(C) 2013 Masaatsu Tanaka. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new echiuran, Arhynchite hayaoi sp. n., is described from newly collected specimens from sandy flats of the Seto Inland Sea, Japan, together with many museum specimens, including those once identified as Thalassema owstoni Ikeda, 1904 or Arhynchite arhynchite (Ikeda, 1924). The new species is clearly distinguishable from its congeners by the smooth margin of gonostomal lips and lack of rectal caecum. Brief references are also made to the morphological distinction between the new species and Thalassema owstoni, originally described from the deep bottom on the Japanese Pacific coast.
Annelida, Echiura, new species, Arhynchite hayaoi, Thalassema owstoni, sandy flat, Seto Inland Sea
Echiurans are a small group of marine coelomates, previously classified as a distinct phylum, but now often included in the Phylum Annelida on the basis of recent molecular phylogenetic analyses (
The echiuran in the present study was first identified as Thalassema mucosum (currently accepted as Anelassorhynchus mucosus (Ikeda, 1904)) by
We have compared echiurans newly collected from a sandy flat near Onomichi Bay with the specimens deposited in the Tohoku University Museum (TUM), collected from the bay in 1931 and labeled as “Thalassema owstoni Ikeda” and “kouji” (probably equivalent to “kouju”), probably by Sato, who then belonged to the Tohoku Imperial University, the predecessor of Tohoku University. We found the old and new specimens to be very similar. Further, we found a significant difference between the specimens and the description of Thalassema owstoni Ikeda, 1904, although we attempted to examine its holotype and collect new specimens from the type locality, we have so far been unsuccessful. Thus, we herein describe “kouju” as a new species of the genus Arhynchite Satô, 1937.
We collected new specimens from a sandy flat, named Hachi-no-higata, at the mouth of the Kamogawa River, Takehara, Hiroshima Prefecture, Japan in 2011. The specimens were relaxed with menthol, and minute pieces of proboscis were taken from the holotype and two paratypes and fixed with pure ethanol for future molecular analyses; all remaining material was fixed in 10% seawater formalin and then stored in 70% ethanol. Further materials have been retained at our Laboratory of Taxonomy, Toho University (LTTU), the Department of Zoology, University Museum, University of Tokyo (UMUTZ), or TUM. The type series, as well as the two non-type ones, are deposited in the National Museum of Nature and Science, Tsukuba, Japan (NSMT).
