(C) 2013 Sergey A. Belokobylskij. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Braconid genera Histeromerus Wesmael, 1838 from subfamily Histeromerinae and Ecclitura Kokujev, 1902 from subfamily Euphorinae are recorded in the fauna of Italy for the first time. The discussions about taxonomic position, morphological characters and composition of these genera as well as the redescriptions of the genus and species of Ecclitura primoris Kokujev are given.
Braconidae, Histeromerus, Ecclitura, parasitoids, new records, redescriptions
Family Braconidae represents one of the largest and diversified families of parasitic Hymenoptera, including, with a few exceptions, mainly primary parasitoids. These occur in very diverse habitats and result linked to a wide range of hosts. As a fact, braconids can represent an interesting ecological resource as sensitive indicators of environmental richness and stability and of local diversity. The regulatory effect which they exert on host insects population derives from a very wide diversity of physiological and behavioural adaptations (
Terminology adopted for morphological features and measurements follows
Histeromerus mystacinus Wesmael, 1838
Histeromerus Wesmael is a type genus of the monotypic subfamily Histeromerinae. The systematic position of this peculiar genus changed many times during its study. For a long time Histeromerus was considered as a member of subfamily Doryctinae and included in the tribe Doryctini or Histeromerini (
Hitherto, four species of Histeromerus are known. Type species Histeromerus mystacinus was recorded only in the Western Palaearctic (including Iran). Histeromerus canadensis Ashmead, 1891, mainly known from U.S.A. and Canada, was also found in the Netherlands. Histeromerus orientalis Chou & Chou, 1991 was described from Taiwan and later was additionally recorded in Japan (Ogasawara Islands). Finally a fourth species, Histeromerus clavatus Austin & Wharton, 1992, was described from Australia (Queensland) (
Histeromerus mystacinus Wesmael is widely distributed in several European countries and also in Caucasus and Central Asia (North Iran). On the other hand, this species practically resulted unknown for the south part of Europe (Mediterranean coast). First discovery of Histeromerus mystacinus in Italy is very interesting, because expands the geographical distribution of this taxon in dry territories. Members of Histeromerus are gregarious ectoparasitoids of concealed living beetles’ larvae from subfamilies Anobiidae, Buprestidae, Cerambycidae, Cisidae, Elateridae, Lucanidae, Lyctidae, and Ptinidae (
http://species-id.net/wiki/Histeromerus_mystacinus
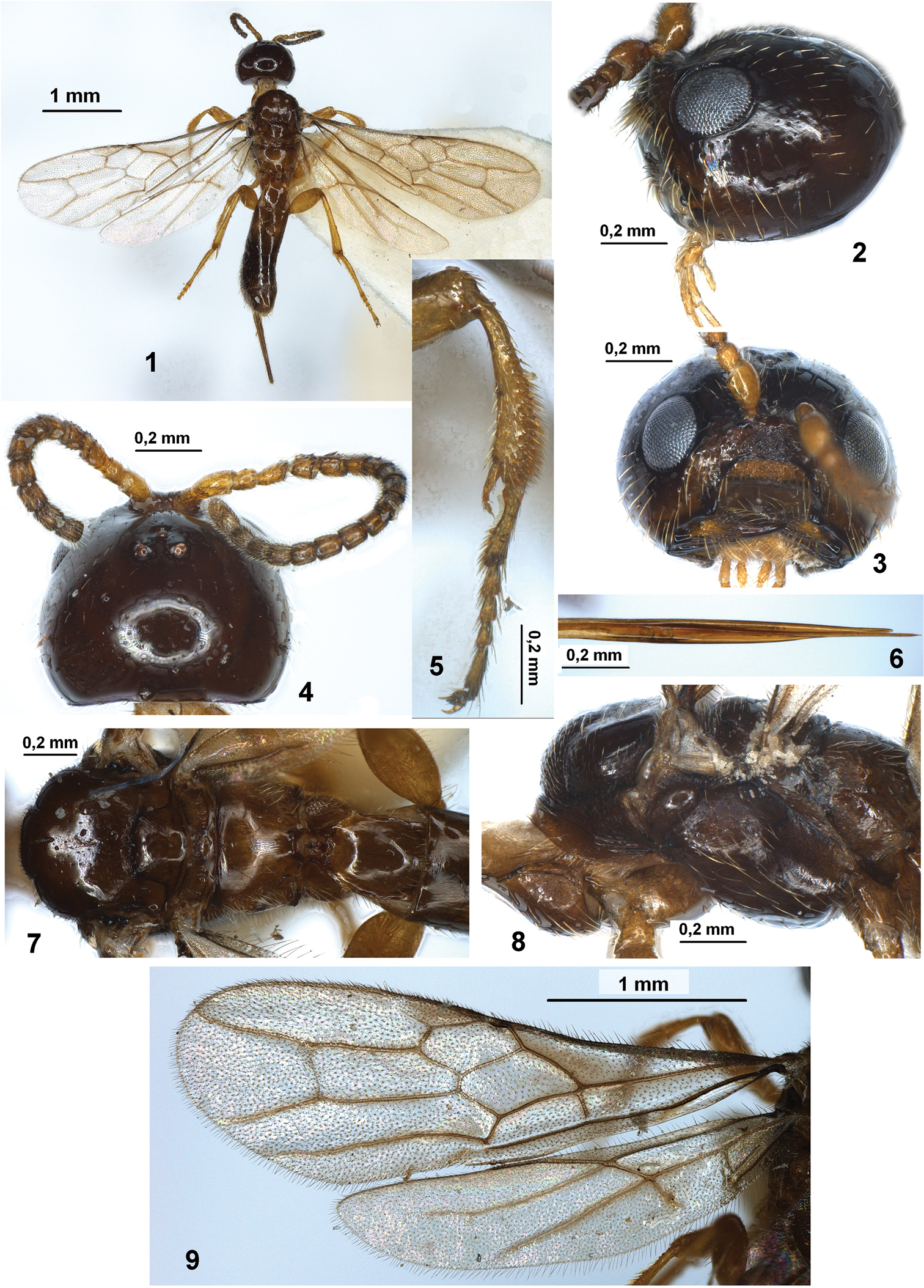
