(C) 2013 Khwanruan Srinui. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Srinui K, Nishida S, Ohtsuka S (2013) A new species of Pseudodiaptomus (Crustacea, Copepoda, Calanoida, Pseudodiaptomidae) from the Prasae River Estuary, Gulf of Thailand. ZooKeys 338: 39–54. doi: 10.3897/zookeys.338.5531
A new species of the calanoid copepod genus Pseudodiaptomus was collected from the Prasae River Estuary, Rayong Province, on the eastern coast of the Gulf of Thailand. This species is definitely assigned to the lobus species group sensu Walter (1986a). The female of the new species differs from other congeners in the elongate genital double-somite with a blunt process ventrally and the second urosomite about 2.54 times as long as wide. The male is also easily distinguished from other congeners by the structure of the right fifth leg.
The present new species is a euryhaline species and occurred in brackish waters with salinity ranging from 0.7 to 23.3. Its breeding season may be from June to October, as indicated by the presence of egg-sacs.
Copepoda, Calanoida, Gulf of Thailand, Prasae River, Pseudodiaptomus, new species
We have been intensively investigating the taxonomy, biology and ecology of gelatinous and crustacean zooplankters in Thailand since 1997 (
During our survey in estuaries of Thailand in 2004–2012 a new species of the calanoid copepod genus Pseudodiaptomus was found at the mouth of Prasae River, Gulf of Thailand. Pseudodiaptomus is broadly distributed in freshwater to marine habitats in the Atlantic and Indo-Pacific regions, and frequently comprises a main component in the zooplankton communities (
