(C) 2013 Ming Zhang. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Agria mihalyii (Rohdendorf and Verves, 1978) is recorded from China for the first time, and both sexes are thoroughly documented using a combination of illustrations, photographs and scanning electron microscopy images. The generic affiliation is corroborated from an expanded definition of genus Agria Robineau-Desvoidy, 1830, and a key to males of the two known species from China is provided. The distribution of coeloconic sensilla on the male pre- and postgonite are shown to possess significant diagnostic and phylogenetic information in this genus.
Paramacronychiinae, Agria, coeloconic sensilla, new record, China
The genus Agria Robineau-Desvoidy is a small genus of the subfamily Paramacronychiinae (Sarcophagidae), occurring in the Holarctic region and comprising eight species worldwide (
Before the present contribution, only Agria affinis (Fallén, 1817) was known from China (
The specimens examined were collected by sweeping from brushwood in mountainous regions and are deposited in the Museum of Beijing Forestry University, Beijing, China.
Methods for the preparation of terminalia, illustrations, photographs and scanning electron microscopy images follow
Terminology of male morphology and terminalia follows
http://species-id.net/wiki/Agria_affinis
Figs 1 and 7China: Beijing: 1 ♂, Xiaolongmen, 39°57'50"N, 115°28'26"E, 1100 m, 6.VII.2009, Coll. R. Bi and F. Li; 1 ♂, Mt. Songshan, 40°30'00"N, 115°49'12"E, 800−1000 m, 30.V.2012, Coll. Y.O. Chen; 1 ♂, Mt. Songshan, 40°30'00"N, 115°49'12"E, 800−1000 m, 30.V.2012, [collector unknown].
China (Beijing, Qinghai, Xinjiang); Mongolia; Kazakhstan; Kyrgyzstan; common throughout Europe.
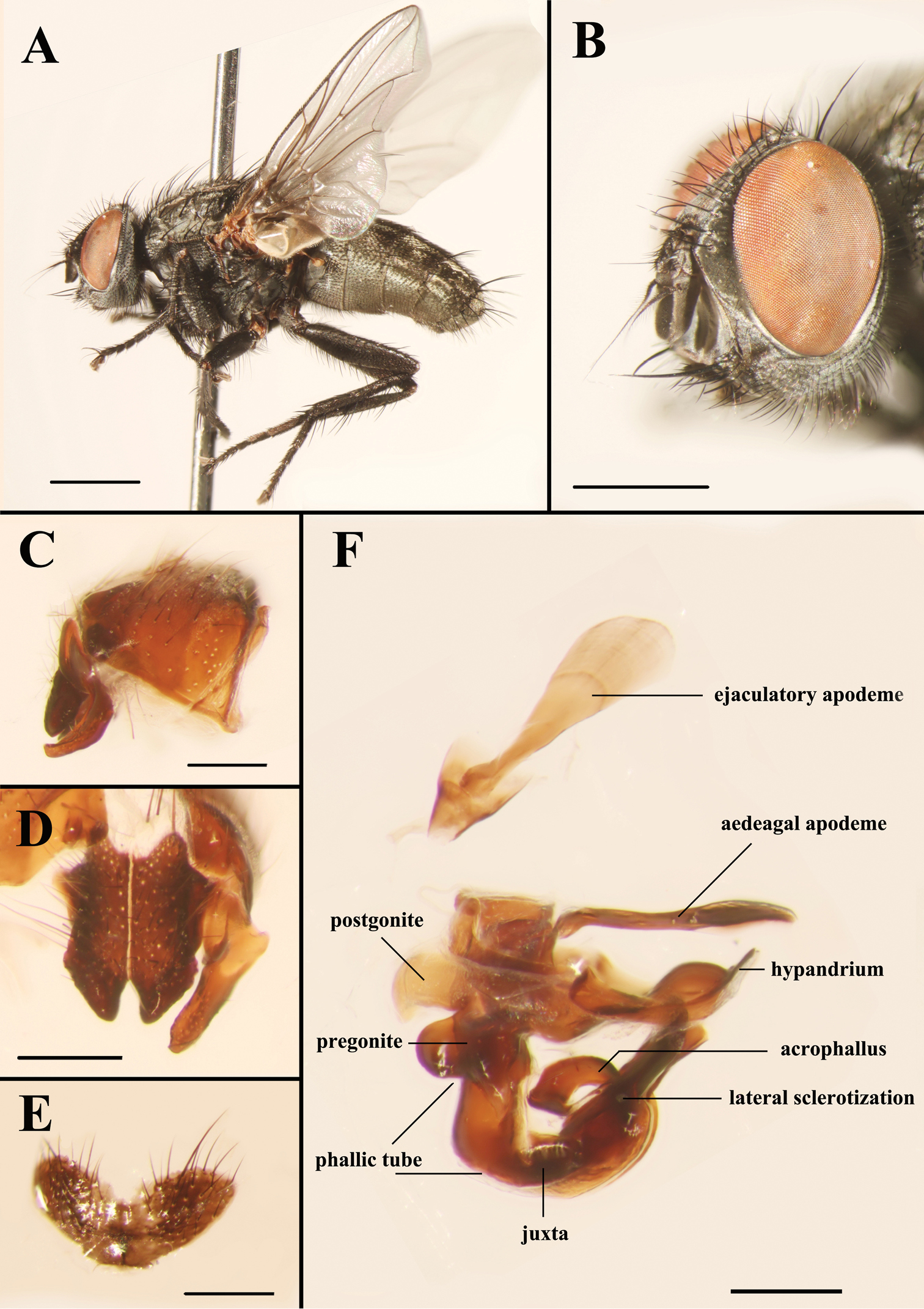
Light micrographs of the male Agria affinis (Fallén, 1817). A Habitus, lateral view B Head, anterolateral view C Terminalia, epandrium, surstylus and cercus, lateral view D Surstylus and cerci, dorsal view E Sternite 5, ventral view F Genitalia, lateral view. Scale bars: A= 2.00 mm, B= 1.00 mm, C−F= 0.25 mm.
