(C) 2013 Tobias Pfingstl. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Three new intertidal oribatid species, Selenoribates elegans sp. n., Selenoribates quasimodo sp. n. and Selenoribates satanicus sp. n. are described from the archipelago of Bermuda. Selenoribates elegans sp. n. is characterized by its slender body shape, Selenoribates quasimodo sp. n. possesses a hunchback in lateral view and Selenoribates satanicus sp. n. exhibits two horn-like projections on its anterior gastronotic region. Based on these new findings, the number of Selenoribates species doubled at once and the distribution of this genus, formerly limited to the Mediterranean and the Red Sea, includes now occurrences in the Atlantic and Indo-pacific Ocean as well. The morphology of Selenoribates quasimodo sp. n. and Selenoribates satanicus sp. n. deviates conspicuously from the other known members of Selenoribates, thus indicating that not only the number of species but also the anatomy of this genus is more diverse than formerly supposed. Nymphs of Selenoribates quasimodo sp. n. show an interesting case of ontogenetic neotrichy, with gastronotic setae being duplicated with each moult.
Bermuda, intertidal, juvenile instars, Selenoribates, biogeography
The family of Selenoribatidae represents a group of littoral oribatid mites. These mites are air-breathing terrestrial organisms, but they have managed to colonize marine associated habitats and are now exclusively confined to intertidal zones of coastal areas (
In the course of a recent study on intertidal oribatid mites from Bermuda, three new Selenoribates species could be discovered and this finding changes biogeographic and morphological aspects of this genus dramatically. Therefore this paper describes the morphology of the three new species, modifies the distribution pattern and tries to answer the question why the genus Selenoribates has vanished into thin air for more than forty years.
Intertidal algae growing on sandy and rocky substrate, as well as on roots of the black mangrove (Avicennia germinans) were collected on the archipelago of Bermuda during low tide and afterwards put in a Berlese-Tullgren apparatus for the extraction of mites. For investigation in transmitted light all animals were stored in ethanol (70% or pure ethanol), then heated in lactic acid (80°C for about 20 minutes) and afterwards embedded in BERLESE mountant. Observations, photographs and drawings were made with an Olympus BH-2 Microscope equipped with a drawing attachment. Image stacks were obtained by an Olympus E1 digital camera and layered with the Combine ZP software. Inscriptions of drawings were done according to
The following diagnosis summarizes the characters provided by
Small sized (198–308 × 119–185 µm) intertidal mites. Cerotegument granular. Interlamellar setae short or minute. Lamellar ridges present but short. Sensillus flagelliform and long. Pedotectum I small but robust, pedotectum II absent. Notogaster with 14 pairs of setae, c3 absent. Obvious depressions, variable in number and shape, present on anterior part of notogaster. Two median epimeral cavities present. Epimeral setal formula 1-0-1-1. Genital plates with three-four pairs of setae. Aggenital setae absent. Two-three pairs of adanal setae and one-three pairs of anal setae. Legs monodactylous; claws with one or two proximoventral teeth. Juveniles plicate with large centrodorsal plate.
urn:lsid:zoobank.org:act:CA3698B8-B235-4EAA-8DC3-E3F1DDDF7D84
Bermuda: Coney Island, 32°21'30"N, 64°42'59"W, lower intertidal area, sand and algae growing on mangrove roots, 18 October 2011.
Holotype: male, preserved in pure ethanol, deposition: Naturhistorisches Museum Wien, collection nr. NHMW 21884. Paratypes: two males, deposition: Senckenberg Museum für Naturkunde Görlitz, collection Nr. 11/48677.
Red-brown sclerotized mites. Average length 228 µm, mean width 139 µm. Notogaster rounded in dorsal view, hunchbacked in lateral view. A large anteriorly arched depression on anterior part of notogaster. Lamellar ridges short. Interlamellar seta of normal length and spiniform. Fourteen pairs of spiniform notogastral setae. Uniform median epimeral cavity. Three pairs of genital, two pairs of adanal and two pairs of anal setae present. Legs monodactylous; claw of each leg large. Claw with one proximoventral and a proximodorsal tooth. No porose areas on femora discernable.
Adult: Females (N=14), length: 222–244 μm (mean 231 μm), width: 136–152 µm (mean 142 µm); males (N=16), length: 212–238 μm (mean 226 μm), width: 131–147 µm (mean 137 µm)
Integument. Colour red brown. Cerotegument appears basically slightly granular. Cerotegument of prodorsum nearly smooth between bothridia, strongly granular anterior and lateral to lamellar ridges. Cerotegument of notogaster and venter slightly granular. Cerotegument of lateral body parts generally finely granular, larger granules in areas surrounding acetabula. Cerotegument of legs slightly granular.
Prodorsum. Rostrum rounded in dorsal view, but slightly projecting anteroventrally in lateral view. Rostral setae (ro) short and smooth. Lamellar setae (le) and interlamellar setae (in) simple, short and smooth. Exobothridial setae (ex) minute. Lamellar ridge conspicuous, but short, not reaching insertions of lamellar setae. Bothridium large cup exhibiting a strongly projecting posterior ridge with three lobe-like protrusions overhanging anterior border of gastronotic region. Sensillus long (ca. 50µm) and flagelliform. Tutorium developed as slightly dorsally curved ridge.
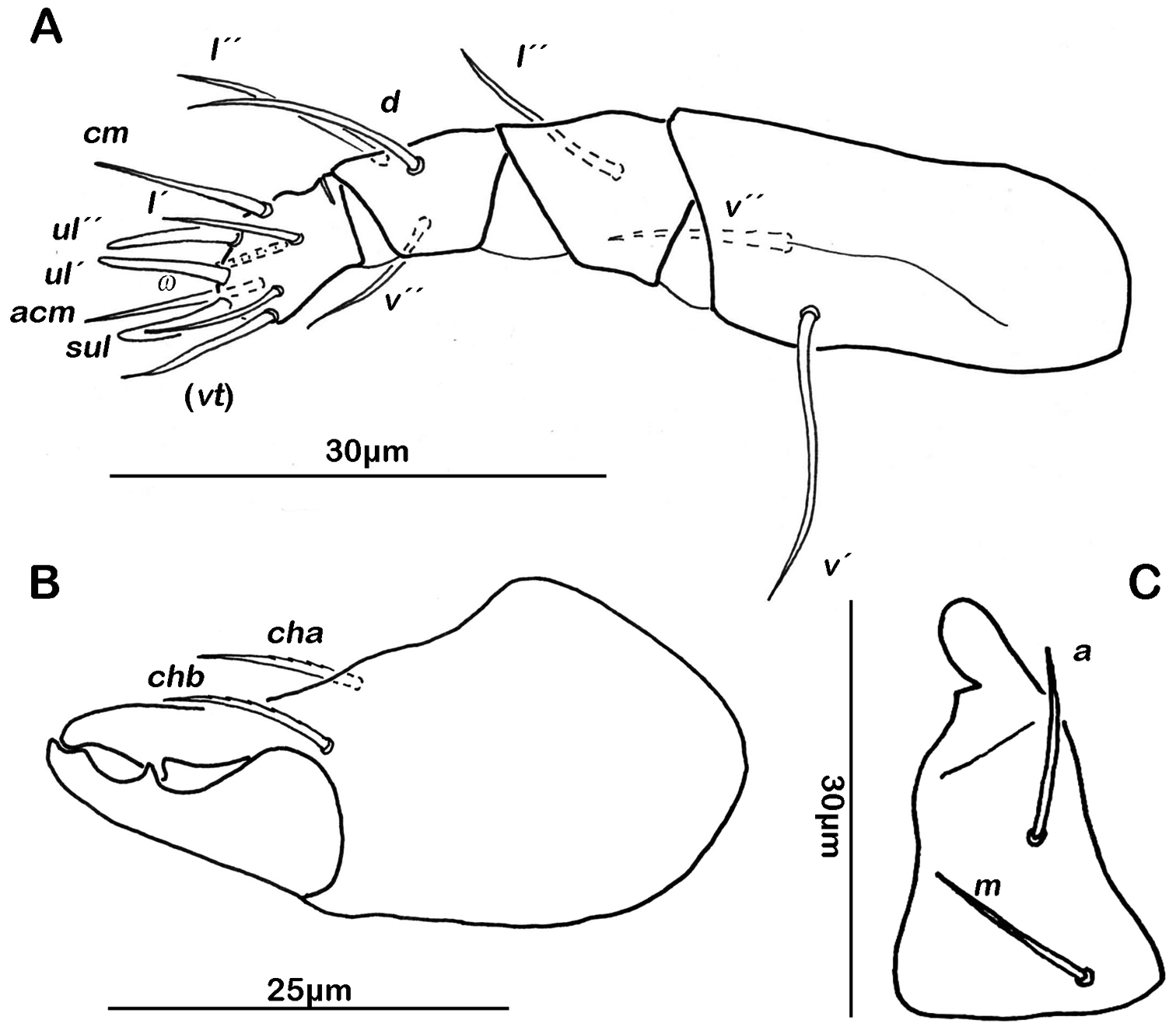
Gnathosoma. Pedipalp pentamerous 0-2-1-3-9 (including solenidion) (Fig. 1A). Solenidion erect, not fused with eupathidium acm. Chelicera chelate, in lateral view forceps-like and each digit with two teeth, whereas from frontal view most distal teeth split into two symmetrical teeth (Fig. 1B). No porose area on proximal part of fixed digit discernable. Seta cha and chb dorsally slightly pectinate, both same length. Distal part of rutellum developed as thin triangular slightly curved inward membrane (Fig. 1C). Setae a and m long and smooth. Mentum regular, setae h simple, thin and long.
Selenoribates quasimodo sp. n. mouthparts. A left pedipalp antiaxial view B left chelicera antiaxial view C right rutellum ventral view.
