(C) 2013 Sergio Leiva. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species, Agistemus aimogastaensis, is described with the aid of optical and Scanning Electron Microscopy. This mite is an important predator of two eriophyid mites (Aceria oleae and Oxycenus maxwelli) in olive orchards (Olea europaea, variety Arauco) in La Rioja Province. The problems related to eriophyids in olive orchards in Argentina are highlighted and photos of the damage on leaves and fruit are included.
Agistemus aimogastaensis, new species, predator, Aceria olea, Oxycenus maxwelli, Olive orchards, Argentina
Species of the genus Agistemus are considered important predators on phytophagous mites, scale insects and their eggs. Recently several studies have been done on agriculturally important crop plants, such as apple, pear and citrus orchards, blackberry fruits, coconut, coffee, and fig trees, grapevines, leguminous plants, Yerba mate trees, medicinal and ornamental plants as well as vegetable crops and the stored products of these plants (
Little is known about Agistemus species as predators of eriophyid mites in Olive orchards (
The genus Agistemus was erected by
All specimens were collected individually from tree surfaces (vegetative buds, leaves, inflorescences, or fruits) and preserved in 70% ethanol. Specimens studied by means of light microscopy were macerated in lactic acid and observed in the same medium, using the open-mount technique (cavity slide and cover slip) as described by
urn:lsid:zoobank.org:act:21DBBD18-3CF8-42B0-9F79-41FEC0BB98D2
The specific epithet is dedicated to the city of Aimogasta, La Rioja, Argentina, where the specimens were found.
Holotype female and 2 female paratypes, Aimogasta, Province de La Rioja, Argentina 11-NOV-2012 deposited in Instituto Nacional de Tecnologia Agropecauria (INTA), Aimogasta, La Rioja Argentina; 4 Paratype females, same date and locality as holotype deposited in Museum National d’Histoire Naturelle, Paris, France and 4 paratypes, same date and locality as holotype deposited in Geneva Natural History Museum, Switzerland. All preserved in 70% ethanol. All type specimens were collected from vegetative buds, leaves, inflorescences and fruit of Olea europaea, variety Arauco.
(adult female). Propodosomal plate: trapezoidal; ornamented with a faintly accentuated, polyhedral reticulate pattern; eyes clearly visible, ovoid convex, smooth; post ocular body triangular, rounded extremities, with series of longitudinally aligned small round-convex elevations, joined by thread–like strands. Metapodosomal plate hexagonal to polyhedral; ornamented with accentuated transverse polyhedral reticulate pattern. Wide area with fine transverse integumental striae, separating propodosomal and metapodosomal plates. Humeral and intercalar plates marginally. Setae g, ps1, ps2 Similarly shaped, finely barbate, sharply tipped; ps3 minutely dentate, truncate g, ps1, ps2 larger than ps3 and very different in shape and appearance in optical and SEM. Legs: genua II, III, IVsetal formula 0-0-0; leg IV lacks solenidion. Ambulacra with two claws and empodium with three pairs of bicapitate, fan shaped Y-raylets.
This species most closely resembles Agistemus collyerae Gonzalez-Rodriguez 1963, principally in relation to the setation of leg IV. However Agistemus aimogastaensis can be easily differentiated from the latter on account of the disposition and shape of propodosomal, metapodosomal, humeral and intercalar plates; as well as the length and disposition of dorsal setae. Specific characters given by Gonzalez-Rodriguez for Agistemus collyerae in relation to the unusual lengths of the ag2 setae (pg2 sensu
Measurements: SEM: 325 (312–351) × 160 (152–173) Light microscopy: 336 (331–339) × 168 (166–174) (n=10).