http://species-id.net/wiki/Agria_mihalyii
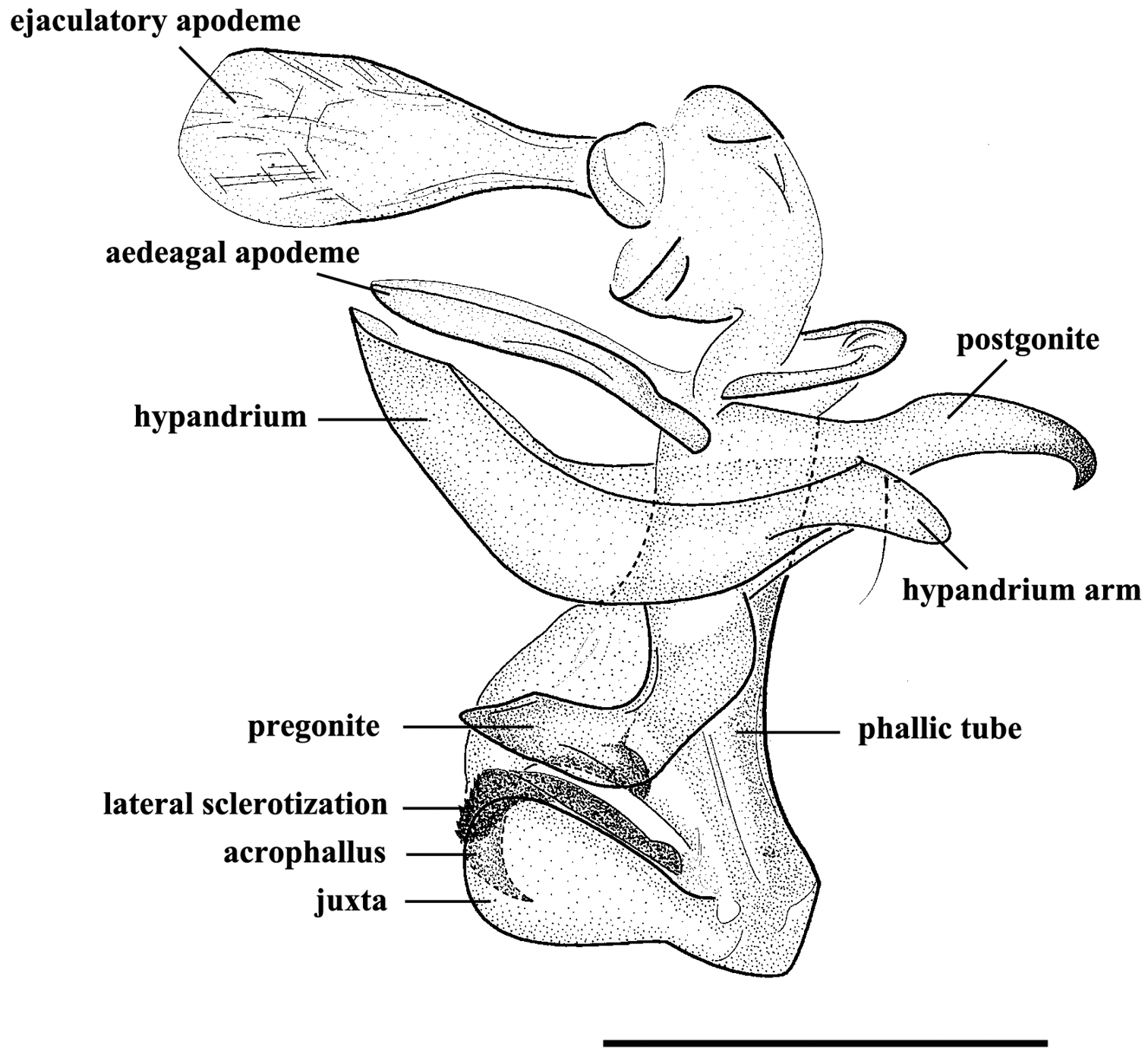
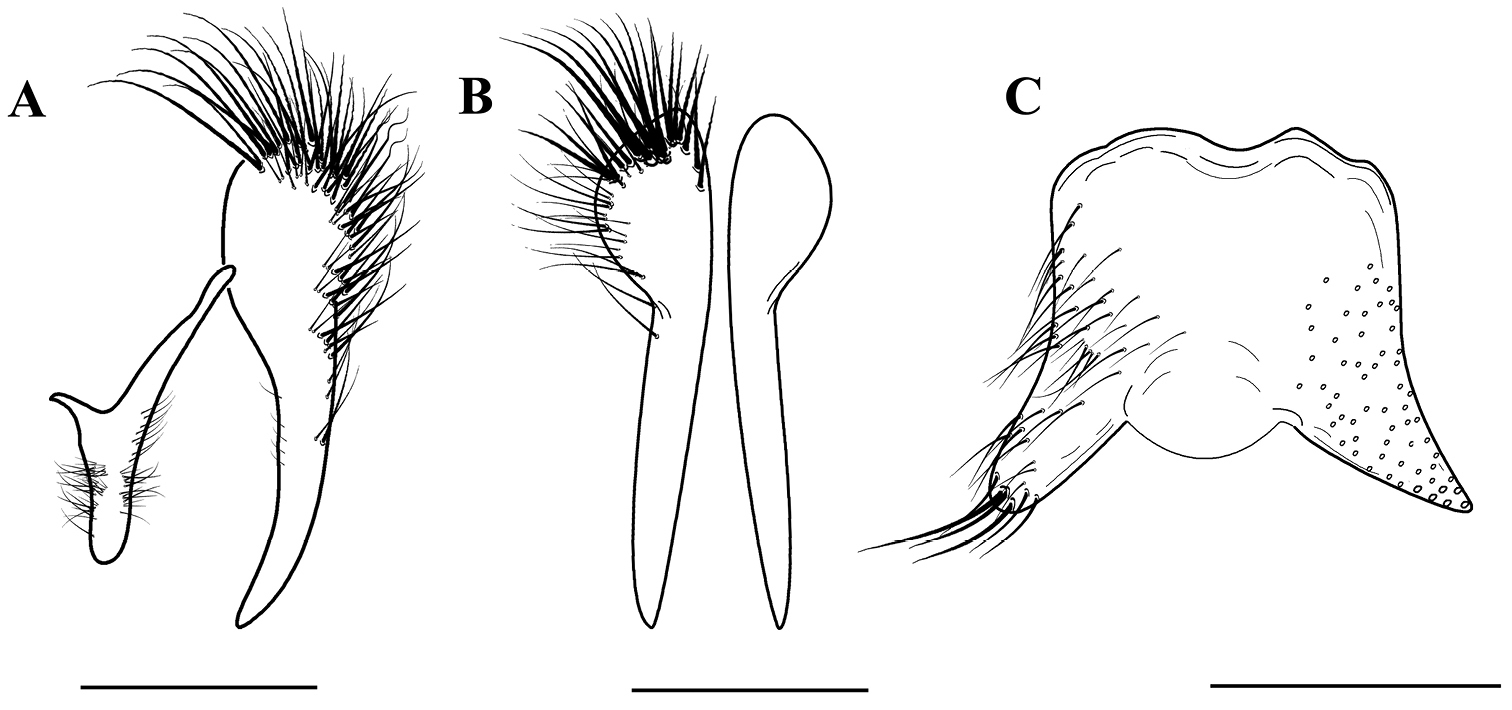
Figs 2−6, 8 and 9MALE. Body length 7.8−10.4 mm. Eyes bare. Fronto-orbital and parafacial plates black with grey pollinosity; postocular strip bare and with silvery grey pollinosity; parafacial plate and fronto-orbital plate with rows of fine bristles. Frontal vitta black, 2.10 × as broad as fronto-orbital plate at the narrowest point; frons at vertex 0.30 × head width; frontal row of 9−12 strong bristles; outer vertical bristle not differentiated from postocular bristles, upper orbital bristle one. One pair of strong ocellar bristles. Gena ground color black, with sparse and short black bristles and silvery grey pollinosity, height 0.47 × eye height in lateral view. Antenna slightly reddish basally, otherwise blackish, not reaching the level of vibrissal insertion, first flagellomere 1.70 × as long as pedicel; arista black brown, short plumose in basal 3/5–2/3. Palpus orange. Thorax ground color black; scutum with three black dorsal vittae. Chaetotaxy: acrostichals 0+1, dorsocentrals 2(3)+3, intra-alars 1(0)+2(3), supra-alars 2, postpronotals 3, postalars 3 or 4, notopleurals 2, scutellum with 1 apical, 1 subapical, 1 basal and 1 discal bristles, with or without prebasal bristle. Pleuron with meropleurals 8−10, katepisternal bristles 2(3)+1, prosternum and metasternum bare, proepisternum bare, proepimeron in lower part with fine bristles, postalar wall bare or with fine bristles. Wing hyaline; subcostal sclerite and basicosta yellow, bare; tegula dark yellow, with black setulae; costal spine not differentiated, several dorsal black bristles at node of R4+5-R2+3. Legs dark; fore femur with one strong row of posterior bristles, and with long and dense bristles along anteroventral, ventral and posteroventral margins, fore tibia with four anterodorsal and one posterior