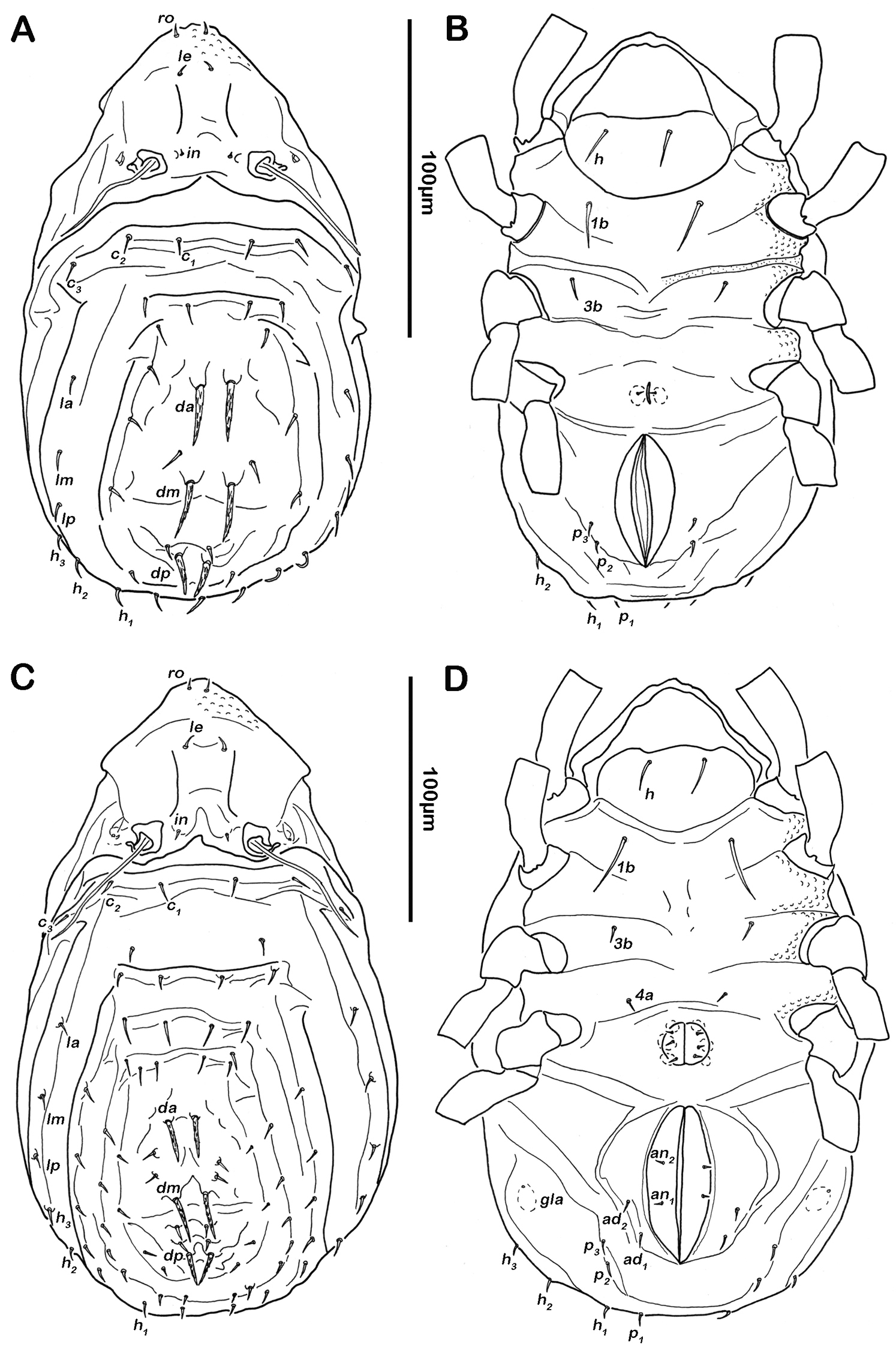
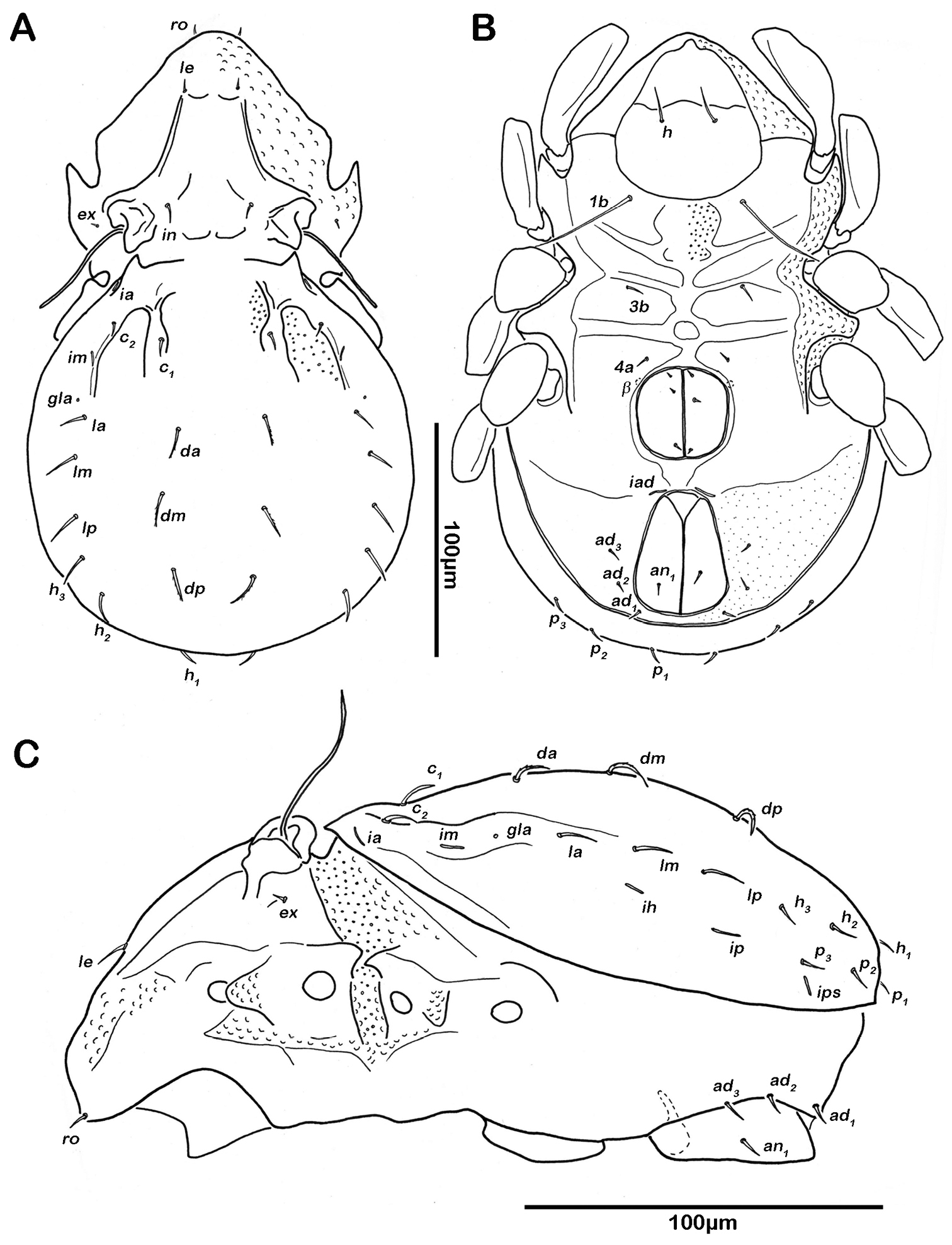
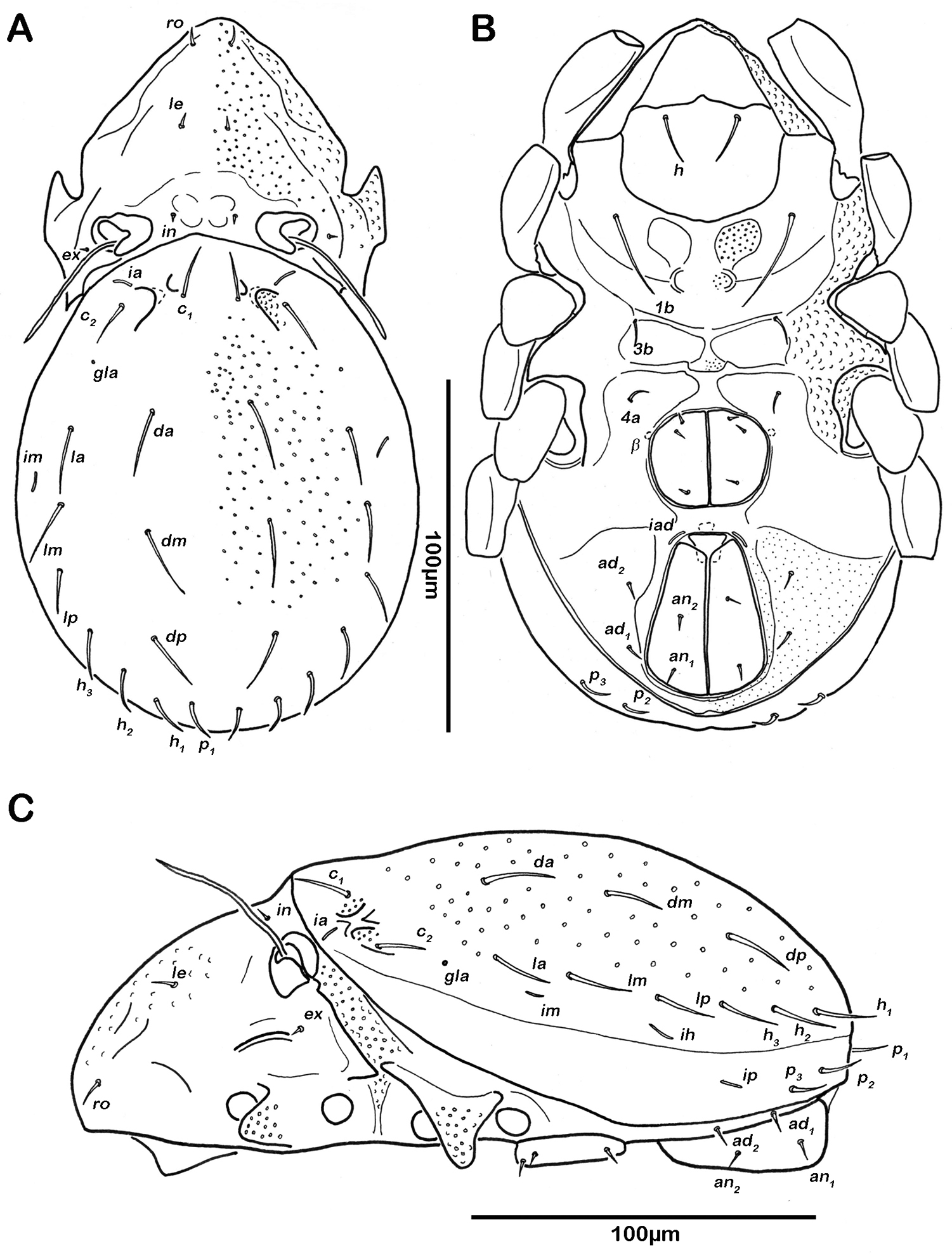
Notogaster (Figs 2A, 3A). Rounded in dorsal view, hunchbacked in lateral view. Anterior margin of notogaster distinct. A large, arched depression on anterior part of notogaster showing obvious granulation. Fourteen pairs of simple notogastral setae (approximate length 5–7 µm), c1-2, da, dm, dp, la, lm, lp, h1-3, p1-3; c3 absent. Notogastral setae sometimes completely covered by a layer of cerotegument. Porose areas or distinct pores absent. Five pairs of notogastral lyrifissures present; ia next to setae c2 close and rectangular to anterior notogastral border; im slightly anterior and laterad of setae la; lyrifissures ih, ip and ips laterally close to lateroventral borders of notogastral plate. Opisthonotal gland openings (gla) located posteriorly to lyrifissures im.
