(C) 2013 Derek S. Sikes. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of Nicrophorus in the nepalensis species-group, Nicrophorus efferens Sikes and Mousseau, is described from Bougainville Island in the Solomon Islands archipelago. It is distinguished from the known species of the genus Nicrophorus and its likely closest relative, Nicrophorus reticulatus Sikes and Madge, based on external morphology. A comparison among the four Nicrophorus species known from the Solomon Island archipelago and Papua New Guinea is presented.
Nicrophorus, Silphidae, burying beetles, taxonomy, Solomon Islands, Papua New Guinea
The nepalensis species group (Coleoptera, Nicrophorus) is the second largest species group within the genus Nicrophorus. Species of this group are primarily montane in regions of eastern Asia and the Malay Archipelago, ranging in longitude from 73°E (Pakistan) to 160°E (Guadalcanal) and latitude from 51°N (Ussuri, Russia) to 9°48'S (Papua New Guinea) (
Only two species of Nicrophorus were known from the Solomon Islands archipelago prior to this study: Nicrophorus reticulatus Sikes and Madge and Nicrophorus kieticus Mroczkowski (
The 6 adult specimens comprising the type series were borrowed from the Bernice Pauahi Bishop Museum of Hawaii (BPBM). One paratype from this series will be deposited in the BMNH. These were compared with specimens from the following collections: FMNH – Field Museum of Natural History, Chicago, IL, USA; DSSC – Derek S. Sikes Collection, University of Alaska Fairbanks, Fairbanks, AK, USA; BMNH – Department of Entomology, The Natural History Museum, Cromwell Rd, London, UK. Four of the type specimens were broken with parts associated on separate pins. All nepalensis-group characters were coded independently by both authors and any differences of opinion were discussed to reach a consensus. Morphological data were managed using MacClade 4.04 OSX (
The data underpinning the analyses reported in this paper are deposited at GBIF, the Global Biodiversity Information Facility, http://ipt.pensoft.net/ipt/resource.do?r=type_specimen_data_for_new_species_nicrophorus_efferens.
urn:lsid:zoobank.org:act:7B4F8F2A-CC50-407A-BD15-CEC84EB706F6
http://species-id.net/wiki/Nicrophorus_efferens
Figs 1, 2A, B, 3, 4Male (in BPBM), here designated, labeled “Bougainville: NE Mutahi. 700m 18 km S. E. Tinputz” / “8–14. III. 1968” / “Tawi Collector BISHOP” / “Nicrophorus kieticus Mroczkouski [sic] det. S.B. Peck. 1993” / “HOLOTYPE Nicrophorus efferens Sikes & Mousseau 2013 BPBM124189Nic” [red paper].
5 Specimens. 2 Females, labeled “Bougainville: NE Mutahi. 700m 18 km S. E. Tinputz” / “15–21. III. 1968” / “Tawi Collector BISHOP” / “Nicrophorus kieticus Mroczkouski [sic] det. S.B. Peck. 1993” / “PARATYPE Nicrophorus efferens Sikes & Mousseau 2013 BPBM124190Nic” [red paper], this specimen’s head is mounted on a card below the body (deposited in London, BMNH), BPBM124191Nic, this specimen’s abdomen (genitalia missing) is mounted on a card on a separate pin and its left elytron is mounted below the body on a card. 1 Female, labeled “Bougainville: NE Mutahi. 700m 18 km S. E. Tinputz” / “8–14. III. 1968” / “Tawi Collector BISHOP” / “Nicrophorus kieticus Mroczkouski [sic] det. S.B. Peck. 1993” / “PARATYPE Nicrophorus efferens Sikes & Mousseau 2013 BPBM124192Nic” [red paper], phoretic mites removed from body into genitalia vial on pin below body. 1 Female, labeled “Bougainville: NE Mutahi. 700m 18 km S. E. Tinputz” / “1–7. III. 1968” / “& R. Straatman Collectors BISHOP MUSEUM” / “Nicrophorus kieticus Mroczkouski [sic] det. S.B. Peck. 1993” / “PARATYPE Nicrophorus efferens Sikes & Mousseau 2013 BPBM124193Nic” [red paper], this specimen’s prothorax and head are mounted on a card below the body. 1 Male, labeled “Bougainville: NE Mutahi. 700m 18 km S. E. Tinputz” / “1–7. III. 1968” / “Tawi Collector BISHOP” / “Nicrophorus kieticus Mroczkouski [sic] det. S.B. Peck. 1993” / “PARATYPE Nicrophorus efferens Sikes & Mousseau 2013 BPBM124194Nic” [red paper], specimen broken and mounted on two pins: hind legs and pygidium on card below body, on pin 2 genitalia in vial with glycerin, head and prothorax on card.