Figures 1–9Italy: 1 female, Campania, Torre del Greco, (NA), Parco del Vesuvio, 3–18.VII.2007, Guerrieri leg.
Ireland, U.K., Belgium, Netherland, Denmark, France, Germany, Czech Republic, Hungary, Italy (first record), Sweden, Poland, Slovakia, Ukraine, Bulgaria, Russia (European part), Georgia, Iran.
Histeromerus mystacinus Wesmael (female) 1 habitus, dorsal view 2 head, lateral view 3 head, front view 4 head and antenna, dorsal view 5 fore tibia and tarsus 6 tip of ovipositor 7 mesosoma and first metasomal tergite, dorsal view 8 mesosoma, lateral view 9 fore and hind wings.
Ecclitura primoris Kokujev, 1902.
Ecclitura Kokujev is until recently monotypic genus originally described from Turkmenistan in the beginning of 20th century (
Taxonomic position of Ecclitura was discussed in several publications.
Knowledge on molecular phylogeny and its implication for classification of subfamily Euphorinae are very limited. A specific publication for this task (
The single described species of this genus, Ecclitura primoris, was recorded in the Western Palaearctic Region. Two other undescribed species of this genus were reported also from U.S.A. (
Four females of Ecclitura were collected by Malaise traps closely to Napoli in the Campania Province. Fourteen females were captured in 2012 in three vineyards in the surroundings of Pisa (Tuscany), by using Malaise traps. These traps worked from the half of May to the half of October. Ecclitura specimens were captured from July 12 to October 4. Hitherto, no males of this species have never been recorded over area of his distribution, and it is possible Ecclitura primoris reproduces by thelytokous modality as already observed for several other species of Euphorinae (
Head strongly transverse (Figs 10, 21). Vertex at least in anterior half densely rugose-reticulate with granulation. Eye with very short and rather dense setae. Ocelli arranged in obtuse triangle with base 1.1–1.3 times its sides. Occipital carina complete dorsally, archedly fused below with hypostomal carina weakly upper base of mandible. Frons with more or less distinct median carina in anterior half. Face weakly convex. Eye distinctly convergent below (Figs 14, 17). Tentorial pits deep, situated upper lower level of eyes. Malar suture distinct. Clypeal suture complete, but shallow. Mandible medium sized, almost not twisted. Palpi short, maxillary palpus 3-segmented, labial palpus 2-segmented. Antennae (Figs 11, 19, 20) weakly claviform, stable 17-segmented. Scape (Figs 12, 14, 17, 22) long, weakly curved, almost as long as maximum diameter of eye. Pedicel rather long, oval. First flagellar segment as long as or weakly longer than second segment. Segments in apical half of antenna weakly thickened. Apical segment pointed, but without spine.
