and more numerous. Ozopores flush, opening on dorsal surface of paraterga to be located at their lateral margin, pore formula normal. Sphaerotrichomes rather often present, usually affecting both tibiae and tarsi.
Gonopod aperture large, transversely ovoid, not extending onto prozonite, but sometimes spreading caudad between coxae 9; gonocoxae subglobose, sometimes with a distolateral projection; prefemoral parts usually lying transversely to main body axis and, together with coxae, taking up most of ventral extent of ♂ ring 7, quite often with an obvious gonocoel formed by enlarged coxae; cannula and seminal groove nearly always present, both absent only exceptionally; entire telopodites or, more usually, acropodites distal to prefemoral parts usually complex, parallel to main body axis and directed forward, but often either crossing each other or, more rarely, directed more or less laterad, dorsolaterad or, exceptionally, even caudad, also exceptionally perforating lateral walls of coxites. A solenomere, an exomere and/or an endomere often present, sometimes these being prominent. An accessory seminal chamber and/or a hairy pulvillus at its orifice only seldom developed, mostly tend to be absent.
All continents except Australia, mostly a Northern Hemisphere group only marginally represented in the Southern Hemisphere (Indonesia + New Guinea and Melanesia, Afrotropical realm north of Malawi and Madagascar, Neotropical realm north of Bolivia, Paraguay and southern Brazil), apparently fully allopatric with the superfamily Dalodesmoidea.
Body polydesmoid, only exceptionally paraterga deeply lobulate laterally. Sphaerotrichomes only rarely present. Antennomere 5 sometimes devoid of sensilla distodorsally. Sphaerotrichomes only sometimes present.
Gonopod aperture large, transversely oval, with exposed to deeply sunken gonocoxae. Gonocoxae subglobose, usually with normal, tube-shaped cannulae, from small to rather large while telopodites from strongly exposed to concealed inside a considerable central gonocoel; prefemoral portion tends to be orientated transversely to main body axis, only occasionally somewhat to clearly shortened and thus resembling the condition observed in Polydesmoidea; acropodite tri-, bi- or uniramous, usually directed cephalad or cephalomesad; solenomere mostly evident, simple, only seldom a short tooth, more often long, distal in location, either stout or slender/flagelliform. Normally neither an accessory seminal chamber nor a hairy pulvillus, only exceptionally with a primordial accessory seminal chamber. Caucasodesmus Golovatch, 1985 (Caucasus and Crimea) is aberrant in having no sensilla on antennomeres 5 and no cannulae or seminal grooves.
Type genus: Trichopolydesmus Verhoeff, 1898.
The family Mastigonodesmidae Attems, 1914, based on Mastigonodesmus Silvestri, 1898 (ca 8 eight species in the western Mediterranean), is sometimes regarded as a synonym of Polydesmidae Leach, 1815 (
The same applies to the family Macrosternodesmidae, which basically fails to differ from Trichopolydesmidae, but simply tends to encompass quite a few genera with small to medium-sized, invariably globose gonocoxae and strongly exposed, often complex telopodites. Since the purely Nearctic nominate family Nearctodesmidae shares the gonopod conformation with Macrosternodesmidae, and it has sometimes been treated as only a subfamily or even a possible synonym of the latter family, I am inclined to treat these two latter families as synonyms of Trichopolydesmidae as well, syn. n.
The diversity of gonopod structural plans in nearctodesmids+macrosternodesmids (
Thus refined, the Trichopolydesmidae currently contains ca 20 genera and about 60 species in the Holarctic, as well as ca 55 genera and about 80 species in the tropics.
Sphaerotrichomes absent. Gonopod aperture large, transversely oval, with small and largely exposed coxites. Telopodites elongate, directed strongly dorsolaterad; seminal groove running along most of telopodite extent on caudal face to terminate distally either on a special branch or tooth (= solenomere), flush on caudal surface, or debauch inside an accessory seminal chamber which normally is supplied with a hairy pulvillus.
Opisotretus Attems, 1907
Only seven genera and 29 described species ranging from the Ryukyu Islands, Japan, Taiwan, southern mainland China and the Himalayas in the North and Northwest, through Indochina and across Indonesia, to Papua New Guinea in the Southeast (
I am greatly obliged to Petar Beron and Pavel Stoev (both NMNHS) for allowing me to study the above interesting material. In addition, Petar Beron most helpfully provided information on some other potential troglobites of Greece. Kirill Makarov (Moscow, Russia) skilfully took all pictures. Robert Mesibov and again Pavel Stoev very kindly reviewed the manuscript. My visit to the NMNHS in August 2013 was partly supported through the Russian-Bulgarian interacademician exchange programme, partly also by a grant issued by the Ministry of Education and Science of Russia.