[Papua New Guinea]: Solomon Islands [Archipelago]: Bougainville: NE Mutahi, SE Tinputz, 700m, [~ 5.716°S, 155.086°E +/- 2 km, WGS84] (Fig. 1).
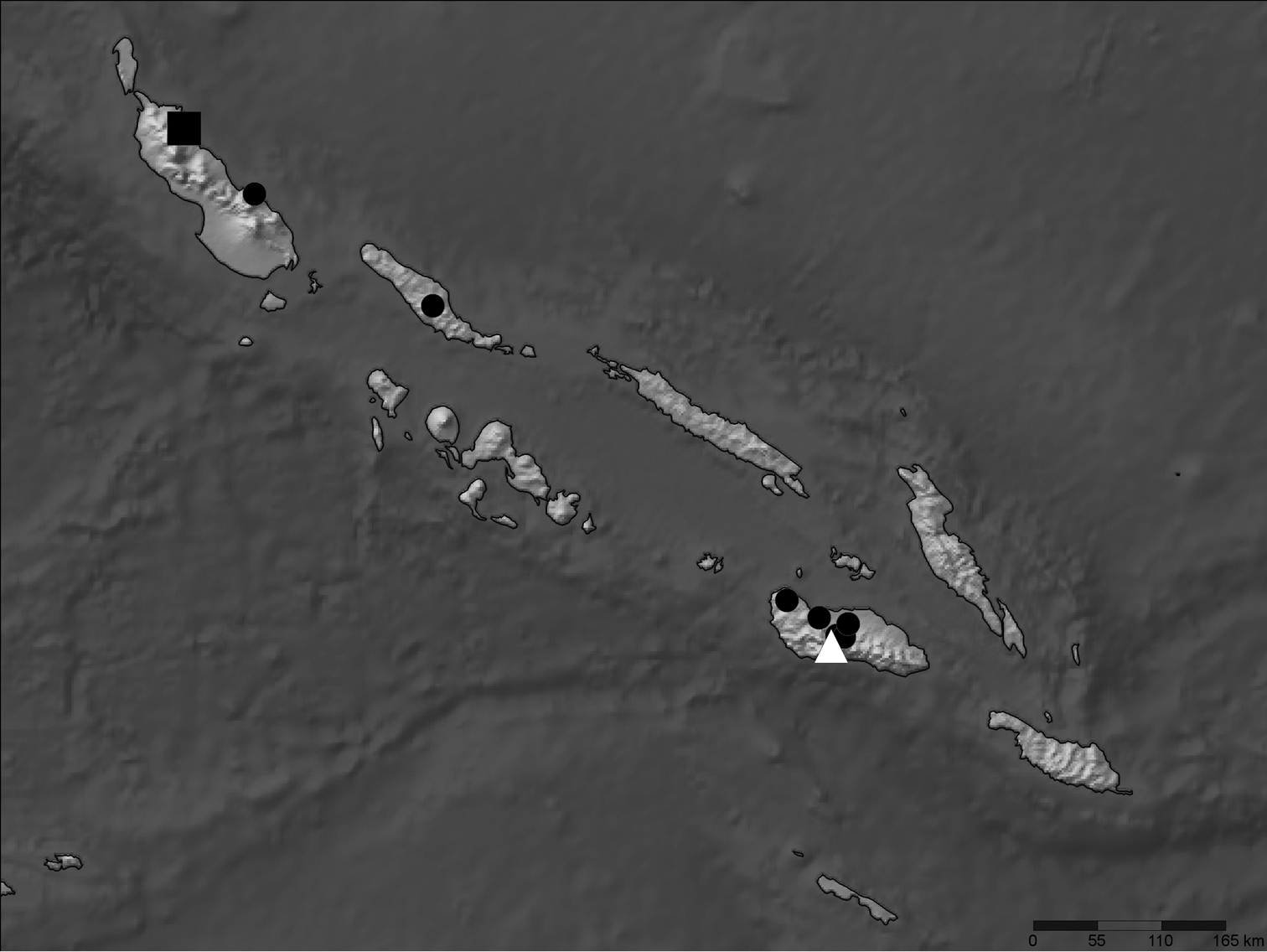
All georeferenced records for Nicrophorus efferens (black square), Nicrophorus kieticus (black circles) and Nicrophorus reticulatus (white triangle) in the Solomon Islands archipelago.
