(C) 2013 William E. Moser. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Moser WE , Briggler JT, Richardson DJ, Schuette CD, Hammond CI, Hopkins WA, Lazo-Wasem EA (2013) Redescription and molecular characterization of Placobdella cryptobranchii (Johnson & Klemm, 1977) (Glossiphoniidae, Hirudinida). ZooKeys 338: 1–10. doi: 10.3897/zookeys.338.5995
Placobdella cryptobranchii (Johnson & Klemm, 1977) was originally described from specimens collected from Ozark Hellbenders (Cryptobranchus alleganiensis bishopi) from the North Fork of the White River in Missouri, U.S.A. Leeches collected during August 2009 to August 2011 from five localities in Missouri (including the type locality) facilitated a redescription and molecular characterization of Placobdella cryptobranchii. Placobdella cryptobranchii has a rusty, reddish-brown dorsum with 2 lateral rows of unpigmented papillae, two unpigmented nuchal bands, unpigmented patches, and pair of four pre-anal papillae. Molecular comparison of CO-I sequence data from Placobdella cryptobranchii revealed a 93–94% similarity to Placobdella ornata and 10–17% difference among other species of Placobdella.
Placobdella cryptobranchii, Batracobdella, Desserobdella, Cryptobranchus bishopi, Cryptobranchus alleganiensis bishopi, Ozark Hellbender, Glossiphoniidae, Hirudinea, Rhychobdellida, Clitellata, leech
The hellbender (Cryptobranchus alleganiensis alleganiensis and Cryptobranchus alleganiensis bishopi) is among the largest salamanders in the world, but is unfortunately imperiled across much of its range in North America. Batracobdella cryptobranchii was described by
In distribution and natural history investigations, Placobdella cryptobranchii was reported from additional localities in Arkansas and Missouri (
As part of a long term monitoring program of Cryptobranchus alleganiensis bishopi populations of by one of the authors (JTB), Ozark hellbenders were captured by hand from 18 August 2009 through 26 August 2011 from the Current River (Carter Co., Ripley Co., and Shannon Co., Missouri), Eleven Point (Oregon Co., Missouri), and the type locality of Placobdella cryptobranchii, North Fork of the White River (Ozark Co., Missouri), and examined for leeches. Due to the sensitive status of Cryptobranchus alleganiensis bishopi, exact localities are not given. Leeches were also removed from Cryptobranchus alleganiensis bishopi specimens collected from the Eleven Point River (Oregon Co., Missouri) and housed at the Saint Louis Zoo for propagation efforts.
Specimens were relaxed, examined, and fixed as described by
Molecular analyses were conducted on newly collected material according to
PCR reactions were prepared using the Illustra PuRe Taq Ready-To-Go PCR beads from GE Health Care (Cat. No. 27-9559-01). Primers were purchased from Invitrogen and were comprised of 2 primers each for cytochrome c oxidase subunit I (CO-I) as specified by
Purified PCR products were sequenced using the HCO2198 primer and the LCO1490 primer for the Cytochrome c oxidase subunit I products by the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale University. The DNA sequences were aligned using Clustal W version 2 (
Examination of the type series of Placobdella cryptobranchii included the Holotype (USNM 54365) and Paratypes (USNM 54366, 10 specimens; MPM 2675-2677). All of the specimens in the type series were collected on 24 September, 1972 from the North Fork of the White River in Ozark County, Missouri, U.S.A. The USNM Holotype and Paratype lots are currently stored in 70% ethanol, however, the museum label in the vial of the Holotype indicates that it was preserved in weak formalin (4% formalin), stained in borax carmine, and cleared and stored in methyl salicylate (Figure 1). The Holotype still has a faint wintergreen odor of methyl salicylate. The museum label in the vial of the Paratype indicates that the specimens were preserved and stored in weak formalin (4% formalin).
Holotype specimen of Placobdella cryptobranchii, USNM 54365, dorsal surface. Scale bar equals 2 mm.
