(C) 2013 Casey H. Richart. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
In
Molecular phylogenetics, morphometrics, Pacific Northwest, northern Idaho, Salmon River, Olympic Peninsula, species delimitation, cybertaxonomy
The genus Acuclavella was described in 1986 by Shear in his revision of the superfamily Ischyropsalidoidea (Opiliones, Dyspnoi). In this work, four new species in the genus were described (
The biogeographic situation in Acuclavella, with several short-range endemic species from a small geographic area, coupled with an apparently widespread (but disjunct) species, clearly invites further investigation. The biogeographic barriers separating the Cascade Mountains from the Rocky Mountains of northern Idaho have promoted speciation in a variety of taxa (e.g., amphibians:
The goal of this paper is to use morphometrics and molecular phylogenetics to investigate the validity of the four species hypothesized by
Most fieldwork was conducted in the summer of 2008, with additional adult specimens collected from May to September in 2006, 2007, and 2009 (Appendix I - Collection Locality Information). Acuclavella are crenophilic denizens of small, perennial water features such as headwater streams and seeps in the Tsuga heterophylla Zone and the coastal Picea sitchensis Zone of the Pacific Northwest (
Genomic DNA was extracted from two legs per specimen using the Qiagen DNeasy kit, per manufacturer’s protocol. Currently, few genes are available for resolving shallow phylogenetic relationships in Opiliones (reviewed in
Bi-directional Sanger reads were assembled into contiguous sequences using Sequencher v4.5 (Gene Codes Corporation, MI). EF-1α haplotypes were reconstructed in PHASE (
Individual gene trees were reconstructed using maximum likelihood and Bayesian inference. Bayesian analyses were implemented using MrBayes v3.1.2 (
All available DNA sequences, with the exception of apparently nuclearized copies of COI (see Results), were concatenated for phylogeny reconstruction. The concatenated matrix was analyzed using both maximum likelihood (RAxML) and Bayesian approaches (MrBayes v3.1.2) using a seven-partition strategy (EF-1α intron + exon, Wnt2, 28S, individual COI codon positions). The Bayesian analysis was run for 1 X 107 generations; tree sampling and burn-in were as above. Outgroup sequences for five of six ischyropsalidoid genera (Ceratolasma, Taracus, Hesperonemastoma, Sabacon, and Ischyropsalis) and two genera from Troguloidea (Dendrolasma and Ortholasma) were used in concatenated phylogenetic analyses. These sequences were generated in-house or downloaded from GenBank (see Table 1).
Specimen measurements were taken using an Olympus SZX12 dissecting microscope with an ocular micrometer. Individuals with missing data (i.e., no Leg II) were excluded from analyses. Standard measurements (
Principle components analyses (PCA) and discriminant function analyses (DFA) were carried out using SYSTAT 12 (Systat Software, Inc.). Though both PCA and DFA are multivariate analyses, DFA is a validation approach that deals specifically with the problem of separating predetermined groups. In taxonomy, DFAs are almost exclusively used to differentiate between morphologically similar species that are difficult to identify from single characteristics (see
In taxonomy, PCAs are regularly employed for species delimitation. This utilization has ranged from comparing the vector angles in a scatter plot of predefined species groups (
Due to sexual dimorphism, male and female specimens were analyzed separately for all morphometric analyses. Since the data used to conduct PCAs were measured in the same units (mm), analyses were conducted on both correlation and covariance matrices. PCAs using covariance matrices tend to be dominated by characters showing the most variability (
We initiated analyses with seven a priori hypothesized species, with species-level distinctions based on geographic criteria or on preliminary specimen sorting (i.e., not morphometrics). Geographic criteria were based on the results of work on amphibians endemic to the disjunct mesic forests of the Pacific Northwest (
North of the Salmon River in Idaho, Acuclavella merickeli and Acuclavella quattuor were distinguished using the diagnostic features outlined by
A cybertaxonomic approach was undertaken for enhanced dissemination of this work (e.g.,
The data underpinning the analyses reported in this paper are deposited in the Dryad Data Repository at doi: 10.5061/dryad.16737.
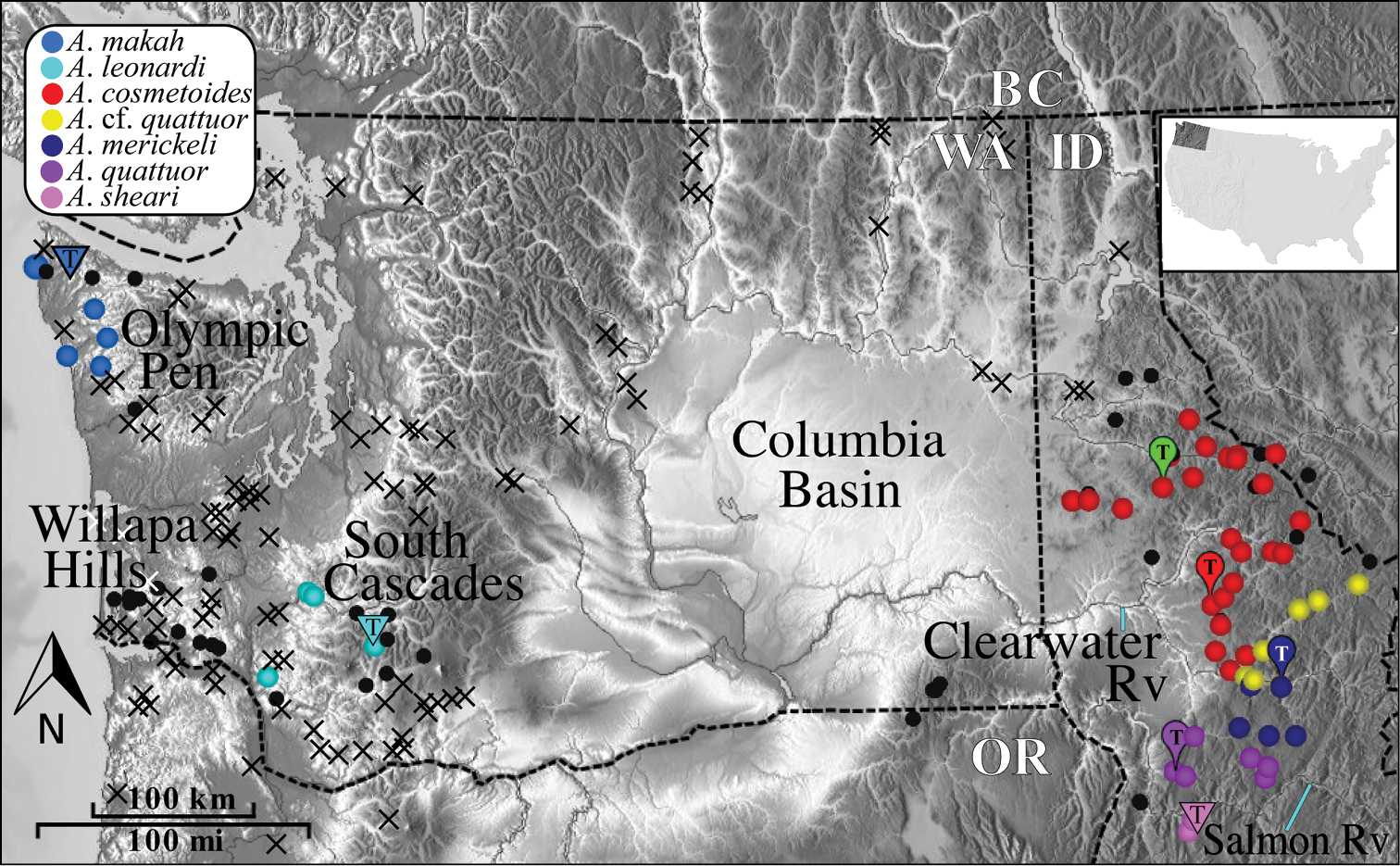
Fieldwork resulted in the collection of 272 Acuclavella specimens from 61 localities. Populations were sampled from throughout northern Idaho, as well as in the Cascade Mountain and Olympic Peninsula physiogeographic regions of western Washington (Figure 1). The first state records for Montana were secured from the Bitterroot Mountains. Specimens were collected from the four type localities (
Distribution of Acuclavella. Black circles are localities where Acuclavella were specifically targeted, but not encountered. Black crosses indicate localities where general surveys of litter invertebrates (e.g., Opiliones, Diplopoda, terrestrial Gastropoda) were conducted and Acuclavella were not encountered. Blue lines point to labeled rivers. Type localities (T) from
Although the overall geographic distribution of Acuclavella is now reasonably well-known, additional collecting efforts in the following areas may prove fruitful: south of known localities in Idaho, east into more inland areas of Montana, the Coast Range of Oregon, and the Willapa Hills of Washington. For example, most animal taxa that include populations in the Olympic Peninsula and Cascade Mountains are also found in the Willapa Hills (e.g. Rhyacotriton,
Mitochondrial COI DNA sequences for a geographical subset of Acuclavella specimens were inconsistent with expectations of protein-coding gene evolution. TheseCOI sequence reads showed ambiguous nucleotides at individual sites, a large number of mutations resulting in replacement amino acid substitutions, as well as insertions and deletions. Some deletions resulted in frame-shift mutations resulting in stop codons. These patterns of variation suggest that nuclearized copies of the mitochondrial COI gene (NUMTs;
Sequences showing evidence for nuclearization were discarded, resulting in the removal of all sequences for individuals collected north of the Middle Fork Clearwater River, including all Acuclavella cosmetoides and Acuclavella shoshone (sensu stricto). In addition to apparent authentic COI sequences, we have multiple nuclear markers providing a similar history for three new species, so our taxonomic conclusions should not be compromised. The final COI matrix included 18 ingroup sequences (L = 1224 basepairs; Table 1). The first, second, and third codon positions included 34, 9, and 282 parsimony-informative characters in the ingroup. GenBank numbers for all mitochondrial gene sequences can be found in Table 1.
Phylogenetic taxon sample and GenBank accession numbers.
| Species / Voucher No. | COI | 28S | EF-1 α | Wnt2 |
|---|---|---|---|---|
| Acfquattuor_OP2230_DeVoto | KF181730 | KF181744 | KF181759 | |
| Acfquattuor_OP2275_SplitCkTr | KF181731 | KF181760 | KF181779 | |
| Acfquattuor_OP2284_SelwayRvRd | KF181732 | KF181761 | KF181780 | |
| Acosmetoides_OP2281_2ShadowsCk | KF181745 | KF181762 | ||
| Acosmetoides_OP2296_FS250 | KF181746 | KF181763 | ||
| Acosmetoides_OP2299_TribOrogrande | KF181781 | |||
| Acosmetoides_OP2319_MeadowCk | KF181747 | |||
| Acosmetoides_OP2341_GooseCk | KF181748 | |||
| Aleonardi_OP2347_IronCk | GQ870648 | KF181749 | GQ872169 | KF181782 |
| Aleonardi_OP2349_NFGobleCk | KF181764 | |||
| Aleonardi_OP2712_KjesbuRd | KF181750 | KF181765 | ||
| Aleonardi_OP2714_UpperIronCk | KF181728 | KF181766 | ||
| Americkeli_OP2237_FS443 | KF181733 | KF181751 | KF181767 | |
| Americkeli_OP2245_FS443 | KF181734 | KF181783 | ||
| Americkeli_OP2250_RedHorseCk | KF181735 | KF181768 | KF181784 | |
| Amakah_OP1699_RubyBeach | KF181736 | KF181769 | ||
| Amakah_OP2345_CedarCk | GQ870647 | KF181752 | GQ872168 | |
| Amakah_OP2715_HokoFalls | KF181737 | KF181753 | KF181770 | KF181785 |
| Amakah_OP2716_BrownesCk | KF181738 | KF181771 | ||
| Amakah_OP2719_YahooLkRd | KF181772 | |||
| Aquattuor_OP2242_GrouseCk | KF181739 | KF181773 | KF181786 | |
| Aquattuor_OP2257_SlateCk | KF181740 | KF181754 | KF181774 | |
| Aquattuor_OP2270_FS221 | KF181729 | KF181787 | ||
| Asheari_OP2708_BurgdorfRd | KF181741 | KF181755 | KF181775 | |
| Asheari_OP2709_BurgdorfRd | KF181742 | KF181776 | ||
| Asheari_OP2720_FS592 | KF181743 | KF181756 | KF181777 | KF181788 |
| Ashoshone_OP2316_EmeraldCkRd | KF181789 | |||
| Ashoshone_OP2323_Hobo | KF181757 | KF181778 | ||
| Ceratolasma | GQ912865 | JX573543 | AF240864 | KF181790 |
| Dendrolasma | KF181727 | GQ912771 | AF240865 | |
| Hesperonemastoma | JX573642 | JX573548 | AF240869 | KF181791 |
| Ischyropsalis | JX573639 | AF240870 | JX573603 | KF181792 |
| Ortholasma | GQ912870 | KF181758 | GQ872161 | |
| Sabacon | JX573670 | JX573551 | AF240877 | KF181793 |
| Taracus | JX573680 | JX573592 | AF240881 | KF181794 |
Gblocks removal of ambiguous sites in the 28S matrix resulted in a reduction of alignment length from 1211 to 1173 positions. The aligned matrix included 26 parsimony informative characters for 14 ingroup sequences. For the EF-1α gene, eight of 22 Acuclavella sequences showed signs of heterozygosity. Three of these individuals were ambiguous at a single base pair, two were ambiguous at two sites, and remaining individuals were ambiguous at 3, 4, or 5 sites respectively. All haplotypes were reconstructed using PHASE. The resulting aligned matrix consisted of 690 bp of exon data, and an 80 bp intron with gaps. The EF-1α exon contained 30 parsimony informative characters in the ingroup. The intron sequences did not have outgroup representatives; there were 18 parsimony informative characters in the intron. The Wnt2 gene matrix (L = 370) included 7 ingroup sequences, with only 4 parsimony informative characters. GenBank numbers for all nuclear gene sequences can be found in Table 1.