The genus Pseudodiaptomus has so far accommodated 77 species (
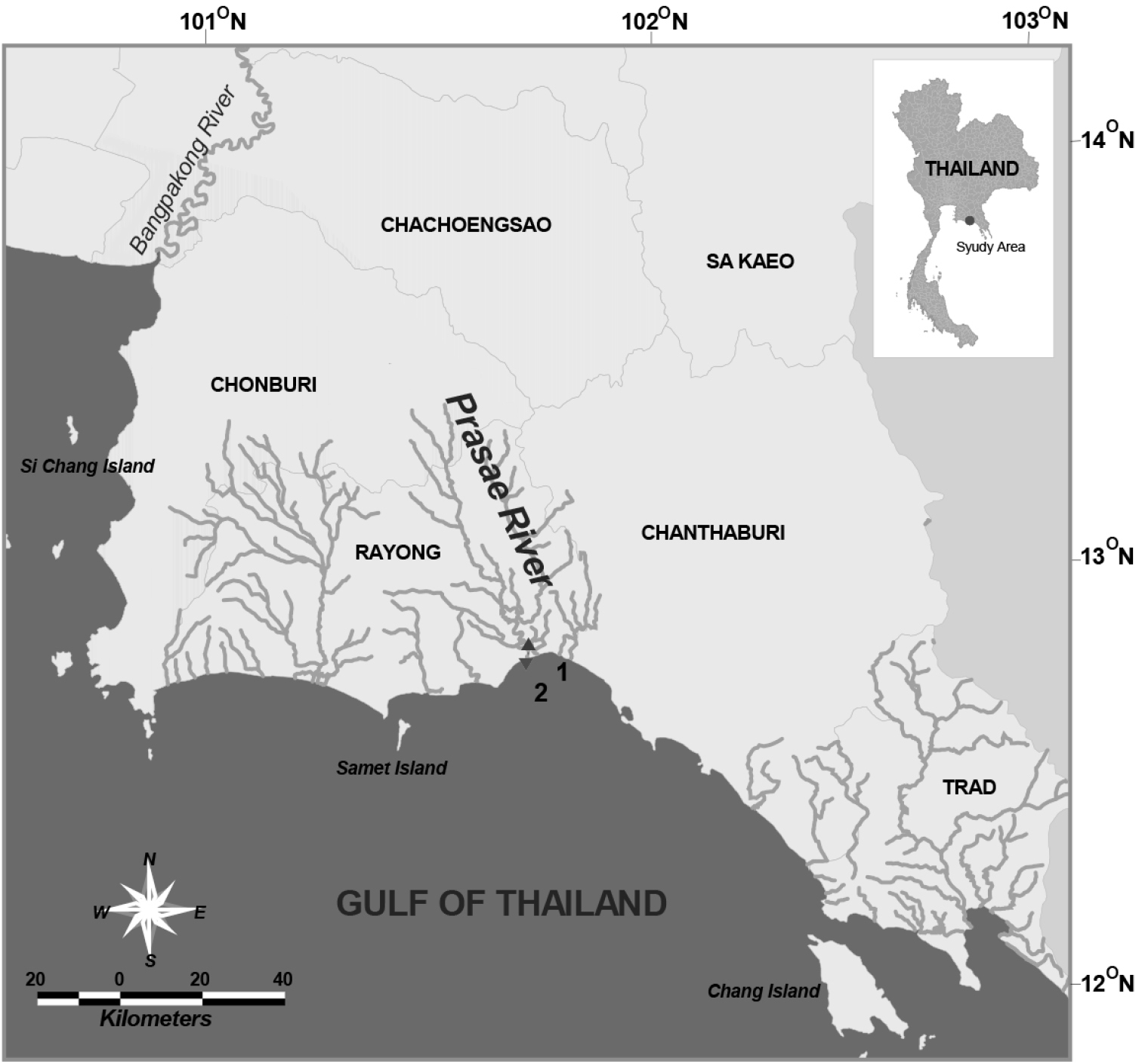
Copepods were collected at 2 stations from the near-bottom of the Prasae River Estuary, Rayong Province, in June 2011 and August 2004, 2012 using a plankton net (0.1 mm in mesh size) and a sledge net (0.3 mm) (Fig. 1). Samples were fixed in 5% neutralized formaldehyde/seawater solution immediately after capture. Calanoid copepods were sorted out of the original samples under a stereo microscope. Copepod specimens were transferred directly from preservative to polyvinyl lactophenol and dissected with a pair of fine needles. All drawings were made with the aid of a camera lucida attached to a compound microscope (Olympus BX50). Each segment and appendage is numbered using Arabic numerals. Terminology follows
Sampling stations in Prasae River Estuary, Rayong Province.
http://zoobank.org/4DE3A857-3BDE-4107-8758-F1B74325C573
http://species-id.net/wiki/Pseudodiaptomus_siamensis
Figs 2–5Prasae River Estuary, the Gulf of Thailand, station1: (12°42.66'N, 101°42.37'E; station 2: 12°41.14'N, 101°42.49'E) (Fig. 1), 23 August 2004 (6♂♂); 4 June 2011 (8♀♀, 6♂♂); 13 August 2012 (11♀♀, 1♂).
Holotype: 1♀ station 1, 4 June 2011, dissected and mounted on 2 glass slides (BIMS-Z00-0130), allotype: 1♂ station 1, 4 June 2011, dissected and mounted on 5 glass slides (BIMS-Z00-0131); paratypes: 4♀♀, station 1, 13 August 2012, 3 ♂♂ station 2, 23 August 2004 partly dissected and mounted on 3 glass slides (BIMS-Z00-0132).