Observations, dissections, and drawings were made under a stereoscopic microscope. Trunk length (TL) and proboscis length (PL) were measured. General terminology was based on
urn:lsid:zoobank.org:act:598901D0-6F77-4F4B-AA50-343FEAD4F9E5
http://species-id.net/wiki/Arhynchite_hayaoi
New Japanese name: Setouchi-dochikuchi-yumushi Figs 1, 2Holotype: NSMT-Ec 100, mature male, TL 25 mm, PL >5 mm (proboscis damaged), Hachi-no-higata sandy flat at the mouth of the Kamogawa River, Hiroshima Pref., Japan (34°19.42'N, 132°53.88'E), May 18, 2011, collected by M. Tanaka and T. Nishikawa. Paratypes: NSMT-Ec 101–105, 3 males + 2 females, TL 28–40 mm, PL 6 mm, collection data as for the holotype. Non-type specimens: TUM Echiurida 2-11, labeled as “Thalassema owstoni Ikeda” probably by H. Sato, 8 males + 6 females + 1 specimen of unknown sex, TL 29–73 mm, proboscis absent, Onomichi Bay, Hiroshima Pref., Japan (34°24'N, 133°12'E), March 7, 1931, collected by Takashi Gamo; TUM Echiurida 1-5, labeled as “Thalassema owstoni Ikeda” and “kouji” probably by H. Sato, 2 males + 3 females + 1 of unknown sex, TL 31–62 mm, PL 16–33 mm, Onomichi Bay, Hiroshima Pref., Japan (34°24'N, 133°12'E), March 7, 1931, by Takashi Gamo; NSMT-Ec 106, 1 of unknown sex, TL 28 mm, proboscis absent, intertidal sandy flat at the mouth of the Kamogawa River, Hiroshima Pref., Japan (34°19.42'N, 132°53.88'E), July 8, 2006, by Masanori Sato; NSMT-Ec 107, 1 of unknown sex, TL 33 mm, proboscis absent, intertidal sandy to muddy flat on Ikarise Islet, located in a channel of Lake Hamana, Shizuoka Pref., Japan (34°41.08'N, 137°35.98'E), April 19, 2003, by Shoichi Kimura; UMUTZ-Ecur-2, 1 of unknown sex, TL 55 mm, proboscis absent, above low-water mark, Tomono-ura, Hiroshima Pref., Japan (34°22'N, 133°23'E), July 1882, by I. Ikeda, reported as Arhynchite arhynchite (Ikeda) by
Trunk up to 80 mm long in preserved specimens. Leaf-like gonostomal lips with smooth margins. Neurointestinal vessel unbifurcated. Ring vessel absent. Rectal caecum absent. Anal vesicles fastened basally to the trunk wall by mesenteries.