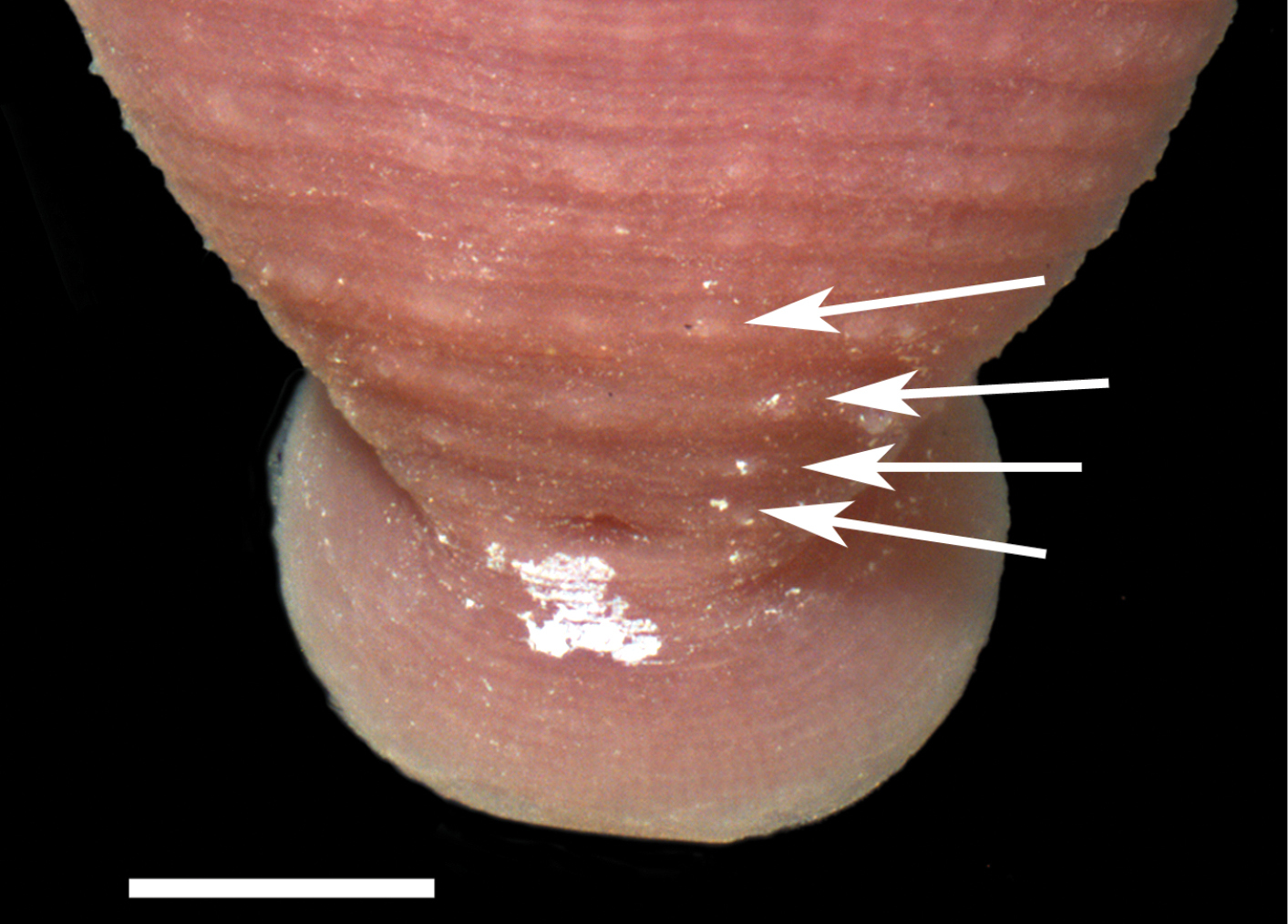
Examination of the Holotype and Paratype specimens revealed a narrowly rhomboid body, caudal sucker on a short pedicel, 2 annuli between the male and female gonopores, mouthpore on the rim/lip of the oral sucker, and a faint outline of two pair of eye spots. The dorsal surface of the Holotype and a few Paratypes had minute papillae. Beginning adjacent to the anus and commencing anteriad on either side of the anus in the Holotype and a few Paratypes were two rows of four papillae (the last row – most anteriad – papillae are medially indented) (Figure 2). Any trace of pigmentation or pigmentation pattern has faded.
Holotype specimen of Placobdella cryptobranchii, USNM 54365, pre-anal papillae (arrows). Scale bar equals 0.5 mm.