bristles; mid femur with two anterior and two posterior bristles, and distal 1/3 with short ventral comb-like posteroventral bristles, mid tibia with two or three anterior and one or two hair-like posterior bristles; hind femur with one row of anterodorsal bristles, and with long and dense bristles along anteroventral, ventral and posteroventral margins, hind tibia with one posterodorsal bristle, one row of anteroventral bristles (7 or 8) and one row of anterodorsal bristles (3 or 4). Abdomen long oval with densely grey pollinosity; tergites each with three distinct black spots; tergite 3 without median marginal bristles, tergite 4 with one pair of median marginal bristles, tergite 5 with strong marginal bristles, tergites 7+8 form a hump-shaped structure, epandrium brownish black, sternites 1−4 with long and dense bristles. Terminalia: Cercus tapering and pointed distally, basal 1/3 with long dense bristles; surstylus long and with oval rounded tip in lateral view (Fig. 5A). Ejaculatory apodeme large (Figs 4 and 8B). Pregonite broad, longer than postgonite, with some fine bristles on the basal part (Fig. 8C), and distal half perpendicular to basal half (Figs 4, 8A, 8C, 9A and 9D); postgonite broad with curved tip and a strong bristle proximally on anterior margin, six coeloconic sensilla (2.10 µm in height, 1.68 µm in width at the base and 1.20 µm at the middle, and originating from a cuticular ring inside a shallow depression) distributed on distal half (Figs 8E and 8F); juxta very large and shell-shaped, apically with a pair of slanting processes covering most of the acrophallus in lateral view (Figs 4, 8A and 9A−C); phallic tube broad, with the dorsal part dark; acrophallus very broad basally, the distal part strongly tapering and recurving between the juxta (Figs 4, 9B and 9C); lateral sclerotizations short, with a serrated distal margin (Figs 4 and 9C).