In life, trunk colored pinkish yellow and proboscis pale yellow (Fig. 1). Coloration fading to pale white or beige after fixation with formalin.
Arhynchite hayaoi sp. n., found at the type locality, with the proboscis extending toward the right.
In preserved specimens, TL ranging from 25 to 73 mm (n = 28) and PL ranging from 6 to 33 mm (n = 8). Proboscis, often detached from trunk, elongated and slightly expanded at its extremity, with its proximal end forming small lower cup around mouth. Trunk wall thickened and covered with numerous distinct papillae, especially prominent (up to 1 mm) at both extremities. Trunk musculature consisting of outermost circular, middle longitudinal, and innermost oblique layers, all of which continuous throughout.
Paired ventral setae of usual form, with strong interbasal muscle (Fig. 2A), but only very rarely single seta without interbasal muscle in 2 of 6 non-type specimens in TUM Echiurida 1-5.
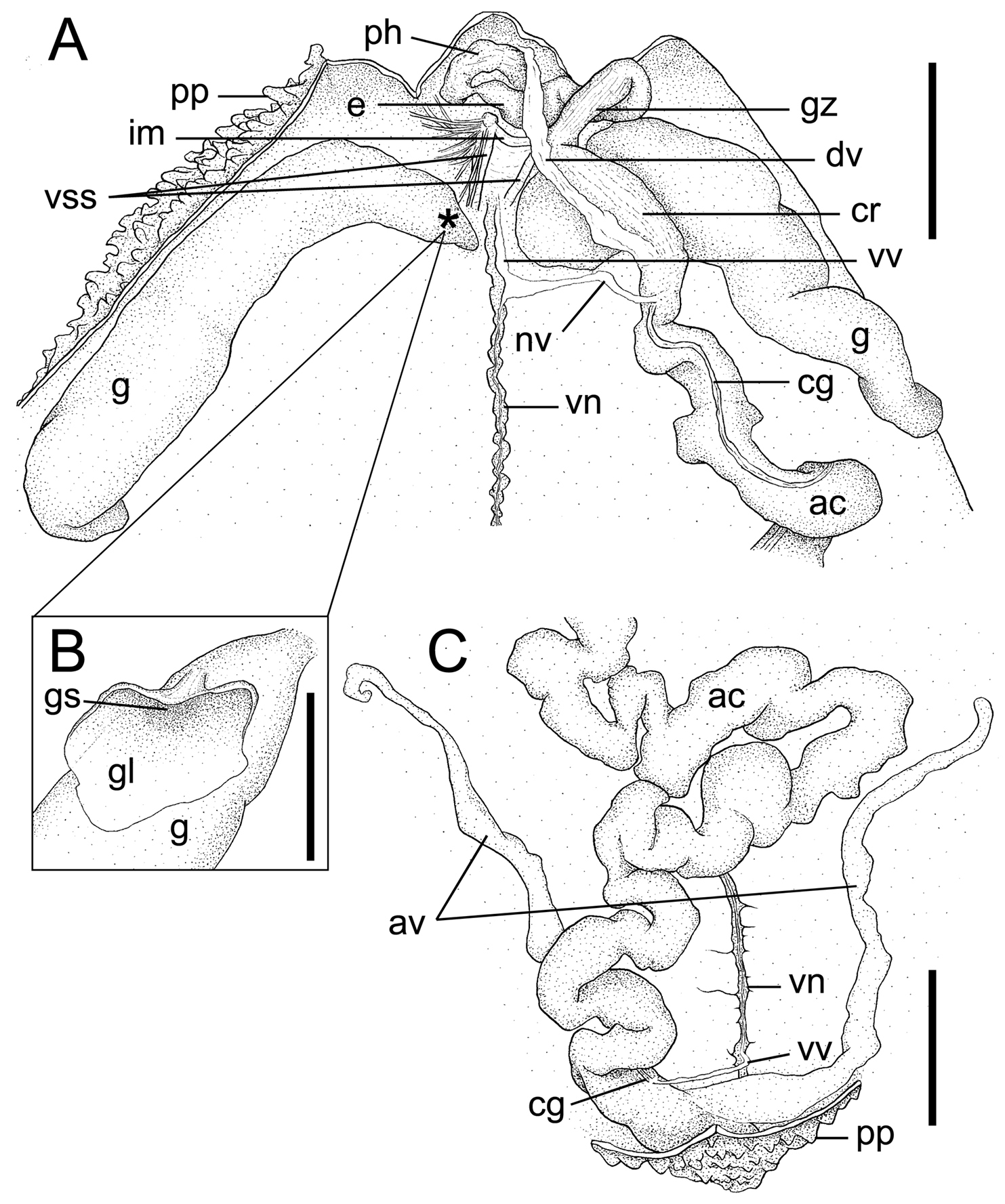
Arhynchite hayaoi sp. n., paratype (NSMT-Ec 104). A internal morphology of the anterior end of the trunk, dorsal view; an asterisk indicates the position of the gonostome B gonostome and gonostomal lip, magnified C internal morphology of the posterior end of the trunk, dorsal view. Abbreviations: ac alimentary canal; av anal vesicle; cg ciliated groove; cr crop; dv dorsal vessel; e esophagus; g gonoduct; gl gonostomal lip; gs gonostome; gz gizzard; im interbasal muscle; nv neurointestinal vessel; ph pharynx; pp papillae; vn ventral nerve cord; vss ventral setal sac; vv ventral vessel. Scales: A and C 5 mm; B 1 mm.
Paired gonoducts situated slightly behind ventral setae (Fig. 2A). Gonostomes situated proximally, each with leaf-like gonostomal lip with smooth margin (Fig. 2B); lip plicated marginally, probably due to fixation in many non-type specimens (TUM Echiurida 1-5, 2-11).
Long and convoluted alimentary canal, filled with sand grains and elliptical fecal pellets, ca 2 mm in long axis (Fig. 2A, C). Anterior part of alimentary canal, so-called foregut, divided into pharynx, esophagus, gizzard, and crop (Fig. 2A). Intestine following foregut fastened to trunk wall with numerous thread-like mesenteries and divided into pre-siphonal, siphonal, and post-siphonal parts (Fig. 2A, C). Pre- and post-siphonal parts having a ciliated groove; elongated pre-siphonal part about twice as long as TL (Fig. 2A). Rectal caecum absent (Fig. 2C).