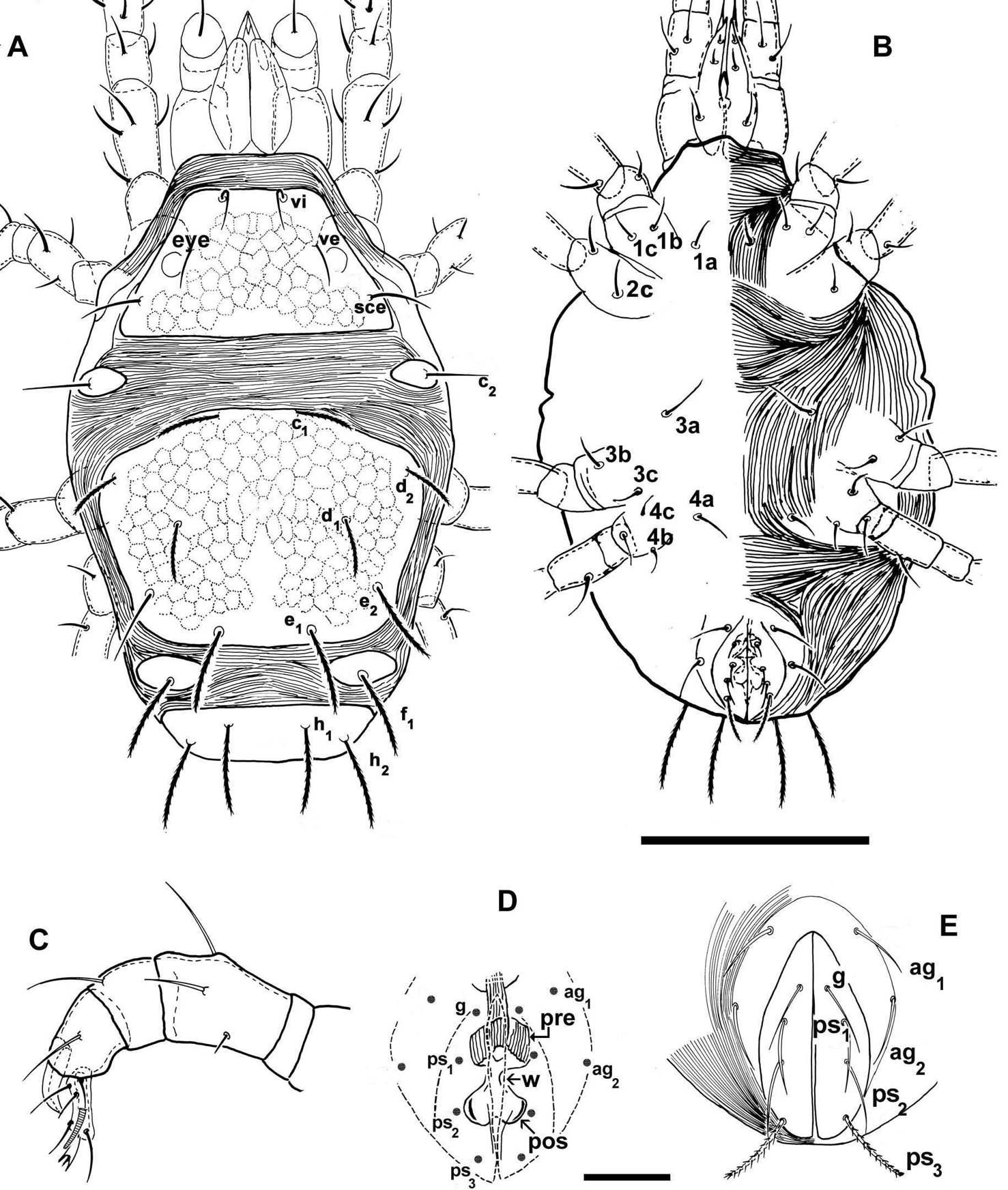
Shape: ovoid (Figures 1A, B).
Agistemus aimogastaensis sp. n. Adult female, optical microscopy. A dorsal view B ventral view C palp D cuticular components of genital chamber; the anogenital covers are presented as indication of its relation to genital organs. E, anogenital covers. Abbreviations see Material and methods. Scale bars: A, B: 100 µm; C, D, E: 15 µm.
Colour: variable. Specimens observed in reflected light: orange-yellow, slightly shiny or white. We studied specimens of different colors and all were female.
Integument: (Figures 1A, B; 2A, D, E)
Microsculpture complicated, varying according to body region.
