(C) 2013 Kai Borkenhagen. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Borkenhagen K, Krupp F (2013) Taxonomic revision of the genus Carasobarbus Karaman, 1971 (Actinopterygii, Cyprinidae). ZooKeys 339: 1–53. doi: 10.3897/zookeys.339.4903
Representatives of the fish genus Carasobarbus Karaman, 1971 (Actinopterygii: Cyprinidae) from the Middle East and North Africa were previously placed in 14 different genus-group taxa (Barbellion, Barbus, Barynotus, Capoeta, Carasobarbus, Cyclocheilichthys, Kosswigobarbus, Labeobarbus, Luciobarbus, Pseudotor, Puntius, Systomus, Tor and Varicorhinus). The generic assignment of several species changed frequently, necessitating a re-evaluation of their taxonomic status. In this study, the genus Carasobarbus is revised based on comparative morphological examinations of about 1300 preserved specimens from collections of several museums and freshly collected material. The species Carasobarbus apoensis, Carasobarbus canis, Carasobarbus chantrei, Carasobarbus exulatus, Carasobarbus fritschii, Carasobarbus harterti, Carasobarbus kosswigi, Carasobarbus luteus and Carasobarbus sublimus form a monophyletic group that shares the following combination of characters: medium-sized barbels with a smooth last unbranched dorsal-fin ray, nine or 10 branched dorsal-fin rays and six branched anal fin-rays; scales large, shield-shaped, with many parallel radii; the lateral line containing 25 to 39 scales; the pharyngeal teeth hooked, 2.3.5-5.3.2 or 2.3.4-4.3.2; one or two pairs of barbels. The species are described in detail, their taxonomic status is re-evaluated and an identification key is provided. A lectotype of Systomus luteus Heckel, 1843 is designated. Carasobarbus Karaman, 1971, Kosswigobarbus Karaman, 1971, and Pseudotor Karaman, 1971 are subjective synonyms, and acting as First Reviser we gave precedence to the name Carasobarbus.
Cyprinidae, SW Asia, NW Africa, taxonomy
The species of the cyprinid genus Carasobarbus Karaman, 1971 are distributed across SW Asia and NW Africa. They occur in all major river systems of the Levant, Mesopotamia, southern Iran, the western and south-western Arabian Peninsula and in northern Morocco. Carasobarbus species are an important element of the ichthyofaunas of these areas.
Research about the Mesopotamian and Levantine representatives of Carasobarbus began as early as the middle of the 19th century. Important ichthyologists of that era, such as A. Valenciennes and J. J. Heckel, were the first to study these fish. One of the most prominent biological collections from the Middle East of this time was made by T. Kotschy from 1836 to 1840. It is stored at the Museum of Natural History of Vienna and encompasses the type specimens of many zoological and botanical taxa (
The objectives of the current study are to (1) define a monophyletic genus that is based on synapomorphic characters, (2) provide a conclusive diagnosis of the genus Carasobarbus, (3) give a detailed re-description of all species based on a sample of specimens large enough to show the intraspecific variability, (4) map the range of each species based on records confirmed by voucher specimens, (5) summarise information on biology, habitat and conservation status of each species, (6) discuss the taxonomic history and current status of each species, (7) provide an identification key. This will form a baseline for a molecular phylogenetic and zoogeographic analysis of Carasobarbus and related genera that is currently in preparation and will be published separately by the first author.
Abbreviations for ichthyological collections follow
Nomenclature of geographic names follows the spelling recommended by the “United States Board on Geographic Names” (http://geonames.usgs.gov/), even though the transcriptions/transliterations of these toponyms are sometimes inconsistent. Wādī or Oued refer to a temporary stream. Nahr, Naẖal, Nehri, Rūdkhāneh or Rūd refer to a permanent river or stream. Buḩayratt, Göl or Daryācheh refer to a lake. ‘Ayn or Aïn refer to a spring. Geographical coordinates in parentheses are original coordinates, given by a publication, the collector or a collection database. Coordinates determined ex post are marked by brackets. Most of these are from the National Geospatial-Intelligence Agency gazetteer (http://geonames.nga.mil/ggmagaz/) and as a consequence, do not refer to the actual site of collection, but to the geographic feature itself. For some of the well known waterbodies and cities the conventional name is used: Euphrates (Nahr al Furāt / Fırat Nehri), Jordan River (HaYarden / Nahr al Urdan), Lake Homs (Buḩayratt Qaţţīnah), Lake Tiberias (Yam Kinneret / Buḩhayratt Ţabarīyā), Orontes (Nahr al ‘Āşī / Asi Nehri), Tigris (Dicle Nehri / Nahr Dijlah), Aleppo (Ḩalab), Damascus (Dimashq), Mosul (Al Mawşil).
Twenty morphometric measurements were taken from specimens straightened whenever necessary; severely damaged and bent specimens were not used. There are some differences in the way of taking measurements (e.g.
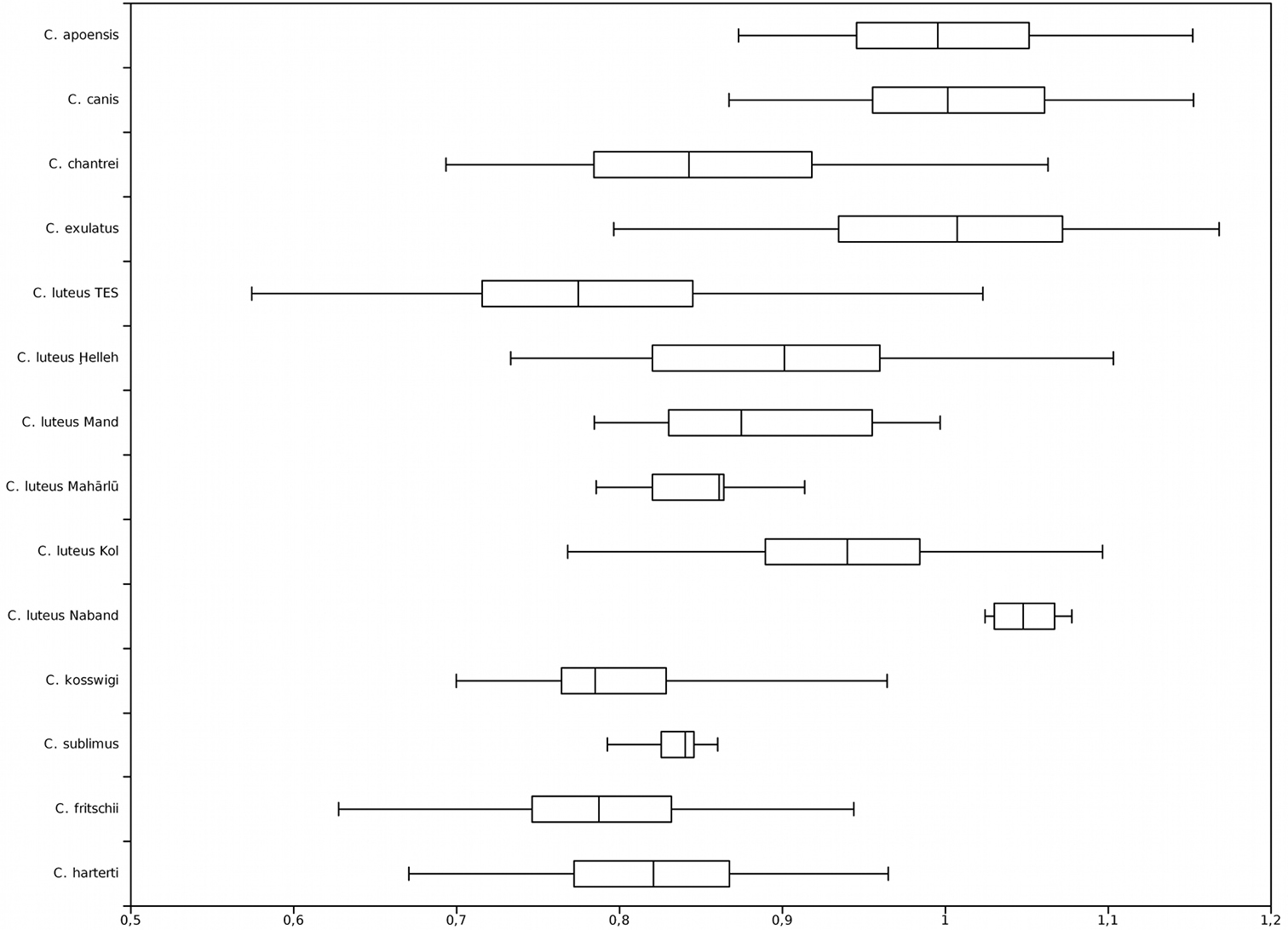
Comparison of morphometric characters of specimens between 50 mm SL and 150 mm SL. All measurements expressed as percentage of SL.
| total length | preanal length | predorsal length | preventral length | head length | caudal peduncle length | body depth | caudal peduncle depth | dorsal fin length | pectoral fin length | ventral fin length | anal fin length | dorsal-fin base length | anal-fin base length | anterior barbel length | posterior barbel length | eye diameter | mouth width | interorbital distance | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carasobarbus apoensis | max | 130.5 | 81.0 | 55.8 | 57.9 | 31.2 | 18.1 | 32.6 | 12.3 | 24.5 | 22.6 | 20.0 | 21.3 | 19.4 | 10.8 | 0.0 | 6.6 | 8.1 | 9.0 | 10.5 |
| min | 121.0 | 76.3 | 48.5 | 51.3 | 26.5 | 11.8 | 25.4 | 10.2 | 16.7 | 18.1 | 16.9 | 16.8 | 15.1 | 7.2 | 0.0 | 2.4 | 5.5 | 5.7 | 8.1 | |
| med | 126.0 | 78.8 | 53.1 | 53.9 | 28.8 | 14.2 | 29.1 | 11.1 | 21.1 | 20.2 | 18.1 | 18.8 | 17.3 | 8.8 | 0.0 | 4.8 | 6.3 | 7.4 | 9.5 | |
| n | 41 | 43 | 44 | 44 | 44 | 44 | 44 | 44 | 42 | 44 | 44 | 43 | 44 | 44 | 0 | 44 | 44 | 44 | 44 | |
| Carasobarbus canis | max | 132.6 | 82.1 | 56.4 | 58.0 | 32.4 | 17.2 | 31.2 | 12.6 | 30.7 | 24.4 | 21.9 | 22.3 | 20.5 | 10.1 | 3.9 | 6.3 | 9.0 | 8.8 | 9.6 |
| min | 121.2 | 75.0 | 47.8 | 50.9 | 26.2 | 12.5 | 26.7 | 9.9 | 18.0 | 18.4 | 16.1 | 14.9 | 16.8 | 7.0 | 0.7 | 2.3 | 5.4 | 6.2 | 7.1 | |
| med | 126.3 | 78.4 | 51.6 | 54.3 | 29.1 | 14.8 | 28.9 | 11.9 | 21.5 | 20.5 | 18.0 | 18.7 | 18.6 | 8.6 | 2.3 | 4.8 | 6.6 | 7.1 | 8.6 | |
| n | 54 | 56 | 56 | 56 | 56 | 56 | 56 | 56 | 46 | 56 | 56 | 56 | 56 | 56 | 52 | 55 | 56 | 54 | 56 | |
| Carasobarbus chantrei | max | 134.8 | 82.3 | 53.9 | 55.3 | 30.0 | 17.1 | 35.8 | 14.0 | 27.5 | 24.4 | 21.8 | 23.8 | 21.2 | 10.2 | 2.5 | 4.7 | 8.9 | 8.1 | 10.3 |
| min | 121.9 | 72.8 | 47.7 | 50.6 | 22.2 | 11.7 | 26.4 | 11.0 | 18.8 | 17.6 | 16.4 | 16.3 | 17.2 | 7.0 | 0.5 | 2.2 | 5.0 | 5.8 | 7.7 | |
| med | 129.7 | 77.9 | 50.6 | 53.0 | 26.1 | 14.5 | 30.8 | 12.6 | 24.6 | 21.7 | 19.8 | 20.4 | 19.2 | 8.8 | 1.1 | 3.4 | 6.9 | 6.9 | 9.2 | |
| n | 81 | 84 | 84 | 84 | 84 | 84 | 84 | 84 | 76 | 82 | 84 | 83 | 84 | 84 | 68 | 84 | 84 | 81 | 84 | |
| Carasobarbus exulatus | max | 134.8 | 82.3 | 53.9 | 55.3 | 30.0 | 17.1 | 35.8 | 14.0 | 27.5 | 24.4 | 21.8 | 23.8 | 21.2 | 10.2 | 2.5 | 4.7 | 8.9 | 8.1 | 10.3 |
| min | 121.9 | 72.8 | 47.7 | 50.6 | 22.2 | 11.7 | 26.4 | 11.0 | 18.8 | 17.6 | 16.4 | 16.3 | 17.2 | 7.0 | 0.5 | 2.2 | 5.0 | 5.8 | 7.7 | |
| med | 129.7 | 77.9 | 50.6 | 53.0 | 26.1 | 14.5 | 30.8 | 12.6 | 24.6 | 21.7 | 19.8 | 20.4 | 19.2 | 8.8 | 1.1 | 3.4 | 6.9 | 6.9 | 9.2 | |
| n | 81 | 84 | 84 | 84 | 84 | 84 | 84 | 84 | 76 | 82 | 84 | 83 | 84 | 84 | 68 | 84 | 84 | 81 | 84 | |
| Carasobarbus fritschii | max | 137.5 | 82.9 | 53.7 | 55.3 | 26.8 | 18.8 | 34.3 | 13.6 | 28.0 | 25.2 | 23.8 | 31.3 | 20.6 | 11.9 | 5.6 | 8.3 | 8.8 | 9.8 | 10.7 |
| min | 121.1 | 73.6 | 44.9 | 47.0 | 18.8 | 10.7 | 24.8 | 10.6 | 19.6 | 19.3 | 15.8 | 18.1 | 15.0 | 6.6 | 1.4 | 2.8 | 4.7 | 5.4 | 6.3 | |
| med | 129.8 | 77.1 | 49.3 | 50.7 | 23.0 | 15.0 | 29.2 | 11.9 | 23.6 | 22.2 | 20.5 | 22.4 | 17.4 | 9.1 | 3.1 | 4.7 | 6.6 | 7.1 | 9.1 | |
| n | 229 | 243 | 244 | 243 | 244 | 244 | 244 | 243 | 196 | 244 | 244 | 242 | 243 | 243 | 242 | 244 | 244 | 244 | 244 | |
| Carasobarbus harterti | max | 140.9 | 78.0 | 53.7 | 55.8 | 27.5 | 18.4 | 31.6 | 13.3 | 31.8 | 25.5 | 24.8 | 24.1 | 19.0 | 10.3 | 9.1 | 9.6 | 9.5 | 7.1 | 9.6 |
| min | 122.6 | 70.9 | 46.5 | 48.5 | 21.2 | 12.3 | 26.8 | 11.8 | 25.8 | 21.5 | 20.4 | 18.9 | 16.5 | 8.4 | 4.5 | 5.5 | 5.9 | 5.2 | 7.4 | |
| med | 131.8 | 75.0 | 49.8 | 51.1 | 24.4 | 16.0 | 29.2 | 12.8 | 28.9 | 23.5 | 22.8 | 21.1 | 18.0 | 9.3 | 6.6 | 7.8 | 7.4 | 6.2 | 8.4 | |
| n | 19 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 11 | 24 | 23 | 23 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | |
| Carasobarbus kosswigi | max | 133.9 | 79.7 | 53.4 | 51.9 | 25.3 | 16.0 | 32.8 | 12.9 | 35.5 | 23.8 | 21.8 | 26.4 | 21.8 | 11.1 | 5.5 | 7.4 | 8.1 | 5.9 | 8.5 |
| min | 127.1 | 75.4 | 47.0 | 48.7 | 22.8 | 11.9 | 26.2 | 10.4 | 26.1 | 20.1 | 19.1 | 20.0 | 18.1 | 8.8 | 2.8 | 3.6 | 4.8 | 3.7 | 7.3 | |
| med | 130.4 | 77.7 | 49.7 | 50.8 | 24.5 | 14.5 | 31.1 | 11.9 | 28.8 | 22.1 | 20.8 | 22.6 | 19.9 | 10.0 | 4.3 | 5.1 | 5.9 | 4.6 | 7.9 | |
| n | 14 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 14 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | |
| Carasobarbus luteus | max | 134.1 | 84.0 | 56.3 | 57.9 | 33.1 | 15.8 | 40.1 | 14.3 | 31.9 | 24.6 | 22.7 | 23.7 | 22.6 | 10.7 | 3.2 | 7.1 | 10.1 | 10.7 | 11.3 |
| min | 120.4 | 74.7 | 47.3 | 48.6 | 21.7 | 8.6 | 26.2 | 11.0 | 17.6 | 17.9 | 16.8 | 15.8 | 14.9 | 6.6 | 0.6 | 2.8 | 0.0 | 5.1 | 7.4 | |
| med | 127.5 | 79.1 | 52.2 | 53.4 | 27.3 | 13.2 | 33.6 | 12.8 | 24.9 | 21.4 | 19.8 | 19.9 | 19.2 | 9.0 | 1.7 | 4.4 | 7.2 | 7.1 | 9.6 | |
| n | 257 | 264 | 268 | 265 | 267 | 266 | 268 | 268 | 241 | 267 | 268 | 266 | 267 | 267 | 41 | 266 | 265 | 233 | 268 | |
| Carasobarbus sublimus | max | 137.9 | 81.4 | 57.0 | 56.8 | 30.1 | 14.8 | 33.4 | 13.8 | 29.9 | 25.5 | 23.8 | 28.4 | 22.1 | 11.5 | 7.0 | 9.7 | 10.0 | 7.6 | 9.0 |
| min | 131.9 | 76.5 | 49.1 | 49.6 | 25.2 | 10.3 | 27.9 | 11.8 | 19.7 | 22.9 | 21.0 | 21.9 | 19.4 | 8.7 | 4.1 | 5.1 | 5.6 | 3.6 | 6.8 | |
| med | 134.4 | 77.9 | 52.5 | 54.2 | 27.7 | 13.0 | 30.6 | 12.8 | 28.3 | 24.3 | 22.2 | 23.9 | 20.7 | 10.2 | 5.4 | 8.0 | 8.9 | 6.4 | 8.4 | |
| n | 16 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 14 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
In addition, seven meristic characters were analysed. The last branched anal- and dorsal-fin rays were counted as one when lying directly adjacent to each other without an interspace. Scales in the lateral line were counted from the first scale with a pore to the last scale on the caudal peduncle (some authors only count to the end of the hypural plate). Scales above the lateral line were counted between the origin of the dorsal fin and the lateral line; the lateral line was not included and a scale on the mid-line of the back was counted as 0.5. Scales below the lateral line were counted between origin of the anal fin to the lateral line; the lateral line was not included and a scale on the mid-line of the belly was counted as 0.5. Scales around the least circumference of the caudal peduncle were counted as one circle of scales around the least circumference of the caudal peduncle. Number of pairs of barbels was counted as two when anterior and posterior pairs are present, counted as one if only posterior pair is present and counted as 1.5 if posterior pair and one single anterior barbel is present.