FEMALE. Body length 7.0−9.0 mm. Frons at vertex 0.40 × as broad as head width; frontal row of 9 or 10 bristles; outer vertical bristle differentiated from postocular bristles, proclinate orbital bristles two. Gena height 0.40 × eye height in lateral view. First flagellomere length 1.40 × as long as pedicel. Thorax chaetotaxy: acrostichals 0+2, intra-alars 1+2. Fore femur with one posterior, one posterodorsal and one posteroventral rows of bristles; mid femur with short and sparse ventral bristles, without apical comb-like posteroventral bristles, mid tibia with two posterodorsal and two posterior bristles, one strong ventral bristle; hind tibia with two or three posterodorsal bristles, one anteroventral bristle. Abdomen oval; tergites 5 and 6 entire, tergite 7 membranous like with several bristles on the anterior margin, tergite 8 divided into two plates and each with two strong bristles (Fig. 6E); sternites 1−6 without long and dense bristles (Fig. 6A); epiproct as a single setose plate, hypoproct and sternite 8 sclerotized (Fig. 6E). Other morphological characteristics are the same as for the male.
China: Shanxi: 3 ♂♂, Tianzhen county, 40°24'00"N, 114°6'00"E, 1600−1700 m, 24.V.1987, Coll. M.F. Wang; 1 ♂, Yuxian county, Mt. Zangshan, 38°6'00"N, 113°24'00"E, 900−1000 m, 23.VI.1999, Coll. M.F. Wang. Beijing: 1 ♂, Mt. Songshan, 40°30'00"N, 115°49'12"E, 800−1000 m, 5.VII.2008, [collector unknown]; 1 ♂, Mt. Songshan, 40°30'00"N, 115°49'12"E, 800−1000 m, 5.VI.2009, [collector unknown]; 1 ♂, Mt. Songshan, Daxigou, 40°31'30"N, 115°46'19"E, 1200 m, 25.VII.2009, Coll. D. Zhang; 1 ♀, Mt. Songshan, Changyugou, 40°30'00"N, 115°48'57"E, 800 m, 28.VII.2009, Coll. D. Zhang; 2 ♂♂, Mt. Songshan, 40°30'00"N, 115°49'12"E, 800−1000 m, 29.VII.2010, [collector unknown];1 ♂, Mt. Songshan, 40°30'00"N, 115°49'12"E, 800−1000 m, 30.V.2012, Coll. Y.O. Chen.
China (Beijing, Shanxi); Mongolia; North Korea; Russia (East Siberia, Far East, West Siberia); Ukraine.