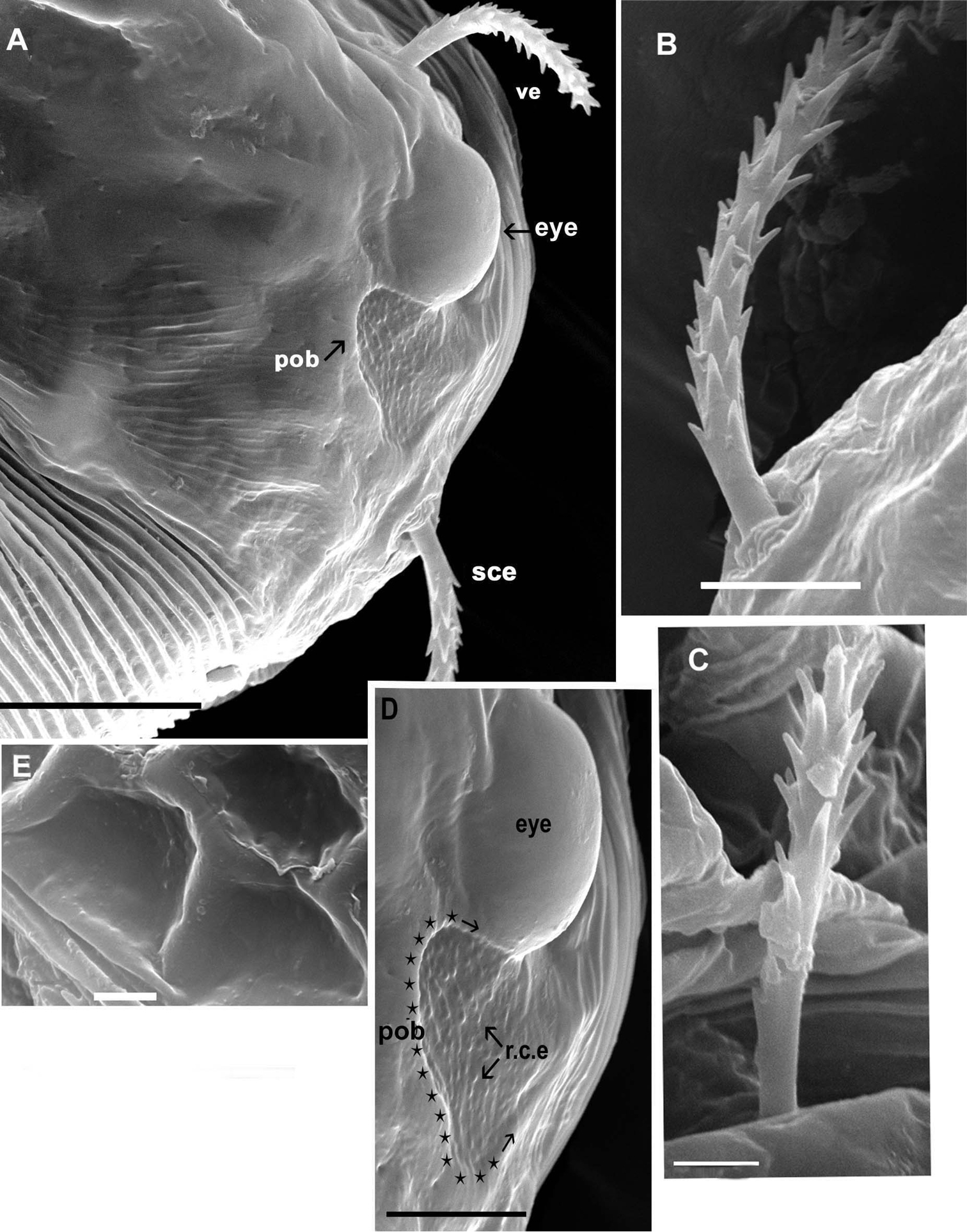
Agistemus aimogastaensis sp. n. Adult female, SEM. A eye and post ocular body, lateral view B external scapular setae (sce), lateral view C internal vertical setae (vi), lateral view D eye and postocular body, detail, lateral view (indicated by stars and arrow) E ornamentation, metapodosomal plate. Abreviations: see Material and Methods. Scale bars: A: 10µm; B, D, E: 5µm; C: 2µm.