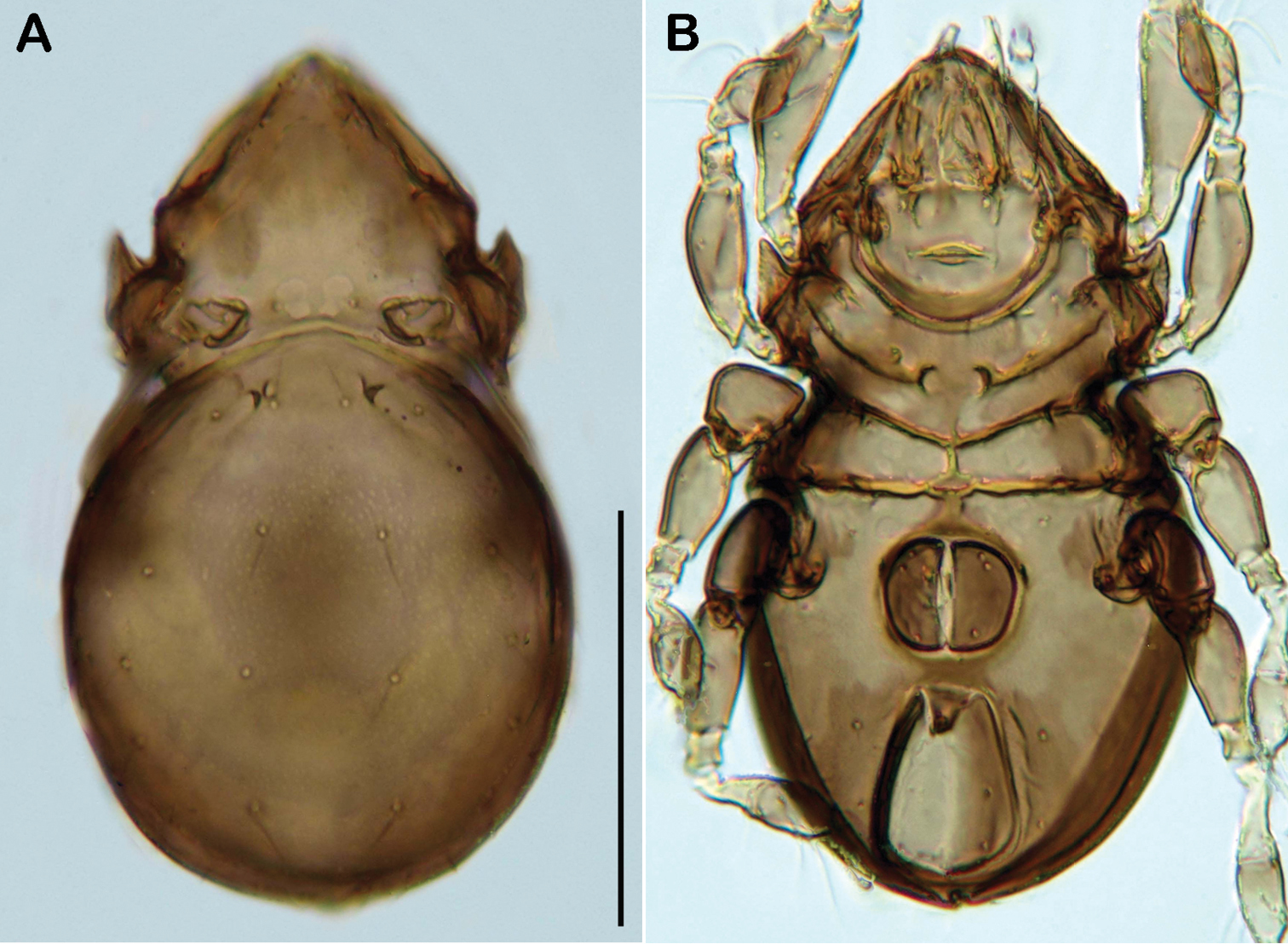
Selenoribates quasimodo sp. n. adult. A dorsal view B ventral view C lateral view.
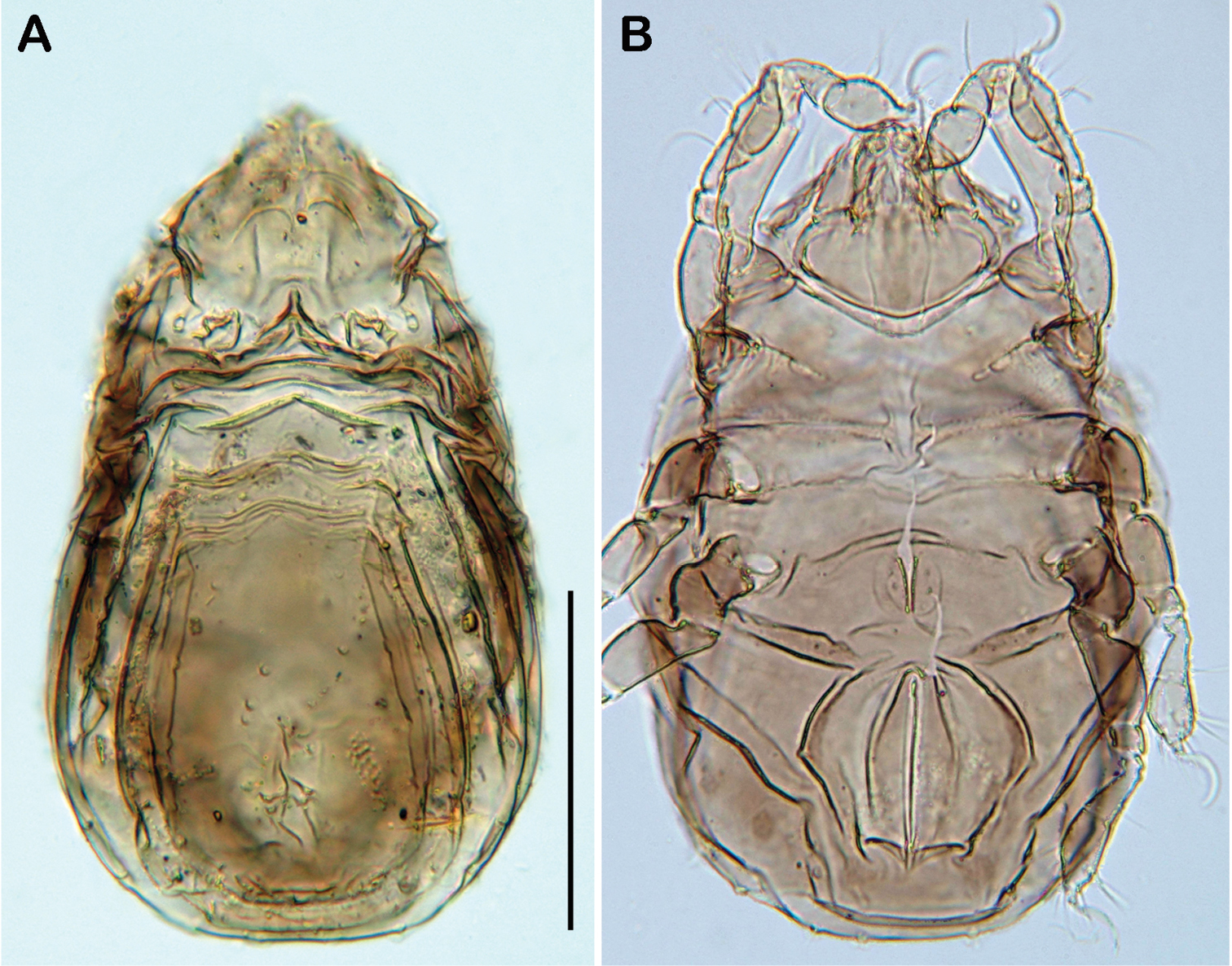
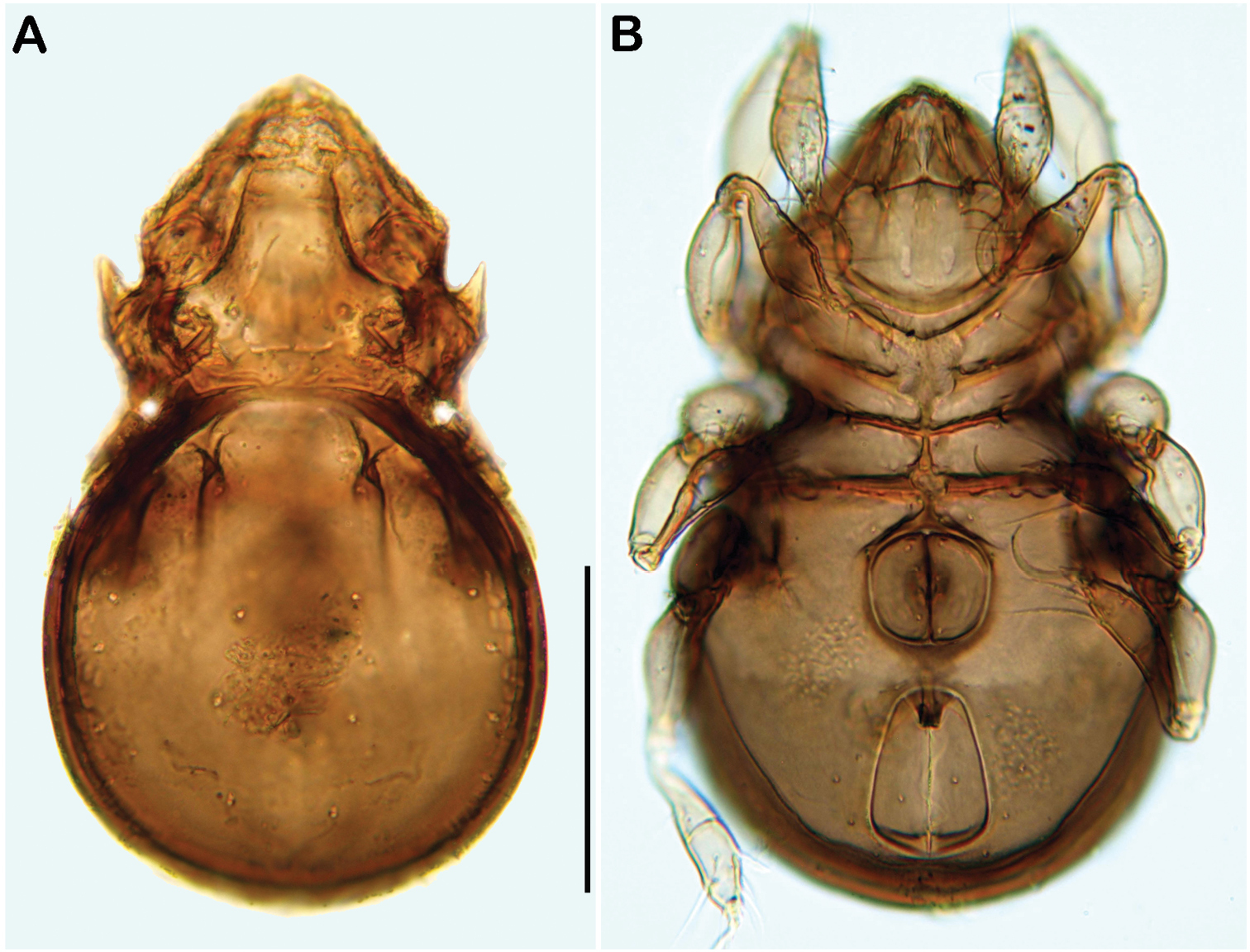
Selenoribates quasimodo sp. n. adult micrographs layered from 5–10 sequentially focused images. A dorsal view B ventral view C lateral view. Scale bars = 100 µm.
Lateral aspect (Figs 2C, 3C). Pedotectum I small but thick, pedotectum II absent. Lateral parts of anterior margin of notogaster broad and deep, showing conspicuous granules. Enantiophyse consisting of two strong triangular teeth orientated against each other. Discidium developed as strong rectangular bulge between acetabulum III and IV.
Ventral region of idiosoma (Figs 2B, 3B). Epimeral setation 1-0-1-1, seta 1b long reaching trochanter II, setae 3b normal length and 4a short. Internal borders of all epimera well visible, sternal apodemes II, sejugal and III well developed. A densely granulated median sternal cavity on epimeron I. Three pairs of short and fine genital setae, arranged in longitudinal rows, anterior two pairs close to each other. Insertion of tendon β next to anterior corners of genital orifice. Aggenital setae absent. Anal plates triangular. Preanal organ triangular in ventral view. Two pairs of short anal setae, an1-2, present. Two pairs of short and simple adanal setae ad1-2 present, ad3 absent. Lyrifissure iad obliquely, adjacent to anterior corners of anal orifice.
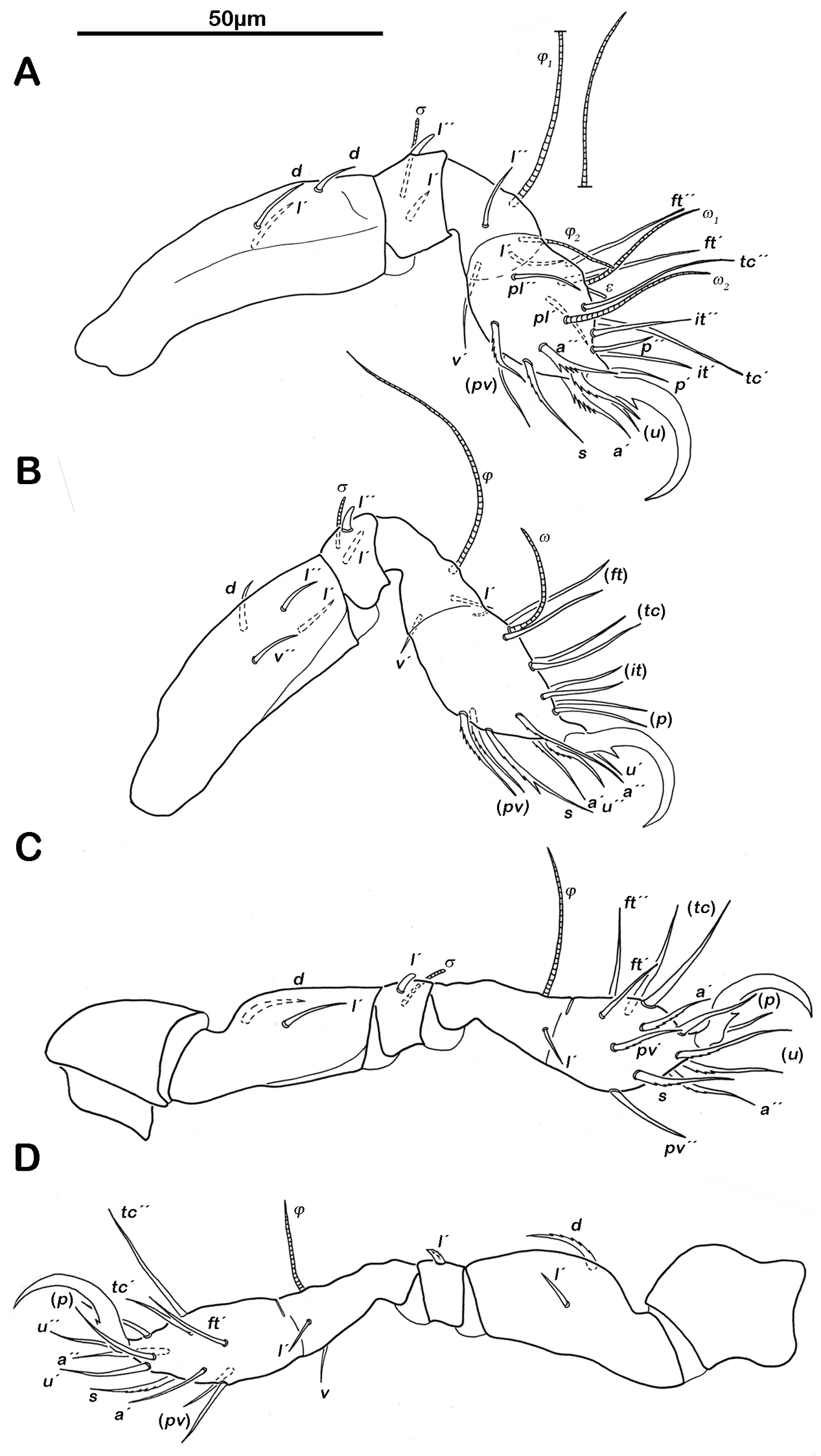
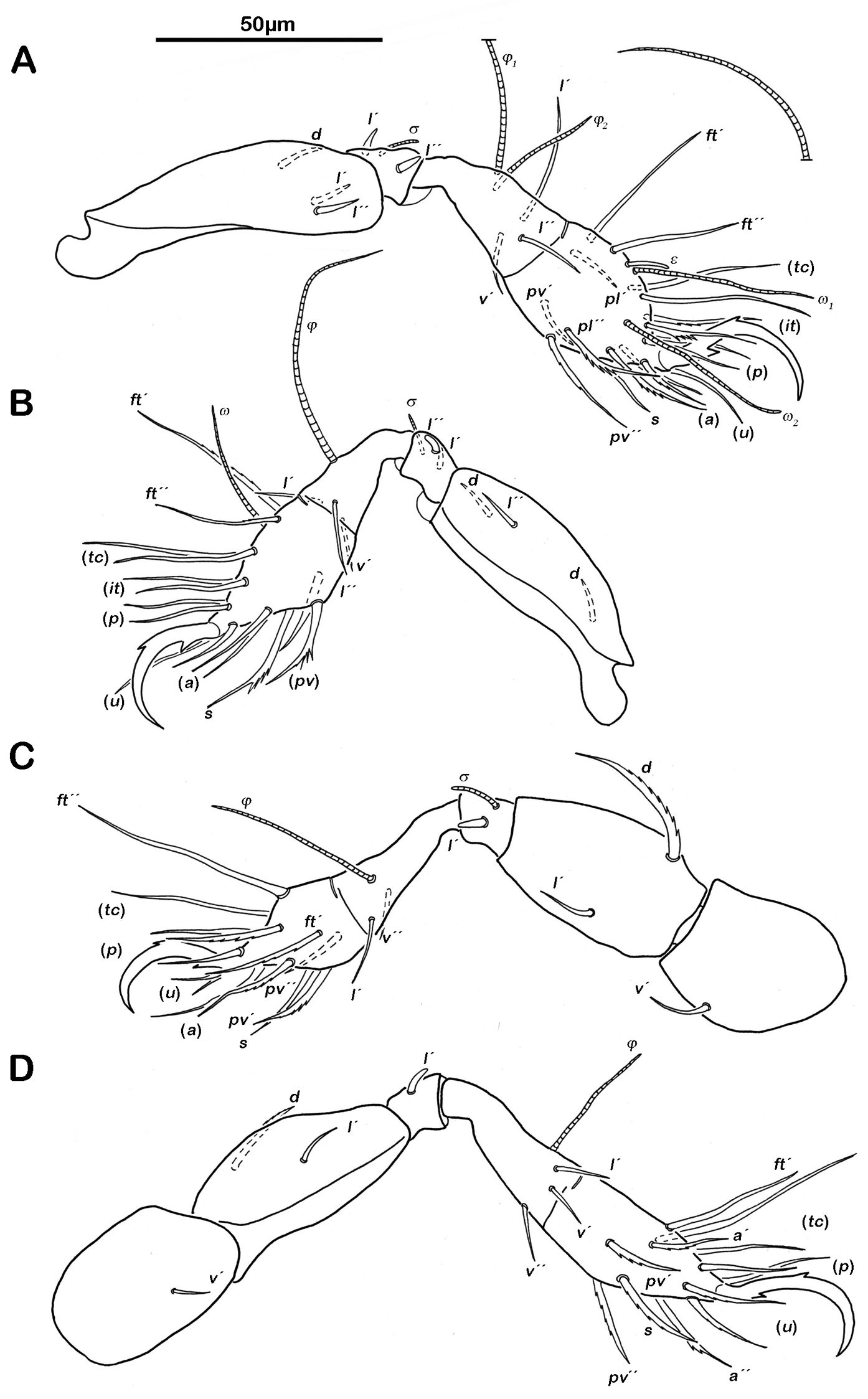
Legs. Monodactylous. Long hook-like claws with one conspicuous proximoventral and one minuscule proximodorsal tooth. Trochanters III and IV with an obvious triangular dorsodistal projection. Femora with slightly projecting ventral carinae. All tarsi with one proximal lyrifissure. No porose areas on femora discernable. Solenidia φ1 on tibia I long and orientated backwards. Chaetome and Solenidia see Table 1.