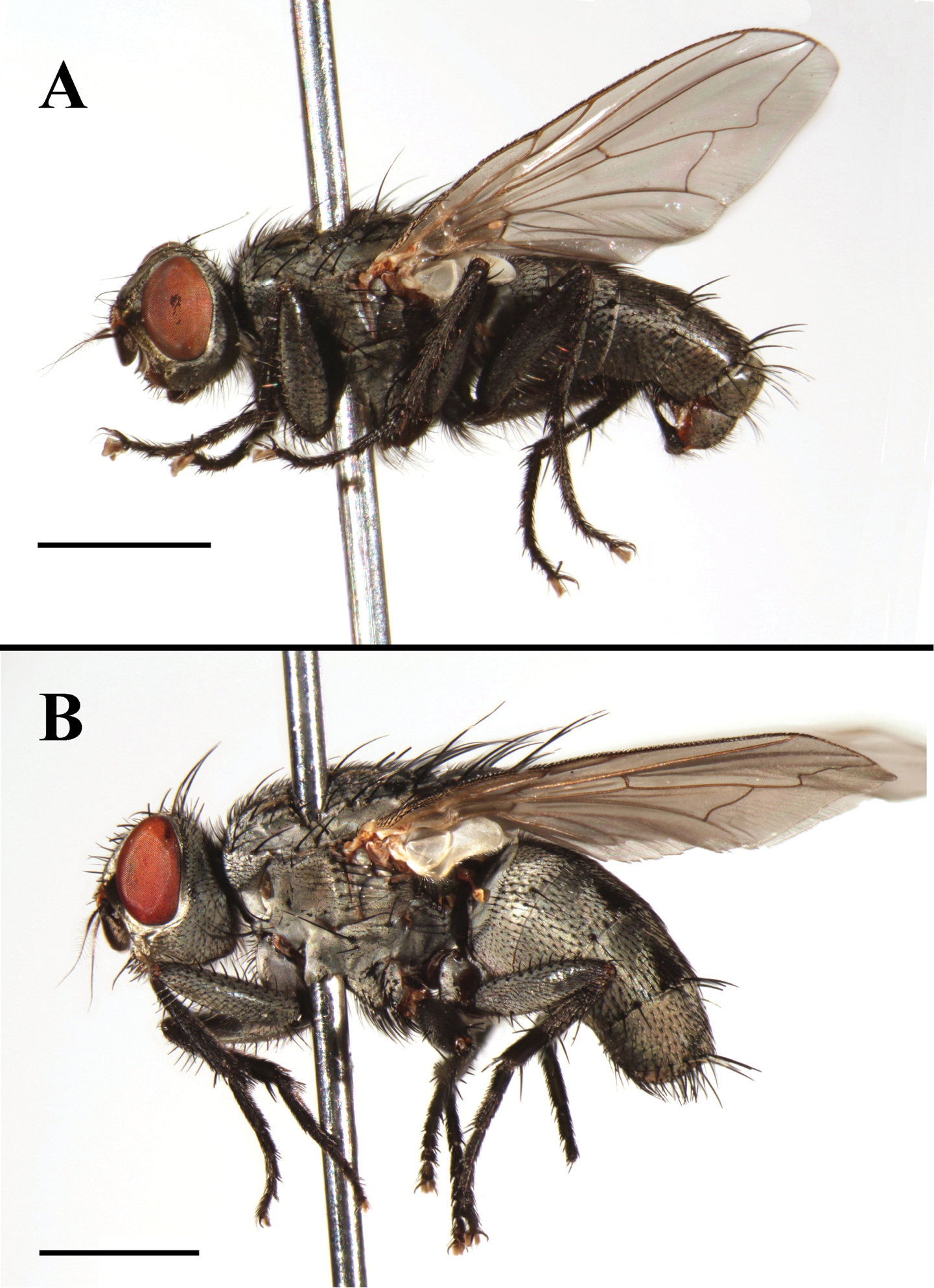
Agria mihalyii (Rohdendorf and Verves, 1978). A Male habitus, lateral view B Female habitus, lateral view. Scale bars: A and B= 2.00 mm.
Agria mihalyii (Rohdendorf and Verves, 1978). A Male head, left anterolateral view B Female head, left anterolateral view C Male abdomen, dorsal view D Female abdomen, dorsal view. Scale bars: A−D= 1.00 mm.
Agria mihalyii (Rohdendorf and Verves, 1978). Male, genitalia, lateral view. Scale bar = 0.50 mm.
Agria mihalyii (Rohdendorf and Verves, 1978). Male, terminalia. A Cercus and surstylus, lateral view B Cerci, dorsal view C Sternite 5, ventral view. Scale bars: A−C = 0.50 mm.