(2 males, 4 females), pronotal width: male 5.02 – 5.87, 5.45 ± 0.6 mm, female 5.34 – 6.42, 5.82 ± 0.45 mm.
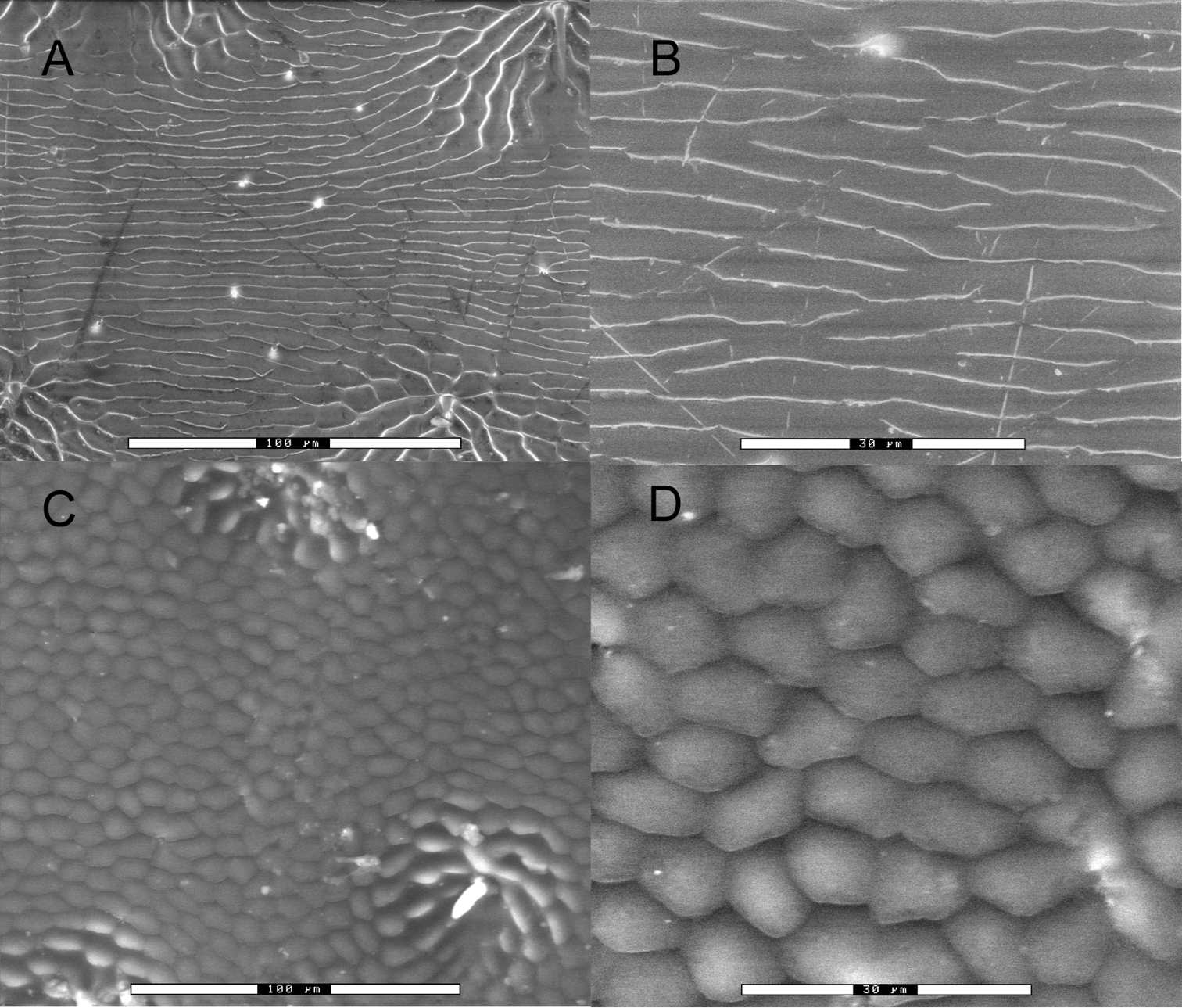
Diagnosis. Nicrophorus with center of abdominal sternite 3 between metacoxae glabrous, with long erect brown setae lateral of center hidden above and exposed posterior of coxae (shared with only four Nicrophorus species: Nicrophorus distinctus Grouvelle, Nicrophorus apo Arnett, Nicrophorus heurni Portevin, and Nicrophorus reticulatus Sikes & Madge); epipleura black or black throughout with some dark orange in the posterior dorsal portion (of these four, shared with only Nicrophorus reticulatus); elytral disc microsculpturing transverse, straight, narrow with breaks (Fig. 2A, B).