Mesosoma (Figs 13, 16, 21, 23, 24). Pronope absent. Notauli complete, shallow and wide, densely rugulose-reticulate. Mesoscutum rugose-striate and setose on wide medioposterior half, smooth and glabrous on anterior and sublateral areas. Prescutellar depression deep and long. Scutellum convex, with lateral carinae, at least partly rugose. Sternaulus (precoxal sulcus) shallow, wide, long, densely rugulose. Prepectal carina distinct. Postpectal carina absent. Metapleural lobe long, wide, rounded apically. Propodeum strongly and abruptly rounded from median part (lateral view), weakly and widely longitudinally concave in medioposterior half (dorsal view), entirely rugulose-areolate, with lateral carinae.
Wings (Fig. 11, 15). Radial vein arising behind middle of pterostigma. Radial cell distinctly shortened. Second radial abscissa evenly and distinctly curved. First medial abscissa absent; as result, discoidal and first radiomedial cells fused. Mediocubital vein entirely sclerotised. Basal and recurrent veins distinctly convergent. Brachial cell short, widely open distally. Basal part of parallel vein weakly curved. In hind wing, first abscissa of mediocubital vein 4.0–5.0 times longer than second abscissa. Third abscissa of costal vein long; fourth abscissa almost straight.
Legs long and slender. Segments of median tarsus long. Fifth tarsal segments slender. Hind femur slender. Tarsal claws long, slender and weakly curved (Fig. 25).
Metasoma (Figs 11, 18). First tergite long, distinctly widened towards apex, its latero-ventral sides not fused, widely separated and with distinct split; dorsope small and very shallow; laterope indistinct. Second suture very shallow and fine. Only second tergite with separated laterotergites. Ovipositor (Figs 26, 27) long, straight, compressed laterally.
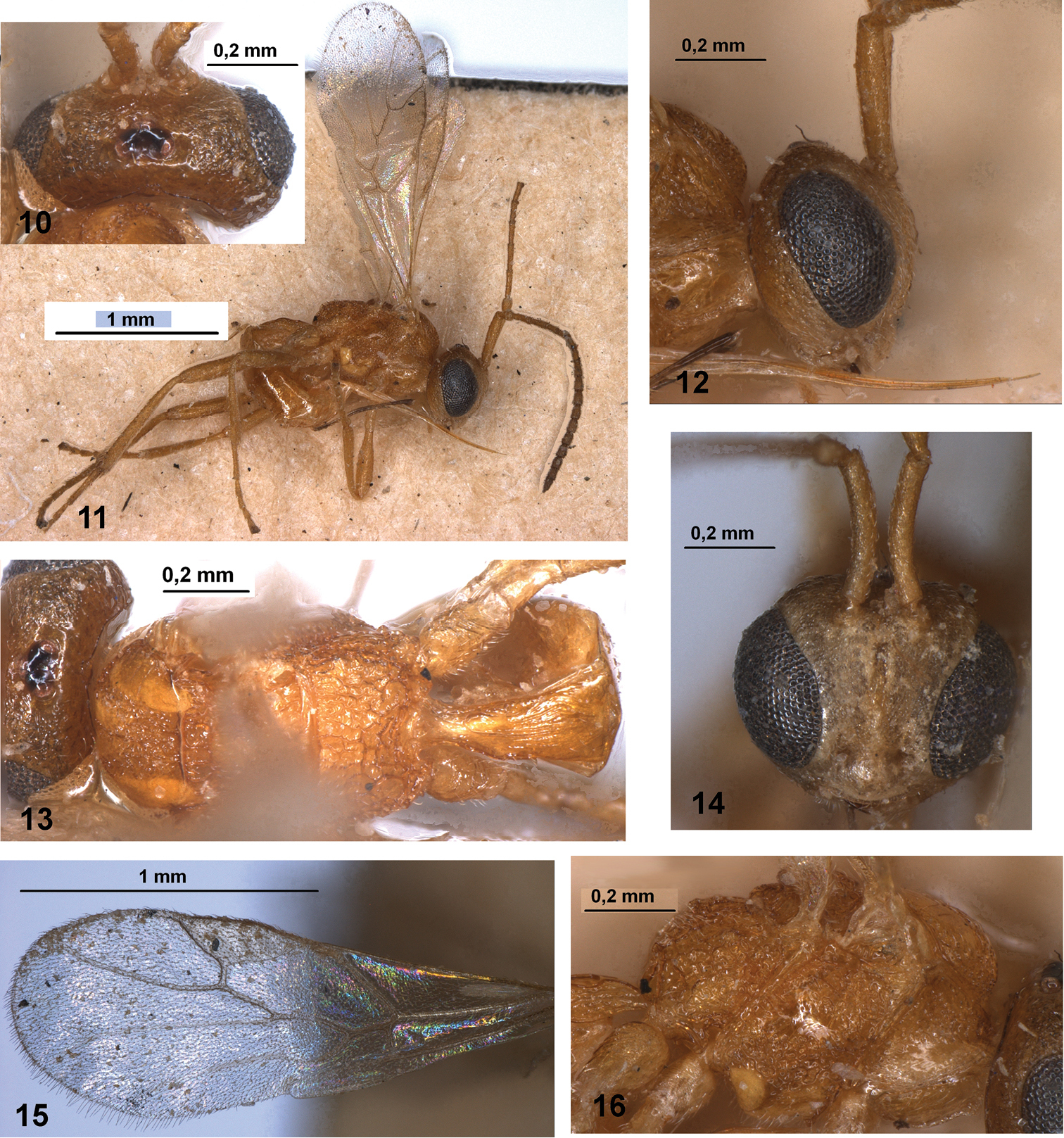