Propodosomal plate (P) polyhedral reticulate pattern: tiny accentuated polyhedral reticulated pattern, extending behind vi setal insertion and paraxially to ve and sce setal insertion, and paraxial to eye (eye) and post ocular body (pob). Near the eye and post ocular body and antiaxially to the ve and sce setal insertion smooth (Figs 2A, D). Existing paraxially to eye and pob, very fine integumental striae.
Metapodosomal (M) plate with polyhedral reticulate pattern, accentuate (Fig. 2D). Humeral plate (H), Intercalary plate (I), and Suranal plate (SA), more or less smooth (Fig. 1A).
Fine integumental striae covering zone between Propodosomal, Metapodosomal, Humeral, Intercalar and Suranal plates (Figs 1A, 2A).
Fine integumental striae covering venter of idiosoma, epimeral zone smooth (Fig. 1B).
Legs: cuticular surface smooth.
Setation. All dorsal setae minutely denticulate and truncate (Fig. 3C, D). Length: vi 12.60 (12.04-13.012); ve 13, 78 (13.05-13.92); sce 18.80 (18.78-18.93); c2 20.70 (19.89-21.01; c1 19.5 (19.56-19.80); d1 16.45 (16.43-16.48); e1 18.1 (18.00-18.09); d2 19.33 (19.23-19.92); e2 17.80 (17.77-17.84); f1 17.85 (16.01-17.69); h1 14.20 (14.18-14.24); h2 17.20 (17.16-17.24).
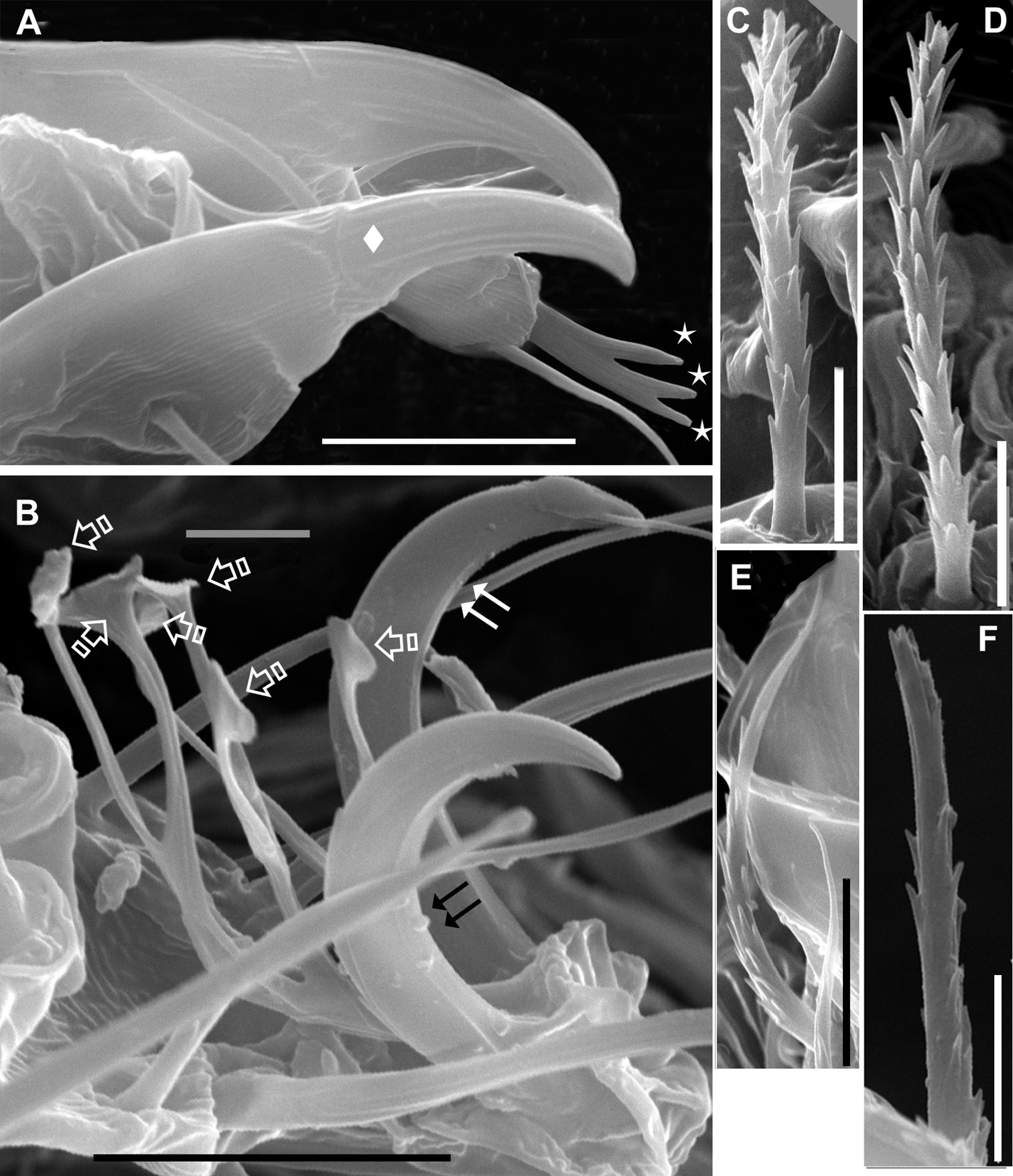
Agistemus aimogastaensis sp. n. Adult female, SEM. A palp, tibia and tarsus lateral view B ambulacrum leg I, lateral view. C. dorsocentral a setae D dorsolateral la setae E ps2 setae. F ps3 setae. Abreviations see material and Methods. Scale bars: B, E: 5µm; A, C, D, F: 5µm. Small stars indicate the association of eupathidia ul’, ul” and sul. Diamond indicates palp tibial claw. Double arrow, indicates claw, and special single arrow indicate capitate fan-shaped raylets.
Ventral setae: epimeric smooth (1a, 1b, 1c, 2a, 3a, 3b, 3c, 4a, 4b, 4c); (ag1, ag2) and g, ps1, ps2, finely barbate (Fig. 3E), sharply tipped; ps3 minutely dentate, truncate (Fig. 3F).