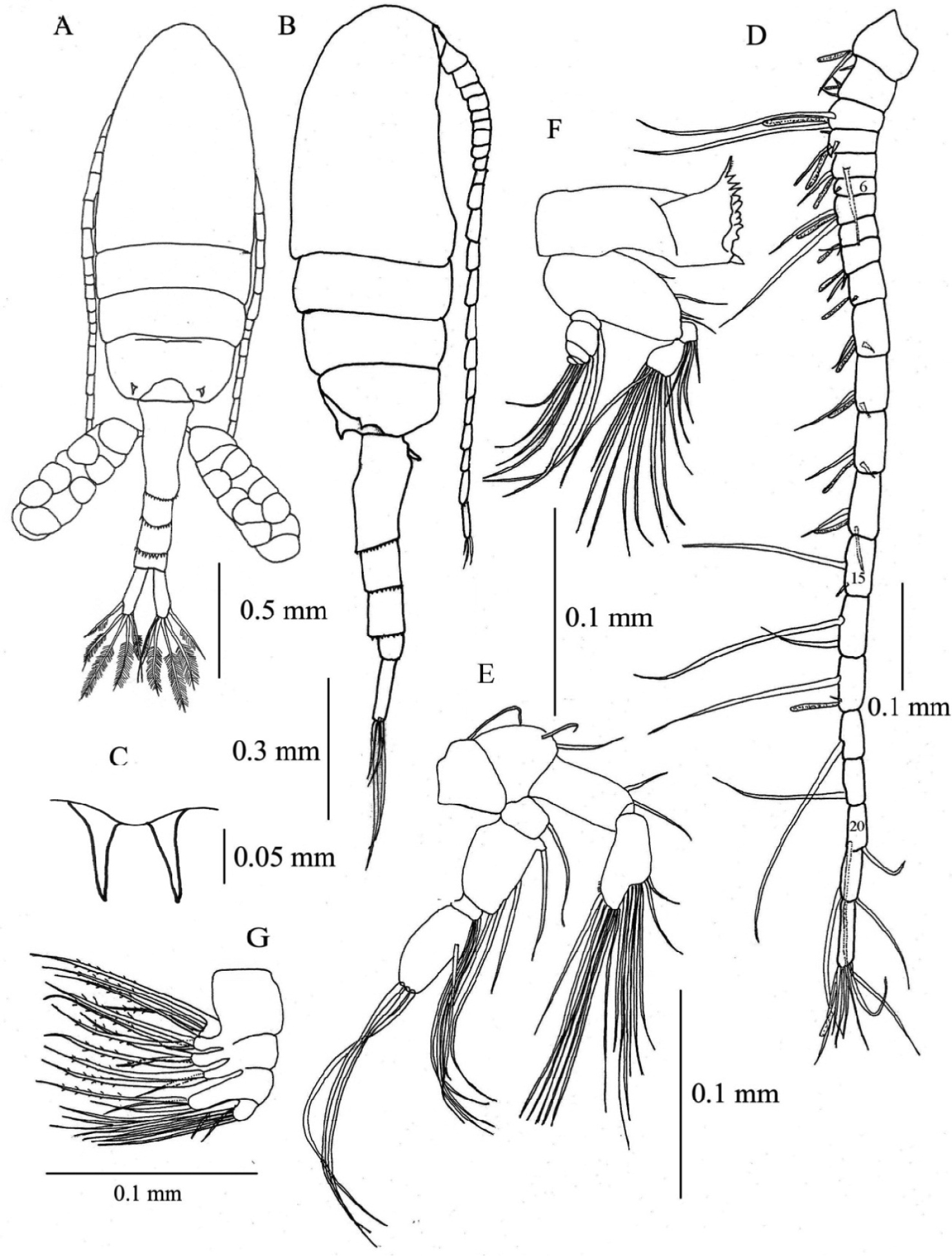
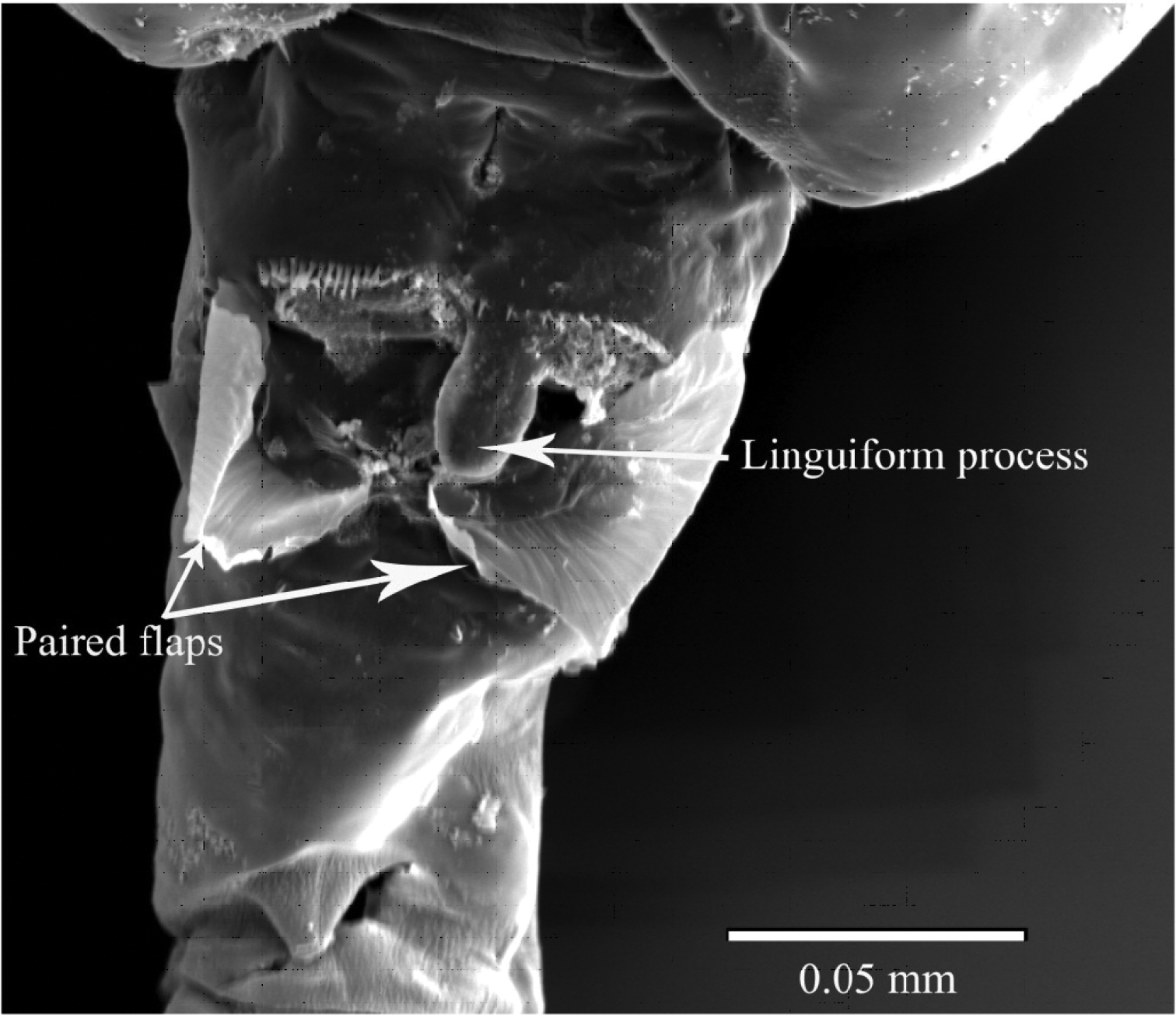
Female. Total length, 1.29–1.41 mm (mean±SD = 1.37±0.04 mm, N=5; holotype, 1.29 mm); prosome length, 0.75–0.82 mm (0.79±0.02 mm; holotype, 0.75 mm); prosome width, 0.31–0.34 mm (0.32±0.01 mm; holotype, 0.32 mm). Habitus (Figs 2A, B) with anterior margin of cephalosome rounded in dorsal view. Rostrum with paired filaments (Fig. 2C). Cephalosome and first pedigerous somite completely fused; fourth and fifth pedigerous somites totally fused. Prosomal ends rounded; dorso-lateral spines on fifth pedigerous somites. Urosome 4-segmented. Genital double-somite asymmetrical in dorsal view, elongate, ca. 2.54 times as long as wide; postero-dorsal and lateral margins with somewhat irregular row of spinules; in ventral view, genital area furnished with blunt, linguiform process midway, transverse rows of spinules anteriorly and paired flaps originating from genital opercula (see Fig. 5); each of paired egg-sacs consisting of 9–14 eggs, attached to lateral of genital opening (Fig. 2A). Proportional lengths of urosomites and caudal ramus 43:15: 15: 7: 20 (=100); length to width ratios 2.5, 1.3, 1.3, 0.4, and 3.6, respectively. Second and third urosomites with row of minute spinules along postero-dorsal and lateral margins. Caudal rami with hair on inner margin and symmetrical with 6 setae: seta I absent, seta II with fine setules only along inner margin; setae III-VI plumose; seta VII located dorsally.
Pseudodiaptomus siamensis, sp. n., female (holotype). A habitus, dorsal view B habitus, lateral view C rostrum, ventral view D right antennule, arabic numerals denote segment numbers E right antenna F mandible G maxilla.