The following redescription of Placobdella cryptobranchii is based on the Holotype (USNM 54365), Paratypes (USNM 54366, 10 specimens), and newly collected specimens consistent with the description of Batracobdella cryptobranchii by
http://species-id.net/wiki/Placobdella_cryptobranchii
Figures 3–5Body very deeply ovoid to obovoid. Length of preserved specimens 3.6–13.3 mm long, mean±SE: 6.6±0.3 mm (n=42), width at widest point (in posterior half of body) 2.1–6.6 mm, mean±SE: 3.8±0.2 mm (n=42). Dorsum rusty, reddish-brown with 2 lateral rows of unpigmented papillae (Figure 3); smaller sensillae on every annulus (absent on poorly-preserved specimens). Apical cephalic region unpigmented, extending and tapering posteriorly through two thin nuchal bands (Figure 3). Two pair of eye spots (one pair much larger than the other) within cephalic unpigmented region. Unpigmented genital bar and anal patch with some specimens possessing unpigmented patch in between unpigmented genital bar and anal patch (Figure 3). Pigmentation gradually fades in ethanol and may not be present in poorly-preserved specimens. Beginning adjacent to the anus, just anterior to the anus furrow, and commencing anteriad are two rows of 4 pre-anal papillae (the last row, most anteriad, papillae are medially indented) (Figure 4). Caudal sucker small, 0.4–1.9 mm in diameter, mean±SE: 1.1±0.1 (n=35), and unpigmented or with large unpigmented patches. No papillae on caudal sucker or 1 row of small papillae on the lateral edge. Ventrum unpigmented with male and female gonopores in furrows and separated by 2 annuli.
Dorsal surface of Placobdella cryptobranchii. A Living, YPM IZ 06339 B Preserved, YPM IZ 06340. Scale bar equals 2 mm.
Pre-anal papillae of Placobdella cryptobranchii (USNM 1223081). Scale bar equals 0.5 mm.
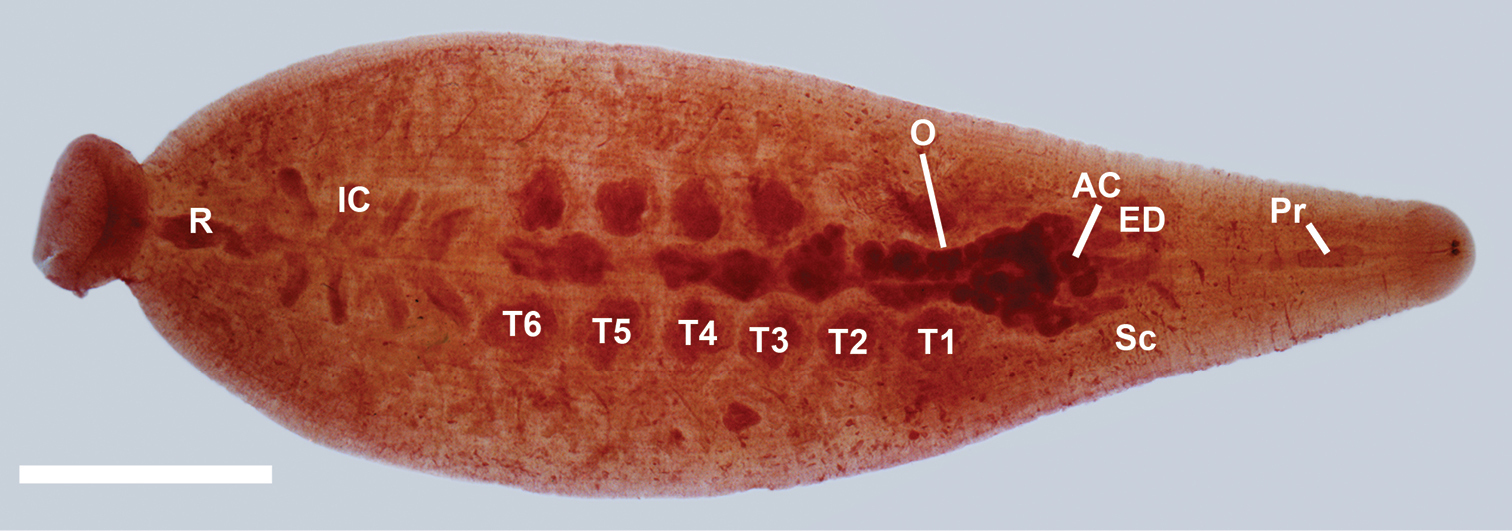
Proboscis pore just posteriad of the rim/lip of the oral sucker. Blunt-tipped proboscis, nearly uniformly cylindrical, slightly enlarged at base, and in membranous sheath (Figure 5). In the anterior third of the leech, salivary cells strewn on either side of the proboscis (Figure 5). Salivary cells most numerous in three somites at the base of the proboscis, and more scattered anteriad and posteriad of that region. Retractor muscle attached to dorsal body wall and joining salivary ductule bundles attaching at each side of the base of the proboscis. Slim, flaccid esophagus extends from the base of the proboscis with one pair sac-like mycetomes [called esophageal diverticulum by
Internal anatomy of Placobdella cryptobranchii (USNM 1223088), ventral view, atrial cornuae (AC), ejaculatory duct (ED), intestinal ceca (IC), ovisac (O), proboscis (Pr), rectum (R), salivary cells (Sc), testisac (T1–T6). Scale bar equals 2 mm.