Length: ag1 17.61 (17.58-17.66); ag2 17.70 (17.68-17.75); g 17.25 (17.17-17.29); ps1 17.40 (17.38-17.43); ps2 18.20 (18.18-18.24); ps3 17.05 (17.00-17.12).
In optical microscopy the dorsal setae and genital ps3 appear as dark, while epimerics, paragenital and genitals (g, ps1, ps2) appear transparent. Scanning Electron Micrographs depicted in Figure 3.
Dorsal region (Figure 1A). Propodosomal plate (P) trapezoidal, with three pairs of setae: vi situated close to the anterior margin of plate; ve situated slightly anteriorly and paraxially to the eye and the postocular body (pob); sce, situated posteriorly and antiaxially to pob. All setae situated on very small protuberances.
Observation of eye and the postocular body (pob) (not shown on Fig. 1A) is complex, because on mites not cleared the eye and the pob can both be observed, but in cleared animals only the eye is visible. Position of ve setae complicating observation in optical microscopy. SEM permits observation of the eye in dorsal view (Fig. 2A, D) as a smooth structure, ovoid and convex in lateral view; length: 9.55 (9.48-9.56); width: 6.28 (6.26-6.29). The pob has a more or less triangular shape with rounded extremities (Fig. 2A, D); 5.81(5.79-5.83) in length and 5.34 (5.32-5.37) in width; a series of longitudinally aligned slightly rounded-convex elevations (r.c.e) present, joined by thread–like strands. In recently mounted specimens (observed in optical microscopy), the pob presenting small red-yellow spots, disappearing quickly; possibly these spots are the r.c.e observed in SEM.
Propodosomal and metapodosal plates separated by a relatively large expanse of fine integumental striae (Fig. 1A, 2A).
Humeral plate (H) ovoid, situated antiaxially to P-plate and slightly antiaxially to M-plate; setae c2 insertion situated slightly paraxially to d2 insertion level (Fig. 1A).
Metapodosomal plate (M) hexagonal to polyhedral.