For counting the number of pharyngeal teeth, the pharyngeal bones were extracted in a subsample of specimens and the pharyngeal teeth counted sometimes only on one side. Lost teeth were counted when the point of insertion was clearly visible. Scales were extracted in the anterior part of the body, above the lateral line.
We did not differentiate between male and female specimens because sex determination was not possible without dissection.
http://species-id.net/wiki/Carasobarbus
Medium-sized cyprinids with an ossified, smooth last unbranched dorsal-fin ray; 9 or 10 branched dorsal-fin rays and 6 branched anal-fin rays; large, shield-shaped scales with numerous parallel radii; the lateral line with 25 to 39 scales; the pharyngeal teeth hooked at their tips, their count being 2.3.5-5.3.2 or 2.3.4-4.3.2; 1 or 2 pairs of barbels present.
Carasobarbus species are evolutionarily hexaploid (
‘Barbus’ grypus, Mesopotamichthys sharpeyi and ‘Barbus’ reinii from the Middle East have five branched rays in the anal fin. The hexaploid species from Africa (Labeobarbus and Varicorhinus), which are the sister group to Carasobarbus and the other species from the Middle East (
Out of the generic names Barbellion, Barbus, Barynotus, Capoeta, Carasobarbus, Cyclocheilichthys, Kosswigobarbus, Labeobarbus, Luciobarbus, Pseudotor, Puntius, Systomus, Tor, and Varicorhinus that were used for this taxon − or its parts − by previous authors, only Carasobarbus, Kosswigobarbus and Pseudotor are available for the genus in question. All other generic names have not been considered, because their type species are not closely related to the species under discussion here (
Within the genus, several species share characters that are potentially synapomorph and elucidate sister group relations. Carasobarbus fritschii and Carasobarbus harterti both have pharyngeal bones with four teeth in the medial row. This character is probably synapomorph, because all other congeners have five teeth in the medial row. This group corresponds to Pseudotor. Carasobarbus kosswigi and Carasobarbus sublimus share the possession of a spatulate lower jaw and a median lobe on the lower lip. The spatulate lower jaw is synapomorph, because no congener and no other closely related species shares this character. The close phylogenetic relationship between Carasobarbus kosswigi and Carasobarbus sublimus is confirmed by genetic analysis (
http://species-id.net/wiki/Carasobarbus_apoensis
Type material. Holotype of Barbus apoensis: BMNH 1976.4.7:166, Saudi Arabia, permanent stream near Khamīs Mushayt (18°17'N, 42°34'E), F. Tippler, 12 Dec 1968.
Paratypes of Barbus apoensis: BMNH 1976.4.7:167-171, 5, same data as holotype. - BMNH 1976.4.7:172-175, 4, Saudi Arabia, upper Wādī Turabah near Aţ Ţā’if (22°56'N, 40°54'E), G. Popov. - BMNH 1971.2.11:1-2, 2, Saudi Arabia, intermittent watercourse in Wādī Adamah (19°53'N, 41°57'E), J. P. Mandaville, 27 Oct 1969.
Non-type material. Endorheic darinages. BMNH 1980.7.1:15, 1, Saudi Arabia, Wādī Habayaba between Aţ Ţā’if and Ash Shafā [N21°11’, E 40°24'], A. Farag, 1980. - SMF 30167, 3; SMF 30170, 10 Saudi Arabia, Wādī Būwah (20°45'N, 41°8'E), F. Krupp and W. Schneider, 21 Mar 1990. - SMF 30169, 6; SMF 33147, 4, Saudi Arabia, Wādī Būwah (20°44'N, 41°7'E), F. Krupp and W. Schneider, 21 Mar 1990. - SMF 30168, 6; SMF 30171, 9, Saudi Arabia, Wādī Turabah (20°32'N, 41°17'E), F. Krupp and W. Schneider, 20 Mar 1990.
Streams draining towards the Red Sea. CMNFI 87-0135, 1; CMNFI 87-0137, 4, Saudi Arabia, Wādī Hadīyah (25°34'N, 38°41'E). - SMF 33149, 1, Saudi Arabia, Wādī Ḩaqqaq (22°49'N, 39°22'E), W. Büttiker, 5/6 May 1983. - SMF 33148, 2, Saudi Arabia, Wādī ‘Ilyab (20°5'N, 40°54'E), H. Felemban and J. Gasparetti, 28 Oct 1983. - SMF 33539, 3, Saudi Arabia, Wādī ‘Ilyab (20°7'N, 40°57E), W. Büttiker, 10−11 Nov 1983.
Unknown drainage system. SMF 33146, 4, Saudi Arabia, Al Ḩijāz, W. Büttiker.
One pair of barbels, usually 10 branched rays in the dorsal fin, 27 to 32 scales in the lateral line, usually 12 scales around the least circumference of the caudal peduncle, last unbranched ray of dorsal fin shorter than head.
The body depth is comparatively low and a nuchal hump is present in adults but not developed in juveniles. The height of the caudal peduncle is relatively low (Table 1). The dorsal and ventral fins are usually positioned behind the middle of the body. The head is elongate with a straight or slightly concave dorsal profile. The ventral profile of the head is slightly convex. (Figs 1, 2). The head length is about equal to the body depth. The mouth is broad and terminal or slightly sub-terminal with one pair of barbels (Fig. 3, Table 2). Only one out of 65 specimens had two pairs of barbels and in one specimen a single anterior barbel was present. The eyes are in the anterior half of the head and slightly protuberant. The morphometric characters are summarised in Table 1.
Carasobarbus apoensis, holotype (BMNH 1976.4.7:166) from a permanent stream near Khamīs Mushayt, © The Natural History Museum, London.
Carasobarbus apoensis, live specimen from Wādī Turabah.
Ventral view of the head and chest. A Carasobarbus apoensis (SMF 30167, 108.6 mm SL) B Carasobarbus canis (SMF 33135, 108.3 mm SL) C Carasobarbus chantrei (SMF 33133, 122.9 mm SL) D Carasobarbus exulatus (SMF 33109, 103.7 mm SL) E Carasobarbus fritschii (SMF 33446, 89.6 mm SL) F Carasobarbus harterti (SMF 33368, 93.6 mm SL) G Carasobarbus kosswigi (SMF 30173, 107.1 mm SL) H Carasobarbus luteus (SMF 30176, 120.7 mm SL) I Carasobarbus sublimus (SMF 33118, 80.2 mm SL), pictures resized to facilitate comparison.
Number of pairs of barbels.
| n | 1 | 1, 5 | 2 | |
|---|---|---|---|---|
| Carasobarbus apoensis | 65 | 63 | 1 | 1 |
| Carasobarbus canis | 89 | 4 | 1 | 84 |
| Carasobarbus chantrei | 157 | 5 | 6 | 146 |
| Carasobarbus exulatus | 83 | 83 | ||
| Carasobarbus fritschii | 299 | 2 | 297 | |
| Carasobarbus harterti | 30 | 30 | ||
| Carasobarbus kosswigi | 23 | 23 | ||
| Carasobarbus luteus | 421 | 365 | 9 | 47 |
| Naband population | 10 | 10 | ||
| Carasobarbus sublimus | 18 | 18 |
The dorsal fin and its base are rather short. It usually has four unbranched and 10 branched rays (Table 3). The last unbranched ray is considerably shorter than the head (Fig. 4), weakly ossified, and its distal part is flexible. The anal fin has three unbranched and six branched rays (Table 4). Pectoral and ventral fins are relatively short (Table 1).
Number of branched dorsal-fin rays.
| n | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|
| Carasobarbus apoensis | 66 | 2 | 63 | 1 | ||
| Carasobarbus canis | 90 | 5 | 85 | |||
| Carasobarbus chantrei | 196 | 21 | 164 | 11 | ||
| Carasobarbus exulatus | 110 | 8 | 99 | 3 | ||
| Carasobarbus fritschii | 297 | 1 | 23 | 268 | 5 | |
| Carasobarbus harterti | 30 | 30 | ||||
| Carasobarbus kosswigi | 23 | 3 | 20 | |||
| Carasobarbus luteus | 441 | 1 | 23 | 411 | 6 | |
| Naband population | 10 | 1 | 9 | |||
| Carasobarbus sublimus | 18 | 2 | 16 |
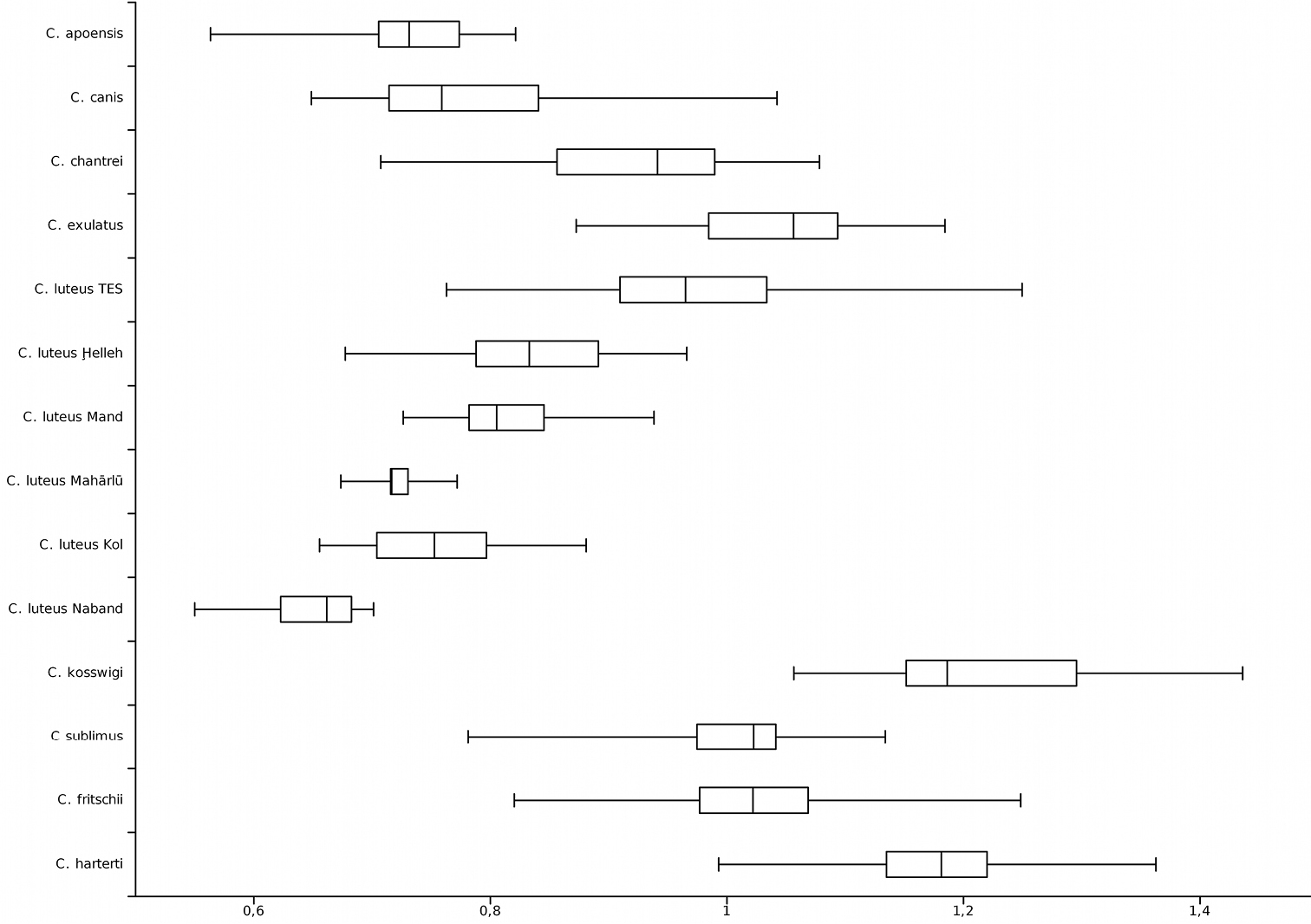
Last unbranched dorsal-fin ray length / head length; TES = Tigris-Euphrates system.