Vascular system composed of dorsal, neurointestinal, and ventral vessels, without ring vessel (Fig. 2A). Dorsal vessel attached to entire length of crop (Fig. 2A). Ventral vessel running along almost entire length of ventral nerve cord and terminating at posterior end of post-siphonal intestine near anus (Fig. 2A, C). Neurointestinal vessel, although injured in holotype, issuing from ventral vessel slightly behind gonostomal level and terminating at anterior end of intestine without bifurcation (Fig. 2A). In 6 non-type specimens (5 in TUM Echiurida 2-11 and 1 in TUM Echiurida 1-5), ventral vessel issuing additional branch forward at ventral origin of neurointestinal vessel, with the branch terminally forming small loop around interbasal muscle. Further, in another non-type specimen of TUM Echiurida 1-5, origin of neurointestinal vessel shifted far forward, crossing interbasal muscle but without additional branches.
Paired simple anal vesicles, ca one-third of TL in holotype, while rarely attaining TL in other specimens, covered wholly with numerous microscopic ciliated funnels and fastened basally to trunk wall by some mesenteries (Fig. 2C).
The specific name is dedicated to the late Dr Hayao Sato who made a significant contribution to the taxonomy of echiurans, sipunculans, and priapulids in Japan and adjacent waters.
Hiroshima Pref. (Seto Inland Sea), Ikarise Islet at the entrance of Lake Hamana, and Misaki (Sagami Bay), Japan, intertidal to subtidal, sandy to muddy bottoms.
Table 1 gives a comparison of all known species and the newly described species assigned to the genus Arhynchite. Arhynchite hayaoi sp. n. is distinguishable from Arhynchite arhynchite, the only congener recorded to date from Japanese coasts, by the absence of such digitiform projections or irregular serrations along the margin of the gonostomal lips as are present in Arhynchite californicus and Arhynchite pugettensis, respectively (
A comparison of all known species referred to the genus Arhynchite.
| Species | Rectal caecum | Ring vessel | Bifurcation of neurointestinal vessel | Margin of gonostomal lip | Mesenteries mainly fastened to | Type locality | Sources |
|---|---|---|---|---|---|---|---|
| Arhynchite hayaoi sp. n. | absent | absent | absent | smooth | body wall | Seto Inland Sea, Japan | This study |
| Arhynchite arhynchite | absent | absent | barely detectable | with digitiform projections | body wall | Probably around Hokkaido, Japan | |
| Arhynchite californicus | absent | absent | absent | with digitiform projections | body wall | Monterey Bay, California, USA | |
| Arhynchite hiscocki | absent | absent | absent | with digitiform projections | intestine | Dunwich, Queensland, Australia | |
| Arhynchite inamoenus | absent | absent | absent | with irregular serrations | unknown | Monterey Bay, California, USA | |
| Arhynchite pugettensis | absent | present | absent | with irregular serrations | body wall | Puget Sound, Washington, USA | |
| Arhynchite paulensis | present | absent | unknown | with irregular serrations | intestine | Araça Beach, São Paulo, Brazil | |
| Arhynchite rugosus | present | unknown | unknown | smooth | unknown | Jiaozhou Bay, Shandong, China |
Thalassema owstoni was established by
Thus, the taxonomic identity of Thalassema owstoni remains unknown.
Thalassema fuscum Ikeda, 1904 is also similar to Arhynchite hayaoi in terms of living coloration (
Our cordial thanks are due to Drs S. Sato (TUM) and R. Ueshima (UMUTZ) for loan of specimens with permission to dissect; Mr K. Okada for help in collecting specimens; Dr M. Sato (Kagoshima University), Messrs Y. Kuwahara (Abashiri Fisheries Research Institute) and S. Kimura (Mie University) for materials; Dr T. Kon (Toho University) for helpful discussions; a reviewer for helpful comments on the manuscript; and to Enago (www.enago.jp) for English language review. This study was partly supported by a Grant-in-Aid for Field Research from Faculty of Science, Toho University to TN.