Scanning electron micrographs of elytral microsculpture. A Nicrophorus efferens paratype BPBM124191Nic, 500×, scale bar is 100 µm B Nicrophorus efferens 1500×, scale bar is 30 µm C Nicrophorus reticulatus 500× scale bar is 100 µm D Nicrophorus reticulatus 1500× scale bar is 30 µm.
Eight character states not shared with Nicrophorus reticulatus, the likely closest relative of Nicrophorus efferens, are indicated below by use of italics.
Head. Mandibles asymmetrical with dent receptive notch on right mandible. Mandibles preapically wide, short, stout; with dorsal, ridged groove. Maxillary palpus not elongate, concealed beneath mandible when mandibles open. Segment 1 of labial palpus as long as segment 2. Labrum with bilateral pair of elongate setae in clusters. Clypeal membrane orange, yellow or brown, not black. Female clypeal membrane fasciform, male clypeal membrane campanulate and produced posteriorly and laterally enclosed by clypeus. Gula bar of large males reduced to thin strip, replaced by membrane, not contiguous with submentum. Frontoclypeal (epistomal) suture present. Gular sutures confluent, reducing gula to small triangle posterior of gula bar. Antennomeres 2 and 3 fused making an 11 segmented antenna appear 10 segmented. Antennal scape with posterior face flattened (fitting against eyes). Ridge surrounding antennal socket forming distinctly ridged lateral edge of clypeus. Antennal club abrupt, large; basal segment black, apical 3 orange. Posterior of antennal club segments unlike anterior, with large raised ridge, with sharp lateral edges. Basal antennomere of club oval, transverse, not circular. Antennal club segment joints at edge of segments. Antennomeres of club weakly emarginate. Frons black, without orange spot. Epicranial sulci (grooves along inner margin of eyes) long, reaching posterior of eyes and usually joining. Postocular bulge present. Lateral margins of head of large males parallel, or subparallel, giving rear of head a square appearance. Width across postocular bulge of large males less than width across eyes. Post-ocular bulge of large males larger than females. Posterior margin of eye of large males in lateral view sinuate. Width of eyes of large males wide (width greater than or equal to half length). Dorsum of neck with two nonpunctate band(s). Microsculpture of frons absent (smooth, polished).