Number of branched anal-fin rays.
| n | 5 | 6 | 7 | |
|---|---|---|---|---|
| Carasobarbus apoensis | 65 | 65 | ||
| Carasobarbus canis | 90 | 2 | 88 | |
| Carasobarbus chantrei | 197 | 3 | 194 | |
| Carasobarbus exulatus | 109 | 3 | 106 | |
| Carasobarbus fritschii | 296 | 3 | 293 | |
| Carasobarbus harterti | 30 | 29 | 1 | |
| Carasobarbus kosswigi | 23 | 23 | ||
| Carasobarbus luteus | 439 | 3 | 435 | 1 |
| Naband population | 10 | 10 | ||
| Carasobarbus sublimus | 18 | 18 |
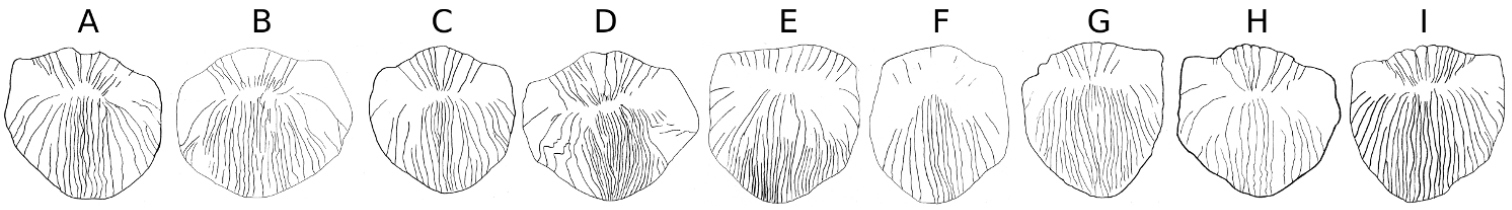
Carasobarbus apoensis has 27 to 32 scales in the lateral line (Table 5), usually 4.5 scales above the lateral line (Table 6), 3.5 or 4.5 scales below the lateral line (Table 7) and 12 scales around the least circumference of the caudal peduncle (Table 8). The scales are shown in Fig. 5.
Lateral line scale count.
| n | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carasobarbus apoensis | 60 | 1 | 9 | 15 | 20 | 14 | 1 | |||||||||
| Carasobarbus canis | 74 | 1 | 3 | 16 | 19 | 12 | 13 | 10 | ||||||||
| Carasobarbus chantrei | 168 | 5 | 11 | 31 | 48 | 36 | 29 | 7 | 1 | |||||||
| Carasobarbus exulatus | 79 | 1 | 3 | 17 | 18 | 24 | 13 | 3 | ||||||||
| Carasobarbus fritschii | 264 | 1 | 12 | 21 | 39 | 75 | 58 | 36 | 15 | 4 | 3 | |||||
| Carasobarbus harterti | 24 | 1 | 5 | 9 | 4 | 4 | 1 | |||||||||
| Carasobarbus kosswigi | 19 | 1 | 7 | 2 | 3 | 5 | 1 | |||||||||
| Carasobarbus luteus | 390 | 11 | 52 | 79 | 120 | 84 | 29 | 9 | 5 | 1 | ||||||
| Naband population | 8 | 1 | 3 | 3 | 1 | |||||||||||
| Carasobarbus sublimus | 11 | 4 | 3 | 4 |
Number of scales above the lateral line.
| n | 3, 5 | 4 | 4, 5 | 5 | 5, 5 | 6 | 6, 5 | 7 | |
|---|---|---|---|---|---|---|---|---|---|
| Carasobarbus apoensis | 60 | 2 | 45 | 7 | 6 | ||||
| Carasobarbus canis | 82 | 48 | 11 | 20 | 3 | ||||
| Carasobarbus chantrei | 171 | 4 | 1 | 147 | 6 | 13 | |||
| Carasobarbus exulatus | 79 | 3 | 70 | 5 | 1 | ||||
| Carasobarbus fritschii | 276 | 15 | 226 | 35 | |||||
| Carasobarbus harterti | 28 | 4 | 24 | ||||||
| Carasobarbus kosswigi | 21 | 8 | 5 | 7 | 1 | ||||
| Carasobarbus luteus | 389 | 6 | 2 | 315 | 19 | 46 | 1 | ||
| Naband population | 8 | 8 | |||||||
| Carasobarbus sublimus | 17 | 16 | 1 |
Number of scales below the lateral line.
| n | 3 | 3, 5 | 4 | 4, 5 | 5 | 5, 5 | 6 | 6, 5 | |
|---|---|---|---|---|---|---|---|---|---|
| Carasobarbus apoensis | 57 | 14 | 41 | 2 | |||||
| Carasobarbus canis | 80 | 2 | 3 | 65 | 1 | 9 | |||
| Carasobarbus chantrei | 173 | 1 | 84 | 3 | 84 | 1 | |||
| Carasobarbus exulatus | 79 | 24 | 1 | 51 | 3 | ||||
| Carasobarbus fritschii | 286 | 7 | 3 | 151 | 5 | 117 | 1 | 2 | |
| Carasobarbus harterti | 29 | 1 | 10 | 18 | |||||
| Carasobarbus kosswigi | 23 | 4 | 3 | 15 | 1 | ||||
| Carasobarbus luteus | 384 | 2 | 125 | 16 | 231 | 9 | 1 | ||
| Naband population | 8 | 8 | |||||||
| Carasobarbus sublimus | 17 | 1 | 13 | 1 | 2 |
Number of scales around the least circumference of the caudal peduncle.
| n | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carasobarbus apoensis | 60 | 58 | 2 | |||||||||
| Carasobarbus canis | 85 | 80 | 1 | 4 | ||||||||
| Carasobarbus chantrei | 168 | 4 | 7 | 110 | 27 | 20 | ||||||
| Carasobarbus exulatus | 87 | 1 | 6 | 80 | ||||||||
| Carasobarbus fritschii | 253 | 3 | 12 | 212 | 26 | 23 | 1 | |||||
| Carasobarbus harterti | 28 | 2 | 4 | 3 | 18 | 1 | ||||||
| Carasobarbus kosswigi | 21 | 1 | 2 | 10 | 3 | 5 | ||||||
| Carasobarbus luteus | 408 | 3 | 2 | 399 | 4 | |||||||
| Naband population | 9 | 8 | 1 | |||||||||
| Carasobarbus sublimus | 17 | 17 |
Striation pattern of scales taken from anteriour part of the boby above lateral line. A Carasobarbus apoensis B Carasobarbus canis C Carasobarbus chantrei D Carasobarbus exulatus E Carasobarbus fritschii F Carasobarbus harterti G Carasobarbus kosswigi H Carasobarbus luteus I Carasobarbus sublimus.
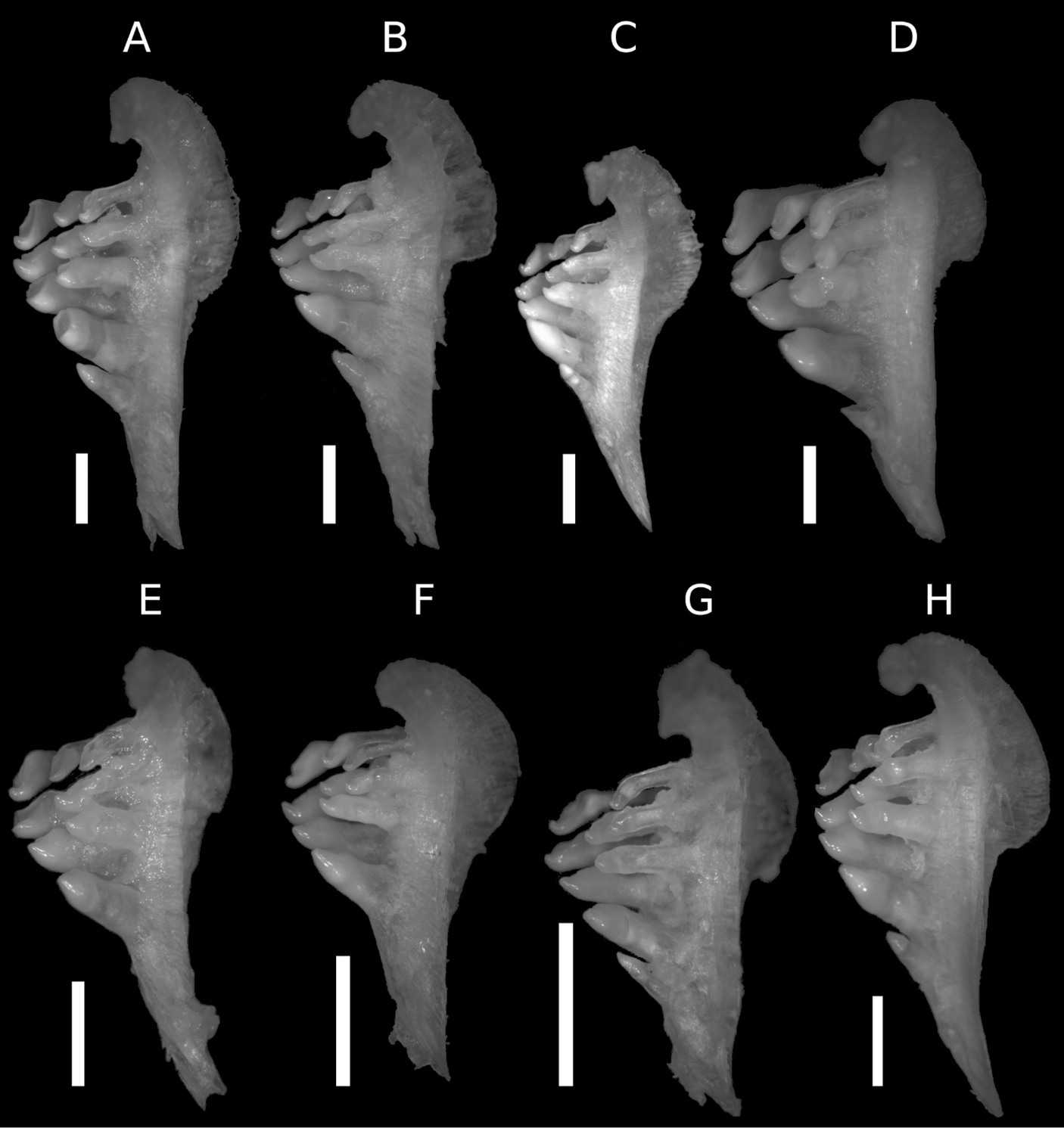
The pharyngeal teeth count is 2.3.5- in 12 specimens, -5.3.2 in two specimens and 1.3.5- in one specimen. The pharyngeal teeth are hooked at their tips (Fig. 6).
Pharyngeal bone. A Carasobarbus apoensis (SMF 30168, 190.1 mm SL) B Carasobarbus canis (SMF 30175, 168.7 mm SL) C Carasobarbus chantrei (SMF 33133, 165.9 mm SL) D Carasobarbus exulatus (SMF 33107, 170.1 mm SL) E Carasobarbus fritschii (SMF 33405, 147.2 mm SL) F Carasobarbus harterti (SMF 33396, 105.9 mm SL) G Carasobarbus kosswigi (SMF 30174, 141.5 mm SL) H Carasobarbus luteus (SMF 30179, 143.4 mm SL). Scale bar = 3 mm.
Live colouration is golden with olive fins. The upper side is darker than the belly (Fig. 2). In ethanol-preserved specimens the upper side is dark, the belly yellow and the fins are grey or yellow (Fig. 1). Juveniles have a dark lateral spot on the caudal peduncle.
The maximum length observed in the material examined is 288 mm SL.
Carasobarbus apoensis differs from all congeners, except Carasobarbus luteus, by having one rather than two pairs of barbels. For a comparison with Carasobarbus luteus populations see below.
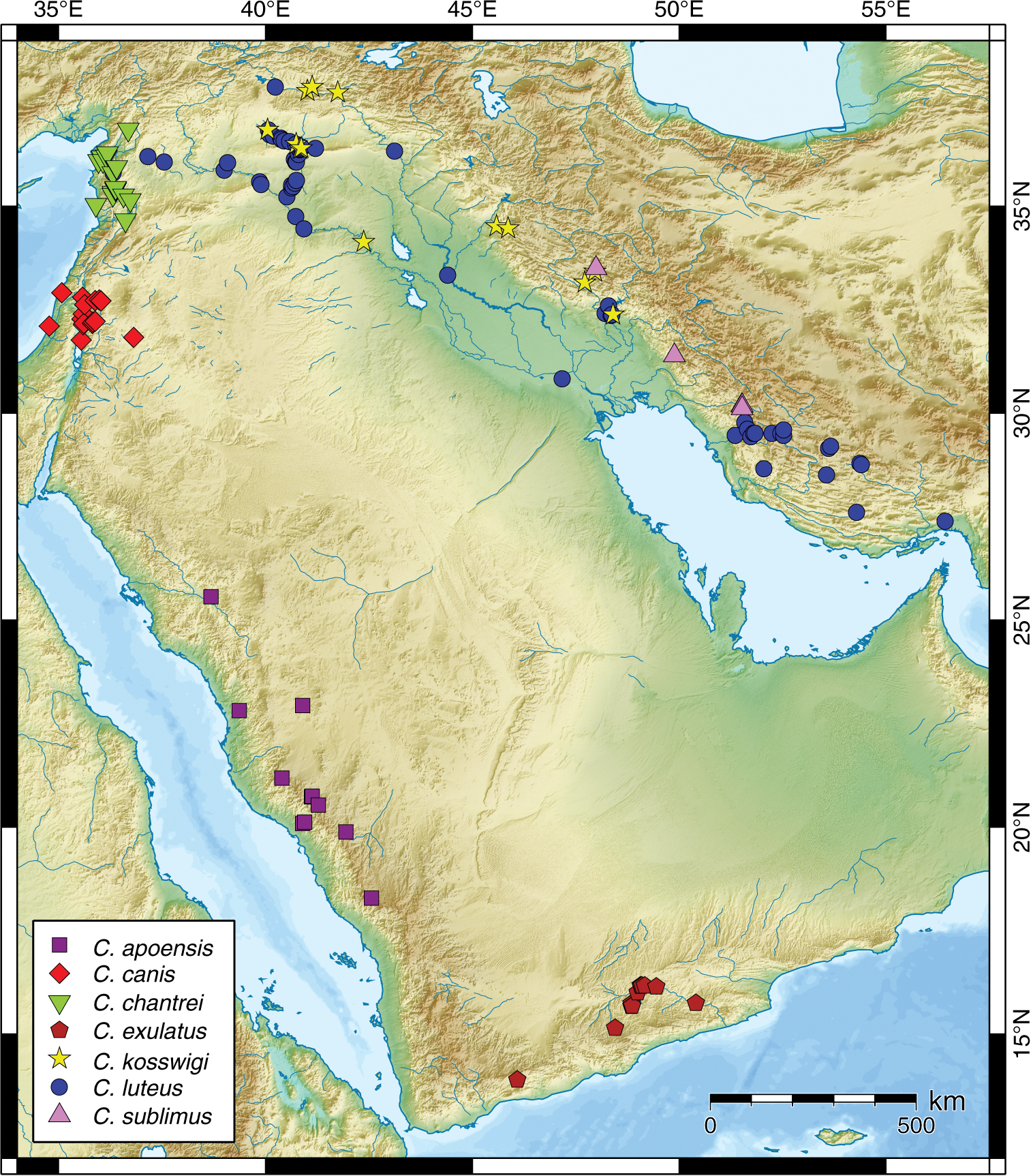
Distribution. Carasobarbus apoensis occurs in the Al Ḩijāz mountain range in wadis draining either inland or towards the Red Sea (Fig. 7). It is endemic to Saudi Arabia.
Map of the distribution of Carasobarbus apoensis, Carasobarbus canis, Carasobarbus chantrei, Carasobarbus exulatus, Carasobarbus kosswigi, Carasobarbus luteus, and Carasobarbus sublimus.
This species inhabits the upper courses of wadis, which are characterised by strong seasonal fluctuations in water levels, temperature and other physiochemical parameters.
Carasobarbus apoensis is rated Least Concern and still occurs in large numbers, but abstraction of large specimens by recreational fishing, water abstraction and habitat loss might become problematic for this species (
Carasobarbus apoensis was originally described from Khamīs Mushayt, Wādī Turabah and Wādī Adamah as a member of the genus Barbus (
Carasobarbus apoensis is very closely related to Carasobarbus luteus (KB unpublished data).
http://species-id.net/wiki/Carasobarbus_canis
Type material. Lectotype of Barbus canis: MNHN 0000-1413, 1, Jordan River [31°46'N, 35°33'E], Bové, 1833 (designated by
Paralectotype of Barbus canis: MNHN 0000-3944, 1, same data as lectotype.
Holotype of Barbus beddomii: BMNH 1863.11.3:5, 1, Lake Tiberias [32°48'N, 35°35'E], T. W. Beddome.