The specific name refers to Quasimodo, the famous bell-ringer of Victor Hugo’s historical novel “Notre-Dame de Paris” (1831). This appellation is due to the hunchback of this species shown in lateral view (that does not necessarily mean the species is as ugly as the bell-ringer was supposed to be). The name is given as noun in apposition.
Apheredermous. Colour light brown. Integument strongly plicate, except for centrodorsal plate. Thick layer of cerotegument covering whole body. Prodorsum triangular, rostrum rounded. Rostral and lamellar setae short and smooth. Exobothridial setae reduced to a circular vestigial structure. Interlamellar setae very short. Sensillus long and flagelliform. Bothridium large cup, laterally opened. Gnathosoma no obvious differences to adult instar. Hysterosoma slightly concave, plateau-like. Slightly plicate centrodorsal plate occupying two thirds of dorsal hysterosoma, bearing centrodorsal setae. Hysterosomal cupules not discernable in any instar. Large folds framing centrodorsal plate completely, showing fine granular surface. Orifice of opisthonotal gland laterad of seta ad2. Integument surrounding anogenital area folded. Dorsal setae of tibiae and genua absent. No porose areas detectable in any stage.
Length (N=3): 172–209 μm (mean 191 μm)
Gastronotic region (Fig. 4A) with 24 pairs of notogastral setae; setae c1-3, da, dm, dp, la, lm and lp duplicated, h1-3 and p1-3 normal. Centrodorsal setae da, dm and dp robust and dorsally serrate, all other setae simple and small.
Ventral region of idiosoma (Fig. 4B). Epimeral setation 1-0-1-0. One pair of short genital setae.
Selenoribates quasimodo sp. n. nymphs. A protonymph dorsal view B protonymph ventral view C tritonymph dorsal view D tritonymph ventral view.
Legs. Chaetome and Solenidia see Table 1.
Chaetome and solenidia of the new Selenoribates species. Presence of setae characterized by letters. () = pairs of setae, – = no change with regard to preceding stage.
| Selenoribates quasimodo sp. n. | instars | trochanter | femur | genu | tibia | tarsus | chaetome | solenidia |
| Leg I | protonymph | - | d, d, l´ | (l), σ | (l), ν´, φ1 | (pv), (pl), s, (a), (u), (p), (tc), (ft), ω 1, ω 2, ε | 0-3-2-3-16 | 1-1-2 |
| tritonymph | - | - | - | φ2 | (it) | 0-3-2-3-18 | 1-2-2 | |