Antennule (Fig. 2D) reaching beyond posterior end of genital double-somite, symmetrical, 22-segmented; segments 6-7 incompletely fused; segments 6, 15, 16, 18-21 each without aesthetasc (ae). Fusion pattern and setal elements as follow: 1 - 1 + ae, 2 - 3 + ae, 3 - 2 + ae, 4 - 3 + ae, 5 - 3 + ae, 6 - (1 spiniform element), 7 - 2 + ae, 8 - 2 + ae, 9 - 2 + ae, 10 - (1 spiniform element) + ae, 11-14 - 2 + ae, 15-16 - 2, 17 - 2 + ae, 18-19 - 1, 20-21 - 2, 22 - 6 + ae.
Antenna (Fig. 2E) coxa with single seta; basis with 2 setae at inner corner; endopod 2- segmented, first segment with 2 setae, second segment with 7 and 8 setae on terminal and subterminal lobes, respectively, and lateral row of fine setules; exopod 4-segmented, first segment with 1 seta, second segment with 1 proximal, 2 medial and 1 terminal setae; third segment with 3 setae; fourth segment with 1 medial and 3 terminal setae.
Mandible (Fig. 2F) with basis bearing 4 setae along inner margin; endopod 2- segmented, first segment with 4 setae, second with 9 setae; exopod 5-segmented, first to fifth segments with 1, 1, 1, 1, 2 setae, respectively. Gnathobase (coxa) with serrate dorsal seta and 3 cuspidate and 4 blunt teeth.
Maxillule (Fig. 3A) with preacoxal arthrite bearing 9 strong and 6 fine setae and small spinules; coxa with 4 setae on endite and 9 setae on epipodite; basis with 4 and 5 setae on proximal and distal endites, respectively; basal exite with 1 seta; endopod 3-segmented, with 4, 4 and 6 setae from first to third segments, respectively; exopod foliaceous with 10 setae along outer margin.