Non-type material. Jordan River Drainage. SMF 14075, 2, Lake Tiberias (32°48'N, 35°35'E), M. Goren, 15 Mar 1968. - SMF 33134, 16, Syria, Nahr al Yarmūk near Jallayn (32°44'21"N, 35°58'56"E), N. Alwan et al., 16 Oct 2008. - SMF 24464, 1, Jordan, Nahr al Yarmūk near Maqārin (32°43'N, 35°53'E), F. Krupp and W. Schneider, 23 Sep 1985. - SMF 30175, 11, Syria, Lake Muzayrīb [32°42'40"N, 36°1'39"E], F. Krupp and W. Schneider, 12 Apr 1989. - SMF 33135, 17, Jordan, Wadi al-‘Arab near the dam (32°37'6"N, 35°37'46"E), N. Alwan et al., 25 Oct 2008. - SMF 17123, 16, Wādī al Yābis (32°24'N, 35°36'E), F. Krupp and W. Schneider, 23 Jul 1980. - ZMH H 2343, 3, Jordan, Wādī Kufrinjah (32°16'25"N, 35°33'42"E). - SMF 24344, 3; SMF 24345, 17, Jordan, Nahr az Zarqā’ (32°12'N, 35°50'E), F. Krupp and W. Schneider, 22 Jul 1980. - SMF 24339, 3, Jordan, Nahr az Zarqā’ (32°10'N, 35°37'E), F. Krupp and W. Schneider, 21 Jul 1980. - SMF 24340, 1, Jordan, Nahr az Zarqā’ near Sadd al Malik Talal (32°10'N, 35°49'E), F. Krupp and W. Schneider, 22 Jul 1980. - SMF 24331, 7; SMF 24346, 3, Jordan, Nahr al Yarmūk channel (32°08'N, 35°36'E), F. Krupp and W. Schneider, 21 Jul 1980. - NMW 53961, 1, Jordan River [31°46'N, 35°33'E], Cenoni, Dec 1898.
Azraq Oasis. BMNH 1956.2.24:15-16, 2; BMNH 1965.11.24:2, 1, Jordan, wetland near Azraq ash Shīshān [31°50'N, 36°49'E].
Coastal rivers of the Mediterranean Sea. BMNH 1949.9.16:124, 1, Israel, Naẖal Na‘aman [32°54'42"N, 35°4'50"E]. - NMW 22367, 1, Israel, Naẖal Na‘aman [32°54'42"N, 35°4'50"E], H. Steinitz, 21 Oct 1955. - SMF 9229, 1, Israel, Naẖal Yarqon [32°6'7"N, 34°46'32"E].
Two pairs of barbels, 29 to 35 scales in the lateral line and usually 12 scales around the least circumference of the caudal peduncle, last unbranched ray of dorsal fin shorter than head.
The body is low. A nuchal hump is present in adults but absent in juveniles. The largest body depth is at the origin of the dorsal fin. The head is long, rather low and fairly narrow with straight dorsal and convex ventral profile (Figs 8, 9). The head length approximately equals the body depth. The mouth is terminal or slightly subterminal. Two pairs of barbels are present (Table 2). The lips are smooth and thin (Fig. 3). The eyes are at the end of the anterior half of the head. The morphometric characters are summarised in Table 1.
Carasobarbus canis, lectotype (MNHN 1413) from Jordan River.
Carasobarbus canis, live specimen from Wadi al-‘Arab.
Pectoral, ventral, dorsal and anal fins are comparatively short (Table 1). The dorsal fin usually has four unbranched and 10 branched rays (Table 3). The last unbranched ray is ossified and its distal part is flexible. It is usually markedly shorter than the head (Fig. 4). The anal fin usually has three unbranched and six branched fin rays (Table 4).
There are 29 to 35 scales in the lateral line (Table 5), usually 4.5 or 5.5 scales above the lateral line (Table 6), usually 4.5 scales below the lateral line (Table 7) and usually 12 scales around the least circumference of the caudal peduncle (Table 8). The scales are shown in Fig. 5.
The pharyngeal teeth count is 2.3.5-5.3.2 in 23 specimens, 2.3.3-5.3.2 in one specimen, 2.3.5- in one specimen and -5.3.2 in one specimen. The pharyngeal teeth are hooked at their tips (Fig. 6).
Live specimens are silvery to bronze coloured. The posterior third of the body and the fins are distinctly yellow in many specimens (Fig. 9). Ethanol-preserved specimens are brownish yellow and the back is only slightly darker than the rest of the body (Fig. 8). The fins are brownish yellow. Juveniles have a dark lateral spot on the caudal peduncle.
Carasobarbus canis differs from Carasobarbus apoensis and Carasobarbus luteus in having two pairs of barbels vs. one, from Carasobarbus kosswigi and Carasobarbus sublimus in having a crescent-shaped lower lip without median lobe vs. a spatulate lower lip with median lobe, from Carasobarbus exulatus in modally having 10 branched dorsal-fin rays vs. nine and from Carasobarbus chantrei, Carasobarbus fritschii and Carasobarbus harterti in modally having 10 scales around the least circumference of the caudal peduncle vs. 14 or 16.
Carasobarbus canis occurs in the Jordan River system (Fig. 7). There are only few records from coastal rivers of the Mediterranean Sea (Naẖal Na‘aman and Naẖal Yarqon). A recent treatment of the inland water fish communities of Israel does not report Carasobarbus canis from coastal rivers (
Carasobarbus canis inhabits a wide range of rivers, lakes and ponds (
Catches in Lake Tiberias are declining (
Carasobarbus canis was described from the Jordan River as a member of the genus Barbus (
http://species-id.net/wiki/Carasobarbus_chantrei
Type material. Lectotype of Labeobarbus chantrei: MNHN A-3866, Turkey, Amik Gölü [36°12'24"N, 36°9'26"E], H. Chantre, 1881 (designated by
Paralectotypes of Labeobarbus chantrei: MNHN A-3937, 1, same data as lectotype. - MNHN A-3938, 2; MNHN A-3939, 3; MNHN A-3940, 1, Syria, Ḩamāh [35°9'0"N, 36°43'59"E], H. Chantre, 1881.
Non-type material. Orontes River drainage. MNHN B-2977, 1, Syria, Orontes, A. Gruvel, 1829. - BMNH 1934.1.25:4, 1, Syria, Orontes. - FSJF 2311, 11, Turkey, Karasu Çayı below dam of Tahtaköprü Barajı (36°51'7"N, 36°41'10"E), M. Özulug and J. Freyhof, 7 Nov 2007. - SMF 17115, 8, Turkey, Orontes, 8 km E of Hatay (36°17'N, 36°11'E), J. Winkler and B. Koster, 20 Sep 1982. - CMNFI 88-0019, 1, Turkey, 8 km southwest of Hatay (36°11'N, 36°3'E). - SMF 17110, 4, Turkey, tributary to Orontes (36°11'N, 36°3'E), F. Krupp, 23 Aug 1978. - SMF 17122, 2, Turkey, 2 km southeast of Samandağı (36°6'N, 35°58'E), F. Krupp, 23 Aug 1978. - FSJF uncatalogued, 16, Turkey, at Sinanlı (36°5'51"N, 36°4'43"E), M. Özulug and J. Freyhof, 8 Nov 2007. - SMF 33130, 40, Syria, near Mashra’a el Būz (35°57'3"N, 36°23'45"E), N. Alwan et al., 8 Oct 2008. - SMF 33131, 58, Syria, ‘Ayn az Zarqa (35°56'40"N, 36°24'9"E), N. Alwan et al., 8 Oct 2008. - SMF 17107, 1, Syria, Jisr ash Shughūr (35°48'N, 36°19'E), F. Krupp, 20 Aug 1980. - SMF 17109, 2, Syria, main bridge at Jisr ash Shughūr (35°48'N, 36°19'E), F. Krupp, 19 Aug 1978. - CMNFI 88-0018, 4, Syria, ‘Ayn Zaqa (35°27'N, 36°23'E). - SMF 17114, 1; SMF 17121, 7, Syria, ‘Ayn Zaqa (35°27'N, 36°23'E), F. Krupp, 25–27 Mar 1979. - BMNH 1968.12.13:188-190, 3, Syria, spring lake at Qal‘at al Maḑīq [35°25'N, 36°23'E]. - SMF 17120, 7, Syria, aquaculture pond near Qal‘at al Maḑīq (35°25'N, 36°23'E), F. Krupp, 8 Aug 1978. - SMF 33132, 5, Syria, stream at Qal‘at al Jarras (35°19'49"N, 36°18'38"E), N. Alwan et al., 12 Oct 2008. - SMF 17111, 6, Syria, ‘Ašārna (35°17'N, 36°19'E), F. Krupp, 11 Aug 1978. - SMF 17117, 5, Syria, near Shayzar (35°16'N, 36°34'E), F. Krupp, 27 Mar 1979. - SMF 24349, 3, Syria, Shayzar (35°16'N, 36°34'E), F. Krupp and W. Schneider, 17 Aug 1980. - SMF 17118, 1, Syria, 200 m below western outlet of Lake Homs (34°40'N, 36°37'E), F. Krupp and W. Schneider, 3 Aug 1978. - SMF 17119, 5, Syria, western outlet of Lake Homs (34°40'N, 36°37'E), F. Krupp and W. Schneider, 3 Aug 1978. - SMF 33133, 24, Syria, Lake Homs at Qaţţīnah (34°39'43"N, 36°37'6"E), N. Alwan et al., 13 Oct 2008.
Mediterranean coastal rivers. SMF 31669, 1; SMF 31670, 1, Syria, Nahr Marqīyah (35°1'50"N, 35°54'18"E), N. Alwan et al., 10 Oct 2008.
Tigris-Euphrates system. SMF 12966, 1, Turkey, Balıklıgöl at Şanlıurfa [37°8'52"N, 38°47'4"E], L. Lortet, 1884.
Two pairs of barbels, 31 to 38 scales in the lateral line and usually 14 to 16 scales around the least circumference of the caudal peduncle, last unbranched dorsal-fin ray equal to or shorter than head.
The body is comparatively high-backed and laterally compressed in mid-sized specimens but low-backed and almost cylindrical in large specimens. In large specimens a pronounced nuchal hump is present, in smaller specimens it is only weakly developed or absent. The maximum body depth is at the origin of the dorsal fin. The head is short and blunt with a convex ventral profile and a slightly convex to straight dorsal profile (Figs 10, 11). The mouth is terminal or slightly sub-terminal with two pairs of short barbels (Table 2).The body depth is usually greater than the head length (Fig. 12).The eyes are slightly protuberant and lie at the end of the anterior half of the head. The morphometric characters are summarised in Table 1.
Carasobarbus chantrei, paralectotype (MNHN A-3939) from Orontes at Ḩamāh.
Carasobarbus chantrei, live specimen from Buḩayratt Qaţţīnah.
Head length / body depth; TES = Tigris-Euphrates system.
The dorsal fin usually has four unbranched and nine to 11 branched rays (Table 3). The last unbranched ray is ossified but not very thick and flexible in its distal part. It is usually shorter than the head (Fig. 4). The anal fin usually has three unbranched and five or six branched rays (Table 4).
There are 31 to 38 scales in the lateral line (Table 5), 4.5 to 6.5 scales above the lateral line (Table 6), four to six scales below the lateral line (Table 7) and 12 to 16 scales around the least circumference of the caudal peduncle (Table 8). The scales are shown in Fig. 5.
The pharyngeal teeth count is 2.3.5-5.3.2 in two specimens, 2.3.5- in 11 specimens, -5.3.2 in two specimens and 1.3.5- in one specimen. The pharyngeal teeth are hooked at their tips (Fig. 6).
Small live specimens are silvery; larger specimens are silvery or bronze coloured and sometimes have yellow pectoral and ventral fins (Fig. 11). Small ethanol-preserved specimens are silvery with a somewhat darker back and a salmon pink hue. Juveniles have a dark lateral spot on the caudal peduncle. Ethanol-preserved adults are yellow-brown and the back is only slightly darker than the rest of the body (Fig. 10).
The maximum length observed in the material examined is 385 mm SL.
Carasobarbus chantrei differs from Carasobarbus apoensis, Carasobarbus canis, Carasobarbus exulatus, Carasobarbus luteus and Carasobarbus sublimus in having 31 to 38 scales in the lateral line vs. 27 to 32, 29 to 35, 26 to 32, 25 to 33 and 27 to 29 respectively and modally 14 scales around the least circumference of the caudal peduncle vs. 12. It differs from Carasobarbus kosswigi and Carasobarbus sublimus in having a crescent-shaped lower lip without median lobe vs. a spatulate lower lip with median lobe and from Carasobarbus exulatus, Carasobarbus fritschii and Carasobarbus harterti in modally having 10 branched dorsal-fin rays vs. nine.
Carasobarbus chantrei occurs in the Orontes river drainage system (Fig. 7). Two juvenile specimens where collected in Nahr Marqīyah, a coastal river in Syria. This species had never before been reported from this location (
Carasobarbus chantrei occurs in a wide range of habitats stretching from stagnant waters of lakes to rapidly flowing river courses.
Carasobarbus chantrei is utilised as food fish locally but is increasingly replaced by carp (
Carasobarbus chantrei was described from the Orontes and placed in Labeobarbus by
http://species-id.net/wiki/Carasobarbus_exulatus
Type material. Holotype of Barbus exulatus: BMNH 1976.4.7:299, Yemen, Wādī Ḩaḑramawt at Qasam (16°10'N, 49°4'E), W. A. King-Webster.
Paratypes of Barbus exulatus: BMNH 1976.4.7:308, 1; BMNH 1976.4.7:300-307, 8, same data as holotype. - BMNH 1976.4.7:328-329, 2; BMNH 1976.4.7:330-331, 2, Yemen, Wādī ‘Idim/Wādī Ḩaḑramawt at Ghuraf (16°0'N, 49°0'E), W. A. King-Webster. - BMNH 1976.4.7:309, 1; BMNH 1976.4.7:310-318, 9; BMNH 1976.4.7:319-327, 9, Yemen, Wādī Ḩaḑramawt at Ghayl ‘Umar (15°44'N, 48°51'E), W. A. King-Webster. - BMNH 1976.4.7:332-333 probably Wādī Marrān in Wādī Aḩwar system [13°53'51"N, 46°05'14"E], G. Popov, 2 Aug 1962.
Non-type material. Wādī Ḩaḑramawt/al Masīlah drainage. BMNH 1976.5.17:9-10, 2, Yemen, Wādī al Khūn (16°10'N, 49°10'E). - SMF 33108, 10, Yemen, Wādī al Khūn (16°9'51"N, 49°6'2"E), F. Krupp et al., 3 Jun 2005. - SMF 33109, 17, Yemen, Wādī al Khūn (16°9'45"N, 49°4'46"E), F. Krupp et al., 3 Jun 2005. - SMF 33110, 14, Yemen, Wādī al Masīlah near Fughmah (16°8'36"N, 49°27'7"E), F. Krupp et al., 4 Jun 2005. - SMF 33111, 1, Yemen, Wādī al Masīlah at al Hind (15°44'53"N, 50°24'32"E), F. Krupp et al., 5 Jun 2005. - SMF 33106, 8, Yemen, Wādī ‘Idim at Ghayl ‘Umar near Arḑ ar Raydah (15°40'51"N, 48°51'59"E), F. Krupp et al., 2 Jun 2005. - SMF 33107, 11, Yemen, Wādī ‘Idim near Ghayl ‘Umar (15°40'10"N, 48°51'4"E), F. Krupp et al., 2 Jun 2005. -SMF 33105, 13, Yemen, Wādī Mara in Wādī Daw‘an system (15°8'36"N, 48°26'58"E), F. Krupp et al., 31 May 2005.
Dorsal fin with 9 branched rays in most specimens; last unbranched ray of dorsal fin as long as or longer than head; 2 pairs of barbels; 26 to 32 scales in the lateral line and usually 12 scales around the least circumference of the caudal peduncle.