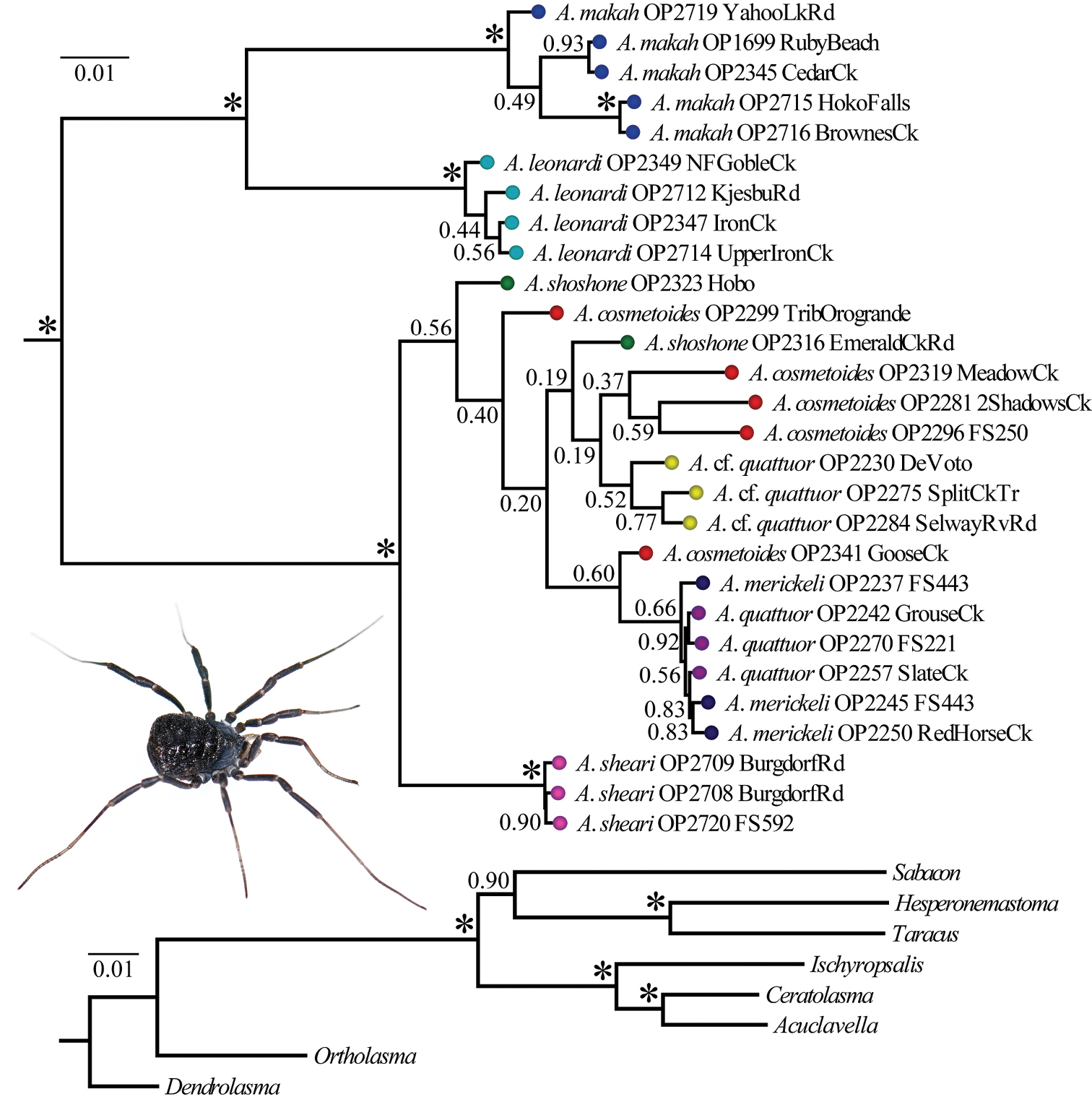
Models of evolution inferred from jModelTest are summarized in Table 2. These partition-specific models were used in a Bayesian analysis of the concatenated matrix. Figure 2 shows results from the Bayesian analysis and associated posterior probabilities; the phylogeny resulting from the RAxML analysis is available in a supplementary file (Appendix VII). The concatenated Bayesian and ML phylograms are congruent in their strong support for a monophyletic Acuclavella. Ceratolasma is recovered as sister to Acuclavella with strong support, confirming the hypothesis of
Models of DNA sequence evolution as determined by jModelTest v0.1.1
| Gene | Model |
| COI 1st partition | GTR+I+G |
| COI 2nd partition | GTR+I+G |
| COI 3rd partition | GTR+G |
| 28S | GTR+I+G |
| EF-1α intron | GTR+G |
| EF-1α exon | SYM+G |
| Wnt2 | SYM+I |
Bayesian phylogram resulting from analysis of concatenated dataset. Numbers at nodes correspond to Bayesian posterior probabilities. The outgroup topology is shown at bottom. The inset picture is a female Acuclavella shoshone (sensu stricto) collected from the type locality (
Within Acuclavella, samples from Washington and Idaho are reciprocally monophyletic in both ML and Bayesian analyses, with regional clades strongly-supported and subtended by relatively long branches (Figure 2, Appendix VIII). Within Washington, both analyses recover samples from the Olympic Peninsula and southern Cascade Mountains as monophyletic lineages separated by long branches with high support. Both ML and Bayesian analyses also strongly support Acuclavella sheari as monophyletic and sister to all other Idaho samples. Acuclavella cf. quattuor was recovered by both analyses, but is not supported by either (PP = 0.52, BS = 13). No other molecular clades are recovered conforming to other described species of northern Idaho Acuclavella. A KML file has been created to more easily visualize where sequences used to construct the Bayesian phylogeny occur in geographic space (Appendix VI).
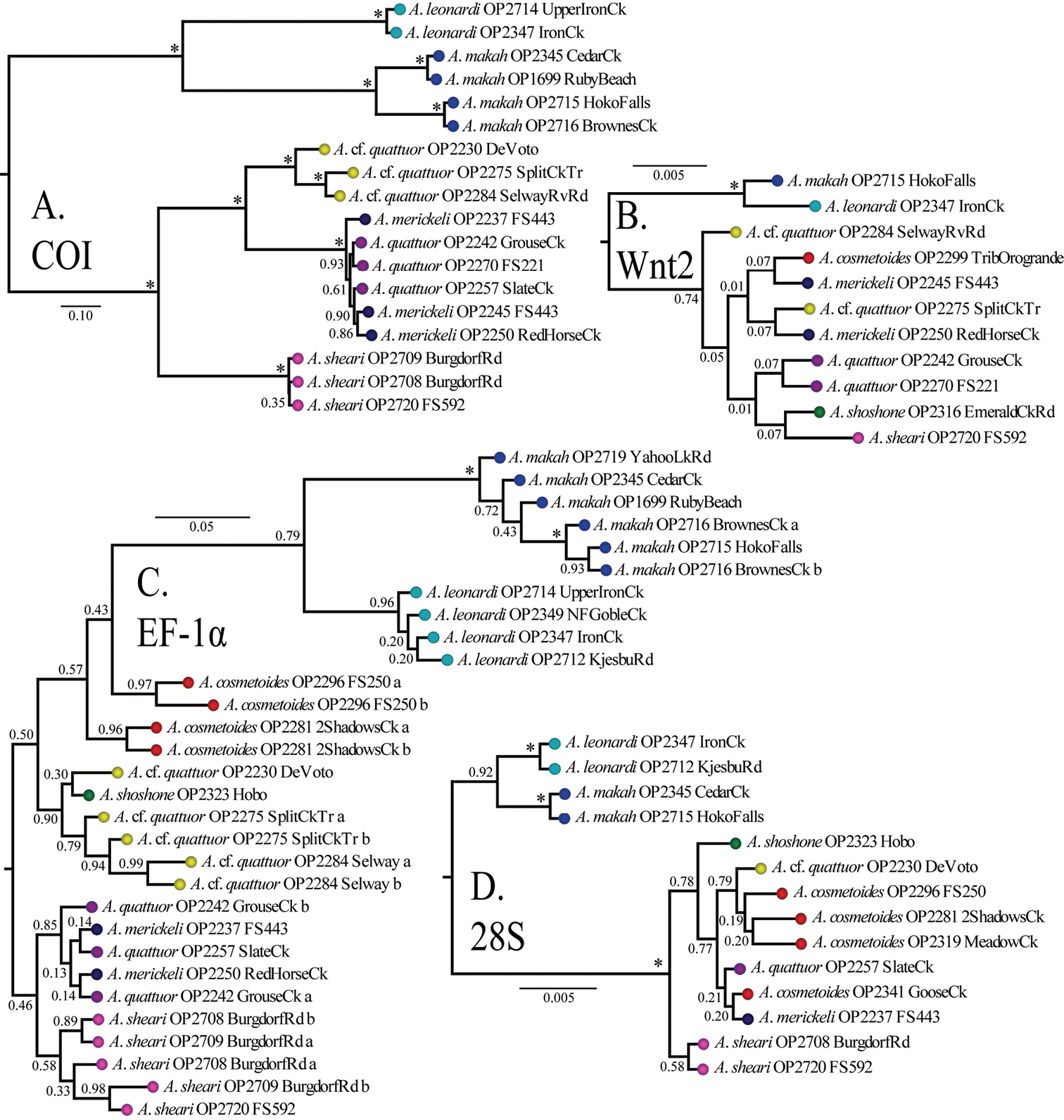
All Bayesian individual gene trees had their longest branch separating Washington and Idaho genetic groups (Figure 3). The COI, EF-1α, and 28S gene trees (Figure 3A, 3C, and 3D respectively) also show a deep split separating monophyletic Acuclavella leonardi and Acuclavella makah samples. Acuclavella sheari was also recovered in each of the individual trees, but with varied support and topological placement within a clade of Idaho samples. The COI gene tree shows significant support for a monophyletic Acuclavella sheari, which is sister to all remaining Idaho sequences. This species is recovered as monophyletic but without support by EF-1α and 28S gene trees. The Wnt2 gene tree (Figure 3B) supports reciprocal monophyly of samples from Washington and Idaho, but lacks phylogenetic signal within Idaho. The lack of structure for shallow evolutionary events within Acuclavella, coupled with high support for most nodes within Ischyropsalidoidea above the generic level, points to a deeper phylogenetic utility for Wnt2 in Opiliones.
Bayesian gene trees with posterior probabilities: A COI B Wnt2 C EF-1α D 28S. All trees outgroup rooted (Appendix VII, Figure 1). RAxML concatenated phylogeny (Appendix VII, Figure 2) and gene trees (Appendix VII, Figures 3-5) are also available.
DFA and PCA morphometric analyses of male and female data sets most frequently recovered Acuclavella sheari, followed by Acuclavella leonardi and Acuclavella makah. Undescribed morphologies from northern Idaho clustered with samples of Acuclavella cosmetoides and Acuclavella shoshone (sensu stricto) of Shear, 1986. Acuclavella merickeli was also frequently recovered in these analyses. The two populations with two pairs of scutal spines on scute areas I and II (Acuclavella quattuor and Acuclavella cf. quattuor) were frequently recovered as distinct from other hypothesized species, but were not recovered as morphometrically distinct from each other.
Since the group frequencies of hypothesized species varied greatly (Table 3), jackknifed classification of some data sets reduced the number of individuals used to define a group to just two or three individuals. An assumption is made here that variation seen within hypothesized species is normal in terms of representing the cluster. Male Acuclavella are robustly discriminated by DFA analysis, with nearly all species correctly classified with 100% accuracy. An exception is Acuclavella cf. quattuor, with two specimens classified as an Acuclavella quattuor (classification error rate of 0.09). Results of DFAs are available Results of DFAs are available in Appendix IV. The analysis was repeated with a jackknife classification resulting in successful discrimination of 94% of male individuals. Considering the high variation in group frequencies of hypothesized species (Table 3), analyses were rerun allowing prior probabilities of group membership. These analyses resulted in very similar groupings (not shown).
Sample sizes for PCA and DFA analyses.
| Species | Males | Females | Total |
|---|---|---|---|
| Acuclavella makah | 10 | 14 | 24 |
| Acuclavella leonardi | 6 | 4 | 10 |
| Acuclavella sheari | 4 | 3 | 7 |
| Acuclavella quattuor | 14 | 17 | 28 |
| Acuclavella merickeli | 19 | 19 | 38 |
| Acuclavella cf. quattuor | 22 | 18 | 40 |
| Acuclavella cosmetoides | 56 | 58 | 114 |
| Total | 131 | 133 | 261 |
The number of individuals from each hypothesized species used in morphometric analyses.