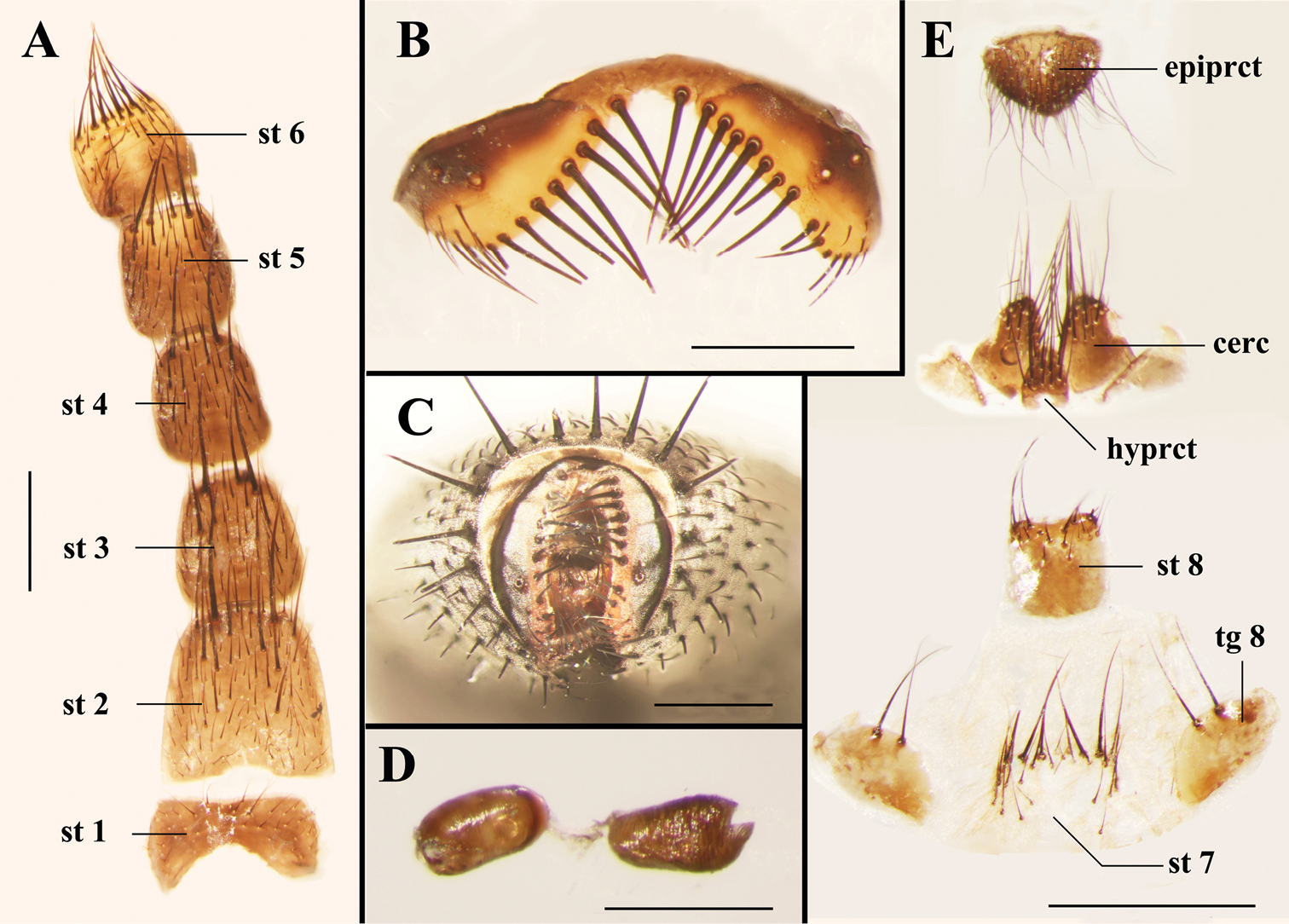
Light micrographs of the female terminalia of Agria mihalyii (Rohdendorf and Verves, 1978). A Sternites 1−6, ventral view B Tergite 6, dorsal view C Terminalia, posterior view D Spermathecae E Terminalia, ventral view. Scale bars: A−C and E= 0.50 mm, D= 0.25 mm. Abbreviations: cercus (cerc); epiproct (epiprct); hypoproct (hyprct); sternite (st); tergite (tg).
Scanning electron micrographs of the male genitalia of Agria affinis (Fallén, 1817). A Lateral view of the genitalia B Ventral view of distiphallus, arrows show the dividing line between phallic tube and juxta C Surstylus, lateral view D Cercus, lateral view E Pregonite and base of postgonite enlarged view, with inset showing highly enlarged view of one of the sockets from a coeloconic sensilla. Abbreviations: acrophallus (acr); cercus (cerc); juxta (j); lateral sclerotization (ls); phallic tube (ph); postgonite (pog); pregonite (prg); surstylus (sur).