The body is not particularly high backed and the maximum body depth is at the origin of the dorsal fin or slightly in front of it (Fig. 13). A nuchal hump is present in adult specimens (Fig. 14) but absent in juveniles (Fig. 15). The caudal peduncle is slender. The head profile is convex ventrally and straight dorsally. The body depth is about the same as the head length (Fig. 12). In specimens below 100 mm SL, the head is rather narrow, in larger specimens it becomes wider. The mouth is subterminal and comparatively narrow. Two pairs of barbels are present (Table 2), the posterior one is rather long. The eyes are at the end of the anterior half of the head and slightly protuberant. The morphometric characters are summarised in Table 1.
Carasobarbus exulatus, holotype (BMNH 1976.4.7:299) from Wādī Ḩaḑramawt at Qasam, © The Natural History Museum, London, photo P. Hurst.
Adult Carasobarbus exulatus, live specimen from Wādī al Khūn.
Juvenile Carasobarbus exulatus, live specimen from Wādī al Khūn.
The dorsal fin is long and usually has four unbranched and eight to 10 branched rays (Table 3). The last unbranched ray is strongly ossified and only the tip is flexible. Its length is about the same as the head length (Fig. 4). The anal fin is long, usually has three unbranched and five or six branched rays (Table 4).
There are 26 to 32 scales in the lateral line (Table 5), 4 to 5.5 scales above the lateral line (Table 6), 3.5 to five scales below the lateral line (Table 7) and 10 to 12 scales around the least circumference of the caudal peduncle (Table 8). The scales are shown in Fig. 5.
The pharyngeal teeth count is 2.3.5-5.3.2 in one specimen, 2.3.5- in 16 specimens, -5.3.2 in one specimen and 2.3.4- in one specimen. The pharyngeal teeth are hooked at their tips (Fig. 6).
In live specimens and freshly preserved specimens the back and the sides are grey to golden, the belly is yellowish white and the fins are sometimes golden to orange (Fig. 15). Preserved specimens have a dark back and a lighter belly, the fins are whitish or greyish. Juveniles have a dark spot on the sides of the caudal peduncle.
The maximum length observed in the material available is 288 mm SL.
Carasobarbus exulatus differs from all congeners, except Carasobarbus fritschii and Carasobarbus harterti in modally having nine instead of 10 branched dorsal-fin rays. It differs from Carasobarbus fritschii and Carasobarbus harterti in modally having 12 scales around the least circumference of the caudal peduncle vs. 16 and in having 26 to 32 scales the lateral line vs. 30 to 39 and 31 to 38 respectively.
This species is endemic to Yemen and occurs in Wādī Ḩaḑramawt / Wādī al Masīlah and its pleistocene tributaries (
Locality data for BMNH 1976.4.7:332-333 is given as “Wadi Maran, E. Yemen” (
The biology of this species is mostly unknown.
During a field expedition in 2005 one of the authors saw large, continuous water bodies in the Wādī Ḩaḑramawt / Wādī al Masīlah area. The species is rated as “Endangered B1a, b; B2a, b” and water extraction is identified as the main threat (
Carasobarbus exulatus was described from Wādī Ḩaḑramawt and Wādī Maran in Yemen and placed in Barbus (
http://species-id.net/wiki/Carasobarbus_fritschii
Type material. Syntypes of Barbus fritschii: BMNH 1874.1.30:27-31, 5, Morocco, Oued Ksob in Oued Igrounzar drainage [31°28'59"N, 9°46'3"W], K. v. Fritsch and J. Rein, 1872.
Syntypes of Barbus paytonii: BMNH 1903.10.29:17-20, 7, Morocco, Oued Oum er Rbia [33°19'40"N, 8°20'2"W], F. W. Riggenbach.
Syntypes Barbus riggenbachi: BMNH 1902.7.28:20-21, 2, Morocco, Oued Oum er Rbia [33°19'40"N, 8°20'2"W], F. W. Riggenbach. - BMNH 1902.7.28:19, 1, Morocco, Oued Talmest [31°52'15"N, 9°18'31"W], F. W. Riggenbach.
Syntypes Barbus rothschildi: BMNH 1901.7.26:6-7, 2, Morocco, Oued Oum er Rbia [33°19'40"N, 8°20'2"W], E. Hartert.
Syntypes of Capoeta atlantica: BMNH 1902.1.4:18-19, 2, Morocco, Oued Nfis at Trigadir-el-hor (Tagadirt n’Bour?) [31°9'21"N, 8°6'2"W], E. G. B. Meade-Waldo.
Syntypes of Capoeta waldoi: BMNH 1902.1.4:16-17, 2, Morocco, Oued Nfis at Trigadir-el-hor (Tagadirt n’Bour?) [31°9'21"N, 8°6'2"W], E. G. B. Meade-Waldo.
Non-type material. Oued al Maleh drainage. SMF 33412, 5; SMF 33510, 1; SMF 33511, 1; SMF 33512, 1, Morocco, Oued al Maleh above the dam (33°33'53"N, 7°22'3"W), K. Borkenhagen and J. Freyhof, 19 Apr 2011. - MNHN 1919-0365, 1; MNHN 1919-0366, 1, Morocco, Oued Bou Asseïla near Chaouia [33°19'34"N, 7°16'46"W], H. Millet, 1919.
Oued Bou Regreg drainage. MNHN 1939-0124, 1, Morocco, Oued Akrech [33°56'7"N, 6°47'41"W], J. M. Pérès, 1939. - SMF 33411, 10; SMF 33503, 1; SMF 33504, 1; SMF 33505, 1, Morocco, Oued Korifla above the dam lake (33°44'0"N, 6°43'43"W), K. Borkenhagen and J. Freyhof, 18 Apr 2011.
Oued Igrounzar drainage. BMNH 1889.7.19:9, 1, Morocco, near Essaouira [31°30'45"N, 9°46'12"W], C. Payton. - SMF 636, 4; SMF 952, 6, Morocco, Oued Ksob [31°28'59"N, 9°46'3"W], K. v. Fritsch and J. Rein, 1872. - SMF 33405, 19; SMF 33446, 1; SMF 33450, 1; SMF 33451, 1, Morocco, Oued Ksob near Essaouira (31°28'0"N, 9°45'32"W), A. Azeroual et al., 11 Apr 2011. - SMF 33388, 1; SMF 33389, 1; SMF 33390, 1; SMF 33404, 20, Oued Igrounzar between Ounara and El Ghazouane (31°27'21"N, 9°41'4"W), A. Azeroual et al., 10 Apr 2011. - SMF 33406, 2, Oued Igrounzar near El Khemis des Meskala (31°21'31"N, 9°24'20"W), A. Azeroual et al., 11 Apr 2011.
Oued Iqem drainage. SMF 33509, 1, Morocco, Oued Iqem near Skhirat (33°53'22"N, 6°59'56"W), K. Borkenhagen and J. Freyhof, 19 Apr 2011.
Oued Kiss drainge. MNHN 1924-0174, 1, Algeria, Oued Kiss at Marsa Ben Mehid (35°4'59"N, 2°10'1"W), C. A. Alluaud, 1924.
Oued Moulouya drainage. SMF 33407, 8; SMF 33408, 4; SMF 33479, 1; SMF 33481, 1; SMF 33484, 1, Morocco, Oued Za near Guefaït (34°13'36"N, 2°23'34"W), K. Borkenhagen and J. Freyhof, 15 Apr 2011. - MNHN 1926-0070, 1, Morocco, Oued Melloulou near Guercif [34°13'32"N, 3°21'13"W], P. M. Pallary, 1926. - NMW 19533, 1, Morocco, Ras el Aïn near Aïn Beni Mathar (=Berguent) [34°0'41"N, 2°1'47"W], F. Werner. - NMW 19532, 1, Morocco, Oued Za [32°57'0"N, 5°12'0"W], F. Werner.
Oued Oum er Rbia drainage. BMNH 1902.7.28:22-26, 5, Morocco, Oued Oum er Rbia [33°19'40"N, 8°20'2"W], F. Riggenbach. - BMNH 1903.7.1:8, 2, Morocco, El Jadida [33°15'18"N, 8°30'22"W], F. Riggenbach. - MNHN 1927-0099, 1; MNHN 1927-0100, 1; MNHN 1989-0535, 1, Morocco, Oued Oum er Rbia near Khenifra [32°56'21"N, 5°40'7"W], A. Gruvel and R. Dollfus, 1927. - MNHN 1928-0054, 1; MNHN 1928-0055, 1, Morocco, Oued Oum er Rbia near Khenifra [32°56'21"N, 5°40'7"W], P. Pallary, 1928. - SMF 33513, 1; SMF 33514, 1; SMF 33515, 1, Morocco, Oued Oum er Rbia near Boulaouane (32°51'33"N, 8°2'41"W), K. Borkenhagen and J. Freyhof, 20 Apr 2011. - SMF 33344, 1; SMF 33345, 1; SMF 33346, 1; SMF 33394, 12, Morocco, Oued Srou at bridge between Tighassaline and Khenifra (32°49'51"N, 5°36'36"W), A. Azeroual et al., 7 Apr 2011. - SMF 33360, 1; SMF 33361, 1; SMF 33362, 1; SMF 33395, 17, Morocco, Oued Derra near Oulad Yaïch (32°26'23"N, 6°19'24"W), A. Azeroual et al., 9 Apr 2011. - SMF 33363, 1; SMF 33364, 1; SMF 33365, 1; SMF 33397, 22, Morocco, Oued Oum er Rbia (32°18'53"N, 6°54'33"W), A. Azeroual et al., 9 Apr 2011.
Oued Sebou drainage. SMF 33410, 9; SMF 33494, 1; SMF 33495, 1; SMF 33496, 1, Morocco, Oued Ouergha between Sidi Qacem and Ouazzane (34°27'52"N, 5°30'39"W), K. Borkenhagen and J. Freyhof, 17 Apr 2011. - MNHN 1939-0125, 1; MNHN 1939-0126, 1; MNHN 1939-0127, 1, Morocco, El Gharb [34°25'N, 6°20'W], J. M. Pérès, 1939. - MNHN 1939-0122, 2; MNHN 1939-0123, 2; MNHN 1939-0145, 1, Morocco, Oued Sebou [34°15'53"N, 6°41'5"W], J. M. Pérès, 1939. - SMF 33409, 15; SMF 33489, 1; SMF 33491, 1; SMF 33493, 1, Morocco, Oued Lahdar near Taza (34°14'35"N, 4°3'55"W), K. Borkenhagen and J. Freyhof, 16 Apr 2011. - MNHN 1924-0191, 3, Morocco, Oued Beth near Dar Bel Hamri [34°11'14"N, 5°57'54"W], C. A. Alluaud, 1924. - MNHN 1920-0061, 1; MNHN 1920-0062, 1, Morocco, Oued Bou Hellou [34°9'19"N, 4°25'33"W], P. M. Pallary, 1920. - MNHN 1922-0065, 1, Morocco, Moulay Yacoub [34°5'17"N, 5°10'54"W], C. A. Alluaud, 1922. - MNHN 1920-0202, 1, Morocco, Faraoun near Volubilis [34°4'25"N, 5°33'25"W], C. A. Alluaud, 1920. - MNHN 1939-0128, 1; MNHN 1939-0129, 1, El Mabbabat [?], J. M. Pérès, 1939.
Oued Tennsift drainage. BMNH 1904.11.28:60, 1; BMNH 1905.11.28:60-63 and BMNH 1904.11.28:57-58, 6, Morocco, Oued Chichaoua [31°43'48"N, 8°49'48"W], F. Riggenbach. - MNHN 1919-0379, 1; MNHN 1919-0380, 1; MNHN 1919-0381, 1; MNHN 1919-0382, 1, Morocco, Oued Nfis near Dar Goundafi [31°43'41"N, 8°21'1"W], P. M. Pallary, 1919. - MNHN 1988-1146, 4, Morocco, Oued Nfis [31°43'41"N, 8°21'1"W], Goubier, VI.1988. - MNHN 1922-0066, 1; MNHN 1922-0067, 1; MNHN 1922-0068, 1, Morocco, Oued Chichaoua near Chichaoua [31°32'37"N, 8°45'46"W], C. A. Alluaud, 1922. - SMF 33371, 1; SMF 33372, 1; SMF 33373, 1; SMF 33374, 1; SMF 33398, 3, Morocco, Oued Nfis near Tameslouht (31°27'2"N, 8°8'22"W), A. Azeroual et al., 10 Apr 2011. - SMF 33378, 1; SMF 33379, 1; SMF 33380, 1; SMF 33399, 14; SMF 33403, 22, Morocco, Oued Nfis near Ouirgane (31°13'24"N, 8°6'50"W), A. Azeroual et al., 10 Apr 2011. - MNHN 1925-0371, 1, Morocco, Oued Nfis near Ouirgane [31°10'40"N, 8°4'24"W], J. Pellegrin, 1925.
Two pairs of barbels, 30 to 39 scales in the lateral line and 14 to 20 scales around the least circumference of the caudal peduncle; dorsal fin usually shorter than anal fin and more than 15 % of its last unbranched ray flexible, dorsal profile of the head convex.
The body is of moderate height and sometimes has a small nuchal hump in larger specimens. The head is round with a convex dorsal profile and convex or straight ventral profile (Figs 16, 17). The head length is shorter than the body depth (Fig. 12), the mouth is inferior with two pairs of barbels (Table 2). The lower lip is crescent shaped and sometimes weakly keratinised. The eyes are in the anterior half of the head. The morphometric characters are summarised in Table 1.
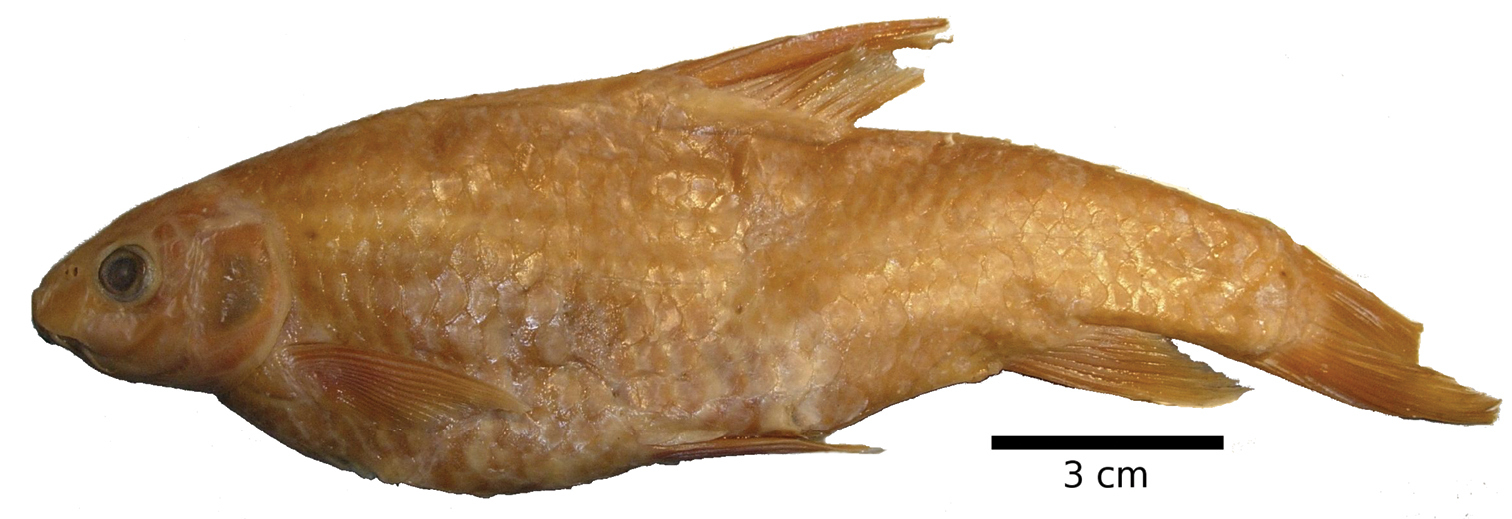
Carasobarbus fritschii, syntype (BMNH 1874.1.30:27-31) from Oued Ksob, © The Natural History Museum, London.
Carasobarbus fritschii, from Oued Ksob.