Groupings based on female specimens were not recovered as frequently as male-based groups. Females of Acuclavella leonardi, Acuclavella merickeli, and Acuclavella sheari were correctly discriminated in the classification matrix. There was support for female samples of Acuclavella makah and Acuclavella cosmetoides, with correct classification 93% and 97% of the time respectively. About 25% of Acuclavella quattuor and Acuclavella cf. quattuor females were misclassified as the other species. Results from the jackknifed classification matrix show similar, if slightly lower percentages of correctly classified individuals. In this matrix, one Acuclavella sheari is misclassified as Acuclavella cosmetoides, and three Acuclavella cosmetoides are misclassified as Acuclavella sheari. This is likely the result of convergent similarities – females of Acuclavella sheari and some Acuclavella cosmetoides (Acuclavella shoshone sensu stricto) lack scutal spines.
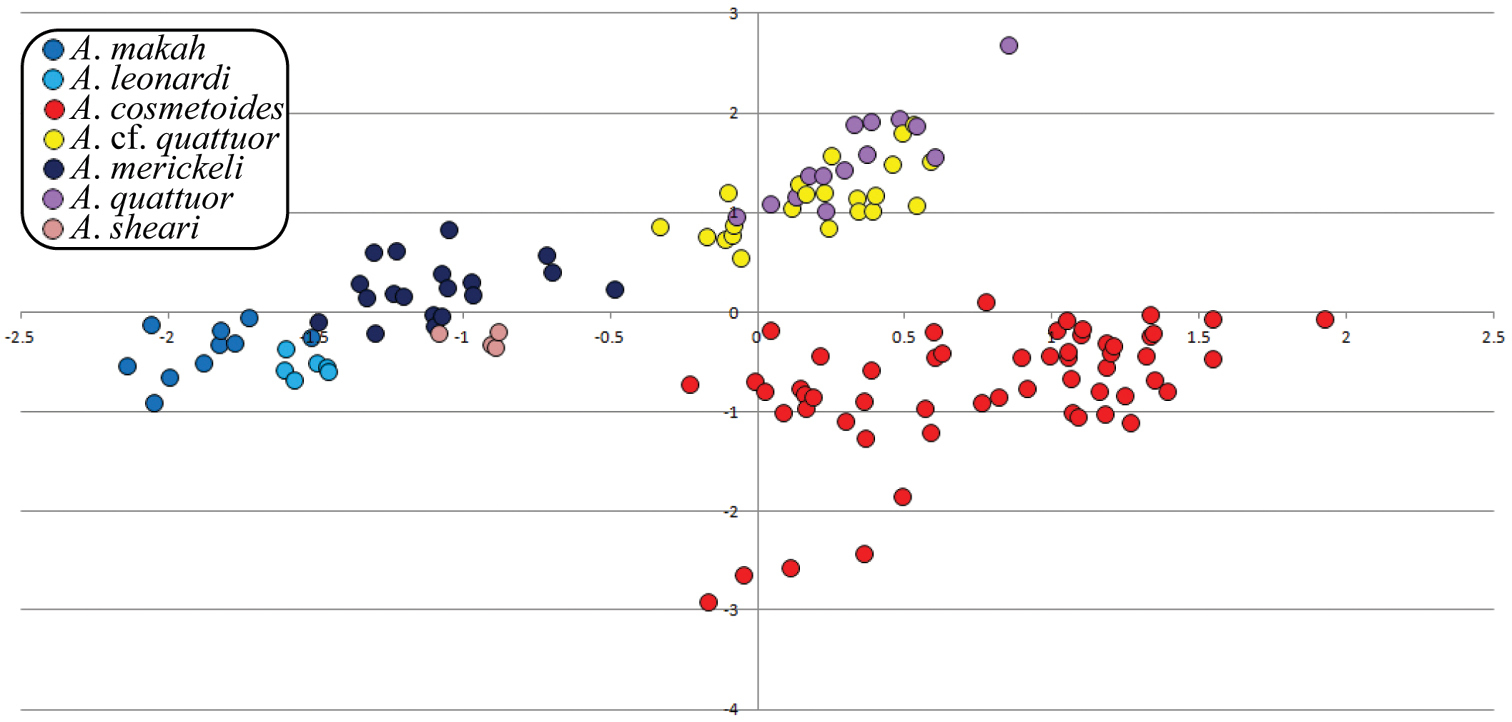
Principle components analyses regularly recovered all hypothesized species of Acuclavella with the exception of Acuclavella cf. quattuor from Acuclavella quattuor. Figure 4 shows a graph recovering or nearly recovering these species. Acuclavella sheari was most frequently recovered in morphospace, with strong evidence for Acuclavella leonardi and Acuclavella makah as morphometrically distinct. Also well-supported, Acuclavella merickeli and Acuclavella cosmetoides were recovered in PCA analyses. Acuclavella cf. quattuor and Acuclavella quattuor were regularly recovered as distinct from other hypothesized species, but were not distinguishable from each other. Generally, analyses run on covariance matrices had more success of clustering hypothesized species than analyses run on a correlation matrix. Similar to DFAs, males tended to be recovered more frequently than females. Graphs plotting principle components from all PCA analyses conducted (n=143), as well as a description of strategies employed to explore morphospace, are available as a supplementary file (Appendix IV).
PCA bi-dimensional plot. PCA results of male data using a covariance matrix; plot of principle components 2 and 3.
Both phylogenetic and morphometric analyses strongly support three new species: Acuclavella leonardi, Acuclavella makah, and Acuclavella sheari, but do not clearly support already recognized taxa within Idaho samples north of the Salmon River. Molecular and morphological data sets agree that phylogeographic structure associated with the Salmon, Selway, and Lochsa Rivers exists, and morphological data suggests differentiation across the South Fork Clearwater River. Although the number of individuals and known localities was greatly improved by this research, further specimen and genetic sampling is needed to adequately test species limits in northern Idaho. In particular, more rapidly-evolving genetic markers are needed for resolution of what is likely a relatively recent evolutionary radiation in this region. A lack of phylogenetic structure within most of northern Idaho, coupled with the finding that some of the undescribed morphologies cluster together with Acuclavella shoshone and Acuclavella cosmetoides, make it possible that Acuclavella cosmetoides is a widespread and morphologically highly variable species that encompasses Acuclavella shoshone. The same hypothesis may apply to the Acuclavella merickeli plus Acuclavella quattuor lineage.
Though they lack molecular support, Acuclavella merickeli and Acuclavella quattuor are readily discriminated by morphometric analyses (Figure 4). Since male Acuclavella quattuor were collected from the type locality and agree with the diagnostics for females (
The Western Hemlock Zone (WHZ) (
Reconstructed molecular phylogenies show that Acuclavella biogeography is largely congruent with that seen in other dispersal-limited taxa of the Pacific Northwest. The deep phylogenetic split between Washington and Idaho (Figures 2, 3) conforms to the ancient vicariance hypothesis (
Acuclavella are riparian obligate forest-dwellers. The vast majority of specimens were collected within the channel wall of perennial headwater streams or in seep-like features. Most specimens were found underneath large woody debris, though excavation of woody sediment wedges (
The primary defense of Acuclavella appears to be one of crypsis. Their dark bodies are cryptic against moist woody debris, onto which specimens adhere with outstretched legs. When detected, specimens are easily collected by grabbing onto an outstretched leg; a hurried scramble is soon followed by limb rigidity. Autospasy was not encountered. A strategy to overcome the crypsis of Acuclavella is to expose appropriate areas and wait a few minutes for animals to come out of thanatosis to scurry for cover and appropriate microclimatic conditions. Acuclavella likely employ mechanical defense via spination. When turning a cover object the first author placed the pad of his finger directly on an individual; it felt like being pricked by a rose. It is likely that heavy sclerotization of the integument and the ubiquitous hemispherical warts add to the structural integrity of these spines. Though circumstantial, this proposal of heavy sclerotization for mechanical defense is consistent with recent findings in other harvestmen (
Acuclavella appear to have an annual life cycle, with seasonal adults. Penultimate stage subadult and adult animals have been collected in April and May; additionally, a young instar was collected in mid October (CHR3435; same locality as CHR 1409; Appendix I). Adults have been found from May to September. Mating was not encountered, nor was feeding. A single individual (CHR2403) (Appendix I) was found dead in the web of a Pimoa spider (CHR2404).
Recent surveys of litter dwelling animals in mesic forests of the Pacific Northwest have led to the discovery of many new species. Taxa include terrestrial gastropods (
The genus Acuclavella, and species Acuclavella merickeli, Acuclavella cosmetoides, Acuclavella shoshone, and female Acuclavella quattuor are described by
http://species-id.net/wiki/Acuclavella
The simple, distally tapering penis (Appendix VIII, Figure I) and short ovipositor (Appendix III, Figure 2) morphologies are conserved across species, with intraspecific variation seemingly as great as interspecific variation. Penis sheath with two sclerotized bands. Metatarsus of leg II with or without false leg articulations (Appendix IX). Distitarsi with three segments on legs I and II; distal end of legs III and IV with two constrictions, each comprised of two segments. All males with raised, glandular (
This dichotomous key should allow users to identify the new species described herein. However, discovery of new morphologies in northern Idaho not described by
| 1a | Setose mounds dorsally on surface of cheliceral article I; males (Fig. 6) | 2 |
| 1b | Without raised mounds on chelicerae; females | 8 |
| 2a (1a) | Paramedian tubercles acute spines on scute area II only (Fig. 9) | 3 |
| 2b | Paramedian tubercles acute spines on multiple scute areas (Fig. 11) | 6 |
| 3a (2a) | Ocularium height (ventral edge of eye to tip of ocularium) ≤ 0.60 mm; area II spine height ≤ 0.50 mm from surface of tergite | Acuclavella sheari sp. n. |
| 3b | Ocularium and area II spine heights ≥ 0.80 mm | 4 |
| 4a (3b) | Distal ends of leg femora, patellae, and tibiae distinctly light, contrasting as light joints; palpi white or nearly so; with or without false leg articulations on leg II metatarsi (Appendix IX); with or without distal, dark, prolateral tubercle on palpal patellae; western Washington | 5 |
| 4b | Distal ends of leg segments not light; palpi light brown or dark; without false leg articulations and palpal tubercles; Idaho | Acuclavella merickeli Shear, 1986 |
| 5a (4a) | Leg II femur ≤ 3.76 mm; Cascade Mountains | Acuclavella leonardi sp. n. |
| 5b | Leg II femur ≥ 3.76 mm; Olympic Peninsula | Acuclavella makah sp. n. |
| 6a (2b) | Paramedian tubercles raised into acute spines on scute areas I and II only (Fig. 11); known distribution bracketed by the Salmon River and South Fork Clearwater River | Acuclavella quattuor Shear, 1986 |
| 6b | Paramedian tubercles with acute spines on three or more scute areas; scute area III always with such tubercles (Appendix V, Figure 1) | 7 |
| 7a (6b) | Paramedian tubercles not raised into spines on scute area I, paired spines on areas II, III, and IV | Acuclavella cosmetoides Shear, 1986 |
| 7b | Paramedian tubercles raised into acute spines on scute areas I – IV (four pairs of abdominal spines | Acuclavella shoshone (Shear, 1986) |
| 8a (1b) | Paramedian tubercles acute spines on scute area II only (Fig. 9) | 9 |
| 8b | Tubercles not as previous; if spines on area II only, greatly reduced | 11 |
| 9a (8a) | As couplet 4a | 10 |
| 9b | As couplet 4b | Acuclavella merickeli |
| 10a (9a) | Known only from the Cascade Mountains of Washington State; no perceived discriminating features | Acuclavella leonardi sp. n. |
| 10b | Known only from the Olympic Peninsula of Washington State; no perceived discriminating features | Acuclavella makah sp. n. |
| 11a (8b) | As couplet 6a | Acuclavella quattuor Shear, 1986 |
| 11b | Paramedian tubercles not as previous | 12 |
| 12a (11a) | As couplet 7a | Acuclavella cosmetoides Shear, 1986 |
| 12b | Paramedian tubercles not enlarged into spines, but paired, low, rounded tubercles on all scutal areas (Fig. 9) | 13 |
| 13a (11b) | Palpal femur ≤ 0.88 mm; leg II tarsus ≤ 3.92 mm; leg II femur ≤ 2.75 mm; currently known south of Salmon River | Acuclavella sheari sp. n. |
| 13b | Palpal femur ≥ 0.90 mm; leg II tarsus ≥ 3.95 mm; leg II femur ≥ 2.72 mm, north of Middle Fork Clearwater River | Acuclavella shoshone (Shear, 1986) |
urn:lsid:zoobank.org:act:3F79C21E-E37A-4FC5-92E3-170CA8D9481C
http://species-id.net/wiki/Acuclavella_leonardi
MorphBank images of specimens considered this species include:
Paratype AMNH, MorphBank Specimen Id: 822643, 1 image
Paratype CASENT9039218, MorphBank Specimen Id: 822508, 4 images
Paratype CASENT9039224, MorphBank Specimen Id: 822516, 2 images
SDSU OP2347, MorphBank Specimen Id: 822509, 1 image
SDSU OP2349, MorphBank Specimen Id: 822511, 3 images
SDSU OP2712, MorphBank Specimen Id: 822515, 1 image
SDSU OP2714, MorphBank Specimen Id: 822517, 3 images
Figure 5 and 6, Appendix VIII: Figure 1, Figure 2Male holotype (AMNH), and male (CAS, CASENT9039218) and female (AMNH) paratypes from a tributary of Iron Creek, Forest Service Rd 25 4.6 miles south of FS Rd 300, Gifford Pinchot National Forest, Lewis County, Washington; male paratype (UWBM, WA2392/6319) and female paratype (CAS, CASENT9039224) from upper Iron Creek, FS Rd 28 0.1 miles E of FS 25, Gifford Pinchot National Forest, Skamania County, Washington; female paratype (UWBM, WA2391/6027) from a tributary of Goble Creek, Cowlitz County, Washington. Further information on type localities can be found in Appendix I with the exception of female paratype from Goble Creek. This specimen was collected by the first author 3 August 2005 and deposited in a research collection; this specimen was not used in morphometric analyses, but is used to characterize the species in the description below. This specimen was collected at a location accessed via S Goble Creek Rd; 2.0 miles east of Rose Valley Rd turn right, 1.6 miles turn right, 0.6 miles park; 46.0963°N, 122.7607°W, elevation 227 meters.