Maxilla (Fig. 2G) with first and second praecoxal endites having 4 and 3 setae, respectively; first coxal endite with 3 long setae, second endite with 1 short strong and 2 long setae; basis with 1 short and 2 long setae; endopod with 9 setae.
Maxilliped (Fig. 3B) with praecoxa and coxa completely fused; endites with 0, 2, 3, 4 setae, respectively; basis with 3 setae; endopodal segment having 6 segments, first segment with 2 setae, second segment with 2 bifurcated setae and 1 seta, third and fourth segments with 1 bifurcated seta and 1 seta, fifth and sixth segments with 3 and 4 setae, respectively.
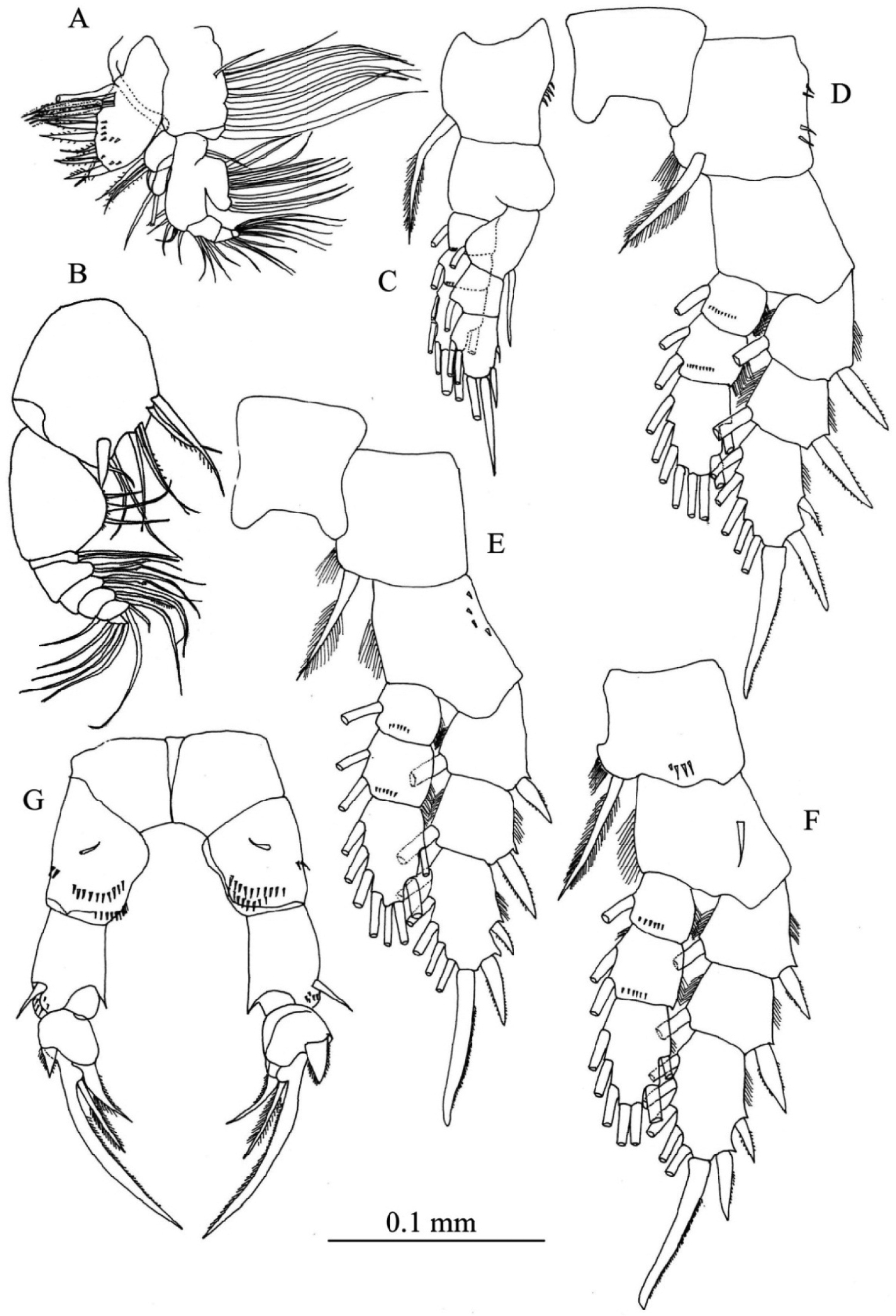
Legs 1–4 (Figs 3C–F) biramous with 3-segmented rami; coxa and basis of both rami with spinules on distal corner. Seta and spine formula as follows:
Leg 5 (Fig. 3G) uniramous and almost symmetrical; in posterior view, basis with short medial seta and spinular rows; exopod 3-segmented, first segment produced into small pointed process at inner subterminal corner, with distolateral spine and one or two rows of spinules; second segment having short and thickned disto-lateral process and medial serrate spine; third segment spiniform, tapering distally with inner spinules and proximo-medial spine.
Pseudodiaptomus siamensis, sp. n., female (holotype). A maxillule B maxilliped C leg 1, posterior view D leg 2, posterior view E leg 3, anterior view F leg 4, posterior view G leg 5, posterior view.