The dorsal fin is short and weakly ossified and more than 15 % of the length of its last unbranched ray is flexible. Its last unbranched ray is about as long as the head (Fig. 4). It usually has four unbranched and seven to 10 branched rays (Table 3). The anal fin usually has three unbranched and five or six branched rays (Table 4). Its length is rather variable in adult specimens. It reaches the base of the caudal fin in some specimens.
Carasobarbus fritschii has 30 to 39 scales in the lateral line (Table 5), usually 5.5 scales above the lateral line (Table 6), usually 4.5 or 5.5 scales below the lateral line (Table 7), and 14 to 20 scales around the least circumference of the caudal peduncle (Table 8). The scales are shown in Fig. 5.
The pharyngeal teeth count is 2.3.4-4.3.2 in two specimens, 2.3.4- in one specimen and -4.3.2 in eight specimens. Pharyngeal teeth are hooked at their tips (Fig. 6).
Live specimens are silvery and usually have a dark longitudinal band above the lateral line. Fins are hyaline to slightly orange (Fig. 17). Ethanol-preserved specimens are yellow-brown, the back is usually distinctly darker than the belly and flanks.
The maximum length observed in the material available is 180 mm SL.
Carasobarbus fritschii differs from all congeners except Carasobarbus exulatus and Carasobarbus harterti in having nine instead of 10 branched dorsal-fin rays. It differs from Carasobarbus exulatus in having 30 to 39 scales in the lateral line vs. 26 to 32 and modally 16 scales around the least circumference of the caudal peduncle vs. 12. It differs from Carasobarbus harterti in having a convex dorsal head profile and a last unbranched dorsal-fin ray that is weakly ossified and flexible for more than 15 % of its length vs. a straight dorsal head profile and a strongly ossified last unbranched dorsal-fin ray that is flexible in less than 15 % of its length.
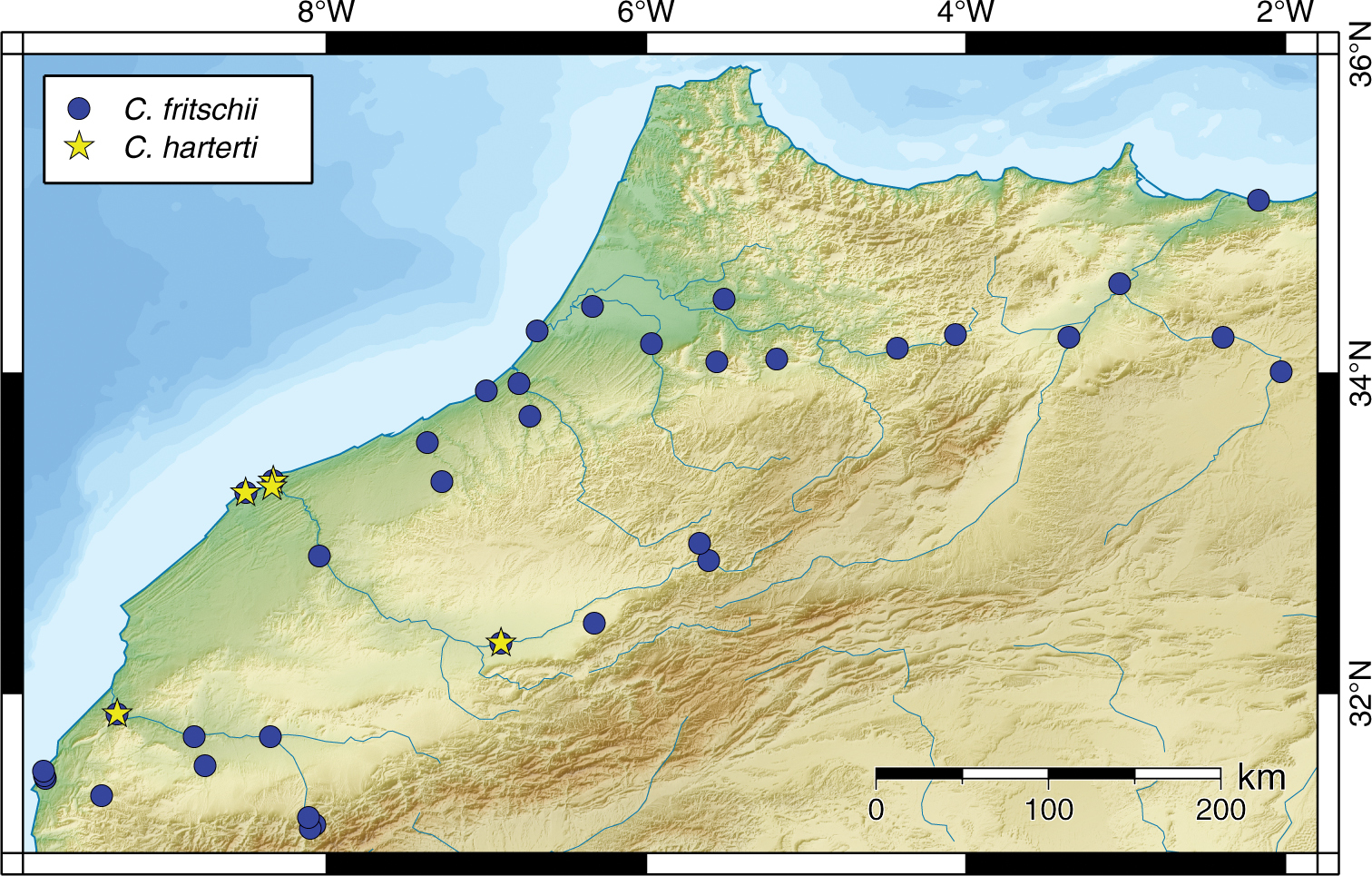
Carasobarbus fritschii is widespread and abundant in Northern and Central Morocco (Fig. 18). It occurs in the Oued al Maleh, Oued Bou Regreg, Oued Igrounzar, Oued Moulouya, Oued Oum er Rbia, Oued Sebou and Oued Tennsift drainage systems, and in numerous small coastal rivers. Most records are from Morocco, but one specimen is from the Oued Kiss in Algeria.
Map of the distribution of Carasobarbus fritschii and Carasobarbus harterti.
Carasobarbus fritschii occurs in a wide range of running water courses and dam lakes.
Carasobarbus fritschii is a hardy species and occurs in near-natural as well as heavily modified habitats. It is tolerant against pollution, damming and the presence of several exotic species (KB pers. obs.). The IUCN rates Carasobarbus fritschii as “Least Concern” and Barbus paytonii (which is treated as a junior synonym in this study) as “Vulnerable B2ab(iii)” (
Carasobarbus fritschii was described from the Oued Ksob as a member of the genus Barbus (
The name of this species is frequently misspelled “Barbus fritschi”.
The ‘Catalog of Fishes’ lists SMF 636 and SMF 952 as types for Carasobarbus fritschii (
http://species-id.net/wiki/Carasobarbus_harterti
Type material. Syntypes: BMNH 1901.7.26:4-5, 2, Morocco, Oued Oum er Rbia [33°19'40"N, 8°20'2"W], E. Hartert.
Non-type material. Oued Oum er Rbia drainage. BMNH 1902.7.28:27-33, 7; BMNH 1903.10.29:11-15, 8, Morocco, Oued Oum er Rbia [33°19'40"N, 8°20'2"W], F. Riggenbach. - BMNH 1903.7.1:5-7, 3, Morocco, Oued Oum er Rbia near El Jadida [33°15'18"N, 8°30'22"W], F. Riggenbach. - MNHN 1912-0089, 1; MNHN 1912-0090, 1; MNHN 1912-0091, 1; MNHN 1912-0092, 1; MNHN 1912-0093, 1, Morocco, Oued Oum er Rbia near Azemmour [33°17'22"N, 8°20'33"W], C. du Gast, 1912. - SMF 33366, 1; SMF 33368, 1; SMF 33370, 1, Morocco, Oued Oum er Rbia (32°18'53"N, 6°54'33"W), A. Azeroual et al., 9 Apr 2011.
Oued Tennsift drainage. BMNH 1902.7.28:34, 1, Morocco, Oued Talmest [31°52'15"N, 9°18'31"W], F. Riggenbach.
Two pairs of long barbels; 31 to 38 scales in the lateral line and 13 to 17 scales around the least circumference of the caudal peduncle; dorsal fin longer than anal fin and less than 15 % of the length of its last unbranched ray is flexible, dorsal profile of the head straight.
The body is of moderate height and without a nuchal hump. The head is triangular with almost straight dorsal and ventral profile (Figs 19, 20). The head length is shorter than the body depth (Fig. 12). The mouth is subterminal with two pairs of long barbels (Table 2). The eyes are in the anterior half of the head and relatively big. The morphometric characters are summarised in Table 1.
Carasobarbus harterti, syntype (BMNH 1901.7.26:4-5) from Oued Oum er Rbia, © The Natural History Museum, London.
Carasobarbus harterti, live specimen from Oued Oum er Rbia.
The dorsal fin is long and strongly ossified and less than 15 % of the length of its last unbranched ray is flexible. Its last unbranched ray is as long as or longer than the head (Fig. 4). It usually has four unbranched and nine branched rays (Table 3). The anal fin usually has three unbranched and six or seven branched rays (Table 4). It does not reach the caudal fin origin.
Carasobarbus harterti has 31 to 38 scales in the lateral line (Table 5), usually 5.5 or 6.5 scales above the lateral line (Table 6), 4.5 to 6.5 scales below the lateral line (Table 7) and 13 to 17 scales around the least circumference of the caudal peduncle (Table 8). The scales are shown in Fig. 5.
The pharyngeal teeth count is -4.3.2 in four specimens examined. The pharyngeal teeth are hooked at their tips (Fig. 6).
Live specimens are silvery with an olive tinge and orange fins (Fig. 20). Ethanol-preserved specimens are yellow-brown, the back is darker than the belly and flanks.
The maximum length observed in the material examined is 250 mm SL.
Carasobarbus harterti differs from all congeners except Carasobarbus exulatus and Carasobarbus fritschii in having nine rather than 10 branched dorsal-fin rays. It differs from Carasobarbus exulatus in having 31 to 38 scales in the lateral line vs 26 to 32 and modally 16 scales around the least circumference of the caudal peduncle vs. 12. It differs from Carasobarbus fritschii in having a straight dorsal head profile and a last unbranched dorsal-fin ray that is strongly ossified and flexible for less than 15 % of its length vs. a convex dorsal head profile and a last unbranched dorsal-fin ray that is weakly ossified and flexible for more than 15 % of its length.
Carasobarbus harterti occurs in the rivers of the Oued Oum er Rbia and Tennsift drainage systems in Morocco (Fig. 18).
Carasobarbus harterti is less common than Carasobarbus fritschii and inhabits only the lower and middle course of big rivers.
The IUCN rates this species as “Vulnerable A2ace“ (
Carasobarbus harterti was described from Oued Oum er Rbia as Barbus harterti (
http://species-id.net/wiki/Carasobarbus_kosswigi
Type material. Holotype of Cyclocheilichthys kosswigi: ZMH H 1148, Turkey, Batman Çayı [37°47'16"N, 41°0'51"E], C. Kosswig, IV.1939.
Non-type material. Tigris-Euphrates system. NMW 90369, 1, Turkey, Batman Çayı near Baschkaja [37°53'15"N, 41°7'56"E], V. Pietschmann, 15 Jul 1914. - NMW 90805, 1, Turkey, Gökçesu Çayı (37°45'N, 41°45'E), 26 Sep 1985. - ZMH 9548, 2, Turkey, Ceylanpınar [36°50'50"N, 40°3'0"E]. - SMF 33119, 1, Syria, Nahr al Khābūr at Al Ḩasakah [36°30'9"N, 40°44'52"E], F. Krupp. - SMF 30172, 1, Syria, Nahr al Khābūr near Tall Budayrī (36°24'N, 40°52'E), F. Krupp, 2–4 Nov 1986. - SMF 30173, 1, Syria, Nahr al Khābūr near Nahāb (36°23'N, 40°50'E), F. Krupp, 23–27 May 1989. - SMF 30174, 1, Syria, Nahr al Khābūr near Nahāb (36°23'N, 40°50'E), F. Krupp, 28 Sep–8 Oct 1988. - CMNFI 79-0290, 2, Iran, Qaşr-e Shīrīn (34°31'N, 45°35'E). - CMNFI 79-0289, 1, Iran, 25–30 km from Qaşr-e Shīrīn (34°28'N, 45°52'E). - BMNH 1974.2.22:1292-1296, 4; BMNH 1974.2.22:1281, 1, Iraq, Euphrates at Ḩadīthah [34°8'23"N, 42°22'41"E], 19 Oct 1953. - CMNFI 79-0275, 1, Iran, Rūdkhāneh-ye Kashgān, 2 km from Ma‘mūlān (33°25'N, 47°58'E). - SMF 33129, 3, Iran, Rūdkhāneh-ye Karkheh at Pol-e Dokhtar (33°9'36"N, 47°43'12"E), N. Alwan et al., 3 Mar 2008. - ZM-CBSU 4153, 1; ZM-CBSU 4154, 1, Iran, Rūdkhāneh-ye Dez at Dezfūl [32°22'57"N, 48°24'7"E], F. Bossaghzadeh, 8 Jun 2005.
Two pairs of barbels; 32 to 38 scales in the lateral line, usually 14 to 16 scales around the least circumference of the caudal peduncle; last unbranched dorsal-fin ray markedly longer than head; mouth narrow, lower lip spatulate and median lobe present.
Body moderately high, laterally compressed and without a nuchal hump. The greatest body depth is at the point of the origin of the dorsal fin. The ventral profile of the head is straight, its dorsal profile has a slight to pronounced hump near the nostrils (Figs 21, 22). The head is short and narrow. The mouth is inferior. The maximum body depth is bigger than the head length (Fig. 12). The lips are comparatively thick and the lower jaw is narrow with a sharp horny sheath and a median lobe. The two pairs of barbels (Table 2) are stout and the anterior pair is quite long. The eyes are rather high in the middle of the head and rather small. The morphometric characters are summarised in Table 1.
Carasobarbus kosswigi, holotype (ZMH 1148) from Batman Çayı.
Carasobarbus kosswigi, live specimen from Rūdkhāneh-ye Karkheh.
The dorsal fin is long and usually has four unbranched and nine or 10 branched rays (Table 3). The last unbranched ray is long and well ossified; only the tip is flexible. It is considerably longer than the head (Fig. 4). The anal fin usually has three unbranched rays and six branched rays (Table 4). Its base is long. The bases of the dorsal and anal fin have a sheath of scales.
There are 32 to 38 scales in the lateral line (Table 5), 5.5 to seven scales above the lateral line (Table 6), 4.5 to 6.5 scales below the lateral line (Table 7) and (12) 14 to 16 scales around the least circumference of the caudal peduncle (Table 8). The scales are shown in Fig. 5.
The pharyngeal teeth count is 2.3.5-5.3.2 in seven specimens, 2.3.5- in one specimen and -4.3.2 in one specimen. The pharyngeal teeth are hooked at their tips (Fig. 6).
Live specimens are silvery. The back is darker than the belly, which is almost white (Fig. 22). Fixed specimens are yellow-brown and some have a darker back.
Carasobarbus kosswigi differs from all congeners, except Carasobarbus sublimus, by having a spatulate lower jaw with a median lobe on the lower lip vs. a crescent-shaped lower jaw and a lower lip without median lobe. It differs from Carasobarbus sublimus by having 32 to 38 scales in the lateral line vs. 27 to 29 and modally 14 scales around the least circumference of the caudal peduncle vs. 12 and by having a longer and more ossified last unbranched ray in the dorsal fin.
Carasobarbus kosswigi occurs in the Euphrates-Tigris system (Fig. 7).
Carasobarbus kosswigi is rare, inhabits fast-flowing reaches of rivers and feeds on small animals (
Little information is available, but because this species is dependent on fast-flowing water, it is probably impacted by the construction of dams.