The specific epithet is a patronym in honor of the naturalist and careful observer William P. Leonard for his work on litter-dwelling organisms in the poor-person’s rainforest of the Pacific Northwest.
Distinguished from all Acuclavella except Acuclavella makah by the combination of having paramedian tubercles as enlarged spines on area II only, and having light, strongly contrasting ends to sclerotized leg segments, giving the appearance of banding. Also distinguished from these taxa in that false leg articulations on the metatarsi of legs II are present, or single dark prolateral tubercles on the palpal patellae are present, but these features are not consistently found in Acuclavella leonardi. Scutes posterior to spines containing many raised mounds bearing warty tubercles, more distinct than in Acuclavella makah. Though the height of scutal spines is similar, the base of the spines in Acuclavella leonardi appears broader than in Acuclavella makah. Diagnostic COI sequences have been uploaded to the Barcode of Life Data Systems (BOLD: ACUOP005-13).
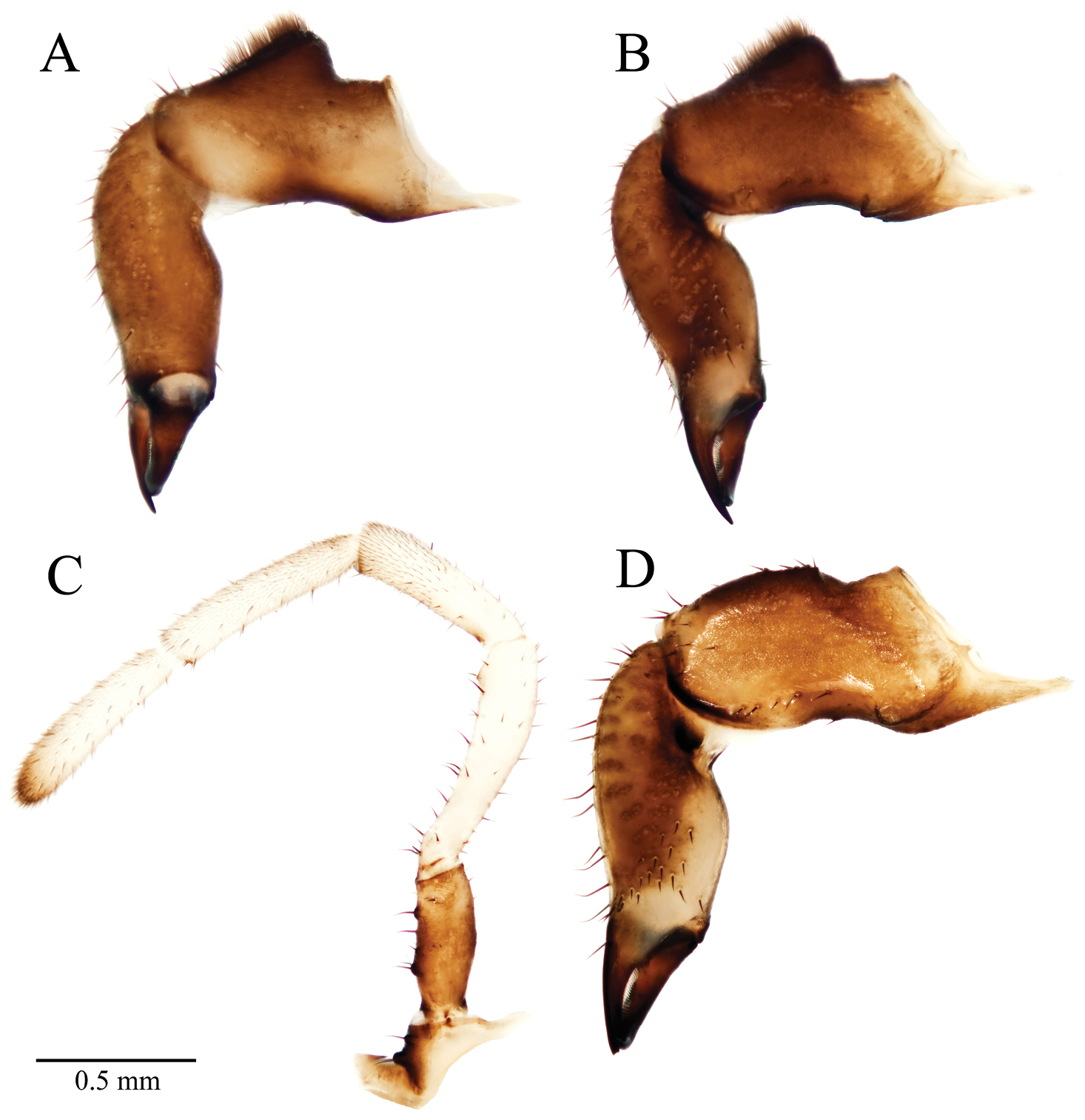
Body arched and convex dorsally (Figure 5); sides parallel or nearly so when broader posteriorly. Nearly all of body heavily sclerotized black or brown, with densely scattered hemispherical warts which irregularly house short setae apically or posteriorly. Total length 4.18 mm (n=3, 3.88–4.35 mm), carapace length 1.22 mm (n=7, 1.10–1.35 mm), carapace width 2.53 mm (n=3, 2.44–2.65 mm), length of fused tergites I-V 2.24 mm (n=7, 2.10–2.41 mm), scutum length 2.73 mm (n=3, 2.65–2.85 mm), scutum width 2.63 mm (n=3, 2.55–2.75 mm).
Acuclavella leonardi. Top: male, CHR3325; bottom: female, CHR 2492.
Acuclavella leonardi chelicerae and pedipalp. A right chelicera, retrolateral view B left chelicera, prolateral view C right pedipalp, retrolateral view (A–C, male CASENT9039218) D left chelicera, retrolateral view (female CASENT9039224).
Eye tubercle at anterior edge of carapace prolonged into an acute spine lacking hemispherical warts on distal half; standing 1.25 mm above the surface of the carapace (n=3, 1.13–1.35 mm), 1.05 mm (n=7, 0.88–1.19 mm) from the ventral edge of the eye to the tip of the tubercle. Eye color brown, brown-gray, or gray, located basally on tubercle. Metapeltidial paramedian sensory cones raised into a sharp, acute spine standing 0.26 mm (n=7, 0.23–0.28 mm) above surface of the carapace, curving slightly towards the midline; lateral to these spines clusters of warts form tubercles.
All scutal tergites with pairs of median tubercles, these prolonged into large spines on area II only; lateral tubercles distinct. Tergite I with paired median tubercles as raised mounds adorned with warts standing 0.09 mm (n=7, 0.08–0.13 mm) above the scutal surface; two additional pairs of warty mounds reduce in size laterally. Median tubercles on area II tergite greatly enlarged into erect spines standing 1.04 mm (n=7, 0.85–1.16 mm) above the surface of the scutum; lateral to these tubercles are raised mounds adorned with warts. Area III tergite with three pairs of relatively large tubercles in the form of raised mounds adorned with warts; these not decreasing in size laterally; apical setae on each mound; median pair 0.06 mm above scutum (n=7, 0.03–0.08 mm). Tergite areas IV and V with three or four (UWBM) pairs of tubercles in the form of raised mounds adorned with warts, these not decreasing in size laterally or posteriorly; setae as previous; area IV median tubercle height 0.06 mm (n=7, 0.05–0.08 mm). First free tergite (VI) with relatively large and numerous tubercles in the form of raised warty mounds. Second free tergite (VII) as previous in the holotype and paratype (CAS); paratype (UWBM) without tubercles. Free tergite VIII without distinguishable tubercles, or with a median pair only (CAS paratype).
Abdominal sternite warty sculpturing strongest laterally and on posterior margins; sternites brown. Sclerotized areas of genital operculum relatively setose. Prosomal sternum (n=1) length 0.19 mm, width 0.37 mm; brown; without setae. Labium weakly to moderately sclerotized, wider than long or longer than wide (CAS); lengths: 0.14, 0.20, 0.08 mm, widths: 0.28, 0.16, 0.18 mm; light brown to brown; without setae. Palpal endites setose, light brown or brown. Leg II endites bearing 1 or 2 (UWBM) setae, leg IV bearing 1 setae in paratype only, all other endites without setae. Horn-shaped process of epistome decurved, projecting 0.40 mm from sulcus (n=1).
Chelicerae brown or light brown (Figure 6); darker dorsally; article II with prolateral and retrolateral striations of darker, more sclerotized cuticle; weakly so in paratypes. Cheliceral measurements (n=3): article I length 1.14 mm (1.03–1.34 mm), width 0.41 mm (0.40–0.44 mm), article II length 1.32 mm (1.20–1.38 mm), width 0.38 mm (0.35–0.38 mm), article III length 0.56 mm (0.53–0.60 mm). Dorsal surface of article I with raised, glandular area dense with setae, these capped with a white secretion in the CAS paratype; proximal end of article I with boss-like tubercles on retrolateral and proventrolateral surfaces. Palpal coxae brown or light brown (UWBM paratype), with 2 seta-bearing tubercles. Palpal measurements given in Table 4. Trochanter (Figure 6) brown, light brown, or pale brown, with 3 or 4 (UWBM paratype) seta-bearing tubercles. Palpal femora brown or white; patella white without a dark diffuse band, with dark, prolateral tubercles distally, bearing small trichia and setae distally; tibia white, with scattered setae and dense microtrichia; tarsus white, darkening distally, with vestiture of microtrichia and setae. Claw rudiment very small.
Acuclavella leonardi palpus and leg measurements.
| Appendage | Segment | Male | Female | ||||
|---|---|---|---|---|---|---|---|
| mean | Range | n | Mean | Range | n | ||
| Palpus | trochanter | 0.55 | 0.52–0.58 | 3 | 0.61 | 0.58–0.65 | 3 |
| femur | 0.94 | 0.84–1.00 | 7 | 0.94 | 0.90–0.98 | 5 | |
| patella | 0.67 | 0.66–0.68 | 3 | 0.71 | 0.70–0.73 | 3 | |
| tibia | 0.74 | 0.73–0.74 | 3 | 0.73 | 0.67–0.80 | 3 | |
| tarsus | 0.69 | 0.67–0.72 | 3 | 0.66 | 0.64–0.68 | 3 | |
| Leg I | trochanter | 0.49 | 0.48–0.52 | 3 | 0.50 | 0.45–0.56 | 3 |
| femur | 2.37 | 2.28–2.44 | 3 | 2.25 | 1.96–2.60 | 3 | |
| patella | 0.88 | 0.88 | 3 | 0.87 | 0.84–0.92 | 3 | |
| tibia | 1.60 | 1.48–1.72 | 3 | 1.46 | 1.44–1.50 | 3 | |
| metatarsus | 2.17 | 1.84–2.36 | 3 | 2.19 | 2.04–2.28 | 3 | |
| tarsus | 2.89 | 2.72–3.08 | 3 | 2.80 | 2.68–2.88 | 3 | |
| Leg II | trochanter | 0.57 | 0.52–0.60 | 7 | 0.55 | 0.52–0.56 | 5 |
| femur | 3.53 | 3.32–3.76 | 7 | 3.46 | 2.88–3.92 | 5 | |
| patella | 1.02 | 0.96–1.08 | 7 | 1.00 | 0.92–1.08 | 5 | |
| tibia | 2.38 | 2.16–2.60 | 7 | 2.36 | 2.12–2.60 | 5 | |
| metatarsus | 3.70 | 3.44–4.05 | 7 | 3.69 | 3.28–3.96 | 5 | |
| tarsus | 4.93 | 4.74–5.19 | 7 | 4.58 | 4.40–4.70 | 5 | |
| Leg III | trochanter | 0.48 | 0.48 | 3 | 0.54 | 0.52–0.60 | 3 |
| femur | 2.23 | 2.12–2.28 | 3 | 2.10 | 1.88–2.25 | 3 | |
| patella | 0.85 | 0.80–0.88 | 3 | 0.84 | 0.80–0.88 | 3 | |
| tibia | 1.57 | 1.56–1.60 | 3 | 1.50 | 1.48–1.52 | 3 | |
| metatarsus | 2.49 | 2.40–2.56 | 3 | 2.38 | 2.24–2.45 | 3 | |
| tarsus | 3.13 | 3.08–3.20 | 3 | 2.95 | 2.80–3.05 | 3 | |
| Leg IV | trochanter | 0.57 | 0.52–0.64 | 3 | 0.58 | 0.55–0.64 | 3 |
| femur | 3.23 | 3.08–3.32 | 3 | 3.09 | 2.76–3.35 | 3 | |
| patella | 1.03 | 0.96–1.08 | 3 | 0.99 | 0.96–1.00 | 3 | |
| tibia | 2.01 | 1.96–2.08 | 3 | 1.99 | 1.96–2.05 | 3 | |
| metatarsus | 4.03 | 3.88–4.12 | 3 | 3.85 | 3.64–4.00 | 3 | |
| tarsus | 4.09 | 4.08–4.12 | 3 | 3.87 | 3.76–4.99 | 3 | |
All measurements in millimeters; n= sample size.