Thorax. Microsculpture of pronotum disc isodiametric. Pronotum of large males orbicular. Anterior margin of pronotum straight or weakly concave. Pronotal anterior impressions complete, with distinct inner arcs. Setae on posterioventral portion of hypomeron long, erect, sparse; filling region extending a third or less length between trochantin and posterior margin, restricted to upper region of sclerite. Anterior corner of hypomeron glabrous. Triangular depression at midpoint posterior margin of pronotum absent. Pronotum disk black. Posterior of pronotum with flat margin or border. Setae present on pronotum, brown to light-brown, short (ca. < 0.1mm), sparse, and restricted to margins. Pair of ‘v’-shaped bumps present on posterior of pronotum. Medial groove of pronotum present. Antemesosternal sclerite cordiform and bilobate. Elytral locking device under apex of scutellum composed of single channel, without distinct, thin, raised median ridge. Humerus immediately dorsal of epipleural ridge black. Humeral setae present, short and not forming row. Epipleuron entirely black or black throughout with some dark orange in the posterior dorsal portion. Posterior epipleural ridge without isolated single-file row of contiguous preapical setae. Epipleural ridge distinct and short, to tip of scutellum. Epipleuron glabrous, or with very sparse, extremely small setae (ca. the size of a puncture). Lateral margin of elytron glabrous, or with very sparse, extremely small setae (ca. the size of a puncture). Elytral surface without long setae. Elytra bicolored and bifasciate. Elytron with costae not distinctly raised but visible to naked eye. Region adjacent to apex of elytral suture black. Profile of elytra in lateral view raised posteriorly. Anterior fascia of elytron without black spot, small, triangular-square, just reaching 2nd costa. Posterior fascia between costae, anterior margin u -shaped between costae 1 and 2 (with bottom of u towards posterior). Posterior fascia not touching lateral or posterior margins of elytron. Elytral posterior fascia without black spot. Elytral posterior fascia greatly reduced, far from suture, jagged. Elytral microsculpture transverse straight, narrow with breaks. Elytral color varation – greatly increased orange and all black forms unknown. Ventral surface of elytron with golden sheen. Row of setae facing inward or downward on inner lateral margin of elytra in posterior quarter along lateral ridge of elytron in a distinct, single file row. Posterior margin of elytron with 5-7 clusters of very short, dark setae. Flange along mesepisternal anterior margin tapering gradually towards mesosternum. Metanotal subalare with sharply rising, internally margined ridge along anteriomedial edge, anteriormedial ridge narrow leaving a wide depression. Metepimeron constricted. Metepisternum with upper third impunctate and glabrous. Metasternal pubescence medially (between mesocoxae) and laterally, long, dark brown. Metasternum with long setae, bald patch posterior of mesocoxae absent. Metepimeral posterior lobe with sparse, short brown setae throughout lobe, or glabrous. Metasternum posterior margin edge glabrous. Furcal arms of metendosternal apophysis directed posteriorly (forming acute angle with main stalk). Laminae of metendosternal apophysis joining furcal arms to anterior projection of stem. Ventral laminae of metendosternal apophysis reaching from anterior projection to bend in main stalk (anterior 3 fourths of entire length). Radial hinge of wing notched. Apex of pterostigma of wing truncate-rectangular.
Abdomen. Pair of stridulatory files on tergite 5 present, files parallel, separated by 2 or more file widths, touching posterior margin of tergite. Stridulatory file scraper on venter of elytron near elytral apex (< 0.2mm). First abdominal spiracle slit short, less than one third length of spiracle, in straight line with center line of spiracle. First abdominal spiracle lobed, with small bulb projecting anteriorly. Posterior margins of abdominal segments 3-6 with short setae (2-3 times longer than the distance between adjacent setae). Center of sternite 3 between metacoxae glabrous, but with long erect setae lateral of center under coxae. Spiracle of tergite six elongate, slit-like, and parallel to lateral margin of tergite, or forming less than 30-degree angle with lateral margin. Tergite seven with short depressed setae. Abdominal setae dark brown. Setae of tergite 9 of males (pygidium) brown.
Legs. Venter of protibia apex with lateral process. Anterior face of protrochantin with regions of dense pubescence composed of short, recumbant golden setae. Anterior of procoxae with short setae on basal half. Lateral margin of anterior of procoxae smooth, without ridge or bump. Meso- and metatibia apical angle produced into lobe. Outer margin of mesotibia straight or curved outwards. Inner margin of metatibia of large males gradually curved outwards. Inner margin of metatibia with inner face not forming wide channel entirely filled with dense long setae. Middle of outer margin of metatibia slightly swollen in large males. Middle of inner face of metatibia of large males greatly widened (2.5 or greater than width at base). Metatibia straight. Inner face of apex of metafemora with circular or oval cluster of short setae occupying 1/4 to 1/2 of apical region. Inner face of base of metafemora with single ridge separating medial setose area (area not depressed) from lateral half. Female metafemora slender (length ≥ 2.5 times greatest width). Posterior/ventral face of metafemora flattened with ridged edges. Metatrochanter spine of males short and subapical, apex pointing parallel (or almost parallel) to leg, straight, not recurved dorsally. Metacoxae wider than long. Metacoxae with anterior line complete for half or more of metacoxa. Posterior margin of metacoxae without white microsetae. Tarsal empodium bisetose. Venter of metatarsomere 1 glabrous medially, especially distally, leaving narrow glabrous channel, with dense cluster of long setae at apex.