Carasobarbus kosswigi was described from the Batman Çayı and placed in the genus Cyclocheilichthys (
Carasobarbus kosswigi is paraphyletic with respect to Carasobarbus sublimus (
http://species-id.net/wiki/Carasobarbus_luteus
Type material. Nahr Quwayq basin. Paralectotypes of Systomus luteus: NMW 54248, 1; NMW 54250:1-2, 2; NMW 54254:1-3, 3; SMF 6784, 1, Syria, Nahr Quwayq near Aleppo [36°12'10"N, 37°9'31"E], T. Kotschy, 17 May 1842.
Syntypes of Systomus albus: NMW 53674-53677, 4; NMW 53680, 1; SMF 812, 1, Syria, Nahr Quwayq near Aleppo [36°12'10"N, 37°9'31"E], T. Kotschy, 18 May 1842.
Rūd-e Mand basin. Syntypes of Systomus albus alpina: NMW 53678, 5; NMW 53679:1-2, 2; NMW 53681:1-2, 2, Iran, Rūdkhāneh-ye Qarah Āghāj near Shīrāz [29°31'3"N, 52°15'0"E], 2 Jan 1844.
Rūdkhāneh-ye Ḩelleh basin. Syntypes of Systomus albus alpina: NMW 53682:1-2, 2, Iran, Daryācheh-ye Parīshān [29°31'7"N, 51°47'47"E].
Tigris-Euphrates system. Lectotype of Systomus luteus (by present designation): NMW 54253:2, Iraq, Tigris near Mosul [36°20'6"N, 43°7'8"E], T Kotschy, 10 Apr 1843.
Paralectotypes of Systomus luteus: NMW 54247:1-2, 2; NMW 54249, 1; NMW 54253:1, 1; NMW 54255:1-2, 2; NMW 80043, 2 same data as lectotype.
Syntype of Systomus albus: NMW 91400, 1, Iraq, Tigris near Mosul [36°20'6"N, 43°7'8"E], 11 Apr 1843.
Unknown drainage system. Paralectotype of Systomus luteus: NMW 10827, 1, Syria, “Damascus”, T. Kotschy, 1837.
Non-type material. Daryācheh-ye Mahārlū basin. CMNFI 79-0047, 1, Iran, source of Ab-e Paravan marshes 19.9 km from Shīrāz University [29°36'N, 52°32'E]. - FSJF 2232, 2, Iran, Pirbano spring about 10 km south of Shīrāz (29°31'8"N, 52°27'56"E), A. Abdoli and J. Freyhof, 21 Apr 2007. - ZM-CBSU 3439, 1; ZM-CBSU 3449, 1; ZM-CBSU uncatalogued, 1, Iran, Pol-e Berenji, southwest of Shīrāz [29°27'30"N, 52°32'0"E], H. R. Esmaeili et al. - CMNFI 79-0347, 1, Iran, Solţānābād marshes near Pol-e Berenji (29°27'30"N, 52°32'0"E).
Orontes basin. MNHN 1977-0255, 1, Syria, Orontes, Gruvel, 1929, only one of two specimen examined. - MNHN 1977-0257, 1, Syria, Orontes, Gruvel, 1930. - SMF 24341, 1, Syria, Orontes at Jisr ash Shughūr (35°48'N, 36°19'E), F. Krupp, 21 Mar 1979 (aberrant specimen).
Rūd-e Mand basin. CMNFI 79-0206, 1, Iran, Qanat 41 km from Estahbān on road to Kharāmeh (29°12'N, 53°40'E). - CMNFI 79-0160, 1, Iran, cement pool near spring along road to Neyrīz (29°9'N, 53°37'E). - ZM-CBSU 4934-4942, 9, Iran, Dareh Daarveshan between Rudbal and Simakan (28°39'10"N, 52°2'27"E), H. R. Esmaeili et al. - ZM-CBSU 101-103, 3; ZM-CBSU 110, 1; ZM-CBSU uncatalogued, 1, Iran, Rūdkhāneh-ye Sīmakān near Jahrom [28°30'0"N, 53°33'38"E], H. R. Esmaeili et al.
Rūdkhāneh-ye Ḩelleh basin. CMNFI 79-0026, 1, Iran, Rūdkhāneh-ye Shāhpūr near Shahr-e Tārīkhī-ye Neyshābūr (29°47'N, 51°35'E). - ZM-CBSU 5180-5190, 10; ZM-CBSU 5192, 1, Iran, Kāzerūn, Sarab Dokhtar [29°37'10"N, 51°39'15"E], H. R. Esmaeili et al. - ZM-CBSU 6508-6517, 10; ZM-CBSU 6574, 1; ZM-CBSU 6602-6607, 6; ZM-CBSU 6610, 1; ZM-CBSU 6614+6615+6617-6619, 5; ZM-CBSU uncatalogued, 12, Iran, Daryācheh-ye Parīshān [29°31'7"N, 51°47'47"E], H. R. Esmaeili et al. - CMNFI 79-0240, 2; CMNFI 79-0304, 3, Iran, Daryācheh-ye Parīshān (29°31'N, 51°50'E). - CMNFI 79-0125, 1, Iran, Rūdkhāneh-ye Dālakī near Dālakī (29°28'N, 51°21'E). - ZM-CBSU 2650-2651, 2; ZM-CBSU 2654-2655, 2, Iran, spring at Palangī Dādīn, near Kāzerūn, Rūdkhāneh-ye Dālakī [29°25'20"N, 51°43'54"E], H. R. Esmaeili et al.
Rūdkhāneh-ye Kol basin. ZM-CBSU 3219-3229, 11; ZM-CBSU 3252-3260, 9, Iran, Golabi spring north of Dārāb [28°47'15"N, 54°22'19"E], H. R. Esmaeili et al. - FSJF 2253, 6, Iran, Golabi spring 35 km north of Dārāb (28°47'15"N, 54°22'19"E), A. Abdoli and J. Freyhof, 21 Apr 2007. - CMNFI 79-0155, 1, Iran, spring at Gavanoo, east of Ḩasanābād [28°47'N, 54°22'E]. - CMNFI 79-0154, 2, Iran, Korsia village on Dārāb-Fasā road (28°45'30"N, 54°24'0"E). - ZM-CBSU 5622-5626, 5, Iran, Tang-e Khūr near Lār [27°36'N, 54°17'E], H. R. Esmaeili et al.
Rūdkhāneh-ye Naband basin. CMNFI 79-0187, 10, Iran, stream and pools at Sarkhūn, Rūdkhāneh-ye Sarzeh (27°23'30"N, 56°26'0"E).
Tigris-Euphrates system. SMF 30208, 1, Turkey, Tigris at Diyarbakır (37°53'N, E40°14’), R. Kinzelbach, 1982. - SMF 30176, 11, Syria, Nahr al Khābūr at Ra’s al ‘Ayn (36°51'N, 40°4'E), F. Krupp, 24–26 May 1989. - SMF 30186, 12, Syria, ‘Ayn Sālūba and ‘Ayn Hamza near Ra’s al ‘Ayn (36°51'N, 40°4'E), F. Krupp, 3 Oct 1988. - SMF 30200, 2, Syria, ‘Ayn Sālūba at Ra’s al ‘Ayn (36°51'N, 40°4'E), F. Krupp, 3 Oct 1988. - SMF 30190, 7, Syria, Nahr al Khābūr 2 km East of Tall Junaydīyah (36°44'N, 40°6'E), F. Krupp, 26 May 1989. - SMF 30197, 2, Syria, Nahr al Khābūr 2 km East of Tall Junaydīyah (36°44'N, 40°6'E), F. Krupp, 5 Oct 1988. - SMF 30179, 3, Syria, Nahr al Khābūr at Tall ʿAtaš (36°42'N, 40°11'E), F. Krupp, 26 May 1989. - SMF 30188, 3, Syria, Nahr al Khābūr at Tall ʿAtaš (36°42'N, 40°11'E), F. Krupp, 6 Oct 1988. - SMF 31317, 1; SMF 33139, 7, Syria, Nahr al Khābūr at Tall Tamr (36°39'7"N, 40°21'51"E), N. Alwan et al., 29 Oct 2008. - SMF 30199, 1, Syria, Nahr al Khābūr at Tall Naşrī (36°37'N, 40°23'E), F. Krupp, 6–7 Oct 1988. - SMF 30178, 1; SMF 30202, 10, Syria, Nahr al Khābūr near Tall Bāz (36°35'N, 40°27'E), F. Krupp, 7 Oct 1988. - SMF 30184, 1; SMF 30193, 3, Syria, Nahr al Khābūr at Tall Bāz (36°35'N, 40°27'E), F. Krupp, 26 May 1989. - SMF 30181, 1; SMF 30192, 3, Syria, Nahr al Khābūr at Tall Umm al Māʿaz (36°34'N, 40°35'E), F. Krupp, 27 May 1989. - SMF 30183, 3, Syria, Nahr al Khābūr at Umm al-Māʿaz (36°34'N, 40°35'E), F. Krupp, 7 Oct 1988. - SMF 30182, 2, Syria, Nahr al Khābūr at Al Ḩasakah (36°30'N, 40°44'E), F. Krupp, 27 May 1989. - SMF 30195, 1, Syria, Nahr al Khābūr at Al Ḩasakah (36°30'N, 40°44'E), F. Krupp, 7 Oct 1988. - SMF 30185, 1; SMF 30213, 6, Syria, Nahr al Khābūr and Wādī Furātī at Tall Tayyiǧ (36°26'N, 40°52'E), F. Krupp, 8 Oct 1988. - SMF 30189, 4, Syria, Nahr al Khābūr at Baḩrat Khātūnīyah (36°24'N, 41°13'E), F. Krupp, 23–24 May 1989. - SMF 30214, 5, Syria, Nahr al Khābūr at Tall Budayrī (36°24'N, 40°49'E), F. Krupp, 26 Sep–8 Oct 1988. - SMF 30206, 7, Syria, Nahr al Khābūr at Tall Budayrī (36°24'N, 40°52'E), F. Krupp, 2–4 Nov 1986. - SMF 30177, 3, Syria, Nahr al Khābūr at Nahāb (36°23'N, 40°50'E), F. Krupp, 28 Sep–8 Oct 1988. - SMF 30201, 23, Syria, Nahr al Khābūr at ‘Ayn Ţābān (36°22'N, 40°50'E), F. Krupp, 28 Sep 1988. - SMF 30191, 2, Syria, Nahr al Khābūr at mouth of Wādī ar Raml (36°15'N, 40°48'E), F. Krupp, 8 Oct 1988. - SMF 30196, 1, Syria, Nahr al Khābūr at Umm Rukaybah (36°8'N, 40°42'E), F. Krupp, 8 Oct 1988. - SMF 30194, 3, Syria, Nahr al Khābūr at Ash Shaddādah (36°4'N, 40°44'E), F. Krupp, 9 Oct 1988. - SMF 31316, 1; SMF 33138, 2, Syria, Nahr al Khābūr at Ash Shaddādah (36°3'46"N, 40°44'30"E), N. Alwan et al., 28 Oct 2008. - SMF 33152, 6, Syria, Jisr Shānīn (36°3'4"N, 39°5'10"E), F. Krupp and W. Schneider, 19 Aug 1980. - SMF 31308, 1, Syria, Mamlaḩat al Jabbūl (36°3'36"N, 37°33'1"E), N. Hamidan, 23 Jun 2008. - SMF 28707, 18, Syria, Euphrates down stream Buḩayratt al Asad (35°51'48"N, 39°0'34"E), R. Beck, Jun 1998. - SMF 30198, 2, Syria, Nahr al Khābūr at Tall ash Shaykh Ḩamad (35°37'N, 40°45'E), F. Krupp, 21 Sep–14 Oct 1988. - SMF 30204, 1; SMF 30205, 4, Syria, Nahr al Khābūr at Tall ash Shaykh Ḩamad (35°37'N, 40°45'E), F. Krupp, 20 Oct–9 Nov 1986. - SMF 33140, 1; SMF 33141, 37, Syria, Euphrates at Harmūshīyah (35°35'52"N, 39°51'25"E), N. Alwan et al., 31 Oct 2008. - SMF 30203, 2, Syria, Nahr al Khābūr 8 km South of Tall ash Shaykh Ḩamad (35°33'N, 40°43'E), F. Krupp, 24 Oct 1986. - SMF 28737, 5, Syria, Euphrates between Ḩalabīyah-Zalābīyah and Dayr az Zawr, R. Beck, Jun 1998. - SMF 28630, 3, Syria, Euphrates upstream Dayr az Zawr (35°31'N, 39°54'E), R. Beck, 23 May 1998. - SMF 28674, 41, Syria, Euphrates upstream Dayr az Zawr [35°31'N, 39°54'E], R. Beck, 30 May 1998. - SMF 33153, 1, Syria, Nahr al Khābūr at Aş Şuwar (35°30'N, 40°38'E), F. Krupp, 15 Mar 1979. - SMF 31315, 1; SMF 33137, 1, Syria, Nahr al Khābūr at Ghawat (35°28'51"N, 40°39'54"E), N. Alwan et al., 28 Oct 2008. - SMF 30187, 2, Syria, Nahr al Khābūr near Ḩarījīyah (35°27'N, 40°38'E), F. Krupp, 10 Oct 1988. - SMF 30180, 5, Syria, Nahr al Khābūr at Mashikh (35°14'N, 40°31'E), F. Krupp, 10 Oct 1988. - SMF 28663, 6, Syria, Euphrates at Qal‘at aş Şāliḩīyah (Dura Europos) [34°45'0"N, 40°43'30"E], R. Beck, 28 May 1998. - SMF 28758, 2, Syria, Euphrates at Abū Kamāl at mouth of Wādī Ratqah [34°26'45"N, 40°56'0"E], R. Beck, 9 Jul 1998. - NMW 93019:1-2, 2, Iraq, Tigris at Baghdād [33°20'26"N, 44°24'3"E], V. Pietschmann, Aug 1910. - SMF 33127, 4, Iran, Rūdkhāneh-ye Bālārūd (32°35'19"N, 48°17'11"E), N. Alwan et al., 3 Mar 2008. - BMNH 1980.8.28:6, 1, Iran, Rūdkhāneh-ye Dez at Dezfūl [32°25'N, 48°13'E]. - SMF 33125, 1, Iran, Rūdkhāneh-ye Dez at Dezfūl (32°22'40"N, 48°22'58"E), N. Alwan et al., 2 Mar 2008. - SMF 33121, 5, Iran, Rūdkhāneh-ye Dez at Dezfūl (32°21'49"N, 48°21'28"E), K. Borkenhagen et al., 3 Nov 2006. - SMF 17303, 1, Iraq, Hawr al Ḩammār (30°50'N, 47°10'E), L. A. J. Al-Hassan, 1986. - SMF 30211, 1, Iraq, ‘Ayn Zālah 50 km west of Mosul, Z. Rahemo, 1990.
Unknown drainage system. SMF 33120, 2, Syria, fish market in Damascus (reported to be from Buḩayratt Ar Rastan [34°56'N, 36°44'E] in Orontes drainage), F. Krupp. - CMNFI 79-0687, 4, Iran, Shīrāz bazar (probably from Rūd-e Mand basin or Daryācheh-ye Mahārlū basin).
The lectotype (NMW 54253:2) is a specimen of 211 mm SL, collected in the Tigris near Mosul on 10 Apr 1843 by T. Kotschy (Fig. 23). It has four unbranched and 10 branched rays in the dorsal fin, three unbranched and six branched rays in the anal fin, 27 scales in the lateral line and one pair of barbels. A bigger specimen (216 mm SL) from the same lot (NMW 54253:1) was not selected as lectotype, because it is atypical in having 11 branched rays in the dorsal fin and two pairs of barbels. The designation of a lectotype became necessary to fix the type locality of Systomus luteus (see Discussion).
Carasobarbus luteus, lectotype (NMW 54253:2) from Tigris near Mosul, © Naturhistorisches Museum Wien, photo E. Lavergne.
One pair of barbels; 25 to 33 scales in the lateral line, and typically 12 scales around the least circumference of the caudal peduncle; last unbranched ray of the dorsal fin about as long as the head or slightly shorter.