Dorsocentral setae: insertions c1 and e1 situated on the same longitudinal level; d1 insertion situated antiaxially to c1 and e1 insertion level. Dorsolateral setae: d2 insertionsituated externally and close to plate margin, posteriorly to c1 insertion level but anteriorly to d1 insertion level; e2 situated slightly paraxially to the d2 insertion level and posteriorly and antiaxially to d1 insertion level (Fig. 1A).
Intercalary plates (I) ovoid, situated near the body margin (Fig. 1A); f1 setal insertion situated paraxially to e2 insertion level and antiaxally to e1 insertion level.
Ventral region. Epimera well defined (Fig. 1B). Setal formulae: 3-1-3-3. Anogenital region clearly discernible. Two pairs of paragenital setae: ag1, ag2; and four pairs of setae: g, and three anal setae ps1, ps2, ps3 (see Setation). g, ps1, ps2 and ps3 differing in shape (See Setation).
Cuticular components of the genital chamber with preatrium (pre), saucer-shaped structure, longitudinal striate and postatrium (post) bilobed; between pre and pos a constriction or waist (w) (Fig. 1D).
Legs (Figure 4A–D). All legs with ambulacrum, composed of two claws with small tooth, and an empodium with three pairs of capitate fan-shaped raylets (resembling leaves of Ginkgo biloba tree) (Fig. 3B).
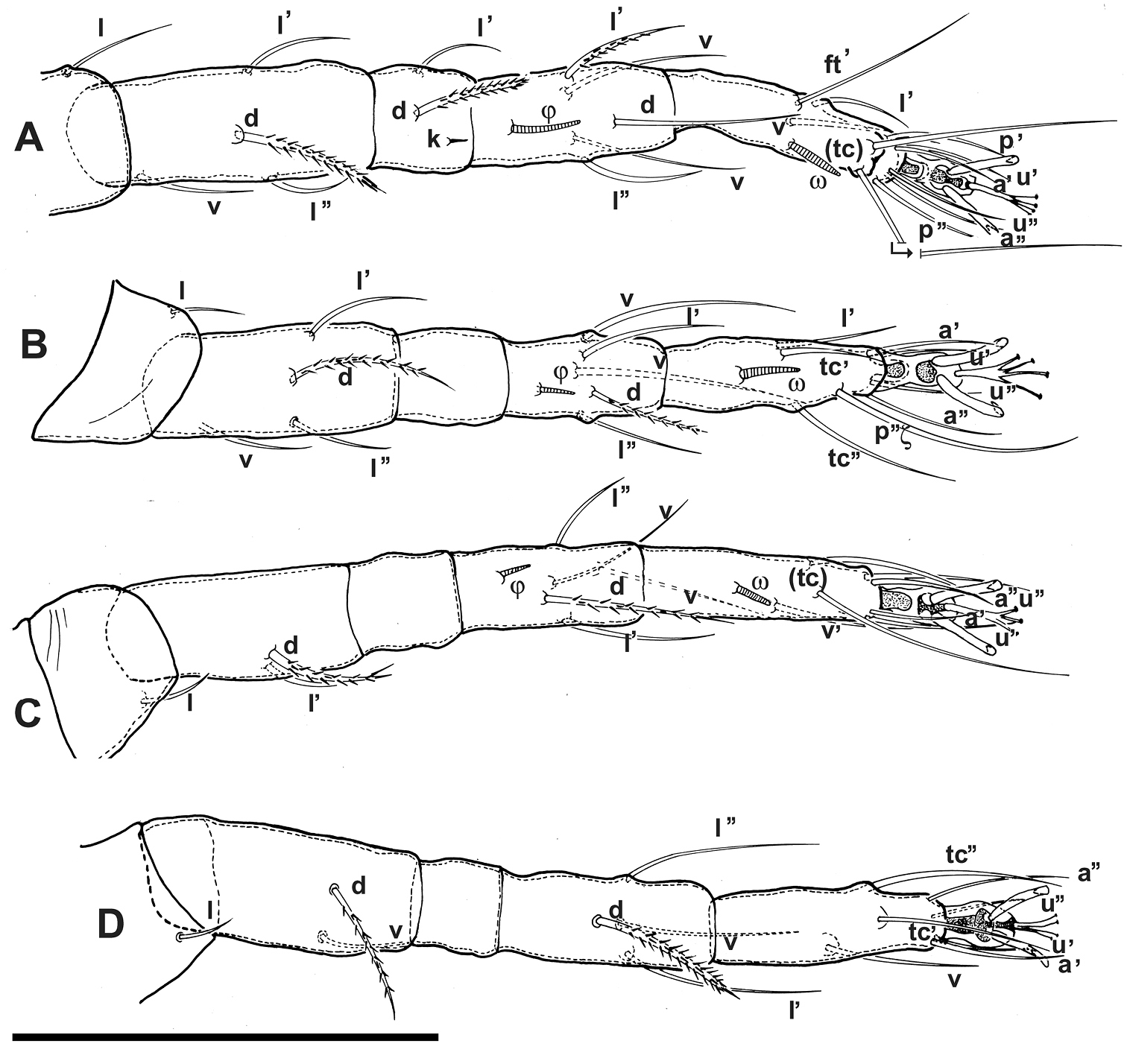
Agistemus aimogastaensis sp. n. Adult female, legs. All legs in dorsal view. Abbreviations: see Materials and Methods. Scale bar A–D: 50 µm.
Setal formulae (solenidia in parentheses) I (1-4-2(1)-5(1)-11(1)); setae k on genu I; II (1-4-0-5(1)-8(1)); III (1-2-0-5(1)-7(1)); IV (1-2-0-4-7).
Setal formulae of palp (3-1-2-8(1)) (Fig. 1C); tarsus with four eupathidia and solenidion ω; (ul) ζ, sul ζ united in fork, with typical characteristics of Stigmaeidae (
The post ocular body, delimited by red-yellow spots, is clearly visible in fresh recently prepared specimens, but these spots disappear quickly making it difficult to view; this situation is similar to observations made on Hydrozetes lemnae (Oribatida, Hydrozetidae) and at the base of the ultrastructural studies of secondary eye(
Our observations on cuticular components of the genital chamber using optical microscopy must be indicated as relative, and we stress that their value for taxonomic studies is limited as their main significance is only to confirm adulthood [as indicated by