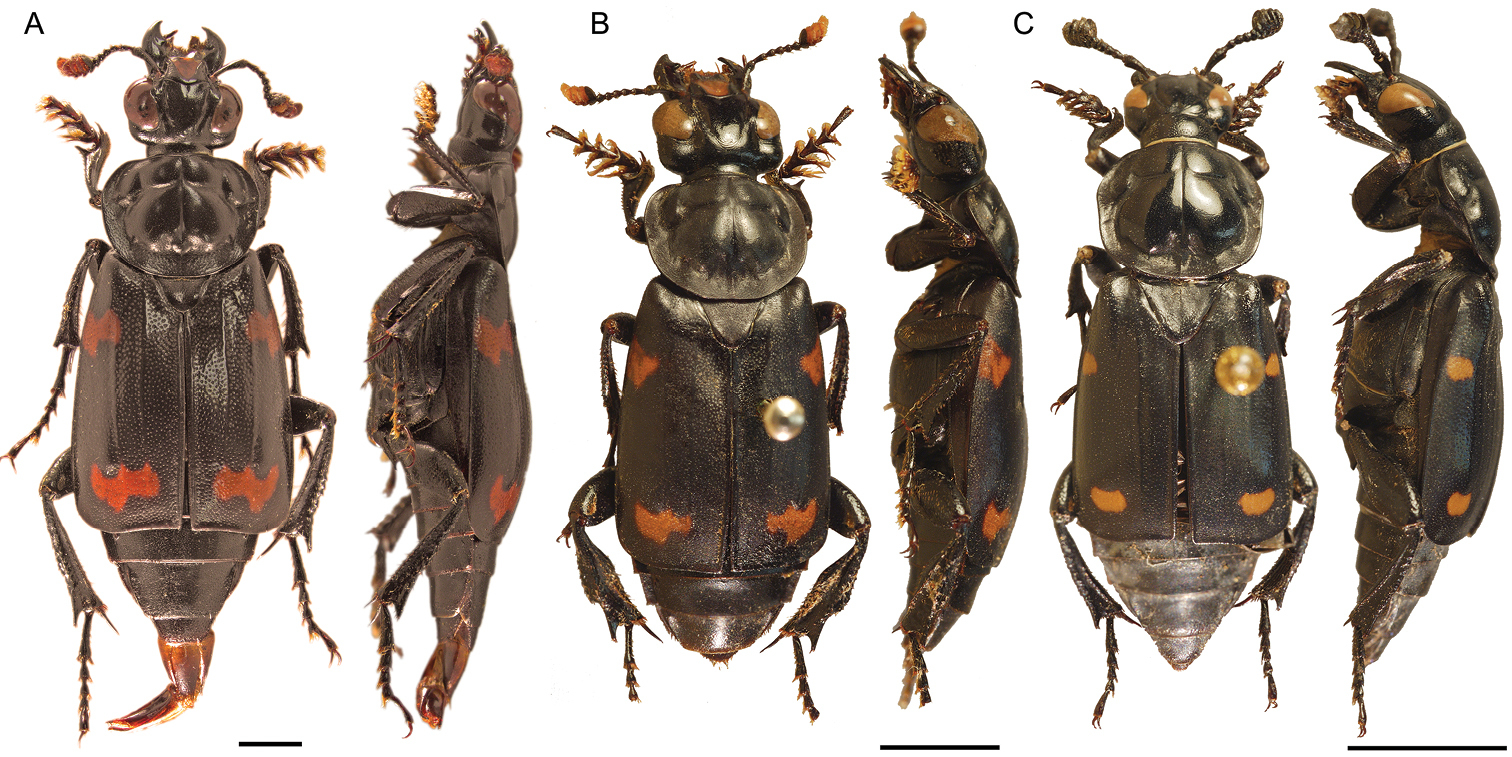
Dorsal and lateral habitus of adult males (at different scales). A small male (pronotal width 5.02 mm) with aedeagus everted, Nicrophorus efferens (holotype BPBM124189Nic), scale bar is 2 mm, fifth protarsomeres are missing B large male (pronotal width 6.6 mm) Nicrophorus reticulatus (paratype BMNH000826), scale bar is 5 mm C small male (pronotal width 5.19 mm) Nicrophorus kieticus (BMNH000809Nic) scale bar is 5 mm.