Specimens from Rūdkhāneh-ye Naband basin were excluded from this species description (see below).
The dorsal profile is convex up to the origin of the dorsal fin and a nuchal hump is present in specimens longer than about 100 mm SL. This species has a high back and caudal peduncle (Figs 23, 24). The ventral profile of the head is convex, its dorsal profile is almost straight to convex and has a hump near the nostrils in juvenile specimens. The mouth is sub-terminal. The barbels are short and stout. The maximum body depth is usually greater than the head length (Fig. 12). Usually one pair of barbels is present, but about 10 % of the specimens have two pairs of barbels (Table 2). The eyes are at the back of the anterior half of the head. They are big and slightly protuberant. The morphometric characters are summarised in Table 1.
Carasobarbus luteus, live specimen from Nahr al Khābūr.
The dorsal fin usually has four unbranched and eight to 11 branched rays (Table 3). In specimens from the Tigris-Euphrates drainage system the last unbranched ray of the dorsal fin is strong with only the tip being flexible and it is about as long as the head. It is shorter and less ossified in Iranian populations (Fig. 4). The anal fin usually has three unbranched rays and five to seven branched rays (Table 4).
There are 25 to 33 scales in the lateral line (Table 5), 3.5 to 6 scales above the lateral line (Table 6), 3 to 5.5 scales below the lateral line (Table 7) and 10 to 13 scales around the least circumference of the caudal peduncle (Table 8). The scales are shown in Fig. 5.
The pharyngeal teeth count is 2.3.5-5.3.2 in 26 specimens, 2.3.4-5.3.2 in two specimens, 2.3.5-4.3.2 in one specimen, 2.3.5-5.3.3 in one specimen, 1.3.5-5.3.2 in one specimen, 2.3.5- in one specimen and 2.3.4- in one specimen. The pharyngeal teeth are hooked at their tips (Fig. 6).
Live specimens are silvery to olive and sometimes have yellowish fins (Fig. 24). Ethanol-preserved specimens are light yellowish brown to grey. In most cases the back is darker than the rest of the body. Some of the lighter coloured specimens have a salmon hue, others are silvery. The fins are yellowish brown to grey. Juveniles have a dark spot on the sides of the caudal peduncle.
Carasobarbus luteus from Ḩelleh, Kol, Mahārlū and Mand populations: The last unbranched ray of the dorsal fin is shorter and less well ossified. It is pronouncedly shorter than the head (Fig. 4). The mouth is wider and the body is not as high-backed as in specimens from the Tigris-Euphrates system (Fig. 12).
Carasobarbus luteus from Rūdkhāneh-ye Naband basin: In this population all specimens examined had two pairs of barbels (Table 2). The anterior pair is longer than in specimens from Tigris-Euphrates system with two pairs. The last unbranched ray in the dorsal fin is considerably shorter than the head (Fig. 4) and comparatively weak. Compared with specimens from Tigris-Euphrates system, the dorsal and ventral fins tend to be slightly further away from the head. The head is longer and the body not as high backed as in specimens from Tigris-Euphrates system (Fig. 12). The general body shape (Fig. 25) resembles that of Carasobarbus apoensis and Carasobarbus canis. Some of the gill rakers are y-shaped in the largest specimen examined.
Carasobarbus luteus, specimen (CMNFI 79-0187) from Rūdkhāneh-ye Sarzeh.
Carasobarbus luteus, except the population from Rūdkhāneh-ye Naband, differs from all congeners, except Carasobarbus apoensis, in having one instead of two pairs of barbels. It differs from Carasobarbus apoensis, Carasobarbus canis, Carasobarbus chantrei, Carasobarbus fritschii, Carasobarbus harterti and Carasobarbus kosswigi in modally having 28 scales in the lateral line vs. 30, 32, 34, 34, 34 and 33 respectively. It differs from Carasobarbus kosswigi and Carasobarbus sublimus in having a crescent-shaped lower lip without median lobe vs. a spatulate lower lip with median lobe and from Carasobarbus exulatus, Carasobarbus fritschii and Carasobarbus harterti in modally having 10 rather than nine branched dorsal-fin rays. All populations, except the one from Rūdkhāneh-ye Naband differ from Carasobarbus apoensis in having a shorter head and a higher back. The population from Rūdkhāneh-ye Naband is very similar to Carasobarbus apoensis in body shape, but differs in having two as compared to one pair of barbels.
Carasobarbus luteus has a much greater range than any of its congeners and its distribution area is fragmented, resulting in several isolated populations. It is widespread all over the Tigris-Euphrates drainage system, and occurs in the rivers of south-western Iran (Fig. 7). The Nahr al Quwayq population, from one of the sites of the type locality, is probably extirpated due to drought and pollution (
Carasobarbus luteus is mainly herbivorous. It feeds on algae, aquatic plants, detritus and small invertebrates, the main feeding period is at noon, but food is also taken at night (
This species can tolerate saline waters to some degree (
There are attempts on aquaculture of this species. The stickiness of the eggs can be lowered by several chemical treatments for this purpose (
Larvae hatch at 64 degree-days in well oxygenated water and the eyes are still without pigments at this stage. The development is similar to that of other cyprinids (
Conservation status. Carasobarbus luteus is widespread and abundant in the Tigris-Euphrates system. Peripheral populations, like those in smaller Iranian rivers and the Nahr al Quwayq in Syria are more threatened or have already been extirpated (see above).
Carasobarbus luteus was described as Systomus luteus by
We do not think that the population at Rūdkhāneh-ye Naband should be elevated to specific rank, because the number of specimens available is too low. We provisionally consider it an atypical population of Carasobarbus luteus that might have been affected by bottleneck effects and accelerated morphological change, due to the restricted size and extreme conditions (high salinity and temperature) of its habitat. It would be very interesting to collect more samples for morphological studies and molecular sequence analysis.
In spite of some morphometric differences, Carasobarbus luteus populations of Tigris-Euphrates system and Iran belong to the same species (
Carasobarbus luteus and Carasobarbus apoensis are closely related to each other (KB, unpublished data) and Carasobarbus apoensis might be the ecologically specialised sister species of Carasobarbus luteus, that is adapted to the environmental conditions of the wadi ecosystems of the Al Ḩijāz mountains.
http://species-id.net/wiki/Carasobarbus_sublimus
Type material. Holotype of Barbus sublimus: CMNFI 1995-0009, Iran, Rūdkhāneh-ye A‘lā near Pol-e Tīghen (31°23'30"N, 49°53'0"E), B. W. Coad et al., 20 Sep 1995, not examined.
Paratypes of Barbus sublimus: CMNFI 95-0009a, 1, same data as holotype. - CMNFI 95-0010, 1, same data as holotype, not examined. - CMNFI 95-0011, 3, Iran, Rūdkhāneh-ye A‘lā near Pol-e Tīghen (31°23'30"N, 49°53'0"E), G. Eskanderi, Dec 1994, only one specimen examined.
Non-type material. Rūdkhāneh-ye Kashgān. CMNFI 79-0277, 1, Iran, Rūdkhāneh-ye Kashgān at Harpul Kashkow, 50 km from Khorramābād (33°30'0"N, 47°59'30"E), K. Evans and H. Assadi, 5 Jul 1977.
Rūdkhāneh-ye Zohreh drainage. ZM-CBSU 5781-5786, 6, Iran, Rūdkhāneh-ye Fahlīān at Nūrābād [30°6'51"N, 51°31'18"E], H. R. Esmaeili et al. - SMF 33117, 3, Iran, Rūdkhāneh-ye Fahlīān (30°11'10"N, 51°31'14"E), K. Borkenhagen et al., 29 Nov 2007. - SMF 33118, 6, Iran, Rūdkhāneh-ye Fahlīān (30°11'9"N, 51°31'15"E), N. Alwan et al., 29 Feb 2008.
Two pairs of barbels; 27 to 29 scales in the lateral line, 12 scales around the least circumference of the caudal peduncle; last unbranched dorsal-fin ray about as long as the head; mouth narrow, lower jaw spatulate and median lobe present on lower lip.
A nuchal hump is not developed. The maximum body depth is at the anterior end of the dorsal fin base. The ventral profile of the head is almost straight; the dorsal profile is convex and evenly curved (Figs 26, 27). The maximum body depth is greater than the head length (Fig. 12). The mouth is inferior, narrow, the lips are thick and the lower jaw is spatulate with a horny sheath and a median lobe on the lower lip. The two pairs of barbels (Table 2) are well developed. The eyes are at the posterior end of the anterior half of the head. Some morphometric characters are summarised in Table 1.
Carasobarbus sublimus, paratype (CMNFI 95-0011) from Rūdkhāneh-ye A‘lā, photo S. Tränkner.
Carasobarbus sublimus, live specimen from Rūdkhāneh-ye Fahlīān.
The dorsal fin usually has four unbranched and nine or 10 branched rays (Table 3). The last unbranched ray of the dorsal fin is weakly ossified and about as long as the head (Fig. 4). The anal fin usually has three unbranched and six branched rays (Table 4) and its base is surrounded by a sheath of scales. Pectoral, ventral and anal fins are longer than in all other Carasobarbus species (Table 1).
There are 27 to 29 scales in the lateral line (Table 5), 4.5 or 5.5 scales above the lateral line (Table 6), 3.5 to 5.5 scales below the lateral line (Table 7) and 12 scales around the least circumference of the caudal peduncle (Table 8). The scales are shown in Fig. 5.
The pharyngeal teeth count is 2.3.4-5.3.2, 2.3.4-5.3.1 or 3.3.4-4.3.3 (
Live specimens from Rūdkhāneh-ye Fahlīān are silvery with hyaline fins (Fig. 27). Live specimens from Rūdkhāneh-ye A‘lā are silvery with a slightly darker back, the scales have dark pigments on their hind margin; pectoral, ventral and anal fins have a yellow to orange hue, which is most obvious with fins folded back; dorsal and caudal fins are grey or hyaline (
Carasobarbus sublimus differs from all congeners, except Carasobarbus kosswigi, by having a spatulate lower jaw with a median lobe on the lower lip vs. a crescent shaped lower jaw and a lower lip without median lobe. It differs from Carasobarbus kosswigi by having 27 to 29 scales in the lateral line vs. 32 to 38 and modally 12 scales around the least circumference of the caudal peduncle vs. 14 and by having a shorter and less ossified unbranched last dorsal-fin ray.
This species is known from Rūdkhāneh-ye A‘lā, Rūdkhāneh-ye Fahlīān and possibly Rūdkhāneh-ye Kashgān (see discussion) in south-western Iran (Fig. 7).
Carasobarbus sublimus is adapted to streams with fast currents with water flowing over hard substrate (
Little is known about the conservation status of Carasobarbus sublimus, but because this species is dependent on fast-flowing water, it is probably impacted by the construction of dams.
Carasobarbus sublimus was described in the genus Barbus and aligned with Carasobarbus apoensis, Carasobarbus canis, Carasobarbus chantrei, Carasobarbus exulatus, Carasobarbus kosswigi and Carasobarbus luteus in the original description (
Locality data for CMNFI 79-0277 is not beyond doubt, because this lot was mentioned as Carasobarbus kosswigi in the original description of Carasobarbus sublimus (
Two putative intergeneric hybrids of Carasobarbus canis with other cyprinids are known, one with Capoeta damascina (Valenciennes in
The hybrids are intermediate in many morphometric and meristic characters (
Carasobarbus canis x Capoeta damascina, aquarium photograph of SMF 17184, originally from Nahr az Zarqā’.
These hybrids are intermediate in many morphometric and meristic characters (
Holotype of Barbus continii = Carasobarbus canis x Barbus longiceps preserved specimen (SL=165 mm), Lake Tiberias (MCSN 22300).
| 1 | Branched dorsal-fin rays 9, Yemen and NW Africa | 2 |
| – | Branched dorsal fin rays 10 | 4 |
| 2 | Scales around least circumference of the caudal peduncle 10−12, Yemen | Carasobarbus exulatus |
| – | Scales around least circumference of the caudal peduncle 13−20, Morocco | 3 |
| 3 | Dorsal profile of head convex, more than 15 % of the last unbranched dorsal-fin ray flexible | Carasobarbus fritschii |
| – | Dorsal profile of head straight, less than 15 % of the last unbranched dorsal-fin ray flexible | Carasobarbus harterti |
| 4 | Lower jaw spatulate and lower lip with a median lobe | 5 |
| – | Lower jaw u-shaped or crescent shaped and lower lip without median lobe | 6 |
| 5 | Scales around the least circumference of the caudal peduncle 12, 27−29 scales in the lateral line, head about as long as dorsal fin | Carasobarbus sublimus |
| – | Scales around the least circumference of the caudal peduncle 12−16, 32−38 scales in the lateral line, dorsal fin longer than the head | Carasobarbus kosswigi |
| 6 | Modally 14 (12−16) scales around the least circumference of the caudal peduncle | Carasobarbus chantrei |
| – | Modally 12 (10−14) scales around the least circumference of the caudal peduncle | 7 |
| 7 | Usually two pairs of barbels, Jordan River and adjacent waterbodies | Carasobarbus canis |
| – | Usually one pair of barbels, Mesopotamia, southern Iran and Arabia | 8 |
| 8 | Head about as long as body depth, dorsal fin markedly shorter than head, modally 30 scales in the lateral line, Western Arabian Peninsula | Carasobarbus apoensis |
| – | Head shorter than body depth, dorsal fin about as long as head (except in Iranian populations), modally 28 scales in lateral line, Mesopotamia and southern Iran | Carasobarbus luteus |
We are grateful to James Maclaine, Oliver Crimmen, Patrick Campbell (BMNH); Hamid Reza Esmaeili (ZM-CBSU); Guy Duhamel, Romain Causse, Patrice Pruvost, Claude Ferrara, Zora Gabsi (MNHN); Ernst Mikschi, Helmut Wellendorf, Christa Prenner, Matthias Reithofer (NMW) and Horst Wilkens (ZMH) for making the collections under their care available for study and to Jörg Freyhof for providing additional specimens.
Phil Hurst, Edouard Lavergne and Sven Tränkner contributed specimen photos. Nina Bogutskaya and Florian Wicker offered valuable comments on the manuscript. Eva Feltkamp prepared the maps.
We thank Nisreen Alwan, Zuhair Amr, Abdelhamid Azeroual, Mehrgan Ebrahimi, Hamid Reza Esmaeili, Jörg Freyhof, Christiane Frosch, Mehdi Ghanbari-Fardi, Zeinab Gholami, Abbas Kazemi, Masoumeh Malek, Majid Moradmand, Hossein Parsa, Sobhan Rahimi, Hassan Rahimian, Bettina Reichenbacher, Hassan Salehi, Alireza Sari, Adwan Shehab, Wolfgang Schneider, Azad Teimori, Florian Wicker and Uwe Zajonz for help with organising and participating in fieldwork in Iran, Jordan, Morocco, Saudi Arabia, Syria and Yemen.
Part of the fieldwork was funded by the German Academic Exchange Service (DAAD), in the framework of the project “Establishment of a Middle Eastern Biodiversity Network”. Field work in Yemen was supported by the Coastal Zone Management Pilot Project of the Environment Protection Authority Yemen and the World Bank and received financial support from grant TF 023492 of the Global Environment Facility. Field research in Saudi Arabia and Yemen was funded by the Saudi Wildlife Authority (SWA, formerly National Commission for Wildlife Conservation and Development, NCWCD) and King Abdulaziz City for Science and Technology (KACST), Riyadh, Saudi Arabia.
This research received support from the SYNTHESYS Project, which is financed by European Community Research Infrastructure Action under the FP6 “Structuring the European Research Area” Programme (grants AT-2427 and FR-3580).
The FAZIT Foundation funded two research visits to the BMNH.
The study was supported by the Biodiversity and Climate Research Centre (BiK-F), Frankfurt, which is part of research funding programme “LOEWE”.
Table of localities for all lots examined. (doi: 10.3897/zookeys.339.4903.app) File format: OpenDocument spreadsheet (ods).
Explanation note: Table of localities for all specimens examined as a spreadsheet to make them more easily available for use in biodiversity databases and geospatial investigations.