| adult | - | - | - | - | - | 0-3-2-3-18 | 1-2-2 | |
| Leg II | protonymph | - | d, l" | (l), σ | l´, ν´, φ | (pv), s, (a), (u), (p), (tc), (ft), ω | 0-2-2-2-13 | 1-1-1 |
| tritonymph | - | d, l´ | - | - | (it) | 0-4-2-2-15 | 1-1-1 | |
| adult | - | - | - | l" | - | 0-4-2-3-15 | 1-1-1 | |
| Leg III | protonymph | - | d, l´ | l´, σ | l´, φ | (pv), s, (a), (u), (p), (tc), (ft) | 0-2-1-1-13 | 1-1-0 |
| tritonymph | - | - | - | - | - | 0-2-1-1-13 | 1-1-0 | |
| adult | ν´ | - | - | v" | - | 1-2-1-2-13 | 1-1-0 | |
| Leg IV | protonymph | - | - | - | - | (pv), (u), (p), ft´ | 0-0-0-0-7 | 0-0-0 |
| tritonymph | - | d, l´ | l´ | l´, ν", φ | s, (a), (tc) | 0-2-1-2-12 | 0-1-0 | |
| adult | ν´ | - | - | - | - | 1-2-1-2-12 | 0-1-0 | |
| Selenoribates satanicus sp. n. | instars | trochanter | femur | genu | tibia | tarsus | chaetome | solenidia |
| Leg I | adult | - | (l), d | (l), σ | (l), ν´, φ1, φ2 | (pv), (pl), s, (a), (u), (p), (it), (tc), (ft), ω 1, ω 2, ε | 0-3-2-3-18 | 1-2-2 |
| Leg II | adult | - | d, d, l" | (l), σ | (l), ν´, φ | (pv), s, (a), (u), (p), (it), (tc), (ft), ω | 0-3-2-3-15 | 1-1-1 |
| Leg III | adult | ν´ | d, l´ | l´, σ | l´, ν´, φ | (pv), s, (a), (u), (p), (tc), (ft) | 1-2-1-2-13 | 1-1-0 |
| Leg IV | adult | ν´ | d, l´ | l´ | l´, (ν), φ | (pv), s, (a), (u), (p), (tc), ft´ | 1-2-1-3-12 | 0-1-0 |
| Selenoribates elegans sp. n. | Instars | trochanter | femur | genu | tibia | tarsus | chaetome | solenidia |
| Leg I | adult | - | (l), d | (l), σ | (l), ν´, φ1, φ2 | (pv), (pl), s, (a), (u), (p), (it), (tc), (ft), ω 1, ω 2, ε | 0-3-2-3-18 | 1-2-2 |
| Leg II | adult | - | d, d, l" | (l), σ | (l), ν´, φ | (pv), s, (a), (u), (p), (it), (tc), (ft), ω | 0-3-2-3-15 | 1-1-1 |
| Leg III | adult | ν´ | d, l´ | l´, σ | l´, ν´, φ | (pv), s, (a), (u), (p), (tc), (ft) | 1-2-1-2-13 | 1-1-0 |
| Leg IV | adult | ν´ | d, l´ | l´ | l´, (ν), φ | (pv), s, (a), (u), (p), (tc), ft´ | 1-2-1-3-12 | 0-1-0 |
Length (N=9): 228–266 μm (mean 243 μm)
Gastronotic region (Fig. 4C, 5A). 44 pairs of notogastral setae, setae of series c, d, l and h-series further multiplied.