Ovipositor. (Fig. 4A, B) Valvifer with claw. Proctiger (T10) apex strongly lobed, spatulate, not bifurcate. Spatula on proctiger (T10) apex narrow (ca. equal to half the width across widest region of metatarsomere 5). Proctiger (T10) lobe with pubescent apex, ventral setae. Proctiger (T10) venter of spatula apex smooth, lacking medial keel-like ridge. Gonocoxite terminal claw absent. Dorsal ridge on proctiger (T10) absent. Paraproct (T9) apex glabrous, with well-defined, raised ridge. Paraproct without process. Valvifer claw glabrous, dentate; dentition composed of small rounded lobe situated in the middle to distal third of the valvifer.
Aedeagus. (Fig. 4C, D). Paramere apex rounded, with setae laterally not reaching the apical curve. Paramere ventral setal patch not composed of 5 evenly spaced setae of identical length and not overlapping with apicolateral patch. Without 3rd paramere setal patch or paramere flange. Parameres not constricted behind apex. Parameres evenly curved, tapering towards apex.
Variation. The holotype male and the largest female paratype has an epipleuron mostly black throughout, but with some dark orange faintly visible in the posterior dorsal portion. Two of the type specimens have no setae along the posterior margin of the elytra, presumably they have been lost to abrasion.
Genitalia. Female ovipositor (paratype BPBM124190Nic), A lateral B dorsal. Scale bar is 1 mm. Male aedeagus (holotype BPBM124189Nic) C lateral D dorsal. Scale bar is 500 µm.
Geographic Distribution. This species is only known from the type locality (Fig. 1).
From the latin verb ‘effero’ to carry out for burial, bear to the grave, bury; present participle in the nominative singular.
This new species is likely closely related to one if not both of the Nicrophorus previously known from the Solomon Islands archipelago (Nicrophorus reticulatus [Figs 2, 3] and Nicrophorus kieticus, [Figs 1, 3]) and may be the key to untangling the mystery of how they are related. Two of its three diagnostic characters are likely synapomorphies between it and Nicrophorus reticulatus. Although the holotype and one paratype of the four known specimens of Nicrophorus reticulatus have entirely black epipleura, the two remaining paratypes have epipleura mostly black throughout, but with a small region of dark orange in the posterior dorsal portion. This weakly present orange region is present on two of the Nicrophorus efferens type specimens. These two species, along with Nicrophorus kieticus, also share greatly shortened setae along the posterior margins of abdominal sterna 3 to 6 and four highly reduced and similar looking fasciae on the elytra (Fig. 3). However, Nicrophorus efferens differs strongly from Nicrophorus kieticus in its possesion of most synapomorphies of the nepalensis group (
| 1 | Elytra with epipleuron partially or entirely black (Fig. 3A, B) | 2 |
| – | Elytra with epipleuron entirely red–orange | 4 |
| 2 | Elytral disc with strongly transverse microsculpturing (Fig. 2A, B) | 2.5 |
| – | Elytral disc with isodiametric microsculpturing (Fig. 2C, D) | Nicrophorus reticulatus |
| 2.5 | Elytra with epipleuron black with entire medial (dorsal) half orange | 3 |
| – | Elytra with epipleuron entirely black (or black with some dark orange in the posterior dorsal portion) | Nicrophorus efferens |
Thank you to Shepherd Myers (BPBM) for loaning the Nicrophorus efferens specimens studied and to Margaret Thayer (FMNH) and Max Barclay (BMNH) for making comparative material available. Thank you to Fred Falcao for the second author’s visit to Hawaii. Thanks to lab manager and SEM technician Betty Strack at the FMNH for help with obtaining the Nicrophorus efferens SEM images. We also thank our long time friend and collaborator, Ronald Madge, with whom we share a passion for silphid systematics, for his input on this new species.