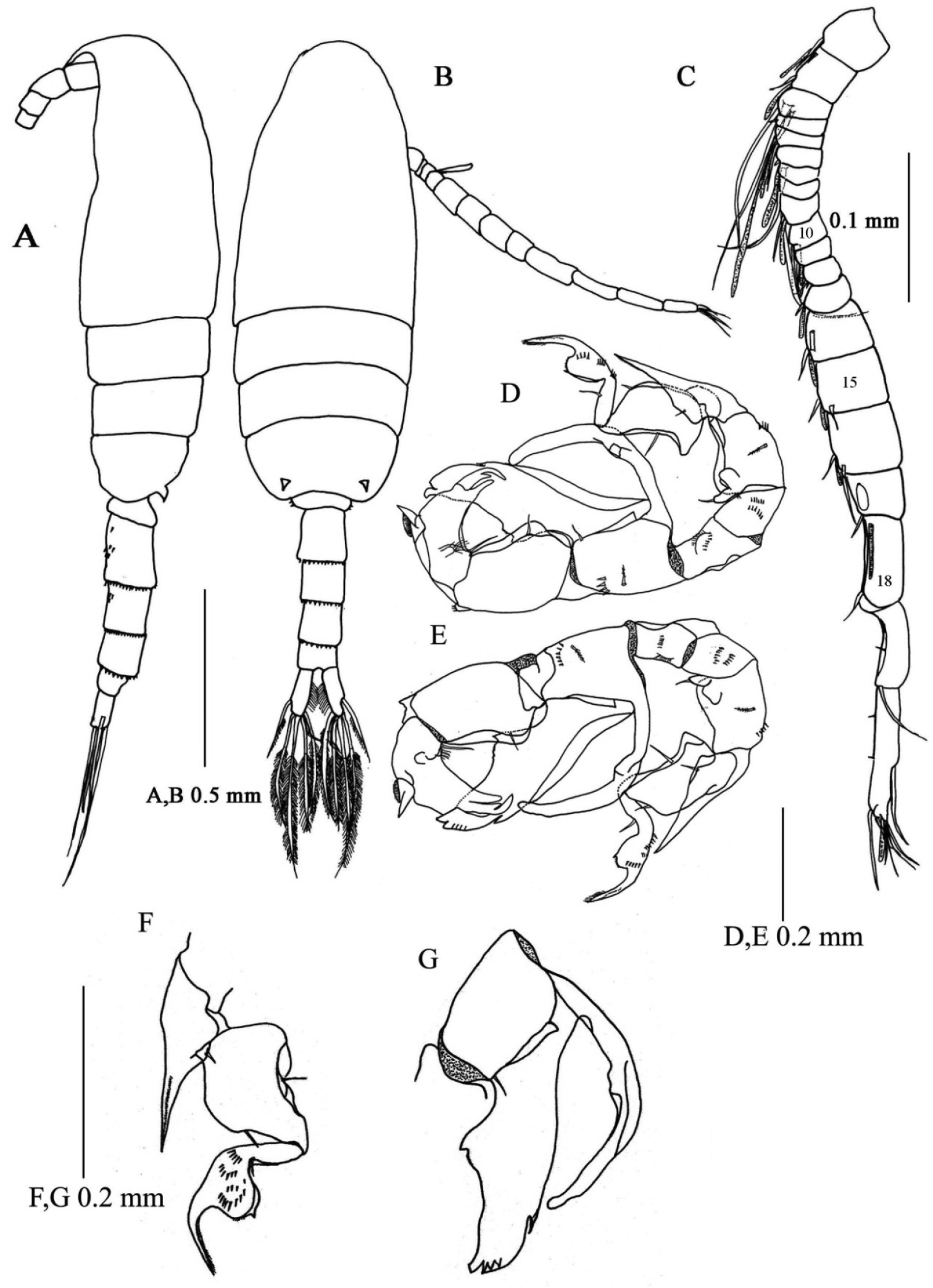
Male. Total length 0.94-1.02 mm (mean±SD = 0.97±0.03, N= 4; allotype, 1.02 mm). Prosome length 0.62-0.66 mm (mean±SD = 0.64±0.01, allotype, 0.66 mm), width 0.26-0.27 mm (mean±SD = 0.26±0.005, allotype, 0.26 mm).
Habitus (Figs 4A, B) similar to that of female, except for urosome. Urosome 5- segmented; proportional lengths of urosomites and caudal ramus 13: 25: 21: 17: 11:13 (=100); length to width ratios 0.5, 1.1, 1.2, 1, 0.6 and 1.7. Genital somite nearly symmetrical with one or two rows of spinules ventrally. Urosomites 2–4 with spinular row along posterior margin. Caudal rami symmetrical, with six setae as in female.
Right antennule (Fig. 4C) geniculate and indistinctly 20-segmented; setal formula as follows: 1 -1 + ae, 2 - 2 + ae, 3 - 2 + ae, 4 - 1, 5 -1 + ae, 6 - (1 spiniform element), 7 - 1 + ae, 8 - (1 spiniform element), 9 - 2 + ae, 10 - (1 spiniform element), 11 - 1 + ae, 12 - (1 spiniform element) + ae, 13 -1 + ae, 14-16 - 2 + ae, 17-18 - 1 + (1 process), 19 - 2 + (1 process), 20 - 9 + ae.
Pseudodiaptomus siamensis, sp. n., male (allotype). A habitus, lateral view B habitus, dorsal view C right antennule, arabic numerals denote segment numbers D leg 5, anterior view E leg 5, posterior view F anterior view of exopod of right leg 5 G posterior view of inner process and outer process of endopod of left leg 5.
SEM micrograph of ventral side of genital double-somite of female Pseudodiaptomus siamensis, showing blunt process anterior to genital area.