Ventral region of idiosoma (Fig. 4D, 5B). Epimeral setation 1-0-1-1. Three pairs of short genital setae in a longitudinal row. Two pairs of adanal setae ad1-2. Two pairs of anal setae an1-2.
Legs (Fig. 6). Chaetome and Solenidia see Table 1.
Selenoribates quasimodo sp. n. tritonymph, micrographs layered from 5–10 sequentially focused images. A dorsal view B ventral view. Scale bar = 100 µm.
Selenoribates quasimodo sp. n. tritonymph legs. A right leg I antiaxial view B right leg II antiaxial view C left leg III antiaxial view D right leg IV antiaxial view.
urn:lsid:zoobank.org:act:30EC290D-FD6A-4E7B-B581-30F627ADC954
Bermuda: Lover’s Lake (marine pond), 32°22'03"N, 64°42'37"W, median eulittoral zone, green algae growing on sandy substrate among mangrove roots, 26 January 2012.
Holotype: female, preserved in pure ethanol, deposition: Naturhistorisches Museum Wien, collection nr. NHMW 21885. Paratypes: 1 female and 1 male, same locality as holotype; deposition: Senckenberg Museum für Naturkunde Görlitz, collection Nr. 12/48678.
Light brown sclerotized mites. Average length 283 µm, mean width 174 µm. Notogaster rounded in dorsal view, concave in lateral view. Lamellar ridges long reaching lamellar setae. On anterior border of notogaster a pair of horn-like structures. Three notogastral depressions, framed by longitudinal ridges. Fourteen pairs of spiniform notogastral setae. Two median epimeral cavities. Three pairs of genital setae. Three pairs of adanal and one pair of anal setae. Legs monodactylous with large claw. Claw with one proximoventral and one proximodorsal tooth.
Adult: Females (N=6), length: 278–308 μm (mean 290 μm), width: 175–188 µm (mean 181 µm); males (N=5), length: 265–291 μm (mean 274 μm), width: 163–172 µm (mean 168 µm).
Integument. Colour light brown. Cuticle appears granular under dissecting microscope. Cerotegument of prodorsum and notogaster granular. Cerotegument of lateral parts generally finely granular, larger granules in areas surrounding acetabula. Ventral cerotegument generally finely granular, areas laterad of anal opening showing stronger granulation.
Prodorsum. Rostrum rounded in dorsal view, but slightly projecting anteroventrally in lateral view. Rostral setae (ro), lamellar setae (le) and interlamellar setae (in) short and simple. One pair of minute exobothridial setae (ex). Lamellar ridges conspicuous, reaching insertions of lamellar setae. Bothridium large cup with a projecting posterior ridge. Sensillus long (ca. 53 µm) and flagelliform. Tutorium weakly developed and small.
Gnathosoma. Pedipalp pentamerous 0-2-1-3-9 (including solenidion). Solenidion erect, not fused with eupathidium acm. Chelicera chelate, in lateral view forceps-like and each digit with two teeth, whereas from frontal view most distal teeth split into two symmetrical teeth. Distal part of rutellum developed as thin triangular slightly curved inward membrane. Setae a and m long and smooth. Mentum regular, setae h simple, thin and long.
Notogaster (Figs 7A, 8A). Rounded in dorsal view, concave in lateral view. Anterior margin of notogaster incomplete, medially interrupted. On anterior border of notogaster a pair of strongly anteriorly projecting horn-like structures, situated directly posterior of bothridia. Three notogastral depressions on anterior third of notogaster, framed by longitudinal ridges reaching transversal line of setae la and da. Cerotegument of depressions strongly granular. Fourteen pairs of notogastral setae, c1-2, da, dm, dp, la, lm, lp, h1-3, p1-3 (approximate length 10- 13 µm); c3 absent. Setae da- dp, slightly serrate, all other setae smooth. Porose areas or distinct pores absent. Five pairs of notogastral lyrifissures present; ia anterior to seta c2, in dorsal view hidden under anterior notogastral horn-like projection; im posterior of seta c2; lyrifissures ih, ip and ips laterally close to lateroventral border of notogastral plate. Orifice of opisthonotal gland (gla) next to setae la.
Selenoribates satanicus sp. n. adult. A dorsal view B ventral view C lateral view.
Selenoribates satanicus sp. n. adult micrographs layered from 5–10 sequentially focused images. A dorsal view B ventral view. Scale bar = 100 µm.
Lateral aspect (Figs 7C, 8C). Pedotectum I small but thick, pedotectum II absent. Lateral sejugal furrow broad and deep, showing conspicuous granulation. Enantiophyse consisting of two strong triangular teeth orientated against each other. Anterior tooth slightly rounded. Discidium developed as strong triangular bulge between acetabulum III and IV.
Ventral region of idiosoma (Figs 7B, 8B). Epimeral setation 1-0-1-1, seta 1b long reaching trochanter III, setae 3b and 4a short. Internal borders of all epimera well visible, sternal apodemes II, sejugal and III well developed. A densely granulated median sternal cavity on epimeron I and a second circular median cavity on a level with apodemes III. Three pairs of short and fine genital setae, arranged in longitudinal rows, anterior two pairs of setae close to each other. Insertion of tendon β next to anterior corners of genital orifice. Aggenital setae absent. Anal valves triangular. Preanal organ shaped triangular in ventral view. One pair of short anal setae, an1, located on posterior half of anal valves. Three pairs of short and simple adanal setae ad1-3. Lyrifissure iad obliquely, adjacent to anterior corners of anal orifice.
Legs (Fig. 9). Monodactylous. Long pointed hook-like claw with one conspicuous proximoventral and a minute proximodorsal tooth. Trochanters III and IV with a triangular dorsodistal projection. Femora exhibiting slightly projecting ventral carinae. All tarsi with one proximal lyrifissure. No porose areas on femora discernable. Solenidion φ1 on tibia I long, orientated backwards. Chaetome and Solenidia see Table 1.
Larva. Length (N=1): 137 μm
Selenoribates satanicus sp. n. legs. A right leg I antiaxial view B right leg II axial view C right leg III antiaxial view D left leg IV antiaxial view.
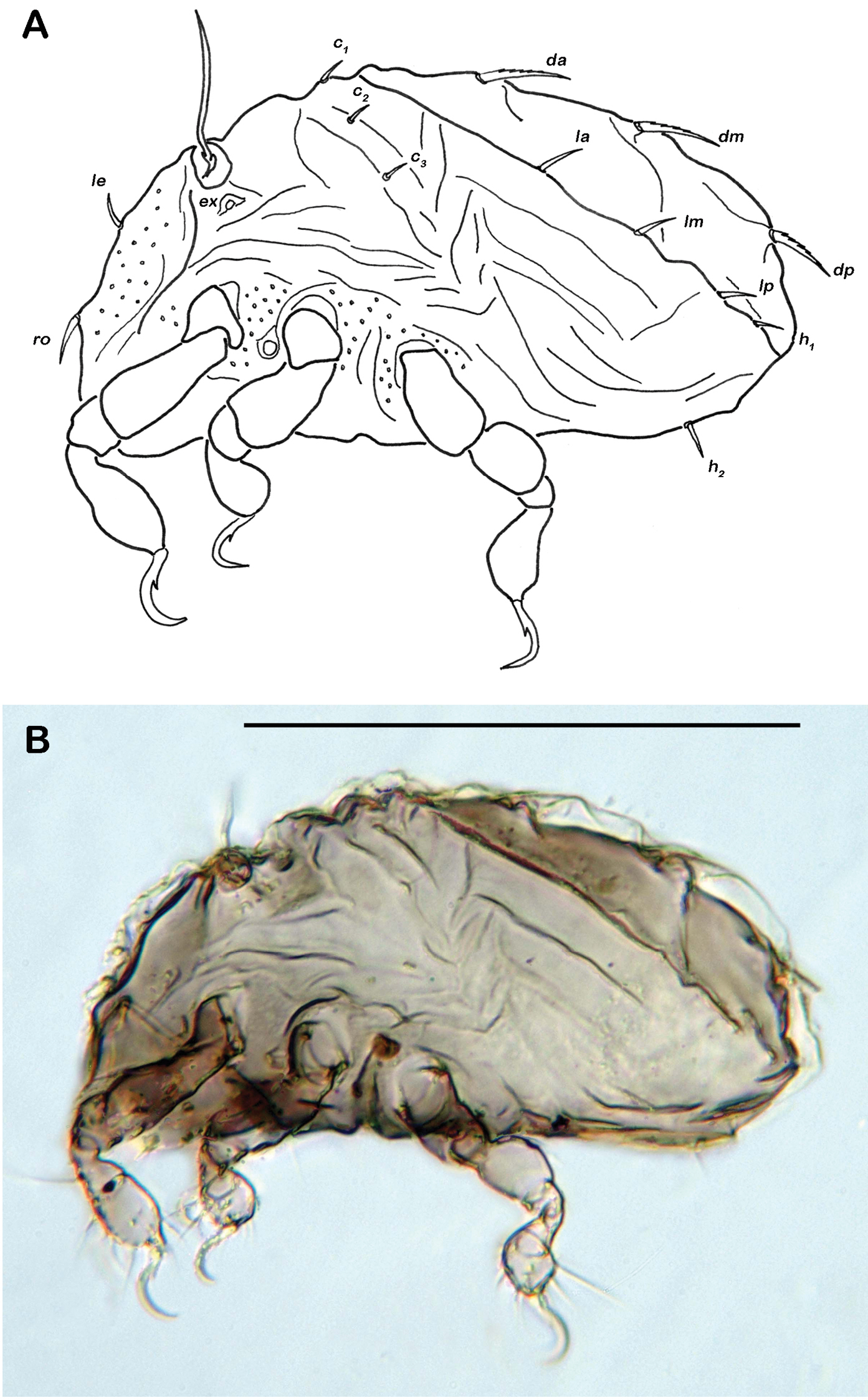
Gastronotic region (Fig. 10). 11 pairs of notogastral setae; setae c1-3, da, dm, dp, la, lm, lp and h1-2, h3 absent. Centrodorsal setae da, dm and dp robust and dorsally serrate, all other setae simple and small.