Leg measurements given in Table 4. Microsculpture of femora, patellae, and tibiae scattered, distally elevated scales, bilobed scales not observed; scales subtend setae, occasionally housing seta apically. Leg trochanters, femora, patellae, tibiae light brown, dark brown, or black, lighter at joints; metatarsi of leg III with proximal one-third to one-half light brown, brown, or black; leg IV with proximal three-quarters light brown, brown, or black; proximal end of metatarsi of legs I and II pale brown, brown, or black; remaining metatarsal areas pale brown; tarsi pale brown, darkening distally. Scaled microsculpture subequal to darkened areas, remainder with setae and microtrichia. Metatarsi of leg II with false leg articulations (n=7).
Penis length 2.48 mm (n=1), glans plate 0.37 mm, stylus 0.09 mm, stylus slightly twisted, not decurved.
Similar to male for nearly all characters. Total length 4.71 mm (n=3, 4.45–5.06 mm); carapace length 1.35 mm (n=5, 1.25–1.44 mm); carapace width 3.01 mm (n=3, 2.81–3.13 mm); scutum length 3.63 mm (n=3, 3.19–4.30 mm); scutum width 3.70 mm (n=3, 3.12–3.50 mm); length of fused sternites I-V 2.96 mm (n=5, 2.81–3.06 mm).
Eye tubercle height above surface of carapace 1.18 mm (n=3, 1.00–1.28 mm); distance from ventral edge of eye to tip of spine 1.06 mm (n=5, 0.90–1.13 mm). Eye color dark brown, pink-gray, or light brown. Metapeltidial spine 0.22 mm (n=5, 0.18–0.25 mm).
Paramedian tubercles or tergite I height 0.08 mm (n=5, 0.05–0.13 mm) above surface of tergite; median tubercles raised mounds adorned with warts, two additional pairs of warty mounds reducing in size laterally. Tergite II paramedian tubercles greatly enlarged into erect spines standing 0.94 mm (n=5, 0.78–1.03 mm) above tergite surface; lateral to these are raised mounds adorned with warts. Paramedian tubercles of tergite III 0.07 mm (n=5, 0.03–0.10 mm); IV 0.06 mm (n=5, 0.03–0.10 mm). Tergite areas III, IV, and V with three pairs of relatively large tubercles in the form of raised mounds adorned with warts; these not decreasing in size laterally, decreasing slightly posteriorly across tergites. First free tergite (VI) adorned with raised warty mounds; tergites VII and VIII without discernable tubercles in paratypes (AMNH, CAS), tergite VII of paratype (UWBM) with tubercles as in VI.
Sternites brown. Transverse furrow and membranous lateral sutures of genital operculum less distinct than in other species. Prosomal sternum (n=1) length 0.18 mm, width 0.39 mm; pale-brown; without setae. Labium wider than long or subequal; lengths: 0.10, 0.19, 0.13 mm; widths: 0.16, 0.18, 0.19 mm; moderately or weakly sclerotized; brown; without setae. Palpal endites light brown. Leg II endites adorned with 2 setae.
Horn-shaped process of epistome decurved, projecting 0.42 mm from sulcus. Chelicerae brown or light brown; article I length 1.18 mm (n=3, 1.16–1.21 mm), width 0.43 mm (0.42–0.44 mm); article II length 1.39 mm (1.34–1.42 mm), width 0.41 mm (0.38–0.43 mm); article III length 0.59 mm (0.58–0.62 mm). Article I without raised glandular mound (Figure 6). Article II with 6 setae on prolateral dark area at cleavage of corpus and fixed finger of chela; 15 setae on ventral surface of article II; these patches discrete. Palpus dimensions in Table 4; coxae light brown or brown with two seta-bearing tubercles; trochanters brown or pale brown with 4 or 5 seta-bearing tubercles; only paratype (CAS) with tubercle on patella, only paratype (UWBM) with partial diffuse band on patella.
Leg measurements given in Table 4. Leg trochanters, femora, patellae, and tibiae brown to dark brown, lighter at joints; metatarsi of legs III with proximal one-half brown, of leg IV with proximal three-quarters light brown to brown, proximal ends of legs I and II light brown to brown, remaining metatarsal areas pale brown; tarsi pale brown, darkening distally.
Ovipositor length 0.80 mm, width 0.44 mm; corona of setae at furcal base surrounding lobes, apical setae on lobes; furca without dorsoventral differentiation.
Acuclavella leonardi is found in the southern Cascade Mountains of Washington State in the Cowlitz River (includes Iron Creek) and Coweeman River (includes Goble Creek) watersheds in Lewis, Cowlitz, and Skamania Counties (Appendix I). Found in coniferous forests with small perennial water-features such as side-slope seeps, springs, and headwater streams; underneath stream-side woody debris.
urn:lsid:zoobank.org:act:8C34D2F8-D211-42B7-B58A-A90285771589
http://species-id.net/wiki/Acuclavella_makah
MorphBank images of specimens considered this species include:
Holotype AMNH, MorphBank Specimen Id: 822644, 2 images
Paratype CASENT9039219, MorphBank Specimen Id: 822645, 3 images
CHR1536, MorphBank Specimen Id: 828519, 2 images
SDSU OP1699, MorphBank Specimen Id: 828516, 1 image
CHR2457.0, MorphBank Specimen Id: 822505, 4 images
CHR2457.1, MorphBank Specimen Id: 822506, 3 images
CHR2457.2, MorphBank Specimen Id: 822507, 3 images
CHR3387.0., MorphBank Specimen Id: 822647, 1 image
CHR3387.1, MorphBank Specimen Id: 822646, 1 image
Figure 7 and 8, Appendix VIII: Figure 1, Figure 2Male holotype and female paratype (AMNH), and male and female paratypes (CAS, CASENT9039219) from Brownes Creek, Clallam County, Washington; male and female paratypes (UWBM, WA2393/7641) from Yahoo Lake Road 1.4 miles east of Hoh-Clearwater Road, Jefferson County, Washington (Appendix I). An additional specimen is housed at UWBM (WA0973/8147): Ahlstroms Prairie, Olympic National Park, Clallam County, Washington, (NAD 1927) 48.157°N, 124.704°W, elevation 40 meters; Rod Crawford (collected under permit), 16–17 July 1984.
The specific epithet refers to the Makah Nation, which historically occupied much of the known distribution of the species. The name Makah was given to these people by their neighbors; it means “generous with food”. These people have shared with many people access to their beautiful land, next to the rocks and gulls. For more information on the Makah Nation see: http://www.makah.com.
Distinguished from all Acuclavella except Acuclavella leonardi by the combination of having paramedian tubercles on area II only, having light, and strongly contrasting ends to sclerotized leg segments, giving the appearance of banding at joints. Though not always present, false leg articulations on the metatarsi of legs II, and single dark prolateral tubercles on the palpal patellae also diagnose it from these species. Scutal tubercles lateral to paramedian tubercles tend to be on more distinctive raised mounds in Acuclavella leonardi. Area II spines more narrow at base than in Acuclavella leonardi. Best diagnosed from Acuclavella leonardi using molecular data. Diagnostic COI sequences have been uploaded to the Barcode of Life Data Systems (BOLD: ACUOP007-13).
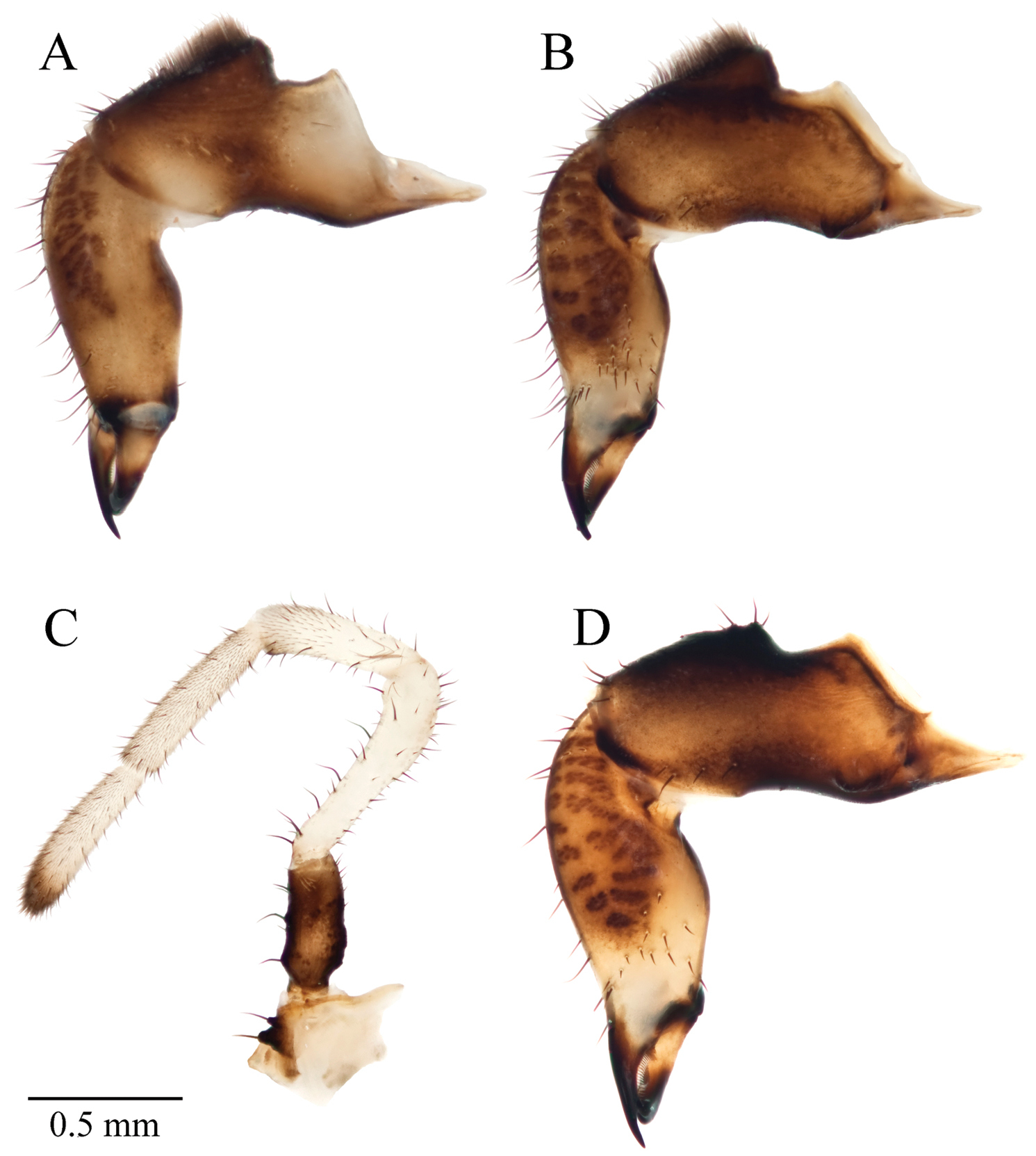
Body arched and convex dorsally (Figure 7), sides parallel with equal scutum and carapace widths; nearly all of body heavily sclerotized, black, with densely scattered hemispherical warts which irregularly house short setae apically or posteriorly. Total length 4.11 mm (n=3; 4.05–4.20 mm), carapace length in midline 1.26 mm (n=10; 1.05–1.44 mm), greatest carapace width 2.57 mm (n=3; 2.50–2.72 mm); length of fused scutes I-V in midline 2.33 (n=10; 2.20–2.48 mm), scutum length in midline 2.74 (n=3; 2.60–2.90 mm) greatest scutum width 2.57 mm (n=3; 2.50–2.72 mm).
Acuclavella makah. Top: male, CHR2457.1; bottom: female, CASENT9039219.
Acuclavella makah chelicerae and pedipalp. A right chelicera, retrolateral view B left chelicera, prolateral view C right pedipalp, retrolateral view (A–C, male CHR2457-0) D left chelicera, retrolateral view (female CHR2457-2).
Eye tubercle at anterior edge of carapace, prolonged anteriad into a sharp conical spine 1.27 mm (n=10; 1.05–1.45 mm) from ventral edge of eye to tip of spine; 1.58 mm above the carapace (n=3; 1.53–1.64 mm). Eyes light-brown to brown, located basally on tubercle. Surface of carapace evenly curved, posterior margin arcuate. Metapeltidial paramedian sensory cones short, acute spines standing 0.19 mm (n=10; 0.13–0.25 mm) above the surface of the metapeltidium, shiny, lacking warts.