Leg 5 (Figs 4D, E, F, G) highly asymmetrical and biramous; intercoxal sclerite and both coxae fused; coxa with fine spinular rows on anterior surface. Right leg (Figs 4D, E) with basis having outer spinular row; endopod rudimentary, represented by knob-like process with fine setule at tip; exopod (Fig. 3F) 3-segmented, first segment protruded into outer process reaching middle of third segment, proximal process with 1 spine and spinular row; second segment expanded midway, each side with spine; third segment curved inward with 3 rows of spinules on anterior surface and middle swelling, distal to which tapering distally. Left leg (Figs 4D, E) with elongated basis having triangular process at midlength; endopod (Fig. 4G) highly developed, bifurcated, inner medial process smoothly curved outward reaching distal tip of second exopod, outer process thickened, foliaceous with 1 subterminal and 4 thin terminal protrusions; exopod 2-segmented, first segment as long as basis, irregularly sinuated along inner margin; second segment triangular with hirsute process proximally and stout serrated protrusion at medio-lateral margin, with 3 processes of unequal length terminally.
The present new species can be definitely assigned to the lobus species group sensu
In the poppei-subgroup the new species is most closely related to Pseudodiaptomus tollingerae from the Indian waters (
The species was named after the type locality “Siam” (an old name of Thailand).
As mentioned above, Pseudodiaptomus siamensis composes a sister group with Pseudodiaptomus tollingerae. Pseudodiaptomus poppei from Celebes (
The habitat of the present new species, the Prasae Estuary was euryhaline, where the salinity widely ranged between 0.7 and 23.3 during the present investigation. Dominant copepods that co-occurred with the new species seasonally differed with salinity: Acartia plumosa Scott, 1894, Bestiolina similis Sewell, 1914, Parvocalanus crassirostris Dahl, 1894, Pseudodiaptomus annandalei Sewell, 1919, and Oithona simplex Farran, 1913, were abundant in the wet season (May–October), while Bestiolina similis, Parvocalanus crassirostris, Oithona simplex, and Oithona dissimilis Lindberg, 1940 in the dry season (November-April) (
Although our collections of planktonic copepods were intermittently carried out, some information of the breeding of the new species was obtained. The ovigerous and/or spermatopore-bearing females of the new species were found during the wet season (June to October). In addition, the density of immature females reached 139 individuals per cubic meter in August 2004, suggesting it was an active breeding season.
Seventy-eight species of Pseudodiaptomus, including the new species Pseudodiaptomus siamensis, have been recorded from the world (
Female
| 1 | First urosomite symmetrical without blunt linguiform process on mid-ventral | 2 |
| – | First urosomite asymmetrical with blunt linguiform process on mid-ventral | Pseudodiaptomus siamensis |
| 2 | First urosomite with pair of anterodorsal spines and posterodorsal cluster of 3 spinules | Pseudodiaptomus smithi |
| – | First urosomite without pair of anterodorsal spines and posterodorsal cluster of 3 spinules | 3 |
| 3 | Prosomal ends with one pair of processes dorsally | Pseudodiaptomus tollingerae |
| – | Prosomal ends with two pairs of processes dorsally | Pseudodiaptomus poppei |
Male
| 1 | Fifth pair of legs without left endopodal segment | Pseudodiaptomus poppei |
| – | Fifth pair of legs with left endopodal segment | 2 |
| 2 | First exopodal segment of fifth right legs with recurved process at distolateral corner | Pseudodiaptomus smithi |
| – | First exopodal segment of fifth right legs with straight process at distolateral corner | 3 |
| 3 | Endopod of fifth left leg with outer process tapering distally | Pseudodiaptomus siamensis |
| – | Endopod of fifth left leg with outer process concave at the tip | Pseudodiaptomus tollingerae |
We express our sincere thanks to Mr. Somjai Srinui and Ms. Rujira Kaewking for their help in the field sampling and other members of the Institute of Marine Science, Burapha University. This study was partly supported by a grant from the Asian CORE Program of Japan Society for the Promotion of Science (JSPS) and the RONPAKU Program of JSPS. We also thank to Mr. T. Hirabayashi for his assistance for SEM observations. This study was partially supported by a grant-in-aid from the Japan Society of the Promotion of Science, awarded to SO (No. 25304031).