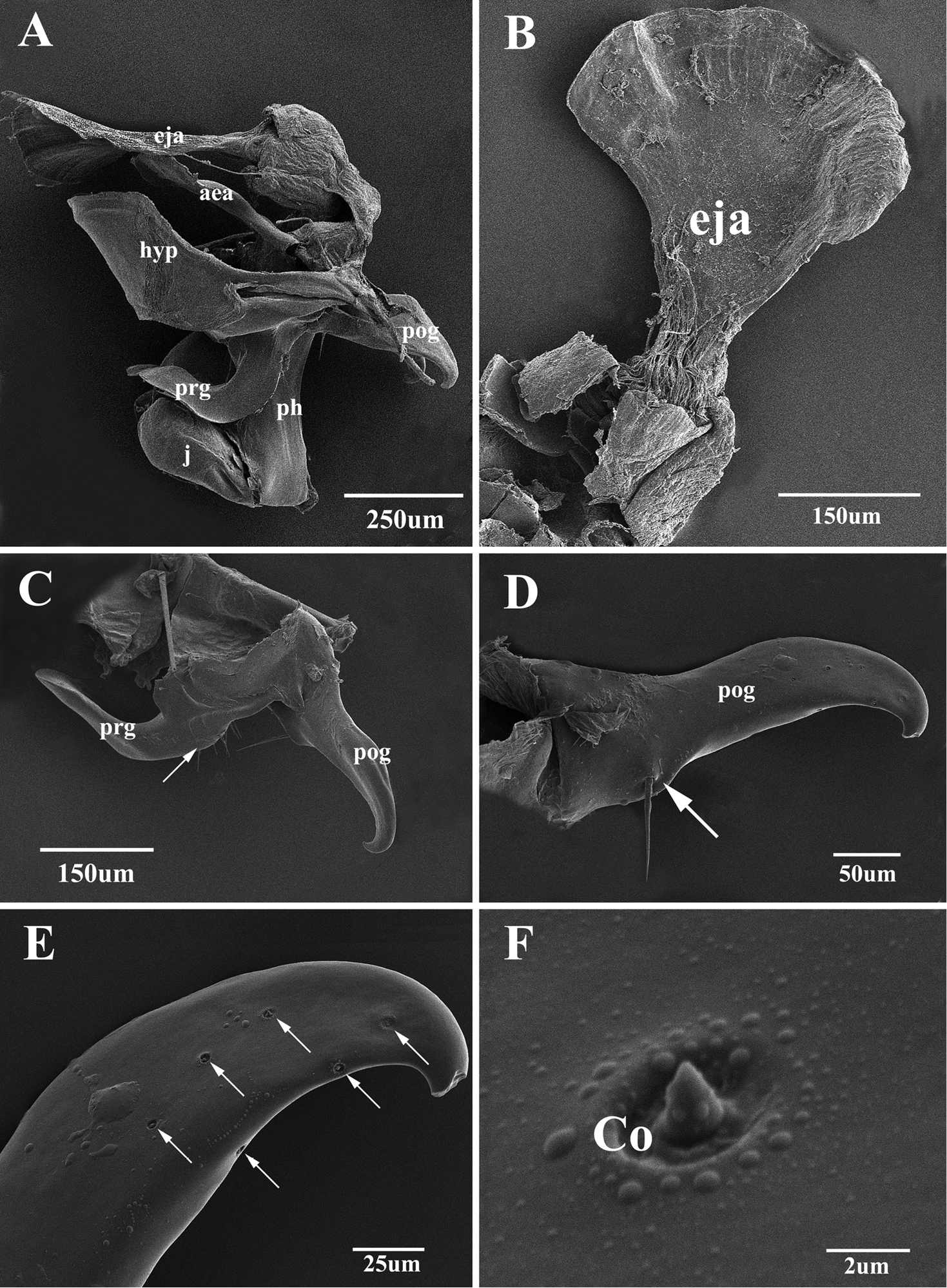
Scanning electron micrographs of the male genitalia of Agria mihalyii (Rohdendorf and Verves, 1978). A Lateral view of the entire genitalia B Ejaculatory apodeme C Pregonite and postgonite, the former with some fine bristles at the dorso-basal edge (arrow) D Postgonite, with one well developed bristle (arrow) near base of anterior margin E Distal half of postgonite (extreme tip broken), arrows show the distribution of coeloconic sensilla F Coeloconic sensilla on the postgonite. Abbreviations: aedeagal apodeme (aea); coeloconic sensillum (Co); ejaculatory apodeme (eja); hypandrium (hyp); juxta (j); phallic tube (ph); postgonite (pog); pregonite (prg).