All scutal areas with pairs of paramedian tubercles. Area I paramedian tubercles cluster of cuticular warts standing 0.04 mm above the surrounding scute (n=10; 0.025–0.075 mm), two additional pairs of warty tubercles reduce in size laterally. Paramedian tubercles of area II rise to form large acute spines standing 1.37 mm above the scutum surface (n=10; 1.13–1.75 mm); lateral to spines a pair of tubercles typically small cluster of warts, though one individual (CHR1536) with lateral pair of short spines. Scute areas III, IV, and V with three pairs of wart-clustered tubercles each; paramedian pair largest, diminishing in size laterally along scute, and posteriorly across scute areas. Paramedian tubercles of area III stand 0.03 mm (n=10, 0.03–0.05 mm) above surface of the tergite; area IV tubercle height 0.04 mm (n=10, 0.03–0.08 mm). Holotype and paratype (CAS) with first free tergite (area VI) with barely distinguishable tubercle as median pair of enlarged warts, tergites VII and VIII without tubercles; paratype (UWBM) tergite VI with median and lateral pair of enlarged warty tubercles, tergite VII with barely distinguishable tubercles, these lacking on tergite VIII. Tergite IX divided, triangular, bracketing tergite X, which forms the anal operculum.
Abdominal sternites with infrequent setae; warty sculpturing strongest on posterior and lateral margins; brown to dark brown. Prosomal sternum length 0.14 mm (n=2, 0.13–0.16 mm), width 0.28 mm (0.26–0.30 mm); brown; without setae. Labium weakly sclerotized, wider than long, length 0.08 mm (n=3; 0.05–0.11 mm), width 0.15 mm (0.13–0.17 mm); light brown to yellow-brown, without setae. Palpal endites brown to yellow-brown, large, free, bearing many setae; leg II endite bearing 3 setae in holotype and paratype (CAS), 2 in paratype (UWBM); legs I, III, IV endites without setae. Epistome with horn-like anteriad projection, decurved or slightly decurved, projecting 0.34 mm (n=2, 0.33–0.36 mm) from sulcus. Chelicerae basal article (I) dark brown dorsally, middle article (II) with prolateral and retrolateral striations of darker, more sclerotized striations. Article I length 1.18 mm (n=3, 1.06–1.30 mm), width (I) at widest point 0.41 mm (0.38–0.43 mm), article II length 1.40 mm (1.34–1.46 mm), width (II) at widest point 0.40 mm (0.38–0.43 mm), article III length 0.53 mm (0.50–0.57 mm). Article I with raised, setose glandular area on dorsal surface (Figure 8). Article I with boss-like tubercles proximally on proventrolateral and retrolateral surfaces, one individual with proventrolateral tubercles bilobed; proventrolateral tubercle may function to macerate food or manipulate food items in conjunction with the epistome process. Cleavage of corpus and fixed finger housing 5 or 6 setae on prolateral side of article II; 12 or 15 setae on ventral surface of article II; these patches discrete. Palpal coxae yellow-brown to brown, with two seta-bearing tubercles ventrally (Figure 8). Palpal measurements given in Table 5. Trochanter brown to dark-brown with 3 (holotype) or 4 seta-bearing tubercles; femur white; patella white without dark band medially, with prolateral, distal darkened tubercles; bearing microtrichia and small setae distally; tibia white with scattered setae and dense microtrichia; tarsus white, usually darkening distally. Claw rudiment very small.
Acuclavella makah palpus and leg measurements.
| Appendage | Segment | Male | Female | ||||
|---|---|---|---|---|---|---|---|
| Mean | Range | n | Mean | Range | n | ||
| Palpus | trochanter | 0.58 | 0.54-0.62 | 3 | 0.57 | 0.54-0.60 | 3 |
| femur | 0.95 | 0.90-0.98 | 10 | 0.98 | 0.88-1.05 | 14 | |
| patella | 0.67 | 0.62-0.72 | 3 | 0.70 | 0.67-0.74 | 3 | |
| tibia | 0.79 | 0.73-0.84 | 3 | 0.72 | 0.68-0.76 | 3 | |
| tarsus | 0.73 | 0.71-0.76 | 3 | 0.68 | 0.67-0.68 | 3 | |
| Leg I | trochanter | 0.57 | 0.56-0.60 | 3 | 0.53 | 0.50-0.56 | 3 |
| femur | 2.67 | 2.60-2.72 | 3 | 2.46 | 2.36-2.56 | 3 | |
| patella | 0.97 | 0.92-1.00 | 3 | 0.90 | 0.88-0.92 | 3 | |
| tibia | 1.75 | 1.72-1.76 | 3 | 1.57 | 1.55-1.60 | 3 | |
| metatarsus | 2.36 | 2.24-2.44 | 3 | 2.13 | 2.08-2.20 | 3 | |
| tarsus | 2.93 | 2.76-3.04 | 3 | 2.72 | 2.56-2.80 | 3 | |
| Leg II | trochanter | 0.60 | 0.56-0.64 | 10 | 0.61 | 0.56-0.64 | 14 |
| femur | 4.01 | 3.76-4.35 | 10 | 3.85 | 3.60-4.20 | 14 | |
| patella | 1.12 | 1.00-1.20 | 10 | 1.14 | 1.04-1.28 | 14 | |
| tibia | 2.65 | 2.40-2.88 | 10 | 2.64 | 2.40-2.92 | 14 | |
| metatarsus | 3.91 | 3.60-4.25 | 10 | 3.80 | 3.52-4.75 | 14 | |
| tarsus | 5.11 | 4.55-5.50 | 10 | 4.62 | 4.25-4.94 | 14 | |
| Leg III | trochanter | 0.55 | 0.52-0.56 | 3 | 0.57 | 0.55-0.60 | 3 |
| femur | 2.47 | 2.32-2.56 | 3 | 2.21 | 2.12-2.32 | 3 | |
| patella | 0.93 | 0.92-0.96 | 3 | 0.96 | 0.88-1.05 | 3 | |
| tibia | 1.64 | 1.60-1.68 | 3 | 1.60 | 1.52-1.65 | 3 | |
| metatarsus | 2.64 | 2.56-2.76 | 3 | 2.48 | 2.40-2.64 | 3 | |
| tarsus | 3.16 | 3.00-3.32 | 3 | 2.95 | 2.85-3.12 | 3 | |
| Leg IV | trochanter | 0.67 | 0.60-0.72 | 3 | 0.65 | 0.60-0.72 | 3 |
| femur | 3.64 | 3.52-3.80 | 3 | 3.34 | 3.16-3.60 | 3 | |
| patella | 1.05 | 0.96-1.12 | 3 | 1.11 | 1.04-1.20 | 3 | |
| tibia | 2.32 | 2.32 | 3 | 2.30 | 2.16-2.44 | 3 | |
| metatarsus | 4.16 | 4.05-4.30 | 3 | 3.99 | 3.76-4.25 | 3 | |
| tarsus | 4.17 | 3.85-4.35 | 3 | 3.86 | 3.68-3.95 | 3 | |
All measurements in millimeters; n= sample size
Leg measurements given in Table 5. Trochanters, femora, patellae, tibiae black, lighter at joints, with scattered, distally elevated scales which subtend short setae, scales occasionally house setae apically or posteriorly. Metatarsi of leg III with proximal half black or darkened; leg IV proximal three-quarters black or darkened; legs I and II with proximal end darkened; remaining metatarsal areas pale-brown to yellow-brown. Scaled-microsculpture subequal to darkened areas. Metatarsi and tarsi without tubercles. Six of ten males with false leg articulations on metatarsi of leg II, including holotype. Leg claws single, black, not toothed, evenly curved.
Penis 2.39 mm in length (n=2, 2.38–2.40 mm); glans plate length 0.34 mm (n=2, both 0.34 mm); stylus length 0.16 mm (0.14–0.17 mm). Shaft evenly tapered, broadening slightly at glans; glans with scattered small setae; stylus spirally twisted, very slightly decurved.
Similar to male for nearly all characters. Total length 5.14 mm (n=3; 5.00–5.31 mm); carapace length 1.37 mm (n=14; 1.25–1.81 mm); carapace width 3.16 mm (n=3; 3.00–3.31 mm); scutum length 3.82 mm (n=3; 3.63–3.94 mm); scutum width 3.33 mm (n=3; 3.19–3.50 mm); length of fused sternites I-V 2.96 mm (n=14; 2.75–3.19 mm).
Eye tubercle height above surface of carapace 1.37 mm (n=3; 1.31–1.46 mm); distance from ventral surface of eye to tip of spine 1.21 mm (n=14; 1.08–1.33 mm). Eye color brown. Metapeltidial spine 0.16 mm (n=14; 0.10–0.23 mm).
Paramedian tubercles of tergite I height 0.06 mm (n=14; 0.03–0.10 mm) above surface of tergite. Tergite II paramedian tubercles greatly enlarged spines standing 1.10 mm (n=14; 0.93–1.28 mm) above tergite surface; tubercles lateral to spines warty mounds. Paramedian tubercle height of tergite III 0.07 mm (n=14; 0.05–0.10 mm); IV 0.06 mm (n=14; 0.03–0.08 mm). Tergites III, IV, V with three pair of tubercles with median pair largest, reducing in size laterally along tergite and posteriorly across tergites; the right median tubercle of tergite III of paratype (CAS) enlarged into mound with diminished frequency of warts standing 0.22 mm above carapace. First free tergite (IV) with median and lateral pair of enlarged warty tubercles, or barely distinguishable median pair of enlarged warts; tergite VII without tubercles, or tubercles barely distinguishable, or more noticeable; tergite VIII without distinguishable tubercles.
Sternites brown. Prosomal sternum length 0.17 mm (n=2, 0.17–0.18 mm), width 0.32 mm (0.30–0.33 mm); dark brown. Labium wider (0.17 mm, 0.16 mm, 0.14 mm) than long (0.09 mm, 0.09 mm, 0.14 mm), or nearly equal; yellow-brown to light brown. Palpal endites brown, yellow-brown, or light brown. Paratypes (AMNH, CAS) with 3 setae on leg II endite; paratype (UWBM) with 2 setae. Horn-shaped process of epistome decurved or projecting downward; length 0.48 mm (n=2, 0.47–0.50 mm).
Chelicerae article I length 1.35 mm (n=3; 1.20–1.47 mm), width 0.45 mm (n=3, 0.44–0.46 mm); article II length 1.49 mm (n=3; 1.48–1.50 mm), width 0.42 mm (0.42–0.43); article III length 0.59 mm (n=3; 0.56–0.60 mm). Article I without raised glandular mound (Figure 8); prolateral side of cheliceral article II with 2 or 4 setae at cleavage of corpus and fixed finger; 12 setae on ventral surface of article II; these patches discrete. Palpus dimensions in Table 5; coxae brown with two seta-bearing tubercles; trochanter brown to dark brown with 4 seta-bearing tubercles; only paratype with tubercle on patella.
Leg measurements given in Table 5. Leg trochanters, femora, patellae, and tibiae black, lighter at joints; metatarsi of leg III with proximal half black or darkened, leg IV with proximal three-quarters black or darkened, proximal end of legs I and II black or darkened, remaining metatarsal areas yellow-brown or pale brown; tarsi yellow-brown or pale brown, darkening distally.
Ovipositor length 0.74 mm (n=2, 0.72–0.77 mm), width 0.46 mm (0.44–0.47 mm); furca without dorsoventral differentiation; corona of setae at furcal base surround lobes; apical setae on lobes.
Known from the northwest areas of the Olympic Peninsula in Clallam and Jefferson Counties, Washington State (Appendix I). Found in coniferous or riparian forests along small, perennial water-features such as headwater streams, springs, and seeps; underneath woody debris and moss.
urn:lsid:zoobank.org:act:FC134B12-DA1C-4184-B9AB-6AC36E990BB5
http://species-id.net/wiki/Acuclavella_sheari
MorphBank images of specimens considered this species include:
Holotype AMNH, MorphBank Specimen Id: 822513, 4 images
Paratype AMNH, MorphBank Specimen Id: 822512, 6 images
SDSU OP2708, MorphBank Specimen Id: 822514, 3 images
SDSU OP2720, MorphBank Specimen Id: 822518, 3 images
Figure 9 and 10, Appendix VIII: Figure 1, Figure 2Male holotype (AMNH) and female paratypes (AMNH; CAS, CASENT9039225; UWBM) from Burgdorf Road 12.4 miles northwest of Warren Wagon Rd, tributary of Fall Creek, Payette National Forest, Idaho County, Idaho; two male paratypes (CAS, CASENT9039217; UWBM, ID0016/5360) from Burgdorf Road 16.6 miles northwest of Warren Wagon Road; otherwise as previous (Appendix I).
The specific epithet honors Dr. William A. Shear, eminent milliped and opilionid taxonomist. His influence has been important to the authors’ aspirations to be systematic biologists, and we thank him sincerely, and with pleasure.
Generally reduced dimensions overall. Males with one pair of scutal spines on area II only; scutal spines ≤ 0.50 mm, distance from ventral edge of eye to tip of ocularium ≤ 0.60 mm distinguishes it from other males with single pair of spines. Females lack scutal spines. Diagnosed from spine-less Acuclavella shoshone (Shear, 1986 sensu stricto) females by having palpal femora ≤ 0.88 mm, leg II tarsi ≤ 3.92 mm, leg II femora ≤ 2.75 mm.