Ventral region of idiosoma. Epimeral setation 1-0-1. Claparède organ bladder-like. No protecting seta detectable.
Selenoribates satanicus sp. n. larva. A lateral view B lateral view, micrograph layered from 5–10 sequentially focused images. Scale bar = 100 µm.
When the author regarded Selenoribates satanicus in dorsal view for the first time, the oval contour of the notogaster with its two anterior horn-like projections reminded him of the silhouette of the devil’s face, therefore the specific name refers to the Hebraic name Satan and is given as adjective in the nominative singular.
urn:lsid:zoobank.org:act:F9258443-DBA2-408F-890D-2DC72CA9938D
Bermuda, Whalebone Bay, 32°21'55"N, 64°42'49"W, lower intertidal area, red algae on rocks, 22 November 2011.
Holotype: female, preserved in pure ethanol, deposition: Naturhistorisches Museum Wien, collection nr. NHMW 21886.
Red-brown sclerotized mites. Average length 202 µm, mean width 115 µm. Notogaster oval in dorsal view, slightly concave in lateral view. Lamellar ridges absent. Interlamellar setae normal and minute. Two X-shaped ridges on anterior part of notogaster. Fourteen pairs of simple long notogastral setae. Two median epimeral cavities. Claw with two proximoventral and one proximodorsal tooth.
Adult: Males (N=2), length: 200–203 μm (mean 201.5 μm), width: 108–122 µm (mean 115 µm).
Integument. Colour light brown. Cuticle showing dotted pattern. Cerotegument of notogaster granular, larger granules in centre of gastronotic plate. Cerotegument of lateral parts granular, with larger granulation in areas surrounding acetabula. Ventral region finely granular, denser granulation laterad of anal orifice.
Prodorsum. Cerotegument strongly granular. Rostrum rounded in dorsal view, but slightly projecting anteroventrally in lateral view. Rostral (ro) and lamellar setae (le) simple and short, interlamellar setae (in) very short. One pair of minute exobothridial setae (ex). Lamellar ridges absent. Bothridium large cup without posterior ridge. Sensillus long (ca. 48 µm), flagelliform.
Gnathosoma. Pedipalp pentamerous 0-2-1-3-9 (solenidion included). Solenidion erect, not fused with eupathidium acm. Chelicera chelate, forceps-like in lateral view, each digit with two teeth, whereas from frontal view most distal teeth split into two symmetrical teeth. Distal part of rutellum a thin triangular slightly inward curved membrane. Setae a and m long and smooth. Mentum regular, setae h simple, thin and long.
Notogaster (Figs 11A, 12A). Rounded in dorsal view, slightly concave in lateral view. Anterior margin of notogaster complete. On anterior part of notogaster a pair of small X-shaped ridges, close to seta c1. Fourteen pairs of simple notogastral setae, c1-2, da, dm, dp, la, lm, lp, h1-3, p1-3 (approximate length 17-20 µm), setae c3 absent. Porose areas or distinct pores absent. Five pairs of notogastral lyrifissures present; ia anterior to seta c2; im posterior and laterad of seta la; lyrifissures ih, ip and ips laterally close to lateroventral border of notogastral plate. Orifice of opisthonotal gland (gla) posterior to seta c2.
Selenoribates elegans sp. n. adult. A dorsal view B ventral view C lateral view.
Lateral aspect. Pedotectum I small but robust, pedotectum II absent. Enantiophyse consisting of two strong triangular and pointed teeth orientated against each other. Discidium developed as strong triangular projection between acetabulum III and IV.
Ventral region of idiosoma (Figs 11B, 12B). Epimeral setation 1-0-1-1, seta 1b long reaching trochanter III, setae 3b and 4a of normal length and simple. Internal borders of all epimera well visible, sternal apodemes III and IV well developed. Median sternal cavity on epimeron I divided into two anterior symmetric parts and one unpaired posterior part, all parts strongly granulated. A second triangular median cavity on epimeron III on a level with apodeme 3. Three pairs of short and fine genital setae, arranged in longitudinal rows, anterior pairs close to each other. Insertion of tendon β adjacent to anterior corners of genital orifice. Aggenital setae absent. Anal plates triangular. Preanal organ triangular. Two pairs of short anal setae, an1-2. Two pairs of short and simple adanal setae ad1-2, ad3 absent. Lyrifissure iad obliquely, next to anterior corners of anal valves.
Legs. Monodactylous. Long acute hook-like claw with two obvious proximoventral teeth, one close to the base of claw and one proximodorsal tooth. Cuticle finely granular. Femora with projecting ventral carinae. All tarsi with one proximal lyrifissure. Porose areas absent. Solenidia φ1 on tibia I long, orientated backwards. Chaetome and solenidia see Table 1.
Selenoribates elegans sp. n. adult micrographs layered from 5–10 sequentially focused images. A dorsal view B ventral view. Scale bar = 100 µm.
The specific name is derived from the Latin word elegans meaning elegant and refers to the slender and delicate shape of the whole body. The name is given as adjective in the nominative singular.
| 1 | Three depressions on anterior part of notogaster separated by two X-shaped ridges | 2 |
| – | Depressions and ridges shaped different | 5 |
| 2 | Three pairs of anal, four pairs of genital setae | Selenoribates ghardaqensis Abd-El-Hamid, 1973 |
| – | Two pairs of anal setae and three pairs of genital setae | 3 |
| 3 | Sensillus spatuliform | Selenoribates mediterraneus Grandjean, 1966 |
| – | Sensillus flagelliform | 4 |
| 4 | Anterior median epimeral cavity simple and shaped circular; lamellar ridges short but conspicuous | Selenoribates foveiventris Strenzke, 1961 |
| – | Anterior median epimeral cavity divided into two anterior symmetric parts and one unpaired posterior part; lamellar ridges absent | Selenoribates elegans sp. n. |
| 5 | Three depressions on anterior part of notogaster separated by longitudinal ridges reaching transversal line of setae la and da; on anterior border of notogaster a pair of horn-like projections | Selenoribates satanicus sp. n. |
| – | A single large depression on anterior part of notogaster, causing a hunchbacked appearance in lateral view | Selenoribates quasimodo sp. n. |
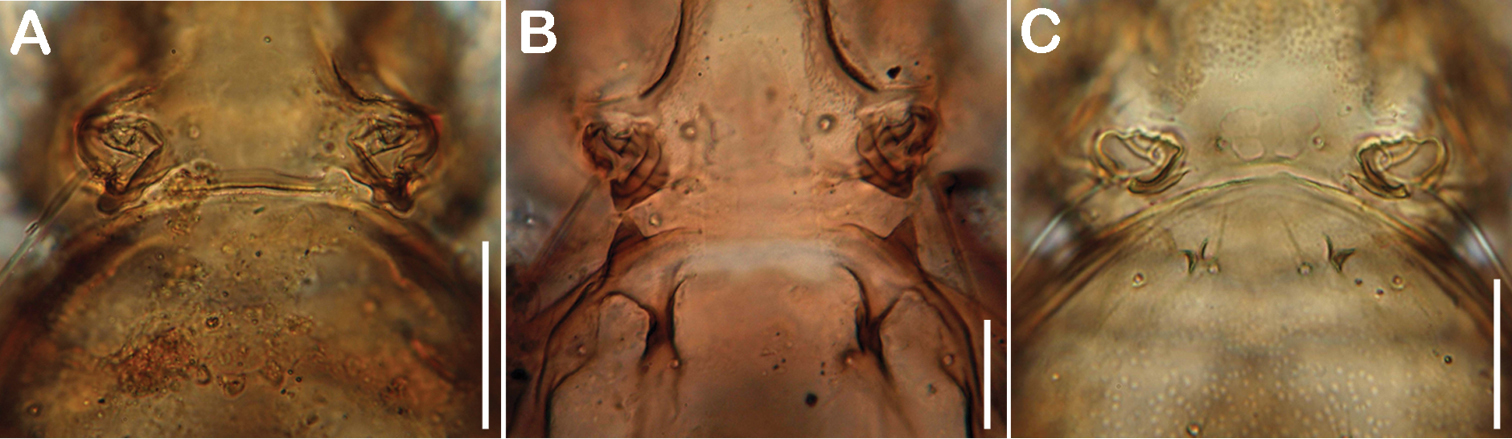
Comparison of cuticular structures on anterior part of gastronotic region. A Selenoribates quasimodo sp. n. B Selenoribates satanicus sp. n. C Selenoribates elegans sp. n. Scale bars = 30 µm.