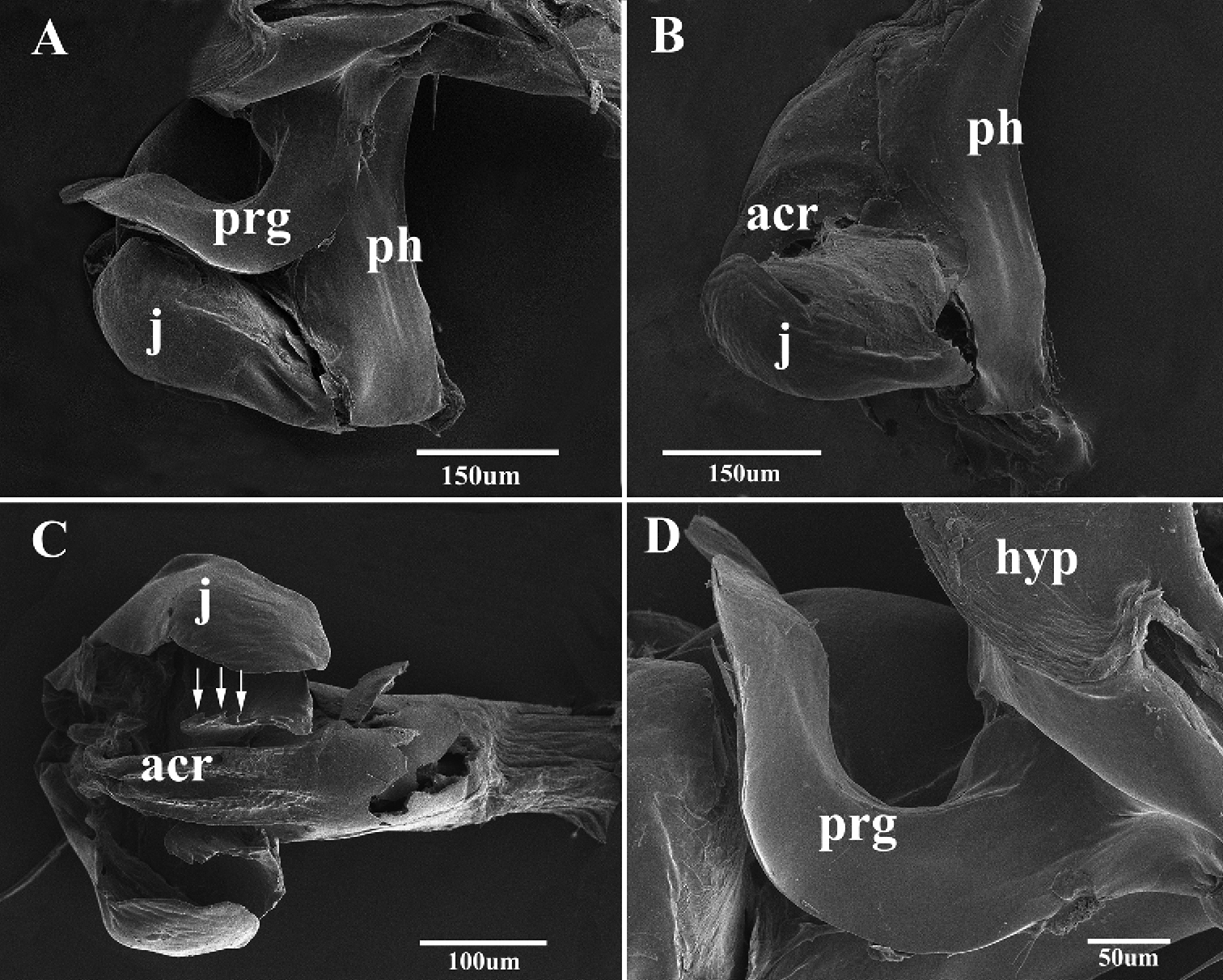
Scanning electron micrographs of the male genitalia of Agria mihalyii (Rohdendorf and Verves, 1978). A Lateral view of the genitalia B Lateral view of distiphallus C Acrophallus in anterior view, apex of the lateral sclerotizations with serrated margin (arrows) D Pregonite, enlarged view. Abbreviations: acrophallus (acr); hypandrium (hyp); juxta (j); phallic tube (ph); postgonite (pog); pregonite (prg).
| 1 | Frons at vertex 0.30 × as broad as head width; antenna slightly reddish basally, palpi orange (Fig. 3A); cerci long, surstylus long and with oval rounded tip (Fig. 5A); pregonite long, distal half perpendicular to basal half (Figs 4 and 9D), postgonite elongate and with a bristle near base of anterior margin (Figs 4, 8D and 8E); lateral sclerotizations short and with serrated distal margin (Figs 4 and 9C); juxta large (Figs 4 and 9A) | Agria mihalyii (Rohdendorf and Verves) |
| – | Frons at vertex 0.16 × as broad as head width; antenna black, palpi at least distally black (Fig. 1B); cerci short, surstylus with apex bent posteriorly (Figs 1C and 1D); pregonite short and strong, almost triangular, postgonite broad and short, without a bristle near base of anterior margin (Fig. 7F); lateral sclerotizations long and without serration (Fig. 1F); juxta small, fused with phallic tube except for an obscure dividing line laterally (Figs 1E, 7A and 7B) | Agria affinis (Fallén) |
The present SEM documentation has revealed the presence of coeloconic sensilla on the distal half of the Agria mihalyii postgonite (Figs 8E and 8F) and on both pre- and postgonite in Agria affinis. This type of sensilla has been proposed to be sensitive to chemo-, thermo-, or hygro-stimulation (
We wish to extend our sincerest thanks to Prof. M.F. Wang (Institute of Entomology, Shenyang Normal University, Shenyang), for donating specimens, and we are thankful to two anonymous reviewers for their constructive comments on the manuscript. This study was supported by the Program for New Century Excellent Talents in University (No. NCET-12-0783), National Science Foundation of China (No. 31201741), and the Chinese Postdoctoral Science Foundation (No. 20100470009, No. SFG-201104059).