Ecclitura primoris Kokujev (holotype, female, Turkmenistan) 10 head, dorsal view 11 habitus, dorsal view 12 head and scape of antenna, lateral view 13 mesosoma and first metasomal tergite, dorsal view 14 head and scape of antenna, front view 15 fore wing 16 mesosoma, lateral view.
Ecclitura primoris Kokujev (female, Italy) 17 head and scape of antenna, front view 18 first metasomal tergite, dorsal view 19 antenna, lateral view 20 basal part of antenna 21 head and mesosoma, dorsal view 22 scape of antenna, dorso-lateral view 23 mesosoma, lateral view 24 head, mesosoma and first metasomal tergite, dorso-lateral view 25 fore tarsus 26 tip of ovipositor, lateral view 27 tip of ovipositor, dorsal view.
Western Palaearctic, Nearctic, Oriental Regions.
Turkmenistan: female (holotype), “K.S. Anger”, “Askhabad, 8. VI. 98, on lamp”, “Ecclitura Megaura primoris ♀ Kok.” (ZISP). Italy: 4 females, Campania, Torre del Greco, (NA), MT, 18–30.VII.2007 (Guerrieri leg.); 7 females, Pisa, Ceppaiano Crespina, MT, 28.VI–12.VII, 12–24.VII, 24.VII–09.VIII, 04–20.IX, 20.IX–04.X.2012 (A. Loni leg.); 7 females, Pisa, Poggio a Casone Crespina, MT, 12–24.VII, 24.VII–09.VIII, 09.VIII–04.IX, 20.IX–04.X.2012 (A. Loni leg.). Turkey: 1 female, “TR Edirne, Trakya, Univ. Biyoloji Bol. (Noct.) 16.9.1999”
Female. Body length 2.3–2.5 mm; fore wing length 1.9–2.2 mm.
Head. Width of head 2.1–2.3 times its median length, 1.4–1.5 times width of mesoscutum. Head behind eye strongly and weakly-curvedly narrowed. Length of eye (dorsal view) 1.8–2.0 times longer than temple. POL 1.0–1.5 times Od, 0.3–0.4 times OOL. Minimum width of face 0.70–0.75 times its height, 0.5–0.6 times its maximum width at level of antennal toruli or level of upper margins of eyes, almost equal to width of clypeus, about 0.9 times minimum (transverse) diameter of eye. Maximum diameter of eye 1.5–1.6 times its minimum diameter. Malar space very short, 0.10–0.13 times maximum diameter of eye, 0.4–0.5 times basal width of mandible. Distance between tentorial pits 2.5–3.0 times distance from pit to eye. Width of cly-peus 1.9–2.0 times its median height.