The Olive industry in Argentina is significant, with several provinces such as Mendoza, San Juan, San Luis, La Rioja and Catamarca producing olive fruit and their derivatives, though levels of production may vary. Olive production plays a very important socio-economic role as principal provider of employment in La Rioja and Catamarca Provinces.
In olive orchards eriophyid mites are considered a secondary pest (
The predominant species of eriophyid mites found in Catamarca and La Rioja Provinces on Olea europaea (variety Arauco) are Aceria oleae and Oxycemus maxwelli. Of these two, Aceria oleae is predominant with a maximum on leaves and fruit in April and November. These two eriophyid mites cause a significant impact on regional economies due to significant fruit and leaf malformations (Figure 5).
Malformations induced by eriophyid mites on leaves and fruit. A affected leaves B affected fruit. The upper left fruit is normal, others with malformations C young fruit attacked by Aceria oleae D detail of attack in C.
The predator Agistemus aimogastaensis was found in these two provinces in large numbers, principally in relation to the population level of eriophyid mites.
The possibility exists of using this predator as biological control measure of problematic eriophyid mites. Our laboratory observations show that Agistemus aimogastaensis is a voracious predator, principally on Aceria oleae. All ontogenetic stages prey on the mites. Several studies on different predation aspects are being conducted.
We thank Prof Dr Yves Coineau (former Director of Arthropod Section at MNHN), for his support in interpreting several morphological aspects of the mite, Prof Eddie Ueckermann (ARC, Plant-Protection Research Institute, Private Bag X134, Queenswood 0121 South Africa) for additional comments, and Leone Hudson for her contribution towards technical and language aspects of the paper.
This work is based on research supported in part by the National Research Foundation of South Africa (UID) 85288. Any opinion, findings and conclusions or recommendations expressed in the material are those of the authors and therefore the NRF does not accept any liability in regard thereto.