Body arched and convex dorsally (Figure 9); sides broader posteriorly. Nearly all of body heavily sclerotized black or dark brown, with densely scattered hemispherical warts which irregularly house short setae apically or posteriorly; bilobed warts sporadic in holotype. Total length 3.90 mm (n=3, 3.68–4.06 mm), carapace length 1.24 mm (n=4, 1.15–1.32 mm), carapace width 2.34 mm (n=3, 2.31–2.36 mm); length of fused tergites I-V 2.26 mm (n=4, 2.16–2.31 mm), scutum length 2.61 mm (n=3, 2.32–2.81 mm), scutum width 2.60 mm (n=3, 2.55–2.63 mm).
Eye tubercle at anterior edge of carapace, hemispherical warts cover entirety of ocular spine, prolonged into an acute spine standing 0.67 mm above the surface of the carapace (n=3, 0.64–0.70 mm), 0.53 mm (n=4, 0.48–0.60 mm) from the ventral edge of the eye to the tip of the tubercle, eye spine less acute than in other species of Acuclavella. Eyes dark brown to brown, located basally on tubercle. Surface of carapace evenly curved, posterior margin arcuate. Metapeltidial paramedian sensory cones raised into sharp, acute spines standing 0.21 mm (n=4, 0.17–0.24 mm) above surface of the carapace, curving slightly towards the midline, lacking warty microsculpture, shiny; lateral to spines, clusters of warts form tubercles, missing in paratype (UWBM).
Acuclavella sheari. Top: male, CHR3254; bottom: female, CHR3404.
Acuclavella sheari chelicerae and pedipalp. A right chelicera, retrolateral view B left chelicera, prolateral view C right pedipalp, retrolateral view (A–C, male CASENT9039217) D left chelicera, retrolateral view (female AMNH).
All scutal tergites with pairs of paramedian tubercles; relatively short, pointed spines on area II only. Fused tergite I with paramedian tubercles as raised mounds, relatively tall but not spike-like, standing 0.125 mm above the surrounding scute (n=4, all 0.125), these adorned with warts; lateral to these, two additional raised mounds become smaller away from the midline, these lacking in holotype. Paramedian tubercles of area II form acute spine 0.39 mm above the scutum surface (n=4, 0.34–0.43 mm), curved slightly posteriad; lateral to these are raised tubercles adorned with warts. Fused tergites III, IV, and V with paramedian tubercles in the form of raised mound adorned with warts; lateral to these, area III with 2 or 3 additional pairs of tubercles, area IV with 1 or 2 additional tubercles, area V with 0 or 2 additional tubercles; these tend to diminish in size away from the midline and posteriorly across tergites. Area III tubercle height above tergite 0.081 mm (n=4, 0.075–0.10 mm); area IV tubercle height 0.075 mm (all 0.075 mm). Free tergites without discernable tubercles, or tubercles occur in single pairs as small warty mounds.
Abdominal sternites with infrequent setae; warty sculpturing strongest laterally and on posterior margin; sternites brown, dark brown, or black. Sclerotized areas of genital operculum relatively setose. Prosomal sternum (n=1) wider than long: length 0.19 mm, width 0.29 mm; dark brown; without setae. Labium weakly to well-sclerotized, wider than long (n=3):, length 0.09 mm (0.08-0.10 mm), width 0.16 mm (0.13-0.19 mm), light brown to dark brown, darkness commensurate to sclerotization, without setae. All coxae with endites. Palpal endites setose, light brown, dark brown, or black. Leg II endite bearing 2 setae, other leg endites unadorned. Horn-shaped process of epistome decurved, projecting 0.34 mm from sulcus.
Chelicerae light brown, dark brown, or black; lighter individuals with article I darker dorsally, article II with darker, more sclerotized striations on prolateral and retrolateral surfaces (Figure 10). Article I length 1.11 mm (n=3, 1.00-1.18 mm), width 0.39 mm (0.36-0.42 mm), article II length 1.21 mm (1.12-1.27 mm), width 0.40 mm (all 0.40 mm), article III length 0.54 mm (0.52-0.56 mm). Dorsal surface of article I with raised, glandular area dense with setae. Palpal coxae pale-brown, light brown, or black, with 2 or 1 (UWBM) seta-bearing tubercles. Palpal measurements given in Table 6. Trochanter (Figure 10) pale-brown or dark brown, with 2 (CAS), 3 (holotype), or 4 (UWBM) seta-bearing tubercles; these reduced on later individual; femur pale-brown, brown, or dark brown; patella pale-brown or brown, with (CAS) or without a diffuse dark band on middle third, without darkened tubercle distally, bearing small setae and microtrichia distally; tibiae pale brown or white (holotype), with scattered setae and dense microtrichia; tarsus pale-brown or white (holotype), darkening to brown distally. Claw rudiment very small to absent.
Acuclavella sheari palpus and leg measurements.
| Appendage | Segment | Male | Female | ||||
|---|---|---|---|---|---|---|---|
| Mean | Range | n | Mean | Range | n | ||
| Palpus | trochanter | 0.51 | 0.49–0.54 | 3 | 0.50 | 0.43–0.54 | 3 |
| Femur | 0.85 | 0.78–0.88 | 4 | 0.85 | 0.83–0.88 | 4 | |
| Patella | 0.63 | 0.62–0.65 | 3 | 0.66 | 0.60–0.70 | 3 | |
| Tibia | 0.74 | 0.72–0.75 | 3 | 0.73 | 0.65–0.79 | 3 | |
| Tarsus | 0.70 | 0.67–0.71 | 3 | 0.65 | 0.59–0.68 | 3 | |
| Leg I | trochanter | 0.53 | 0.48–0.56 | 3 | 0.45 | 0.40–0.50 | 3 |
| Femur | 1.80 | 1.76–1.84 | 3 | 1.73 | 1.48–1.94 | 3 | |
| Patella | 0.87 | 0.84–0.92 | 3 | 0.81 | 0.68–0.91 | 3 | |
| Tibia | 1.33 | 1.28–1.44 | 3 | 1.30 | 1.16–1.40 | 3 | |
| metatarsus | 1.97 | 1.96–2.00 | 3 | 1.88 | 1.68–2.09 | 3 | |
| Tarsus | 2.29 | 2.24–2.32 | 3 | 2.22 | 1.88–2.41 | 3 | |
| Leg II | trochanter | 0.57 | 0.56–0.60 | 4 | 0.52 | 0.44–0.63 | 4 |
| Femur | 2.53 | 2.48–2.64 | 4 | 2.52 | 2.16–2.75 | 3 | |
| Patella | 0.98 | 0.88–1.12 | 4 | 0.93 | 0.76–1.03 | 3 | |
| Tibia | 1.96 | 1.88–2.08 | 4 | 1.90 | 1.64–2.09 | 3 | |
| metatarsus | 2.99 | 2.88–3.12 | 4 | 3.00 | 2.56–3.24 | 3 | |
| Tarsus | 3.67 | 3.64–3.72 | 4 | 3.65 | 3.20–3.92 | 3 | |
| Leg III | trochanter | 0.53 | 0.52–0.56 | 3 | 0.52 | 0.44–0.59 | 3 |
| Femur | 1.72 | 1.64–1.80 | 3 | 1.71 | 1.56–1.84 | 3 | |
| Patella | 0.85 | 0.84–0.88 | 3 | 0.79 | 0.48–0.97 | 3 | |
| Tibia | 1.33 | 1.20–1.48 | 3 | 1.43 | 1.32–1.56 | 3 | |
| metatarsus | 2.21 | 2.16–2.28 | 3 | 2.18 | 1.84–2.38 | 3 | |
| Tarsus | 2.56 | 2.52–2.64 | 3 | 2.53 | 2.20–2.84 | 3 | |
| Leg IV | trochanter | 0.59 | 0.52–0.68 | 3 | 0.59 | 0.52–0.69 | 3 |
| Femur | 2.41 | 2.32–2.56 | 3 | 2.38 | 2.12–2.63 | 3 | |
| Patella | 0.96 | 0.88–1.04 | 3 | 0.89 | 0.80–0.94 | 3 | |
| Tibia | 1.84 | 1.76–1.88 | 3 | 1.77 | 1.56–2.03 | 3 | |
| metatarsus | 3.31 | 3.28–3.32 | 3 | 3.24 | 2.80–3.48 | 3 | |
| Tarsus | 3.23 | 3.08–3.32 | 3 | 3.29 | 2.80–3.68 | 3 | |
All measurements in millimeters; n=sample size
Leg measurements given in Table 6. Microsculpture of femora, patellae, and tibiae scattered, distally elevated scales, bilobed scales not observed; scales subtend setae, occasionally housing seta apically. Trochanters, femora, patellae, tibiae black, or in holotype, trochanters light brown, femora, patellae, and tibiae brown; metatarsi of leg III proximal one-third to one-quarter brown or black; leg IV with proximal half black or brown; proximal end of metatarsi on legs I and II black or brown, remaining metatarsal areas pale brown or brown; all tarsi pale brown or brown, darkening distally. Scaled microsculpture subequal to darkened areas, remainder with setae and microtrichia. False leg articulations not observed. Leg claws single, black, not toothed, evenly curved.
Penis length 2.94 mm (n=1); glans plate 0.31 mm, scattered small setae; stylus 0.13 mm, slightly spirally twisted and decurved.
Similar to male, differing in dimensions, secondary sexual characteristics, and dorsal armature. Total length 4.25 mm (n=3; 4.05-4.65 mm); carapace length 1.325 mm (n=4, 1.05-1.45 mm), width 2.62 mm (n=3, 2.35-3.05 mm); scutum length 3.03 (n=3, 2.90-3.25 mm), width 3.23 mm (n=3, 2.85-3.55 mm); fused tergites I-V length in midline 2.81 mm (n=4, 2.20-3.10 mm).
Eye tubercle height above surface of carapace 0.67 mm (n=3, 0.56-0.73 mm), distance from ventral surface of eye to tip of spine 0.54 mm (n=4, 0.46-0.60 mm). Eye color brown or gray. Metapeltidial spine 0.11 mm (n=4, 0.08-0.15 mm), curving slightly posteriad and towards the midline, lateral to these spines clusters of warts form tubercles.
Dorsal armature lacking. Dorsal adornment generally with median tubercles as raised mounds adorned with warts, decreasing in size away from midline and posteriorly across tergites. Two lateral pairs of warts on areas III and IV, area V with median pair of tubercles barely discernable, two pairs of lateral tubercles, or shallowly raised mounds adorned with warts. Free tergites with tubercles in the form of clusters of warts, or without discernable tubercles. Paramedian tubercles heights above the surface of the tergite (n=4): of area I 0.07 mm (0.05-0.08 mm); area II 0.10 mm (0.08-0.15 mm); area III 0.06 mm (0.05-0.08 mm); area IV 0.05 mm (0.03-0.08 mm).
Sternites brown, black, or light brown. Prosomal sternum (n=1) length: 0.26 mm, width 0.31 mm; light brown, without setae. Labium wider (0.13, 0.17, 0.16 mm) than long (0.08, 0.09, 0.08 mm); light brown or pale brown. Palpal endites brown or pale brown. Endites of leg II adorned with two or one (CAS) setae.
Chelicerae article I length 0.98 mm (n=3, 0.92-1.06 mm), width 0.41 mm (0.37-0.44 mm); article II length 1.31 mm (1.14-1.40 mm), width 0.43 mm (0.40-0.46 mm); article III length 0.57 mm (0.52-0.62 mm). Article I without raised glandular mound (Figure 10). Article I darker dorsally, chelicerae light brown or black with areas of dark brown. Palpus dimensions in Table 6; coxae light brown, dark brown, or white, with two seta-bearing tubercles; trochanters light brown, dark brown, or white, with three (AMNH) or four (CAS, UWBM) seta-bearing tubercles; femora and patella pale brown, brown, or white, patella without both distal tubercles and diffuse bands; tibiae white or pale-brown; tarsus white or pale-brown, fading to brown distally. Claw rudiment very small (AMNH, UWBM), or absent.
Leg measurements given in Table 6. Leg trochanters brown, black, or pale-brown; femora, patellae, and tibiae dark brown, back, or pale brown; metatarsi of legs III with proximal third brown, black, or pale-brown; leg IV metatarsi with proximal half brown, black, or pale-brown; proximal end of legs I and II metatarsi brown, black, or pale-brown; remaining metatarsal areas pale-brown, brown, or white; tarsi pale brown or white, darkening distally.
Ovipositor length 0.82 mm, width 0.46 mm (n=1); otherwise as Acuclavella makah.