(Male) Male gonopores slightly raised. Male atrium opening into paired very broadly orbicular atrial cornuae extending laterally and anteriorly from male gonopore into robust, coiled, muscular ejaculatory ducts, recurving posteriorly to robust seminal vesicles and narrow vas deferentia connecting to testisacs (Figure 5). Six pair of testisacs, each testisac located in the space between a pair of crop ceca (Figure 5). (Female) Female gonopore simple, opening to pair of bifurcated ovisacs and located within coelomic space that is attached on the ventral body wall (Figure 5). Ovisac length depends on the reproductive condition of the leech. In the specimens examined in this study, the ovisac extended posteriad to the sixth testisac or past the sixth testisac. Anterior, cecum-like extensions of the ovisacs are smaller and more delicate than those of the main posterior section.
6 May, 2013: Presented Placobdella cryptobranchii with brood to Necturus maculosus in 5 gallon tank. No reaction; 2 others no reaction after one hour; 2 others left over-night–did not respond.
4 June, 2013: Presented 2 Placobdella cryptobranchii to Necturus maculosus in 5 gal tank. – one did not respond and one exhibited vigorous host seeking behavior.: Leech examined skin of Necturus maculosus for about first 10 minutes but did not feed. Host seeking behavior subsided over the next few moments and the leech then exhibited no further interest.
Intraspecific comparison of 639 nucleotides of CO-I revealed differences of 0.3% to 3.3% (2–21 nucleotides) among seven specimens of Placobdella cryptobranchii (GenBank KF601755–KF601761) collected from the Current River (Carter Co., Ripley Co., and Shannon Co., Missouri), Eleven Point (Oregon Co., Missouri), and the type locality of Placobdella cryptobranchii, North Fork of the White River (Ozark Co., Missouri). In contrast, CO-I sequence data among seven specimens of Placobdella cryptobranchii revealed interspecific differences of 5.8% to 6.8% (37–43 nucleotides) when compared to five specimens of Placobdella ornata (Verrill, 1872) (GenBank JQ8128–JQ8132) collected from the type locality (West River, New Haven County, Connecticut), differences of 6.3% to 7.1% (40–45 nucleotides) among four specimens of Placobdella ornata collected from the type locality (Shivericks Pond, Falmouth, Barnstable County, Massachusetts) of Placobdella phalera (Graf, 1899) (junior synonym of Placobdella ornata) (GenBank JQ812133–JQ812136), differences of 10.4% to 12.1% (66–77 nucleotides) among two specimens of Placobdella translucens Sawyer and Shelley, 1976 (GenBank AY047328, JX122778), differences of 14.8% to 15.4% (94–98 nucleotides) from 1 specimen of Placobdella picta (Verrill, 1872) (GenBank AF116020), and differences of 16.2% to 16.9% (103–109 nucleotides) from 1 specimen of Placobdella biannulata (Moore, 1900) (GenBank AF116021).
Placobdella cryptobranchii is morphologically and molecularly similar to Placobdella ornata as described by
Based on the life history experiments, it was concluded that Placobdella cryptobranchii does not utilize Necturus maculosus as a normal host, suggesting that the occurrence of four Placobdella cryptobranchii on a Red River mudpuppy from the Eleven Point River in Missouri by
Lourdes M. Rojas, Division of Invertebrate Zoology, Peabody Museum of Natural History, Yale University provided valuable assistance in specimen management. Dr. Allen Collins (National Systematics Laboratory, National Marine Fisheries Service, Washington, DC) provided valuable assistance and insight with molecular data analyses. We complied with all applicable institutional animal care guidelines and Missouri state regulations.