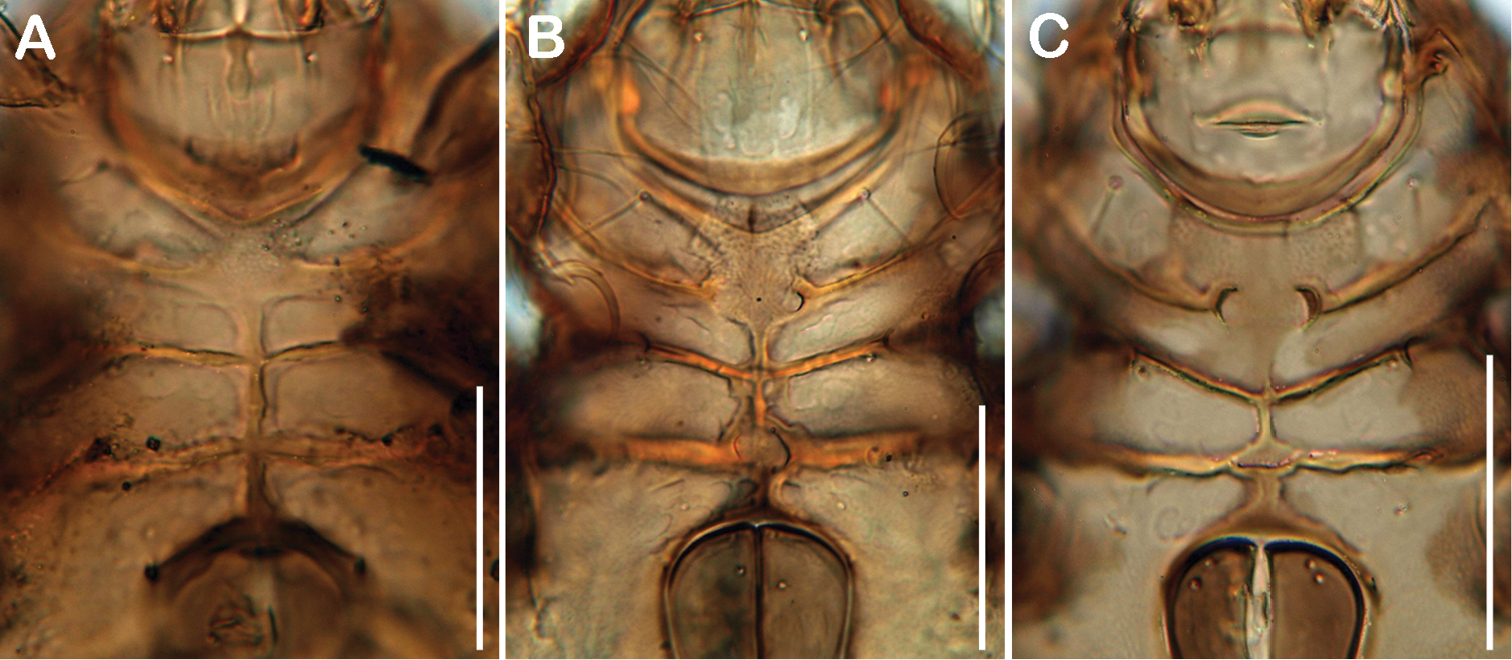
Comparison of ventral cavities. A Selenoribates quasimodo sp. n. B Selenoribates satanicus sp. n. C Selenoribates elegans sp. n. Scale bars 50 µm.
Comparison of diagnostic morphological features of all Selenoribates species. ? = no information available; depr. = depression; X = X-shaped ridge.
| Selenoribates foveiventris | Selenoribates mediterraneus | Selenoribates ghardaqensis | Selenoribates quasimodo | Selenoribates satanicus | Selenoribates elegans | |
|---|---|---|---|---|---|---|
| size (µm) | 240-250 | 242-251 | 198-218 | 212-244 | 265-308 | 200-203 |
| exobothridial seta | vestigial | minute | ? | minute | minute | minute |
| lamellar ridges | short | short | short | short | long | absent |
| gastronotic structures | 3 depr. 2X | 3 depr. 2X | 3 depr. 2X | 1 depr. | 3 depr. | 3 depr. 2X |
| notogastral setae | 14 | 14 | 14 | 14 | 14 | 14 |
| epimeral setae | 1-0-1-1 | 1-0-1-1 | 1-0-1-1 | 1-0-1-1 | 1-0-1-1 | 1-0-1-1 |
| genital setae | 3 | 3 | 4 | 3 | 3 | 3 |
| anal setae | 2 | 2 | 3 | 2 | 1 | 2 |
| adanal setae | 2 | 2 | 2 | 2 | 3 | 2 |
| claw / ventral teeth | 2 | 2 | 2 | 1 | 1 | 2 |
Knowledge about juvenile morphology of this taxon is largely incomplete and only
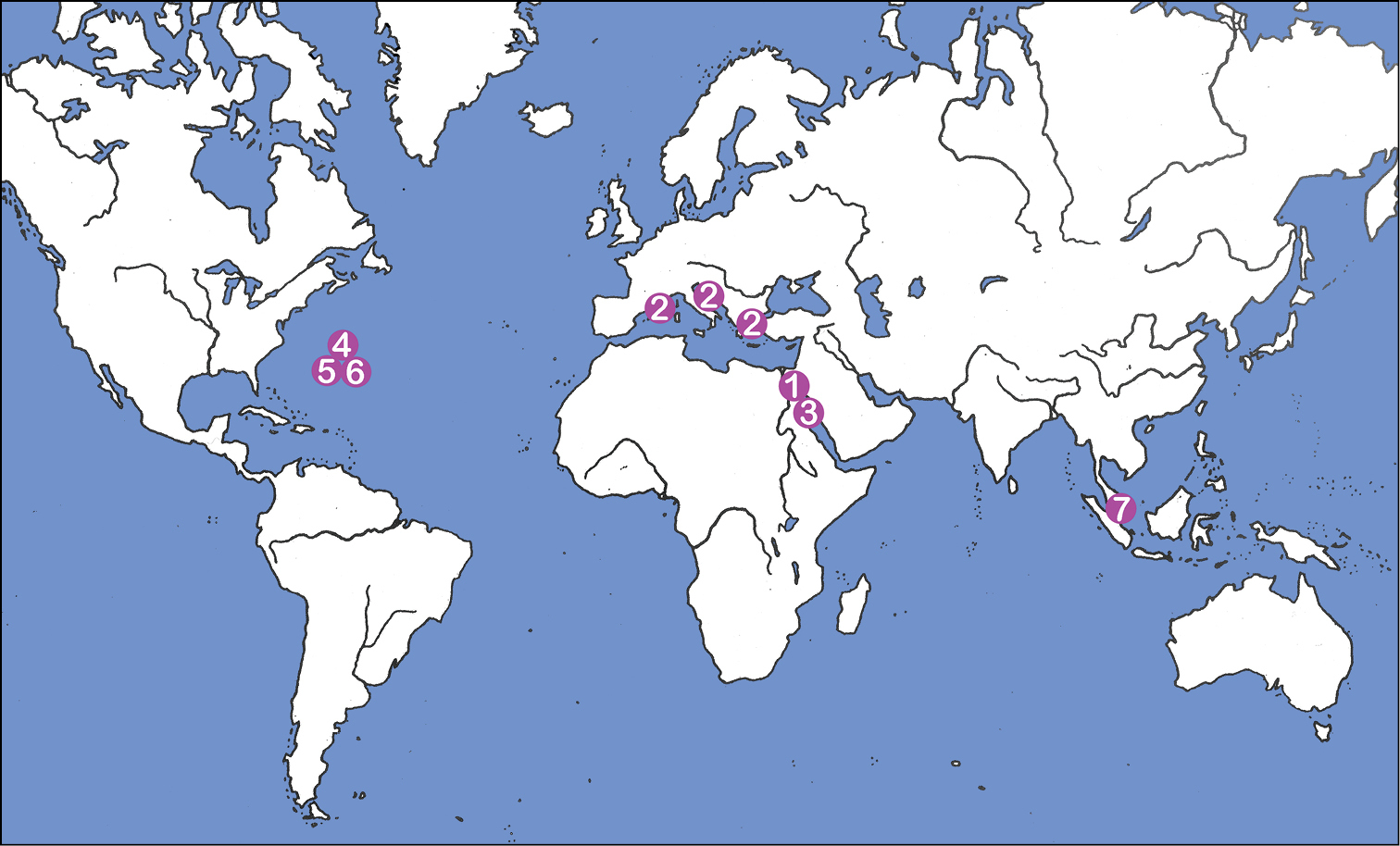
The biogeographic distribution of the genus Selenoribates was formerly limited to the Mediterranean and the Red Sea, but the records of new species from Bermuda clearly demonstrate that members of this genus also exist on coasts of the Western Atlantic. Moreover, littoral samples from Singapore, kindly provided by Ilse Bartsch, also contained specimens of a yet undescribed Selenoribates species. These new findings suggest that members of this genus show a much wider distribution than formerly supposed (Fig. 15) probably occurring on most coasts of tropic and subtropic regions. However, not only the biogeography, but also the diversity of Selenoribates must be reconsidered based on the present data. Bermuda is, with ca. 55 km2, one of the smallest countries of the world and harbours just as much Selenoribates species as the whole Mediterranean and the Red Sea together. Of course the Bermudian intertidal mite fauna may be derived from the Caribbean region and the species found on Bermuda may show a much wider distribution, but this clearly indicates that the real number of species may exceed the presently documented number by far.
Map showing world distribution of the genus Selenoribates: 1 Selenoribates foveiventris, Egypt (
I am indebted to the Bermuda Institute of Ocean Sciences (BIOS) Inc. and its staff for the provision of research facilities and accommodation. I want to thank Wolfgang Sterrer for his valuable and continuous support. Special thanks to Reinhart Schuster, who discovered the first specimens of Selenoribates quasimodo 40 years ago, for his important advices and help in all respects. Thanks also to Ilse Bartsch providing samples from Singapore; the samples were collected during the Comprehensive Marine Biodiversity Survey of Singapore: International Workshops 2012/2013: Johor Strait Workshop organised by Tan Koh Siang, Peter Ng, Tan Heok Hui and Joelle Lai, National Parks Board and sponsored by Shell Eastern Petroleum (Pte Ltd), Asia Pacific Breweries (Singapore), Care-for-Nature Trust Fund and Hong Kong & Shanghai Bank. The present research was funded by the Austrian Science Fund (FWF): [J3150].
Table of localities. (doi: 10.3897/zookeys.312.5478.app) File format: Microsoft Excel file (xls).
Explanation note: Detailed information on sampling localities of the three new Selenoribates species.