Known from north-facing, horizontal band of Abies grandis forest above south shore of Salmon River. This habitat is mainly roadless, though intersected by FS 592 and Burgdorf Road in Payette National Forest, Idaho County, Idaho. Adults collected in June and September. Abies grandis, Pseudotsuga menziesii, and Picea engelmannii associated canopy cover; collected under woody debris adjacent to headwater streams.
http://species-id.net/wiki/Acuclavella_quattuor
MorphBank images of specimens considered this species include:
AMNH, MorphBank Specimen Id: 822462, 3 images
CHR2146.2, MorphBank Specimen Id: 822463, 3 images
CHR2146.5, MorphBank Specimen Id: 822464, 3 images
CHR2147.7, MorphBank Specimen Id: 822604, 1 image
SDSU OP2256, MorphBank Specimen Id: 822496, 3 images
SDSU OP2266, MorphBank Specimen Id: 822605, 1 image
CHR2180.0, MorphBank Specimen Id: 822606, 2 images
CHR2180.3, MorphBank Specimen Id: 822607, 1 image
Figure 11 and 12, Appendix VIII: Figure 1, Figure 2Males (AMNH; CAS, CASENT9039220; UWBM, ID0013/5661) from the type locality: Slate Creek Road 10.2 miles east of US 95, Nez Perce National Forest, Idaho County, Idaho.
Diagnosed from all Acuclavella species except for Acuclavella cf. quattuor by having two pairs of erect spines on tergites I and II; similar morphologically to Acuclavella cf. quattuor, and is best diagnosed using molecular data: basepair 23 of the EF-1α intron cytosine in Acuclavella cf. quattuor, adenine in Acuclavella quattuor; basepair 44 guanine in Acuclavella quattuor, thymine or adenine in Acuclavella cf. quattuor (thymine if sequences aligned due to a 3 basepair deletion in 40% of Acuclavella cf. quattuor at basepair 39–41).
Body arched and convex dorsally (Figure 11); sides parallel or slightly broader posteriorly. Nearly all of body heavily sclerotized, black, with densely scattered hemispherical warts which irregularly house short setae apically or posteriorly. Total length 4.22 mm (n=3, 4.06–4.45 mm); carapace length 1.35 mm (n=14, 1.25–1.50 mm), width 2.60 mm (n=3, 2.56–2.64 mm); scutum length 2.84 mm (n=3, 2.56–3.25 mm), width 2.80 mm (n=3, 2.69–2.88 mm); length fused tergites I-V 2.69 mm (n=14, 2.50–3.10 mm).
Eye tubercle at anterior edge of carapace, erect, spine-like, standing 0.98 mm above the surface of the carapace (n=3, 0.94–1.05 mm), distance from ventral edge of eye to tip of spine 0.84 mm (n=14, 0.65–0.93 mm). Eye color brown, on basal part of ocularium. Surface of carapace evenly curved, posterior margin arcuate. Metapeltidial paramedian sensory cones acute or blunt spines standing 0.28 mm (n=14, 0.18-0.38 mm) above surface of carapace.
Acuclavella quattuor. Male AMNH.
Acuclavella quattuor chelicerae and pedipalp. Male CHR2146.5, A right chelicera, retrolateral view B left chelicera, prolateral view C right pedipalp, retrolateral view.
Scutum of opisthosoma rounded anteriorly, squared of posteriorly. All fused tergites with paramedian pair of tubercles, these in the form of erect spines on areas I and II. Spines of tergites I and II curve posteriad, stand 0.82 mm (n=14, 0.65-1.05 mm) and 0.91 (n=14, 0.70-1.12 mm) above the surface of the tergite respectively; tubercles lateral to spines as small raised wart mounds; one of 14 males with lateral spines on area II. Tergites III, IV, and V with four pairs of tubercles as raised areas adorned with warts; paramedian tubercles of area III height 0.06 mm (n=14, 0.03-0.20 mm); area IV paramedian tubercle height 0.05 mm (n=14, 0.03-0.08 mm); two individuals with one of paramedian tubercles of area III spine-like, left side on one, right side on other. First free tergite (VI) with small median tubercles in the form of enlarged shiny wart; remaining free tergites without discernable tubercles.
Abdominal sternite warty sculpturing strongest laterally and on posterior margin; sternites brown or dark brown. Genital operculum tongue-shaped, clearly delineated by transverse furrow, lateral ends of furrow membranous suture, distal margin rebordered, glossy. Prosomal sternum wider than long; length 0.18 mm (n=3, 0.17-0.21 mm), width 0.29 mm (n=3, 0.26-0.31 mm); pale brown or brown; without setae. Labium moderately sclerotized, wider than long or dimensions subequal; length 0.11 mm (n=3, 0.09-0.13 mm), width 0.14 mm (0.13-0.16 mm); brown or dark brown; without setae. Palpal endites setose; brown. Leg II endites bearing 2 (AMNH, CAS) or 3 setae (UWBM), other leg endites glabrous. Horn-shaped process of epistome strongly decurved; projecting 0.43 mm (n=4, 0.41-0.44 mm) from sulcus.
Chelicerae light brown or brown; darker dorsally; article II with darker, more sclerotized striations on prolateral and retrolateral surfaces (Figure 12). Article I length 1.21 mm (n=3, 1.18-1.25 mm), width 0.44 mm (n=3, 0.42-0.45 mm); article II length 1.38 mm (n=3, 1.35-1.40 mm), width 0.41 mm (n=3, 0.38-0.45 mm); article III length 0.59 mm (n=3, 0.58-0.60 mm). Article I dorsal surface with raised, glandular area densely setose (Figure 12). Prolateral side of article II with dark sclerotization at cleavage of corpus and fixed finger housing 6, 6, 5, 5 setae; ventral surface of article with 19, 20, 16, 23 setae; these patches discrete in three of four individuals examined. Palpal measurements given in Table 7. Palpal coxae light brown or brown (Figure 12), with 2 seta-bearing tubercles. Palpal trochanters light brown, brown, or dark brown; with 4 seta-bearing tubercles. Femora and patella white or yellow-brown; patella without distal prolateral tubercle, without diffuse dark band, bearing small setae and microtrichia distally. Tibia white or pale yellow, with scattered setae and dense microtrichia. Tarsi white or pale yellow, darkening distally; with setae and dense microtrichia. Claw rudiment very small.
Acuclavella quattuor male palpus and leg measurements.
| Palpus | Leg I | Leg II | Leg III | Leg IV | ||
|---|---|---|---|---|---|---|
| Trochanter | Mean | 0.53 | 0.61 | 0.61 | 0.51 | 0.64 |
| Range | 0.52–0.54 | 0.60–0.62 | 0.56–0.68 | 0.48–0.56 | 0.56–0.75 | |
| n | 3 | 3 | 14 | 3 | 3 | |
| Femur | Mean | 1.01 | 2.31 | 3.14 | 2.00 | 2.88 |
| Range | 0.95–1.10 | 2.20–2.44 | 2.72–3.44 | 1.96–2.04 | 2.76–3.00 | |
| n | 14 | 3 | 14 | 3 | 3 | |
| Patella | Mean | 0.67 | 0.97 | 1.08 | 0.95 | 1.10 |
| Range | 0.64–0.73 | 0.92–1.03 | 1.00–1.20 | 0.92–1.00 | 0.96–1.20 | |
| n | 3 | 3 | 14 | 3 | 3 | |
| Tibia | Mean | 0.83 | 1.54 | 2.21 | 1.54 | 2.11 |
| Range | 0.80–0.88 | 1.48–1.58 | 2.00–2.41 | 1.48–1.64 | 2.00–2.20 | |
| n | 3 | 3 | 14 | 3 | 3 | |
| Metatarsus | Mean | - | 2.36 | 3.55 | 2.61 | 3.98 |
| Range | - | 2.16–2.63 | 2.96–4.08 | 2.48–2.80 | 3.68–4.25 | |
| n | - | 3 | 14 | 3 | 3 | |
| Tarsus | Mean | 0.77 | 2.74 | 4.71 | 3.10 | 4.04 |
| Range | 0.73–0.85 | 2.48–3.03 | 3.90–5.25 | 2.76–3.28 | 3.56–4.31 | |
| n | 3 | 3 | 14 | 3 | 3 |
All measurements in millimeters, n=sample size.
Leg measurements given in Table 7. Microsculpture of femora, patellae, and tibiae scattered, distally elevated scales, bilobed scales not observed; scales infrequently house seta apically or distally. Trochanters, femora, patellae, and tibiae dark brown to black; metatarsi of leg III with proximal one-quarter brown or black, leg IV with proximal one-half brown or black, proximal end of metatarsi of I and II brown or black, remaining metatarsal areas brown; tarsi brown, darkening distally. Leg claws single, black, not toothed, evenly curved.
Penis length 2.49 mm (n=4, 2.35–2.55 mm); shaft evenly tapered, broadening slightly at glans. Glans bearing scattered small setae; glans length 0.32 mm (n=3, 0.29–0.36 mm). Stylus length 0.18 mm (n=3, 0.16–0.19 mm); stylus spirally twisted, decurved.
For female description see
Acuclavella quattuor populations are bracketed by the Salmon River to the south and the South Fork Clearwater River to the north, whereas Acuclavella cf. quattuor is found north of the Selway River and south of the Lolo Trail Ridge. Acuclavella quattuor habitats are dominated by Abies grandis and Picea englemannii; microhabitat in litter, moss, and moist woody debris adjacent to headwater streams.
http://species-id.net/wiki/Acuclavella_merickeli
MorphBank images of specimens considered this species include:
CHR2100.1, MorphBank Specimen Id: 822457, 2 images; type locality
CHR2100.2, MorphBank Specimen Id: 822597, 1 image
CHR2121.2, MorphBank Specimen Id: 822601, 3 images
CHR2121.4, MorphBank Specimen Id: 822602, 2 images
CHR2121.5, MorphBank Specimen Id: 822459, 3 images
CHR2140.0, MorphBank Specimen Id: 822460, 3 images
CHR2140.2, MorphBank Specimen Id: 822603, 1 image
Figures: Appendix IXFor species descriptions see
Acuclavella merickeli populations are bracketed to the south by the South Fork Clearwater River and to the north by the Selway River; all localities in Nez Perce National Forest, Idaho County, Idaho. Coniferous habitat dominated by Picea englemannii or Thuja plicata and Pseudotsuga menziesii; microhabitats include moist woody debris and moss adjacent to small perennial water features such as side-slope seeps and headwater streams.
The American Arachnological Society (Vincent Roth Fund for Systematic Research) and the American Museum of Natural History (Theodore Roosevelt Memorial Grant) provided funding to CHR. Several institutional scholarships (Harry E. Hamber Memorial Scholarship, Frank Alverson Memorial Scholarship, and Jordan Dale Covin Memorial Scholarship) to CHR also helped contribute to this work. Specimens were collected with help from Daniel Richart, William Leonard, Shahan Derkarabetian, Jeffery Underwood, Adrienne Richart, Paul Marek, and Dean Levitt. Hours of morphological measurements were expedited with help from Kristen Emata, David Carlson, Nicasia O’Neal, Adrienne Richart, and Kyle Wilson. Nicasia O’Neal, Roy Larimer, and Joseph Warfel helped with images. Maureen McCormack, Shahan Derkarabetian, and Robin Keith Hedin helped with laboratory training. Stas Vidyakin helped with accessioning MorphBank images. This research was enriched from discussions of concepts and methodologies with Ricardo Carretero, Rod Crawford, Shahan Derkarabetian, Andrew Gottscho, William Leonard, Dean Levitt, Maureen McCormack, Tod Reeder, Jordan Satler, Axel Schönhofer, Peter Scott, William Shear, Jack Sullivan, and others. The Wnt2 Ischyropsalis sequence came from the collection of Jochen Martens via Axel Schönhofer, which was collected by S. Huber. The manuscript was improved via suggestions and edits by Angela DiDomenico, Kristen Emata, Ryan Fitch, Erika Garcia, Marc Hayes, Jordan Satler, Axel Schönhofer, Prashant Sharma, William Shear, and an anonymous reviewer.
Collection Locality Information (doi: 10.3897/zookeys.311.2920.app1) File format: Adobe PDF file (pdf).
PCR Primer Information. (doi: 10.3897/zookeys.311.2920.app2) File format: Adobe PDF file (pdf).
Male and Female Morphometric Data and Measurement Scheme. (doi: 10.3897/zookeys.311.2920.app3) File format: Microsoft Office Excel (xlsx).
PCA Methods and Results. (doi: 10.3897/zookeys.311.2920.app4) File format: Adobe PDF file (pdf).
Morphologies of Acuclavella cf. cosmetoides and Geography of Morphotypes. Figure 1: male morphologies. Figure 2: female morphologies. Figure 3: geographic distribution of male morphologies. Figure 4:geographic distribution of female morphologies. (doi: 10.3897/zookeys.311.2920.app5) File format: Adobe PDF file (pdf).
Acuclavella distribution. KMZ file of Acuclavella sampling effort, species distributions, and distribution of individuals used in concatenated phylogenetic reconstruction. (doi: 10.3897/zookeys.311.2920.app6) File format: Google Earth Keyhole Markup (kmz).
Phylogenetic Trees. Figure 1: Outgroup relationships from Bayesian gene trees. Figure 2: RAxML concatenated phylogeny. Figure 3: RAxML COI gene tree. Figure 4: RAxML EF-1α gene tree. Figure 5: 28S and Wnt2 RAxML gene trees. (doi: 10.3897/zookeys.311.2920.app7) File format: Adobe PDF file (pdf).
Acuclavella genitalia. Figure 1: penises. Figure 2: ovipositors. (doi: 10.3897/zookeys.311.2920.app8) File format: Adobe PDF file (pdf).
K. Leg II Morphologies. With and without femoral false leg articulations. (doi: 10.3897/zookeys.311.2920.app9) File format: Adobe PDF file (pdf).