Antennae 17-segmented, weakly claviform. Scape 1.0–1.2 times as long as height of face, 5.5–6.5 times longer than maximum width anteriorly, 4.0–5.0 times longer than maximum width laterally. Pedicel 1.3–1.5 times longer than wide. First flagellar segment 4.0–5.0 times longer than its maximum apical width, about as long as second segment. Penultimate segment 1.5–1.8 times longer than wide, 0.50–0.55 times as long as first segment, 0.5–0.8 times as long as apical segment.
Mesosoma. Length 1.4–1.5 times its median width. Mesoscutum highly and subvertically elevated above pronotum. Scutellum distinctly convex. Prescutellar depression long, with high median and often two to four lateral carinae, finely rugulose between carinae, depression about 0.3 times as long as scutellum. Scutellum 1.1–1.3 times longer than its anterior width. Subalar depression shallow, rather wide, densely rugulose-reticulate. Sternaulus complete, shallow, curved, coarsely rugulose-reticulate. Posterior mesopleural furrow (along mesopleural suture) coarsely crenulate.
Wings. Length of fore wing 2.7–2.8 times its width. Metacarp (inside radial cell) 0.7–0.9 times as long as pterostigma, 2.0–2.4 times longer than distance between apex of radial cell and apex of wing. Radial cell 2.4–2.5 times longer than maximum width. First radial abscissa about 0.5 times as long as maximum width of pterostigma. First radiomedial vein weakly sinuate or straight, 2.5–3.0 times longer than first radial abscissa, 0.3–0.4 times as long as second radial abscissa, 0.7–0.9 times as long as recurrent and second abscissa of medial veins combined. Distance between nervulus and basal vein 0.5–0.8 times nervulus length. Parallel vein basally sclerotised and weakly curved, unsclerotised its most apical part. Hind wing 3.8–4.1 times longer than maximum width.
Legs. Hind femur 4.9–5.5 times longer than maximum width. Hind tarsus almost as long as hind tibia. Hind basitarsus 0.6–0.7 times as long as second-fifth segments combined. Second segment of hind tarsus 0.45–0.50 times as long as basitarsus, 1.15–1.25 times longer than fifth segment.
Metasoma. First tergite almost regularly and distinctly widened from base to apex, with fine spiracular tubercle in basal third. Apical width of first tergite 2.3–2.8 times its minimum width; length of tergite 1.8–2.0 times its apical width. Length of second and third tergites combined 1.1–1.3 times basal width of second tergite, 0.9 times their maximum width. Ovipositor sheath 0.6–0.7 times as long as metasoma, 1.4–1.5 times longer than first tergite, 0.7–0.8 times as long as mesosoma, and 0.30–0.35 times as long as fore wing.
Sculpture and pubescence. Head mainly and densely rugose-reticulate with granulation, vertex sometimes almost smooth posteriorly. Mesoscutum smooth in anterior half of median lobe and on wide median areas of lateral lobes, rugose-reticulate wide along notauli and rugose-striate on wide area in medioposterior half. Scutellum almost entirely distinctly rugose-reticulate, partly with granulation, sometimes smooth or almost smooth on medio-anterior third or anterior half. Mesopleuron mainly reticulate-rugulose, smooth or almost smooth medially on rather small area. Propodeum without areas, coarsely and rather densely reticulate-areolate, with fine rugulosity. Hind femur densely and rather distinctly rugulose-granulate. First metasomal tergite densely striate in basal 0.6–0.8, smooth in apical 0.2–0.4. Remaining tergites entirely smooth. Mesoscutum glabrous on smooth areas, coved by short setae on sculptured areas. Face densely and very shortly setose.
Colour. Body mainly brownish yellow or yellow, metasoma often almost entirely yellow. Antennae yellow, infuscate in apical half. Palpi yellow. Legs yellow, all legs brownish. Ovipositor sheath almost black. Wings hyaline. Pterostigma entirely yellow.
Male. Unknown.
Tajikistan, Turkmenistan, Iran, Azerbaijan, Russia (Dagestan), Turkey (first record), Albania, Italy (first record).
This work was partly supported by the grant of the research project within the framework of the Scientific Cooperation Agreement between CNR (Italy) and RAS (Russia) for first and fourth authors, grant of the Russian Foundation for Basic Research (No. 13-04-00026) for the first author and by Fondi di Ateneo 2012 (Pisa University) for the second and third authors. We are very thankful to Dr Emilio Guerrieri (Portici) for his interesting material collected in Campania and to Dr. Piergiorgio Castellani for hosting our surveys in his farm of Crespina (Pisa).